New Experimental Evidence for Drying Shrinkage of Alkali-Activated Slag with Sodium Hydroxide
Abstract
:1. Introduction
- -
- A study on the drying shrinkage in different relative humidity (RH) conditions and the autogenous shrinkage for different AAS compositions in comparison with OPC;
- -
- A study on the creep compliance for one of the compositions with different curing histories;
- -
- A study on the E-modulus, flexural strength, compressive strength, and carbonation for one of the compositions.
2. Drying and Autogenous Deformation and Mass Variation
2.1. Mortar Composition
2.2. Experimental Procedure
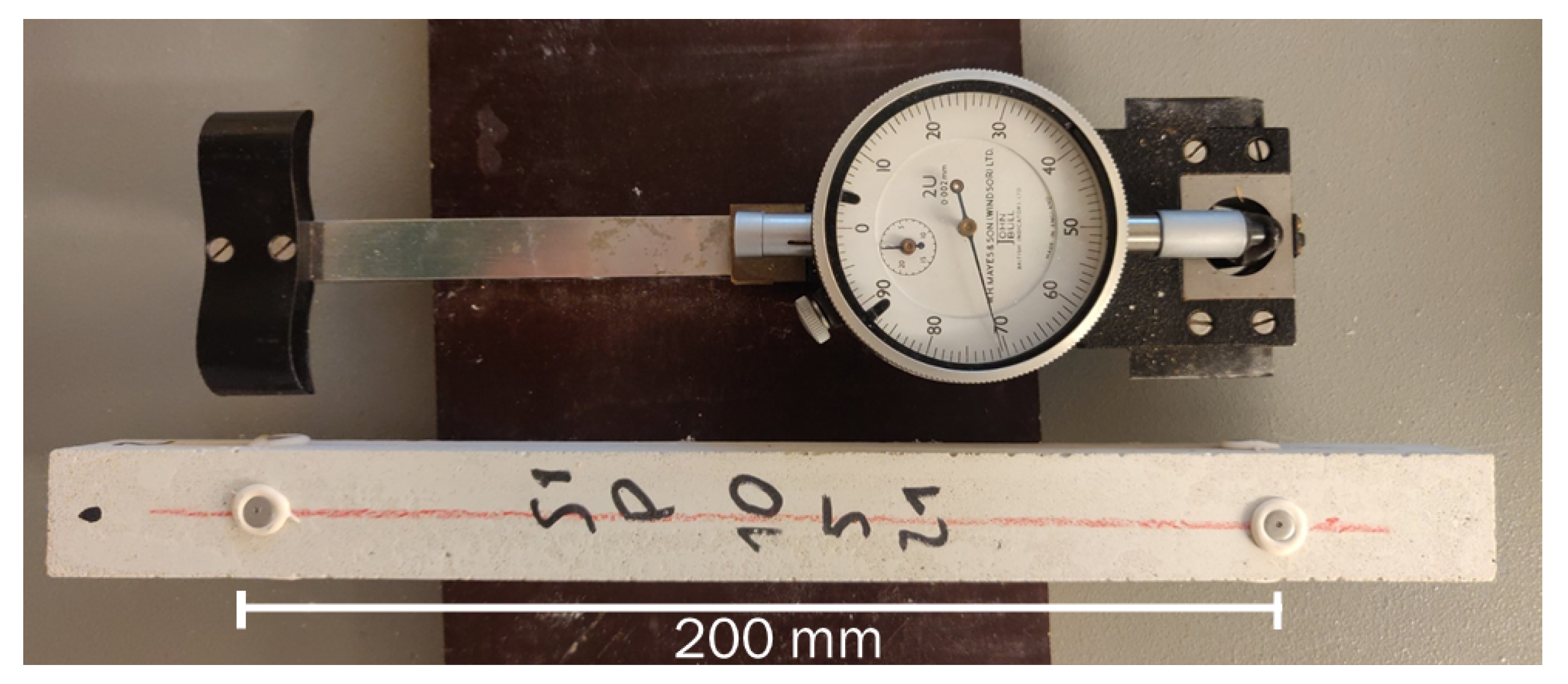
2.2.1. Drying Deformation
2.2.2. Autogenous Deformation
2.2.3. Mass Variation
2.3. Results and Discussion
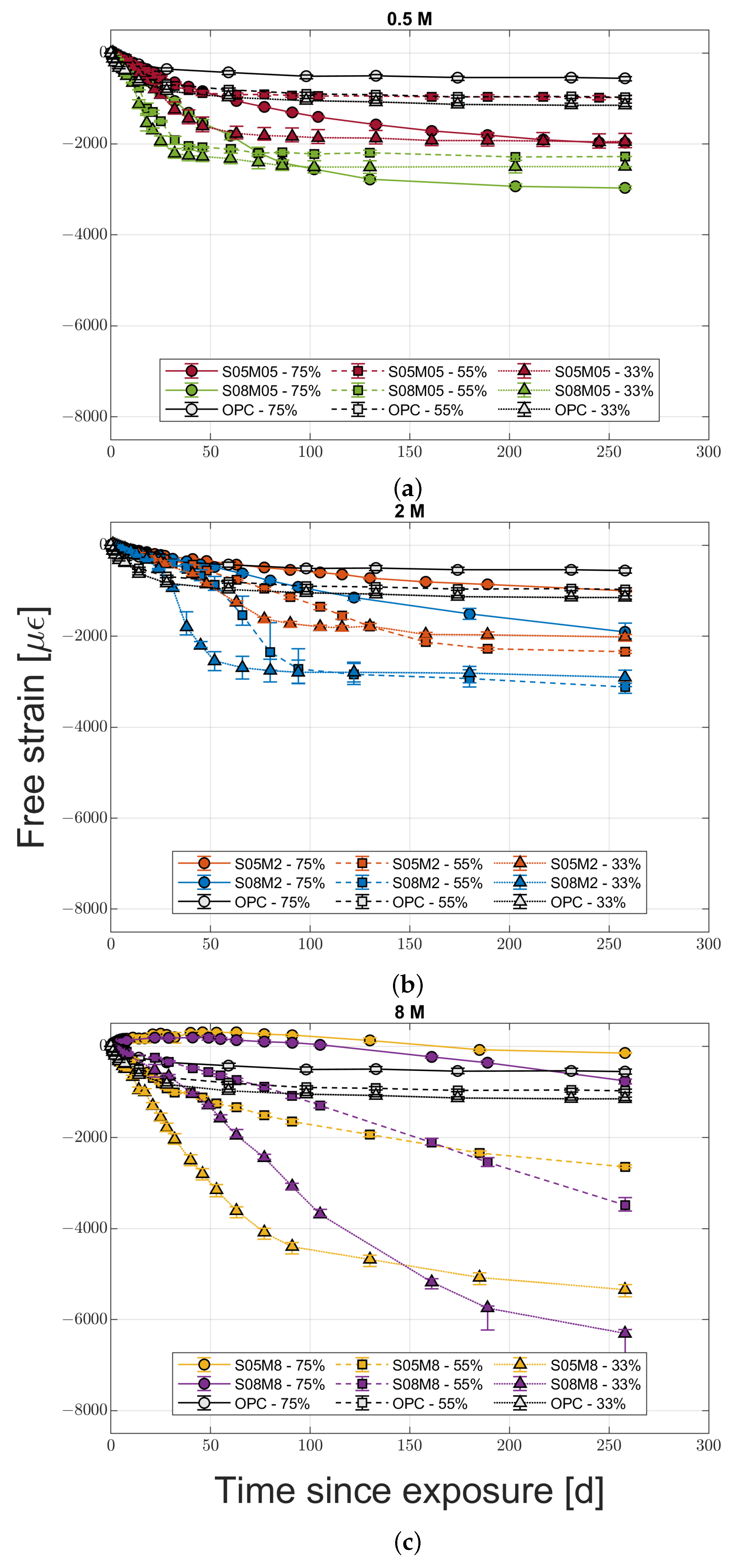
2.3.1. Drying Deformation
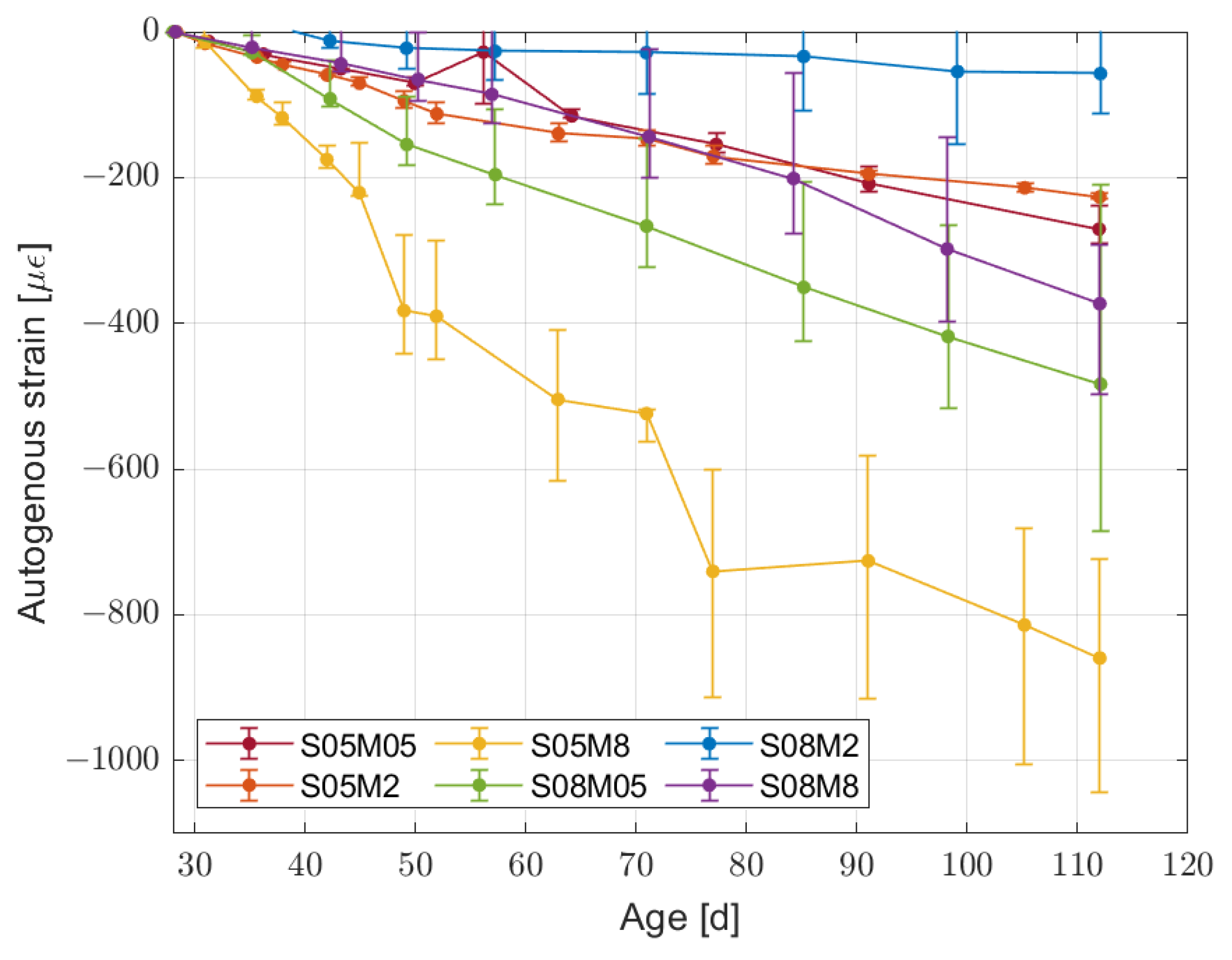
2.3.2. Autogenous Deformation
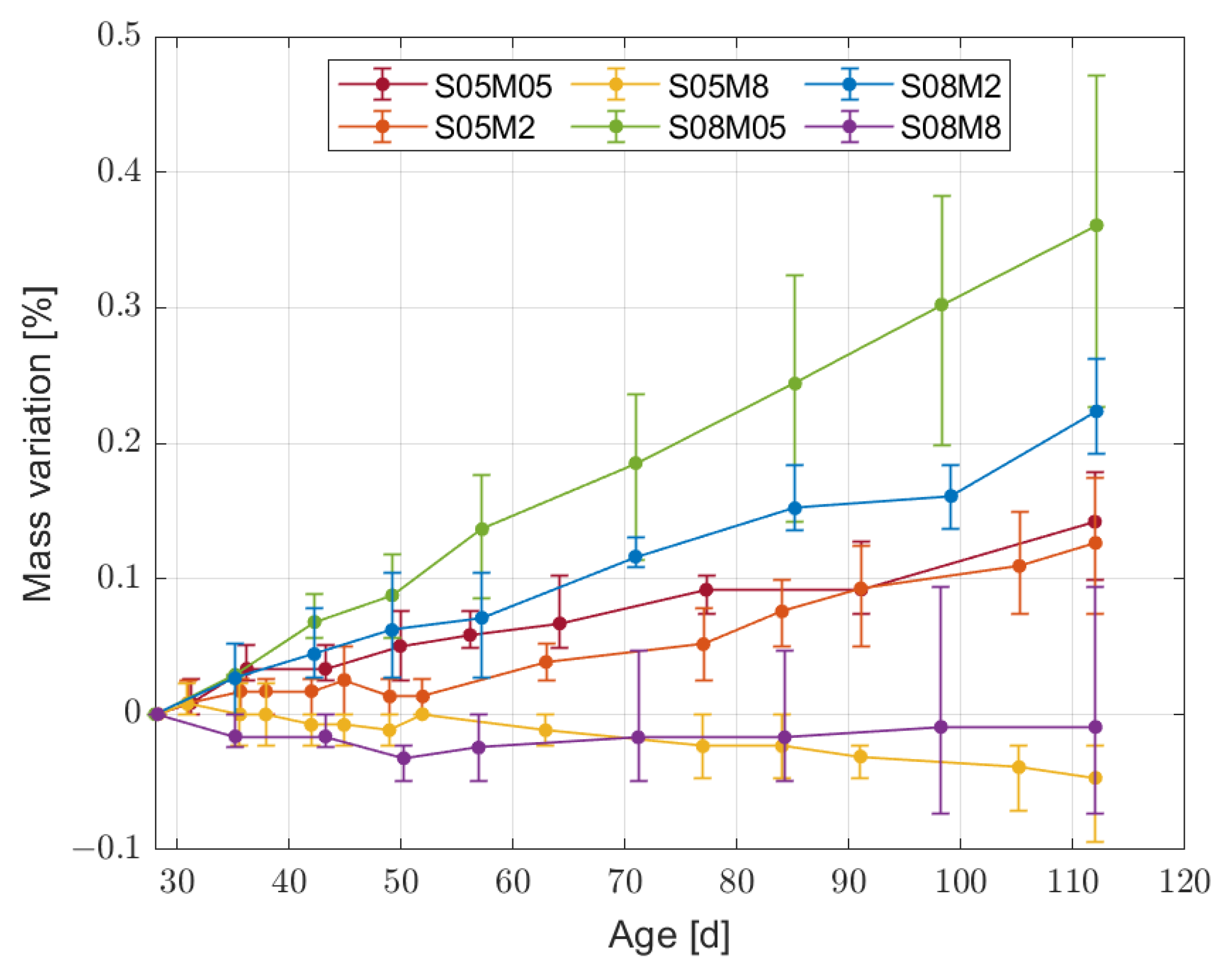
2.3.3. Mass Variation
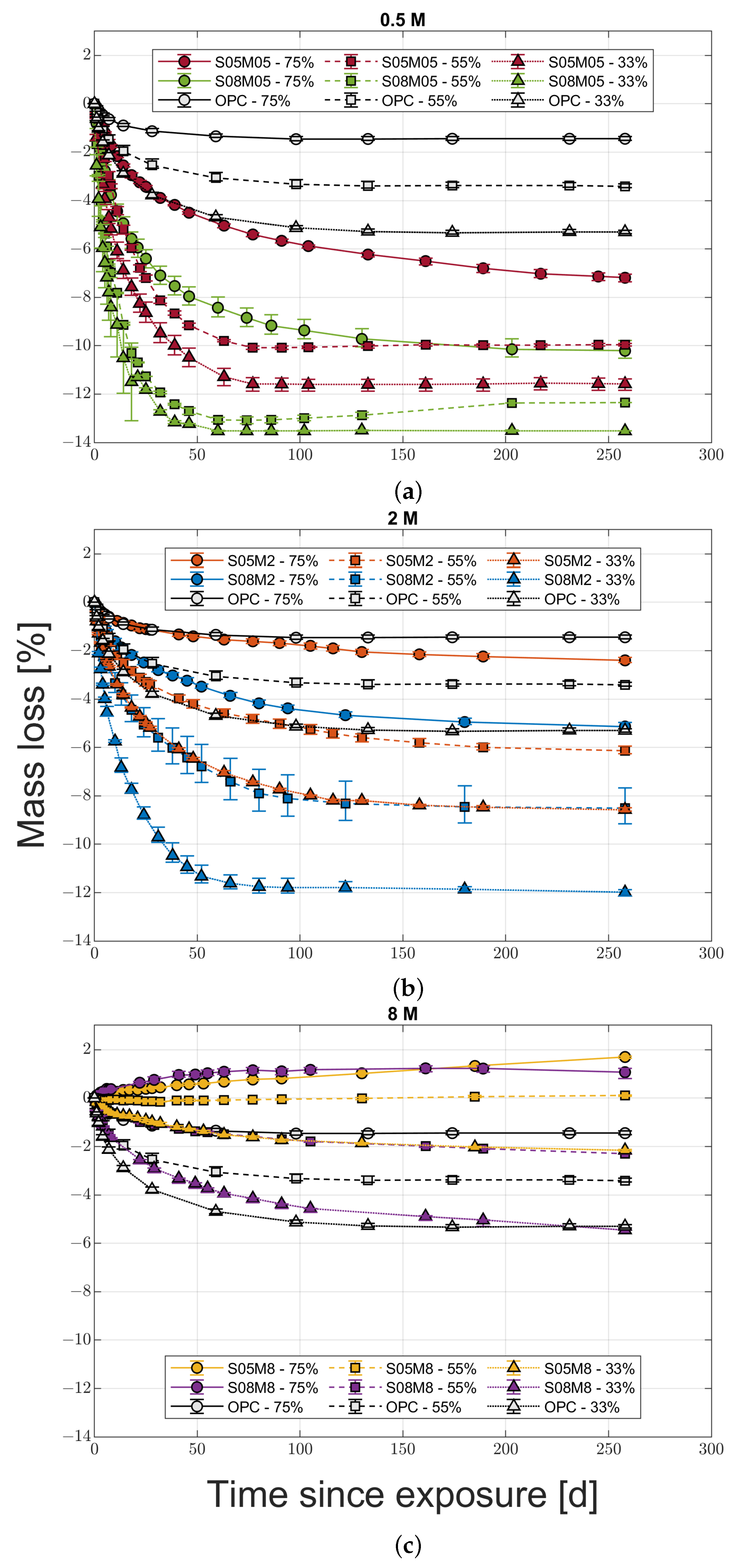
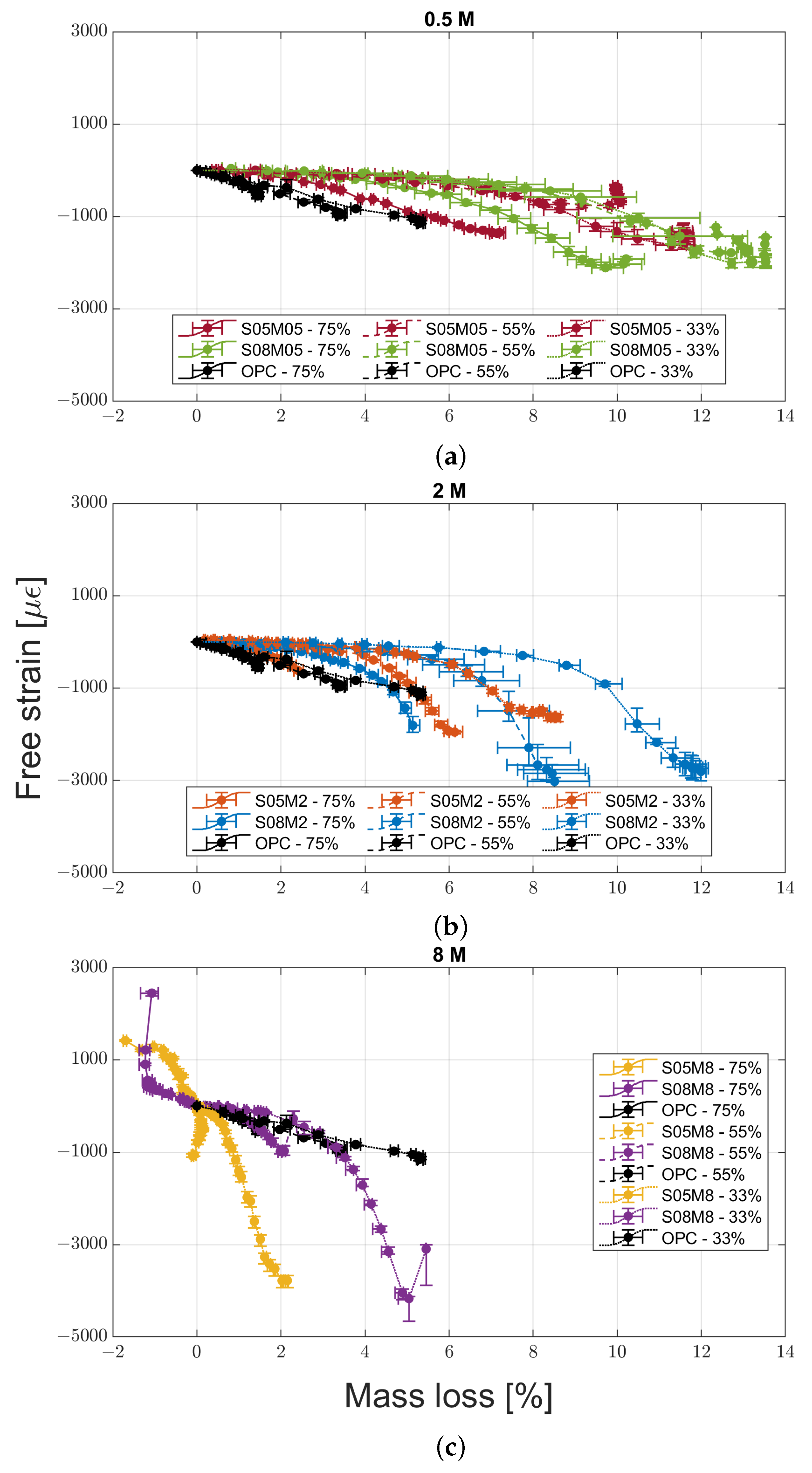
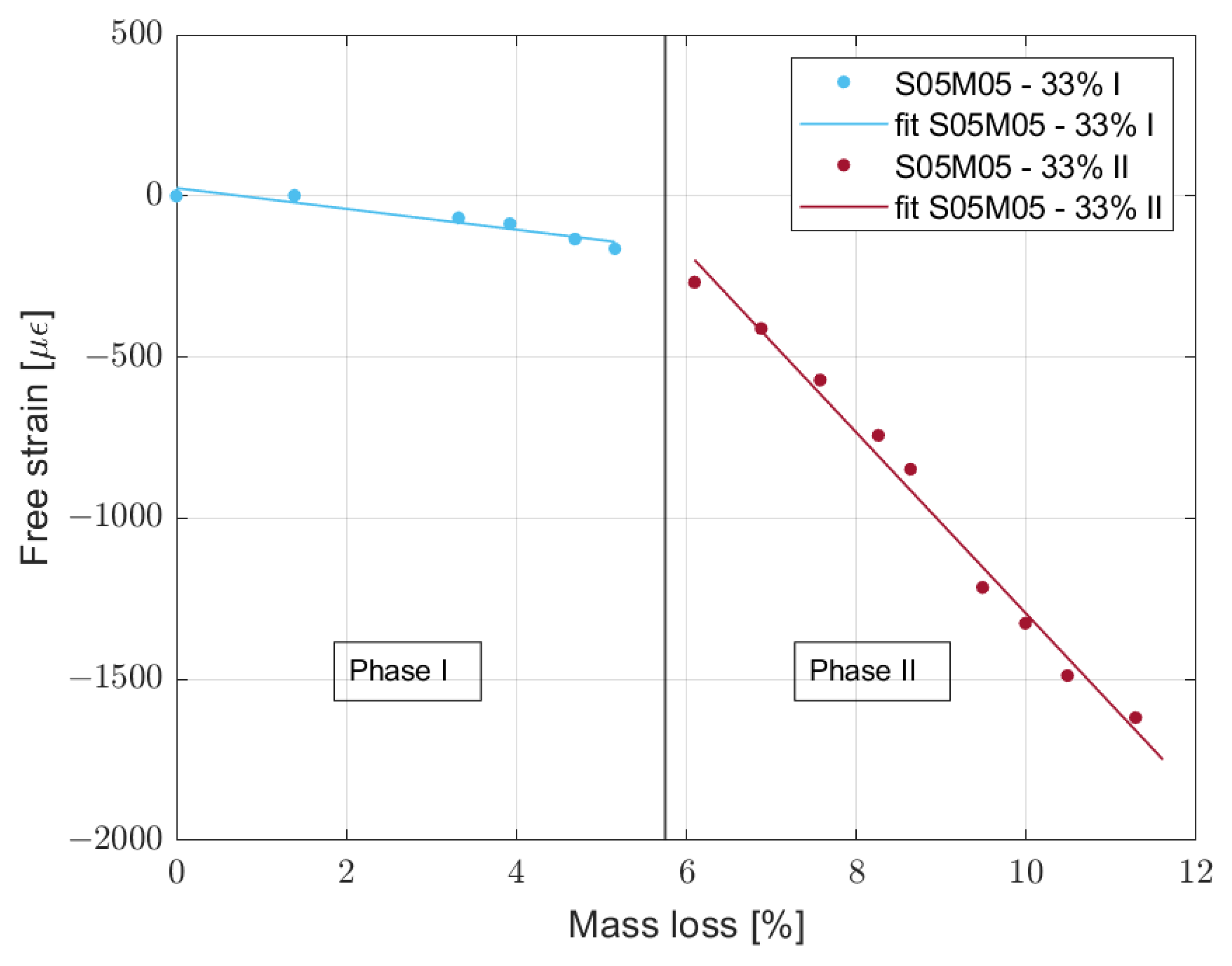
2.3.4. Mass Loss Versus Drying Shrinkage
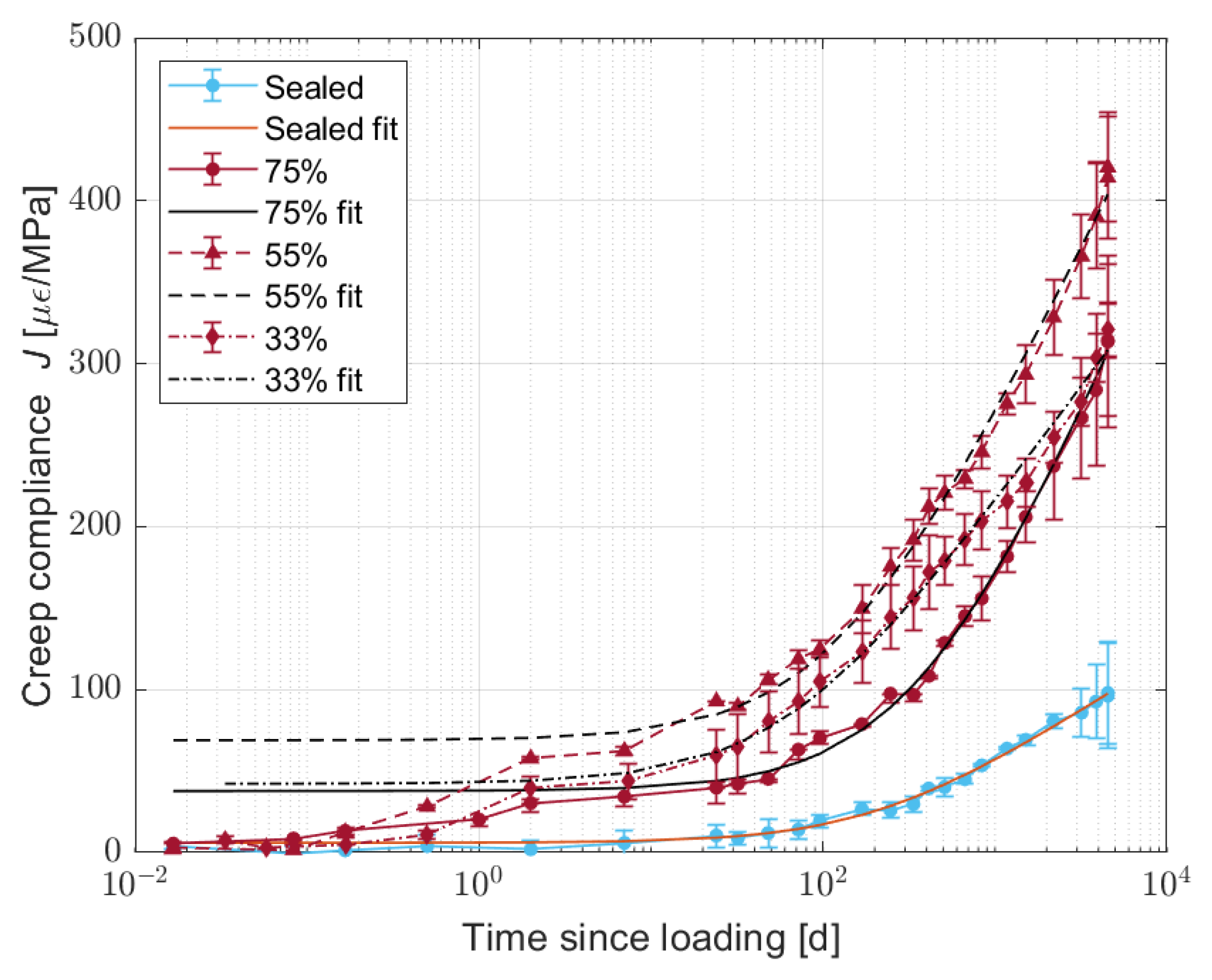
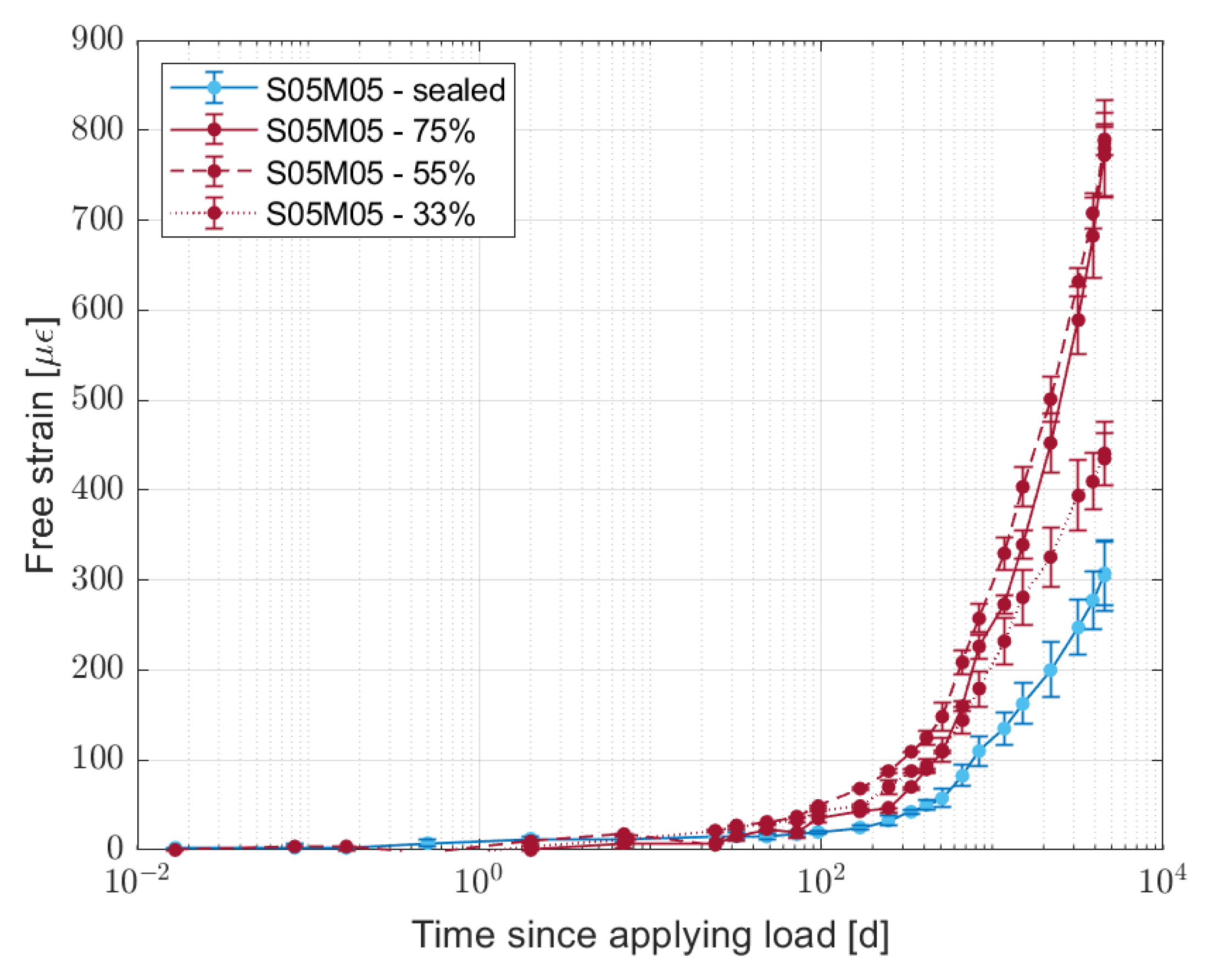
3. Effect of Water Content on Creep
3.1. Mortar Composition
3.2. Experimental Procedure
Creep Test
3.3. Results and Discussion
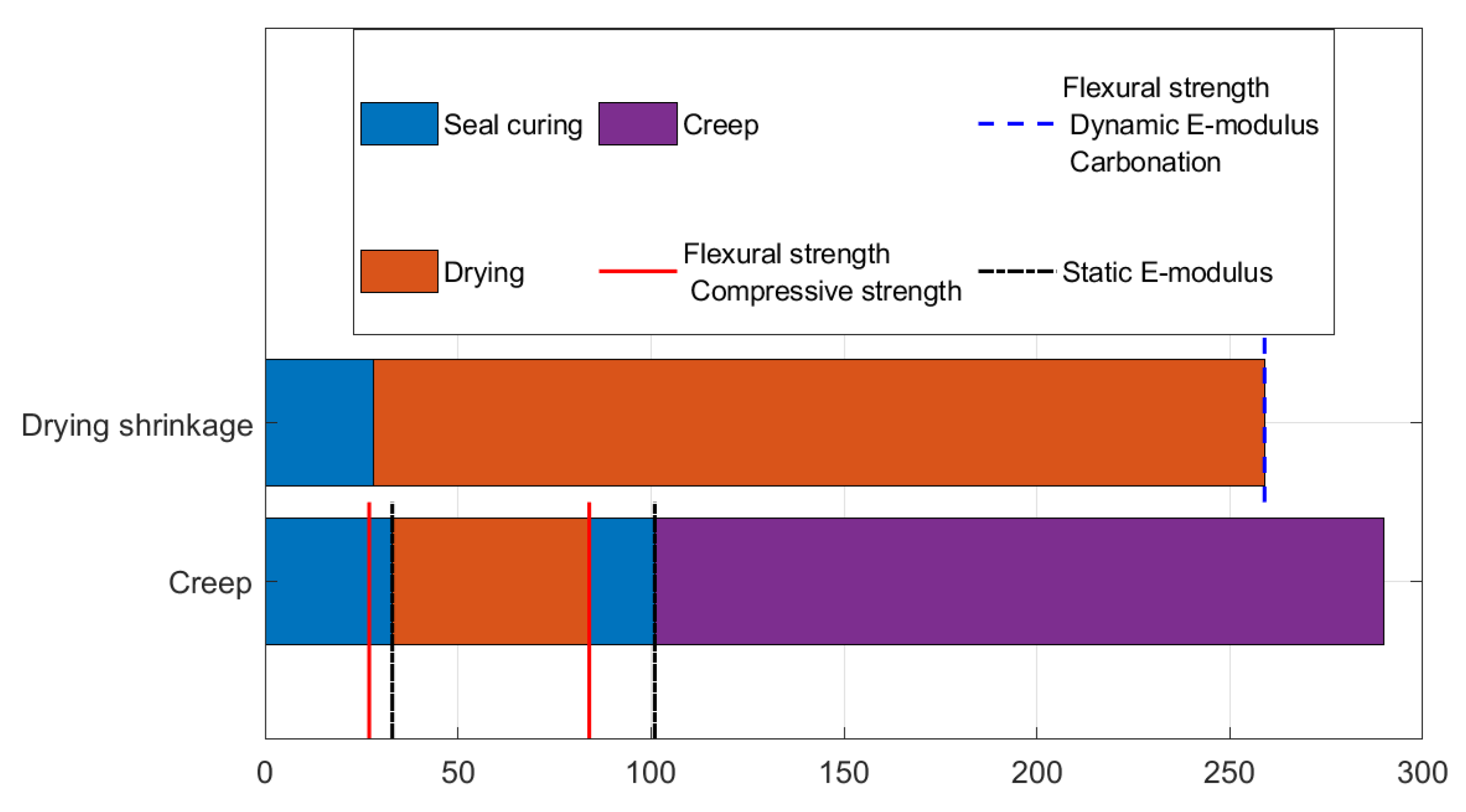
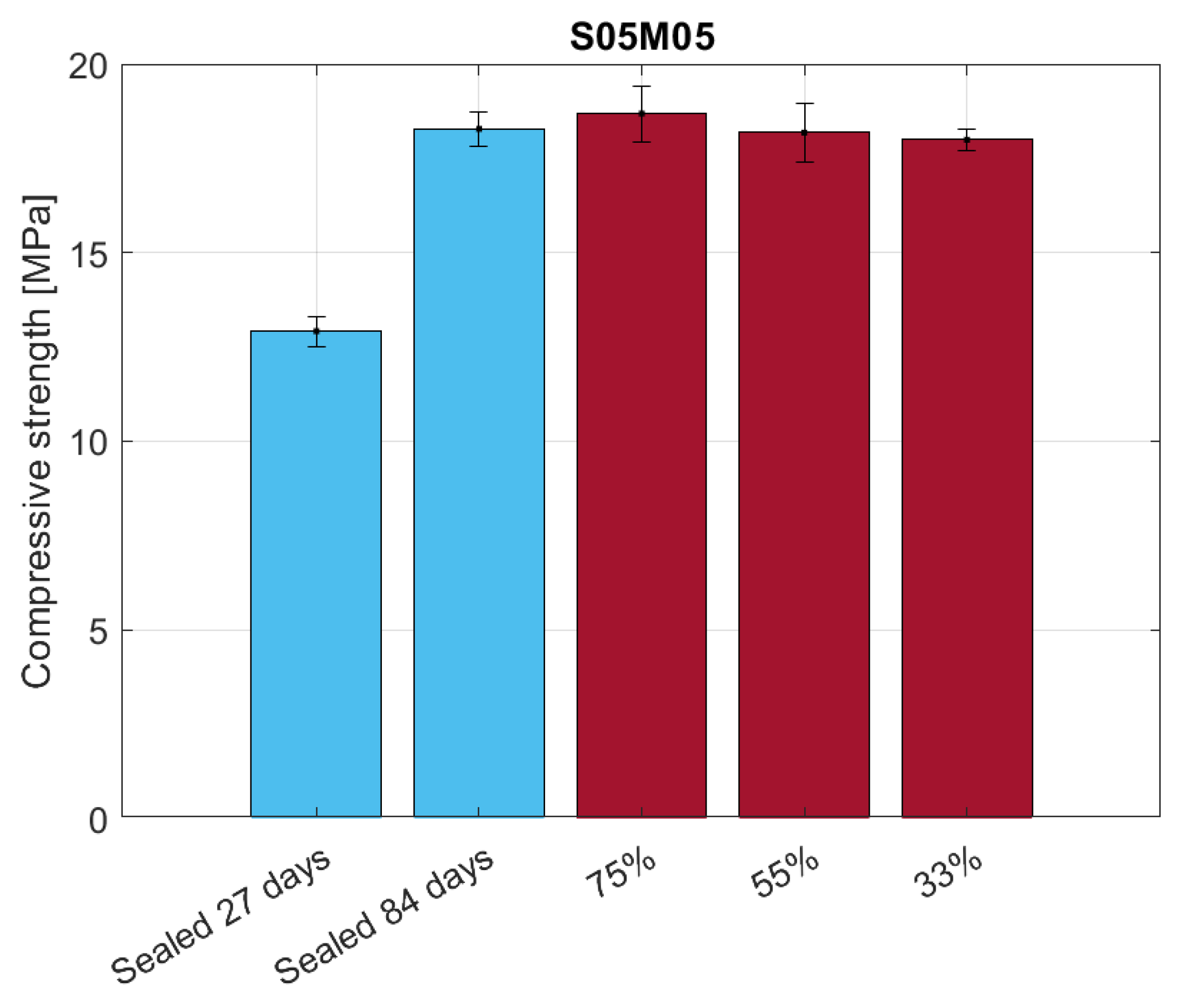
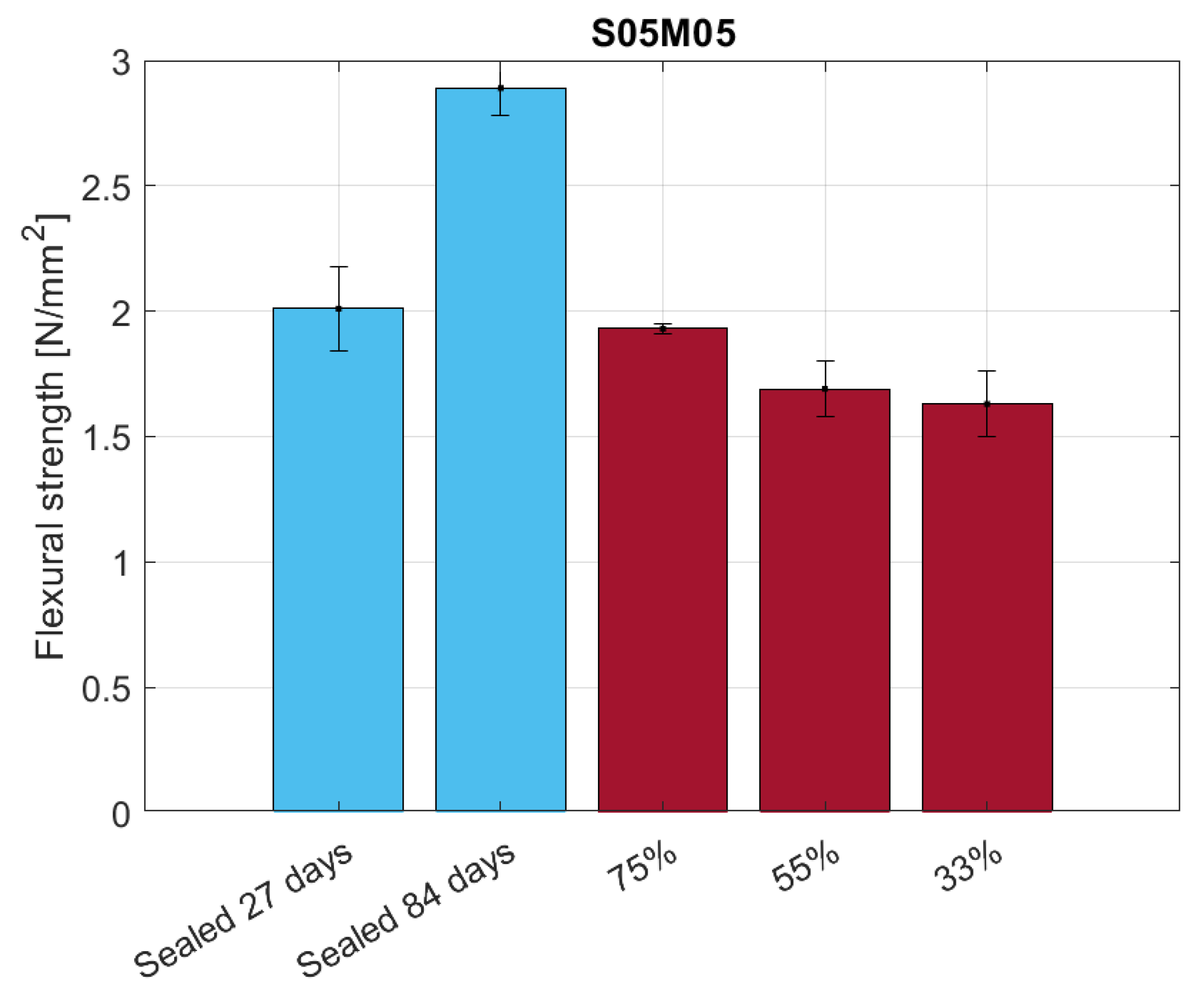
4. Flexural Strength, E-Modulus, and Carbonation
4.1. Mortar Composition
4.2. Experimental Procedure
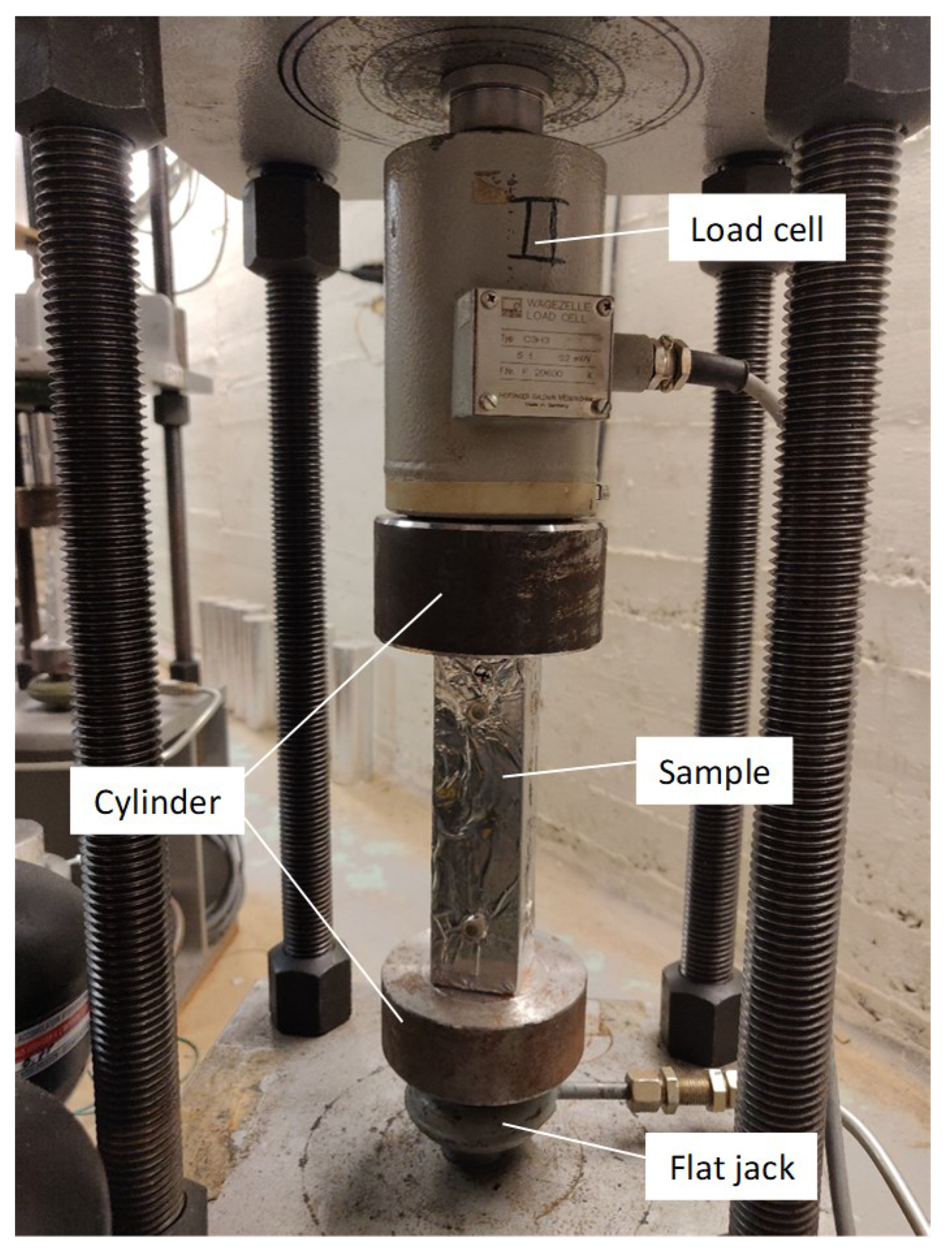
4.2.1. Flexural Strength
4.2.2. Static E-Modulus
4.2.3. Dynamic E-Modulus
4.2.4. Carbonation Depth
4.3. Results and Discussion
4.3.1. Compressive Strength
4.3.2. Flexural Strength
4.3.3. Dynamic and Static E-Modulus
4.3.4. Carbonation Depth
5. Conclusions
- Increasing the molarity increases the drying shrinkage of the material only in medium to low relative humidity conditions, while the opposite is true for high RH conditions.
- For the different compositions, the mass loss is not a good indicator of the shrinkage magnitude, contrary to what has been observed for OPC so far.
- Increasing the solution-to-binder ratio increases the drying shrinkage and the mass loss regardless of the molarity and the relative humidity, indicating that the water content affects the drying shrinkage more than the coarsening of the pore structure, at least for the compositions studied.
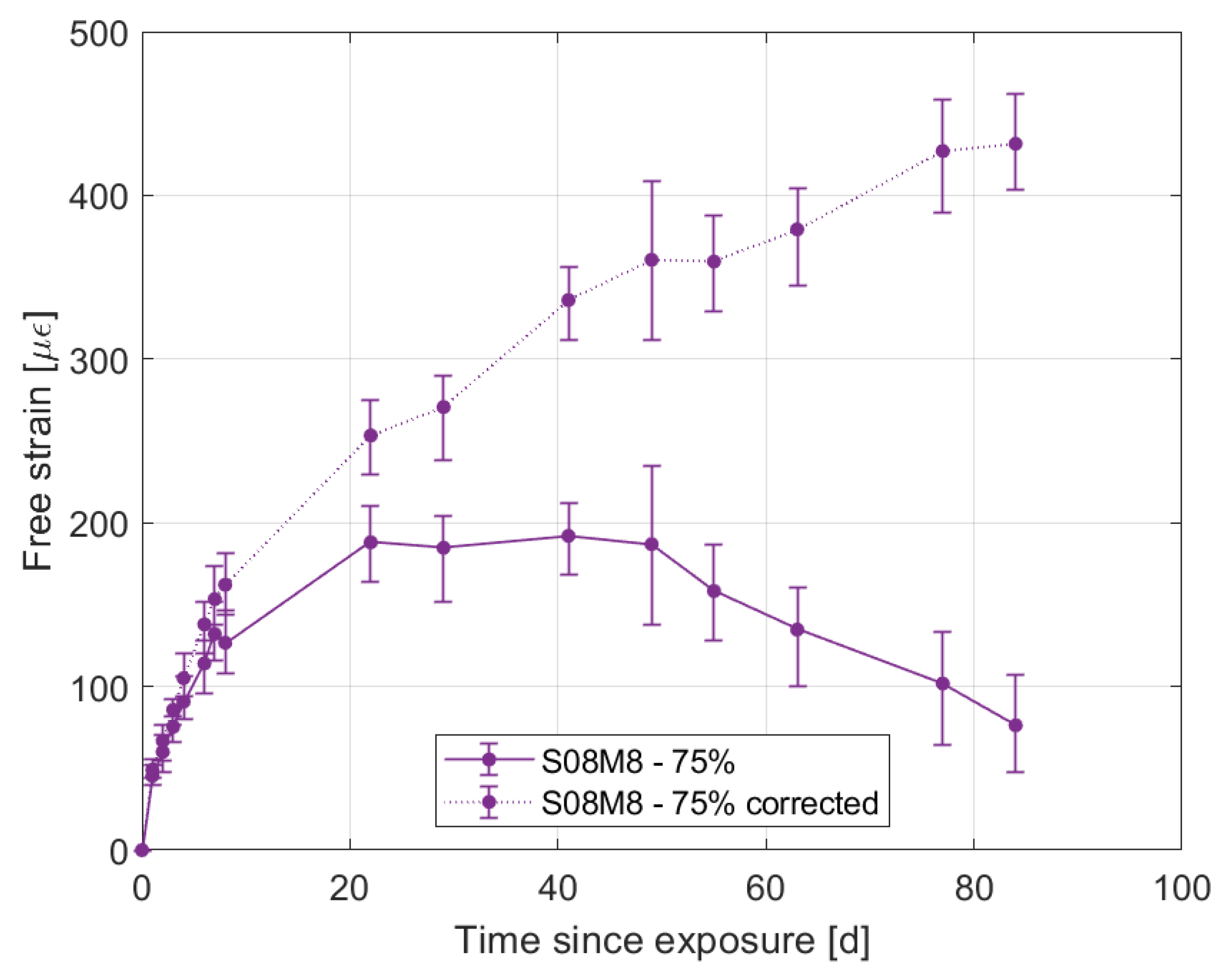
- High molarity compositions at high relative humidity present an initial swelling due to water vapour absorption, with a subsequent shrinkage that does not agree with the mass stabilisation or increase observed. The cause for this phenomenon is likely to be the autogenous deformation that is still ongoing. In addition, they also present strong efflorescence that forms a salt layer on the surface of the samples.
- The influence of autogenous shrinkage on the total deformation is not negligible even after 84 days. The autogenous shrinkage itself does not seem to follow any pattern linked to the molarity or the solution-to-binder ratio.
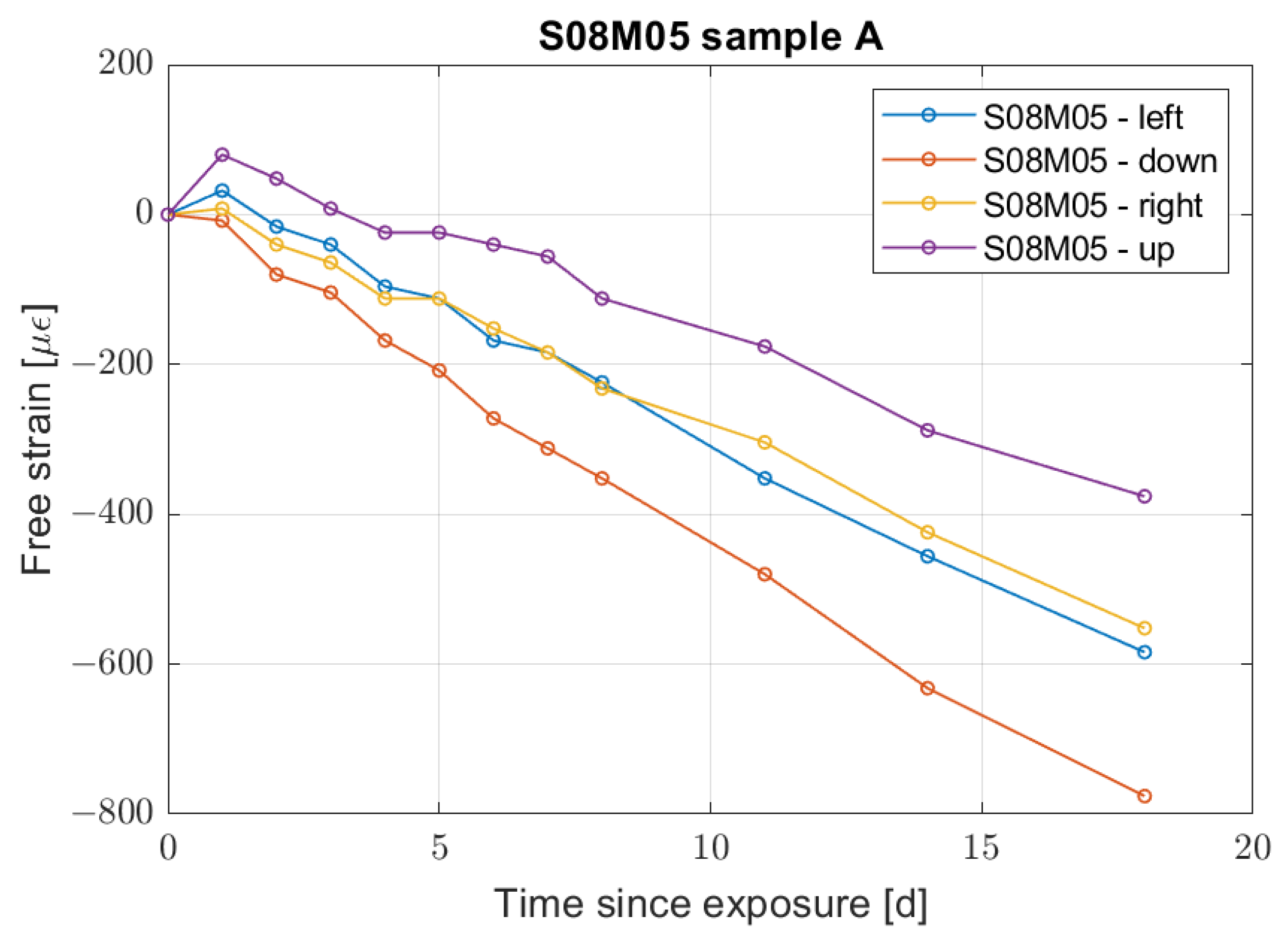
- Compositions with a low molarity and a high solution-to-binder ratio present bending caused by the segregation taking place at the time of casting.
- Reducing the amount of water in the material increases the creep compliance compared to the sealed condition, even though the results do not present a clear relationship with the mass loss or the relative humidity or with the change in the mechanical properties. Further investigation is needed in order to understand how the water content of the material affects its creep compliance.
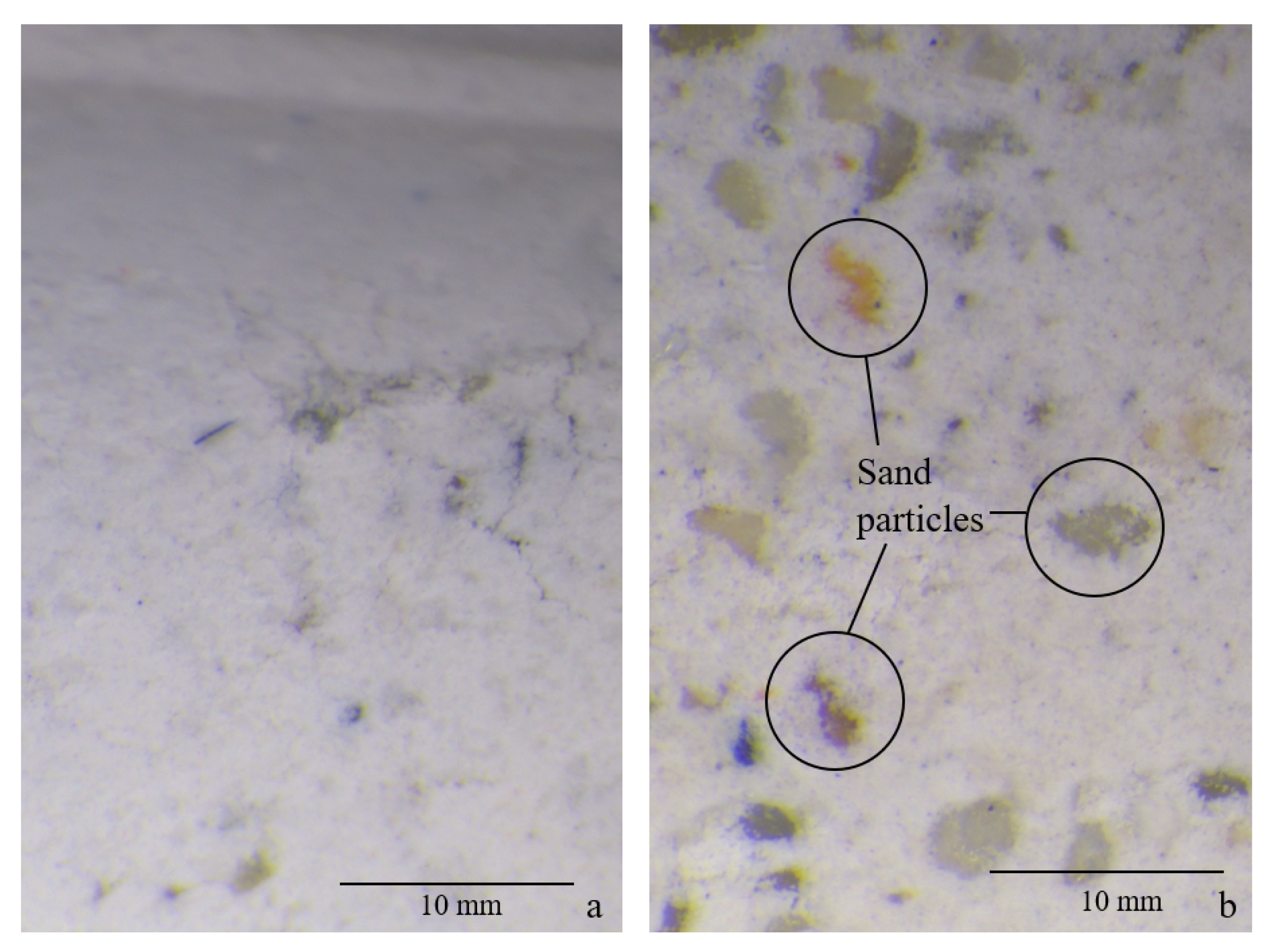
- The minimum shrinkage deformation for the composition with low molarity and a low solution-to-binder ratio occurs at 55% RH due to the higher presence of microcracks, which was observed when testing the flexural strength and the dynamic E-modulus using the UPV method.
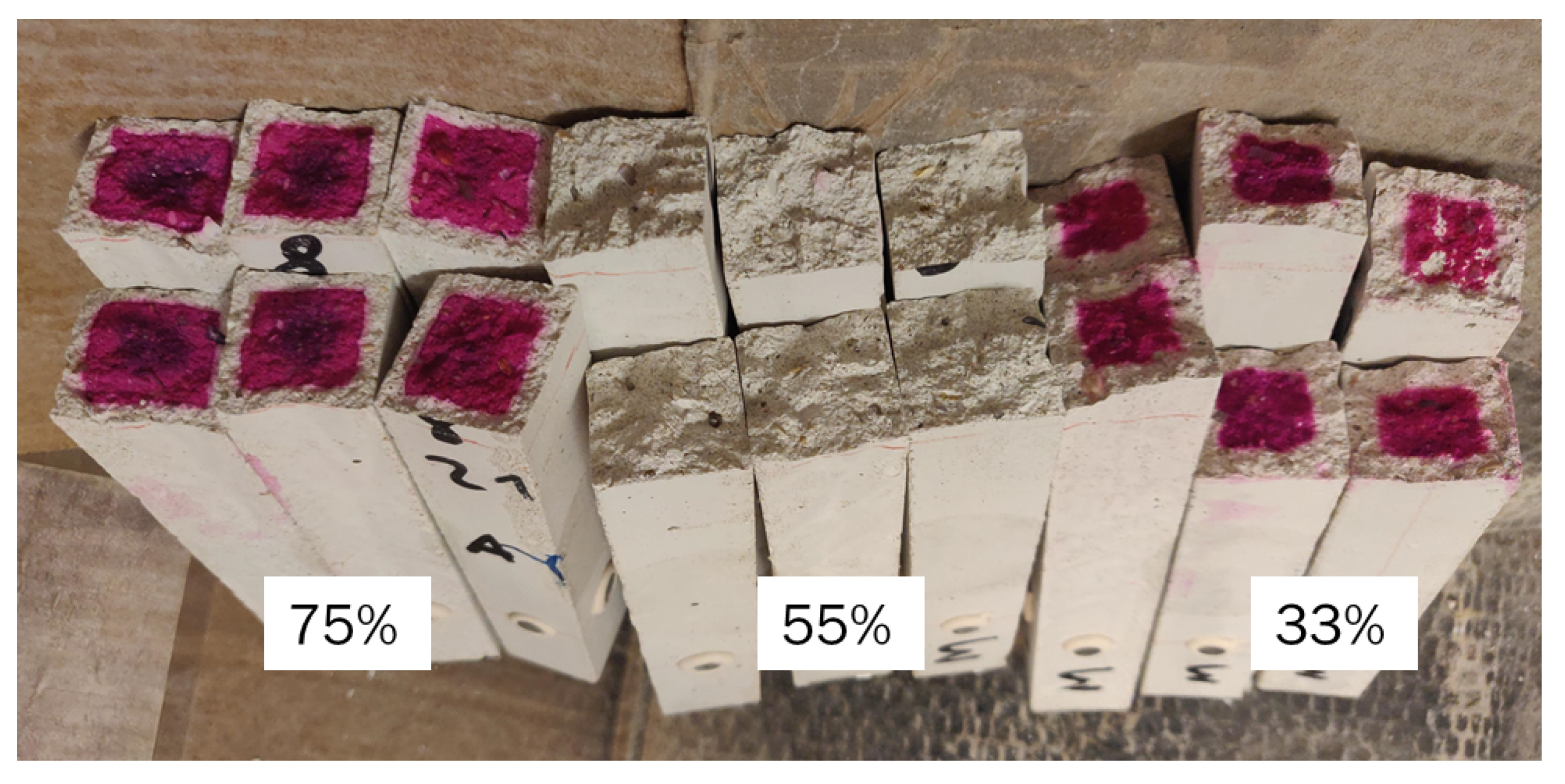
- The carbonation depth presents a maximum at 55% relative humidity, as is the case for OPC, even though the cause seems to be the high presence of microcracks that facilitate the penetration of CO2 in the material, rather than the optimal conditions of diffusivity and the presence of a high dissolution interface. In order to understand the effect of carbonation on the volume stability, further investigation is required.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAS | alkali-activated slag |
| OPC | Ordinary Portland Cement |
| s/b | solution-to-binder ratio |
| w/b | water-to-binder ratio |
| RH | relative humidity |
| UPV | Ultrasonic Pulse Velocity |
References
- Turner, L.K.; Collins, F.G. Carbon Dioxide Equivalent (CO2-e) Emissions: A Comparison between Geopolymer and OPC Cement Concrete. Constr. Build. Mater. 2013, 43, 125–130. [Google Scholar] [CrossRef]
- Benhelal, E.; Zahedi, G.; Shamsaei, E.; Bahadori, A. Global Strategies and Potentials to Curb CO2 Emissions in Cement Industry. J. Clean. Prod. 2013, 51, 142–161. [Google Scholar] [CrossRef]
- Naqi, A.; Jang, J.G. Recent Progress in Green Cement Technology Utilizing Low-Carbon Emission Fuels and Raw Materials: A Review. Sustainability 2019, 11, 537. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Kirkpatrick, R.J. Innovation in Use and Research on Cementitious Material. Cem. Concr. Res. 2008, 38, 128–136. [Google Scholar] [CrossRef]
- Tian, B.; Li, X.; Lv, Y.; Xu, J.; Ma, W.; He, C.; Chen, Y.; Jian, S.; Wang, W.; Zhang, C.; et al. Effect of Rice Husk Ash on the Properties of Alkali-Activated Slag Pastes: Shrinkage, Hydration and Mechanical Property. Materials 2023, 16, 3148. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage Mechanisms of Alkali-Activated Slag. Cem. Concr. Res. 2016, 88, 126–135. [Google Scholar] [CrossRef]
- Thomas, R.J.; Ye, H.; Radlińska, A.; Peethamparan, S. Alkali-Activated Slag Cement Concrete. Concr. Int. 2016, 38, 33–38. [Google Scholar]
- Juenger, M.C.G.; Winnefeld, F.; Provis, J.L.; Ideker, J.H. Advances in Alternative Cementitious Binders. Cem. Concr. Res. 2011, 41, 1232–1243. [Google Scholar] [CrossRef]
- Jang, J.; Ahn, Y.; Souri, H.; Lee, H. A Novel Eco-Friendly Porous Concrete Fabricated with Coal Ash and Geopolymeric Binder: Heavy Metal Leaching Characteristics and Compressive Strength. Constr. Build. Mater. 2015, 79, 173–181. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Effect of Pore Size Distribution on Drying Shrinking of Alkali-Activated Slag Concrete. Cem. Concr. Res. 2000, 30, 1401–1406. [Google Scholar] [CrossRef]
- Melo Neto, A.A.; Cincotto, M.A.; Repette, W. Drying and Autogenous Shrinkage of Pastes and Mortars with Activated Slag Cement. Cem. Concr. Res. 2008, 38, 565–574. [Google Scholar] [CrossRef]
- Duran Atiş, C.; Bilim, C.; Çelik, O.; Karahan, O. Influence of Activator on the Strength and Drying Shrinkage of Alkali-Activated Slag Mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Ye, H.; Cartwright, C.; Rajabipour, F.; Radlińska, A. Understanding the Drying Shrinkage Performance of Alkali-Activated Slag Mortars. Cem. Concr. Compos. 2017, 76, 13–24. [Google Scholar] [CrossRef]
- Hojati, M.; Rajabipour, F.; Radlińska, A. Drying Shrinkage of Alkali Activated Fly Ash: Effect of Activator Composition and Ambient Relative Humidity. In Proceedings of the 4th International Conference on Sustainable Construction Materials and Technologies (SCMT4), Las Vegas, NV, USA, 7–11 August 2016; p. 11. [Google Scholar]
- Hojati, M. Shrinkage and Creep of Alkali-Activated Binders. Doctoral Thesis, Pennsylvania State University, State College, PA, USA, 2017. [Google Scholar]
- Puertas, F.; Palacios, M.; Vázquez, T. Carbonation Process of Alkali-Activated Slag Mortars. J. Mater. Sci. 2006, 41, 3071–3082. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Carbonation-Induced Volume Change in Alkali-Activated Slag. Constr. Build. Mater. 2017, 144, 635–644. [Google Scholar] [CrossRef]
- Shi, C. Strength, Pore Structure and Permeability of Alkali-Activated Slag Mortars. Cem. Concr. Res. 1996, 26, 1789–1799. [Google Scholar] [CrossRef]
- Ben Haha, M.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of Activator Type on Hydration Kinetics, Hydrate Assemblage and Microstructural Development of Alkali Activated Blast-Furnace Slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H. Analysing the Relation between Pore Structure and Permeability of Alkali-Activated Concrete Binders. In Handbook of Alkali-Activated Cements, Mortars and Concretes; Pacheco-Torgal, F., Labrincha, J., Leonelli, C., Palomo, A., Chindaprasirt, P., Eds.; Woodhead Publishing: Sawston, UK, 2015; pp. 235–264. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Alkali Activation of Australian Slag Cements. Cem. Concr. Res. 1999, 29, 113–120. [Google Scholar] [CrossRef]
- Krizan, D.; Zivanovic, B. Effects of Dosage and Modulus of Water Glass on Early Hydration of Alkali–Slag Cements. Cem. Concr. Res. 2002, 32, 1181–1188. [Google Scholar] [CrossRef]
- Thomas, R.; Lezama, D.; Peethamparan, S. On Drying Shrinkage in Alkali-Activated Concrete: Improving Dimensional Stability by Aging or Heat-Curing. Cem. Concr. Res. 2017, 91, 13–23. [Google Scholar] [CrossRef]
- Cui, P.; Wan, Y.; Shao, X.; Ling, X.; Zhao, L.; Gong, Y.; Zhu, C. Study on Shrinkage in Alkali-Activated Slag–Fly Ash Cementitious Materials. Materials 2023, 16, 3958. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Aydın, S.; Yardımcı, M.Y.; Lesage, K.; De Schutter, G. Effects of Activator Properties and GGBFS/FA Ratio on the Structural Build-up and Rheology of AAC. Cem. Concr. Res. 2020, 138, 106253. [Google Scholar] [CrossRef]
- ASTM C490; Standard Practice for Use of Apparatus for the Determination of Length Change of Hardened Cement Paste, Mortar, and Concrete. ASTM International: West Conshohocken, PA, USA, 2021.
- Greenspan, L. Humidity Fixed Points of Binary Saturated Aqueous Solutions. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1977, 81A, 89. [Google Scholar] [CrossRef]
- Granger, L. Comportement différé du béton dans les enceintes de centrales nucléaires: Analyse et modélisation. Doctoral Thesis, Ecole Nationale des Ponts et Chaussées, Marne-La-Vallée, France, 1996. [Google Scholar]
- Benboudjema, F.; Meftah, F.; Torrenti, J. Interaction between Drying, Shrinkage, Creep and Cracking Phenomena in Concrete. Eng. Struct. 2005, 27, 239–250. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Quantitative Analysis of Phase Assemblage and Chemical Shrinkage of Alkali-Activated Slag. J. Adv. Concr. Technol. 2016, 14, 245–260. [Google Scholar] [CrossRef]
- Powers, T.C. The Thermodynamics of Volume Change and Creep. Matériaux et Construction 1968, 1, 487–507. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J.M. Concrete: Microstructure, Properties, and Materials, 3rd ed.; McGraw-Hill: New York, NY, USA, 2006. [Google Scholar]
- Madge, D.S. The Control of Relative Humidity with Aqueous Solutions of Sodium Hydroxide. Entomol. Exp. Appl. 1961, 4, 143–147. [Google Scholar] [CrossRef]
- Saludung, A.; Azeyanagi, T.; Ogawa, Y.; Kawai, K. Effect of Silica Fume on Efflorescence Formation and Alkali Leaching of Alkali-Activated Slag. J. Clean. Prod. 2021, 315, 128210. [Google Scholar] [CrossRef]
- Škvára, F.; Šmilauer, V.; Hlaváček, P.; Kopecký, L.; Cílová, Z. A Weak Alkali Bond in (N, K)–A–S–H Gels: Evidence from Leaching and Modeling. Ceramics-Silikáty 2012, 56, 9. [Google Scholar]
- Tang, D.; Yang, C.; Li, X.; Zhu, X.; Yang, K.; Yu, L. Mitigation of Efflorescence of Alkali-Activated Slag Mortars by Incorporating Calcium Hydroxide. Constr. Build. Mater. 2021, 298, 123873. [Google Scholar] [CrossRef]
- Kang, S.P.; Kwon, S.J. Effects of Red Mud and Alkali-Activated Slag Cement on Efflorescence in Cement Mortar. Constr. Build. Mater. 2017, 133, 459–467. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Fly Ash-Based Geopolymers: The Relationship between Composition, Pore Structure and Efflorescence. Cem. Concr. Res. 2014, 64, 30–41. [Google Scholar] [CrossRef]
- Staquet, S. Analyse et Modelisation du Comportement Differe du Beton Application aux Poutres Mixtes, Preflechies et Precontraintes. Doctoral Thesis, Université libre de Bruxelles, Brussels, Belgium, 2004. [Google Scholar]
- Wittmann, F.H. Creep and Shrinkage Mechanisms. In Creep and Shrinkage in Concrete Structures; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1982. [Google Scholar]
- Bažant, Z.P. Mathematical Modeling of Creep and Shrinkage of Concrete. In Mathematical Modeling of Creep and Shrinakge of Concrete; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 1982. [Google Scholar]
- Azenha, M. Numerical Simulation of the Structural Behaviour of Concrete since Its Early Ages. Doctoral Thesis, Universidade do Porto, Porto, Portugal, 2009. [Google Scholar]
- Parrott, L. Basic Creep, Drying Creep and Shrinkage of a Mature Cement Paste after a Heat Cycle. Cem. Concr. Res. 1977, 7, 597–604. [Google Scholar] [CrossRef]
- Day, R.; Cuffaro, P.; Illston, J. The Effect of Rate of Drying on the Drying Creep of Hardened Cement Paste. Cem. Concr. Res. 1984, 14, 329–338. [Google Scholar] [CrossRef]
- Bažant, Z.P.; Raftshol, W.J. Effect of Cracking in Drying and Shrinkage Specimens. Cem. Concr. Res. 1982, 12, 209–226. [Google Scholar] [CrossRef]
- Zhang, Y.; Hubler, M. Role of Early Drying Cracks in the Shrinkage Size Effect of Cement Paste. J. Eng. Mech. 2020, 146, 04020128. [Google Scholar] [CrossRef]
- Bažant, Z.P.; Planas, J. Fracture and Size Effect in Concrete and Other Quasibrittle Materials; New Directions in Civil Engineering; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Neville, A.M. Properties of Concrete, 5th ed.; Pearson: London, UK, 2011. [Google Scholar]
- Bissonnette, B.; Pigeon, M. Le comportement viscoélastique du béton en traction et la compatibilité déformationnelle des réparations. Mater. Struct. 2000, 33, 108–118. [Google Scholar] [CrossRef]
- Wittmann, F.; Roelfstra, P. Total Deformation of Loaded Drying Concrete. Cem. Concr. Res. 1980, 10, 601–610. [Google Scholar] [CrossRef]
- Rossi, P.; Acker, P. A New Approach to the Basic Creep and Relaxation of Concrete. Cem. Concr. Res. 1988, 18, 799–803. [Google Scholar] [CrossRef]
- Ma, J.; Dehn, F. Shrinkage and Creep Behavior of an Alkali-Activated Slag Concrete. Struct. Concr. 2017, 18, 801–810. [Google Scholar] [CrossRef]
- Delsaute, B.; Torrenti, J.M.; Staquet, S. Modeling Basic Creep of Concrete since Setting Time. Cem. Concr. Compos. 2017, 83, 239–250. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Cracking Tendency of Alkali-Activated Slag Concrete Subjected to Restrained Shrinkage. Cem. Concr. Res. 2000, 30, 791–798. [Google Scholar] [CrossRef]
- Schuab, M.R. On the Development of MK/BFS Alkali-Activated Materials as Repair Mortars: Performance under Free and Restrained Shrinkage Tests. Constr. Build. Mater. 2021, 275, 122109. [Google Scholar] [CrossRef]
- Bažant, Z.P.; Şener, S.; Kim, J.K. Effect of Cracking on Drying Permeability and Diffusivity of Concrete. ACI Mater. J. 1987, 84, 351–357. [Google Scholar]
- Hammad, N.; Elnemr, A.; Shaaban, I.G. State-of-the-Art Report: The Self-Healing Capability of Alkali-Activated Slag (AAS) Concrete. Materials 2023, 16, 4394. [Google Scholar] [CrossRef]
- Srinivasan, S.S.; Rung, M.; Ferron, R.D. Factors Affecting Loss in Durability in Prestressed-Concrete Girders with Microcracking. J. Bridge Eng. 2020, 25, 04020068. [Google Scholar] [CrossRef]
- Srinivasan, S.S.; Ferron, R.D. Effect of Crack Width, Density, and Depth on Strength and Durability of Concrete-Equivalent Mortar. Aci Mater. J. 2021, 118, 65–78. [Google Scholar] [CrossRef]
- Nakamura, H.; Nanri, T.; Miura, T.; Roy, S. Experimental Investigation of Compressive Strength and Compressive Fracture Energy of Longitudinally Cracked Concrete. Cem. Concr. Compos. 2018, 93, 1–18. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z. Carbonation Induced Phase Evolution in Alkali-Activated Slag/Fly Ash Cements: The Effect of Silicate Modulus of Activators. Constr. Build. Mater. 2019, 223, 566–582. [Google Scholar] [CrossRef]
- EN 12390-13; Testing Hardened Concrete—Part 13: Determination of Secant Modulus of Elasticity in Compression. British Standard Institute: London, UK, 2013.
- Delsaute, B. New approach for Monitoring and Modelling of the Creep and Shrinkage Behaviour of Cement Pastes, Mortars and Concretes since Setting Time. Doctoral Thesis, Université libre de Bruxelles, Brussels, Belgium, 2016. [Google Scholar]
- EN 14630:2006; Products and Systems for the Protection and Repair of Concrete Structures—Test Methods—Determination of Carbonation Depth in Hardened Concrete by the Phenolphthalein Method. British Standard Institute: London, UK, 2006.
- Un, H.; Baradan, B. The Effect of Curing Temperature and Relative Humidity on the Strength Development of Portland Cement Mortar. Sci. Res. Essays 2011, 6, 2504–2511. [Google Scholar]
- Bazant, Z.P. Creep and Damage in Concrete. Mater. Sci. Concr. 1995, 4, 355–389. [Google Scholar]
- Bendimerad, A.; Delsaute, B.; Rozière, E.; Staquet, S.; Loukili, A. Advanced Techniques for the Study of Shrinkage-Induced Cracking of Concrete with Recycled Aggregates at Early Age. Constr. Build. Mater. 2020, 233, 117340. [Google Scholar] [CrossRef]
- Papadakis, V.; Vayenas, C.; Fardis, M. Experimental investigation and mathematical modeling of the concrete carbonation problem. Chem. Eng. Sci. 1991, 46, 1333–1338. [Google Scholar] [CrossRef]
- Galan, I.; Andrade, C.; Castellote, M. Natural and Accelerated CO2 Binding Kinetics in Cement Paste at Different Relative Humidities. Cem. Concr. Res. 2013, 49, 21–28. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Myers, R.J.; San Nicolas, R.; van Deventer, J.S. Role of Carbonates in the Chemical Evolution of Sodium Carbonate-Activated Slag Binders. Mater. Struct. 2015, 48, 517–529. [Google Scholar] [CrossRef]
- Rashad, A.M. A Synopsis of Carbonation of Alkali-Activated Materials. Green Mater. 2019, 7, 118–136. [Google Scholar] [CrossRef]
- Humad, A.M.; Provis, J.L.; Habermehl-Cwirzen, K.; Rajczakowska, M.; Cwirzen, A. Creep and Long-Term Properties of Alkali-Activated Swedish-Slag Concrete. J. Mater. Civ. Eng. 2021, 33, 04020475. [Google Scholar] [CrossRef]





| Oxide | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | K2O |
|---|---|---|---|---|---|---|
| Content (%) | 36.2 | 12.4 | 0.6 | 39.8 | 7.3 | 0.5 |
| Composition | Solution-to-Binder Ratio | NaOH Concentration (M) | Sand-to-Paste Ratio | Water-to-Binder Ratio |
|---|---|---|---|---|
| S05M05 | 0.5 | 0.5 | 1 | 0.49 |
| S05M2 | 2 | 0.44 | ||
| S05M8 | 8 | 0.29 | ||
| S08M05 | 0.8 | 0.5 | 0.77 | |
| S08M2 | 2 | 0.69 | ||
| S08M8 | 8 | 0.43 |
| Saturated Salt Solution | Temperature (C) | ||||
| 10 | 15 | 20 | 25 | 30 | |
| RH over the Salt Solution (%) | |||||
| Magnesium chloride—MgCl2 | 33 | 33 | 33 | 33 | 32 |
| Magnesium nitrate—Mg(NO3)2 | 57 | 56 | 54 | 53 | 51 |
| Sodium chloride—NaCl | 76 | 76 | 75 | 75 | 75 |
| Composition | 33% | 55% | 75% |
|---|---|---|---|
| S05M05 | 13.1% | 28.2% | 20.0% |
| S05M2 | 17.1% | 22.1% | 46.1% |
| S05M8 | 21.9% | 54.8% | −336.5% |
| S08M05 | 19.5% | 22.1% | 20.0% |
| S08M2 | 2.0% | 2.3% | 7.0% |
| S08M8 | 13.8% | 35.4% | −595.1% |
| Composition | RH | Delay (d) | Slope Phase I | Slope Phase II |
|---|---|---|---|---|
| S05M05 | 33% | 10.6 | −42.77 | −281.38 |
| 55% | 10.2 | −36.69 | −119.04 | |
| 75% | 10.8 | −122.49 | −398.45 | |
| S05M2 | 33% | 30.4 | −75.26 | −457.30 |
| 55% | 47.9 | −73.20 | −894.68 | |
| 75% | 30.9 | −149.65 | −375.48 | |
| S05M8 | 33% | 5.9 | −603.32 | −2438.6 |
| 55% | ||||
| 75% | ||||
| S08M05 | 33% | 7.4 | −56.50 | −315.56 |
| 55% | 10.8 | −53.03 | −290.37 | |
| 75% | 22.9 | −97.42 | −464.01 | |
| S08M2 | 33% | 22.7 | −38.11 | −769.13 |
| 55% | 39.4 | −69.13 | −1088.0 | |
| 75% | 64.9 | −128.86 | −943.98 | |
| S08M8 | 33% | 39.9 | −206.56 | −2021.7 |
| 55% | 21.9 | −143.19 | −783.18 | |
| 75% |
| RH | A () | C () | (d) |
|---|---|---|---|
| Sealed | −5.978 | −0.0337 | 216.471 |
| 75% | −37.7245 | −0.00928 | 404.793 |
| 55% | −68.8956 | −0.01068 | 130.265 |
| 33% | −42.1892 | −0.01595 | 65.9869 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sirotti, M.; Delsaute, B.; Staquet, S. New Experimental Evidence for Drying Shrinkage of Alkali-Activated Slag with Sodium Hydroxide. Materials 2023, 16, 5659. https://doi.org/10.3390/ma16165659
Sirotti M, Delsaute B, Staquet S. New Experimental Evidence for Drying Shrinkage of Alkali-Activated Slag with Sodium Hydroxide. Materials. 2023; 16(16):5659. https://doi.org/10.3390/ma16165659
Chicago/Turabian StyleSirotti, Marco, Brice Delsaute, and Stéphanie Staquet. 2023. "New Experimental Evidence for Drying Shrinkage of Alkali-Activated Slag with Sodium Hydroxide" Materials 16, no. 16: 5659. https://doi.org/10.3390/ma16165659
APA StyleSirotti, M., Delsaute, B., & Staquet, S. (2023). New Experimental Evidence for Drying Shrinkage of Alkali-Activated Slag with Sodium Hydroxide. Materials, 16(16), 5659. https://doi.org/10.3390/ma16165659






