Environment-Induced Degradation of Shape Memory Alloys: Role of Alloying and Nature of Environment
Abstract
:1. Introduction
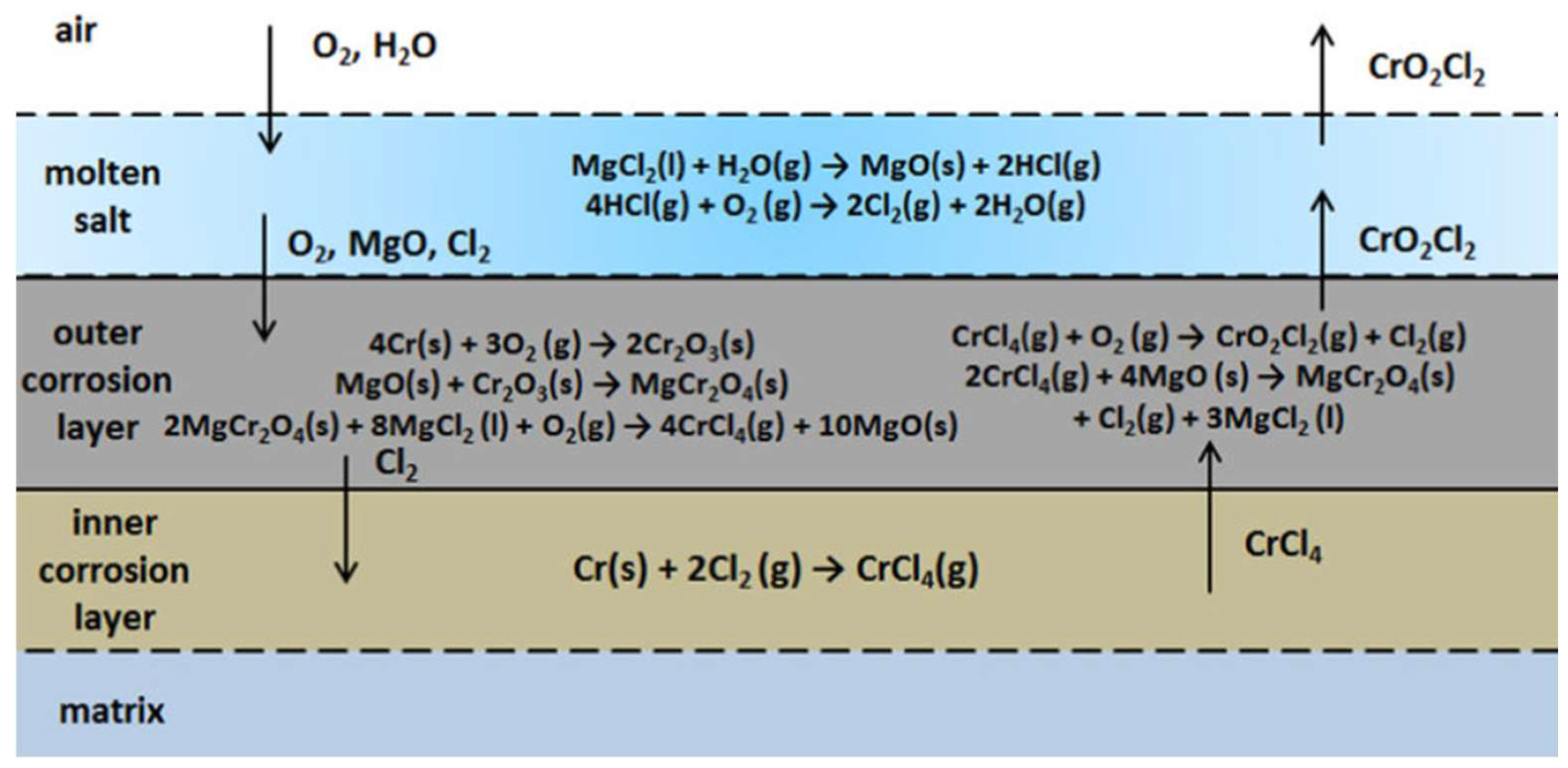
2. Environment-Induced Degradation and Its Mechanism
3. Various Corrosion Resistance Tests and Their Procedures
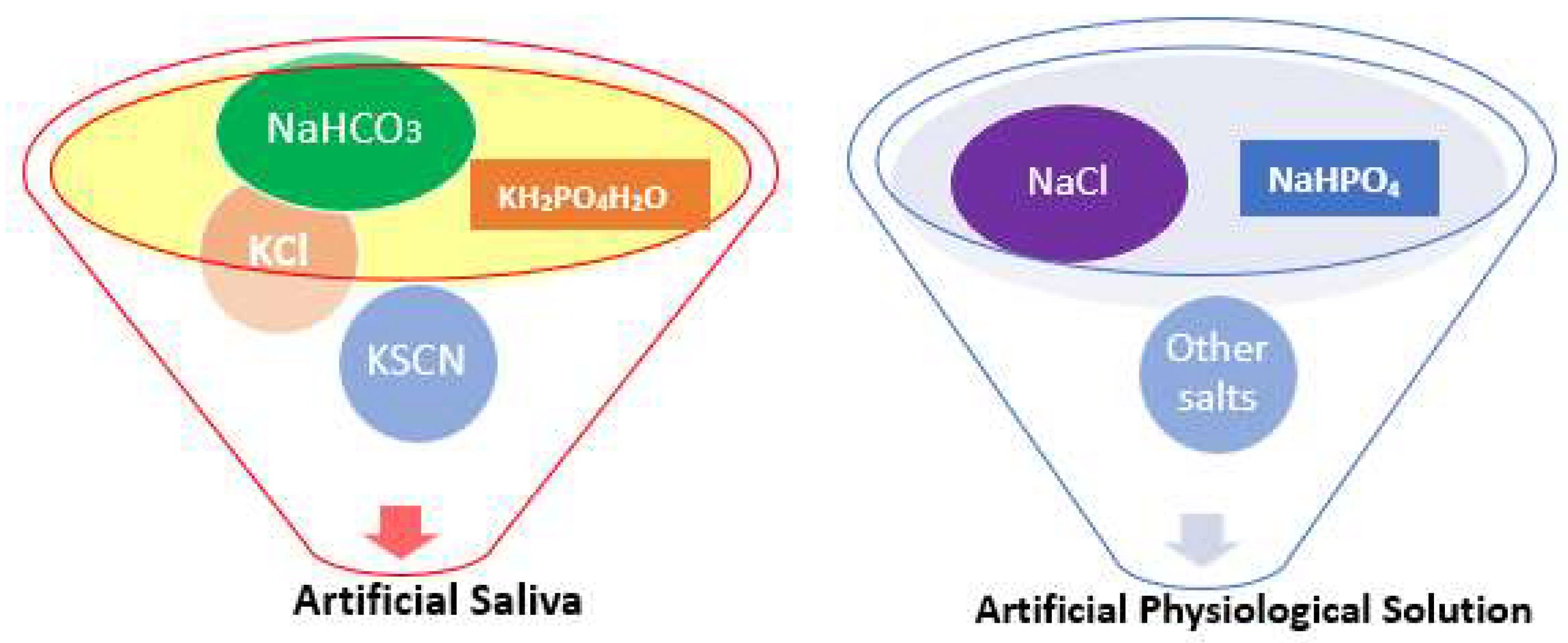
3.1. Various Test Solutions Used
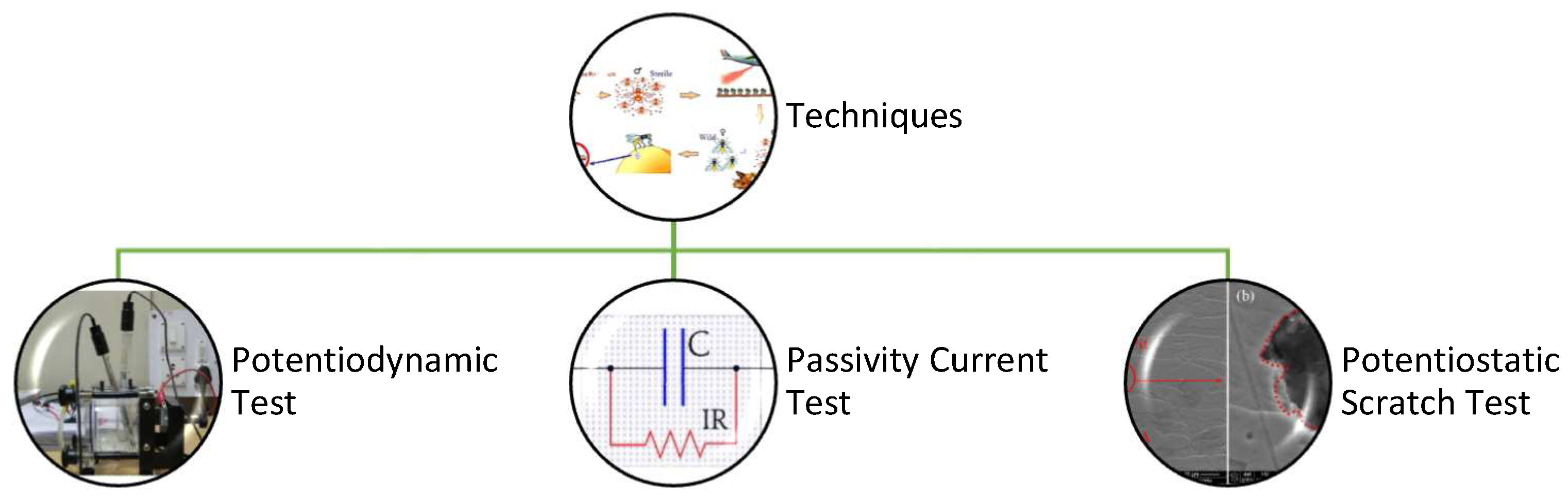
3.2. Techniques
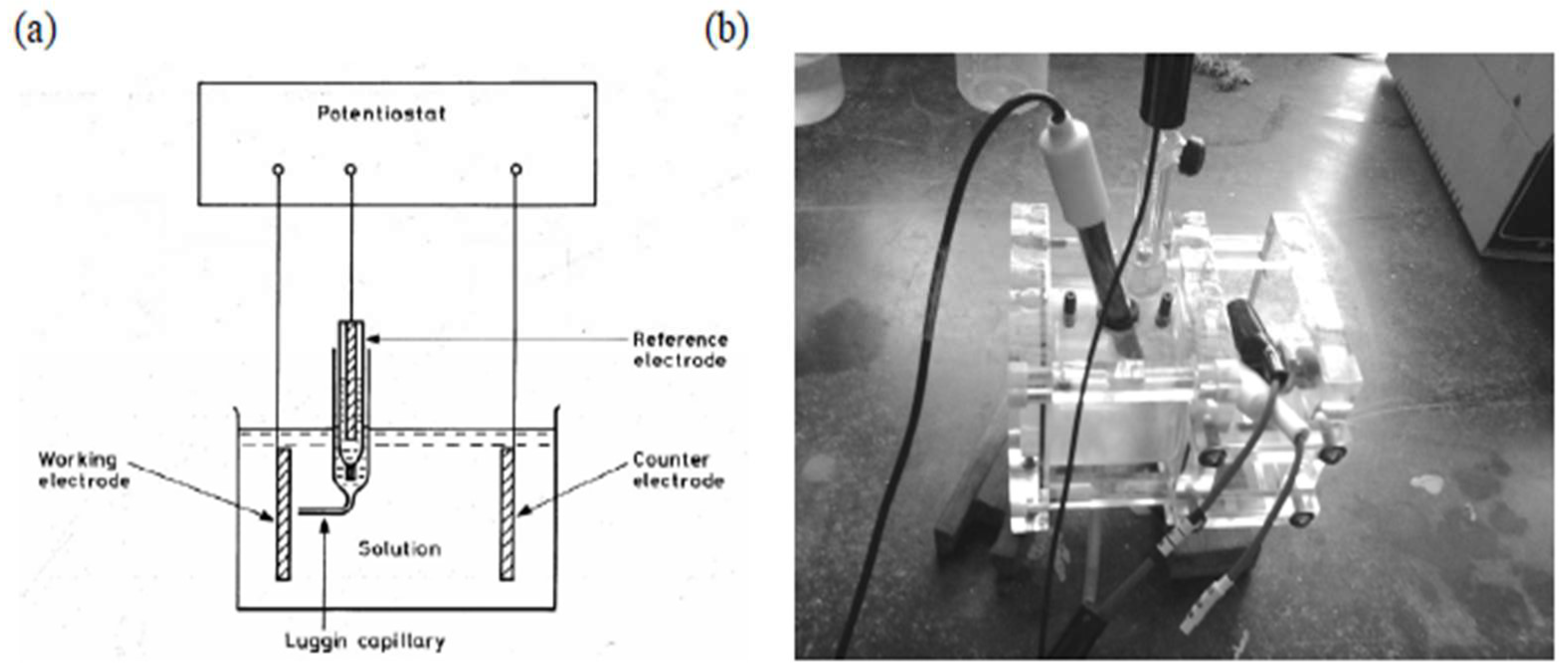
3.3. The Potentiodynamic Test
3.4. Passivity Current Test
3.5. Potentiostatic Scratch Test
4. The Copper-Aluminum-Beryllium (Cu-Al-Be)-Based Shape Memory Alloy
4.1. Experimental Work
- (i)
- Betatized for a full 30 min at 900 °C;
- (ii)
- Step-cooled in water that was boiling (100 °C);
- (iii)
- Finally cooled in a tub containing water at approximately 30 °C [25].
- (a)
- Maintaining the desired voltage in the circuit;
- (b)
- Estimating the characteristics of the current;
- (c)
- Displaying the yield as a voltage (E) versus current (I) plot.
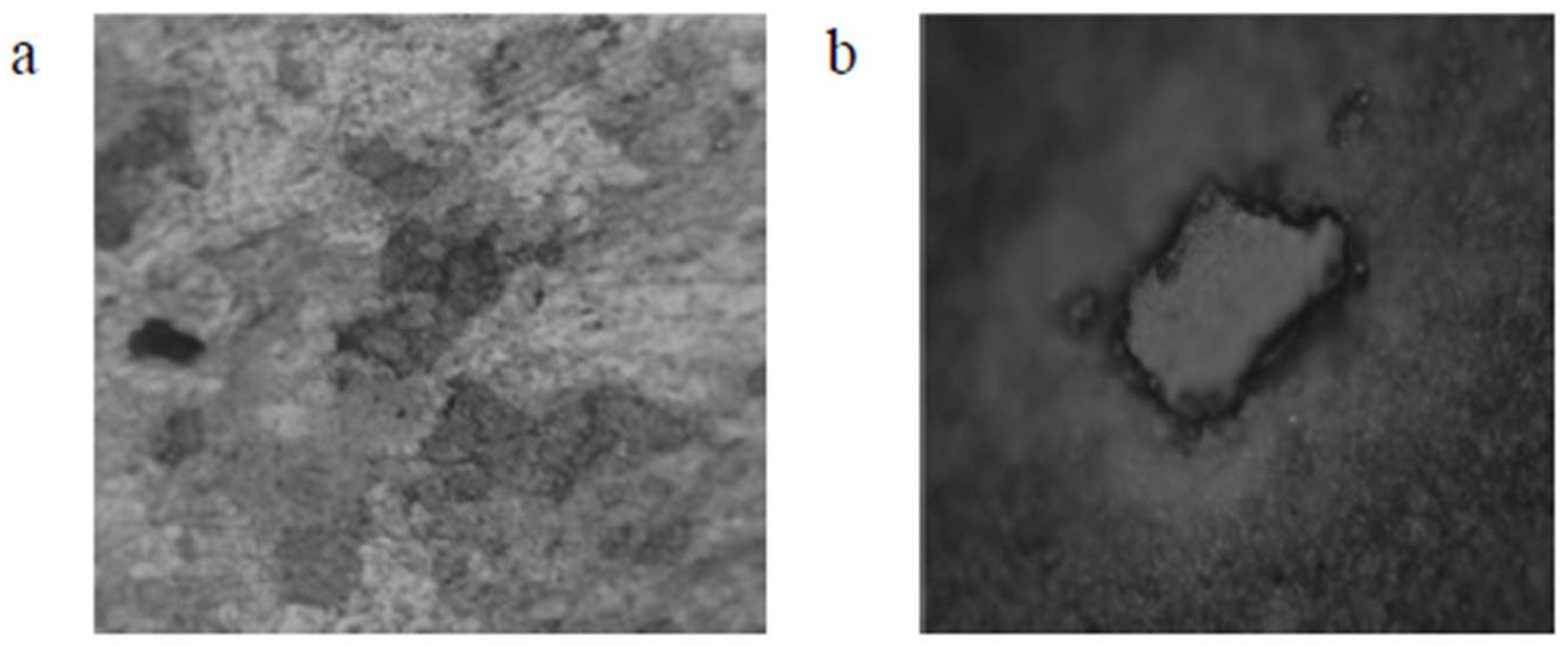
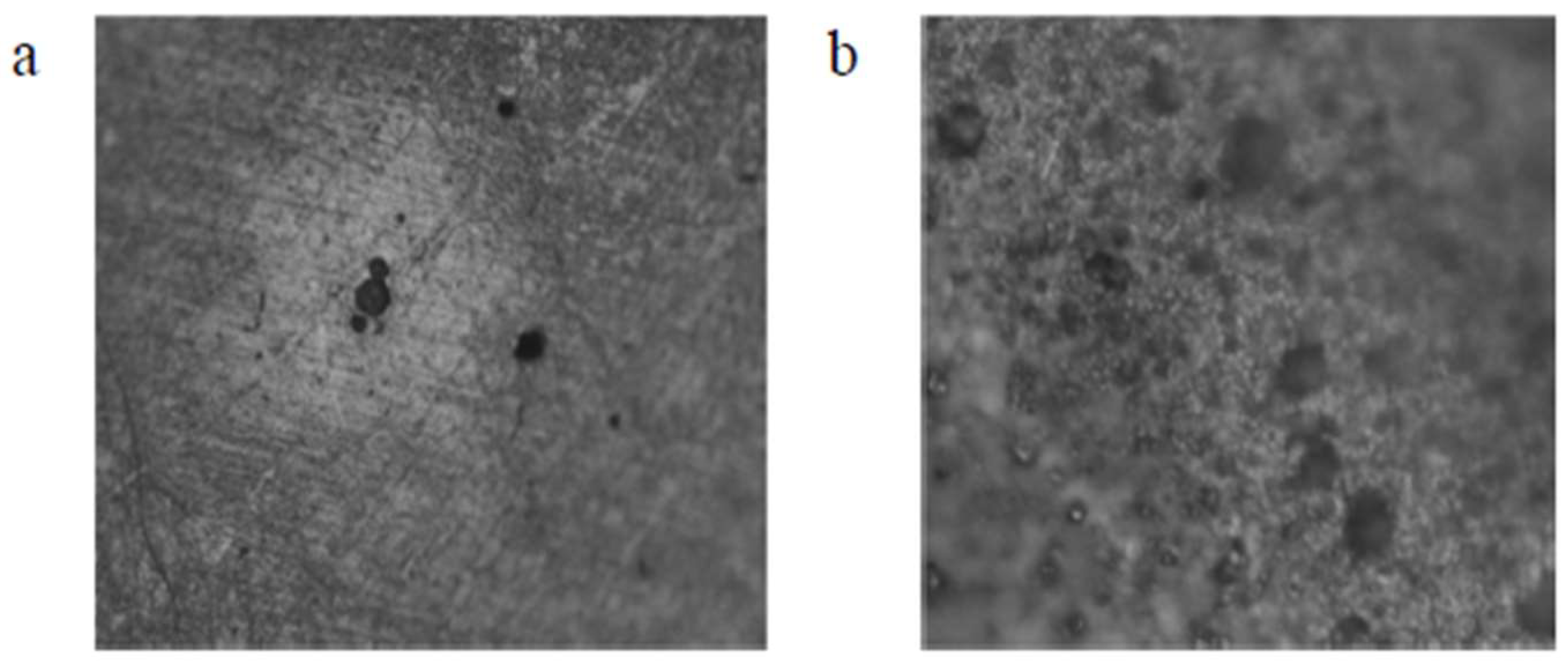
4.2. Observed Differences
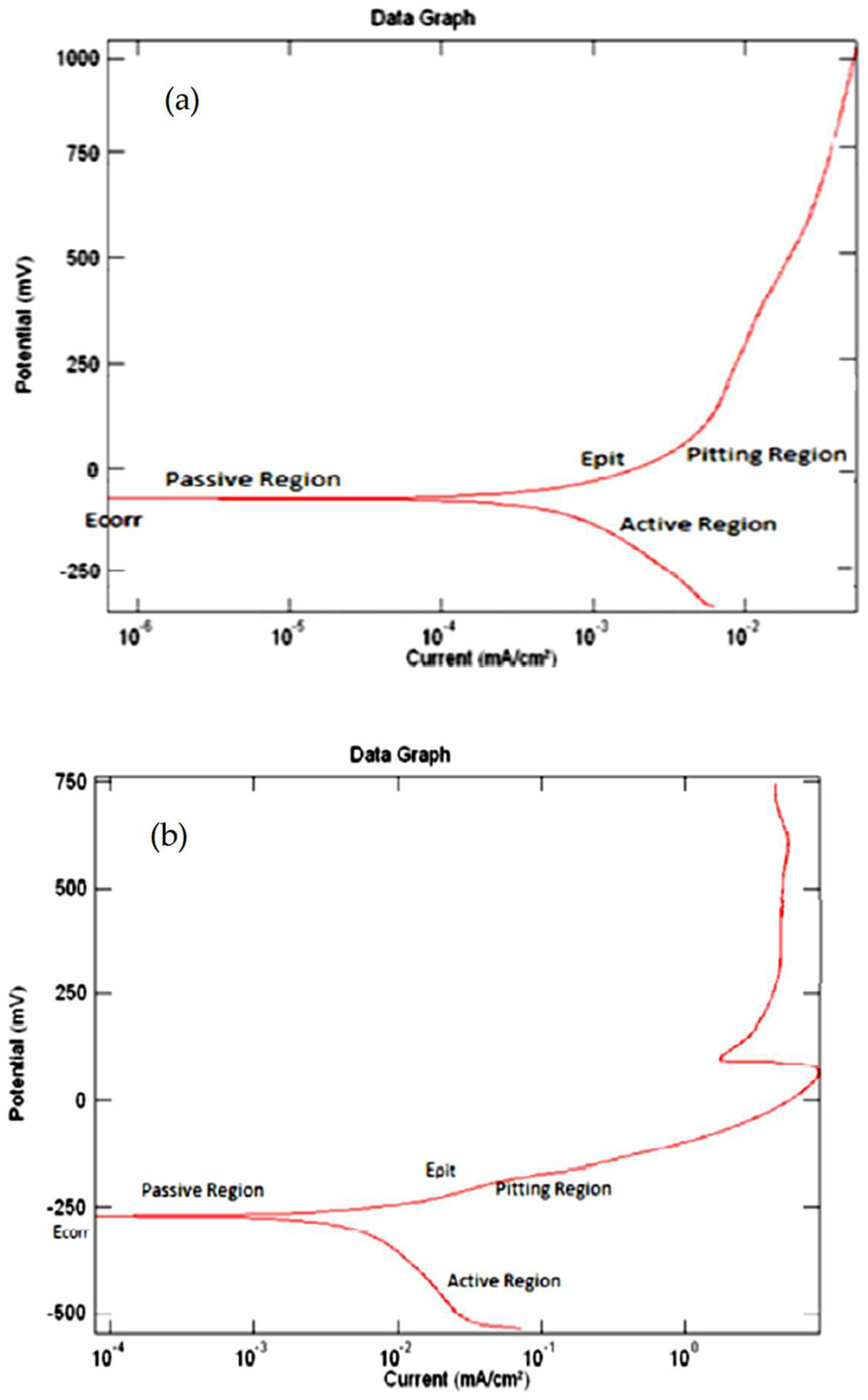
4.3. The Samples’ Tafel Plot
5. Cu-Al-Be-Mn Tetrad Memory Alloys
- (a)
- Excellent shape remembrance;
- (b)
- Appreciable mechanical strength;
- (c)
- Enough immerge ability due to martensitic transfiguration and pseudo-elasticity;
- (d)
- Speculative uniqueness to absorb sound, vibrations, and mechanical waves due to coarse grain.
- (a)
- Filters for embolic protection;
- (b)
- Tooth aligning wires;
- (c)
- Cardiovascular stents;
- (d)
- Microsurgical and endoscopic devices.
5.1. Test Procedure for Evaluating the Influence of Environment
- (i)
- Ocean water (H2O);
- (ii)
- Fresh water (H2O);
- (iii)
- Hank’s solution.
5.2. Results
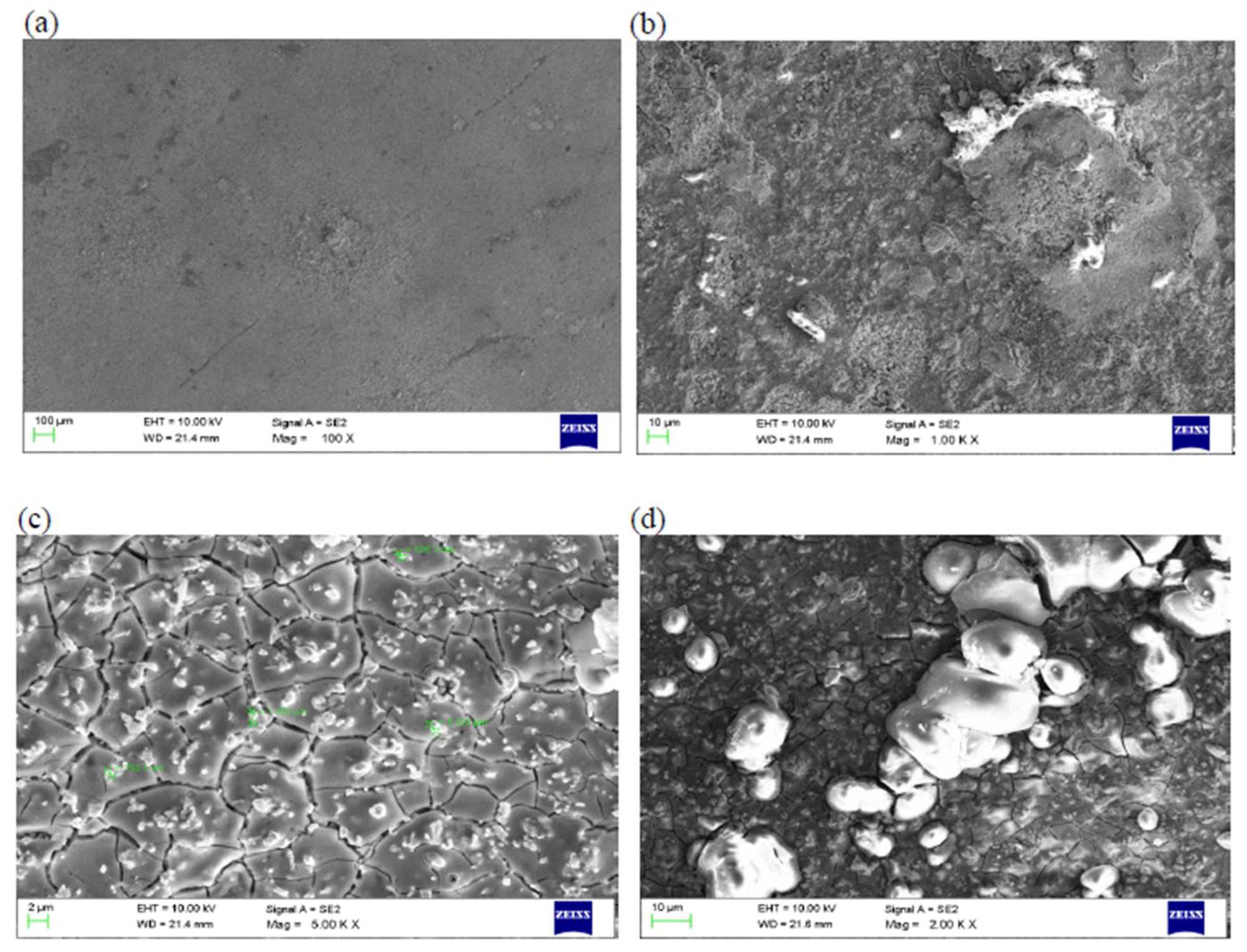
5.2.1. Study of Microstructure Following Exposure to Chosen Environment
5.2.2. Shape Memory Effect
5.2.3. Analysis of Rate of Degradation Due to the Environment
The Tafel Plots
- (i)
- The passivation region.
- (ii)
- The elementary passive zone.
- (iii)
- The initiation of passivation.
- (iv)
- The active region.
- (v)
- The trans-passivation zone.
5.2.4. Observations
- Exposure to freshwater resulted in a lower rate of corrosion for the copper-aluminum-beryllium–manganese shape memory alloy (SMA) when compared with Hank’s solution and ocean water.
- Hank’s solution had a stronger resistance to environment-induced degradation on the copper-aluminum-beryllium–manganese shape memory alloys (SMAs) than ocean water.
- By adding trace amounts of beryllium to the alloy, the copper-aluminum-beryllium–manganese quaternary shape memory alloys (SMAs) revealed an improved resistance to degradation induced by the aqueous environment.
- The Cu-Al-Be-Mn alloy had a remarkable 88 percent shape memory effect (SME).
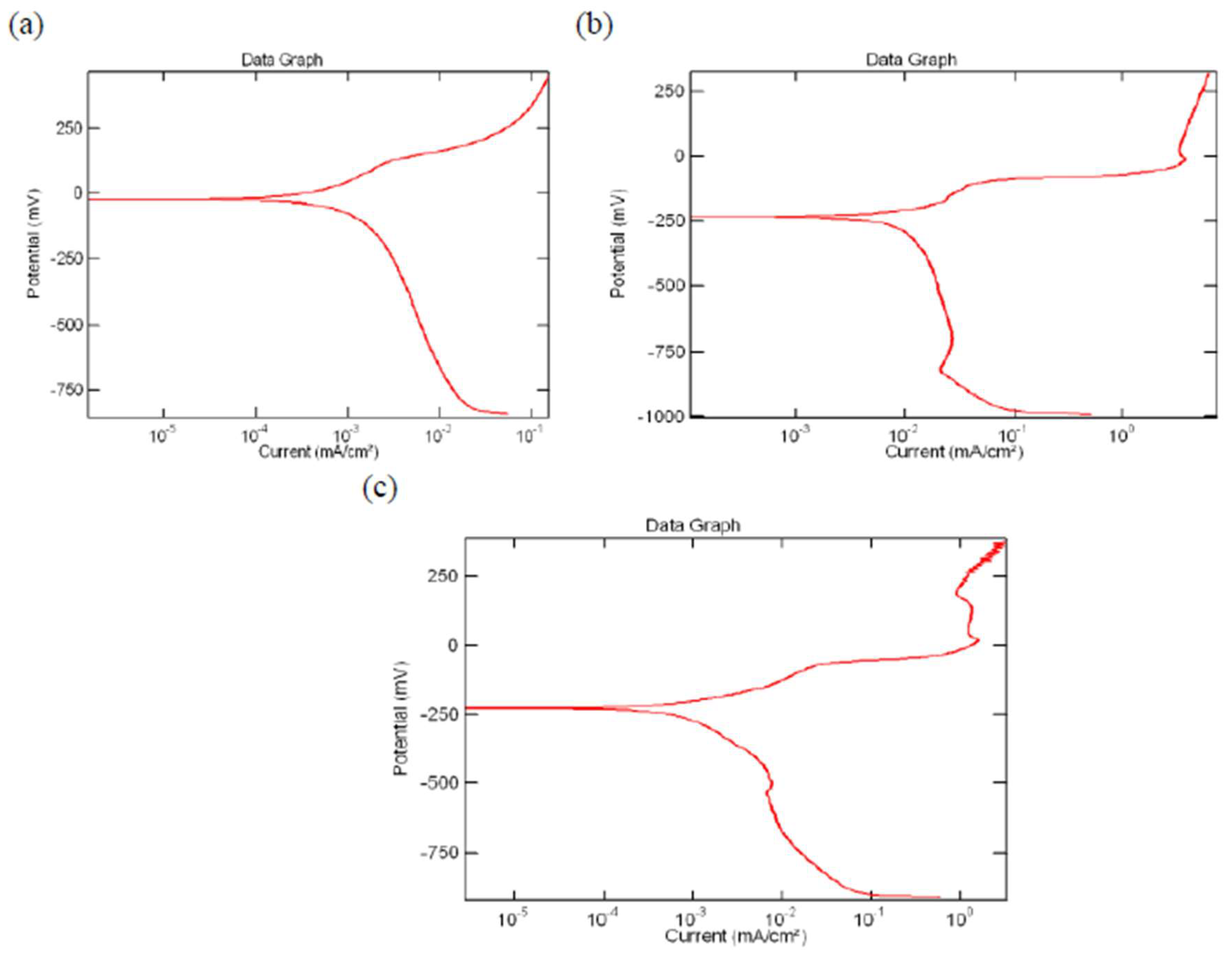
6. The Cu-Al-Ni-xCo Shape Memory Alloys Biformed with Low-Carbon Steel
6.1. Electrochemical Test on Sample Prepared
- (i)
- Copper-aluminum-nickel.
- (ii)
- Copper-aluminum-nickel-1.0 wt% Co.
- (iii)
- Copper-aluminum-nickel-0.4 wt% Co.
6.2. Microstructural Analysis
6.3. Performance upon Exposure to an Aqueous Environment
6.4. Interpretation on the Experimental Findings
- Ageing treatment of the copper-aluminum-nickel alloy with the addition of cobalt as the fourth element promoted grain refinement and produced fine precipitates of the Al75Co22Ni3 phase that contributed to increasing both the compactness and stability due to the formation and presence of a passive film and thereby improved the corrosive resistance.
- After being adjusted with the addition of 1 weight percent of cobalt along with ageing treatment, the linked copper-aluminum-nickel and low-carbon steel shape memory alloy (SMA) revealed an optimum value of corrosion resistance. This resulted in reducing the rate of environment-induced degradation, or corrosion, by well over 50 percent when compared with the low-carbon steel sample (uncoupled).
- After the addition of 1 weight percent of cobalt and ageing at 250 °C for 48 h, the copper-aluminum-nickel shape memory alloy revealed a microhardness of 340 Hv.
7. The Cu-Zn-Al Shape Memory Alloy in Monitored Ambience
- (a)
- X-ray diffraction;
- (b)
- Electronic microscopy;
- (c)
- Optical microscopy after chemically etching the polished surfaces using a chemical reagent. The reagent used was ferric chloride (FeCl3) in hydrochloric acid (HCl) solution.
- (a)
- 3.5% weight sodium chloride (NaCl) aqueous solution that simulated a marine environment.
- (b)
- Acid solutions of 1 M, 0.1 M, and 0.01 M nitric acid (HNO3) that replicated acid rain in a metropolitan setting.
- (c)
- Acid solutions of 1 M, 0.1 M, and 0.01 M sulfuric acid (H2SO4) that replicated acid rain in an industrial setting.
The Test Results
8. Nature of Degradation When Cobalt Is Added to the Nickel–Titanium Shape Memory Alloy (SMA) in Normal Saline Solution
- (a)
- A 30 percent higher modulus than the NiTi alloys.
- (b)
- A distinct loading plateau and an unloading plateau.
- (c)
- Non-reactivity in two tests, namely hemolysis and cytotoxicity.
- (i)
- Electrochemical tests;
- (ii)
- XPS;
- (iii)
- Scanning electron microscopy (SEM) observations;
- (iv)
- Energy dispersive X-ray (EDX) analysis.
8.1. Electrochemical Setup and Solutions
8.2. Role of Addition of Cobalt
Study of Microstructure
8.3. Summary of the Results
- (a)
- Overall homogeneity of the surface electrochemical characteristics;
- (b)
- Reduced activity of the microgalvanic cells.
9. The Nickel-Titanium Shape Memory Alloy Sintered by Spark Plasma Sintering (SPS)
9.1. Development of the Sintered Nickel-Titanium Shape Memory Alloy (SMA)
9.2. Characteristics upon Exposure to an Aggressive Environment
10. Biocompatibility of Shape Memory Alloys and Its Progress
10.1. Methods to Improve Biocompatibility
10.1.1. Grain Refinement
10.1.2. Surface Coatings
10.2. Challenges in Employing Shape Memory Alloys at Bioapplications
- (1)
- To fully comprehend the causes and mechanisms underlying temperature- or stress-induced phase changes between austenite and martensite, further microstructural insights into the mechanisms underlying SMA properties are needed. Understanding these mechanisms can help design SMA and manipulate corrosion with beneficial recommendations. Through in situ studies of SMA deformation and mechanical reactions during the phase transition, advanced microscopy is anticipated to play a significant role in this respect [106]. At the austenite–martensite contacts, crucial information may include the lattice resistance, steps, and dislocation arrays.
- (2)
- These materials must be modified to be suited for more nuanced biological applications, which calls for precise control of the SMA transition temperature and stress. There is evidence that alloy composition and thermomechanical treatments can adjust SMA transition temperatures [107]. The difficulty in designing a functional device stem from the fact that such modulation is not precise enough to achieve the precise needed values. In order to overcome this issue, computational intelligence may be useful, as topological models, artificial neural networks, and Gaussian process regression may all be used to anticipate transformation temperature and stress.
- (3)
- More research on passive films and film–metal interactions may provide new insights into the behavior of SMA corrosion. According to reports, TiO2 is crucial in preventing NiTi corrosion and the passive coatings on NiTi SMA display n-type semiconductor characteristics. While efficient TiO2 dissolution may be achieved by lowering the pH of the corrosion environment, doping levels can be enhanced by donor production at the metal–film interface to reduce the corrosion resistance of passive films [108]. Future work may focus on altering the chemical makeup of passive films to control their corrosion resistances or adjusting the doping levels of semiconducting passive films to produce metastable or stable pits and voids at the metal–film interface to speed up corrosion.
11. Conclusions
- The trend towards the use of less invasive techniques and microscopic applications will continue.
- Processing capabilities are being noticeably improved and the shape memory alloys (SMAs) are gradually gaining for themselves a dominant place for due consideration by all engineers for selection and use in medical design-related applications and even technologies specific to emerging smart materials.
- An increase in the selection and use of the shape memory alloys (SMAs) both in medicine and sensor technology can be expected. The shape memory alloys are currently being chosen for use in critical environments, such as high temperature vital fluids, i.e., the blood stream.
- The nature of environment-induced degradation, or corrosion, of the shape memory alloys (SMAs) is presented and examined in this paper based on results obtained from tests conducted in aggressive aqueous environments.
- Improving the resistance to environment-induced degradation of the shape memory alloys (SMAs) will pave the way for their selection and use in a sizeable number of applications. Further, discovering ways to resist degradation induced by the environment, through the development of passive films and coatings is both essential and desirable.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otsuka, C.M.K. Wayman: Shape Memory Materials; Cambridge University Press: Cambridge, UK, 1998; pp. 1–90. [Google Scholar]
- Santosh, S.; Praveen, R.; Sampath, V. Influence of Cobalt on the Hot Deformation Characteristics of an NiTi Shape Memory Alloy. Trans. Indian. Inst. Met. 2019, 72, 1465–1468. [Google Scholar] [CrossRef]
- Santosh, S.; Nithyanandh, G.; Ashwath, J.; Kishore, K.L. Comparison of Internal friction measurements on Ni-Ti reinforced smart composites prepared by Additive Manufacturing. J. Alloys Compd. 2022, 924, 166027. [Google Scholar] [CrossRef]
- Evirgen, A.; Ma, J.; Karaman, I.; Luo, Z.P.; Chumlyakov, Y.I. Effect of aging on the super elastic response of a single crystalline FeNiCoAlTa shape memory alloy. Scr. Mater. 2012, 67, 475–478. [Google Scholar] [CrossRef]
- Liu, B.; Wei, X.; Wang, W.; Lu, J.; Ding, J. Corrosion behavior of Ni-based alloys in molten NaCl-CaCl2-MgCl2 eutectic salt for concentrating solar power. Sol. Energy Mater. Sol. Cells 2017, 170, 77–86. [Google Scholar] [CrossRef]
- Fadlallah, S.A.; El-Bagoury, N.; El-Rab, S.M.F.G.; Ahmed, R.A.; El-Ousamii, G. An ov view of NiTi shape memory alloy: Corrosion resistance and antibacterial inhibition for dental application. J. Alloys Compd. 2014, 583, 445–464. [Google Scholar] [CrossRef]
- Mallik, U.S.; Sampath, V. Influence of Aluminum and Manganese Concentration on the Shape Memory Characteristics of Cu-Al-Mn Shape Memory Alloys. J. Alloys Compd. 2008, 459, 142–147. [Google Scholar] [CrossRef]
- Castleman, L.S.; Motzki, S.M.; Williams, D.F. Biocompatibility of nitinol. In Biocompatibility of Clinical Implant Material; CRC Press: Boca Raton, FL, USA, 1981; Volume 1, pp. 129–154. [Google Scholar]
- Buehler, J.; Wang, F.E. Summary research in the nitinol alloys and their potential applications in ocean engineering. Ocean. Eng. 1968, 1, 105–108. [Google Scholar] [CrossRef]
- Spruit, D.; Bongaarts, P.J.M.; Malden, K.E. Dermatological effects of nickel. In Nickel in the Environment; Nriagu, J.O., Ed.; Wiley: New York, NY, USA, 1980; pp. 601–609. [Google Scholar]
- Revie, R.W.; Green, N.D. Corrosion behaviour of surgical implant materials: I Effect of sterilization. Corms Sci. 1969, 10, 755–762. [Google Scholar] [CrossRef]
- ASTM International. Annual Book of ASTM Standard; American Society for Testing and Materials: Philadelphia, PA, USA, 1986; Volume 03.02, pp. 560–575. [Google Scholar]
- Pessal, N.; Liu, C. Determination of critical potentials of stainless steels in aqueous chloride environments. Electrochem. Acta. 1971, 16, 1987–2003. [Google Scholar] [CrossRef]
- Malki, B.; Berthomé, G.; Souier, T.; Boissy, C.; Guillotte, I.; Baroux, B. A Combined Experimental and Computational Approach to Study Crevice Corrosion of Stainless Steels. J. Electrochem. Soc. 2021, 168, 101504. [Google Scholar] [CrossRef]
- Sohmura, T.; Kimura, H. Shape recovery in Ti-V-Fe- Al alloy and its application to dental implant. In Proceedings of the International Conference on Martensitic Transformations, Nara, Japan, 26–30 August 1986; The Japan Institute of Metals: Nara, Japan, 1986; pp. 1065–1070. [Google Scholar]
- Edie, J.W.; Andreasen, G.F.; Zaytoun, M.P. Surface corrosion of nitinol and stainless steel under clinical conditions. Angle Orthod. 1981, 51, 319–324. [Google Scholar]
- Sarkar, N.K.; Redmond, W.; Schwaninger, W. The chloride corrosion behaviour of four orthodontic wires. J. Dent. Res. 1979, 10, 53–98. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, Y.; Yamamuro, T.; Kotoura, Y.; Oka, M. Electrochemical corrosion study of surgical implant metals in rabbit back muscle with comparison to Ringer’s solution. In Proceedings of the Third World Biomaterials Congress, Kyoto, Japan, 21–25 April 1988; p. 202. [Google Scholar]
- Nakayama, Y.; Yamamuro, T.; Kotoura, Y.; Oka, M. In vivo measurement of anodic polarization of orthopaedic implant alloys: Comparative study of in viva and in vitro experiments. Biomaterials 1989, 10, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, A.M.; Spires, W.P.; Klawitter, J.J.; Clemow, A.J.T.; Edmunds, J.O. Orthopaedic implant retrieval and analysis study. In Corrosion and Degradation of Implant Materials; Syrett, B.C., Acharya, A., Eds.; ASTM International: West Conshohocken, PA, USA, 1979; pp. 212–228. [Google Scholar]
- Prashanth, S.; Shivasiddaramaiah, A.G.; Mallikarjun, U.S. Corrosion Behaviour of Cu-Al-Be Based Shape Memory Alloy With and Without Coating. Mater. Today Proc. 2019, 17, 147–154. [Google Scholar] [CrossRef]
- Shivasiddaramaiah, A.G.; Manjunath, S.Y.; Singh, P.; Mallikarjun, U.S. Synthesis and Evaluation of Mechanical Properties of Cu-Al-Be- Mn Quaternary Shape Memory Alloys. Int. J. Appl. Eng. Res. 2015, 55, 356–390. [Google Scholar]
- Priyanga, G.S.; Mattur, M.N. Mattur, Structural and mechanical properties of NiTiAg shape memory alloys: ab-initio study. Modelling Simul. Mater. Sci. Eng. 2023, 31, 065004. [Google Scholar] [CrossRef]
- Chen, B.; Liang, C.; Fu, D. Pitting Corrosion of Cu-Zn-Al Shape Memory Alloy in Simulated Uterine Fluid. J. Mater. Sci. Technol. 2005, 21, 226–230. [Google Scholar]
- Shivasiddaramaiah, A.G.; Das, B.R.; Singh, P. Study on Corrosion Behaviour of Cu-Al-Be-Mn Quaternary Shape Memory Alloy At Room Temperature. Int. J. Appl. Eng. Res. 2015, 55, 1115–1130. [Google Scholar]
- Shivasiddaramaiah, A.G.; Singh, P.; Manjunath, S.Y.; Mallikarjun, U.S. Microstructure and Shape Memory Effect of Cu-Al-Be-Mn Quaternary Shape Memory Alloys. Appl. Mech. Mater. 2015, 813–814, 213–217. [Google Scholar] [CrossRef]
- Wu, S.; Lin, H. Recent development of TiNi-based shape memory alloys in Taiwan. Mater. Chem. Phys. 2011, 64, 81–92. [Google Scholar] [CrossRef]
- Duerig, T.W.; Melton, K.N.; Stockel, D.; Wayman, C.M. Engineering Aspects of Shape Memory Alloys, Books on Demand; Butterworth Heinemann Publishing: London, UK, 1990. [Google Scholar]
- Lopez, G.A.; Barrado, M.; Bocanegra, E.H.; San Juan, J.M.; No, M.L. Cu–Al–Ni shape memory alloy composites with very high damping capacity. In International conference on martensitic transformations (ICOMAT); John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; Volume 75, pp. 231–238. [Google Scholar]
- Song, G.; Patil, D.; Kocurek, C.; Bartos, J. Earth and Space 2010: Engineering, Science, Construction, and Operations in Challenging Environments; ASCE Publications: League City, TX, USA, 2010; pp. 1551–1567. [Google Scholar]
- Prashanth, S.; Shivasiddaramaiah, A.G. Shape memory effect in Cu-Sn-Mn ternary shape memory alloy processed by ingot metallurgy. Int. J. Metall. Mater. Sci. 2012, 75, 850–895. [Google Scholar]
- Wei, Z.G.; Sandstrom, R. Shape-memory materials and hybrid composites for smart systems. J. Mater. Sci. 1998, 33, 3743–3762. [Google Scholar] [CrossRef]
- Davis, J.R. Copper and Copper Alloys. ASM Spec. Handb. 2001, 2, 759–780. [Google Scholar]
- Abid, K.A.R.A.; Al-Tai, T.Z. The Effect of Iron Addition on the Dry Sliding Wear and Corrosion Behavior of Cu Al Ni Shape Memory Alloy. Eng. Technol. J. 2010, 28, 6888–6902. [Google Scholar]
- Christian, L. Shape-Memory Alloys Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2013; Volume 8, pp. 61–108. [Google Scholar]
- Chenxu, Z. Shape Memory Stainless Steel. In Advanced Materials & Processing; Xi’an Jiao tong University: Xi’an, China, 2011; pp. 2015–2045. [Google Scholar]
- Sevost’yanov, M.A.; Nasakina, E.O.; Baikin, A.S.; Sergienko, K.V.; Konushkin, S.V.; Kaplan, M.A.; Seregin, A.V.; Leonov, A.V.; Kozlov, V.A.; Shkirin, A.V.; et al. 2018 Biocompatibility of new materials based on nano-structured nitinol with titanium and tantalum composite surface layers: Experimental analysis in vitro and in vivo. J. Mater. Sci. Mater. Med. 2012, 29, 33–60. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, J.; Zhang, X.; Wang, P.; Shi, J.; Huo, F. Corrosion Behaviour of Ti–6Al–4V Alloy as Dental Implant Containing Fluoride Ions. Int. J. Electrochemical. Sci. 2017, 12, 10308–10316. [Google Scholar] [CrossRef]
- Sevostyanov, M.A.; Baikin, A.S.; Shatova, L.A.; Nasakina, E.O.; Berezhnov, A.V.; Gudkov, S.V.; Sergienko, K.V.; Konushkin, S.V.; Baskakova, M.I. Biocompatibility of the Ti81Nb13Ta3Zr3 Alloy Doklady Chemistry. J. Biomed. Mater. Res. 2018, 52, 395–403482. [Google Scholar]
- Kim, J.I.; Kim, H.Y.; Inamura, T.; Hosoda, H.; Miyazaki, S. Shape memory characteristics of Ti– 22Nb–(2–8)Zr(at.%) biomedical alloys. Mater. Sci. Eng. A 2005, 403, 334–339. [Google Scholar] [CrossRef]
- Dubinsky, S.M.; Proboski, S.D.; Brailovski, V.; Inaekyan, K.; Korotitskiy, A.V.; Filonov, M.R.; Petrzhik, M.I. Structure Formation During Thermomechanical Processing of Ti-Nb-(Zr, Ta) Alloys and the Manifestation of the Shape-Memory Effect. Phys. Met. Metallogr. 2011, 112, 529–542. [Google Scholar] [CrossRef]
- de Salazar, J.M.G.; Soria, A.; Barrena, M.I. Corrosion behavior of Cu-based shape memory alloys diffusion bonded. J. Alloys Compd. 2005, 387, 109–114. [Google Scholar] [CrossRef]
- Heusler, K.E.; Landolt, D.; Trasaiti, S. Electrochemical Corrosion Nomenclature. Pure Appl. Chem. 1989, 61, 19–22. [Google Scholar] [CrossRef]
- Trethewey, K.R.; Chamberlain, J. Corrosion for Students of Science and Engineering, 3rd ed.; John Wiley & Sons Inc: Hoboken, NJ, USA, 1995; Volume 18, pp. 2000–2045. [Google Scholar]
- Shaw, B.A.; Kelly, R.G. What is corrosion? Electrochem. Soc. Interface 2006, 15, 24–26. [Google Scholar] [CrossRef]
- Zhang, Q.; Xu, P.; Pang, C.; Yub, K.C.C.; Huang, L. A superhydrophobic surface with a synergistic abrasion–corrosion resistance effect prepared by femtosecond laser treatment on an FeMnSiCrNiNb shape memory alloy coating. New J. Chem. 2022, 46, 19188. [Google Scholar] [CrossRef]
- Velmurugan, C.; Kumar, V.S.; Kamala, P.S. Microstructure and corrosion behavior of NiTi shape memory alloys sintered in the SPS process. Int. J. Miner Metall. Mater. 2019, 42, 1311. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhu, T. Phase-field modelling of martensitic microstructure in NiTi shape memory alloys. Acta Mater. 2014, 75, 337. [Google Scholar] [CrossRef]
- Elayaperumal, K.; Raja, V.S.; Failures, C.; Studies, C. Corrosion Failures: Theory, Case Studies, and Solutions, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015; Volume 24, p. 26. [Google Scholar]
- Mallik, A.G.S.U.S.; Mahato, R.; Shekar, C.S. Evaluation of corrosion behaviour of Cu-Al-Be-Mn quaternary shape memory alloys. Mater. Today Proc. 2017, 4, 10971–10977. [Google Scholar]
- Stergioudi, F.; Vogiatzis, C.A.; Pavlidou, E.; Skolianos, S.; Michailidis, N. Corrosion resistance of porous NiTi biomedical alloy in simulated body fluids. Smart Mater. Struct. 2016, 25, 322–345. [Google Scholar] [CrossRef]
- Jani, J.M.; Leary, M.; Subic, A.; Gibson, M.A. A review of shape memory alloy research, applications and opportunities. Mater. Des. 2014, 56, 1078. [Google Scholar] [CrossRef]
- Novák, P.; Pokorný, P.; Vojtěch, V.; Knaislová, A.; Školáková, A.; Čapek, J.; Karlík, M.; Kopecek, J. Formation of Ni–Ti intermetallic during reactive sintering at 500–650 °C. Mater. Chem. Phys. 2015, 155, 113. [Google Scholar] [CrossRef]
- Elahinia, M.H.; Hashemi, M.; Tabesh, M.; Bhaduri, S.B. Manufacturing, and processing of NiTi implants: A review. Prog. Mater. Sci. 2012, 57, 911. [Google Scholar] [CrossRef]
- Elahinia, M.; Moghaddam, N.S.; Andani, M.T.; Amerinatanzi, A.; Bamber, B.A.; Hamilton, R.F. Fabrication of NiTi through additive manufacturing: A review. Prog. Mater. Sci. 2016, 83, 630. [Google Scholar] [CrossRef]
- Morgan, N.B.; Broadley, M. Taking the art out of smart-Forming processes and durability issues for the application of NiTi shape memory alloys in medical devices. In Proceedings of the Materials and Processes for Medical Devices Conference, St. Paul, MN, USA, 25–27 August 2004; Volume 6, p. 247. [Google Scholar]
- Zhang, L.; Ren, D.; Ji, H.; Ma, A.; Daniel, E.F.; Li, S.; Jin, W.; Zheng, Y. Study on the corrosion behavior of NiTi shape memory alloys fabricated by electron beam melting. NPJ Mater Degrad 2022, 6, 79. [Google Scholar] [CrossRef]
- Yang, C.; Liu, L.; Cheng, Q.; You, D.; Li, Y. Equiaxed grained structure: A structure in titanium alloys with higher compressive mechanical properties. Mater. Sci. Eng. A 2013, 580, 397–405. [Google Scholar] [CrossRef]
- Hang, R.Q.; Ma, S.L.; Ji, V.; Chu, P.K. Corrosion behavior of NiTi alloy in foetal bovine serum. Electrochim. Acta 2010, 55, 5551. [Google Scholar]
- Dong, H.; Ju, X.; Yang, H.; Qian, L.; Zhou, Z. Effect of ceramic conversion treatments on the surface damage and nickel ion release of NiTi alloys under fretting corrosion conditions. J. Mater. Sci. Mater. Med. 2008, 19, 937. [Google Scholar] [CrossRef] [PubMed]
- Chembath, M.; Balaraju, J.N.; Sujata, M. In vitro corrosion studies of surface modified NiTi alloy for biomedical applications. Adv. Biomater. 2014, 20, 229–263. [Google Scholar]
- Kim, J.; Park, J.K.; Kim, H.K.; Unnithan, A.R.; Kim, C.S.; Park, C.H. Optimization of electropolishing on NiTi alloy stents and its influence on corrosion behaviour. J. Nanosci. Nanotechnol. 2017, 17, 460–470. [Google Scholar]
- Hosseini, S.A.; Akbarinia, S.; Mohammadyani, D.; Sadrnezhaad, S.K. Enhanced corrosion resistance of porous NiTi with plasma sprayed alumina coating. Corros. Eng. Sci. Technol. 2015, 50, 595. [Google Scholar]
- Velmurugan, C.; Kumar, V.S. The effect of Cu addition on the morphological, structural and mechanical characteristics of nanocrystalline NiTi shape memory alloys. J. Alloys Compd. 2018, 767, 944. [Google Scholar] [CrossRef]
- Fu, Y.Q.; Gu, Y.W.; Sherwood, C.; Luo, J.K.; Flewitt, A.J.; Milne, W.I. Spark plasma sintering of TiNi nano-powders for biological application. Nanotechnology 2006, 17, 5293. [Google Scholar] [CrossRef]
- Verdian, M.M.; Raeissi, K.; Salehi, M.; Sabooni, S. Characterization and corrosion behavior of NiTi–Ti2Ni–Ni3Ti multiphase intermetallic produced by vacuum sintering. Vacuum 2011, 86, 91. [Google Scholar] [CrossRef]
- Chen, J.; Qiao, Y.; Meng, F.; Wang, Y. Corrosion and Degradation of Materials. Coatings 2022, 2005, 12–969. [Google Scholar]
- Chen, J.; Rasmussen, R.M.; Zagidulin, D.; Noel, J.J.; Shoesmith, D.W. Electrochemical and corrosion behavior of a 304 stainless-steel-based metal alloy waste form in dilute aqueous environments. Corros. Sci. 2013, 66, 142–152. [Google Scholar] [CrossRef]
- Qiao, Y.; Wang, X.; Yang, L.; Wang, X.; Chen, J.; Wang, Z.; Zhou, H.; Zou, J.; Wang, F. Effect of aging treatment on microstructure and corrosion behavior of a Fe-18Cr-15Mn-0.66N stainless steel. J. Mater. Sci. Technol. 2022, 107, 197–206. [Google Scholar]
- Hu, T.; Chu, C.L.; Xin, Y.; Paul, K.C. Corrosion products and mechanism on NiTi shape memory alloy in physiological environment. J. Mater. Res. 2019, 25, 350–358. [Google Scholar] [CrossRef]
- Widu, F.; Drescher, D.; Junker, R.; Bourauel, C. Corrosion and biocompatibility of orthodontic wires. J. Mater. Sci.-Mater. Med. 1999, 10, 275. [Google Scholar] [CrossRef]
- Cruz, J.C.; Nascimentoa, M.A.; Amaral, H.A.V.; Lima, D.S.D.; Teixeira, A.P.C.; Renata, P. Lopes Synthesis and characterization of cobalt nanoparticles for application in the removal of textile dye. J. Environ. Manag. 2019, 242, 220–228. [Google Scholar] [CrossRef]
- Huang, H.H.; Chiu, Y.H.; Lee, T.H.; Wu, S.C.; Yang, H.W.; Su, K.H.; Hsu, C.C. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials 2003, 24, 3585. [Google Scholar] [CrossRef]
- Mediha, K.; Zardari, H.S.A.; Qader, I.N.; Kanca, M.S. The effects of cobalt elements addition on Ti2Ni phases, thermodynamics parameters, crystal structure and transformation temperature of NiTi shape memory alloys. Eur. Phys. J. Plus 2019, 134, 197. [Google Scholar]
- Santosh, S.; Sampath, V. Effect of Ternary Addition of Cobalt on Shape Memory Characteristics of Ni–Ti Alloys. Trans. Indian Inst. Met. 2019, 72, 1481–1484. [Google Scholar] [CrossRef]
- Najib, A.S.M.; Saud, S.N.; Hamzah, E. Corrosion Behavior of Cu–Al–Ni–xCo Shape Memory Alloys Coupled with Low-Carbon Steel for Civil Engineering Applications. J. Bio-Tribo-Corros. 2019, 5, 47. [Google Scholar] [CrossRef]
- Tang, C.Y.; Zhang, L.N.; Wong, C.T.; Chan, K.C.; Yue, T.M. Fabrication and characteristics of porous NiTi shape memory alloy synthesized by microwave sintering. Mater. Sci. Eng. A 2011, 528, 6006–6011. [Google Scholar] [CrossRef]
- Kapanen, A.; Ryann, J.; Danilov, A.; Tuukkanen, J. Effect of nickel–titanium shape memory metal alloy on bone formation. Biomaterials 2001, 22, 2475–2480. [Google Scholar] [CrossRef] [PubMed]
- Mentz, J.; Frenzel, J.; Wagner, M.F.-X.; Neuking, K.; Eggeler, G.; Buchkremer, H.P.; Stover, D. Powder metallurgical processing of NiTi shape memory alloys with elevated transformation temperatures. Mater. Sci. Eng. A 2008, 491, 270–278. [Google Scholar] [CrossRef]
- Velmurugan, C.; Kumar, V.S.; Biswas, K.; Yadav, S. Densification and microstructural evolution of spark plasma sintered NiTi shape memory alloy. Adv. Powder Technol. 2018, 45, 2456–2462. [Google Scholar] [CrossRef]
- Diouf, S.; Molinari, A. Densification mechanisms in spark plasma sintering: Effect of particle size and pressure. Powder Technol 2012, 221, 220–227. [Google Scholar] [CrossRef]
- Cheng, Y.; Cui, Z.; Cheng, L.; Gong, D.; Wang, W. Effect of particle size on densification of pure magnesium during spark plasma sintering. Adv. Powder Technol. 2017, 28, 1129–1135. [Google Scholar] [CrossRef]
- Falodun, O.E.; Obadele, B.A.; Oke, S.R.; Maja, M.E.; Olubambi, P.A. Effect of sintering parameters on densification and microstructural evolution of nano- sized titanium nitride reinforced titanium alloys. J. Alloys Comp. 2018, 736, 202–210. [Google Scholar] [CrossRef]
- Lee, G.; McKittrick, J.; Ivanov, E.; Olevsky, E.A. Densification mechanism and mechanical properties of tungsten powder consolidated by spark plasma sintering. Int. J. Refract. Met. Hard Mater. 2016, 61, 22–29. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.Q.; Jiang, Y.H.; Zhou, R. Super elastic behaviours of biomedical porous NiTi alloy with high porosity and large pore size prepared by spark plasma sintering. J. Alloys Comp. 2015, 644, 513–522. [Google Scholar] [CrossRef]
- Chiyasak, P.; Kalnaowakul, P.; Rodchanarowan, A. The investigation of magnetic property and corrosion resistance of the assisted Sol-Gel synthesis of Ti/Ni/Co nanocomposites. Surf. Coat. Technol. 2020, 393, 2125–2150. [Google Scholar] [CrossRef]
- Nam, T.H.; Lee, J.H.; Jung, D.W.; Yu, C.A.; Liu, Y.; Kim, Y.W. Transformation behaviour of Ti-Ni and Ti-Ni-Cu alloy ribbons with nano Ti2Ni particles. Mater. Sci. Eng. A 2007, 449–451, 1041–1044. [Google Scholar] [CrossRef]
- Alqarni, N.D.; Wysocki, J.; El-Bagoury, N.; Reel, J.; Mohammed, A. Amin Rabah Boukherroub Effect of cobalt addition on the corrosion behavior of near equiatomic NiTi shape memory alloy in normal saline solution: Electrochemical and XPS studies. RSC Adv. 2018, 8, 19289. [Google Scholar] [CrossRef]
- Han, Y.; Kong, F.L.; Han, F.F.; Inoue, A.; Zhu, S.L.; Shalaan, E.; Al-Marzouki, F. New Fe-based soft magnetic amorphous alloys with high saturation magnetization and good corrosion resistance for dust core application. Intermetallic 2016, 76, 18–25. [Google Scholar] [CrossRef]
- de Araújo, C.J.; da Silva, N.J.; da Silva, M.M.; Gonzalez, C.H. A comparative study of Ni–Ti and Ni–Ti–Cu shape memory alloy processed by plasma melting and injection molding. Mater. Des. 2011, 32, 4925–4930. [Google Scholar] [CrossRef]
- da Silva, N.J.; Grassi, E.N.D.; de Araújo, C.J. Dynamic properties of NiTi shape memory alloy and classic structural materials: A comparative analysis. Mater. Sci. Forum 2010, 643, 37–41. [Google Scholar] [CrossRef]
- Machado, L.G.; Savi, M.A. Medical applications of shape memory alloys. Braz. J. Med. Biol. Res. 2002, 36, 683–691. [Google Scholar] [CrossRef]
- Gonzalez, C.H.; Oliveira, C.A.N.; Pina, E.A.C.; Urtiga Filho, S.L.; Araújo Filho, O.O.; de Araújo, C.J. Heat treatments and thermomechanical cycling influences on the R-phase in Ti–Ni shape memory alloys. Mater. Res. 2010, 13, 325–331. [Google Scholar] [CrossRef]
- Cai, W.; Men, X.L.; Zhao, L.C. Recent development of TiNi-based shape memory alloys. Solid. State Mater. Sci. 2005, 9, 296–302. [Google Scholar] [CrossRef]
- Pasebani, S.; Dutt, A.K.; Burns, J.; Charit, I.; Rajiv, S. Mishra Oxide dispersion strengthened nickel-based Alloys via spark plasma sintering. Mater. Sci. Eng. A 2015, 45, 1506–1530. [Google Scholar]
- Makena, I.M.; Shongwe, M.B.; Ramakokovhu, M.M.; Moipone, L. Letha bane A Review on Sintered Nickel based Alloys. In Proceedings of the World Congress on Engineering, London, UK, 5–7 July 2017. [Google Scholar]
- Songwe, M.B.; Diouf, S.; Durowoju, M.O.; Olubambi, P.A. Effect of sintering temperature on the microstructure and mechanical properties of Fe-30%Ni alloys produced by spark plasma sintering. J. Alloys Compd. 2015, 649, 824–832. [Google Scholar] [CrossRef]
- Mignanelli, P. Processing nickel-base superalloys. Naked Sci. 2012, 51, 4015–4020. [Google Scholar]
- Metikos-Hukovic, M.; Babic, R. Passivation and corrosion behaviours of cobalt and cobalt–chromium–molybdenum alloy. Corros. Sci. 2007, 49, 3570–3579. [Google Scholar] [CrossRef]
- Shao, X.; Guo, X.; Han, Y.; Lin, Z.; Qin, J.; Lu, W.; Zhang, D. Preparation of TiNi films by diffusion technology and the study of the formation sequence of the intermetallic in Ti-Ni systems. J. Mater. Res. 2014, 29, 2707–2716. [Google Scholar] [CrossRef]
- Nazeer, A.A.; Madkour, M. Potential use of smart coatings for corrosion protection of metals and alloys: A review. J. Mol. Liq. 2018, 253, 11–22. [Google Scholar] [CrossRef]
- Kawahara, Y. High temperature corrosion mechanisms and effect of alloying elements for materials used in waste incineration environment. Corros. Sci. 2002, 44, 223–245. [Google Scholar] [CrossRef]
- Liu, M.; Zheng, J.Y.; Lu, Y.L.; Li, Z.J.; Zou, Y.; Yu, X.H.; Zhou, X.T. Investigation on corrosion behavior of Ni-based alloys in molten fluoride salt using synchrotron radiation techniques. J. Nucl. Mater. 2013, 440, 124–128. [Google Scholar] [CrossRef]
- Walther, G.; Kl, B.; Büttner, T.; Weißg, T.; Kieback, B.; Böhm, A.; Naumann, D.; Saberi, S.; Timberg, L. A new class of high temperature and corrosion resistant nickel-based open-cell foams. Adv. Eng. Mater. 2008, 10, 803–811. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, X.; Han, E.; Ke, W.; Yang, K.; Jiang, Z. Influence of cold work on pitting corrosion behavior of a high nitrogen stainless steel. J. Electrochem. Soc. 2008, 155, 8. [Google Scholar] [CrossRef]
- Brook, G.B. Applications of titanium-nickel shape memory alloys. Mater. Des. 1983, 4, 835–840. [Google Scholar] [CrossRef]
- Kujala, S.; Ryh, J.; Jämsä, T.; Danilov, A.; Saaranen, J.; Pramila, A.; Tuukkanen, J. Bone modeling controlled by a nickel–titanium shape memory alloy intramedullary nail. Biomaterials 2002, 23, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Kasano, F.; Morimitsu, T. Utilization of nickel-titanium shape memory alloy for stapes prosthesis. Auris Nasus Larynx 1997, 24, 137–142. [Google Scholar] [CrossRef] [PubMed]


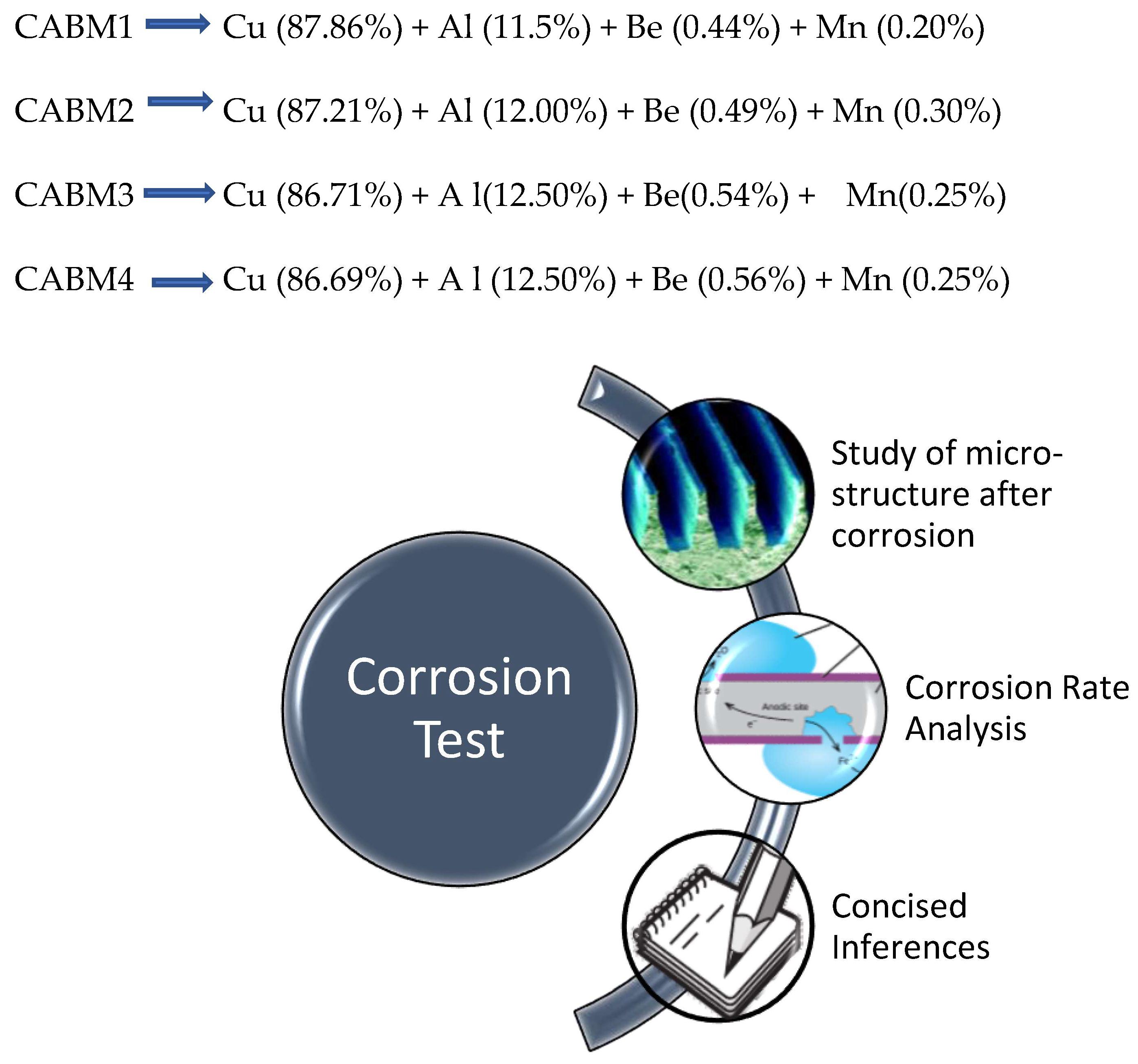
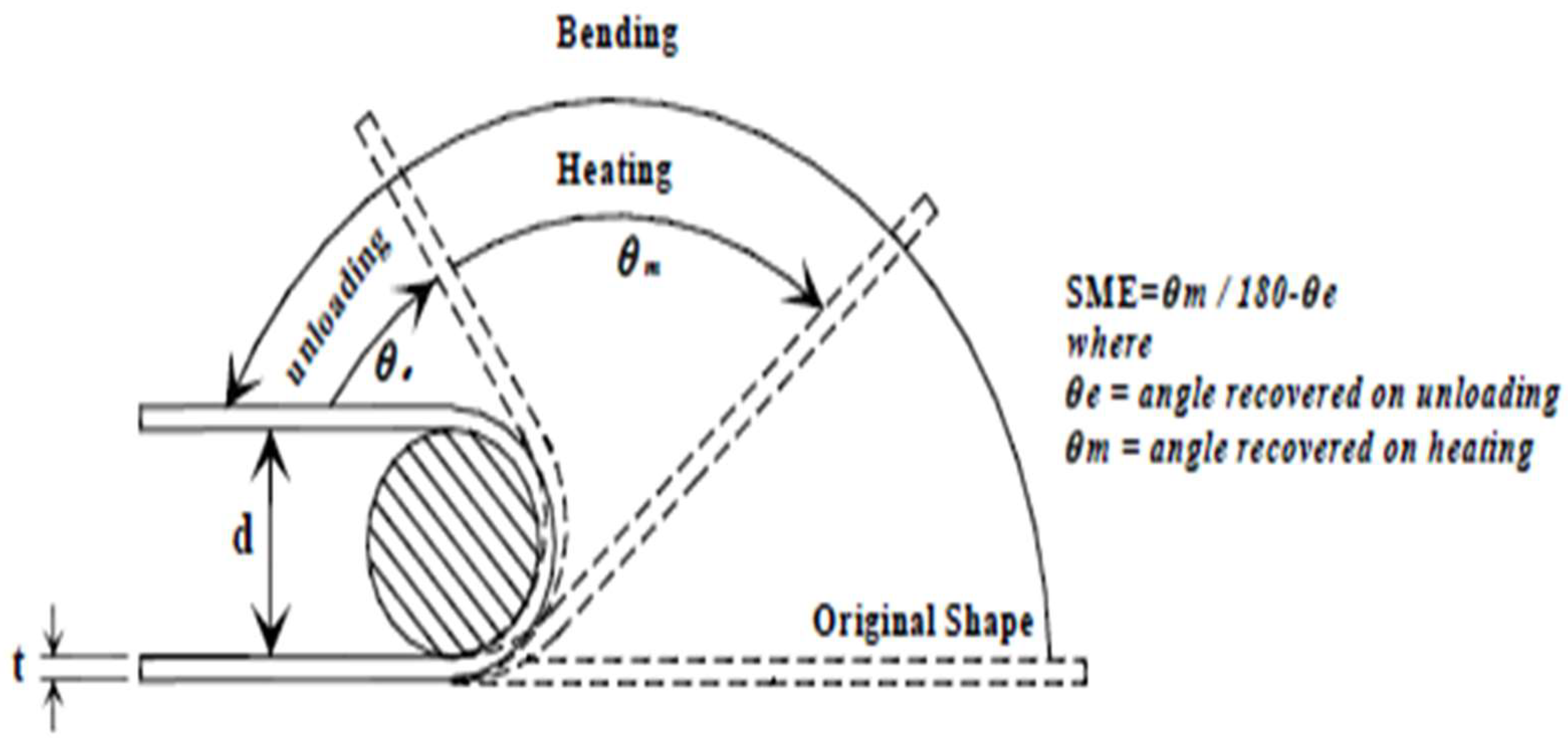

| Sample | Diameter | Thickness | Angle Recovered | SME% |
|---|---|---|---|---|
| CAB1 | 32 mm | 1 | 72 | 81 |
| CAB2 | 32 mm | 1 | 80 | 88 |
| CAB3 | 32 mm | 1 | 60 | 65 |
| CAB4 | 32 mm | 1 | 66 | 75 |
| CAB1 → Cu (88.01%) + Al (11.5%) + Be (0.44%) [Without coating] CAB2 → Cu (88.05%) + Al (11.5%) + Be (0.45%) [Without coating] CAB3 → Cu (88.01%) + Al (11.5%) + Be (0.44%) [With coating] CAB4 → Cu (88.05%) + Al (11.5%) + Be (0.45%) [With coating] | ||||
| Sample | Diameter | Thickness | Angle Recovered | SME% |
|---|---|---|---|---|
| CABM1 | 32 mm | 1 | 155 | 82.39 |
| CABM2 | 32 mm | 1 | 148 | 77.93 |
| CABM3 | 32 mm | 1 | 157 | 83.57 |
| CABM4 | 32 mm | 1 | 163 | 88.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santosh, S.; Harris, W.B.J.; Srivatsan, T.S. Environment-Induced Degradation of Shape Memory Alloys: Role of Alloying and Nature of Environment. Materials 2023, 16, 5660. https://doi.org/10.3390/ma16165660
Santosh S, Harris WBJ, Srivatsan TS. Environment-Induced Degradation of Shape Memory Alloys: Role of Alloying and Nature of Environment. Materials. 2023; 16(16):5660. https://doi.org/10.3390/ma16165660
Chicago/Turabian StyleSantosh, S., W. B. Jefrin Harris, and T. S. Srivatsan. 2023. "Environment-Induced Degradation of Shape Memory Alloys: Role of Alloying and Nature of Environment" Materials 16, no. 16: 5660. https://doi.org/10.3390/ma16165660
APA StyleSantosh, S., Harris, W. B. J., & Srivatsan, T. S. (2023). Environment-Induced Degradation of Shape Memory Alloys: Role of Alloying and Nature of Environment. Materials, 16(16), 5660. https://doi.org/10.3390/ma16165660









