Properties of Gangue Powder Modified Fly Ash-Based Geopolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimens
2.3. Test Procedure
3. Results and Analyses
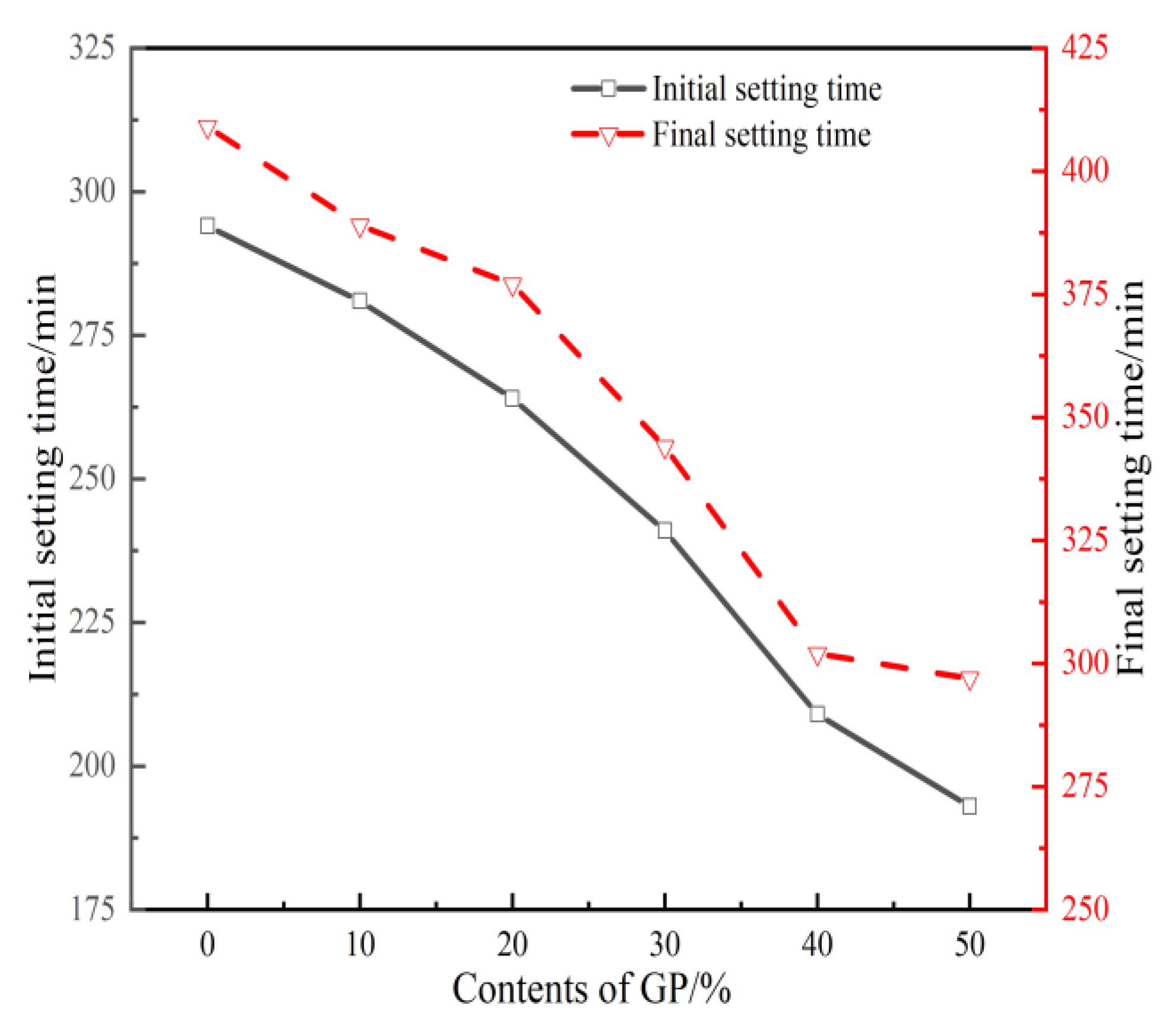
3.1. Setting Time
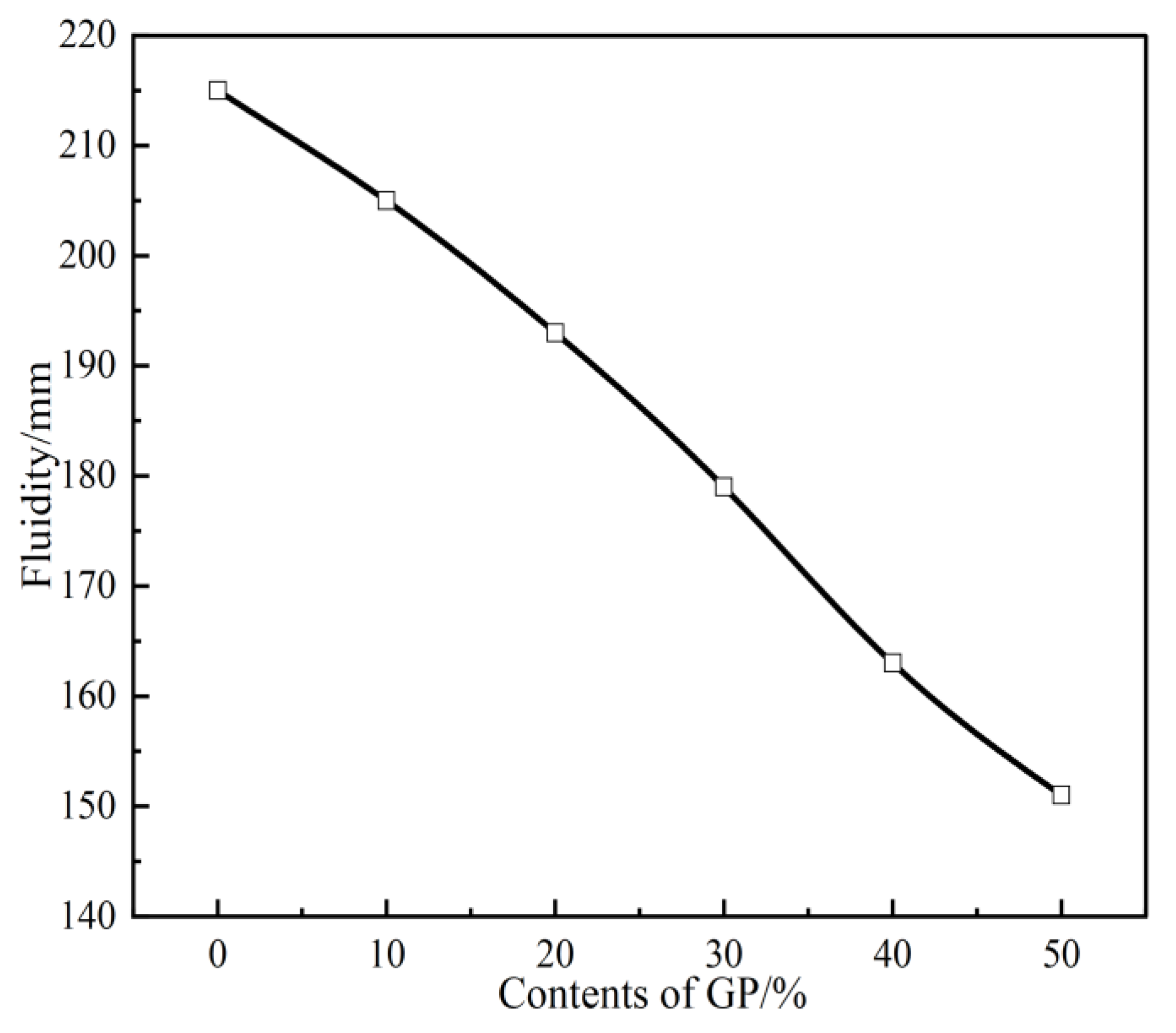
3.2. Fluidity
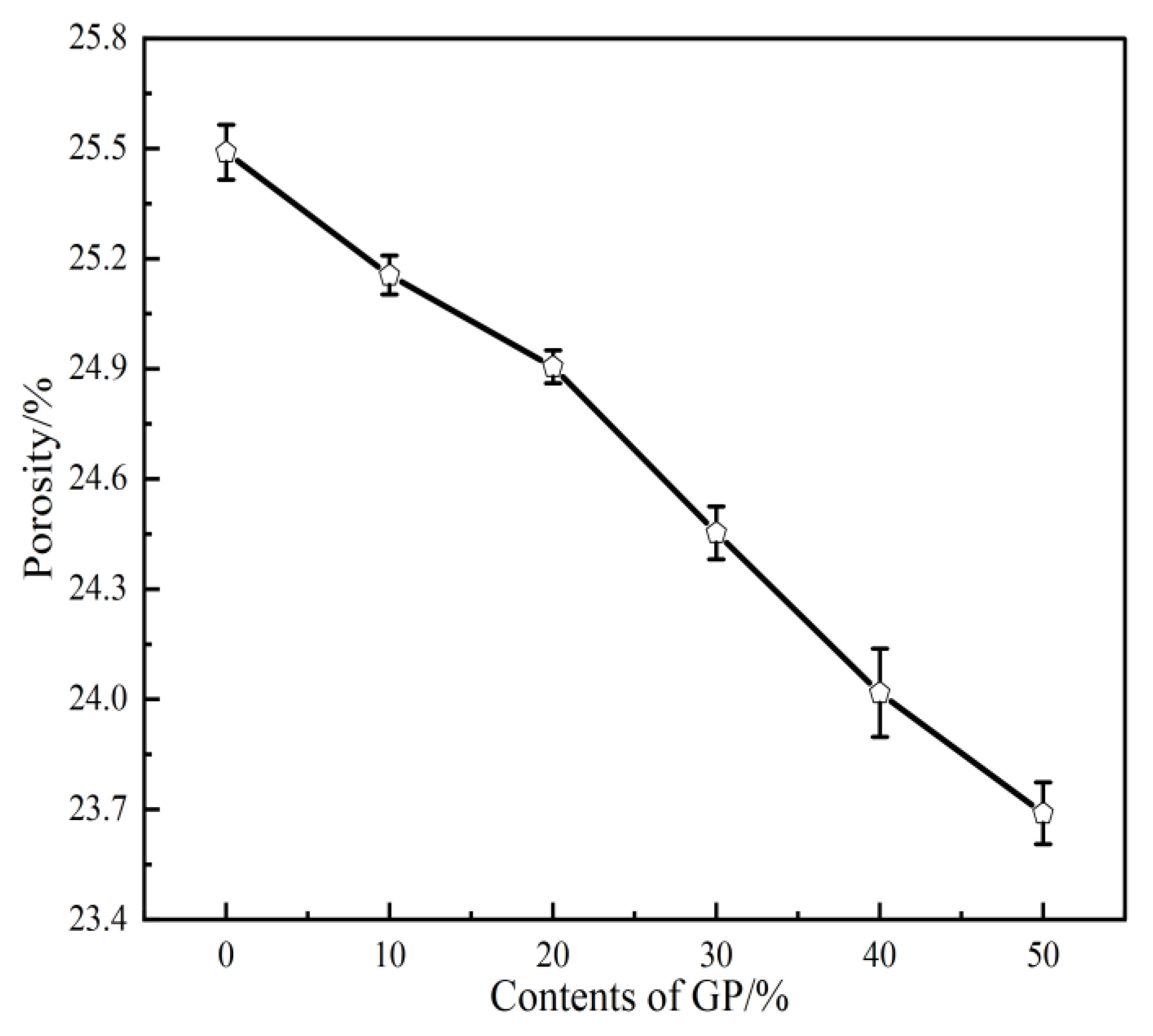
3.3. Porosity
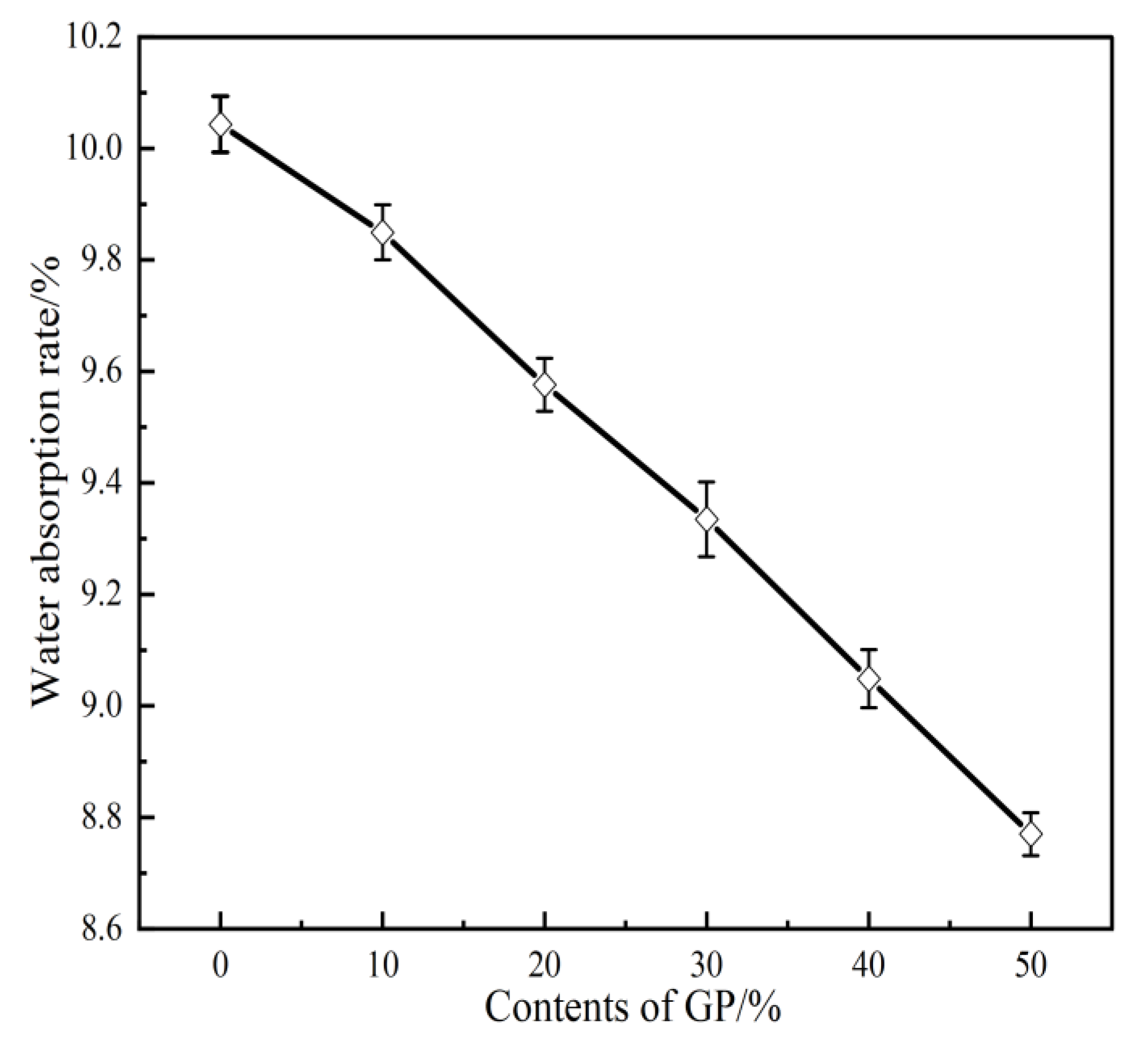
3.4. Water Absorption
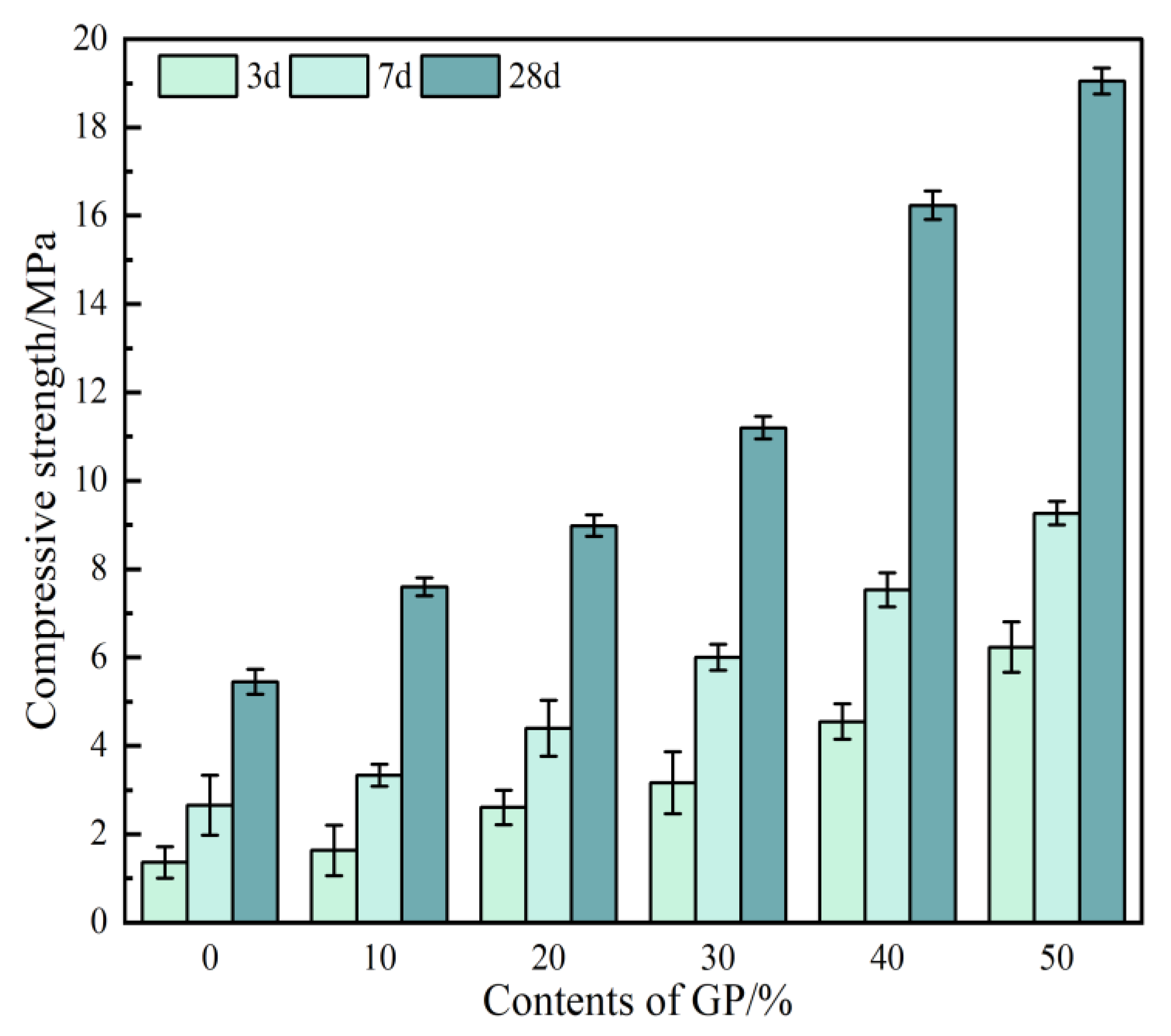
3.5. Compressive Strength
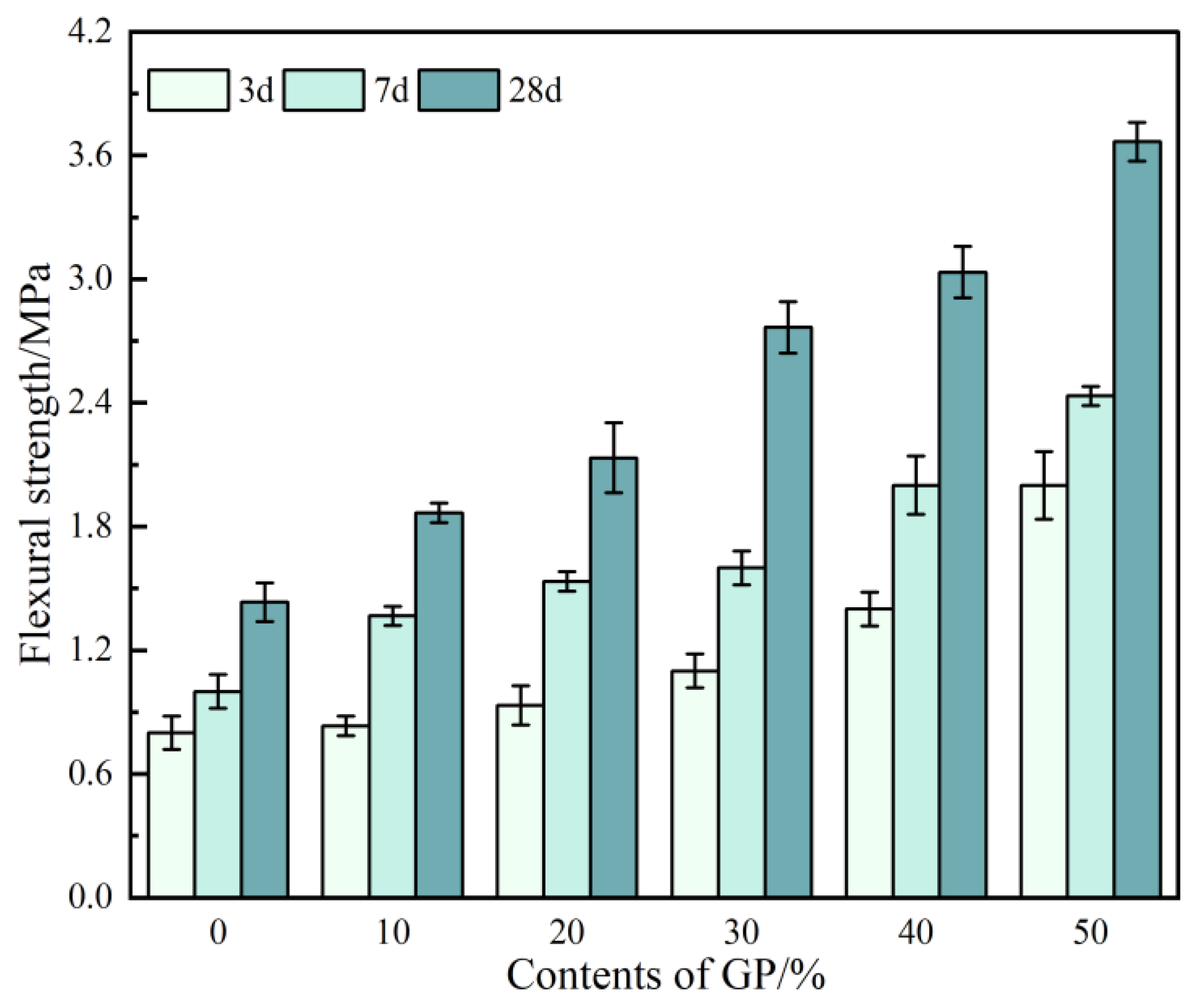
3.6. Flexural Strength
3.7. Drying Shrinkage of Geopolymer Specimens
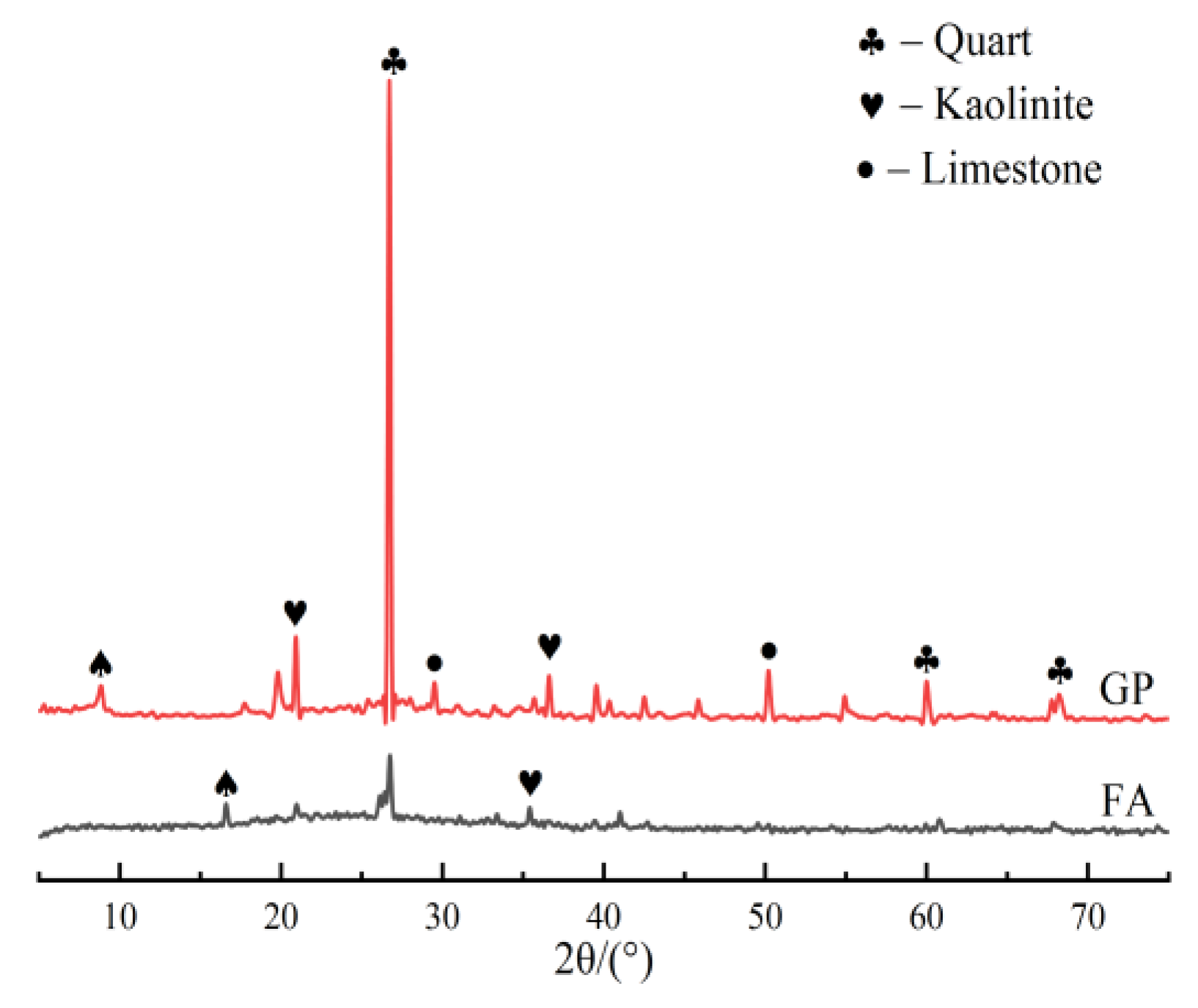
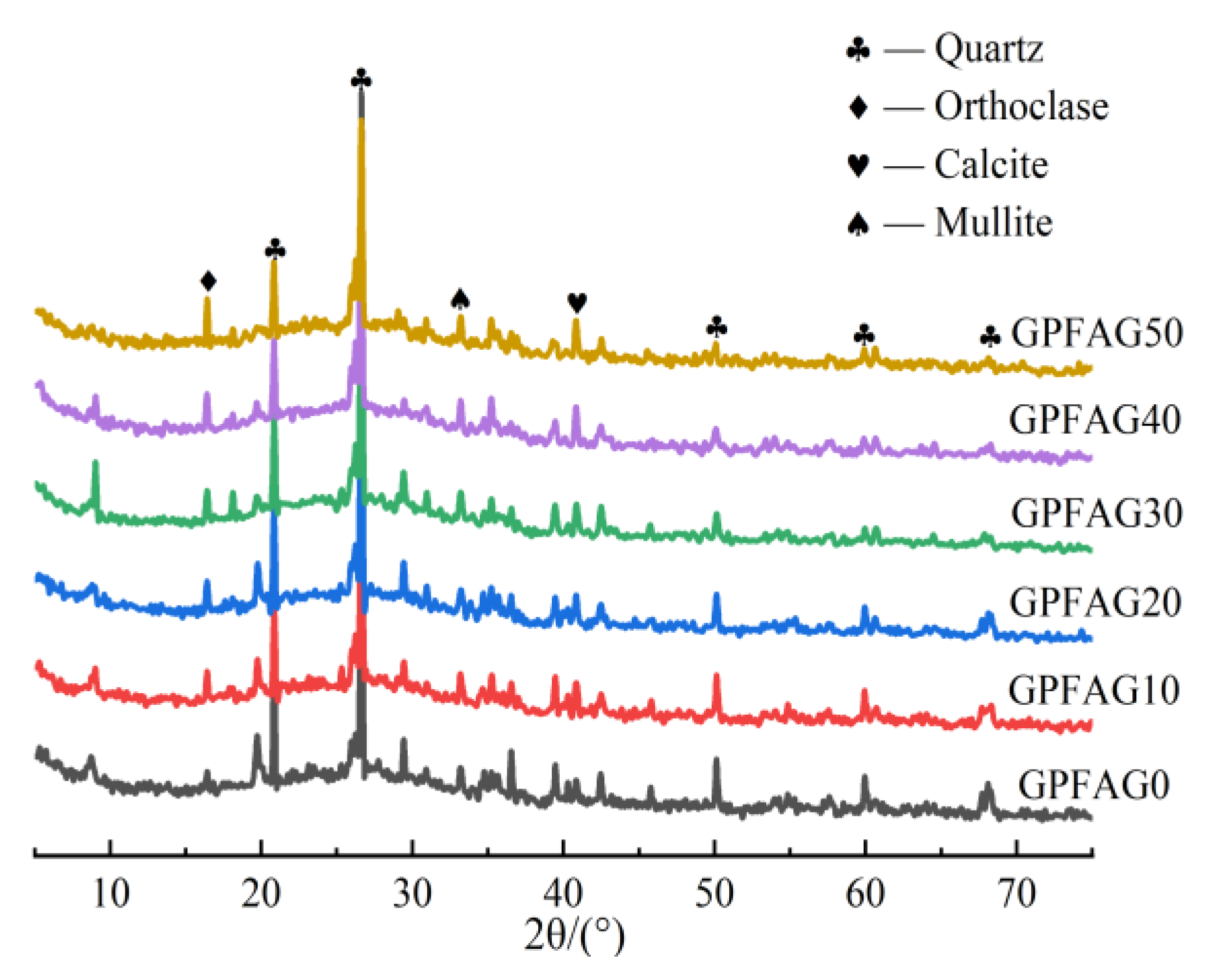
3.8. XRD Analysis
3.9. FTIR Analysis
4. Conclusions
- (1)
- The setting time of GPFAG can be improved by adding GP; it significantly decreases with increasing GP content. Influenced by the liquid–solid ratio, the addition of 50% GP (i.e., GPFAG50) reduces the fluidity, porosity, and water absorption rates by 29.8%, 1.8%, and 1.2%, respectively, compared with those of GPFAG0.
- (2)
- The incorporation of GP improves the mechanical properties of GPFAG, and strength increases with the GP content. The 28-d flexural and compressive strengths of GPFAG with 10–50% (at 10% intervals) GP content compared with those of GPFAG0 increase by 136.8% and 246.4%, respectively.
- (3)
- Based on the drying shrinkage results, the addition of GP induces an increase in the drying shrinkage of FAG due to a simultaneous increase in water demand and water loss within the matrix under drying conditions.
- (4)
- The XRD and FTIR analyses show that the incorporation of GP reduces the amount of unreacted quartz phase inside the matrix. The addition of GP substantially enhances the depolymerization–condensation reaction inside the matrix. Moreover, GP doping generates a considerable amount of amorphous silica–aluminate gels, which contain substantial amounts of Si-O-Si, Si-O-Al and chemically bound water. This contributes to the formation of a dense, continuous, three-dimensional network structure, which enhances the strength of GPFAG.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Q. China’s citizens must act to save their environment. Nature 2013, 497, 159. [Google Scholar] [CrossRef]
- Yuan, H.J. The future of coal in China. Resour. Conserv. Recycl. 2018, 129, 290–292. [Google Scholar] [CrossRef]
- Wang, Q.; Li, R.R. Journey to burning half of global coal: Trajectory and drivers of China’s coal use. Renew. Sustain. Energy Rev. 2016, 58, 341–346. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1611–1656. [Google Scholar] [CrossRef]
- Davidovits, J. Process for the Fabrication of Sintered Panels and Panels Resulting from the Application of This Process. U.S. Patent 05/410490, 13 April 1976. [Google Scholar]
- Zhang, Y.; Ling, T.C. Reactivity activation of waste coal gangue and its impact on the properties of cement-based materials—A review. Constr. Build. Mater. 2020, 234, 117424. [Google Scholar] [CrossRef]
- Magdalena, M.K.; Fabiańska, M.J. Application of organic petrology and geochemistry to coal waste studies. Int. J. Coal Geol. 2011, 88, 1–23. [Google Scholar]
- Laura, C.M.; Medina, C.; Rojas, I.M.S.; Frías, M. Water transport in binary eco-cements containing coal mining waste. Cem. Concr. Compos. 2019, 104, 103373. [Google Scholar]
- Chen, P.; Zhang, L.; Wang, Y.; Fang, Y.; Zhang, F.; Xu, Y. Environmentally friendly utilization of coal gangue as aggregates for shotcrete used in the construction of coal mine tunnel. Case Stud. Constr. Mater. 2021, 15, e00751. [Google Scholar] [CrossRef]
- Frías, M.; Rojas, I.M.S.; García, R.; Valdés, A.J.; Medina, C. Effect of activated coal mining wastes on the properties of blended cement. Cem. Concr. Compos. 2012, 34, 678–683. [Google Scholar] [CrossRef]
- Gruber, K.A.; Ramlochan, T.; Boddy, A.; Hooton, R.D.; Thomas, M.D.A. Increasing concrete durability with high-reactivity metakaolin. Cem. Concr. Compos. 2001, 23, 479–484. [Google Scholar] [CrossRef]
- Moghadam, M.J.; Ajalloeian, R.; Hajiannia, A. Preparation and application of alkali-activated materials based on waste glass and coal gangue: A review. Constr. Build. Mater. 2019, 221, 84–98. [Google Scholar] [CrossRef]
- Koshy, N.; Dondrob, K.; Hu, L.M.; Wen, Q.B.; Meegoda, J.N. Synthesis and characterization of geopolymers derived from coal gangue, fly ash and red mud. Constr. Build. Mater. 2019, 206, 287–296. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Q.; Zhou, M.; Fang, Y.; Zhang, Z. Mechanical properties of concrete with coarse spontaneous combustion gangue aggreagate (SCGA): Experimental investigation and prediction methodology. Constr. Build. Mater. 2020, 255, 119337. [Google Scholar] [CrossRef]
- Mahmood, K.; Dabbaghi, F.; Sadeghi-Nik, A.; Dehestani, M. Mechanical performance of green concrete produced with untreated coal waste aggregates. Constr. Build. Mater. 2020, 233, 117264. [Google Scholar]
- García-Giménez, R.; Frías, M.; Arribas, I.; Vegas, I.; de la Villa, R.V.; Rubio, V. Freeze-thaw effect on the durability of binary cements containing activated coal-mining waste. Constr. Build. Mater. 2018, 190, 140–149. [Google Scholar] [CrossRef]
- Mejia-Ballesteros, J.E.; Rodier, L.; Filomeno, R.; Savastano Jr, H.; Fiorelli, J.; Rojas, M.F. Effect of activated coal waste and treated Pinus fibers on the physico-mechanical properties and durability of fibercement composites. Constr. Build. Mater. 2023, 392, 132038. [Google Scholar] [CrossRef]
- Seiffarth, T.; Hohmann, M.; Posern, K.; Kaps, C. Effect of thermal pre-treatment conditions of common clays on the performance of clay-based geopolymeric binders. Appl. Clay Sci. 2013, 73, 35–41. [Google Scholar] [CrossRef]
- Sedira, N.; Castro-Gomes, J.; Kastiukas, G.; Zhou, X.; Vargas, A. A review on mineral waste for chemical-activated binders: Mineralogical and chemical characteristics. Min. Sci. 2017, 24, 29–58. [Google Scholar]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Dellisanti, F.; Valdrè, G. The role of microstrain on the thermostructural behaviour of industrial kaolin deformed by ball milling at low mechanical load. Int. J. Miner. Process. 2012, 1012, 69–77. [Google Scholar] [CrossRef]
- Ma, H.; Zhu, C.; Wu, H.; Chen, J.; Sun, J.; Liu, J. Study on compressive strength and durability of alkali-activated coal gangue-slag concrete and its mechanism. Powder Technol. 2020, 368, 112–124. [Google Scholar] [CrossRef]
- Liu, Y.; Ling, C.T.; Wang, M.; Wu, Y.Y. Synergic performance of low-kaolinite calcined coal gangue blended with limestone in cement mortars. Constr. Build. Mater. 2021, 300, 124012. [Google Scholar] [CrossRef]
- Al-Akhras, N.M. Durability of metakaolin concrete to sulfate attack. Cem. Concr. Res. 2006, 36, 1727–1734. [Google Scholar] [CrossRef]
- Figila, B.; Korniejenko, K.; Bulut, A.; Şahin, B.; Azizağaoğlu, G.; Plawecka, K.; Kozub, B. Influence of the Size of Milled Coal Gangue Particles on the Mechanical Properties of Geopolymers. Mater. Proc. 2023, 13, 4. [Google Scholar]
- Sitarz, M.; Figiela, B.; Lach, M.; Korniejenko, K.; Mróz, K.; Castro-Gomes, J.; Izabela, H. Mechanical Response of Geopolymer Foams to Heating—Managing Coal Gangue in Fire-Resistant Materials Technology. Energies 2022, 15, 3363. [Google Scholar] [CrossRef]
- Qiu, J.S.; Cheng, K.; Zhang, R.Y.; Gao, Y.; Guan, X. Study on the influence mechanism of activated coal gangue powder on the properties of filling body. Constr. Buuld. Master. 2022, 345, 128071. [Google Scholar] [CrossRef]
- Frías, M.; Villa, R.; Rojas, M.; Medina, C.; Valdes, A.; Jantzen, C. Scientific Aspects of Kaolinite Based Coal Mining Wastes in Pozzolan/Ca(OH)2 System. J. Am. Ceram. Soc. 2012, 95, 386–391. [Google Scholar] [CrossRef]
- Li, C.; Wan, J.H.; Sun, H.H.; Li, L.T. Investigation on the activation of coal gangue bya new compound method. J. Hazard. Mater. 2010, 179, 515–520. [Google Scholar] [CrossRef]
- Zhao, Y.B.; Yang, C.Q.; Li, K.F.; Qu, F.; Yan, C.Y.; Wu, Z.R. Towardunderstanding the activation and hydration mechanisms of composite activated coal gangue geopolymer. Constr. Build. Mater. 2022, 318, 125999. [Google Scholar] [CrossRef]
- Frasson, B.J.; Rocha, J.C. Drying shrinkage behavior of geopolymer mortar based on kaolinitic coal gangue. Case. Stud. Constr. 2023, 18, e01957. [Google Scholar] [CrossRef]
- Guo, Z.H.; Xu, J.J.; Xu, Z.H.; Gao, J.M.; Zhu, X.L. Performance of cement-based materials containing calcined coal gangue with different calcination regimes. J. Build. Eng. 2022, 56, 104821. [Google Scholar] [CrossRef]
- Dong, Z.C.; Xia, J.W.; Fan, C.; Cao, J.C. Activity of calcined coal gangue fine aggregate and its effect on the mechanical behavior of cement mortar. Constr. Build. Mater. 2015, 100, 63–69. [Google Scholar] [CrossRef]
- JGJ/T 70-2009; Construction Mortar Basic Performance Test METHOD Standards. Ministry of Construction of the People’s Republic of China: Beijing, China, 2009. (In Chinese)
- GB/T 2419-2005; Test Method for Fluidity of Cement Mortar. National Standardization Technical Committee Cement: Beijing, China, 2005. (In Chinese)
- ASTM C 642-13; Standard Test Method For Density, Absorption, and Voids in Hardened Concrete. ASTM: West Conshohocken, PA, USA, 2022.
- GB/T 17671-1999; Method of Testing Cements—Determination of Strength. National Standardization Technical Committee Cement: Beijing, China, 1999. (In Chinese)
- Li, X.; Bai, C.; Qiao, Y.; Wang, X.; Yang, K.; Colombo, P. Preparation, properties and applications of fly ash-based porous geopolymers: A review. J. Clean. Prod. 2022, 359, 132043. [Google Scholar] [CrossRef]
- Zhang, L.Y.; Ahmari, S.; Zhang, J.H. Synthesis and characterization of fly ash modified mine tailings-based geopolymers. Constr. Build. Mater. 2011, 25, 3773–3781. [Google Scholar] [CrossRef]
- Ji, X.M.; Ji, D.P.; Yang, Z.X.; Wang, G.L.; Huang, X.F.; Ma, S.H.; Li, W.F. Study on the phase composition and structure of hardened cement paste during heat treatment. Constr. Build. Mater. 2021, 310, 125267. [Google Scholar] [CrossRef]
- Han, Q.; Wang, A.N.; Zhang, J. Research on the early fracture behavior of fly ash-based geopolymers modified by molybdenum tailings. J. Clean. Prod. 2022, 365, 132759. [Google Scholar] [CrossRef]
- Liew, Y.M.; Heah, C.Y.; Li, L.Y.; Jaya, N.A.; Abdullah, M.M.A.; Jin, T.S.; Hussin, K. Formation of one-part-mixing geopolymers and geopolymer ceramics from geopolymer powder. Constr. Build. Mater. 2017, 156, 9–18. [Google Scholar] [CrossRef]
- Trincal, V.; Multon, S.; Benavent, V.; Lahalle, H.; Balsamo, B.; Caron, A.; Bucher, R.; Caselles, L.D.; Cyr, M. Shrinkage mitigation of metakaolin-based geopolymer activated by sodium silicate solution. Cem. Concr. Res. 2022, 162, 106993. [Google Scholar] [CrossRef]
- Popovics, S. Verification of relationships between mechanical properties of concrete like materials. AJER 1975, 8, 83–191. [Google Scholar] [CrossRef]
- Ryu, G.S.; Lee, Y.B.; Koh, K.T.; Chung, Y.S. The mechanical properties of fly ash-based geopolymer concrete with alkaline activators. Constr. Build. Mater. 2013, 47, 409–418. [Google Scholar] [CrossRef]
- Ibrahim, M.; Johari, A.M.M.; Rahman, M.K.; Maslehuddin, M. Effect of alkaline activators and binder content on the properties of natural pozzolan-based alkali activated concrete. Constr. Build. Mater. 2017, 147, 648–660. [Google Scholar] [CrossRef]
- Cheng, Y.; Ma, H.Q.; Chen, H.Y.; Shi, J.X.; Shi, J.; Li, Z.H.; Yu, M.K. Preparation and characterization of coal gangue geopolymers. Constr. Build. Mater. 2018, 187, 318–326. [Google Scholar] [CrossRef]
- Boke, N.D.; Birch, G.M.; Nyale, S.; Petrik, L.F. New synthesis method for the production of coal fly ash-based foamed geopolymers. Constr. Build. Mater. 2015, 75, 189–199. [Google Scholar] [CrossRef]
- Luo, Y.; Klima, K.M.; Brouwers, J.H.H.; Yu, Q. Effects of ladle slag on Class F flyash geopolymer: Reaction mechanism and high temperature behavior. Cem. Concr. Compos. 2022, 129, 104468. [Google Scholar] [CrossRef]
- Onutai, S.; Osugi, T.; Sone, T. Alumino-Silicate Structural Formation during Alkali-Activation of Metakaolin: In-Situ and Ex-Situ ATR-FTIR Studies. Mater. Proc. 2023, 16, 985. [Google Scholar] [CrossRef]
- Garcia-Lodeiro, I.; Palomo, A.; Fernandez-Jimenez, A.; Macphee, D.E. Compatibility studies between N-A-S-H and C-A-S-H gels. Study in the ternary diagram Na2O-CaO-Al2O3-SiO2-H2O. Cem. Concr. Res. 2011, 41, 923–931. [Google Scholar] [CrossRef]





| Materials | Al2O3 | SiO2 | Fe2O3 | CaO | Na2O | K2O | SO3 |
|---|---|---|---|---|---|---|---|
| FA | 26.38 | 47.85 | 8.43 | 5.81 | 1.11 | 2.22 | 1.94 |
| GP | 25.1 | 58.5 | 5.81 | 4.25 | 0.551 | 2.56 | 0.445 |
| Code | FA | GP | Sand | Alkali Exciter | Water | |
|---|---|---|---|---|---|---|
| Na2SiO3 | NaOH | |||||
| GPFAG0 | 480 | 0 | 1320 | 164.24 | 27.76 | 119.9 |
| GPFAG10 | 432 | 48 | ||||
| GPFAG20 | 384 | 96 | ||||
| GPFAG30 | 336 | 144 | ||||
| GPFAG40 | 288 | 192 | ||||
| GPFAG50 | 240 | 240 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, T.; Yang, Z.; Zhang, D.; Yang, Q. Properties of Gangue Powder Modified Fly Ash-Based Geopolymer. Materials 2023, 16, 5719. https://doi.org/10.3390/ma16165719
Zhang T, Yang Z, Zhang D, Yang Q. Properties of Gangue Powder Modified Fly Ash-Based Geopolymer. Materials. 2023; 16(16):5719. https://doi.org/10.3390/ma16165719
Chicago/Turabian StyleZhang, Tianhao, Zhenghui Yang, Dongsheng Zhang, and Qiuning Yang. 2023. "Properties of Gangue Powder Modified Fly Ash-Based Geopolymer" Materials 16, no. 16: 5719. https://doi.org/10.3390/ma16165719
APA StyleZhang, T., Yang, Z., Zhang, D., & Yang, Q. (2023). Properties of Gangue Powder Modified Fly Ash-Based Geopolymer. Materials, 16(16), 5719. https://doi.org/10.3390/ma16165719









