Cyclodextrins for Lithium Batteries Applications
Abstract
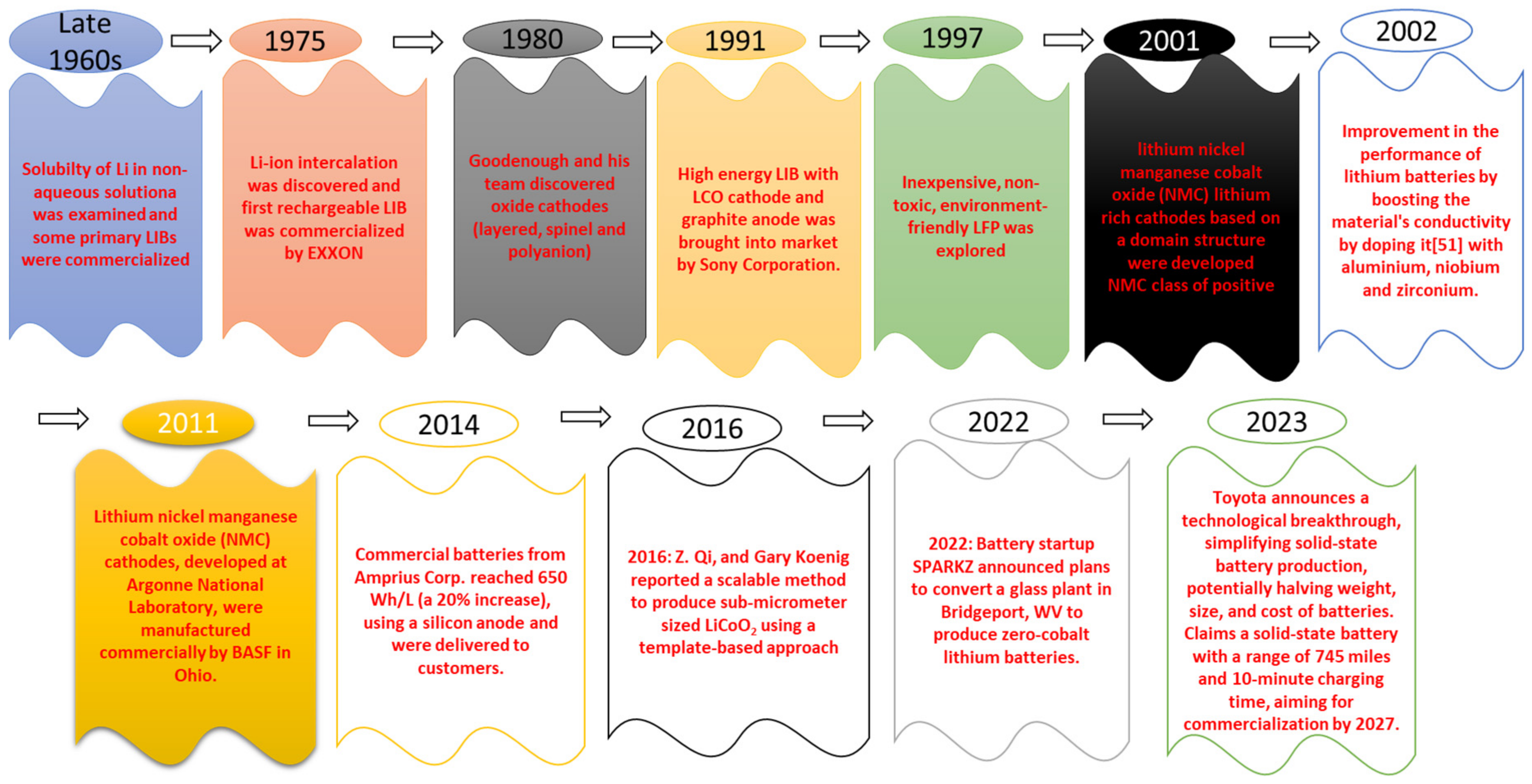
:1. Introduction
2. Cyclodextrin for Lithium-Battery Improvements
2.1. Cyclodextrin-Based Binders
2.1.1. Anode Binders
2.1.2. Cathode Binders
2.1.3. Anode and Cathode Binders
2.2. Cyclodextrin-Based Electrolytes
2.3. Cyclodextrin-Based Separators
3. Limitation
- Scalability: Although cyclodextrins have shown promising results at the laboratory level, it is still difficult to scale them up for mass battery production. Future studies should examine ways to guarantee the reliable and effective production of cyclodextrin-based products on a larger scale. To meet the requirements of high-volume battery production, this would entail creating scalable synthesis routes and optimizing manufacturing procedures.
- Cost-effectiveness: Another crucial factor to consider is the economic viability of cyclodextrin-based technologies. To compete with current battery materials, the price of cyclodextrins and the methods used in their synthesis should be reduced. To lower production costs and increase their commercial viability, future studies should investigate cost-effective manufacturing techniques, such as utilizing renewable feedstocks or enhancing the efficiency of cyclodextrin synthesis.
- Recyclability: Given the growing emphasis on sustainability and the principles of the circular economy, the ability to recycle battery parts is crucial. Materials based on cyclodextrin must be created with recycling in mind. Future studies should consider the recycling potential of cyclodextrin-based products and how they affect the entire battery-recycling process. Reducing waste and environmental impact would be helped by the development of cyclodextrin-based technologies that can be easily recycled or integrated into already-existing recycling processes.
4. Future Research Opportunities
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| CD | Cyclodextrin |
| α-CD | Alpha Cyclodextrin |
| β-CD | Beta Cyclodextrin |
| γ-CD | Gama Cyclodextrin |
| C- β-CD | Carbonate beta cyclodextrin |
| LIB | Lithium Ion Battery |
| PVDF | Polyvinylidene fluoride |
| NMP | N-Methyl-1-2pyrrolidine |
| Li+ | Lithium ions |
| Na+ | Sodium ions |
| H+ | Hydrogen ions |
| OH- | Hydroxide ions |
| e− | electron ions |
| FeF2 | Iron(II) Fluoride |
| CoFe | A binary intermetallic compound made up of cobalt (Co) and iron (Fe) |
| LiCoO2 | A layered oxide material made up of lithium (Li), cobalt (Co) and oxygen (O2) |
| Li0.5CoO2 | A layered oxide material made up of lithium (Li), cobalt (Co) and oxygen (O2), 0.5″ in the formula refers to the stoichiometry of the compound, meaning there is half as much lithium as cobalt in the compound. |
| Li | Lithium |
| LiMn2O4 or (LMO) | A chemical compound made up of lithium (Li), manganese (Mn), and oxygen (O4). It is commonly referred to as Lithium Manganese Oxide (LMO) |
| Ti | Titanium |
| V | Vanadium |
| Cr | Chromium |
| Mn | Manganese |
| Fe | Iron |
| Co | Cobalt |
| Ni | Nickel |
| Cu | Copper |
| Mo | Molybdenum |
| W | Tungsten |
| Ru | Ruthenium |
| O | Oxygen |
| N | Nitrogen |
| F | Fluorine |
| S | Sulphur |
| Si | Silicon |
| Ge | Germanium |
| Sn | Tin |
| Al | Aluminium |
| Sb | Antimony |
| PAA | Polyacrylic acid |
| H2O2 | Hydrogen peroxide |
| NH4VO3 | ammonium vanadate salt |
| Li2CO3 | Lithium Carbonate |
| V | Volt |
| Si β-CDp | Silicon anode using beta cyclodextrin polymer as binder |
| XRD | X-ray diffraction |
| TEM | Transmission electron microscope |
| SEM | Scanning electron microscope |
| CO3O4 | Cobalt Oxide |
| mAh g −1 | milliampere-hours per gram |
| C | Capacity |
| GiTT | Galvanostatic intermittent titration technique |
| CV | Cyclic voltammetry |
| EIS | Electrochemical impedance spectroscopy |
| 1D | one dimensional |
| °A | Angstrom |
| rGO | Reduced graphene oxide |
| CFP | Carbon Foam with Porous |
| C/S | Carbon and sulfur mixture |
| 1D NN | one-dimension nanonets |
| 1D-NP | one-dimension nanoparticle |
| TFSI | Bis (Trifluoromethylesulonyl)imide |
| SQM | Semi-empirical quantum mechanics |
| RMβCD | Randomly Methylated beta cyclodextrin |
| Li-S | lithium and sulfur |
| COSMO-RS: | Conductor like screening model for real solvent |
| 1D | one dimensional |
| 2D | Two dimensional |
| 1H NMR | Proton nuclear magnetic resonance |
| DME | 1,2-Dimethoxyethane |
| MeOH | Methanol |
| nm | Nanometer |
| Mβ-CD | Methyl-beta-cyclodextrin |
| PVP | Polyvinylpyrrolidone |
| ACS | Amylose corn starch |
| DFT | Density function theory |
| PEG | polyethylene glycol |
| PTMC | Trimethyl carbonate |
| CDTPEs | Cyclodextrin based triblock polymer electrolyte. |
| PEO | Poly(ethylene oxide) |
| SSE | Solid state electrolytes |
| ASSLB | All- Solid- Sate Lithium Batteries |
| LiTFSI | Lithium Trifluoromethanesulfonimide |
| CSE | Composite solid electrolyte |
| 3D | Three dimensional |
| CMC | Carboxymethlcellulose |
| LZTO | Is a layer oxide material with chemical formula Li2ZnTi3O8 |
| ECH | Epichlorohydrin |
| NS | nanosponge |
| Si-S | silicon and sulfur |
| CNS | Carbon nanosponges |
References
- Bradley, D. Building Better Batteries. Educ. Chem. 2010, 47, 124–125. [Google Scholar]
- Zhu, Z.; Jiang, T.; Ali, M.; Meng, Y.; Jin, Y.; Cui, Y.; Chen, W. Rechargeable Batteries for Grid Scale Energy Storage. Chem. Rev. 2022, 122, 16610–16751. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.-S. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Tarascon, J.-M.; Armand, M. Issues and Challenges Facing Rechargeable Lithium Batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Service, R. New Generation of Lithium-Ion Batteries Could Hold More Charge—Without Catching Fire. Science 2019, 80, aax9731. [Google Scholar] [CrossRef]
- Scrosati, B. Lithium Batteries: From Early Stages to the Future. In Lithium Batteries; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 21–38. ISBN 9781118615515. [Google Scholar]
- Sun, Y.; Liu, N.; Cui, Y. Promises and Challenges of Nanomaterials for Lithium-Based Rechargeable Batteries. Nat. Energy 2016, 1, 16071. [Google Scholar] [CrossRef]
- Ashworth, C. Interrogating the Interphase. Nat. Rev. Mater. 2018, 3, 1. [Google Scholar] [CrossRef]
- Deng, H.; Aifantis, K.E. Applications of Lithium Batteries. In Rechargeable Ion Batteries; Wiley: Hoboken, NJ, USA, 2023; pp. 83–103. [Google Scholar]
- Chen, Y.; Kang, Y.; Zhao, Y.; Wang, L.; Liu, J.; Li, Y.; Liang, Z.; He, X.; Li, X.; Tavajohi, N.; et al. A Review of Lithium-Ion Battery Safety Concerns: The Issues, Strategies, and Testing Standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Gordon, R.; Smith, A. Towards More Realistic Li-Ion Battery Safety Tests Based on Li-Plating as Internal Cell Error. J. Energy Storage 2023, 72, 108200. [Google Scholar] [CrossRef]
- Khan, F.M.N.U.; Rasul, M.G.; Sayem, A.S.M.; Mandal, N.K. Design and Optimization of Lithium-Ion Battery as an Efficient Energy Storage Device for Electric Vehicles: A Comprehensive Review. J. Energy Storage 2023, 71, 108033. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Li, K.; Zhao, G.; Chen, Z. Perspectives and Challenges for Future Lithium-Ion Battery Control and Management. eTransportation 2023, 18, 100260. [Google Scholar] [CrossRef]
- Zhou, G.; Li, F.; Cheng, H.-M. Progress in Flexible Lithium Batteries and Future Prospects. Energy Environ. Sci. 2014, 7, 1307–1338. [Google Scholar] [CrossRef]
- Scrosati, B. History of Lithium Batteries. J. Solid State Electrochem. 2011, 15, 1623–1630. [Google Scholar] [CrossRef]
- Placke, T.; Kloepsch, R.; Dühnen, S.; Winter, M. Lithium Ion, Lithium Metal, and Alternative Rechargeable Battery Technologies: The Odyssey for High Energy Density. J. Solid State Electrochem. 2017, 21, 1939–1964. [Google Scholar] [CrossRef]
- Padhi, A.K.; Nanjundaswamy, K.S.; Goodenough, J.B. Phospho-olivines as Positive-Electrode Materials for Rechargeable Lithium Batteries. J. Electrochem. Soc. 1997, 144, 1188–1194. [Google Scholar] [CrossRef]
- Zero-Cobalt Li-Ion Battery Maker SPARKZ Announces Site for W Va Gigafactory—Green Car Congress. Available online: https://www.greencarcongress.com/2022/09/20220901-sparkz.html (accessed on 26 July 2023).
- Ziegler, M.S.; Trancik, J.E. Re-Examining Rates of Lithium-Ion Battery Technology Improvement and Cost Decline. Energy Environ. Sci. 2021, 14, 1635–1651. [Google Scholar] [CrossRef]
- Chung, S.-Y.; Bloking, J.T.; Chiang, Y.-M. Electronically Conductive Phospho-Olivines as Lithium Storage Electrodes. Nat. Mater. 2002, 1, 123–128. [Google Scholar] [CrossRef]
- In Search of the Perfect Battery. Available online: https://www.economist.com/technology-quarterly/2008/03/08/in-search-of-the-perfect-battery (accessed on 26 July 2023).
- BASF Breaks Ground for Lithium-Ion Battery Materials Plant in Ohio. Available online: https://www.reliableplant.com/Read/27186/BASF-lithium-ion-battery (accessed on 26 July 2023).
- Toyota Claims Battery Breakthrough in Potential Boost for Electric Cars|Automotive Industry|The Guardian. Available online: https://www.theguardian.com/business/2023/jul/04/toyota-claims-battery-breakthrough-electric-cars (accessed on 26 July 2023).
- Song, Y.; Zavalij, P.Y.; Whittingham, M.S. ε-VOPO[Sub 4]: Electrochemical Synthesis and Enhanced Cathode Behavior. J. Electrochem. Soc. 2005, 152, A721. [Google Scholar] [CrossRef]
- Qi, Z.; Koenig, G.M. High-Performance LiCoO2 Sub-Micrometer Materials from Scalable Microparticle Template Processing. ChemistrySelect 2016, 1, 3992–3999. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief History of Early Lithium-Battery Development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Wan, J.; Xiang, Y.; Zhu, J.; Leng, Q.; Wang, M.; Xu, L.; Yang, Y. Recent Advances and Historical Developments of High Voltage Lithium Cobalt Oxide Materials for Rechargeable Li-Ion Batteries. J. Power Sources 2020, 460, 228062. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, X.; Chong, Y.; Yuan, H.; Huang, J.Q.; Zhang, Q. Advanced Electrode Processing of Lithium Ion Batteries: A Review of Powder Technology in Battery Fabrication. Particuology 2021, 57, 56–71. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, G.; Liu, G.; Yuan, Z.; Huang, X.; Li, Y. A Review on Electrode and Electrolyte for Lithium Ion Batteries under Low Temperature. Electroanalysis 2023, 15, e202300042. [Google Scholar] [CrossRef]
- Cengiz, E.C.; Rizell, J.; Sadd, M.; Matic, A.; Mozhzhukhina, N. Review—Reference Electrodes in Li-Ion and Next Generation Batteries: Correct Potential Assessment, Applications and Practices. J. Electrochem. Soc. 2021, 168, 120539. [Google Scholar] [CrossRef]
- Bhatt, M.D.; O’Dwyer, C. Recent Progress in Theoretical and Computational Investigations of Li-Ion Battery Materials and Electrolytes. Phys. Chem. Chem. Phys. 2015, 17, 4799–4844. [Google Scholar] [CrossRef] [Green Version]
- Lopez, J.; Mackanic, D.G.; Cui, Y.; Bao, Z. Designing Polymers for Advanced Battery Chemistries. Nat. Rev. Mater. 2019, 4, 312–330. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cai, Y.; Wu, H.; Yu, Z.; Yan, X.; Zhang, Q.; Gao, T.Z.; Liu, K.; Jia, X.; Bao, Z. Polymers in Lithium-Ion and Lithium Metal Batteries. Adv. Energy Mater. 2021, 11, 2003239. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y.; Ye, M.; Tang, Y.; Wen, Z.; Liu, X.; Li, C.C. Renewable Lignin and Its Macromolecule Derivatives: An Emerging Platform toward Sustainable Electrochemical Energy Storage. Green Chem. 2023, 25, 4154–4179. [Google Scholar] [CrossRef]
- Costa, C.M.; Lizundia, E.; Lanceros-Méndez, S. Polymers for Advanced Lithium-Ion Batteries: State of the Art and Future Needs on Polymers for the Different Battery Components. Prog. Energy Combust. Sci. 2020, 79, 100846. [Google Scholar] [CrossRef]
- Sen, S.; Trevisanello, E.; Niemöller, E.; Shi, B.X.; Simon, F.J.; Richter, F.H. The Role of Polymers in Lithium Solid-State Batteries with Inorganic Solid Electrolytes. J. Mater. Chem. A 2021, 9, 18701–18732. [Google Scholar] [CrossRef]
- Barbosa, J.C.; Gonçalves, R.; Costa, C.M.; Lanceros-Méndez, S. Toward Sustainable Solid Polymer Electrolytes for Lithium-Ion Batteries. ACS Omega 2022, 7, 14457–14464. [Google Scholar] [CrossRef]
- Pham, Q.T.; Chern, C.S. Applications of Polymers in Lithium-Ion Batteries with Enhanced Safety and Cycle Life. J. Polym. Res. 2022, 29, 124. [Google Scholar] [CrossRef]
- Wang, W.; Li, C.; Zeng, X.; Chen, J.; Sun, R. Application of Polymer-Based Phase Change Materials in Thermal Safety Management of Power Batteries. J. Energy Storage 2022, 55, 105646. [Google Scholar] [CrossRef]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Cheng, J.; Khin, K.T.; Jensen, G.S.; Liu, A.; Davis, M.E. Synthesis of Linear, β-Cyclodextrin-Based Polymers and Their Camptothecin Conjugates. Bioconjug. Chem. 2003, 14, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Harada, A.; Takashima, Y.; Yamaguchi, H. Cyclodextrin-Based Supramolecular Polymers. Chem. Soc. Rev. 2009, 38, 875. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Liu, J. The Classification and Application of Cyclodextrin Polymers: A Review. New J. Chem. 2020, 44, 9137–9148. [Google Scholar] [CrossRef]
- Yao, X.; Huang, P.; Nie, Z. Cyclodextrin-Based Polymer Materials: From Controlled Synthesis to Applications. Prog. Polym. Sci. 2019, 93, 1–35. [Google Scholar] [CrossRef]
- Ito, K. Slide-Ring Materials Using Cyclodextrin. Chem. Pharm. Bull. 2017, 65, 326–329. [Google Scholar] [CrossRef] [Green Version]
- Mocanu, G.; Vizitiu, D.; Carpov, A. Cyclodextrin Polymers. J. Bioact. Compat. Polym. 2001, 16, 315–342. [Google Scholar] [CrossRef]
- Wüpper, S.; Lüersen, K.; Rimbach, G. Cyclodextrins, Natural Compounds, and Plant Bioactives—A Nutritional Perspective. Biomolecules 2021, 11, 401. [Google Scholar] [CrossRef]
- Sá Couto, A.; Salústio, P.; Cabral-Marques, H. Cyclodextrins. In Polysaccharides; Springer International Publishing: Cham, Switzerland, 2015; pp. 247–288. [Google Scholar]
- Saini, K.; Pathak, V.M.; Tyagi, A.; Gupta, R. Microbial Cyclodextrin Glycosyltransferases: Sources, Production, and Application in Cyclodextrin Synthesis. Catal. Res. 2022, 2, 29. [Google Scholar] [CrossRef]
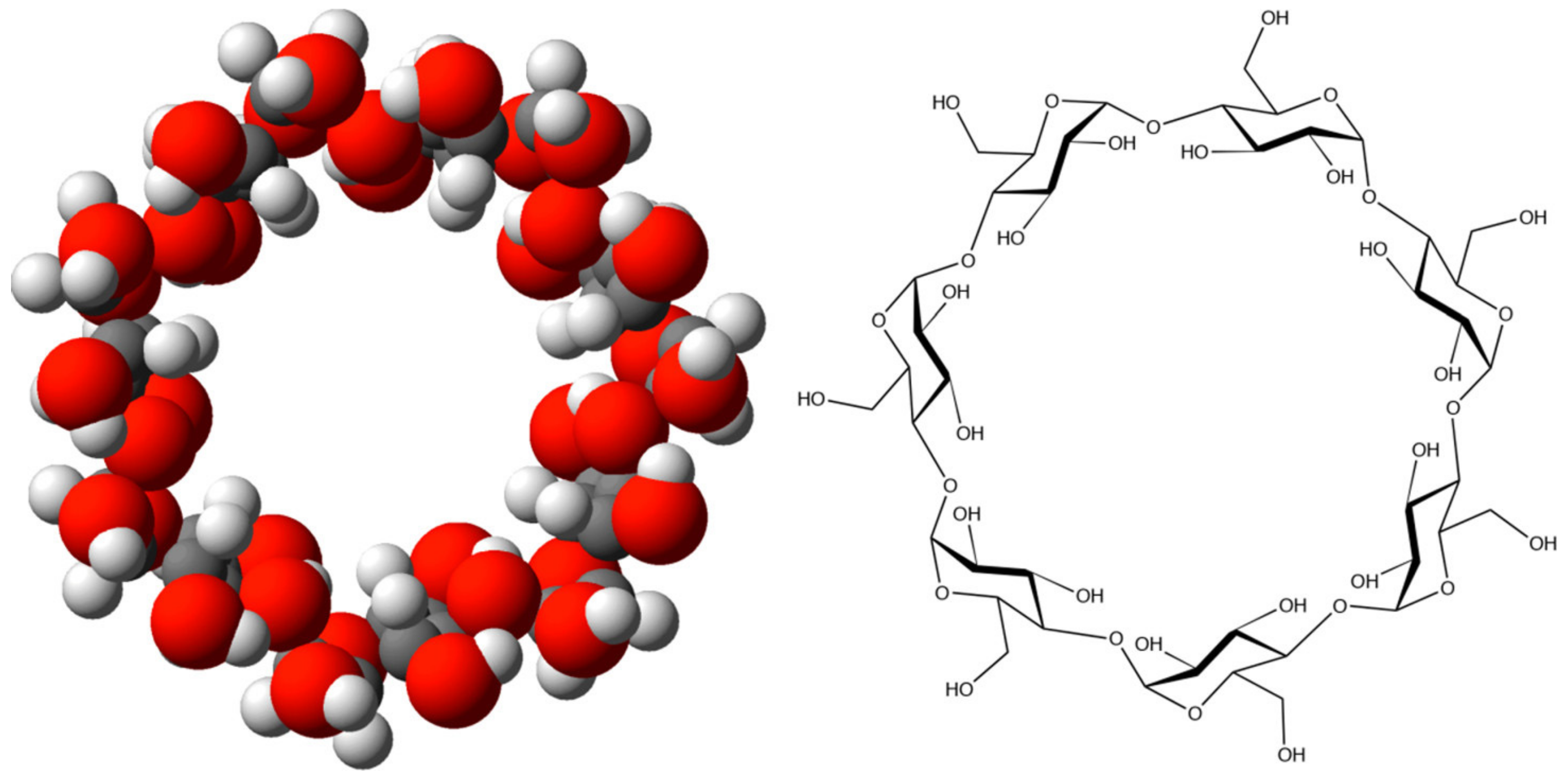
- Poulson, B.G.; Alsulami, Q.A.; Sharfalddin, A.; El Agammy, E.F.; Mouffouk, F.; Emwas, A.-H.; Jaremko, L.; Jaremko, M. Cyclodextrins: Structural, Chemical, and Physical Properties, and Applications. Polysaccharides 2021, 3, 1–31. [Google Scholar] [CrossRef]
- Nagaraju, M.; Narahari Sastry, G. Theoretical Studies on Inclusion Complexes of Cyclodextrins. J. Phys. Chem. A 2009, 113, 9533–9542. [Google Scholar] [CrossRef] [PubMed]
- Marques, H.M.C. A Review on Cyclodextrin Encapsulation of Essential Oils and Volatiles. Flavour Fragr. J. 2010, 25, 313–326. [Google Scholar] [CrossRef]
- Utzeri, G.; Matias, P.M.C.; Murtinho, D.; Valente, A.J.M. Cyclodextrin-Based Nanosponges: Overview and Opportunities. Front. Chem. 2022, 10, 859406. [Google Scholar] [CrossRef]
- Sherje, A.P.; Dravyakar, B.R.; Kadam, D.; Jadhav, M. Cyclodextrin-Based Nanosponges: A Critical Review. Carbohydr. Polym. 2017, 173, 37–49. [Google Scholar] [CrossRef]
- Wang, Q. Industrial Applications of Cyclodextrins. In Handbook of Macrocyclic Supramolecular Assembly; Springer: Berlin/Heidelberg, Germany, 2020; Volume 2665, pp. 1665–1697. [Google Scholar] [CrossRef]
- Harada, A.; Takashima, Y.; Nakahata, M. Supramolecular Polymeric Materials via Cyclodextrin-Guest Interactions. Acc. Chem. Res. 2014, 47, 2128–2140. [Google Scholar] [CrossRef]
- Esmaeilpour, D.; Broscheit, J.A.; Shityakov, S. Cyclodextrin-Based Polymeric Materials Bound to Corona Protein for Theranostic Applications. Int. J. Mol. Sci. 2022, 23, 13505. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Wimmer, T. Cyclodextrins. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Angelova, S.; Nikolova, V.; Pereva, S.; Spassov, T.; Dudev, T. α-Cyclodextrin: How Effectively Can Its Hydrophobic Cavity Be Hydrated? J. Phys. Chem. B 2017, 121, 9260–9267. [Google Scholar] [CrossRef] [PubMed]
- Xu, K. Electrolytes and Interphases in Li-Ion Batteries and Beyond. Chem. Rev. 2014, 114, 11503–11618. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Chen, G.; Zhang, N.; Ma, R.; Liu, X. β-Cyclodextrin as Lithium-Ion Diffusion Channel with Enhanced Kinetics for Stable Silicon Anode. Energy Environ. Mater. 2021, 4, 72–80. [Google Scholar] [CrossRef]
- Liu, W.; Jiang, Y.; Wang, N.; Fu, W. Recent Progress in Flame Retardant Technology of Battery: A Review. Resour. Chem. Mater. 2023, 2, 80–99. [Google Scholar] [CrossRef]
- Wang, Y.B.; Yang, Q.; Guo, X.; Yang, S.; Chen, A.; Liang, G.J.; Zhi, C.Y. Strategies of Binder Design for High-Performance Lithium-Ion Batteries: A Mini Review. Rare Met. 2022, 41, 745–761. [Google Scholar] [CrossRef]
- Wu, B.; Lu, W. Mechanical Modeling of Particles with Active Core-Shell Structures for Lithium-Ion Battery Electrodes. J. Phys. Chem. C 2017, 121, 19022–19030. [Google Scholar] [CrossRef]
- Ali, Y.; Iqbal, N.; Lee, S. Role of SEI Layer Growth in Fracture Probability in Lithium-Ion Battery Electrodes. Int. J. Energy Res. 2021, 45, 5293–5308. [Google Scholar] [CrossRef]
- Zhao, Y.; Stein, P.; Bai, Y.; Al-Siraj, M.; Yang, Y.; Xu, B.X. A Review on Modeling of Electro-Chemo-Mechanics in Lithium-Ion Batteries. J. Power Sources 2019, 413, 259–283. [Google Scholar] [CrossRef]
- Zou, F.; Manthiram, A. A Review of the Design of Advanced Binders for High-Performance Batteries. Adv. Energy Mater. 2020, 10, 2002508. [Google Scholar] [CrossRef]
- Kannan, S.K.; Joseph, J.; Joseph, M.G. Review and Perspectives on Advanced Binder Designs Incorporating Multifunctionalities for Lithium–Sulfur Batteries. Energy Fuels 2023, 37, 6302–6322. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.-M.; Zhang, Y.-C.; Song, Y.; Wang, G.-K.; Liu, Y.-X.; Wu, Z.-G.; Zhong, B.-H.; Zhong, Y.-J.; Guo, X.-D. A Review of Rational Design and Investigation of Binders Applied in Silicon-Based Anodes for Lithium-Ion Batteries. J. Power Sources 2021, 485, 229331. [Google Scholar] [CrossRef]
- Luiso, S.; Fedkiw, P. Lithium-Ion Battery Separators: Recent Developments and State of Art. Curr. Opin. Electrochem. 2020, 20, 99–107. [Google Scholar] [CrossRef]
- Yu, X.; Chen, R.; Gan, L.; Li, H.; Chen, L. Battery Safety: From Lithium-Ion to Solid-State Batteries. Engineering 2023, 21, 9–14. [Google Scholar] [CrossRef]
- Spotte-Smith, E.W.C.; Blau, S.M.; Xie, X.; Patel, H.D.; Wen, M.; Wood, B.; Dwaraknath, S.; Persson, K.A. Quantum Chemical Calculations of Lithium-Ion Battery Electrolyte and Interphase Species. Sci. Data 2021, 8, 203. [Google Scholar] [CrossRef]
- Li, Q.; Chen, J.; Fan, L.; Kong, X.; Lu, Y. Progress in Electrolytes for Rechargeable Li-Based Batteries and Beyond. Green Energy Environ. 2016, 1, 18–42. [Google Scholar] [CrossRef] [Green Version]
- Kwon, T.W.; Choi, J.W.; Coskun, A. The Emerging Era of Supramolecular Polymeric Binders in Silicon Anodes. Chem. Soc. Rev. 2018, 47, 2145–2164. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Zhou, X.; Yu, G. Material and Structural Design of Novel Binder Systems for High-Energy, High-Power Lithium-Ion Batteries. Acc. Chem. Res. 2017, 50, 2642–2652. [Google Scholar] [CrossRef] [Green Version]
- Miranda, A.; Sarang, K.; Gendensuren, B.; Oh, E.S.; Lutkenhaus, J.; Lutkenhaus, J.; Verduzco, R.; Verduzco, R. Molecular Design Principles for Polymeric Binders in Silicon Anodes. Mol. Syst. Des. Eng. 2020, 5, 709–724. [Google Scholar] [CrossRef]
- Magasinski, A.; Zdyrko, B.; Kovalenko, I.; Hertzberg, B.; Burtovyy, R.; Huebner, C.F.; Fuller, T.F.; Luzinov, I.; Yushin, G. Toward Efficient Binders for Li-Ion Battery Si-Based Anodes: Polyacrylic Acid. ACS Appl. Mater. Interfaces 2010, 2, 3004–3010. [Google Scholar] [CrossRef]
- Kovalenko, I.; Zdyrko, B.; Magasinski, A.; Hertzberg, B.; Milicev, Z.; Burtovyy, R.; Luzinov, I.; Yushin, G. A Major Constituent of Brown Algae for Use in High-Capacity Li-Ion Batteries. Science 2011, 334, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lewis, R.B.; Dahn, J.R. Sodium Carboxymethyl Cellulose: A Potential Binder for Si Negative Electrodes for Li-Ion Batteries. Electrochem. Solid-State Lett. 2007, 10, A17. [Google Scholar] [CrossRef]
- Kwon, T.; Jeong, Y.K.; Deniz, E.; AlQaradawi, S.Y.; Choi, J.W.; Coskun, A. Dynamic Cross-Linking of Polymeric Binders Based on Host–Guest Interactions for Silicon Anodes in Lithium Ion Batteries. ACS Nano 2015, 9, 11317–11324. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kwon, T.; Coskun, A.; Choi, J.W. Highly Elastic Binders Integrating Polyrotaxanes for Silicon Microparticle Anodes in Lithium Ion Batteries. Science 2017, 357, 279–283. [Google Scholar] [CrossRef] [Green Version]
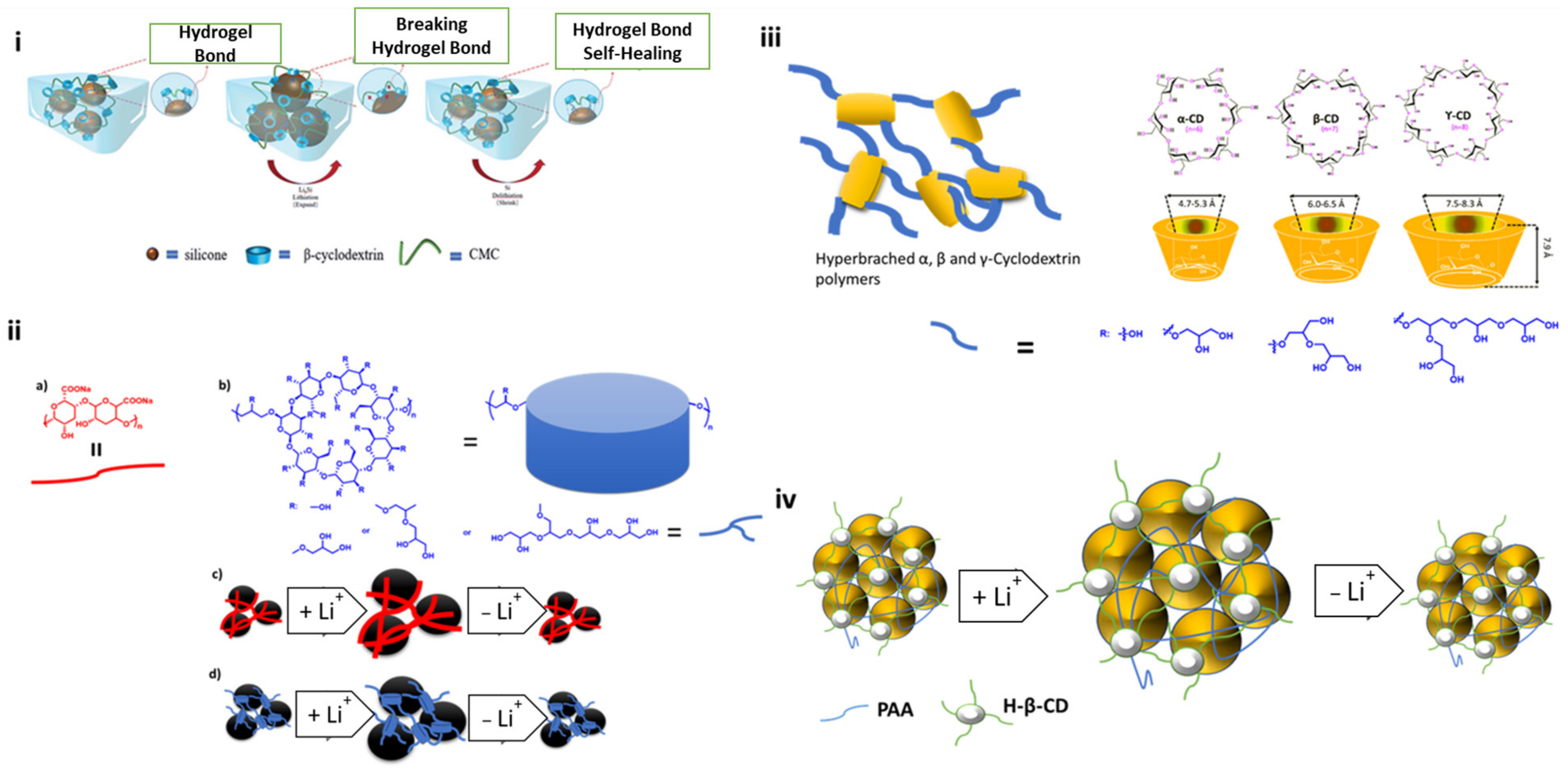
- Jeong, Y.K.; Kwon, T.; Lee, I.; Kim, T.-S.; Coskun, A.; Choi, J.W. Hyperbranched β-Cyclodextrin Polymer as an Effective Multidimensional Binder for Silicon Anodes in Lithium Rechargeable Batteries. Nano Lett. 2014, 14, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.H.; Rong, M.Z.; Zhang, M.Q. Dynamically Cross-Linked Polymeric Binder-Made Durable Silicon Anode of a Wide Operating Temperature Li-Ion Battery. ACS Appl. Mater. Interfaces 2021, 13, 28737–28748. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.; Kim, J.; Elabd, A.; Choi, S.; Park, K.; Kwon, T.; Lee, J.; Char, K.; Coskun, A.; Choi, J.W. A Pyrene–Poly(Acrylic Acid)–Polyrotaxane Supramolecular Binder Network for High-Performance Silicon Negative Electrodes. Adv. Mater. 2019, 31, e1905048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoo, D.J.; Elabd, A.; Choi, S.; Cho, Y.; Kim, J.; Lee, S.J.; Choi, S.H.; Kwon, T.; Char, K.; Kim, K.J.; et al. Highly Elastic Polyrotaxane Binders for Mechanically Stable Lithium Hosts in Lithium-Metal Batteries. Adv. Mater. 2019, 31, e1901645. [Google Scholar] [CrossRef] [Green Version]
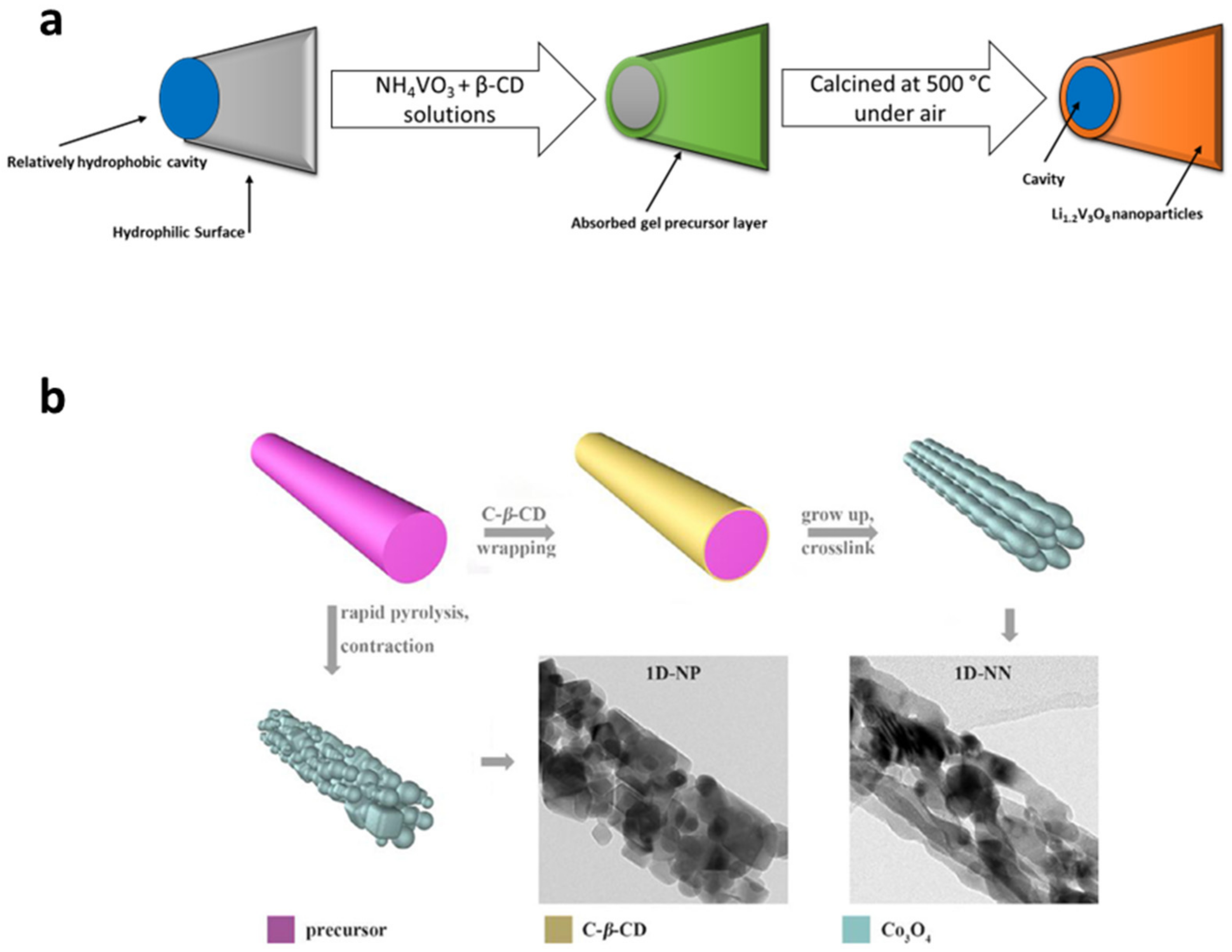
- Cheng, C.; Li, Z.H.; Zhan, X.Y.; Xiao, Q.Z.; Lei, G.T.; Zhou, X.D. A Macaroni-like Li1.2V3O8 Nanomaterial with High Capacity for Aqueous Rechargeable Lithium Batteries. Electrochim. Acta 2010, 55, 4627–4631. [Google Scholar] [CrossRef]
- Hu, Y.; Yan, C.; Chen, D.; Lv, C.; Jiao, Y.; Chen, G. One-Dimensional Co3O4 Nanonet with Enhanced Rate Performance for Lithium Ion Batteries: Carbonyl-Β-Cyclodextrin Inducing and Kinetic Analysis. Chem. Eng. J. 2017, 321, 31–39. [Google Scholar] [CrossRef]
- Jiang, H.W.; Yang, Y.; Nie, Y.M.; Su, Z.F.; Long, Y.F.; Wen, Y.X.; Su, J. Cross-Linked β-CD-CMC as an Effective Aqueous Binder for Silicon-Based Anodes in Rechargeable Lithium-Ion Batteries. RSC Adv. 2022, 12, 5997–6006. [Google Scholar] [CrossRef]
- Lin, S.; Wang, F.; Hong, R. Polyacrylic Acid and β-Cyclodextrin Polymer Cross-Linking Binders to Enhance Capacity Performance of Silicon/Carbon Composite Electrodes in Lithium-Ion Batteries. J. Colloid Interface Sci. 2022, 613, 857–865. [Google Scholar] [CrossRef]
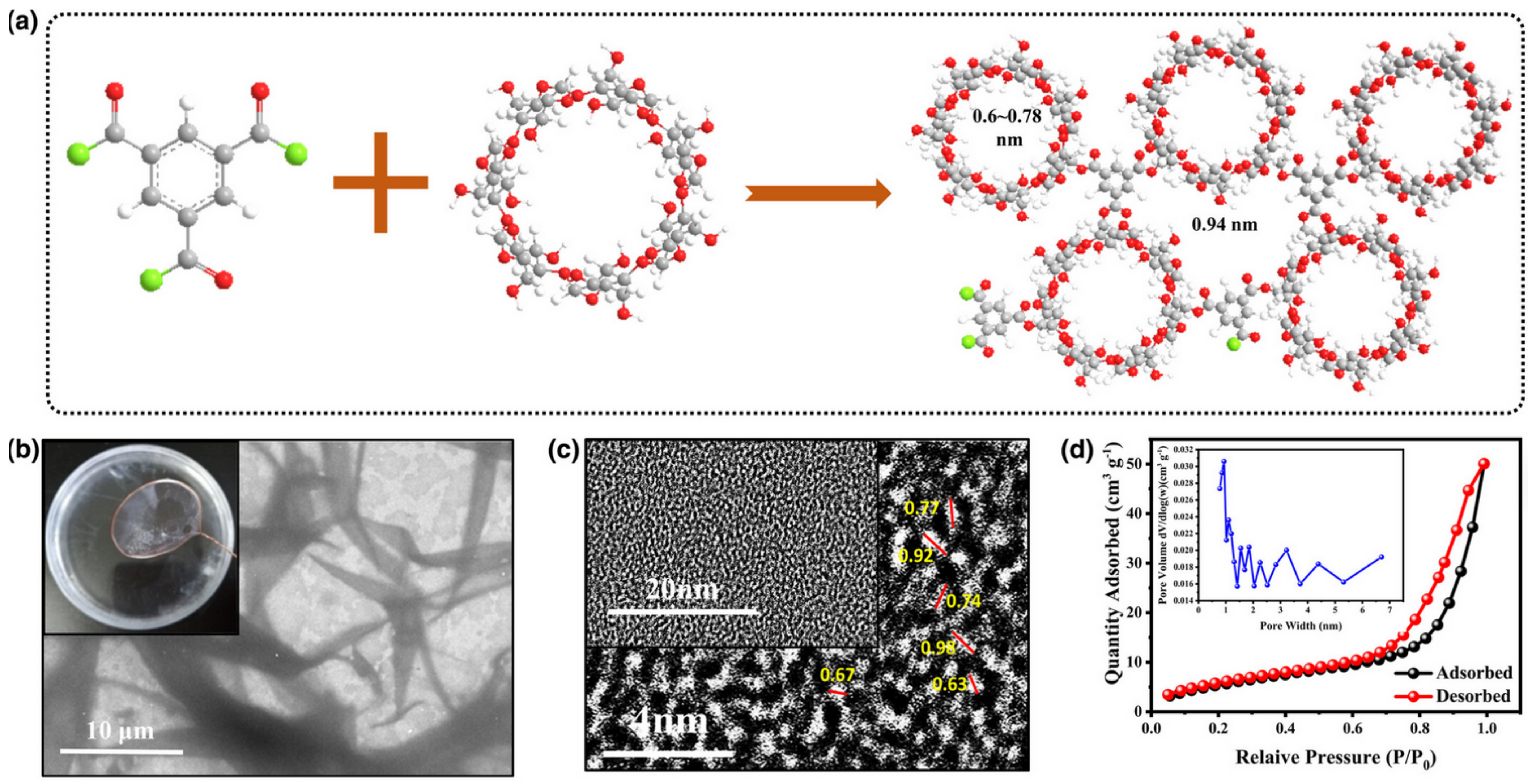
- Alidoost, M.; Mangini, A.; Caldera, F.; Anceschi, A.; Amici, J.; Versaci, D.; Fagiolari, L.; Trotta, F.; Francia, C.; Bella, F.; et al. Micro-Mesoporous Carbons from Cyclodextrin Nanosponges Enabling High-Capacity Silicon Anodes and Sulfur Cathodes for Lithiated Si-S Batteries. Chem. A Eur. J. 2022, 28, e202104201. [Google Scholar] [CrossRef] [PubMed]
- Bhargav, A.; He, J.; Gupta, A.; Manthiram, A. Lithium-Sulfur Batteries: Attaining the Critical Metrics. Joule 2020, 4, 285–291. [Google Scholar] [CrossRef]
- Ding, B.; Wang, J.; Fan, Z.; Chen, S.; Lin, Q.; Lu, X.; Dou, H.; Kumar Nanjundan, A.; Yushin, G.; Zhang, X.; et al. Solid-State Lithium–Sulfur Batteries: Advances, Challenges and Perspectives. Mater. Today 2020, 40, 114–131. [Google Scholar] [CrossRef]
- Soni, R.; Spadoni, D.; Shearing, P.R.; Brett, D.J.L.; Lekakou, C.; Cai, Q.; Robinson, J.B.; Miller, T.S. Deploying Proteins as Electrolyte Additives in Li–S Batteries: The Multifunctional Role of Fibroin in Improving Cell Performance. ACS Appl. Energy Mater. 2023, 6, 5671–5680. [Google Scholar] [CrossRef]
- Soni, R.; Robinson, J.B.; Shearing, P.R.; Brett, D.J.L.; Rettie, A.J.E.; Miller, T.S. Lithium-Sulfur Battery Diagnostics through Distribution of Relaxation Times Analysis. Energy Storage Mater. 2022, 51, 97–107. [Google Scholar] [CrossRef]
- Wu, Y.L.; Yang, J.; Wang, J.L.; Yin, L.C.; Nuli, Y.N. Composite Cathode Structure and Binder for High Performance Lithium-Sulfur Battery. Wuli Huaxue Xuebao/Acta Phys. Chim. Sin. 2010, 26, 283–290. [Google Scholar] [CrossRef]
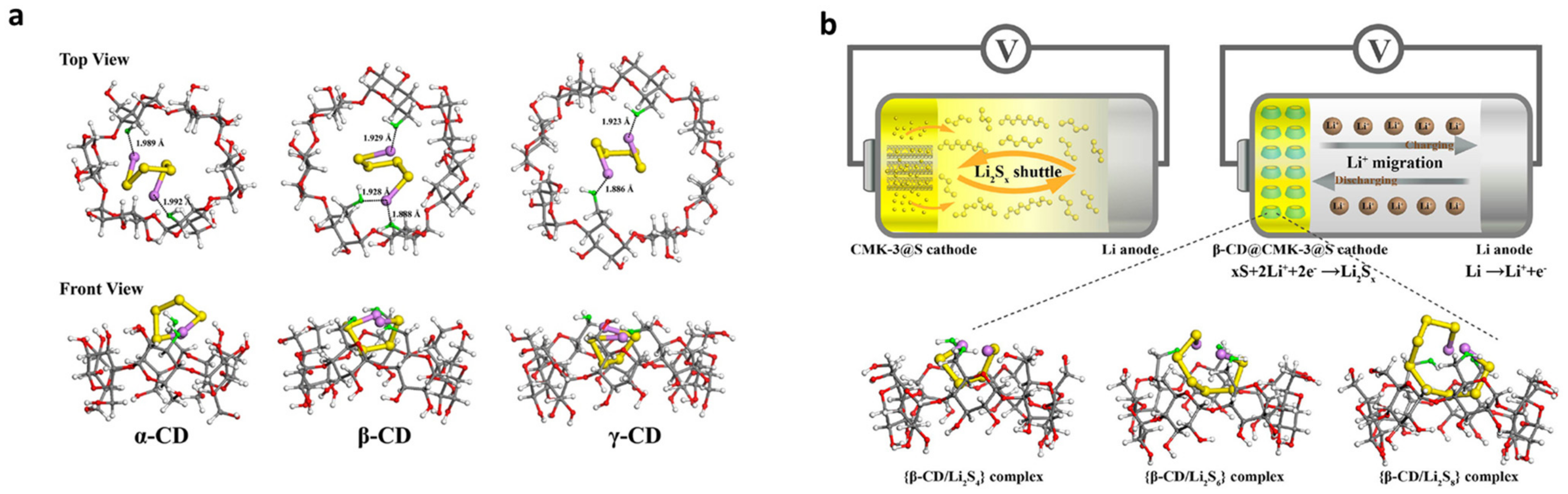
- Wang, J.; Yao, Z.; Monroe, C.W.; Yang, J.; Nuli, Y. Carbonyl-β-Cyclodextrin as a Novel Binder for Sulfur Composite Cathodes in Rechargeable Lithium Batteries. Adv. Funct. Mater. 2013, 23, 1194–1201. [Google Scholar] [CrossRef]
- Zeng, F.; Wang, W.; Wang, A.; Yuan, K.; Jin, Z.; Yang, Y.S. Multidimensional Polycation β-Cyclodextrin Polymer as an Effective Aqueous Binder for High Sulfur Loading Cathode in Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2015, 7, 26257–26265. [Google Scholar] [CrossRef] [PubMed]
- Tegman, R. The Crystal Structure of Sodium Tetrasulphide, Na2S4. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1973, 29, 1463–1469. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- Zubair, U.; Anceschi, A.; Caldera, F.; Alidoost, M.; Amici, J.; Francia, C.; Zanetti, M.; Trotta, F.; Bodoardo, S.; Penazzi, N. Dual Confinement of Sulphur with RGO-Wrapped Microporous Carbon from β-Cyclodextrin Nanosponges as a Cathode Material for Li–S Batteries. J. Solid State Electrochem. 2017, 21, 3411–3420. [Google Scholar] [CrossRef]
- Chen, R.; Lai, J.; Li, Y.; Cao, M.; Chen, S.; Wu, F. β-Cyclodextrin Coated Lithium Vanadium Phosphate as Novel Cathode Material for Lithium Ion Batteries. RSC Adv. 2016, 6, 103364–103371. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, M.; Shen, B.; Xia, Z.; Li, Y.; Wu, Y.; Li, Q. Facile Synthesis of Mesoporous NH4V4O10 Nanoflowers with High Performance as Cathode Material for Lithium Battery. J. Mater. Sci. 2018, 53, 2045–2053. [Google Scholar] [CrossRef]
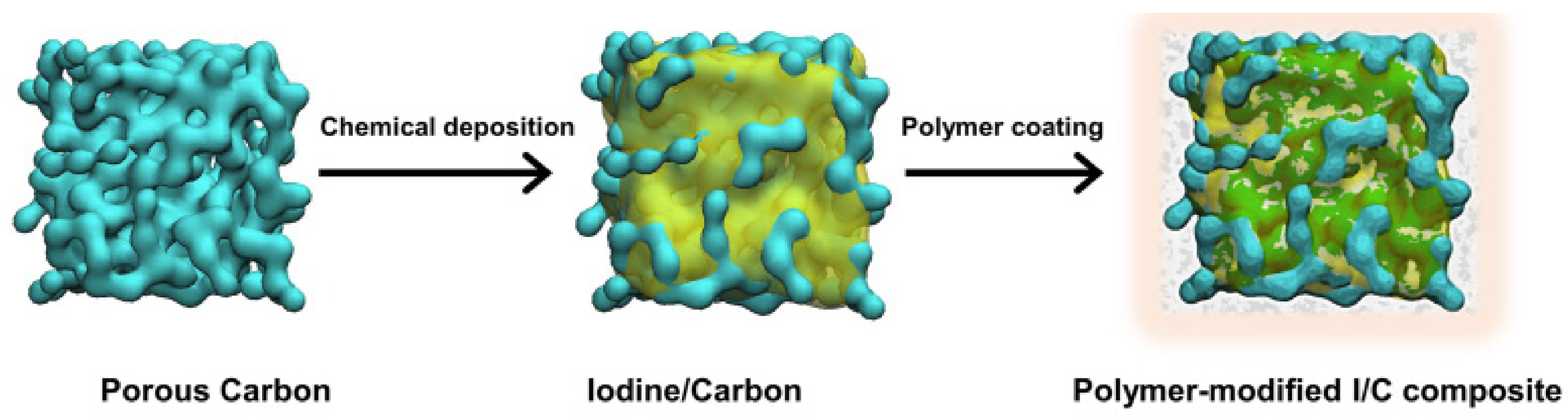
- Cai, F.S.; Duan, Y.Q.; Yuan, Z.H. Iodine/β-Cyclodextrin Composite Cathode for Rechargeable Lithium-Iodine Batteries. J. Mater. Sci. Mater. Electron. 2018, 29, 11540–11545. [Google Scholar] [CrossRef]
- Zhang, Q.; Zeng, Y.H.; Ye, S.H.; Liu, S. Inclusion Complexation Enhanced Cycling Performance of Iodine/Carbon Composites for Lithium–Iodine Battery. J. Power Sources 2020, 463, 228212. [Google Scholar] [CrossRef]
- Chen, J.; Liu, J.; Qi, Y.; Sun, T.; Li, X. Unveiling the Roles of Binder in the Mechanical Integrity of Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2013, 160, A1502–A1509. [Google Scholar] [CrossRef] [Green Version]
- Feng, X.; Fang, H.; Wu, N.; Liu, P.; Jena, P.; Nanda, J.; Mitlin, D. Review of Modification Strategies in Emerging Inorganic Solid-State Electrolytes for Lithium, Sodium, and Potassium Batteries. Joule 2022, 6, 543–587. [Google Scholar] [CrossRef]
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A Conceptual Review on Polymer Electrolytes and Ion Transport Models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Diederichsen, K.M.; Buss, H.G.; McCloskey, B.D. The Compensation Effect in the Vogel-Tammann-Fulcher (VTF) Equation for Polymer-Based Electrolytes. Macromolecules 2017, 50, 3831–3840. [Google Scholar] [CrossRef]
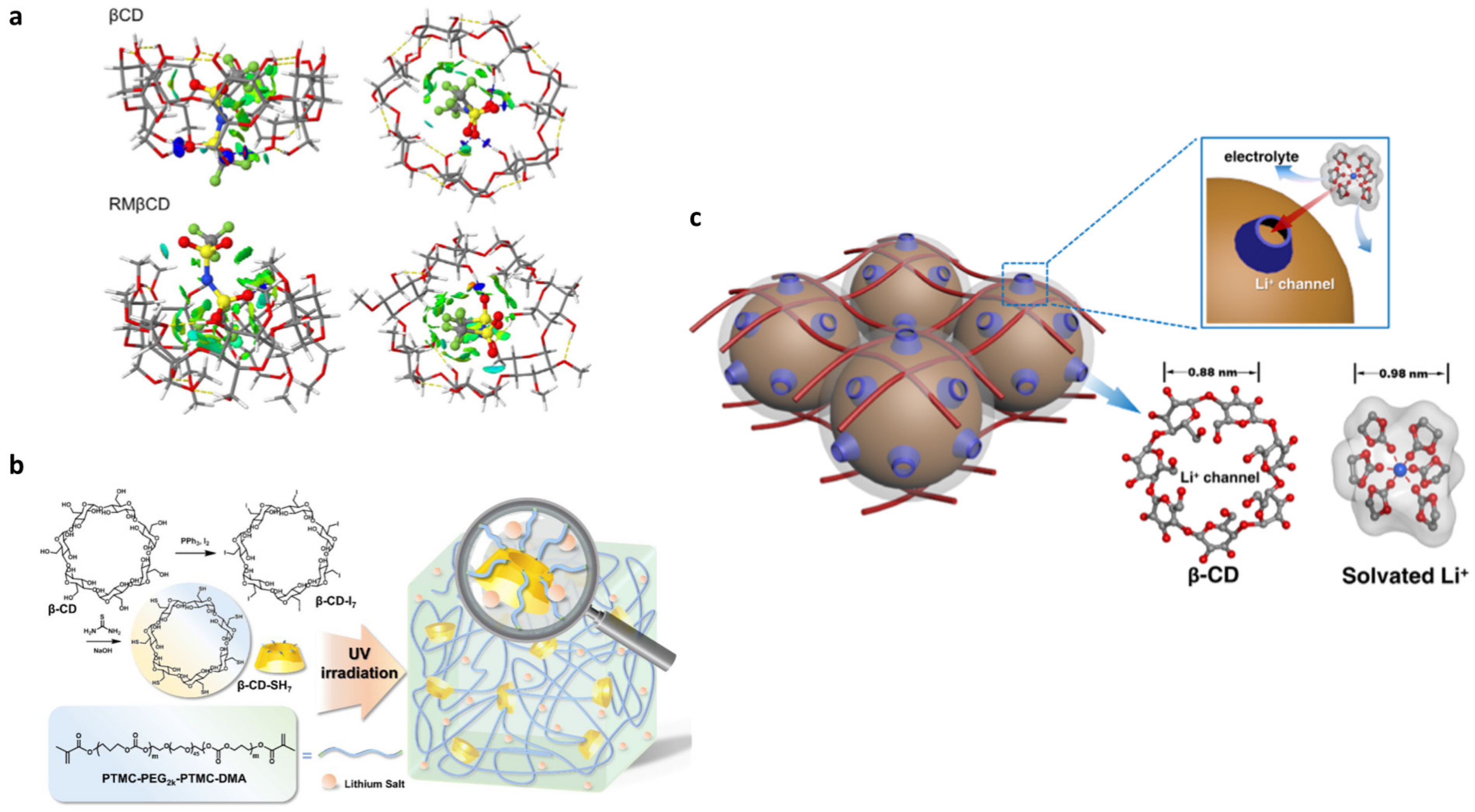
- Jeschke, S.; Jankowski, P.; Best, A.S.; Johansson, P. Catching TFSI: A Computational–Experimental Approach to Β-Cyclodextrin-Based Host–Guest Systems as Electrolytes for Li-Ion Batteries. ChemSusChem 2018, 11, 1942–1949. [Google Scholar] [CrossRef]
- Imholt, L.; Dong, D.; Bedrov, D.; Cekic-Laskovic, I.; Winter, M.; Brunklaus, G. Supramolecular Self-Assembly of Methylated Rotaxanes for Solid Polymer Electrolyte Application. ACS Macro Lett. 2018, 7, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Imholt, L.; Dörr, T.S.; Zhang, P.; Ibing, L.; Cekic-Laskovic, I.; Winter, M.; Brunklaus, G. Grafted Polyrotaxanes as Highly Conductive Electrolytes for Lithium Metal Batteries. J. Power Sources 2019, 409, 148–158. [Google Scholar] [CrossRef]
- Zuo, C.; Li, H.; Chen, G.; Yang, J.; Xu, Z.; Xue, Z. Fabrication of Elastic Cyclodextrin-Based Triblock Polymer Electrolytes for All-Solid-State Lithium Metal Batteries. ACS Appl. Energy Mater. 2021, 4, 9402–9411. [Google Scholar] [CrossRef]
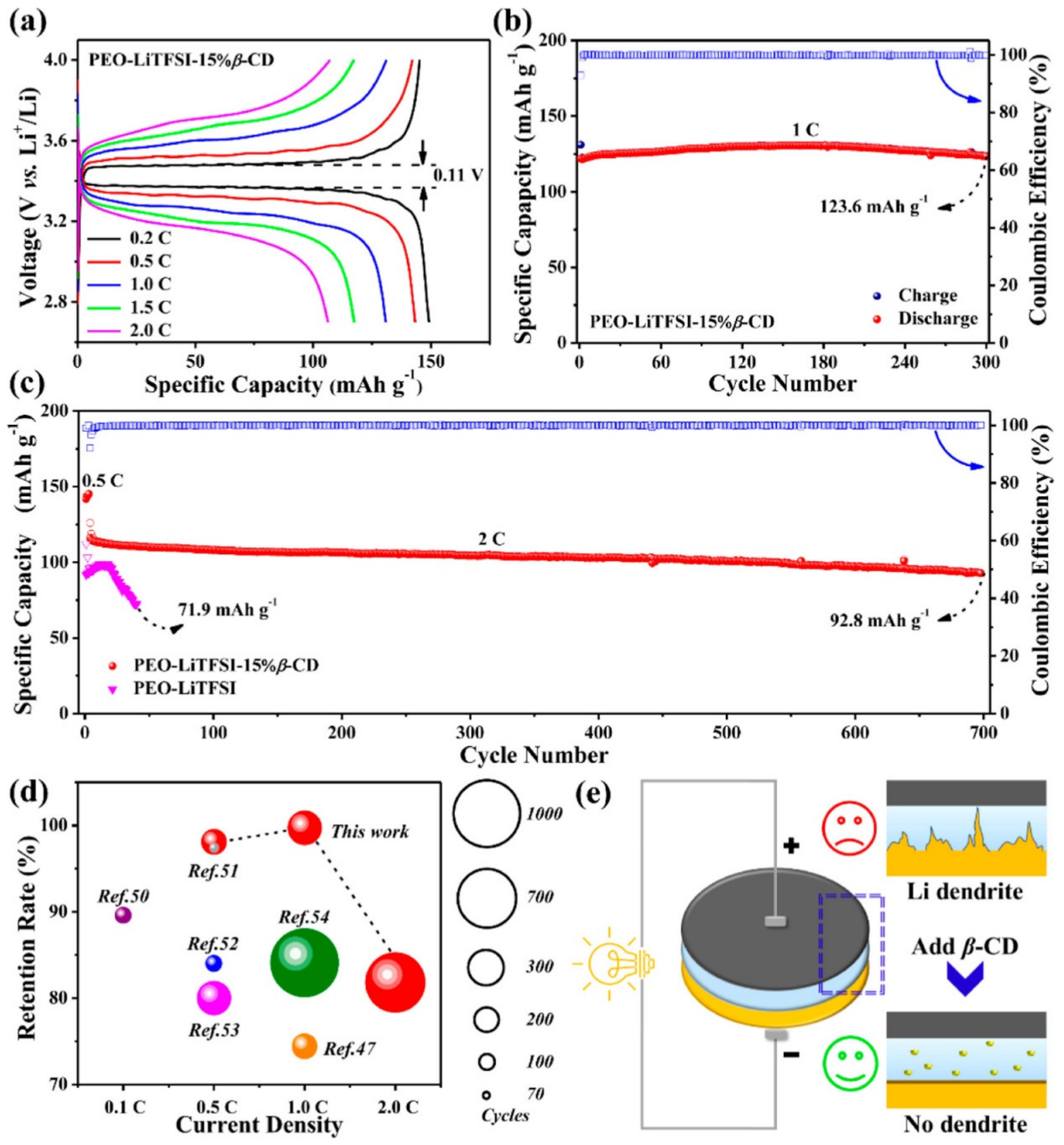
- Duan, H.; Li, L.; Zou, K.; Deng, Y.; Chen, G. Cyclodextrin-Integrated PEO-Based Composite Solid Electrolytes for High-Rate and Ultrastable All-Solid-State Lithium Batteries. ACS Appl. Mater. Interfaces 2021, 13, 57380–57391. [Google Scholar] [CrossRef]
- Huang, J.Q.; Zhang, B.; Xu, Z.L.; Abouali, S.; Akbari Garakani, M.; Huang, J.; Kim, J.K. Novel Interlayer Made from Fe3C/Carbon Nanofiber Webs for High Performance Lithium-Sulfur Batteries. J. Power Sources 2015, 285, 43–50. [Google Scholar] [CrossRef]
- Tian, D.; Song, X.; Qiu, Y.; Sun, X.; Jiang, B.; Zhao, C.; Zhang, Y.; Xu, X.; Fan, L.; Zhang, N. Heterogeneous Mediator Enabling Three-Dimensional Growth of Lithium Sulfide for High-Performance Lithium–Sulfur Batteries. Energy Environ. Mater. 2022, 5, 1214–1221. [Google Scholar] [CrossRef]
- Zhou, G.; Li, L.; Wang, D.-W.; Shan, X.; Pei, S.; Li, F.; Cheng, H.-M. A Flexible Sulfur-Graphene-Polypropylene Separator Integrated Electrode for Advanced Li-S Batteries. Adv. Mater. 2015, 27, 641–647. [Google Scholar] [CrossRef]
- Pang, Y.; Wei, J.; Wang, Y.; Xia, Y. Synergetic Protective Effect of the Ultralight MWCNTs/NCQDs Modified Separator for Highly Stable Lithium-Sulfur Batteries. Adv. Energy Mater. 2018, 8, 1702288. [Google Scholar] [CrossRef]
- Zhao, H.; Kang, W.; Deng, N.; Liu, M.; Cheng, B. A Fresh Hierarchical-Structure Gel Poly-m-Phenyleneisophthalamide Nanofiber Separator Assisted by Electronegative Nanoclay-Filler towards High-Performance and Advanced-Safety Lithium-Ion Battery. Chem. Eng. J. 2020, 384, 123312. [Google Scholar] [CrossRef]
- Deng, N.; Liu, Y.; Li, Q.; Yan, J.; Zhang, L.; Wang, L.; Zhang, Y.; Cheng, B.; Lei, W.; Kang, W. Functional Double-Layer Membrane as Separator for Lithium-Sulfur Battery with Strong Catalytic Conversion and Excellent Polysulfide-Blocking. Chem. Eng. J. 2020, 382, 122918. [Google Scholar] [CrossRef]
- Zhao, H.; Deng, N.; Kang, W.; Wang, G.; Hao, Y.; Zhang, Y.; Cheng, B. The Significant Effect of Octa(Aminophenyl)Silsesquioxane on the Electrospun Ion-Selective and Ultra-Strong Poly-m-Phenyleneisophthalamide Separator for Enhanced Electrochemical Performance of Lithium-Sulfur Battery. Chem. Eng. J. 2020, 381, 122715. [Google Scholar] [CrossRef]
- Wang, X.; Hao, X.; Hengjing, Z.; Xia, X.; Tu, J. 3D Ultraviolet Polymerized Electrolyte Based on PEO Modified PVDF-HFP Electrospun Membrane for High-Performance Lithium-Sulfur Batteries. Electrochim. Acta 2020, 329, 135108. [Google Scholar] [CrossRef]
- Zhu, P.; Zhu, J.; Zang, J.; Chen, C.; Lu, Y.; Jiang, M.; Yan, C.; Dirican, M.; Kalai Selvan, R.; Zhang, X. A Novel Bi-Functional Double-Layer RGO-PVDF/PVDF Composite Nanofiber Membrane Separator with Enhanced Thermal Stability and Effective Polysulfide Inhibition for High-Performance Lithium-Sulfur Batteries. J. Mater. Chem. A 2017, 5, 15096–15104. [Google Scholar] [CrossRef]
- Liao, H.; Zhang, H.; Hong, H.; Li, Z.; Qin, G.; Zhu, H.; Lin, Y. Novel Cellulose Aerogel Coated on Polypropylene Separators as Gel Polymer Electrolyte with High Ionic Conductivity for Lithium-Ion Batteries. J. Memb. Sci. 2016, 514, 332–339. [Google Scholar] [CrossRef]
- Huang, F.; Xu, Y.; Peng, B.; Su, Y.; Jiang, F.; Hsieh, Y.L.; Wei, Q. Coaxial Electrospun Cellulose-Core Fluoropolymer-Shell Fibrous Membrane from Recycled Cigarette Filter as Separator for High Performance Lithium-Ion Battery. ACS Sustain. Chem. Eng. 2015, 3, 932–940. [Google Scholar] [CrossRef] [Green Version]
- Chien, Y.-C.; Pan, R.; Lee, M.-T.; Nyholm, L.; Brandell, D.; Lacey, M.J. Cellulose Separators With Integrated Carbon Nanotube Interlayers for Lithium-Sulfur Batteries: An Investigation into the Complex Interplay between Cell Components. J. Electrochem. Soc. 2019, 166, A3235–A3241. [Google Scholar] [CrossRef]
- Seh, Z.W.; Zhang, Q.; Li, W.; Zheng, G.; Yao, H.; Cui, Y. Stable Cycling of Lithium Sulfide Cathodes through Strong Affinity with a Bifunctional Binder. Chem. Sci. 2013, 4, 3673–3677. [Google Scholar] [CrossRef]
- Yu, X.; Joseph, J.; Manthiram, A. Polymer Lithium-Sulfur Batteries with a Nafion Membrane and an Advanced Sulfur Electrode. J. Mater. Chem. A 2015, 3, 15683–15691. [Google Scholar] [CrossRef]
- Bauer, I.; Thieme, S.; Brückner, J.; Althues, H.; Kaskel, S. Reduced Polysulfide Shuttle in Lithium-Sulfur Batteries Using Nafion-Based Separators. J. Power Sources 2014, 251, 417–422. [Google Scholar] [CrossRef]
- Li, C.; Ward, A.L.; Doris, S.E.; Pascal, T.A.; Prendergast, D.; Helms, B.A. Polysulfide-Blocking Microporous Polymer Membrane Tailored for Hybrid Li-Sulfur Flow Batteries. Nano Lett. 2015, 15, 5724–5729. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Liu, X.; Zhu, K.; Wu, S.; Zhou, H. Metal–Organic Framework-Based Separator for Lithium–Sulfur Batteries. Nat. Energy 2016, 1, 16094. [Google Scholar] [CrossRef]
- Wu, S.; Shi, J.; Nie, X.; Yao, Y.; Jiang, F.; Wei, Q.; Huang, F. Microporous Cyclodextrin Film with Funnel-Type Channel Polymerized on Electrospun Cellulose Acetate Membrane as Separators for Strong Trapping Polysulfides and Boosting Charging in Lithium–Sulfur Batteries. Energy Environ. Mater. 2022, 6, e12319. [Google Scholar] [CrossRef]



| Binder | Linker | ICE (%) | Capacity Retention | Inclusion | Cross-Linking |
|---|---|---|---|---|---|
| β-CD | - | 78 | 69 | no | no |
| β-CD | 6AD | 84 | 90 | Strong | Strong |
| β-CD | 1AD | 83 | 23 | Strong | no |
| α-CD | - | 79 | 64 | no | no |
| α-CD | 6AD | 84 | 19 | no | no |
| α-CD | 1AD | 87 | 29 | no | no |
| γ-CD | - | 79 | 53 | no | no |
| γ-CD | 6AD | 85 | 35 | Weak | Weak |
| γ-CD | 1AD | 84 | 23 | Weak | no |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desoky, M.M.H.; Caldera, F.; Brunella, V.; Ferrero, R.; Hoti, G.; Trotta, F. Cyclodextrins for Lithium Batteries Applications. Materials 2023, 16, 5540. https://doi.org/10.3390/ma16165540
Desoky MMH, Caldera F, Brunella V, Ferrero R, Hoti G, Trotta F. Cyclodextrins for Lithium Batteries Applications. Materials. 2023; 16(16):5540. https://doi.org/10.3390/ma16165540
Chicago/Turabian StyleDesoky, Mohamed M. H., Fabrizio Caldera, Valentina Brunella, Riccardo Ferrero, Gjylije Hoti, and Francesco Trotta. 2023. "Cyclodextrins for Lithium Batteries Applications" Materials 16, no. 16: 5540. https://doi.org/10.3390/ma16165540
APA StyleDesoky, M. M. H., Caldera, F., Brunella, V., Ferrero, R., Hoti, G., & Trotta, F. (2023). Cyclodextrins for Lithium Batteries Applications. Materials, 16(16), 5540. https://doi.org/10.3390/ma16165540








