Nonlinear Optical Properties of Zn(II) Porphyrin, Graphene Nanoplates, and Ferrocene Hybrid Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. EFISH Measurements
2.3. Computational Details
3. Results and Discussion
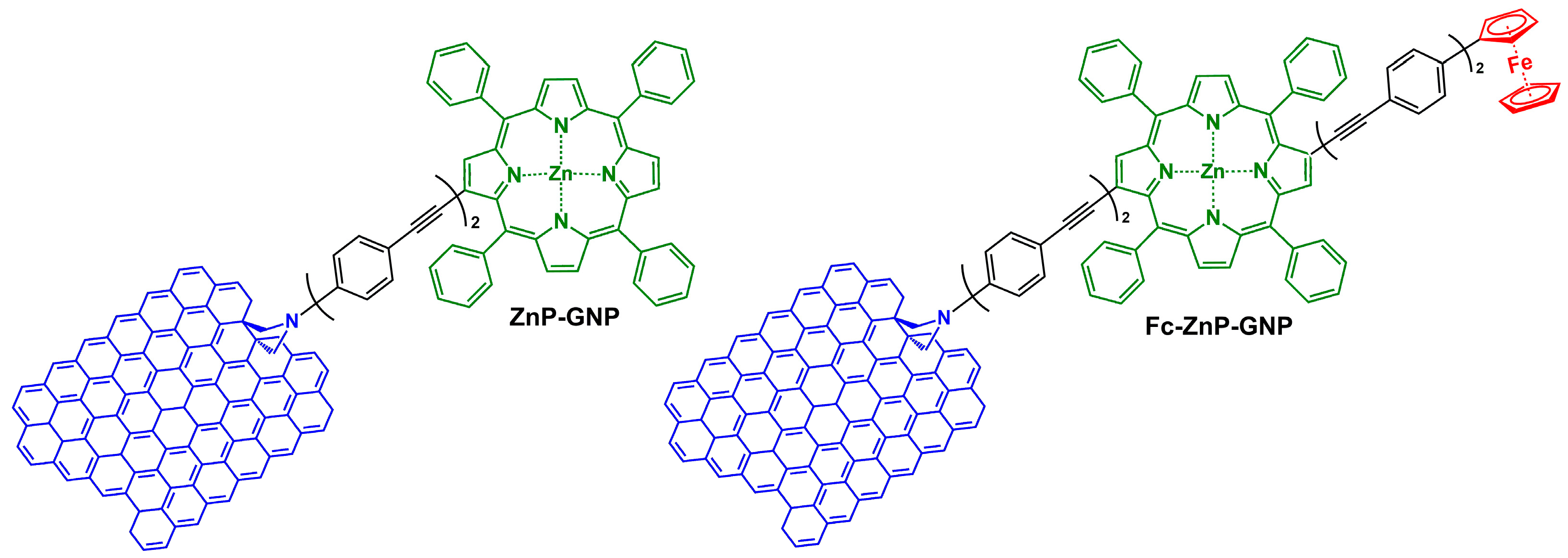
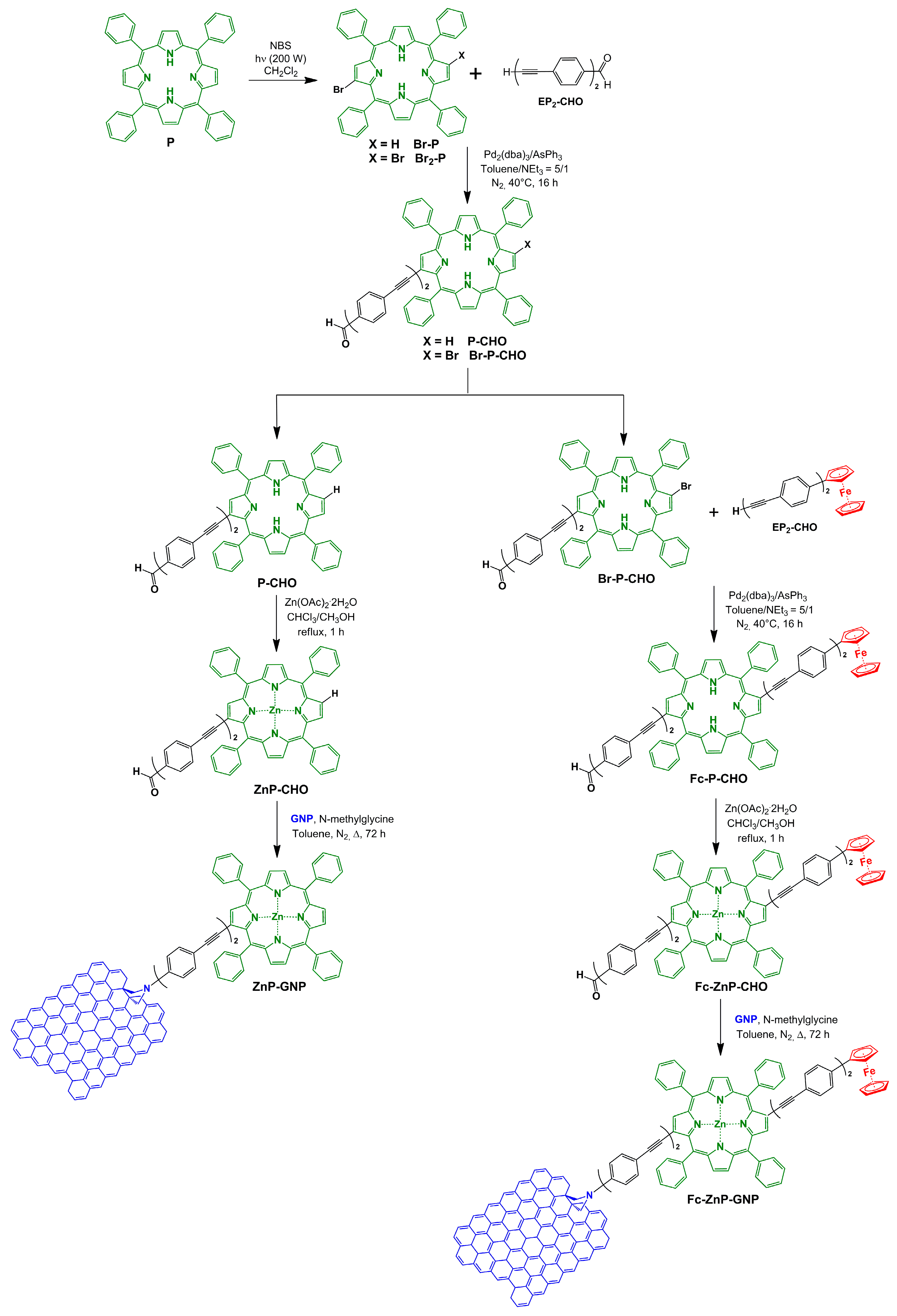
3.1. Synthesis
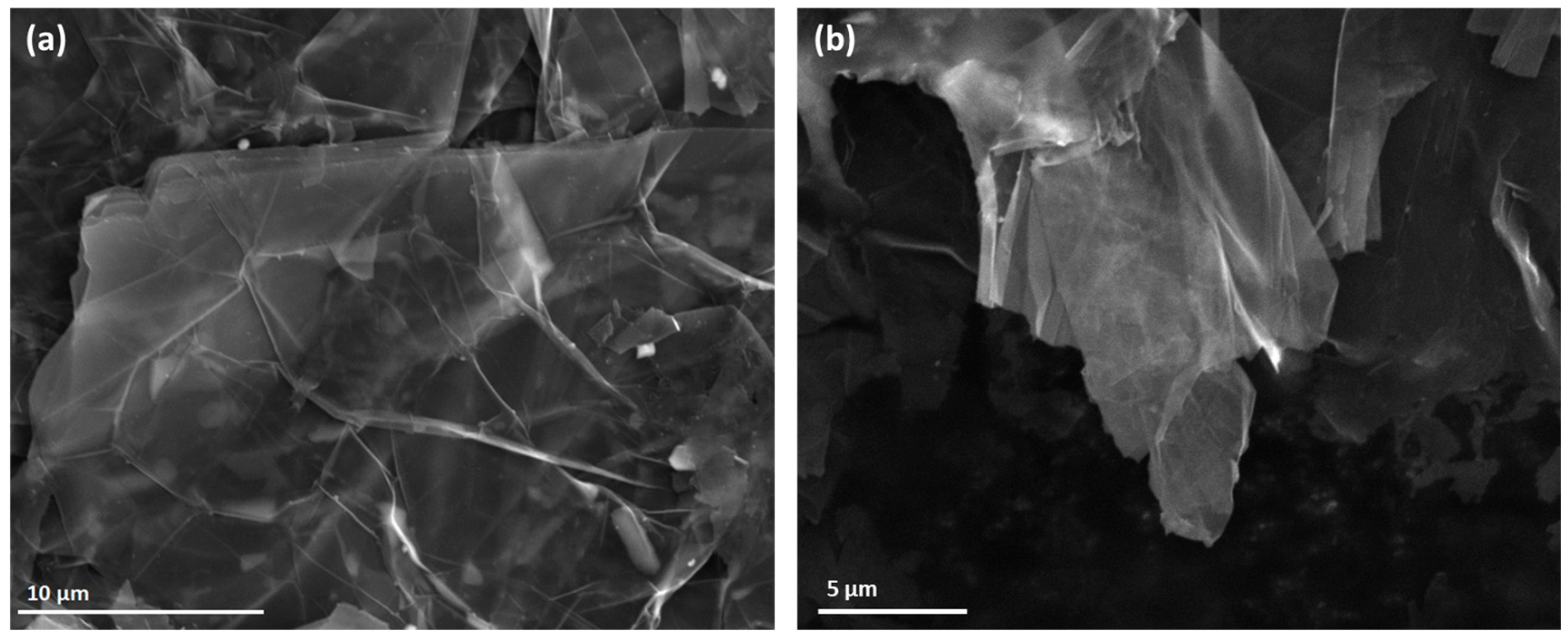
3.2. SEM of GNP
3.3. UV–Vis Spectroscopy in Solution
3.4. EFISH and CP-DFT Investigation of the Second Order NLO Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V. Linear and Nonlinear Optical Properties of Graphene: A Review. J. Electron. Mater. 2021, 50, 3773–3799. [Google Scholar] [CrossRef]
- You, J.W.; Bongu, S.R.; Bao, Q.; Panoiu, N.C. Nonlinear optical properties and applications of 2D materials. Theor. Exp. Asp. 2019, 8, 63–97. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Loboda, O.; Zaleśny, R.; Avramopoulos, A.; Luis, J.-M.; Kirtman, B.; Tagmatarchis, N.; Reis, H.; Papadopoulos, M.G. Linear and Nonlinear Optical Properties of [60]Fullerene Derivatives. J. Phys. Chem. A 2009, 113, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Kajzar, F.; Taliani, C.; Zamboni, R.; Rossini, S.; Danieli, R. Nonlinear optical properties of fullerenes. Synth. Met. 1996, 77, 257–263. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, L.T. Nonlinear optical properties of fullerenes and charge-transfer complexes of fullerenes. J. Phys. Chem. 1992, 96, 1530–1532. [Google Scholar] [CrossRef]
- Limosani, F.; Carcione, R.; Antolini, F. Formation of CdSe quantum dots from single source precursor obtained by thermal and laser treatment. J. Vac. Sci. Technol. B 2019, 38, 12802–12811. [Google Scholar] [CrossRef]
- Carcione, R.; Limosani, F.; Antolini, F. Cadmium Telluride Nanocomposite Films Formation from Thermal Decomposition of Cadmium Carboxylate Precursor and Their Photoluminescence Shift from Green to Red. Crystals 2021, 11, 253. [Google Scholar] [CrossRef]
- Antolini, F.; Limosani, F.; Carcione, R. Direct Laser Patterning of CdTe QDs and Their Optical Properties Control through Laser Parameters. Nanomaterials 2022, 12, 1551. [Google Scholar] [CrossRef]
- Lu, H.; Kobayashi, N. Optically Active Porphyrin and Phthalocyanine Systems. Chem. Rev. 2016, 116, 6184–6261. [Google Scholar] [CrossRef]
- Collini, E.; Mazzucato, S.; Zerbetto, M.; Ferrante, C.; Bozio, R.; Pizzotti, M.; Tessore, F.; Ugo, R. Large two photon absorption cross section of asymmetric Zn(II) porphyrin complexes substituted in the meso or β pyrrolic position by -C-C≡C-C6H4X moieties (X = NMe2, NO2). Chem. Phys. Lett. 2008, 454, 70–74. [Google Scholar] [CrossRef]
- Limosani, F.; Remita, H.; Tagliatesta, P.; Bauer, E.M.; Leoni, A.; Carbone, M. Functionalization of Gold Nanoparticles with Ru-Porphyrin and Their Selectivity in the Oligomerization of Alkynes. Materials 2022, 15, 1207. [Google Scholar] [CrossRef]
- Bonin, J.; Maurin, A.; Robert, M. Molecular catalysis of the electrochemical and photochemical reduction of CO2 with Fe and Co metal based complexes. Recent advances. Coord. Chem. Rev. 2017, 334, 184–198. [Google Scholar] [CrossRef]
- Azcarate, I.; Costentin, C.; Robert, M.; Savéant, J.M. Through-Space Charge Interaction Substituent Effects in Molecular Catalysis Leading to the Design of the Most Efficient Catalyst of CO2-to-CO Electrochemical Conversion. J. Am. Chem. Soc. 2016, 138, 16639–16644. [Google Scholar] [CrossRef]
- Rao, H.; Schmidt, L.C.; Bonin, J.; Robert, M. Visible-light-driven methane formation from CO2 with a molecular iron catalyst. Nature 2017, 548, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Veselov, A.; Thür, C.; Efimov, A.; Guina, M.; Lemmetyinen, H.; Tkachenko, N. Acidity sensor based on porphyrin self-assembled monolayers covalently attached to the surfaces of tapered fibres. Meas. Sci. Technol. 2010, 21, 115205–115216. [Google Scholar] [CrossRef]
- Garg, K.; Majumder, C.; Gupta, S.K.; Aswal, D.K.; Nayak, S.K.; Chattopadhyay, S. Stable negative differential resistance in porphyrin based σ–π–σ monolayers grafted on silicon. RSC Adv. 2015, 5, 50234–50244. [Google Scholar] [CrossRef][Green Version]
- Guo, P.; Zhao, G.; Chen, P.; Lei, B.; Jiang, L.; Zhang, H.; Hu, W.; Liu, M. Porphyrin Nanoassemblies via Surfactant-Assisted Assembly and Single Nanofiber Nanoelectronic Sensors for High-Performance H2O2 Vapor Sensing. ACS Nano 2014, 8, 3402–3411. [Google Scholar] [CrossRef]
- Carbone, M.; Micheli, L.; Limosani, F.; Possanza, F.; Abdallah, Y.; Tagliatesta, P. Ruthenium and manganese metalloporphyrins modified screen-printed electrodes for bio-relevant electroactive targets. J. Porphyr. Phthalocyanines 2018, 22, 491–500. [Google Scholar] [CrossRef]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for Chemical Sensor Applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef]
- Li, L.L.; Diau, E.W.G. Porphyrin-sensitized solar cells. Chem. Soc. Rev. 2013, 42, 291–304. [Google Scholar] [CrossRef]
- Covezzi, A.; Orbelli Biroli, A.; Tessore, F.; Forni, A.; Marinotto, D.; Biagini, P.; Di Carlo, G.; Pizzotti, M. 4D-π-1A type β-substituted ZnII-porphyrins: Ideal green sensitizers for building-integrated photovoltaics. Chem. Commun. 2016, 52, 12642–12645. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, G.; Orbelli Biroli, A.; Tessore, F.; Caramori, S.; Pizzotti, M. β-Substituted ZnIIporphyrins as dyes for DSSC: A possible approach to photovoltaic windows. Coord. Chem. Rev. 2018, 358, 153–177. [Google Scholar] [CrossRef]
- Campbell, W.M.; Burrell, A.K.; Officer, D.L.; Jolley, K.W. Porphyrins as light harvesters in the dye-sensitised TiO2 solar cell. Coord. Chem. Rev. 2004, 248, 817–833. [Google Scholar] [CrossRef]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, M.K.; Grätzel, M. Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef]
- Yang, G.; Tang, Y.; Li, X.; Ågren, H.; Xie, Y. Efficient Solar Cells Based on Porphyrin Dyes with Flexible Chains Attached to the Auxiliary Benzothiadiazole Acceptor: Suppression of Dye Aggregation and the Effect of Distortion. ACS Appl. Mater. Interfaces 2017, 9, 36875–36885. [Google Scholar] [CrossRef]
- Song, H.; Liu, Q.; Xie, Y. Porphyrin-sensitized solar cells: Systematic molecular optimization, coadsorption and cosensitization. Chem. Commun. 2018, 54, 1811–1824. [Google Scholar] [CrossRef] [PubMed]
- O’Regan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Di Carlo, G.; Orbelli Biroli, A.; Pizzotti, M.; Tessore, F. Efficient sunlight harvesting by A4 β-Pyrrolic Substituted ZnII Porphyrins: A Mini-Review. Front. Chem. 2019, 7, 177–198. [Google Scholar] [CrossRef]
- Berardi, S.; Caramori, S.; Benazzi, E.; Zabini, N.; Niorettini, A.; Orbelli Biroli, A.; Pizzotti, M.; Tessore, F.; Di Carlo, G. Electronic Properties of Electron-Deficient Zn(II) Porphyrins for HBr Splitting. Appl. Sci. 2019, 9, 2739. [Google Scholar] [CrossRef]
- Orbelli Biroli, A.; Tessore, F.; Di Carlo, G.; Pizzotti, M.; Benazzi, E.; Gentile, F.; Berardi, S.; Bignozzi, C.A.; Argazzi, R.; Natali, M.; et al. Fluorinated ZnII Porphyrins for Dye-Sensitized Aqueous Photoelectrosynthetic Cells. ACS Appl. Mater. Interfaces 2019, 11, 32895–32908. [Google Scholar] [CrossRef] [PubMed]
- Materna, K.L.; Jiang, J.; Regan, K.P.; Schmuttenmaer, C.A.; Crabtree, R.H.; Brudvig, G.W. Optimization of Photoanodes for Photocatalytic Water Oxidation by Combining a Heterogenized Iridium Water-Oxidation Catalyst with a High-Potential Porphyrin Photosensitizer. ChemSusChem 2017, 10, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- LeCours, S.M.; Guan, H.W.; DiMagno, S.G.; Wang, C.H.; Therien, M.J. Push-pull arylethynyl porphyrins: New chromophores that exhibited large molecular first-order hyperpolarizabilities. J. Am. Chem. Soc. 1996, 118, 1497–1503. [Google Scholar] [CrossRef]
- Karki, L.; Vance, F.W.; Hupp, J.T.; LeCours, S.M.; Therien, M.J. Electronic stark effect studies of a porphyrin-based push-pull chromophore displaying a large first hyperpolarizability: State-specific contributions to β. J. Am. Chem. Soc. 1998, 120, 2606–2611. [Google Scholar] [CrossRef]
- Ray, P.C.; Leszczynski, J. Nonlinear optical properties of highly conjugated push-pull porphyrin aggregates: Role of intermolecular interaction. Chem. Phys. Lett. 2006, 419, 578–583. [Google Scholar] [CrossRef]
- Pizzotti, M.; Tessore, F.; Orbelli Biroli, A.; Ugo, R.; De Angelis, F.; Fantacci, S.; Sgamellotti, A.; Zuccaccia, D.; Macchioni, A. An EFISH, theoretical, and PGSE NMR investigation on the relevant role of aggregation on the second order response in CHCl3 of the push-pull chromophores [5-[[4′-(dimethylamino)phenyl]ethynyl]-15-[(4″-nitrophenyl)ethynyl]-10,20diphenylporphyri. J. Phys. Chem. C 2009, 113, 11131–11141. [Google Scholar] [CrossRef]
- Orbelli Biroli, A.; Tessore, F.; Righetto, S.; Forni, A.; Macchioni, A.; Rocchigiani, L.; Pizzotti, M.; Di Carlo, G. Intriguing Influence of −COOH-Driven Intermolecular Aggregation and Acid-Base Interactions with N,N-Dimethylformamide on the Second-Order Nonlinear-Optical Response of 5,15 Push-Pull Diarylzinc(II) Porphyrinates. Inorg. Chem. 2017, 56, 6438–6450. [Google Scholar] [CrossRef] [PubMed]
- Tessore, F.; Biroli, A.O.; Di Carlo, G.; Pizzotti, M. Porphyrins for Second Order Nonlinear Optics (NLO): An Intriguing History. Inorganics 2018, 6, 81. [Google Scholar] [CrossRef]
- Di Carlo, G.; Pizzotti, M.; Righetto, S.; Forni, A.; Tessore, F. Electric-Field-Induced Second Harmonic Generation Nonlinear Optic Response of A4 β-Pyrrolic-Substituted ZnII Porphyrins: When Cubic Contributions Cannot Be Neglected. Inorg. Chem. 2020, 59, 7561–7570. [Google Scholar] [CrossRef]
- Tessore, F.; Di Carlo, G.; Forni, A.; Righetto, S.; Limosani, F.; Orbelli Biroli, A. Second Order Nonlinear Optical Properties of 4-Styrylpyridines Axially Coordinated to A4 ZnII Porphyrins: A Comparative Experimental and Theoretical Investigation. Inorganics 2020, 8, 45. [Google Scholar] [CrossRef]
- Stegarescu, A.; Cabrera, H.; Budasheva, H.; Soran, M.-L.; Lung, I.; Limosani, F.; Korte, D.; Amati, M.; Borodi, G.; Kacso, I.; et al. Synthesis and Characterization of MWCNT-COOH/Fe3O4 and CNT-COOH/Fe3O4/NiO Nanocomposites: Assessment of Adsorption and Photocatalytic Performance. Nanomaterials 2022, 12, 3008. [Google Scholar] [CrossRef] [PubMed]
- Scarselli, M.; Limosani, F.; Passacantando, M.; D’Orazio, F.; Nardone, M.; Cacciotti, I.; Arduini, F.; Gautron, E.; De Crescenzi, M. Influence of Iron Catalyst in the Carbon Spheres Synthesis for Energy and Electrochemical Applications. Adv. Mater. Interfaces 2018, 5, 1800070–1800079. [Google Scholar] [CrossRef]
- Cinti, S.; Limosani, F.; Scarselli, M.; Arduini, F. Magnetic carbon spheres and their derivatives combined with printed electrochemical sensors. Electrochim. Acta 2018, 282, 247–254. [Google Scholar] [CrossRef]
- Pierantoni, L.; Mencarelli, D.; Bozzi, M.; Moro, R.; Moscato, S.; Perregrini, L.; Micciulla, F.; Cataldo, A.; Bellucci, S. Broadband Microwave Attenuator Based on Few Layer Graphene Flakes. IEEE Trans. Microw. Theory Tech. 2015, 63, 2491–2497. [Google Scholar] [CrossRef]
- Campidelli, S.; Sooambar, C.; Lozano Diz, E.; Ehli, C.; Guldi, D.M.; Prato, M. Dendrimer-Functionalized Single-Wall Carbon Nanotubes: Synthesis, Characterization, and Photoinduced Electron Transfer. J. Am. Chem. Soc. 2006, 128, 12544–12552. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, Z.; Zhang, X.; Wang, Y.; Tian, J.; Huang, Y.; Ma, Y.; Zhang, X.; Chen, Y. A Graphene Hybrid Material Covalently Functionalized with Porphyrin: Synthesis and Optical Limiting Property. Adv. Mater. 2009, 21, 1275–1279. [Google Scholar] [CrossRef]
- Flavin, K.; Chaur, M.N.; Echegoyen, L.; Giordani, S. Functionalization of Multilayer Fullerenes (Carbon Nano-Onions) using Diazonium Compounds and “Click” Chemistry. Org. Lett. 2010, 12, 840–843. [Google Scholar] [CrossRef]
- Imahori, H.; Sekiguchi, Y.; Kashiwagi, Y.; Sato, T.; Araki, Y.; Ito, O.; Yamada, H.; Fukuzumi, S. Long-Lived Charge-Separated State Generated in a Ferrocene–meso,meso-Linked Porphyrin Trimer–Fullerene Pentad with a High Quantum Yield. Chem. A Eur. J. 2004, 10, 3184–3196. [Google Scholar] [CrossRef]
- Kaur, R.; Possanza, F.; Limosani, F.; Bauroth, S.; Zanoni, R.; Clark, T.; Arrigoni, G.; Tagliatesta, P.; Guldi, D.M. Understanding and Controlling Short- and Long-Range Electron/Charge-Transfer Processes in Electron Donor–Acceptor Conjugates. J. Am. Chem. Soc. 2020, 142, 7898–7911. [Google Scholar] [CrossRef]
- Limosani, F.; Possanza, F.; Ciotta, E.; Pepi, F.; Salvitti, C.; Tagliatesta, P.; Pizzoferrato, R. Synthesis and characterization of two new triads with ferrocene and C60 connected by triple bonds to the beta-positions of meso-tetraphenylporphyrin. J. Porphyr. Phthalocyanines 2017, 21, 364–370. [Google Scholar] [CrossRef]
- Possanza, F.; Limosani, F.; Tagliatesta, P.; Zanoni, R.; Scarselli, M.; Ciotta, E.; Pizzoferrato, R. Functionalization of Carbon Spheres with a Porphyrin–Ferrocene Dyad. ChemPhysChem 2018, 19, 2243–2249. [Google Scholar] [CrossRef] [PubMed]
- Limosani, F.; Tessore, F.; Di Carlo, G.; Forni, A.; Tagliatesta, P. Nonlinear Optical Properties of Porphyrin, Fullerene and Ferrocene Hybrid Materials. Materials 2021, 14, 4404. [Google Scholar] [CrossRef]
- Limosani, F.; Kaur, R.; Cataldo, A.; Bellucci, S.; Micciulla, F.; Zanoni, R.; Lembo, A.; Wang, B.; Pizzoferrato, R.; Guldi, D.M.; et al. Designing Cascades of Electron Transfer Processes in Multicomponent Graphene Conjugates. Angew. Chemie Int. Ed. 2020, 59, 23706–23715. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, G.; Orbelli Biroli, A.; Pizzotti, M.; Tessore, F.; Trifiletti, V.; Ruffo, R.; Abbotto, A.; Amat, A.; De Angelis, F.; Mussini, P.R. Tetraaryl ZnII Porphyrinates Substituted at β-Pyrrolic Positions as Sensitizers in Dye-Sensitized Solar Cells: A Comparison with meso-Disubstituted Push–Pull ZnII Porphyrinates. Chem. A Eur. J. 2013, 19, 10723–10740. [Google Scholar] [CrossRef]
- Lembo, A.; Tagliatesta, P.; Guldi, D.M.; Wielopolski, M. Porphyrin-b-oligo-ethynylenephenylene-è60]fullerene triads: Synthesis, electrochemical and photophysical characterization of the new porphyrin-oligo-PPE-[60]fullerene systems. J. Phys. Chem. A 2009, 113, 1779–1793. [Google Scholar] [CrossRef] [PubMed]
- Oudar, J.L.; Chemla, D.S. Hyperpolarizabilities of the nitroanilines and their relations to the excited state dipole moment. J. Chem. Phys. 1977, 66, 2664–2668. [Google Scholar] [CrossRef]
- Oudar, J.L. Optical nonlinearities of conjugated molecules. Stilbene derivatives and highly polar aromatic compounds. J. Chem. Phys. 1977, 67, 446–457. [Google Scholar] [CrossRef]
- Willetts, A.; Rice, J.E.; Burland, D.M.; Shelton, D.P. Problems in the comparison of theoretical and experimental hyperpolarizabilities. J. Chem. Phys. 1992, 97, 7590–7599. [Google Scholar] [CrossRef]
- Ernzerhof, M.; Scuseria, G.E. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. J. Chem. Phys. 1999, 110, 5029–5036. [Google Scholar] [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Scalmani, G.; Frisch, M.J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110–114124. [Google Scholar] [CrossRef] [PubMed]
- de Wergifosse, M.; Champagne, B. Electron correlation effects on the first hyperpolarizability of push–pull π-conjugated systems. J. Chem. Phys. 2011, 134, 74113–74125. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, H.A.; Dudis, D.S. Quantum Mechanical Methods for Predicting Nonlinear Optical Properties. Rev. Comput. Chem. 2007, 12, 241–279. [Google Scholar] [CrossRef]
- Pielak, K.; Tonnelé, C.; Sanguinet, L.; Cariati, E.; Righetto, S.; Muccioli, L.; Castet, F.; Champagne, B. Dynamical Behavior and Second Harmonic Generation Responses in Acido-Triggered Molecular Switches. J. Phys. Chem. C 2018, 122, 26160–26168. [Google Scholar] [CrossRef]
- Krishna, M.B.M.; Kumar, V.P.; Venkatramaiah, N.; Venkatesan, R.; Rao, D.N. Nonlinear optical properties of covalently linked graphene-metal porphyrin composite materials. Appl. Phys. Lett. 2011, 98, 81106. [Google Scholar] [CrossRef]
- Liu, Z.-B.; Xu, Y.-F.; Zhang, X.-Y.; Zhang, X.-L.; Chen, Y.-S.; Tian, J.-G. Porphyrin and Fullerene Covalently Functionalized Graphene Hybrid Materials with Large Nonlinear Optical Properties. J. Phys. Chem. B 2009, 113, 9681–9686. [Google Scholar] [CrossRef]
- Xiao, X.; Nagahara, L.A.; Rawlett, A.M.; Tao, N. Electrochemical Gate-Controlled Conductance of Single Oligo(phenylene ethynylene)s. J. Am. Chem. Soc. 2005, 127, 9235–9240. [Google Scholar] [CrossRef]
- Lewis, P.A.; Inman, C.E.; Maya, F.; Tour, J.M.; Hutchison, J.E.; Weiss, P.S. Molecular Engineering of the Polarity and Interactions of Molecular Electronic Switches. J. Am. Chem. Soc. 2005, 127, 17421–17426. [Google Scholar] [CrossRef]
- Yu, C.J.; Chong, Y.; Kayyem, J.F.; Gozin, M. Soluble Ferrocene Conjugates for Incorporation into Self-Assembled Monolayers. J. Org. Chem. 1999, 64, 2070–2079. [Google Scholar] [CrossRef]
- Stewart, M.P.; Maya, F.; Kosynkin, D.V.; Dirk, S.M.; Stapleton, J.J.; McGuiness, C.L.; Allara, D.L.; Tour, J.M. Direct Covalent Grafting of Conjugated Molecules onto Si, GaAs, and Pd Surfaces from Aryldiazonium Salts. J. Am. Chem. Soc. 2004, 126, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, G.; Orbelli Biroli, A.; Tessore, F.; Rizzato, S.; Forni, A.; Magnano, G.; Pizzotti, M. Light-Induced Regiospecific Bromination of meso-Tetra(3,5-di-tert-butylphenyl)Porphyrin on 2,12 β-Pyrrolic Positions. J. Org. Chem. 2015, 80, 4973–4980. [Google Scholar] [CrossRef] [PubMed]
- Maggini, M.; Scorrano, G.; Prato, M. Addition of azomethine ylides to C60: Synthesis, characterization, and functionalization of fullerene pyrrolidines. J. Am. Chem. Soc. 1993, 115, 9798–9799. [Google Scholar] [CrossRef]
- Gouterman, M. Spectra of porphyrins. J. Mol. Spectrosc. 1961, 6, 138–163. [Google Scholar] [CrossRef]
- Albert, I.D.L.; Marks, T.J.; Ratner, M.A. Large Molecular Hyperpolarizabilities in “Push-Pull” Porphyrins. Molecular Planarity and Auxiliary Donor—Acceptor Effects. Chem. Mater. 1998, 10, 753–762. [Google Scholar] [CrossRef]
- Planells, M.; Pizzotti, M.; Nichol, G.S.; Tessore, F.; Robertson, N. Effect of torsional twist on 2nd order non-linear optical activity of anthracene and pyrene tricyanofuran derivatives. Phys. Chem. Chem. Phys. 2014, 16, 23404–23411. [Google Scholar] [CrossRef] [PubMed]
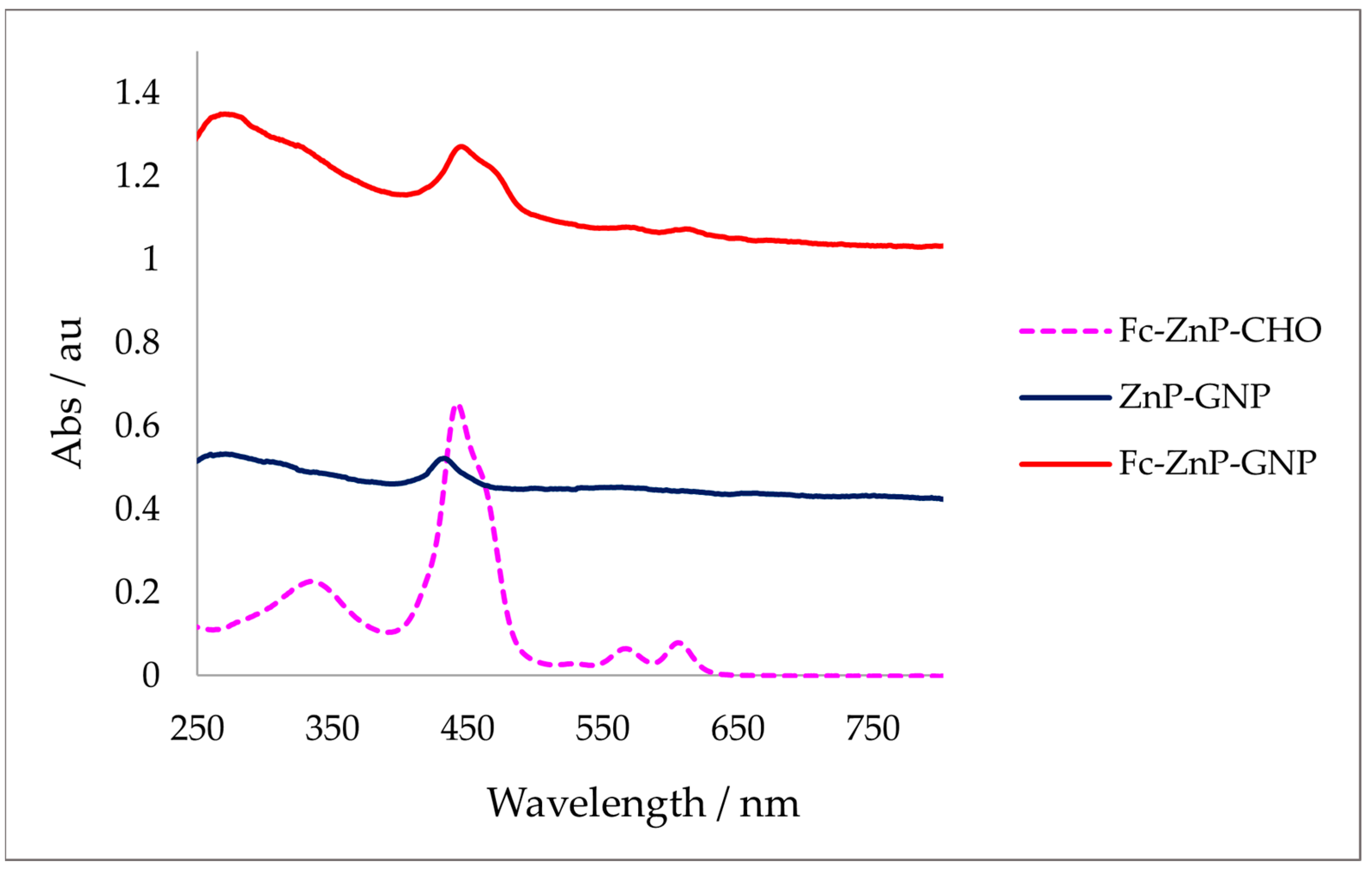
| Compound | Soret Band λmax (nm) | Qα Band λmax (nm) | Qβ Band λmax (nm) |
|---|---|---|---|
| ZnP-GNP | 433 | nd | nd |
| Fc-ZnP-GNP | 445 | 567 | 611 |
| Fc-ZnP-CHO | 442 | 567 | 605 |
| 6(Zn)-C60 1 | 434 | 560 | 598 |
| 10b(Zn)-C60 | 438 | 565 | 603 |
| ZnP 1 | 420 | 548 | 589 |
| Compound | μ (D) | γEFISH (×10−36 esu) | µβ1907 (×10−48 esu) (β1907 × 10−30 esu) | β‖ (×10−30 esu) | μβ‖/5 kT (×10−36 esu) | γ‖ (×10−36 esu) | Dipolar vs. Cubic Contribution % 3 |
|---|---|---|---|---|---|---|---|
| ZnP-GNP | 1.23 | −3160 | −650 (−528) 2 | 20 | 120 | −1890 | 6.3 |
| Fc-ZnP-GNP | 1.17 | −8800 | −1880 (−1607) 2 | 9 | 75 | −4388 | 1.7 |
| Fc-ZnP-CHO | 5.31 | −4130 | −850 (−160) 2 | 94 | 2920 | −5484 | 53 |
| 6(Zn)-C60 1 | 4.77 | −3470 | −720 (−151) 2 | 30 | 696 | −1543 | 45 |
| 10b(Zn)-C60 1 | 4.14 | −6410 | −1330 (−321) 2 | 42 | 845 | −3225 | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limosani, F.; Tessore, F.; Forni, A.; Lembo, A.; Di Carlo, G.; Albanese, C.; Bellucci, S.; Tagliatesta, P. Nonlinear Optical Properties of Zn(II) Porphyrin, Graphene Nanoplates, and Ferrocene Hybrid Materials. Materials 2023, 16, 5427. https://doi.org/10.3390/ma16155427
Limosani F, Tessore F, Forni A, Lembo A, Di Carlo G, Albanese C, Bellucci S, Tagliatesta P. Nonlinear Optical Properties of Zn(II) Porphyrin, Graphene Nanoplates, and Ferrocene Hybrid Materials. Materials. 2023; 16(15):5427. https://doi.org/10.3390/ma16155427
Chicago/Turabian StyleLimosani, Francesca, Francesca Tessore, Alessandra Forni, Angelo Lembo, Gabriele Di Carlo, Cecilia Albanese, Stefano Bellucci, and Pietro Tagliatesta. 2023. "Nonlinear Optical Properties of Zn(II) Porphyrin, Graphene Nanoplates, and Ferrocene Hybrid Materials" Materials 16, no. 15: 5427. https://doi.org/10.3390/ma16155427
APA StyleLimosani, F., Tessore, F., Forni, A., Lembo, A., Di Carlo, G., Albanese, C., Bellucci, S., & Tagliatesta, P. (2023). Nonlinear Optical Properties of Zn(II) Porphyrin, Graphene Nanoplates, and Ferrocene Hybrid Materials. Materials, 16(15), 5427. https://doi.org/10.3390/ma16155427












