Fabrication of Nitrogen-Doped Carbon@Magnesium Silicate Composite by One-Step Hydrothermal Method and Its High-Efficiency Adsorption of As(V) and Tetracycline
Abstract
1. Introduction
2. Experimental
2.1. Materials and Chemicals
2.2. Sample Synthesis
2.3. Materials Characterization
2.4. Adsorption Capacity and Removal Efficiency Measurement
3. Results and Discussion
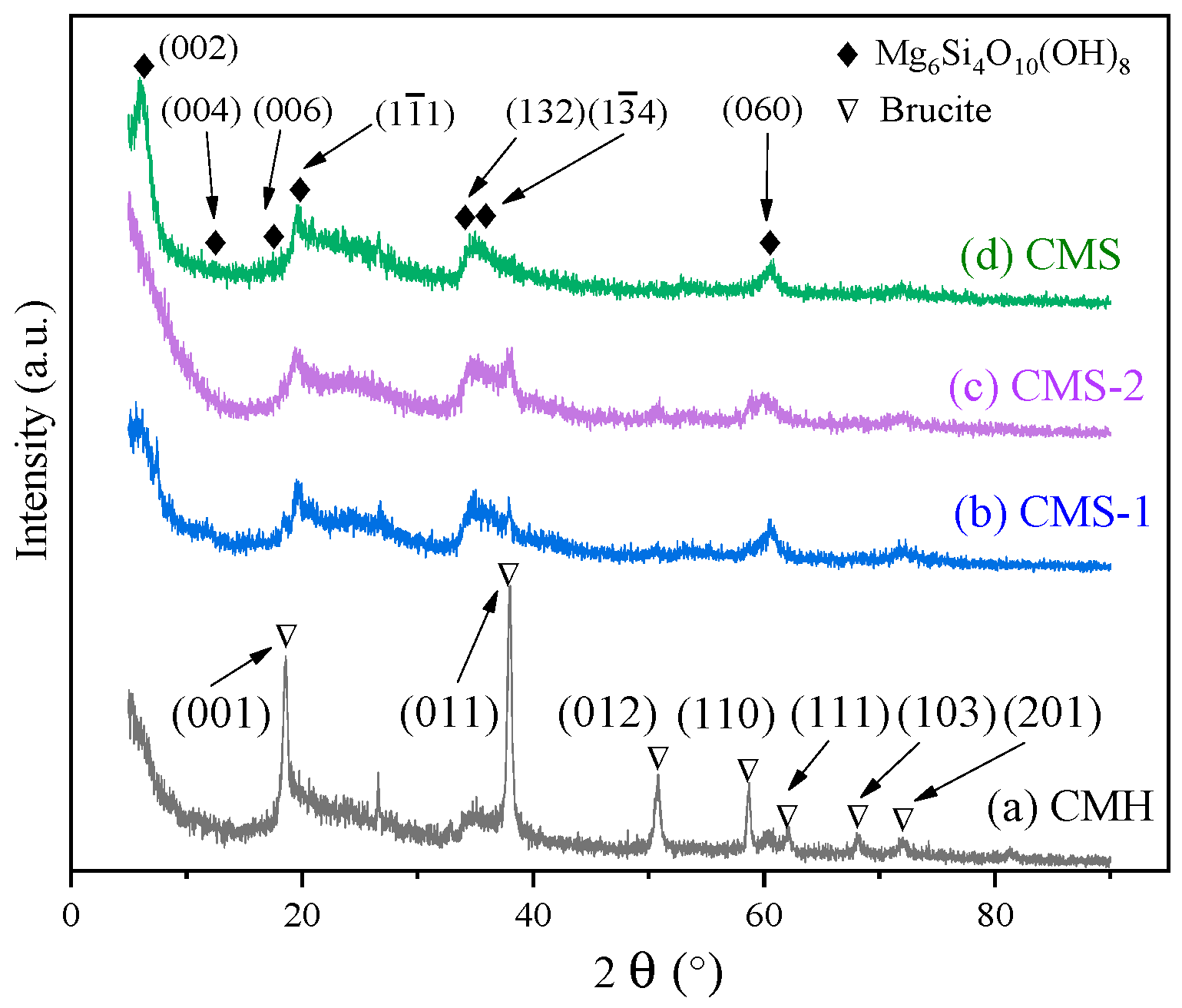
3.1. Phase Composition of the Samples
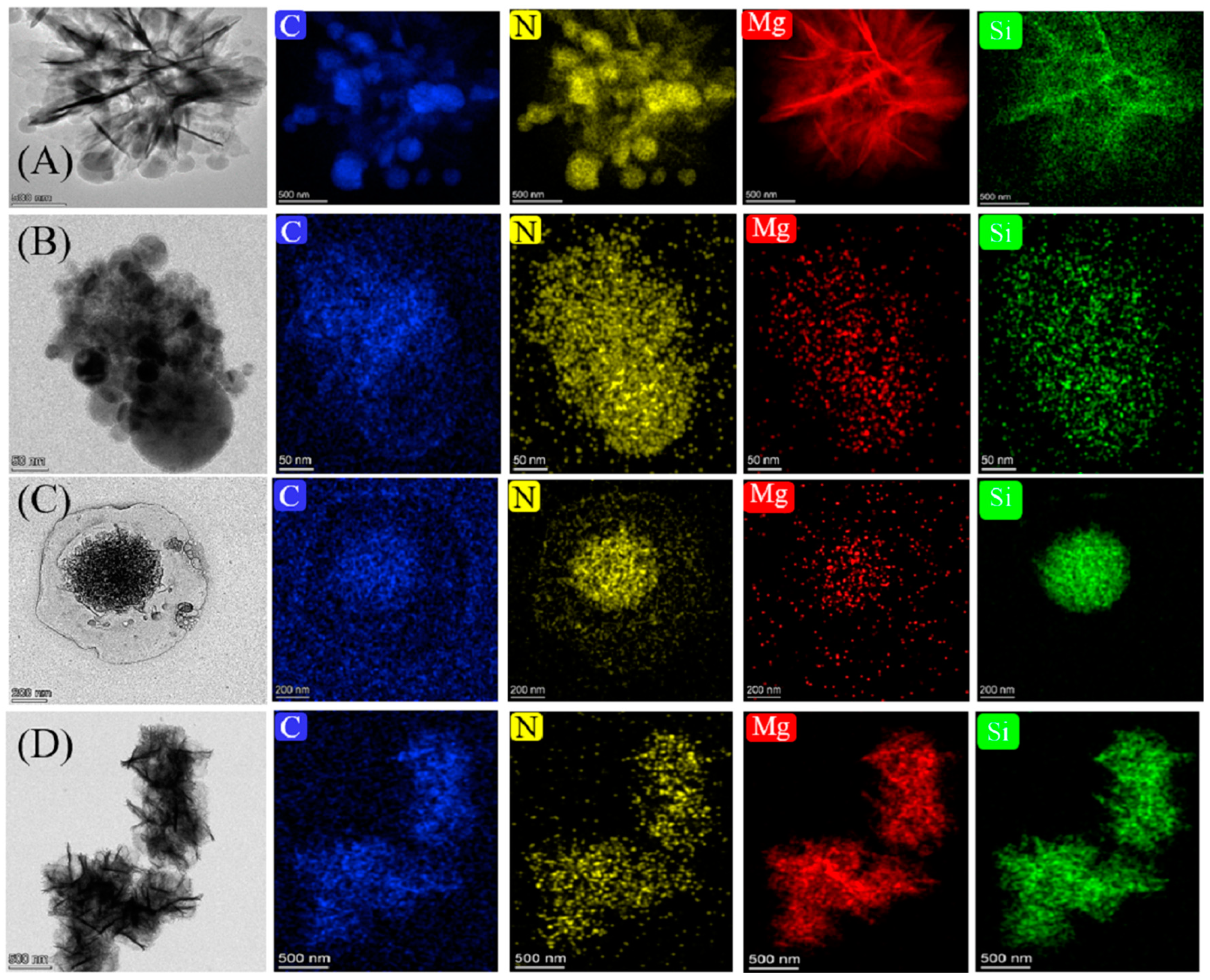
3.2. Surface Morphologies and Pore Structures
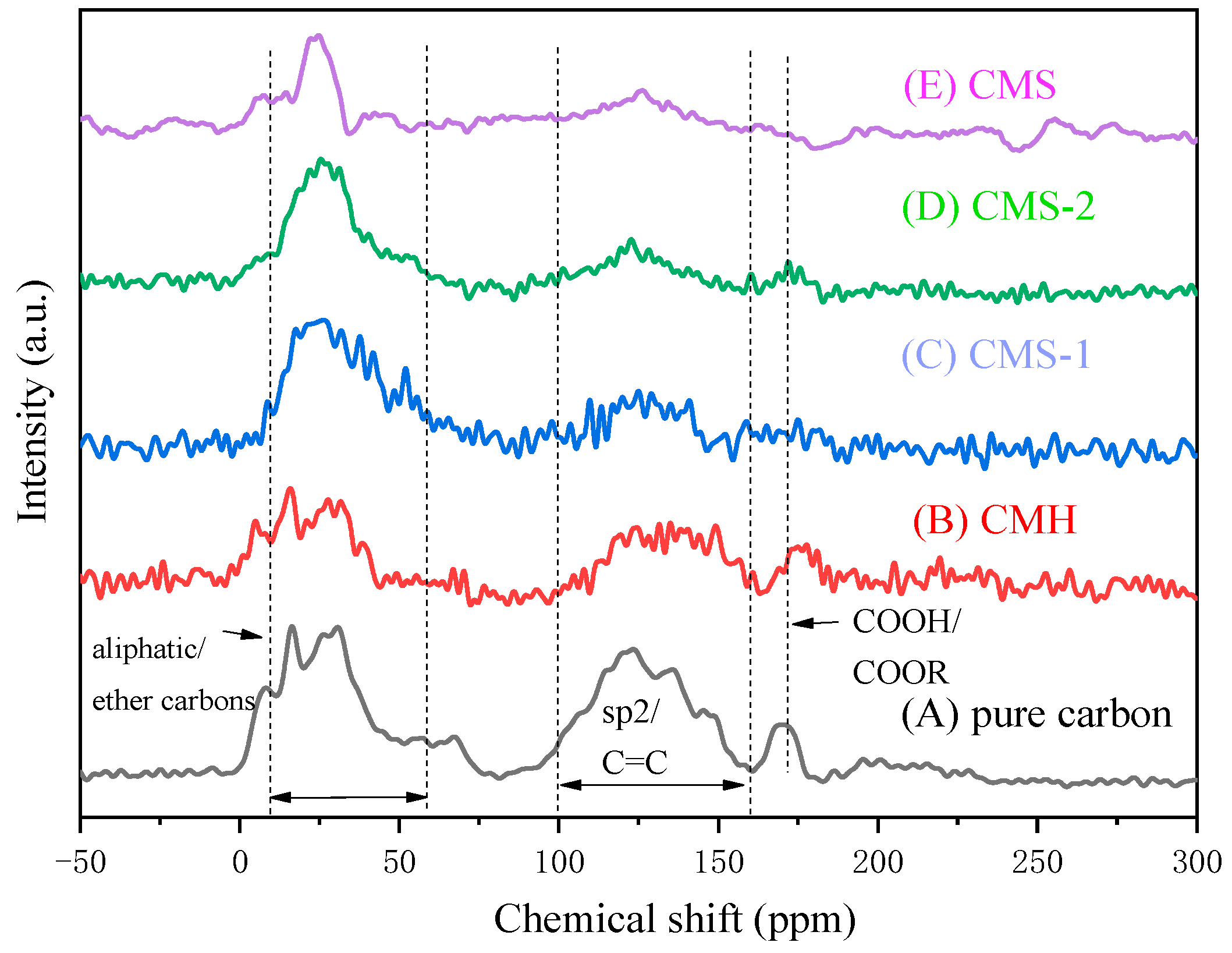
3.3. Morphology Formation Mechanism of Carbon Species
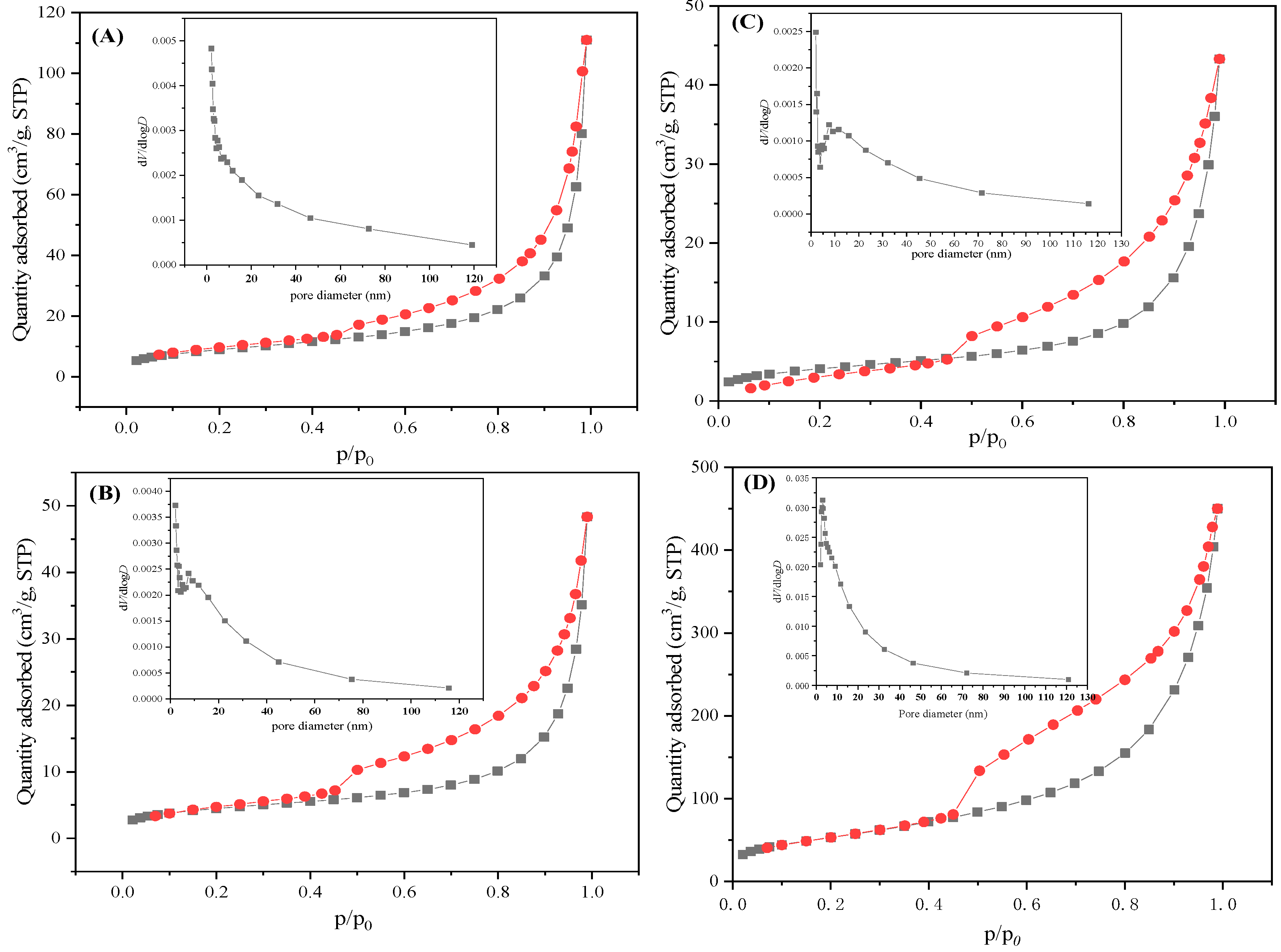
3.4. Surface Areas and Pore-Size Distributions of the Samples
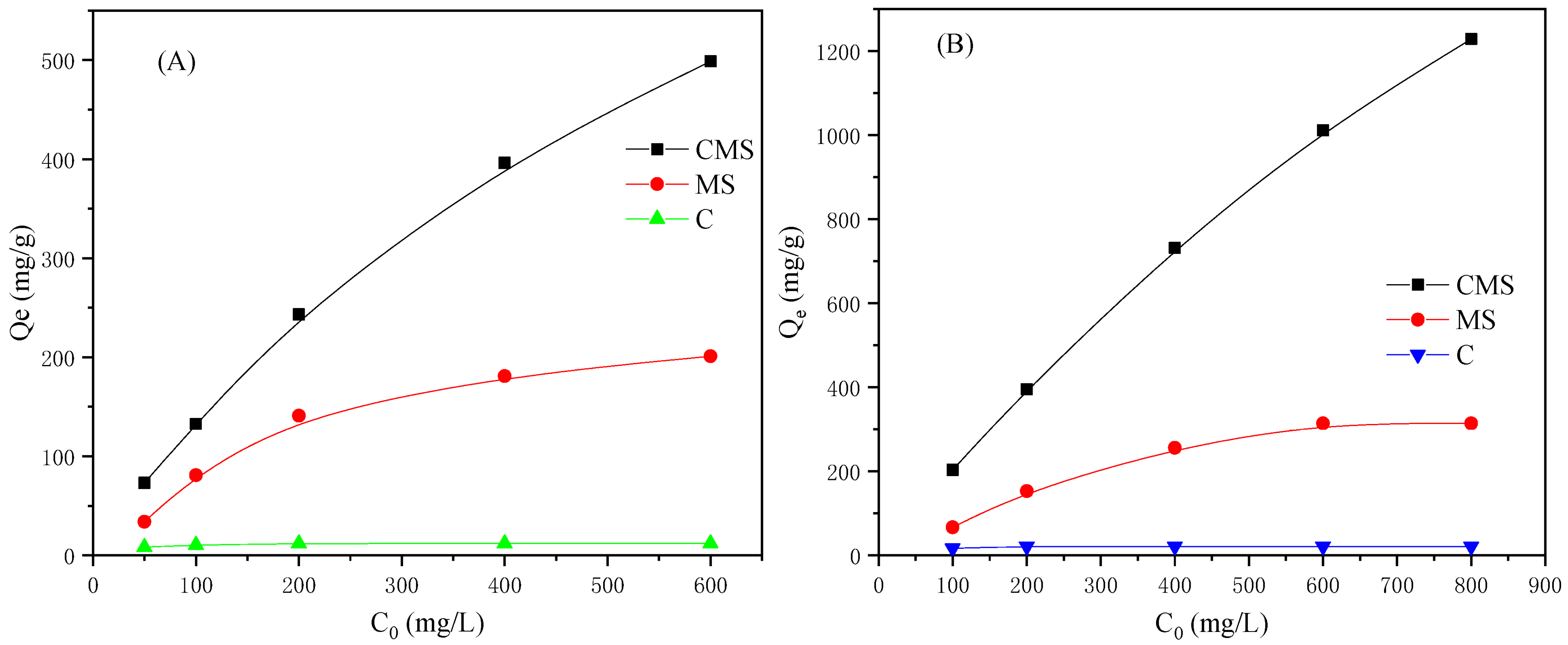
3.5. Adsorption Capacity of CMS towards As(V) and TC
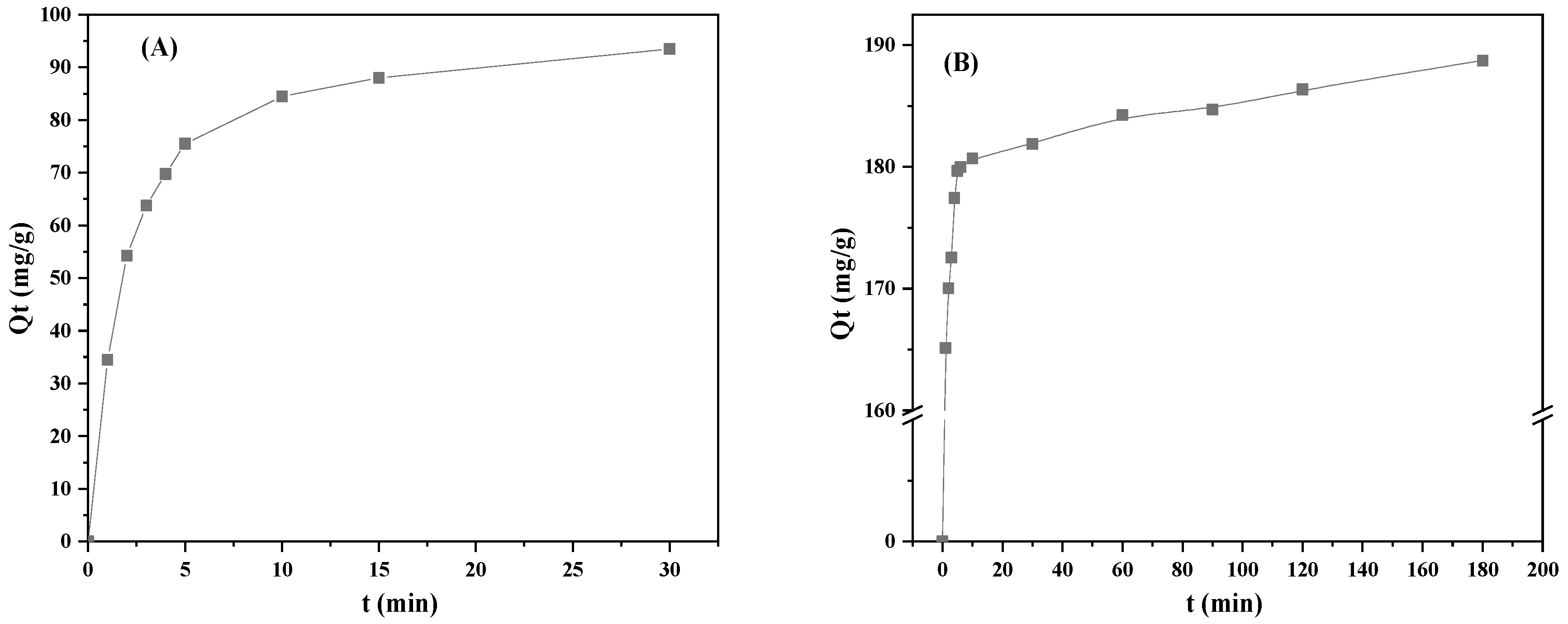
3.6. Adsorption Isotherms and Kinetics
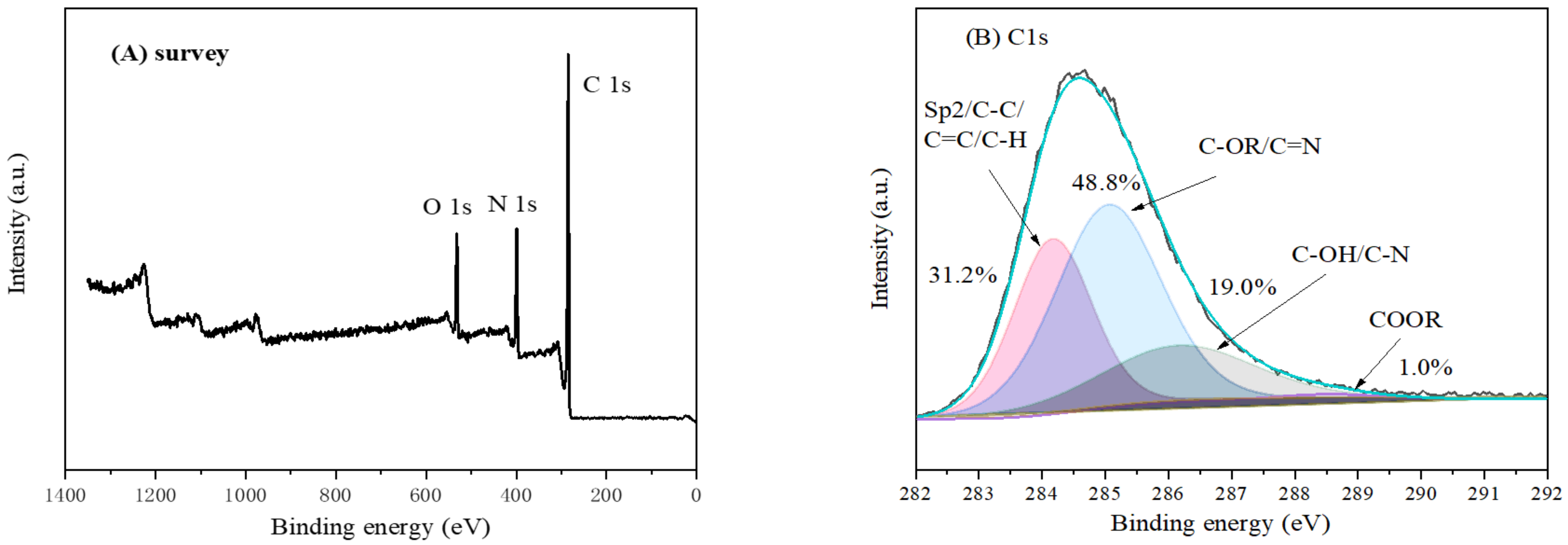
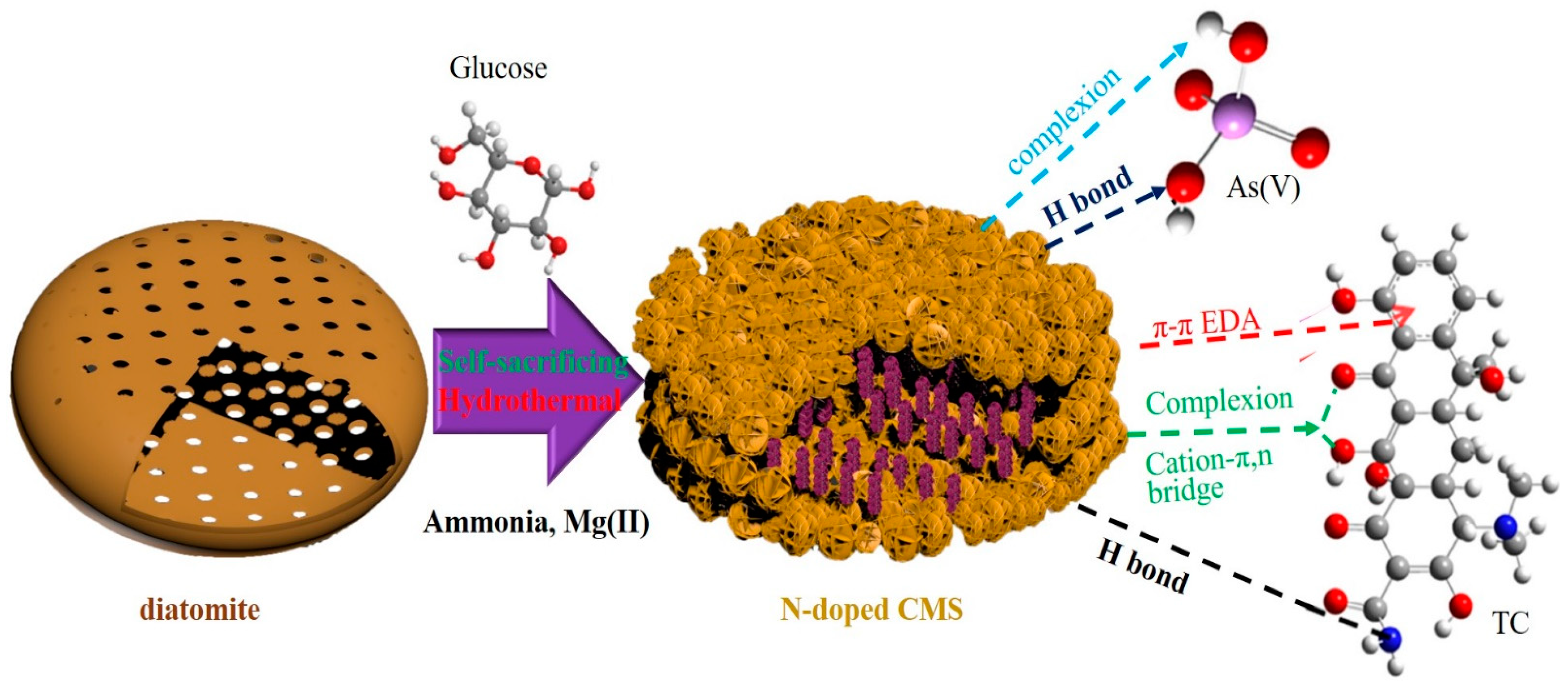
3.7. Adsorption Mechanism of CMS towards As(V) and TC
3.8. Synergism in Construction and Adsorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Zainab, S.M.; Junaid, M.; Xu, N.; Malik, R.N. Antibiotics and antibiotic resistant genes (ARGs) in groundwater: A global review on dissemination, sources, interactions, environmental and human health risks. Water Res. 2020, 187, 116455. [Google Scholar] [CrossRef] [PubMed]
- Anh, H.Q.; Le, T.P.Q.; Da Le, N.; Lu, X.X.; Duong, T.T.; Garnier, J.; Rochelle-Newall, E.; Zhang, S.; Oh, N.H.; Oeurng, C.; et al. Antibiotics in surface water of east and southeast Asian countries: A focused review on contamination status, pollution sources, potential risks, and future perspectives. Sci. Total Environ. 2021, 764, 142865. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Safan, A.; Duan, Y.; Kaushik, V.; Batchelor, B.; Abdel-Wahab, A. Removal of arsenite by reductive precipitation in dithionite solution activated by UV light. J. Environ. Sci. 2018, 74, 168–176. [Google Scholar] [CrossRef]
- Zhu, M.; Kurniawan, T.A.; Avtar, R.; Othman, M.H.D.; Ouyang, T.; Huang, Y.; Zhang, X.; Setiadi, T.; Iswanto, I. Applicability of TiO2(B) nanosheets@hydrochar composites for adsorption of tetracycline (TC) from contaminated water. J. Hazard. Mater. 2020, 405, 123999. [Google Scholar]
- Yu, X.; Lin, X.; Li, W.; Feng, W. Effective Removal of Tetracycline by Using Biochar Supported Fe3O4 as a UV-Fenton Catalyst. Chem. Res. Chin. Univ. 2019, 35, 79–84. [Google Scholar] [CrossRef]
- Ohore, O.E.; Zhang, S.; Guo, S.; Manirakiza, B.; Addo, F.G.; Zhang, W. The fate of tetracycline in vegetated mesocosmic wetlands and its impact on the water quality and epiphytic microbes. J. Hazard. Mater. 2021, 417, 126148. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, Y.; Wang, P.; Wang, Z.; Zuo, C.; Chen, W.; Ao, T. Facile fabrication of N-doped hierarchical porous carbons derived from soft-templated ZIF-8 for enhanced adsorptive removal of tetracycline hydrochloride from water. J. Hazard. Mater. 2022, 423, 127103. [Google Scholar] [CrossRef]
- Agrafioti, E.; Kalderis, D.; Diamadopoulos, E. Arsenic and chromium removal from water using biochars derived from rice husk, organic solid wastes and sewage sludge. J. Environ. Manag. 2014, 133, 309–314. [Google Scholar] [CrossRef]
- Deng, R.; Huang, D.; Zeng, G.; Wan, J.; Xue, W.; Wen, X.; Liu, X.; Chen, S.; Li, J.; Liu, C.; et al. Decontamination of lead and tetracycline from aqueous solution by a promising carbonaceous nanocomposite: Interaction and mechanisms insight. Bioresour. Technol. 2019, 283, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Karki, B.; Pandey, P.; Rajbhandari, R.; Joshi, S.; Koirala, A.R.; Sharma, R.K.; Pant, H.R. Facile Synthesis of Magnetic Activated Carbon Composite for Arsenic Adsorption. J. Inst. Eng. 2019, 15, 71–78. [Google Scholar] [CrossRef]
- Pi, Z.; Hou, K.; Yao, F.; He, L.; Chen, S.; Tao, Z.; Zhou, P.; Wang, D.; Li, X.; Yang, Q. In-situ regeneration of tetracycline-saturated hierarchical porous carbon by peroxydisulfate oxidation process: Performance, mechanism and application. Chem. Eng. J. 2022, 427, 131749. [Google Scholar] [CrossRef]
- Prabhu, S.M.; Chuaicham, C.; Sasaki, K. A Mechanistic Approach for the Synthesis of Carboxylate-Rich Carbonaceous Biomass-Doped Lanthanum-Oxalate Nanocomplex for Arsenate Adsorption. ACS Sustain. Chem. Eng. 2018, 6, 6052–6063. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, X.; Hou, Y.N.; Pan, X.; Zhao, Z.; Qiu, J. Nitrogen-doped mesoporous carbon nanosheets derived from metalorganic frameworks in a molten salt medium for efficient desulfurization. Carbon 2017, 117, 376–382. [Google Scholar] [CrossRef]
- Kan, X.; Chen, X.; Chen, W.; Mi, J.; Zhang, J.Y.; Liu, F.; Zheng, A.; Huang, K.; Shen, L.; Au, C.; et al. Nitrogen-Decorated, Ordered Mesoporous Carbon Spheres as High-Efficient Catalysts for Selective Capture and Oxidation of H2S. ACS Sustain. Chem. Eng. 2019, 7, 7609–7618. [Google Scholar] [CrossRef]
- Lan, H.; Li, J.; Sun, M.; An, X. Efficient degradation of dimethylarsinate and adsorption of arsenic simultaneously over FeCx/N-doped carbon fiber composite in an electro-Fenton process. Water Res. 2016, 100, 57–64. [Google Scholar] [CrossRef]
- Duan, J.; Fan, H.; Shen, W. Nitrogen-Doped Carbon Materials Prepared from Polyurethane Foams. ChemistrySelect 2016, 1, 3204–3207. [Google Scholar] [CrossRef]
- Duan, X.; Sun, H.; Wang, Y.; Jian, K.; Wang, S. N-doping-Induced nonradical reaction on single-walled carbon nanotubes for catalytic phenol oxidation. ACS Catal. 2015, 5, 553–559. [Google Scholar] [CrossRef]
- Franciele, D.S.B.; Cristian, M.L.; Ivana, Z.D.S.; Guilherme, L.D.; Cristiano, R.B.R. A DFT theoretical and experimental study about tetracycline adsorption onto magnetic graphene oxide. J. Mol. Liq. 2022, 353, 118837. [Google Scholar]
- Shen, Y.; Zhu, K.E.; He, D.; Huang, J.; He, H.; Lei, L.; Chen, W. Tetracycline removal via adsorption and metal-free catalysis with 3D macroscopic N-doped porous carbon nanosheets: Non-radical mechanism and degradation pathway. J. Environ. Sci. 2022, 16, 351–366. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Du, Y.; Wu, J.; Teng, W.; He, H. A diatomite-template self-sacrificing route for Mg-chlorite and its adsorption behavior towards Pb(II)/Cd(II). Surf. Interfaces 2022, 29, 101775. [Google Scholar] [CrossRef]
- He, J.; Ni, F.; Cui, A.; Chen, X.; Deng, S.; Shen, F.; Huang, C.; Yang, G.; Song, C.; Zhang, J.; et al. New insight into adsorption and co-adsorption of arsenic and tetracycline using a Y-immobilized graphene oxide-alginate hydrogel: Adsorption behaviours and mechanisms. Sci. Total Environ. 2019, 701, 134363. [Google Scholar] [CrossRef] [PubMed]
- Demir-Cakan, R.; Baccile, N.; Antonietti, M.; Titirici, M.M. Carboxylate-Rich Carbonaceous Materials via One-Step Hydrothermal Carbonization of Glucose in the Presence of Acrylic Acid. Chem. Mater. 2009, 21, 484–490. [Google Scholar] [CrossRef]
- Liang, H.; Sun, R.; Song, B.; Sun, Q.; Peng, P.; She, D. Preparation of nitrogen-doped porous carbon material by a hydrothermal-activation two-step method and its high-efficiency adsorption of Cr(VI). J. Hazard. Mater. 2019, 387, 121987. [Google Scholar] [CrossRef] [PubMed]
- Xiong, T.; Yuan, X.; Chen, X.; Wu, Z.; Wang, H.; Leng, L.; Wang, H.; Jiang, L.; Zeng, G. Insight into highly efficient removal of cadmium and methylene blue by eco-friendly magnesium silicate-hydrothermal carbon composite. Appl. Surf. Sci. 2018, 427, 1107–1117. [Google Scholar] [CrossRef]
- Higgins, L.J.; Brown, A.P.; Harrington, J.P.; Ross, A.B.; Kaulich, B.; Mishra, B. Evidence for a core-shell structure of hydrothermal carbon. Carbon 2020, 161, 423–431. [Google Scholar] [CrossRef]
- Chen, C.; Li, X.; Deng, J.; Wang, Z.; Wang, Y. Shape Engineering of Biomass-Derived Nanoparticles from Hollow Spheres to Bowls via Solvent-Induced Buckling. ChemSusChem 2018, 11, 2540–2546. [Google Scholar] [CrossRef]
- Liu, J.; Shi, W.; Ni, B.; Yang, Y.; Li, S.; Zhuang, J.; Wang, X. Incorporation of clusters within inorganic materials through their addition during nucleation steps. Nat. Chem. 2019, 11, 839–845. [Google Scholar] [CrossRef]
- Liang, J.; Wu, M.; Liu, Y. Progress in carbon microspheres prepared from biomass by hydrothermal carbonization. New Chem. Mater. 2011, 39, 1–4. [Google Scholar]
- Zhang, X.; Lin, X.; He, Y.; Chen, Y.; Luo, X.; Shang, R. Study on adsorption of tetracycline by Cu-immobilized alginate adsorbent from water environment. Int. J. Biol. Macromol. 2019, 124, 418–428. [Google Scholar] [CrossRef]
- Yu, L.; Ma, Y.; Ong, C.N.; Xie, J.; Liu, Y. Rapid adsorption removal of arsenate by hydrous cerium oxide-graphene composite. RSC Adv. 2015, 5, 64983–64990. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Vo, D.V.N.; Nguyen, T.T.; Nguyen, T.T.T.; Nguyen, L.T.; Van Tran, T. Optimization of tetracycline adsorption onto zeolitic–imidazolate framework-based carbon using response surface methodology. Surf. Interfaces 2022, 28, 101549. [Google Scholar] [CrossRef]
- Zhang, D.; Niu, H.; Zhang, X.; Meng, Z.; Cai, Y. Strong adsorption of chlorotetracycline on magnetite nanoparticles. J. Hazard. Mater. 2011, 192, 1088–1093. [Google Scholar] [CrossRef]
- Gu, W.; Huang, X.; Tian, Y.; Cao, M.; Zhou, L.; Zhou, Y.; Lu, J.; Lei, J.; Zhou, Y.; Wang, L.; et al. High-efficiency adsorption of tetracycline by cooperation of carbon and iron in a magnetic Fe/porous carbon hybrid with effective Fenton regeneration. Appl. Surf. Sci. 2021, 538, 147813. [Google Scholar] [CrossRef]
- Chen, H.; Liu, W.; Cheng, L.; Meledina, M.; Meledin, A.; Van Deun, R.; Leus, K.; Van Der Voort, P. Amidoxime-functionalized covalent organic framework as simultaneous luminescent sensor and adsorbent for organic arsenic from water. Chem. Eng. J. 2022, 429, 132162. [Google Scholar] [CrossRef]
- Shi, X.; Hong, J.; Wang, C.; Kong, S.; Li, J.; Pan, D.; Lin, J.; Jiang, Q.; Guo, Z. Preparation of Mg,N-co-doped lignin adsorbents for enhanced selectivity and high adsorption capacity of As(V) from wastewater. Particuology 2021, 58, 206–213. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Wang, M.; Huang, J. Preparation of activated carbons from Iris tectorum employing ferric nitrate as dopant for removal of tetracycline from aqueous solutions. Ecotoxicol. Environ. Saf. 2013, 98, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yu, L.; Shih, K.; Chen, J.P. Yttrium-doped iron oxide magnetic adsorbent for enhancement in arsenic removal and ease in separation after applications. J. Colloid Interface Sci. 2018, 521, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, Q.M.; Li, W.Q. Engineering magnetic N-doped porous carbon with super-high ciprofloxacin adsorption capacity and wide pH adaptability. J. Hazard. Mater. 2020, 388, 122059. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Xu, L.; Jiang, X.; Zhang, H.; Zhu, W. Facile and Green One-Pot Hydrothermal Formation of Hierarchical Porous Magnesium Silicate Microspheres as Excellent Adsorbents for Anionic Organic Dye Removal. Ind. Eng. Chem. Res. 2019, 58, 2945–2957. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, H.; Wang, Q.; Xu, C.; Geng, Z.; She, D.; Du, X. A novel glucose-based highly selective phosphate adsorbent. Sci. Total Environ. 2021, 792, 148452. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wang, B.; Shen, J.; Yan, P.; Kang, J.; Wang, W.; Bi, L.; Zhu, X.; Li, Y.; Wang, S.; et al. Preparation of novel N-doped biochar and its high adsorption capacity for atrazine based on π–π electron donor-acceptor interaction. J. Hazard. Mater. 2022, 432, 128757. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, E.B.; de Oliveira, L.M.; Wilkie, A.C.; Liu, Y.; Ma, L.Q. Arsenic removal from As-hyperaccumulator Pteris vittata biomass: Coupling extraction with precipitation. Chemosphere 2018, 193, 288–294. [Google Scholar] [CrossRef] [PubMed]


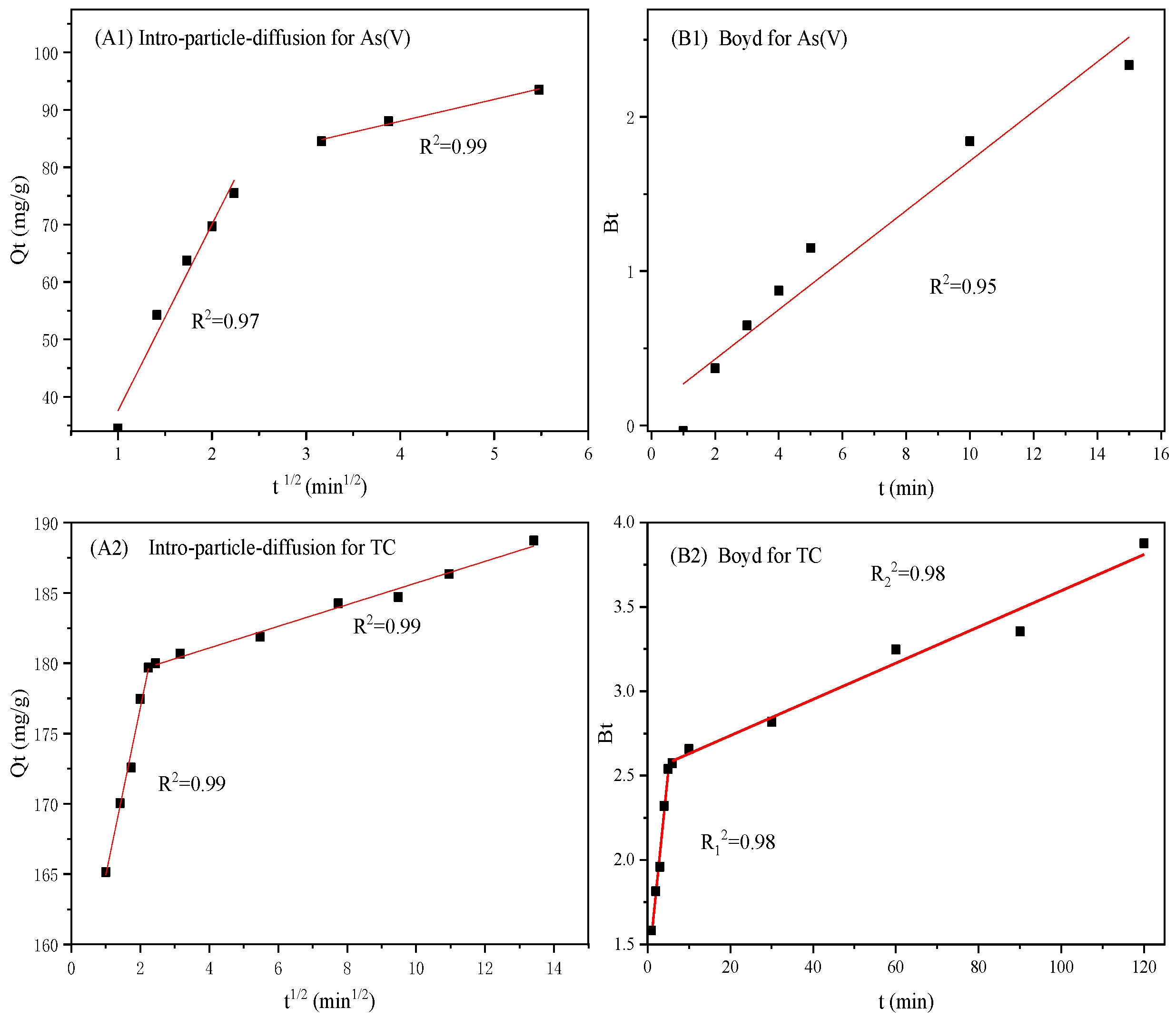


| Item | Glucose (g) | Ammonia (mL) | Ethanol (mL) | Diatomite (g) | MgCl2·6H2O (g) | SBET (m2/g) | Sample | |
|---|---|---|---|---|---|---|---|---|
| No. | ||||||||
| 1# | 1 | 20 | 0 | 1 | 5 | 32 | CMH | |
| 2# | 1 | 40 | 0 | 1 | 5 | 27.7 | CMS-1 | |
| 3# | 1 | 40 | 5 | 1 | 5 | 33.7 | CMS-2 | |
| 4# | 0.5 | 40 | 5 | 1 | 5 | 201 | CMS | |
| 5# | 1 | 20 | 0 | 0 | 0 | / | C2 | |
| 6# | 1 | 20 | 5 | 0 | 0 | / | C1 | |
| 7# | 0 | 40 | 0 | 1 | 5 | 337 | Magnesium silicate [21] | |
| Adsorbent | pH | Qmax(As(V)) (mg/g) | Qmax(TC) (mg/g) | Ref. |
|---|---|---|---|---|
| Magnetic graphene oxide | 5 | - | 149 | [31] |
| Yttrium-doped iron oxide | 7 | 170.4 | - | [38] |
| N-doped carbon | 8 | - | 339 | [32] |
| Y-GO-SA | 5 | 273 | 478 | [22] |
| Mg–N-co-doped lignin | 7 | 687 | - | [36] |
| Fe-doped activated carbon | 4.35 | - | 625 | [33] |
| Boron nitride with N-defects | 7 | - | 1101 | [34] |
| CMS | 7 | 498.75 | 1228.5 | Present work |
| Fe/porous carbon | 7 | - | 1301 | [35] |
| Covalent organic frameworks | 7 | 787 | - | [37] |
| Model | Pseudo-First-Order | Pseudo-Second-Order | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Species | qe,exp (mg/g) | qe,cal (mg/g) | k1 (min−1) | R2 | qe,exp (mg/g) | qe,cal (mg/g) | k2 (min−1) | R2 | |
| As(V) | 93.5 | 50.995 | 0.16055 | 0.95 | 93.5 | 98.619 | 6.023·10−3 | 1.00 | |
| TC | 188.725 | 13.17 | 0.0149 | 0.79 | 188.725 | 187.970 | 0.0108 | 1.00 | |
| Model | Weber–Morris | Boyd | ||||||
|---|---|---|---|---|---|---|---|---|
| Species | K1d (mg·g−1·min−0.5) | R12 | C1 | K2d (mg·g−1·min−0.5) | C2 | R22 | R2 | |
| As(V) | 32.508 | 0.97 | 5.052 | 3.810 | 72.777 | 0.99 | 0.95 | |
| TC | 11.875 | 0.99 | 153.059 | 0.7702 | 178.004 | 0.99 | 0.98/0.98 | |
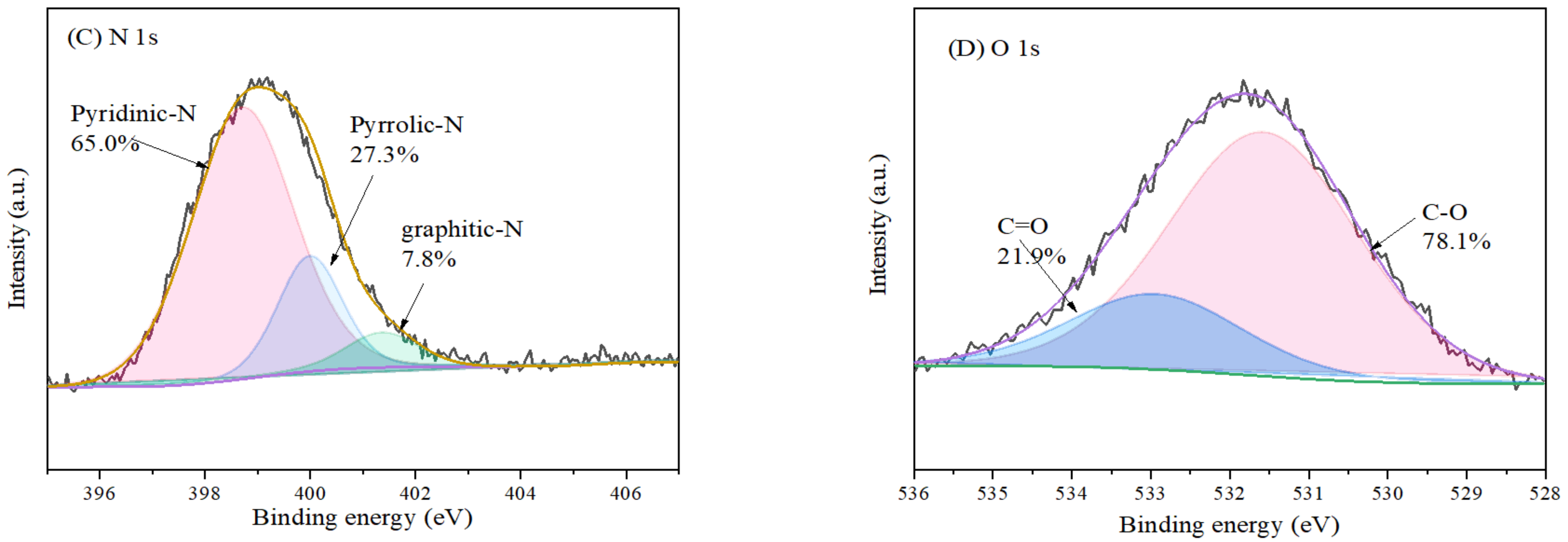
| Bond/Species | Binding Energy (eV) | Concentration (%) | |||
|---|---|---|---|---|---|
| Pure C | CMS | CMS after As(V) Adsorption | CMS after TC Adsorption | ||
| C-C/C=C/CHx | 284.6 | 31.2 | 24.6 | 42.1 | 34.6 |
| C-OR/C=N | 285.7 | 48.8 | 53.2 | 51.3 | 37.6 |
| C-OH/C-N | 287.1 | 19.0 | 11.5 | 3.6 | 8.6 |
| COOR | 289 | 1.0 | 10.8 | 3.1 | 19.2 |
| Pyridinic-N | 399 | 65.0 | 62.9 | 49.5 | 54.3 |
| Pyrrolic-N | 400.3 | 27.3 | 19.5 | 22.8 | 38.0 |
| Graphitic-N | 401.4 | 7.8 | 17.6 | 27.7 | 7.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wang, J.; Li, J.; Du, Y.; Wu, J.; He, H. Fabrication of Nitrogen-Doped Carbon@Magnesium Silicate Composite by One-Step Hydrothermal Method and Its High-Efficiency Adsorption of As(V) and Tetracycline. Materials 2023, 16, 5338. https://doi.org/10.3390/ma16155338
Wang X, Wang J, Li J, Du Y, Wu J, He H. Fabrication of Nitrogen-Doped Carbon@Magnesium Silicate Composite by One-Step Hydrothermal Method and Its High-Efficiency Adsorption of As(V) and Tetracycline. Materials. 2023; 16(15):5338. https://doi.org/10.3390/ma16155338
Chicago/Turabian StyleWang, Xuekai, Jinshu Wang, Jianjun Li, Yucheng Du, Junshu Wu, and Heng He. 2023. "Fabrication of Nitrogen-Doped Carbon@Magnesium Silicate Composite by One-Step Hydrothermal Method and Its High-Efficiency Adsorption of As(V) and Tetracycline" Materials 16, no. 15: 5338. https://doi.org/10.3390/ma16155338
APA StyleWang, X., Wang, J., Li, J., Du, Y., Wu, J., & He, H. (2023). Fabrication of Nitrogen-Doped Carbon@Magnesium Silicate Composite by One-Step Hydrothermal Method and Its High-Efficiency Adsorption of As(V) and Tetracycline. Materials, 16(15), 5338. https://doi.org/10.3390/ma16155338






