Ureido Hyperbranched Polymer Modified Urea-Formaldehyde Resin as High-Performance Particleboard Adhesive
Abstract
1. Introduction
2. Experimental Sections
2.1. Chemicals and Materials
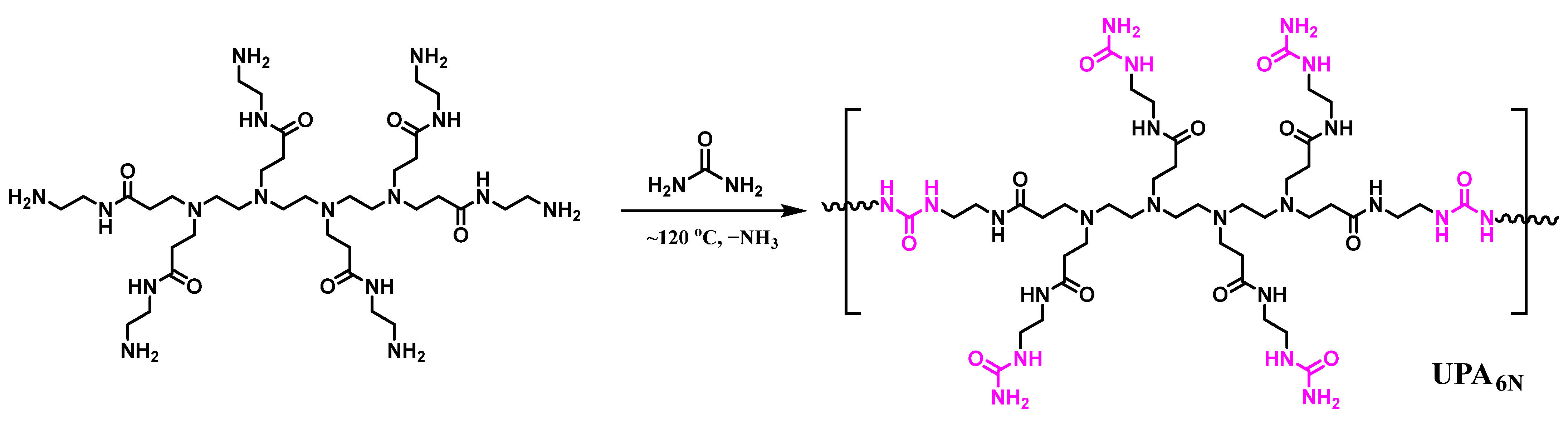
2.2. Synthesis of UPA6N Ureido Polymer
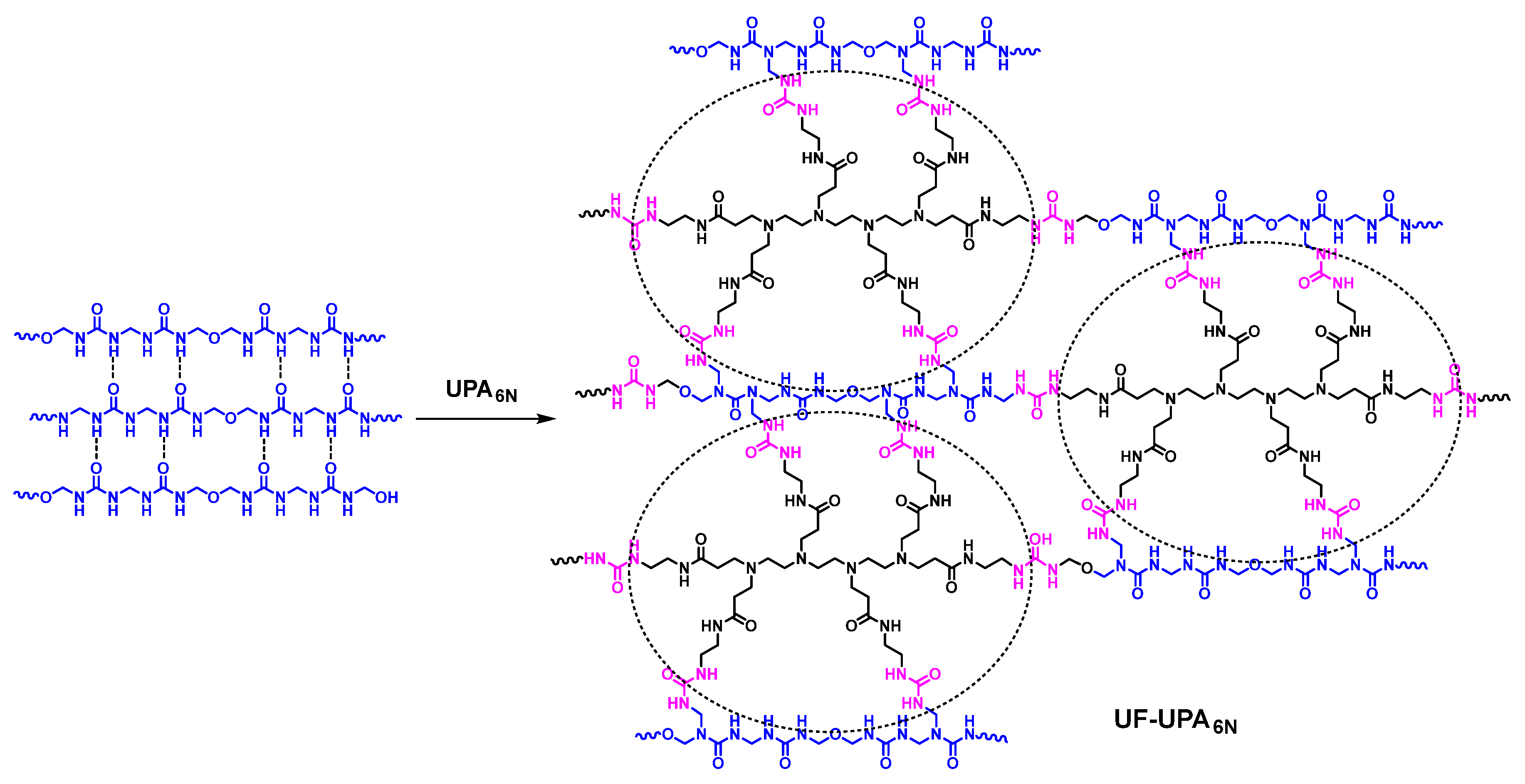
2.3. Preparation of UPA6N Modified UF Resins
2.4. Characterization Methods
2.5. Preparation of Particleboard
2.6. Particleboard Performance Testing
3. Results and Discussion
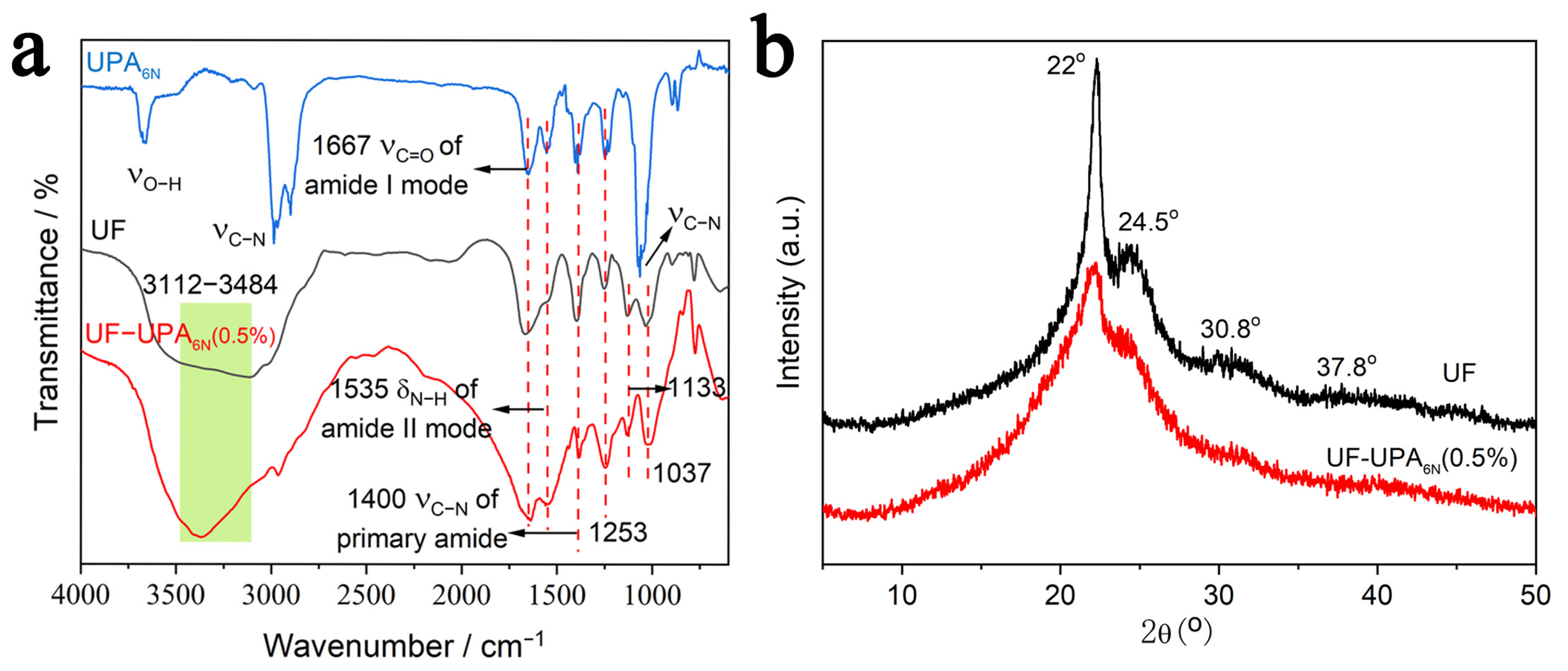
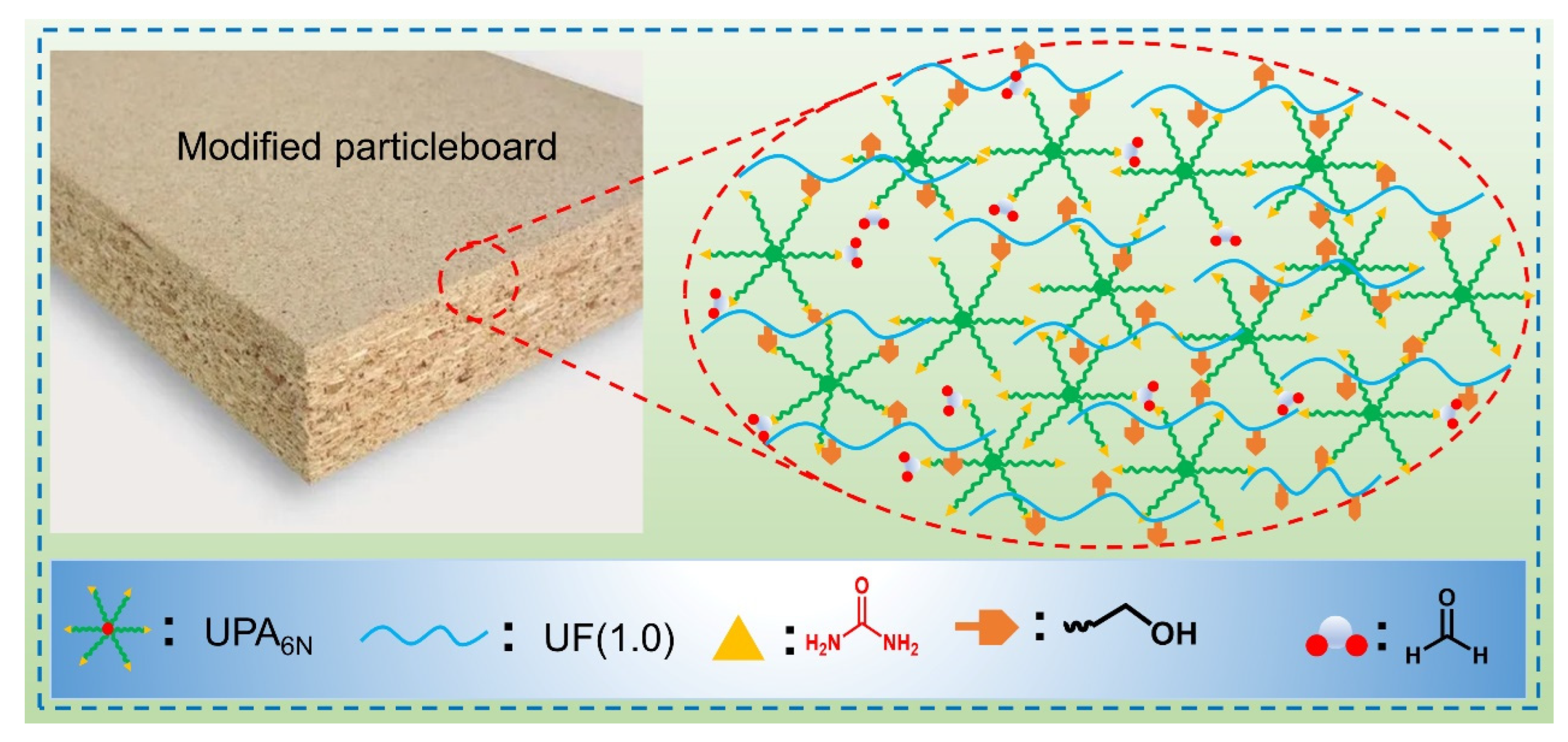
3.1. Structure Analysis of UF Resin and UF-UPA6N Resin
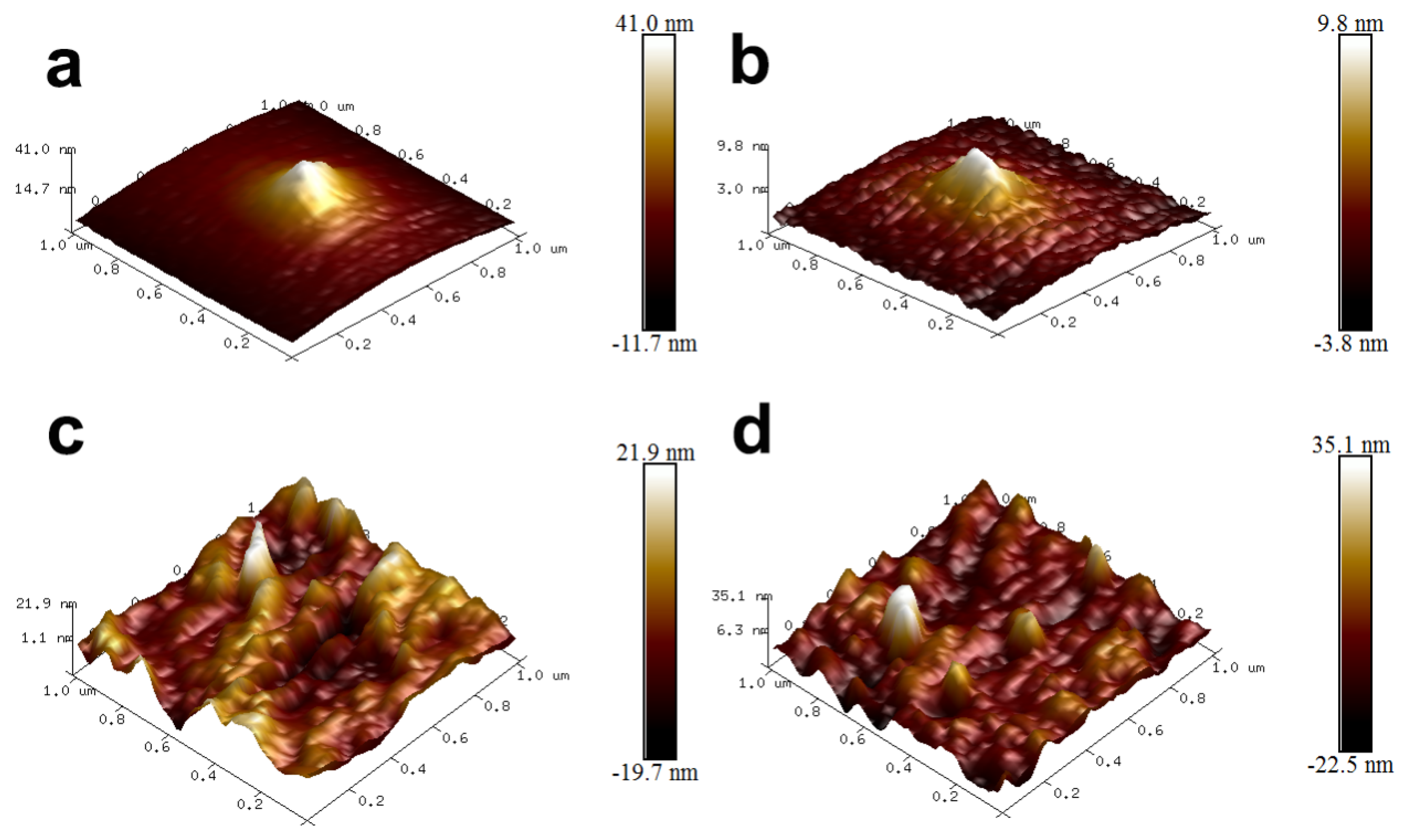
3.2. AFM Images of UF Resin and UPA6N-UF Resin
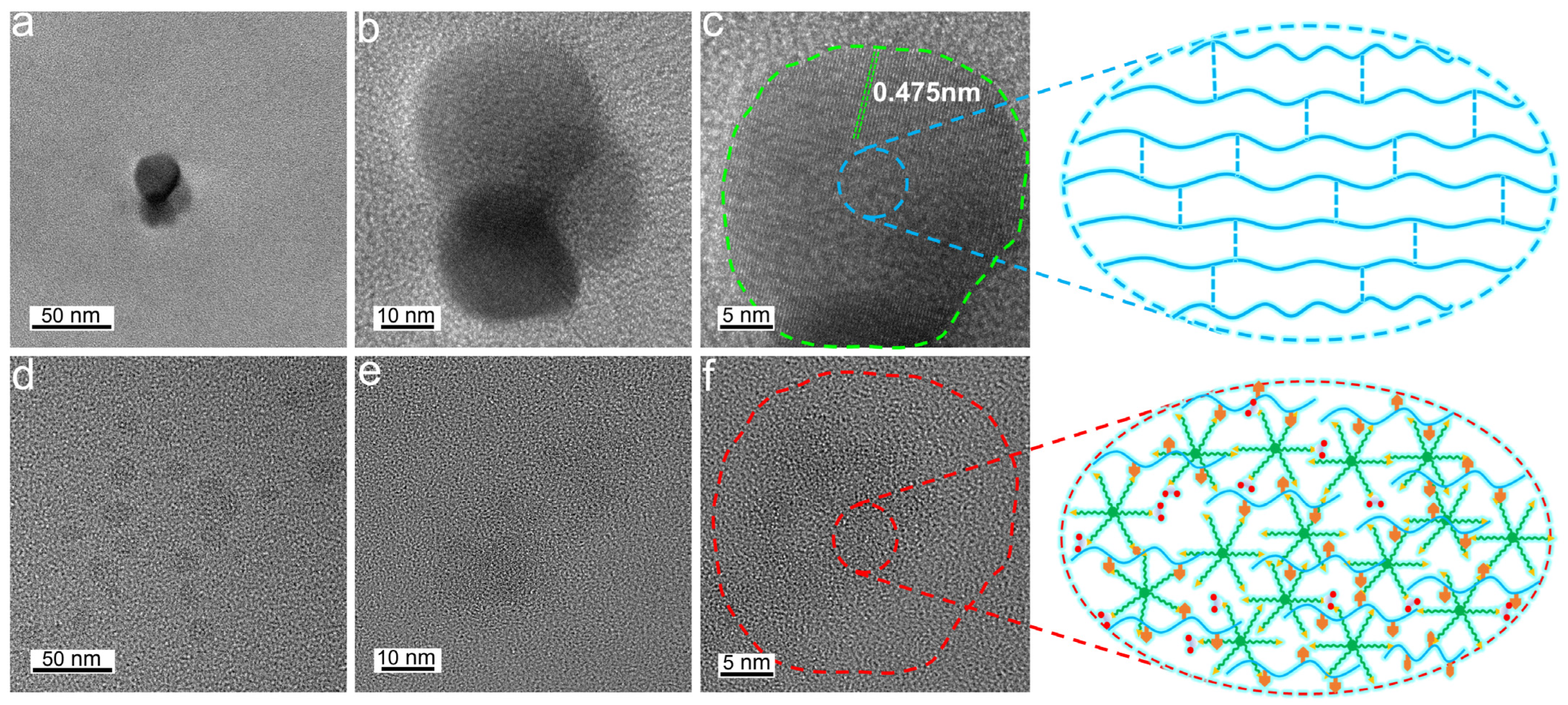
3.3. TEM Images of UF Resin and UF-UPA6N Resin
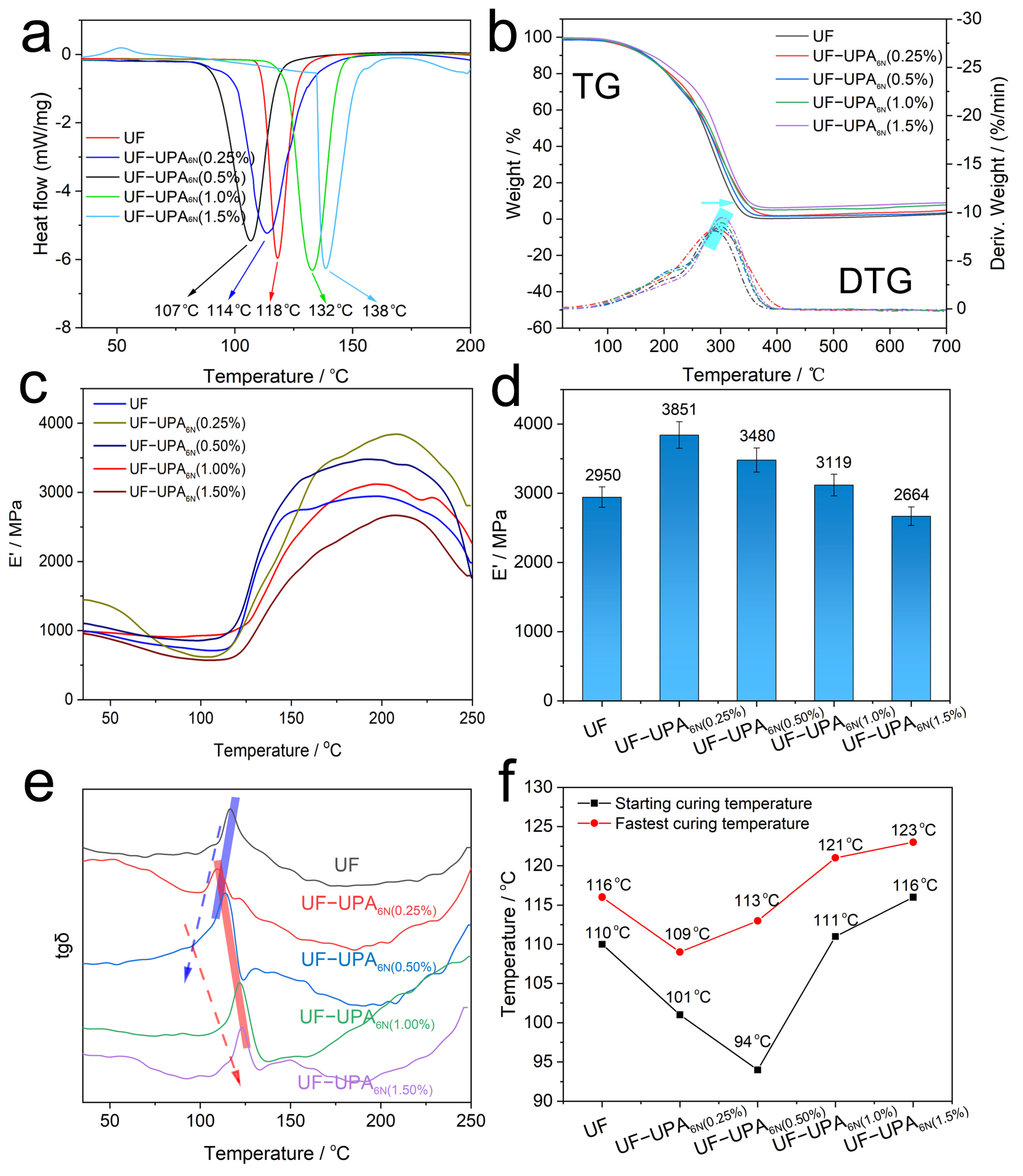
3.4. Thermodynamic Analysis of UF Resin and UF-UPA6N Resin
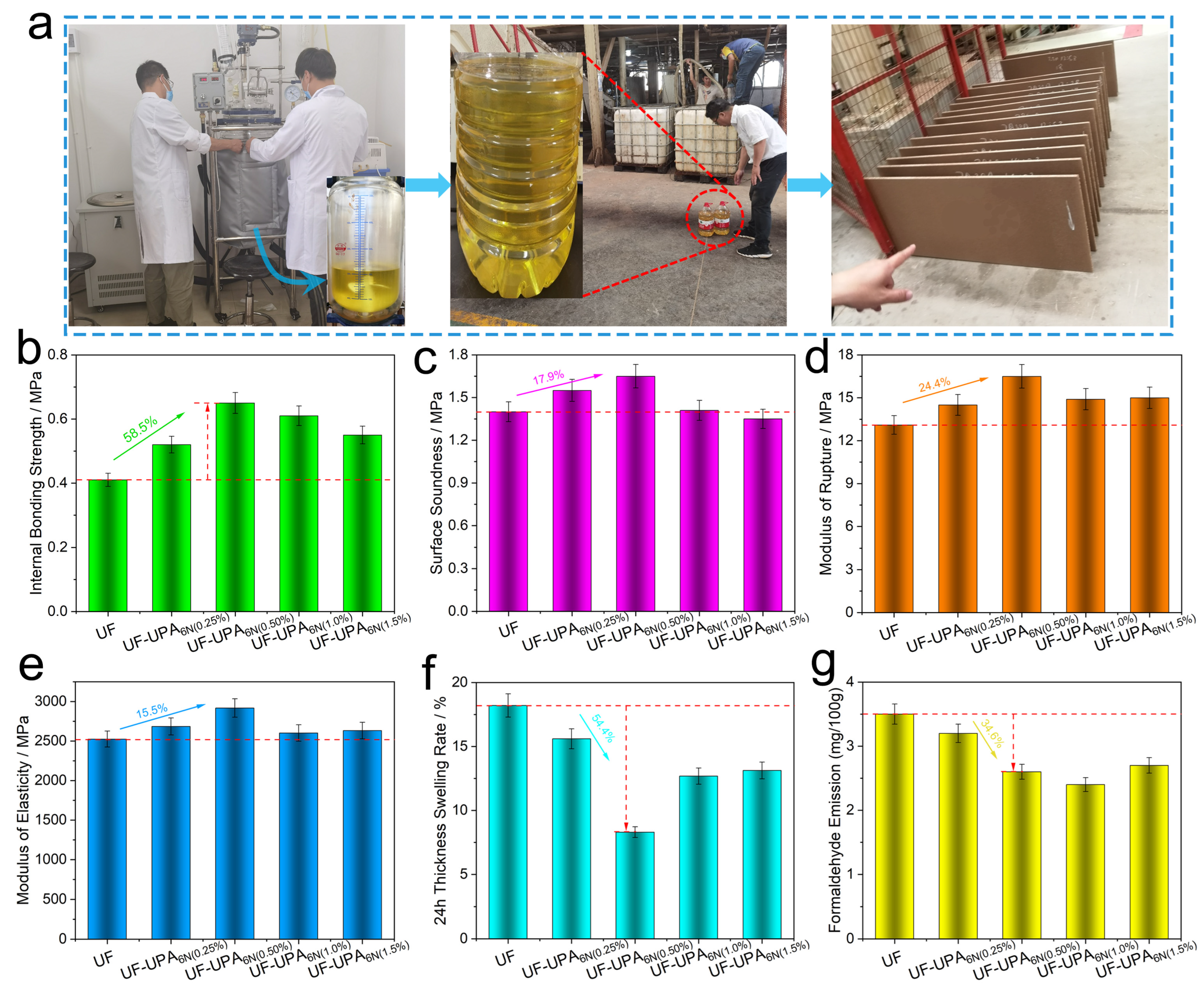
3.5. Physical and the Mechanical Performance Analysis of Particleboard for the UF Resin and UF-UPA6N Resin Adhesives
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mennani, M.; Benhamou, A.A.; Kasbaji, M.; Boussetta, A.; Ablouh, E.H.; Kassab, Z.; Achaby, M.E.; Boussetta, N.; Grimi, N.; Moubarik, A. Insights on the Physico-Chemical Properties of Alkali Lignins from Different Agro-Industrial Residues and Their Use in Phenol-Formaldehyde Wood Adhesive Formulation. Int. J. Biol. Macromol. 2022, 221, 149–162. [Google Scholar] [CrossRef]
- Kelleci, O.; Koksal, S.E.; Aydemir, D.; Sancar, S. Eco-Friendly Particleboards with Low Formaldehyde Emission and Enhanced Mechanical Properties Produced with Foamed Urea-Formaldehyde Resins. J. Clean. Prod. 2022, 379, 134785. [Google Scholar] [CrossRef]
- Lubis, M.A.R.; Park, B.-D.; Lee, S.-M. Modification of Urea-Formaldehyde Resin Adhesives with Blocked Isocyanates Using Sodium Bisulfite. Int. J. Adhes. Adhes. 2017, 73, 118–124. [Google Scholar] [CrossRef]
- Nakanishi, E.Y.; Cabral, M.R.; de Souza Gonçalves, P.; dos Santos, V.; Junior, H.S. Formaldehyde-Free Particleboards Using Natural Latex as the Polymeric Binder. J. Clean. Prod. 2018, 195, 1259–1269. [Google Scholar] [CrossRef]
- Nuryawan, A.; Park, B.D. Quantification of Hydrolytic Degradation of Cured Urea-Formaldehyde Resin Adhesives Using Confocal Laser Scanning Microscopy. Int. J. Adhes. Adhes. 2017, 74, 1–5. [Google Scholar] [CrossRef]
- Park, S.; Park, B.-D. Sustainable Bio-based Dialdehyde Cellulose for Transforming Crystalline Urea–Formaldehyde Resins into Amorphous Ones to Improve Their Performance. J. Ind. Eng. Chem. 2022, 113, 142–152. [Google Scholar] [CrossRef]
- Nemli, G.; Ozturk, I. Influences of some Factors on the Formaldehyde Content of Particleboard. Build. Environ. 2006, 41, 770–774. [Google Scholar] [CrossRef]
- Li, T.; Zhang, B.; Jiang, S.; Zhou, X.; Du, G.; Wu, Z.; Cao, M.; Yang, L. Novel Highly Branched Polymer Wood Adhesive Resin. ACS Sustain. Chem. Eng. 2020, 8, 5209–5216. [Google Scholar] [CrossRef]
- Tuerp, D.; Bruchmann, B. Dendritic polyurea polymers. Macromol. Rapid Commun. 2015, 36, 138–150. [Google Scholar] [CrossRef]
- Dennis, J.M.; Steinberg, L.I.; Pekkanen, A.M.; Maiz, J.; Hegde, M.; Muller, A.J.; Long, T.E. Synthesis and Characterization of Isocyanate-Free Polyureas. Green Chem. 2018, 20, 243–249. [Google Scholar] [CrossRef]
- Gu, J.; Hu, C.; Zhong, R.; Tu, D.; Yun, H.; Zhang, W.; Leu, S.-Y. Isolation of Cellulose Nanocrystals from Medium Density Fiberboards. Carbohydr. Polym. 2017, 167, 70–78. [Google Scholar] [CrossRef]
- Park, B.D.; Kim, J.W. Dynamic Mechanical Analysis of Urea–Formaldehyde Resin Adhesives with Different Formaldehyde-to-Urea Molar Ratios. J. Appl. Polym. Sci. 2008, 108, 2045–2051. [Google Scholar] [CrossRef]
- Kueh, C.S.L.; Martinez-Hermosilla, G.A.; Jamsari, M.A.; Dahm, K.; Bronlund, J.E. Effects of Perforation Design on Corrugated Fiberboard Panel Compression. Food Packag. Shelf Life 2021, 30, 100755. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Park, B.-D. Crystalline Lamellar Structure of Thermosetting Urea-Formaldehyde Resins at a Low Molar Ratio. Macromolecules 2021, 54, 2366–2375. [Google Scholar] [CrossRef]
- Sun, Q.-N.; Hse, C.-Y.; Shupe, T.F. Characterization and Performance of Melamine Enhanced urea Formaldehyde Resin for Bonding Southern Pine Particleboard. J. Appl. Polym. Sci. 2011, 119, 3538. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, J.; Tan, H.; Jiang, X.; Shi, J.; Zuo, Y.; Weng, X. Fabrication, Performances, and Reaction Mechanism of Urea-Formaldehyde Resin Adhesive with Isocyanate. J. Adhes. Sci. Technol. 2013, 27, 2191–2203. [Google Scholar]
- No, B.Y.; Kim, M.G. Syntheses and Properties of Low-Level Melamine-Modified Urea−Melamine−Formaldehyde Resins. J. Appl. Polym. Sci. 2004, 93, 2559. [Google Scholar] [CrossRef]
- Park, B.D.; Lee, S.M.; Roh, J.K. Effects of Formaldehyde/Urea Mole Ratio and Melamine Content on the Hydrolytic Stability of Cured Urea-Melamine Formaldehyde Resin. Eur. J. Wood Wood Prod. 2009, 67, 121. [Google Scholar] [CrossRef]
- Voit, B.I.; Lederer, A. Hyperbranched and Highly Branched Polymer Architectures-Synthetic Strategies and Major Characterization Aspects. Chem. Rev. 2009, 109, 5924–5973. [Google Scholar] [CrossRef]
- Essawy, H.A.; Moustafa, A.-A.B.; Elsayed, N.H. Improving the Performance of Urea-Formaldehyde Wood Adhesive System Using Dendritic Poly(amidoamine)s and Their Corresponding Half Generations. J. Appl. Polym. Sci. 2009, 114, 1348–1355. [Google Scholar]
- Zhou, X.; Essawy, H.A.; Pizzi, A.; Li, X.; Rode, K.; Radke, W.; Du, G. Upgrading of MUF Wood Adhesives for Particleboard production using oligomers of hyperbranched poly(amine-ester). J. Adhes. Sci. Technol. 2013, 27, 1058–1068. [Google Scholar] [CrossRef]
- Zhou, X.; Essawy, H.A.; Pizzi, A.; Zhang, J.; Li, X.; Du, G. First/Second Generation of Dendritic Ester-co-Aldehyde-Terminated Poly(amidoamine) as Modifying Components of Melamine Urea Formaldehyde (MUF) Adhesives: Subsequent Use in Particleboards Production. J. Polym. Res. 2014, 21, 379. [Google Scholar] [CrossRef]
- Amirou, S.; Zhang, J.; Essawy, H.A.; Pizzi, A.; Zerizer, A.; Li, J.; Delmotte, L. Utilization of Hydrophilic/Hydrophobic Hyperbranched Poly(amidoamine)s as Additives for Melamine Urea Formaldehyde Adhesives. Polym. Compos. 2015, 36, 2255–2264. [Google Scholar] [CrossRef]
- Pizzi, A.; Lipschitz, L.; Valenzuela, J. Theory and Practice of the Preparation of Low Formaldehyde Emission UF Adhesives. Holzforschung 1994, 48, 254–261. [Google Scholar] [CrossRef]
- Wibowo, E.S.; Park, B.-D.; Causin, V. Hydrogen-Bond-Induced Crystallization in Low-Molar-Ratio Urea−Formaldehyde Resins during Synthesis. Ind. Eng. Chem. Res. 2020, 59, 13095–13104. [Google Scholar] [CrossRef]
- Jikei, M.; Kakimoto, M. Hyperbranched Polymers: A Promising New Class of Materials. Prog. Polym. Sci. 2001, 26, 1233–1285. [Google Scholar] [CrossRef]
- Gao, C.; Yan, D. Hyperbranched Polymers: From Synthesis to Applications. Prog. Polym. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Wang, D.; Jin, Y.; Zhu, X.; Yan, D. Synthesis and Applications of Stimuli-Responsive Hyperbranched Polymers. Prog. Polym. Sci. 2017, 64, 114–153. [Google Scholar] [CrossRef]
- Lu, Y.; Nemoto, T.; Tosaka, M.; Yamago, S. Synthesis of Structurally Controlled Hyperbranched Polymers Using a Monomer Having Hierarchical Reactivity. Nat. Commun. 2017, 8, 1863. [Google Scholar] [CrossRef]
- Yang, H.; Du, G.; Li, Z.; Ran, X.; Zhou, X.; Li, T.; Gao, W.; Li, J.; Lei, H.; Yang, L. Superstrong Adhesive of Isocyanate-Free Polyurea with a Branched Structure. ACS Appl. Polym. Mater. 2021, 3, 1638–1651. [Google Scholar] [CrossRef]
- Liu, S.; Du, G.; Yang, H.; Su, H.; Ran, X.; Li, J.; Zhang, L.; Gao, W.; Yang, L. Developing High-Performance Cellulose-Based Wood Adhesive with a Cross-Linked Network. ACS Sustain. Chem. Eng. 2021, 9, 16849–16861. [Google Scholar] [CrossRef]
- Fu, Q.; Yan, M.; Jungstedt, E.; Yang, X.; Li, Y.; Berglund, L.A. Transparent Plywood as a Load-Bearing and Luminescent Biocomposite. Compos. Sci. Technol. 2018, 164, 296–303. [Google Scholar] [CrossRef]
- Wang, W.; Zammarano, M.; Shields, J.R.; Knowlton, E.D.; Kim, I.; Gales, J.A.; Hoehler, M.S.; Li, J. A novel Application of Silicone-Based Flame-Retardant Adhesive in Plywood. Constr. Build. Mater. 2018, 189, 448. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Wang, Y.; Li, T.; Wang, S.; Yang, S.; Qing, Y.; Li, X.; Wu, Y.; Liu, M. Calcium Carbonate Modified Urea–Formaldehyde Resin Adhesive for Strength Enhanced Medium Density Fiberboard Production. RSC Adv. 2021, 11, 25010–25017. [Google Scholar] [CrossRef] [PubMed]
- Samarzija-Jovanovic, S.; Jovanovic, V.; Konstantinovic, S.; Markovic, G.; Marinovic-Cincovic, M. Thermal Behavior of Modified Urea–Formaldehyde Resins. J. Therm. Anal. Calorim. 2011, 104, 1159–1166. [Google Scholar] [CrossRef]
- Zhu, P.; Gu, Z.; Hong, S.; Lian, H. Preparation and Characterization of Microencapsulated LDHs with Melamine-Formaldehyde Resin and its Flame Retardant Application in Epoxy Resin. Polym. Adv. Technol. 2018, 29, 2147–2160. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Y.; Du, G.; Yang, H.; Ni, K.; Su, H.; Ran, X.; Zhou, X.; Gao, W.; Yang, L. H2O2-Mediated Tannin Bio-Based Wood Adhesive Inspired by Starfish Structure and Phenol-Amine Synergy. Ind. Crops Prod. 2022, 188, 115714. [Google Scholar] [CrossRef]
- Deng, S.; Du, G.; Li, X.; Pizzi, A. Performance and Reaction Mechanism of Zero Formaldehyde-Emission Urea-Glyoxal (UG) Resin. J. Taiwan Inst. Chem. Eng. 2014, 45, 2029–2038. [Google Scholar] [CrossRef]
- Wibowo, E.; Park, B. Two-Dimensional Nuclear Magnetic Resonance Analysis of Hydrogen-Bond Formation in Thermosetting Crystalline Urea–Formaldehyde Resins at a Low Molar Ratio. ACS Appl. Polym. Mater. 2022, 4, 1084–1094. [Google Scholar] [CrossRef]
- Ying, H.; Cheng, J. Hydrolyzable Polyureas Bearing Hindered Urea Bonds. J. Am. Chem. Soc. 2014, 136, 16974–16977. [Google Scholar] [CrossRef]
- Liu, S.; Li, N.; Ling, Y.; Kang, B.; Geng, S.; Li, N.; Luo, H. pH-Mediated Fluorescent Polymer Particles and Gel from Hyperbranched Polyethylenimine and the Mechanism of Intrinsic Fluorescence. Langmuir 2016, 32, 1881–1889. [Google Scholar] [CrossRef] [PubMed]

| Sample | UF (kg) | UPA6N (kg) | Hot Pressing Time (s) | Hot Pressing Temperature (°C) |
|---|---|---|---|---|
| UF | 1000 | 0.0 | 180 | 200 |
| UPA6N (0.25%) | 1000 | 2.5 | 180 | 200 |
| UPA6N (0.50%) | 1000 | 5.0 | 180 | 200 |
| UPA6N (1.00%) | 1000 | 10.0 | 180 | 200 |
| UPA6N (1.50%) | 1000 | 15.0 | 180 | 200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Wang, H.; Du, G.; Ni, K.; Wu, Y.; Su, H.; Gao, W.; Tan, X.; Yang, Z.; Yang, L.; et al. Ureido Hyperbranched Polymer Modified Urea-Formaldehyde Resin as High-Performance Particleboard Adhesive. Materials 2023, 16, 4021. https://doi.org/10.3390/ma16114021
Yang H, Wang H, Du G, Ni K, Wu Y, Su H, Gao W, Tan X, Yang Z, Yang L, et al. Ureido Hyperbranched Polymer Modified Urea-Formaldehyde Resin as High-Performance Particleboard Adhesive. Materials. 2023; 16(11):4021. https://doi.org/10.3390/ma16114021
Chicago/Turabian StyleYang, Hongxing, Hao Wang, Guanben Du, Kelu Ni, Yingchen Wu, Hang Su, Wei Gao, Xiaoping Tan, Zhaojin Yang, Long Yang, and et al. 2023. "Ureido Hyperbranched Polymer Modified Urea-Formaldehyde Resin as High-Performance Particleboard Adhesive" Materials 16, no. 11: 4021. https://doi.org/10.3390/ma16114021
APA StyleYang, H., Wang, H., Du, G., Ni, K., Wu, Y., Su, H., Gao, W., Tan, X., Yang, Z., Yang, L., & Ran, X. (2023). Ureido Hyperbranched Polymer Modified Urea-Formaldehyde Resin as High-Performance Particleboard Adhesive. Materials, 16(11), 4021. https://doi.org/10.3390/ma16114021






