Abstract
Ammonia (NH3) is a highly important industrial chemical used as fuel and fertilizer. The industrial synthesis of NH3 relies heavily on the Haber–Bosch route, which accounts for roughly 1.2% of global annual CO2 emissions. As an alternative route, the electrosynthesis of NH3 from nitrate anion (NO3−) reduction (NO3−RR) has drawn increasing attention, since NO3−RR from wastewater to produce NH3 can not only recycle waste into treasure but also alleviate the adverse effects of excessive NO3− contamination in the environment. This review presents contemporary views on the state of the art in electrocatalytic NO3− reduction over Cu-based nanostructured materials, discusses the merits of electrocatalytic performance, and summarizes current advances in the exploration of this technology using different strategies for nanostructured-material modification. The electrocatalytic mechanism of nitrate reduction is also reviewed here, especially with regard to copper-based catalysts.
1. Introduction
As an important industrial chemical, ammonia (NH3) is widely used in synthetic drugs, fertilizers, dyes, plastics, and so on [1,2]. NH3 is also regarded as a carbon-free and environmentally friendly clean-energy carrier of hydrogen, since NH3 is easier to liquefy, store, and transport than hydrogen [3,4]. To date, the industrial synthesis of NH3 has relied heavily on the Haber–Bosch route. This route requires harsh conditions of high temperature (400–600 °C) and high pressure (200–350 atm) [5]. In fact, the energy consumption of the Haber–Bosch process accounts for 1.4% of the world’s total annual energy consumption, and the resulting carbon dioxide emissions are as high as 450 million tons, accounting for approximately 1.2% of global annual carbon dioxide emissions [6]. Therefore, researchers began to look for low-carbon and environmentally friendly alternatives for producing NH3 that can be realized under normal temperature and pressure conditions. Among many alternatives, the method of producing NH3 from nitrogen (N2) and H2O driven by renewable electric energy (NRR) has gradually attracted the researchers’ attention [7,8,9]. However, due to the high bond energy (941 kJ mol−1) of the N≡N bond and the low solubility of N2 in water, the energy utilization of NRR in the aqueous environment is low, and its production yield of NH3 is 2–3 orders of magnitude lower than the Haber–Bosch process. As the “Renewable Energy to Fuels through Utilization of Energy-dense Liquids” (REFUEL) program of the Department of Energy (DOE) demonstrates, the electrochemical synthesis of NH3 requires the realization of an NH3-production Faraday efficiency exceeding 90%, an NH3 yield of 10−6 mol s−1 cm−2, a current density of 300 mA cm−2, and an energy efficiency of 60% for industrial production [10]. It is very difficult to achieve these indicators through the NRR process, which limits the further development of NRR [11]. For this reason, researchers have tried to apply other nitrogen-containing compounds as nitrogen sources to synthesize NH3 [12]. Nitrate anion (NO3−) is a good candidate, since the solubility of NO3− in water is high and the bond energy of N=O bond (204 kJ mol−1) is low [13]. The NO3− reduction process is a solid–liquid interface reaction, which is more conducive to mass transfer than a gas–liquid–solid interface reaction during NRR and has the potential to realize the target of the REFUEL program. NO3− is a pollutant that widely exists in industrial and agricultural wastewater and domestic sewage [14]. The residual NO3− in the environment will not only harm the water body ecosystem and lead to water eutrophication but will also pose a serious threat to human health [15]. Therefore, using NO3− as a nitrogen source for NH3 synthesis is not only conducive to improving energy utilization efficiency and reducing greenhouse gas emissions; it can also alleviate the problem of NO3− pollution in the environment.
In the natural environment, the conversion of NO3− to NH3 can be biocatalyzed by a nitrate/nitrite reductase (such as Shewanella oneidensis cytochrome c nitrite reductase) under mild environmental conditions (a process known as bio-NRA) [16]. NH3 can then be concentrated by physicochemical methods such as ion exchange adsorption and struvite precipitation and used for further industrial applications [6]. However, the biocatalytic process of bio-NRA takes a long time to effect the conversion, and the efficiency of NO3− removal is low. In addition, the complex structures of the bio-enzymes are difficult to synthesize artificially. Therefore, scientists began to simulate the function of microbial enzymes to design and develop artificially synthesized catalytic materials to satisfy the demands of industrial production [17]. Research on the electrocatalytic reduction of NO3− (NO3−RR) to NH3 is also facing great challenges. NO3−RR involves the transfer of 8e− and 9 protons, during which various byproducts such as nitrite (NO2−), nitrogen monoxide (NO), nitrogen (N2), and nitrous oxide (N2O) are produced as well [18]. Meanwhile, there is competition between the hydrogen evolution reaction (HER) and NO3−RR. With the increase in the overpotential, HER will be increasingly strengthened, which further reduces the energy utilization efficiency of NH3 formation. Therefore, it is of great significance to carry out in-depth research on the mechanism of electrocatalytic NO3−RR synthesis of NH3 to rationally guide the development and design of catalysts with high catalytic activity and high NH3 formation selectivity.
2. Reaction Mechanism of Electrocatalytic NO3−RR
N has a variety of stable forms of hydrides and oxides from −3 to +5 valence states. The thermodynamic relationship between these nitrides is shown in the Frost–Ebsworth diagram (Figure 1) [13]. A series of reaction intermediate species and final products would be formed during NO3− reduction, and N2 and NH3 are the two most thermodynamically stable products, which can be obtained through the following Equations (1) and (2).
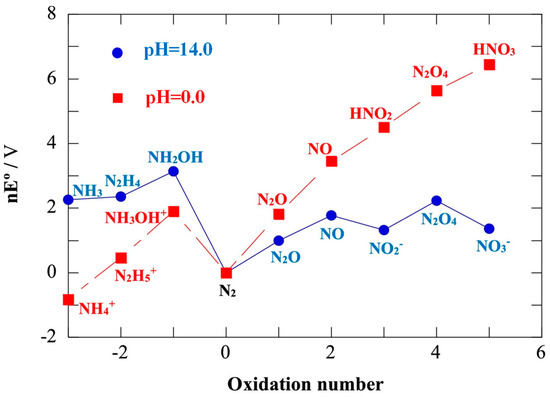
Figure 1.
Frost–Ebsworth diagram [19].
According to the literature, NO3− reduction to NO2− is the rate-determining step of NO3−RR (Figure 2) [20]. NO3− in the solution is first adsorbed onto the cathode surface (Equation (3)), during which some disturbing anions in the solution may compete with NO3− adsorption and inhibit NO3− reduction [21]. The mass transfer rate of NO3− from the solution to the electrode surface will also affect the adsorption process [22,23]. On the other hand, the number of active sites on the electrode’s surface will affect the reaction rate [23]. Currently, researchers propose two reaction mechanisms for NO3−RR to NH3: a direct reduction mechanism and an indirect autocatalytic reduction mechanism [19,24].
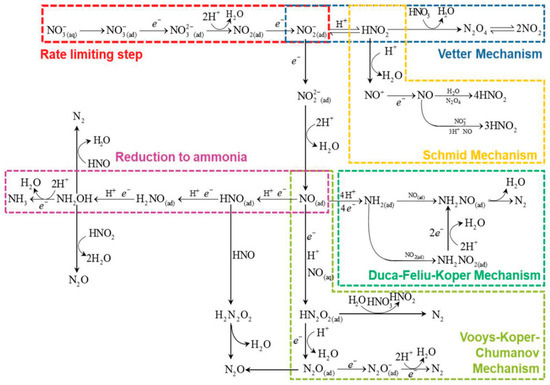
Figure 2.
Schematic diagram of the mechanism of NO3−RR in aquatic media [13].
2.1. Direct Reduction Pathway
In the direct reduction pathway, the adsorbed NO3− can be reduced by proton-coupling electrons or by adsorbed H atoms (*H) [19]. As shown in Equations (4)–(6), in the proton-coupling electron reduction pathway, the process of NO3− being reduced to NO2− involves three steps: (a) electrochemical (Equation (4)), (b) chemical (Equation (5)), and (c) electrochemical process (Equation (6)) [25,26,27]. Koper et al. [20] found that the Tafel slope of NO3−RR over transition metals in acidic solution is approximately 120 mV dec−1, and then they speculated that the first electron-transfer step is the rate-determining step. The slow kinetics of this step was attributed to the high energy of the lowest unoccupied π* orbital (LUMO π*) of NO3−, which made it difficult for electron transfer from the electrode surface into the LUMO π* of *NO3− [28]. Cu with highly occupied d orbitals has a closed energy level with LUMO π* of NO3−. The electrons are easily transferred from the unclosed d orbital of Cu to the adsorbed NO3−, which is conducive to the electrochemical reduction of NO3−.
NO3− adsorption:
NO3−→NO2−:
NO2− is a relatively stable intermediate which will become an unstable anion radical NO22− through a direct electron transfer reaction pathway (Equation (7)) [29]. Similarly to NO32−, NO22− will be rapidly hydrolyzed into adsorbed NOad through Equation (8) (k = 1.0 × 105 s−1) [30].
NO2−→NO:
NOad is the key intermediate species that determines the selectivity of the final products, N2 or NH3. In the Vooys–Koper pathway, NOad reacts with NOaq in solution to form a short-term dimer byproduct, dinitrogen dioxide (HN2O2) (Equation (9)) [31], which is then reduced to dinitrogen oxide (N2O) by secondary electron transfer (Equation (10)) [23,32]. N2O can be further reduced to the unstable N2O− (Equation (11)) [33]. This species will be readily transformed into the final product N2 (Equation (12)) [34].
NO→N2—Vooys–Koper pathway:
Based on the NO3−RR study on the Pt (100) crystal plane, researchers proposed the Duca–Feliu–Koper pathway, through which NO3− will be electrochemically reduced to N2. In this pathway, stable NH2ad will be generated by the reduction of NOad (Equation (13)) and coexists with NOad [35,36]. NOad and NH2ad will recombine according to the Langmuir–Hinshelwood reaction to generate the transient substance nitrosoamide (NONH2) (Equation (14)), which will be rapidly decomposed to N2 (Equation (15)).
NO→N2—Duca–Feliu–Koper pathway:
Among the mechanism studies for NH3 formation, the electrochemical–electrochemical (EE) reaction mechanism consisting of several sequential direct electron-transfer reactions is the main pathway [31]. NO successively undergoes reductive hydrogenation to several intermediates, forming nitroxyl (HNO) (Equation (16)) [37], H2NO (Equation (17)) [31], and hydroxylamine (H2NOH) (Equation (18)) [38]. Hydroxylamine will be protonated (Equation (19)) and then rapidly electrochemically reduced into NH3 (Equation (20)) [39].
NO→NH3:
In addition to the above direct reduction pathway, another hydrogenation pathway using adsorbed active hydrogen species (Had) as a reductant is considered to be an important pathway to promote the production of NH3 [40,41]. In the potential region where hydrogen adsorption occurs on the Pt catalyst, the formation of NH3 is highly promoted [42], since Had is stable on the electrode surface in that potential window. Had will efficiently deoxygenate NO3− and then hydrogenate the intermediates into NH3. A hydrogenation mechanism is postulated according to the theoretical calculation results (Equations (21)–(26)): [43]
However, how Had participates in the NO3−RR process is still not clear, and the key intermediates of the reaction have not been experimentally proved. The intrinsic mechanism of the hydrogenation pathway remains to be further explored.
2.2. Indirectly Autocatalytic Reduction Pathway
The indirectly autocatalytic reduction pathway occurs only in the strongly acidic solution of high NO3− concentrations (1–4 mol L−1) with the presence of HNO2 [23] and mainly affects the step involving the reduction of HNO3 to HNO2. In this pathway, NO3− itself is not the electroactive species and does not participate in the electron transfer process. NO3− and HNO2− will be transformed into NO2 or NO+ that is postulated as the electroactive species. With the accumulation of electroactive intermediates, the reaction will be highly enhanced. These two active substances can be generated via the Vetter pathway and the Schmid pathway, respectively (Equations (27)–(34)) [19]:
Vetter pathway:
Schmid pathway:
The following pathway of HNO2 to NH3 is similar to the direct reduction pathway mentioned previously. The indirect autocatalytic reduction mechanism could effectively accelerate the reaction rate of NO3−RR, which plays an important role in accelerating the conversion rate and reducing the reaction cost.
3. Research Status of Electrocatalytic NO3−RR to NH3 Production
3.1. Evaluation Criteria for NO3−RR Performance
Since nitrogen oxides and NH3 widely exist in air and water, N species pollution during the tests must be strenuously avoided to guarantee the reproducibility of the research results. MacFarlane and Ib Chorkendorff et al. [44,45] proposed a set of testing procedures for NH3 detection during NRR to ensure the accuracy of experimental results. The interference of N species pollution must be eliminated, and it must be determined that N in the formed NH3 is derived from N2 rather than from pollution from other N sources (catalysts, electrolytes, air, etc.) [11]. Similarly, the source of N in the formed NH3 in the NO3−RR process should also be strictly verified to obtain the real experimental results. Therefore, we need to have a unified understanding of the performance evaluation criteria for NH3 synthesis during the NO3−RR process.
There are two main factors affecting the NO3−RR test results: (1) pollution from other N sources and (2) the accuracy of the NH3 detection method. The pollution from other N sources mainly comes from air, reaction devices, electrolytes, and catalysts. Strict requirements on the cleanliness of the experimental equipment and accessories are essential to absolutely exclude these contaminations. Gases and chemicals used in experiments need to be at least analytically pure. The N element in the catalyst is another common contaminant affecting the experimental results. Many researchers who study single-atom catalyst systems should pay special attention to this if they use the MNC as the single-atom catalyst (M is the metal). It is necessary to verify whether the N content in the catalyst is consistent before and after the reaction. In addition, the 15N isotope labeling experiment is also one of the methods of confirming that the 15N in the product 15NH3 comes from 15NO3− instead of pollution from other N sources. The Macfarlane and Zhang groups [46,47] proposed strict experimental methods for the 15N isotope labeling experiment for NRR. Quantitative detection methods for NH3 include UV–Vis spectroscopic/colorimetric methods (Nessler reagent and indophenol blue method) [47,48], the o-phthalaldehyde fluorescence method [49], ion chromatography (IC) [50], ion selective electrode (ISE) [51], hydrogen nuclear magnetic resonance spectroscopy (1H NMR) [46,52], etc. The accuracy of these detection methods can be affected by environmental factors. For example, the pH will affect the complexation of the chromogenic agent with NH3, thereby affecting the coloration. The presence of other cations may affect the peak signal of NH4+ when using ion chromatography detection. Two different detection methods are usually required for a simultaneous detection to ensure the accuracy of the experimental results. This involves using isotope-labeled 15NO3− as the N source for the reaction and then using 1H NMR to detect the 15NH3 product so that we can track the source of N, which is currently considered to be the safest measurement.
The production yield per unit area (mmol h−1 cm−2) and the production yield per unit mass (mmol h−1 g−2) are the key indicators in evaluating the NH3 production rate. Faradaic efficiency (%) and partial current density (mA cm−2) are important indicators for the measurement of the energy utilization efficiency of the electrocatalytic process. In addition, the overpotential (V vs. RHE), stability, and NO3− removal rate (%) of the reaction are also important factors in evaluating the overall catalytic performances.
3.2. Electrocatalysts Designed for NO3−RR to NH3
Noble metal catalysts have shown good electrocatalytic performances in many electrocatalytic reactions, such as hydrogen evolution, alcohol oxidation, nitrogen reduction, and carbon dioxide reduction reactions. In their study of electrocatalytic NO3−RR, DIMA et al. [20] analyzed the catalytic performance of the Pt group catalysts and found that the corresponding NO3−RR activity decreased following the sequence Rh > Ru > Ir > Pd > Pt (Figure 3a–e). The adsorption of NO3− over Pt and Pd is weak, while the adsorption of *H is too strong, making it difficult for NO3−RR to compete with HER. Rh, Ru, and Ir have a higher ability to adsorb NO3− and so those catalysts show better catalytic activity. Li et al. [53] reported that nitrate electroreduction overstrained Ru nanoclusters gave a high rate of NH3 formation (5.56 mol gcat−1 h−1). The primary contributor to such performance is the *H, which are generated by suppressing H-H dimerization during the water splitting enabled by the tensile lattice strains. The *H expedited NO3−-to-NH3 conversion by hydrogenating intermediates of the rate-limiting steps at lower kinetic barriers. The strained nanostructures can maintain nearly 100% NH3 selectivity at >120 mA cm−2 current densities for 100 h. Feliu et al. [42] found that electrocatalytic nitrate reduction was hardly perceptible on Pt (111), while electrocatalytic activity was improved with an increase in the step density of the Pt electrode surface. Inactivation was observed on the step sites in the presence of adsorbed NO3−-derived reduced adsorbates, i.e., adsorbed NO. It was, therefore, concluded that the electrocatalytically active NO3− species did not adsorb on the (111) terraces but on the (111) monoatomic steps (as shown in Figure 3f,g). In addition, Han et al. [54] prepared Pd nanocrystalline with well-designed facets that could act as an efficient NO3−RR electrocatalyst for ambient NH3 synthesis and found that Pd (111) exhibited excellent activity and selectivity in reducing NO3− to NH3 with a Faradaic efficiency of 79.91% and an NH3 production of 0.55 mmol h−1 cm−2 (2.74 mmol h−1 mg−1) in 0.1 M Na2SO4 (containing 0.1 M NO3−), which was 1.4 times higher than Pd (100) and 1.9 times higher than Pd (110), respectively. However, although many studies have been conducted on noble metals and some interesting results have been obtained, more effort should be made to lower the cost of the fabrication of catalysts and apply them to the industrial production of NH3 formation. Therefore, many researchers have turned their attention to the development and design of non-precious metal catalysts.
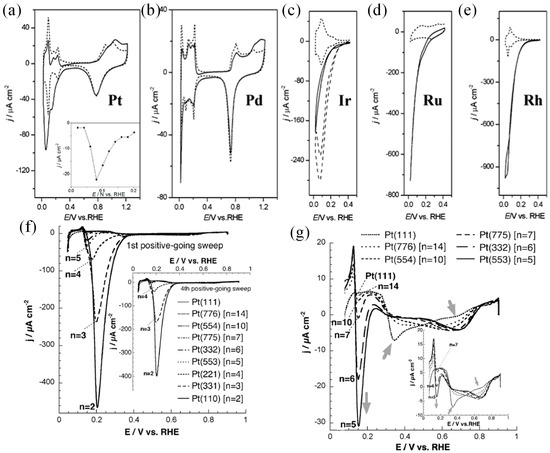
Figure 3.
Cyclic voltammograms on (a) Pt, (b) Pd, (c) Ir, (d) Ru, and (e) Rh in 0.5 M H2SO4 with (solid line) or without (dashed line) the presence of 0.1 M NaNO3. Inset in (a) is the steady-state NO3− reduction current at different potentials [20]. (f) Cyclic voltammograms at Pt(1 1 1) and Pt(S)[n(111) × 111]] electrodes (n = 14, 10, 7, 6, 5, 4, 3, 2) in 0.1 M 10 mM KNO3 + HClO4. (g) The cyclic voltammograms of the expansion of current density scale of (f) [42].
Many transition metal catalysts exhibit a good electrocatalytic activity for NO3−RR. Rossmeissl et. al. [55] studied the thermodynamics of various transition metal catalysts based on DFT calculations and found that Cu has a good adsorption capability for the intermediate species such as *NO3−, *NO2−, and *NO (Figure 4a–d), which is conducive to the continuous reduction and transformation of NO3−. In addition, the adsorption of *H over Cu is weak, which hinders HER competition. Compared with other catalysts (as shown in Figure 4e), *N on Cu is more easily hydrogenated to NOH, which indicates that it has a better NH3 selectivity [12,56]. Jiao et al. [57] experimentally investigated various transition metals (Fe, Co, Ni, Cu) for the electrochemical reduction of NO and N2O to reveal the role of electrocatalysts in determining the product selectivity. Specifically, Cu was highly selective toward NH3 formation with >80% Faradaic efficiency in NO electroreduction. Furthermore, a high NO coverage facilitated the N–N coupling reaction. In acidic electrolytes, the formation of NH3 is greatly favored, whereas N2 production is suppressed.
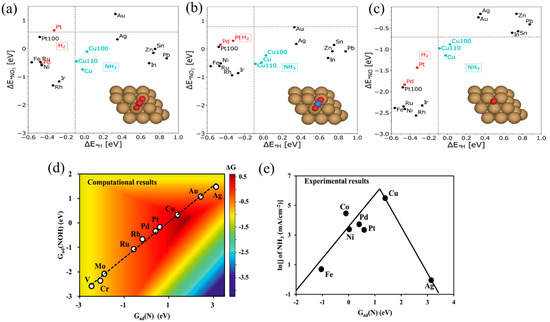
Figure 4.
The adsorption energies of the intermediates (a) ; (b) and (c) corresponding to ΔE*H [55]. (d) A two-dimensional activity map for producing NH3, where all the reaction free energies are shown at 0 V vs. RHE [12]. (e) The logarithm of partial current density of NH3 for NORR on different metal electrodes at 0 V vs. RHE [56].
4. Cu-Based Catalyst for NO3−RR
4.1. Modifications for Cu-Based Catalysts
Currently, the NH3 production rate over Cu-based catalysts is far lower than the traditional Haber–Bosch method (200 mmol gcat−1 h−1), which hinders its further development in industrial applications. Due to the complex reaction process of NO3−RR to NH3 and the low kinetics of NO3−RR, the current density of NO3− reduction over Cu-based electrocatalysts is mostly below 100 mA cm−2, especially in solutions with a low NO3− concentration. Therefore, it is urgent to improve the intrinsic activity of Cu-based catalysts for NH3 formation. Research published in the literature describes the following methods of modification for Cu-based catalysts, which have been studied extensively; they are summarized below.
4.1.1. Size Modulation
The catalytic activity of the catalyst is highly affected by its particle size [58]. By optimizing the particle size of the catalyst, the catalytic activity of the catalyst can be significantly improved. As shown in Figure 5, from nanoparticles to nanoclusters and even single-atom catalysts, the density of active sites per unit mass increases, normally giving an increased catalytic activity [58]. Fu et al. [59] reported that the current density of NO3− reduction over Cu nanoparticles is 13 times higher than that over Cu foil, and the Faraday efficiency of NH3 production has also increased from 30% over Cu foil to approximately 61% over Cu nanoparticles. In addition, the particle size of the catalyst will affect the interaction of reactants with the catalyst’s surface [60]. Yang et al. [61] prepared a Cu single-atom catalyst (Cu SAC) for NO3−RR during which the maximum NH3 yield reached 0.26 mmol cm−2 h−1 (12.5 mol gCu−1 h−1) and the Faraday efficiency of NH3 production reached 84.7%. According to in situ X-ray absorption spectroscopy coupled with advanced electron microscopy technology, it was found that Cu SAC would be reconstructed into Cu nanoparticles (Cu13) with a particle size of approximately 5 nm during NO3−RR. DFT calculation results also showed that there was a high thermodynamic energy barrier of *NO2 reduction over Cu SAC, while the thermodynamic energy barrier of *NO3 to NH3 on a Cu13 cluster presented a continuous downward trend. These results showed that it was the Cu nanoparticles that were the active sites and not the Cu single atom sites.
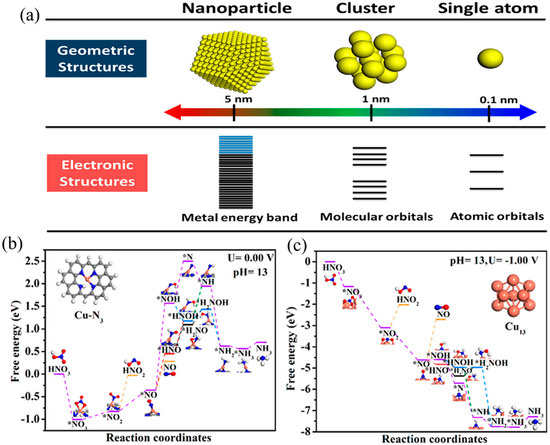
Figure 5.
(a) Schematic diagrams of the geometric structures and electronic structures of single atoms, clusters, and nanoparticles [58]. Free energy diagrams of the electrochemical NO3−RR to NO2−/NH3 on (b) Cu-N3 and (c) Cu13 sites [61].
4.1.2. Crystalline Facet Engineering
Feliu and Koper [42,62] found that NO3−RR on the Pt-based catalyst was highly affected by the surface crystalline facet. Recent NO3−RR production also proved that NO3−RR on the Cu-based catalyst was sensitive to the crystalline facet. Hu et al. [44] studied the NO3−RR and HER processes on different basal facets of Cu by using theoretical calculations and found that the competition between NO3−RR and HER was affected by the solution pH. Cu (100) showed a catalytic performance of NO3− reduction to NH3 in a strong acidic environment that was higher than that of the other basal facet, while Cu (111) had a higher catalytic activity in a neutral or alkaline environment (Figure 6). The difference in NO3−RR activities between the different crystalline facets of Cu are attributed to the different atomic spacing and electronic state of the surface atom. The Cu–Cu bond length on Cu (111) was closer to the distance of two oxygen atoms in NO3−, which is more conducive to the adsorption of NO3− on Cu (111). In addition, in the theoretical calculation, the d-band center on the Cu (111) was higher than that of Cu (100), due to which there was a stronger adsorption ability to NO3− on Cu (111). The electronic state of the catalyst surface affected its adsorption energy to NO3− and other intermediate species that affected the NO3−RR activity. At a potential of 0 V vs. RHE, *NO→*NOH was theoretically proved as the rate-limiting step. The low adsorption energy of *NOH can reduce the reaction energy barrier. Fu et al. [59] prepared the Cu catalysts with different basal facets and found that Cu (111) exhibited its highest catalytic activity in alkaline electrolytes. Koper et al. [63] studied the performance of the electrocatalytic NO3− over Cu single crystal electrode in different pH media. In acidic and alkaline media, the onset potential of Cu (111) was slightly more positive than Cu (100). However, the HER activity on Cu (111) was relatively higher than Cu (100), leading to better NO3−RR performances over the latter crystalline facet within a wide electrode potential window. Based on the advantages of electrochemically controllable technology in nanocrystals synthesis, our research group prepared Cu nanocrystals with different crystal plane, including high-index faceted Cu tetrahexahedron (THH Cu NCs, mainly composed of (211) and (311) crystal planes), low-index faceted Cu cube (100), and Cu octahedron (111) [64]. We found that, compared with Cu cube (100) and Cu octahedron (111), the THH Cu NCs with higher surface stepped sites had a higher reduction current density and a higher Faraday efficiency of NH3 formation of 98.3% (Figure 7). This is consistent with the results on the Pt catalyst reported by Feliu et al. [42]. The surface stepped sites of the catalyst are the main active center. The higher the density of the steps, the better the NO3−RR activity.

Figure 6.
Competing relationship between NRA and HER on (a) Cu (111) and (b) comparison between Cu (111), Cu (110) and Cu (100). (c) Surface structure, (d) d-band centers of Cu (111), Cu (120), and Cu (100), and (e) intermediate adsorption energies on Cu (111), Cu (110), and Cu (100) [44].

Figure 7.
SEM images of (a) THH Cu NCs, (b) Cu nanocubes, and (c) Cu octahedrons; (d) FE of NH3 on THH Cu NCs, Cu nanocubes and Cu octahedrons [64].
4.1.3. Surface Defects Engineering
By constructing the defective structure and decreasing the coordination number of the active site on the catalysts surface, NO3− adsorption and activation on the surface can be significantly enhanced and the intrinsic activity of the catalysts can then be improved. Hu et al. [65] studied the effect of defect exposure on the surface of Cu (100) on NO3− reduction activity. They found that under alkaline conditions, ultra-thin two-dimensional CuO (111) nanoribbons reconstituted into Cu (100) nanoribbons (Cu-NBS -100) in the process of NO3− electrochemical reduction, and, due to the high surface energy of ultra-thin nanoribbons, certain surface defects were formed in the process of structure reconstruction. Cu (100) -D1~7 models with different defects were constructed for DFT calculation. After comprehensive study, it was found that the d-band center of Cu (100)-D upshifted towards the Fermi level after the construction of surface defects on which the adsorption of intermediate species was enhanced. The Gibbs free energy of Cu (100)-D7 for NO3− was significantly lower than that of Cu (100). The defective sites on Cu (100)-D7 had a strong adsorption effect on *H intermediates, which makes *H difficult to desorb from the Cu surface, thus inhibiting HER. Xu et al. [66] also proved that Cu nanosheets with rich surface defects had a higher catalytic activity than those with smooth surfaces. At the potential of −1.3 V vs. SCE, the production rate of NH3 reached 781.25 μg h−1 mg−1, and the Faraday efficiency arrived at 81.99%. Surface defects were able to change charge distribution on the catalyst surface and effectively improved electron transfer between the catalyst and the adsorbed species. They were able to enhance the adsorption and conversion of reaction intermediates on the catalyst surface [67]. In addition, surface atomic defects also brought more irregular edge steps to the catalyst surface and increased the density of surface-active sites.
4.1.4. Interfacial Engineering
In photocatalysis, constructing heterojunction structures to apply interfacial interactions to improve photocatalytic activity is a common catalyst-design strategy [68,69,70]. In electrocatalysis, the interfacial interaction between different phases also has a significant impact on catalytic performances [71,72]. Deng et al. [73] proved that the electrocatalytic NO3−RR activity of CoP can be effectively enhanced by constructing a p-n heterojunction of a CoP/TiO2 nanoarray on titanium plate (CoP/TiO2@TP). According to DFT, the p-n heterojunction in CoP/TiO2 could induce the charge redistribution of CoP and TiO2, enhance the electron transport between the interfaces, effectively reduce the free energy of each reaction step, and improve the performance of NO3−RR. Benefiting from the interaction of heterostructures, such CoP/TiO2@TP exhibited an excellent Faradaic efficiency of 95.0% and a large NH3 yield as high as 499.8 μmol h−1 cm−2, which was superior to CoP@TP and TiO2@TP. For a Cu-based catalyst, Liu et al. [74] grew Pd particles on CuO nano-olives (Pd/CuO NOs) to form a Pd/CuO NOs catalyst with a unique Pd/CuO heterogeneous interface for nitrite reduction (NO2−RR). The heterogeneous interface formation of Pd/CuO NOs was analyzed by means of XPS characterization and DFT calculation. CuO and Pd show the characteristics of negative charge and positive charge, respectively, because of the transfer of electrons. Additionally, the built-in electric field constructed at the Pd/CuO heterointerface promotes local charge polarization. The formation of a Pd/CuO heterointerface optimizes the electronic structure of Cu, which is more favorable for the adsorption of NO2− and reaction intermediates. Pd/CuO NOs showed an NH3 yield of 906.4 μg h−1 mg−1 cat and a Faraday efficiency of 91.8%. Wang et al. [75] designed the Cu/Cu2O nanowire array and revealed that CuO would be electrochemically converted into Cu/Cu2O, which was observed by performing in situ Raman and ex situ experiments. The Cu/Cu2O interface was proved as the active site. The combined results of online differential electrochemical mass spectrometry (DEMS) and DFT calculations demonstrated that the electron transfer from Cu2O to Cu at the interface could facilitate the formation of *NOH intermediate and suppress the HER, leading to 95.8% of NH3 Faraday efficiency and 0.2449 mmol h−1 cm−2 NH3.
In summary, the optimization of Cu catalysts by various modification methods can significantly improve the Faraday efficiency of NH3 production, but it was also found that the current density of NO3−RR and the NH3 production rate were still too low to be applied in industrial applications (Table 1). Moreover, the required overpotential was also too high and was not conducive to being powered by regeneration energy. To this end, researchers have gradually designed Cu-based bimetallic catalysts by introducing second metals, and tried to further improve the catalytic activity of Cu-based bimetallic catalysts by regulating their electronic structure and synergistic effect, and they have promoted the further development of NO3−RR.

Table 1.
Recently reported Cu-based metallic catalysts for NO3− reduction to NH3.
4.1.5. Electronic Structure Modulation
Wang et al. [82] designed Cu100−xNix alloy with different proportions of the Cu and Ni elements and investigated the adsorption energy of intermediate products over the Cu100−xNix alloy. Since the NO3−RR activity on Ni is weak, the increase in the Ni element proportion should decrease the number of active sites on the surface, possibly resulting in a decreased performance. However, compared with pure Cu, Cu50Ni50 showed an increase of 0.12 V in the half-wave potential and a sixfold increase in activity at 0 V vs. RHE. The electronic structure of Cu100−xNix was analyzed by performing X-ray photoelectron spectroscopy (XPS), X-ray adsorption spectroscopy (XAS), and ultraviolet photoelectron spectroscopy (UPS). It was found that the binding energy of Cu 2p in Cu100−xNix alloy shifted to a lower binding energy with the increase in the Ni proportion. The binding energy of the Ni 2p shifted towards a higher binding energy, indicating that the charge in the Cu100−xNix alloy was redistributed and electrons were transferred from the Ni to the Cu. At the same time, the Cu d-band center upshifted, which enhanced the adsorption of the intermediate products on the Cu. Further study by means of DFT calculation found that the adsorption energy of the intermediate products showed a volcanic distribution with the overall reactivity. When the Ni accounted for 50%, the catalyst had moderate adsorption on all intermediate species, and then the highest reaction performances arrived. The establishment of the relationship between the electronic structure of the catalysts and NO3−RR activity provided an idea for guiding the design of novel NO3−RR catalysts. Ge et al. [83] reported polyallylamine (PA) functionalized RhCu bimetallic nanocubes (PA-RhCu cNCs) as a robust electrocatalyst for the reduction of NO3− to NH3. PA-RhCu cNCs showed a remarkable NH3 production yield of 2.40 mg h−1 mgcat−1 and a high Faradaic efficiency of 93.7% at 0.05 V. DFT calculations and experimental results indicated that Cu and PA (adsorbed amino) co-regulated the Rh d-band center, which slightly weakens the adsorption energy of reaction-related species on Rh. These findings may open an avenue to construct other advanced catalysts based on organic molecule-mediated interfacial engineering. Goldsmith’s group [84] predicted NO3−RR activity by calculating the adsorption energy of N and O atoms on transition metals. They proposed that alloyed Fe3Ru, Fe3Ni, Fe3Cu, and Pt3Ru have a strong adsorption capability to N and O atoms and suggested that those bimetals should be the potential NO3− reduction catalysts.
Based on the above analysis, the appropriate adsorption energy of catalysts for intermediate species such as *NO3, *H, and *NH3 is the key factor affecting their performance, which can be achieved by modulating the electronic structure of bimetal alloys to affect the d-band center or by forming a poor/rich-electron center.
4.1.6. Synergistic Effect
Protons (8e−) are involved in the process of NO3− reduction to NH3, giving a series of elementary reactions before the final products are formed. The active sites on single metallic catalysts are not well adapted to simultaneous multiple elementary steps. To this end, the construction of a synergistic effect between multiple active sites become one of the research hotspots in the field of NO3−RR. Precious metals alloying with non-precious metals is one of the most useful strategies for fabricating bimetallic catalysts (Table 2). The introduction of precious metals into Cu metal was proved to facilitate a much higher catalytic activity than that of pure Cu [85,86,87,88]. Kerkeni et al. [89] modified the platinum catalyst’s surface through a deposition on the Cu (sub)monolayer of a certain amount of Cu (Cuads/PT) using electrochemical methods. They found that the intrinsic catalytic activity (at t = 0) and the NH3 selectivity of Cuads/Pt depended on the fraction of the platinum surface occupied by copper adatoms (θCu). The highest activities and selectivity of NH3 were obtained with θCu ≈ 0.4–0.6. Moreover, higher activities were obtained in the reduction of NO3− than in the reduction of NO2−. Barrabés [85] and Wang et al. [90] believed that the existence of Cu was conducive to NO3− adsorption and reduction into NO2− and that NO2− would then be transferred to Pt/Pd to continue being hydrogenated. The presence of Pt/Pd would produce *H, which was conducive to maintaining the existence of Cu0 activity and promoting the intermediate species to further hydrogenation to N2 or NH3. Luo et al. [91] designed the Cu nanowire-supported Rh cluster and single-atom electrocatalyst (Rh@Cu) for a NO3− reduction reaction. Benefiting from the catalytic cooperation between Rh and Cu, whereby Rh activates the hydrogenation of the *NO intermediate on Cu, Rh@Cu systems achieved a Faradaic efficiency up to 93% at −0.2 V vs. an RHE and NH3 yield rate of 1.27 mmol h−1 cm−2. Detailed investigations by means of electron paramagnetic resonance, in situ infrared spectroscopy, differential electrochemical mass spectrometry, and DFT calculations suggested that the high activity originates from the synergistic cooperation between the Rh and Cu sites, whereby adsorbed hydrogen on the Rh site transferred to vicinal *NO intermediate species adsorbed on the Cu. Schuhmann et al. [92] presented a design concept of tandem catalysts, which involves coupling intermediate phases of different transition metals, existing at low applied overpotentials, as cooperative active sites that enable cascade NO3−-to-NH3 conversion, in turn, avoiding the generally encountered scaling relations. They implemented the concept by means of the electrochemical transformation of Cu−Co binary sulfides into potential-dependent core−shell Cu/CuOx and Co/CoO phases. Electrochemical evaluation, kinetic studies, and in situ Raman spectra reveal that the inner Cu/CuOx phases preferentially catalyzed NO3− reduction to NO2−, which was rapidly reduced to NH3 at the nearby Co/CoO shell. This unique tandem catalyst system led to a NO3−-to-NH3 Faradaic efficiency of 93.3 ± 2.1% in a wide range of NO3− concentrations at pH 13, a high NH3 yield rate of 1.17 mmol cm−2 h−1 in 0.1 M NO3− at −0.175 V vs. RHE, and a half-cell energy efficiency of ~36%, surpassing most previous reports.

Table 2.
Recently reported Cu-based bimetallic catalysts for NO3− reduction to NH3.
4.2. Study on Mechanism of Electrocatalysis of NO3−RR Synthesis of NH3 by Cu-Based Catalyst
Hu et al. [44] studied the pathway of the electroreduction of NO3− to NH3 by employing DFT calculation. According to their comprehensive thermodynamic and dynamic analysis, the pathway of NO3−RR on Cu should be as follows: NO3− → *NO3 → *NO2 → *NOH → *NHOH → *NH → *NH2 → *NH3 → NH3 (g) (Figure 8). Butcher et al. [99] detected a series of intermediates in the NO3−RR process in the acid media by means of SHINERS analysis, including NO2− and HNO, NH2, NH4+, etc. This was consistent with DFT calculation results. In an alkaline medium, Koper et al. [63] observed the appearance of hydroxylamine on the Cu catalyst using Fourier infrared spectra analysis, indicating that hydroxylamine was an intermediate species in the alkaline environment.

Figure 8.
(a) The three possible NO3−RR pathways on Cu (111) and (b) the activation energy diagram of each step of NO3−RR synthesis of NH3 on Cu (111) [44].
Gewirth et al. [100] analyzed the structural evolution of the adsorption intermediates on the surface of the Cu (100) by using an in situ electrochemical scanning tunnel microscope (EC-STM). They intuitively observed the process of adsorption of NO3− being transformed into NO2−. The adsorption of oxyanions was always accompanied by the synergistic adsorption of H2O or H3O+ [101]. H2O or H3O+ can stabilize the structure of oxyanions by forming hydrogen bonds with the lone electron pairs of oxygen atoms [102]. Researchers proposed that the NO3− adsorption layer on Cu (100) was also involved with H3O+. In addition, DFT calculation results showed that NO3− and NO2− were adsorbed on the Cu (100) surface by the bridging of their two oxygen atoms. Koper et al. [20] conducted an electrochemically dynamic analysis of the NO3−RR process using cyclic voltammetry and found that the Tafel slope on the Cu-based catalyst was slightly higher than the 120 mV dec−1. Therefore, they proposed that the first electron transfer step was the rate-limiting step. There are two possible mechanisms: *H reduction (Equations (35) and (36)) and the proton-coupling electron reduction mechanism (Equations (37) and (38)).
*H reduction:
Proton-coupling electron reduction:
The HER performance over the Cu catalyst is relatively poor, so it is difficult to produce *H species. Therefore, it is generally believed that the proton-coupling electron reduction mechanism is most effective on a pure Cu catalyst. For catalysts that are more susceptible to activating protons, such as the precious metals contained in bimetals, *H was more conducive to NO3− reduction.
The existence of other anions/cations in electrolytes usually affects the target catalytic reactions. For example, the existence of Cl− ions would lead to different final products. Butcher et al. [99], analyzing to Raman spectra, found that there were more bands of N-Hx species on the Cu basal facets after adding 10 mmol L−1 Cl−, revealing that the presence of Cl− was beneficial for NH3 formation. Gao et al. [103] found that the amount of NH3 formation was dramatically decreased, although a similar removal efficiency of NO3− was obtained when 100 mg L−1 Cl− was added. As the Cl− ion concentration increased, the NH3 selectivity rapidly decreased. This was due to the Cl− indirectly oxidizing NH3 into N2. The Cl− was oxidized by the anode to produce Cl2 (Equation (39)), which was then transformed into HOCl with strong oxidation (Equation (40)) [104]. The HOCl could efficiently oxidize the NH3 into N2 (Equation (41)).
Currently, mechanistic studies of NO3−RR over Cu-based catalysts are relatively scarce, and most of them have followed the results from Pt-based catalysts. Therefore, the mechanism of electrocatalytic NO3− reduction to NH3 over Cu-based catalysts requires further study.
5. Conclusions and Perspectives
Electrocatalytic NO3−RR is a clean and pollution-free process for NH3 production, which has the potential to realize large-scale industrial NH3 production and could solve the problem of NO3− pollution at the same time. This review mainly presents contemporary views on the state of the art in electrocatalytic NO3− reduction over Cu-based nanostructured materials. The effective methods, which were reported to modify the performance of Cu-based catalysts for electrocatalytic NO3−RR to NH3, include size modulation, crystalline facet engineering, surface defect engineering, interfacial engineering, electronic structure modulation, and synergistic effect. A reduction in metal catalyst particle size can effectively improve the utilization efficiency of atoms and enhance reactivity. Theoretically, the single-atom catalyst has nearly 100% atomic utilization efficiency. However, for Cu single-atom catalysts, the active sites were Cu nanoparticles instead of the Cu single atom sites. For the optimization of crystal surfaces, Cu nanocrystalline catalysts with a high crystal index have higher activity than those with a low crystal index. Cu catalysts with a high crystal index can be constructed to further improve the performance of NH3 synthesis. Moreover, by constructing the defective structure and decreasing the coordination number of the active site on the catalyst’s surface, NO3− adsorption and activation on the surface can be significantly enhanced, and the intrinsic activity of the catalysts can then be improved. Cu active sites can be optimized effectively by the three methods mentioned above. However, since the reduction of NO3− to NH3 involves the transfer of nine protons and eight electrons, it is difficult for the single active site to work effectively with simultaneous multiple elementary steps. To this end, catalysts with multiple active sites were designed. The heterogeneous interface between the Cu-based catalysts and other materials could promote local charge polarization by means of the formation of a built-in electric field and change the adsorption of intermediate species of NO3−RR. The alloying of Cu with a secondary metal can regulate the electronic structure of Cu and the synergistic effect. Additionally, different elements/active sites with different activities may have synergistic effects by providing a reduction in intermediate species or tandem reaction mechanisms to effectively reduce the reaction energy barrier. In conclusion, there are broad development spaces in the design and development of catalysts for the electrocatalytic NO3−RR of synthetic NH3, and this paper can provide a reference for subsequent research.
The research of nitrate reduction reaction has developed vigorously and made some progress in recent years, but there are still many problems. Below, we list some of the shortcomings and further research directions.
Research on the mechanism of NO3−RR is still insufficient. The reaction pathway of NO3−RR is affected by many environmental parameters, such as other ions, pH, NO3− concentration, and applied potential. Different systems accrue different basic pathways, which require a more systematic and detailed analysis, including the identification of key reaction intermediates by means of various in situ spectra and the postulation of basic reaction steps using theoretical calculations.
Electrocatalysts that are more economical, efficient, and stable need to be developed. At present, although modified Cu-based catalysts show improved electrocatalytic reduction activities, there are oxidation, dissolution, and leaching problems in catalysts, and these lead to the decay of electrochemical activity and could cause adverse effects on the environment. In addition, structural evolution during electrocatalysis makes it more difficult to identify the real active sites and design strategies for protecting these active sites. Various electrochemical in situ spectral analyses and electron microscopic observations can help track the evolution of active sites during electrocatalysis and provide guidance and assistance for catalyst design. Furthermore, the predictive power of machine learning based on the analysis of large experimental data will help to develop the rapid screening of suitable and efficient NO3−RR catalysts.
There are differences between the actual sewage and the experimental test electrolyte. The potential and limitations of electrocatalytic NO3−RR in practical water-remediation systems should be investigated by fully considering the effects of possible ion pollution, organic compound pollution, and solid sediment on NO3−RR in actual sewage and then designing the corresponding solutions. For example, pretreatment devices could be designed to purify the pollution.
It is necessary to design and build a reasonable and efficient reaction system and device. First, in order to form a complete chemical reaction process with NO3−RR, another set of electrocatalytic reactions (such as oxygen evolution) would be required, which would not only increase the complexity and cost of the reaction but also mean that the oxidation process and reaction byproducts might interfere with NO3− reduction. Therefore, we need to optimize the electrolytic cell. In addition, it is necessary to overcome the influence of mass transfer on scale-up experiments. The design of more efficient flow electrolytic cells is also one of the keys to improving the efficiency of NO3−RR.
The design and evaluation of the whole chain of the NH3 synthesis process by electrochemical NO3−RR require further work. At present, the low efficiency of NH3 extraction is an important factor hindering the development of NH3 production by NO3−RR. Therefore, the overall efficiency and the feasibility of NO3−RR in practical applications still need to be further evaluated. For example, the efficiency and cost of electrocatalytic NO3−RR technology and the Haber–Bosch process, as well as other important parameters related to scalability and commercial feasibility, should be investigated to make a comprehensive technological and economic evaluation.
Funding
This research was financially supported by the National Natural Science Foundation of China (NSFC) (Nos. 22002131) and the China Postdoctoral Science Foundation (Grant No 2020 M671963).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| Full name | Abbreviation |
| ammonia | NH3 |
| nitrate | NO3− |
| nitrate reduction reaction | NO3−RR |
| nitrogen | N2 |
| nitrogen reduction reaction | NRR |
| nitrite | NO2− |
| nitrogen monoxide | NO |
| nitrous oxide | N2O |
| dinitrogen dioxide | HN2O2 |
| nitroxyl | HNO |
| hydroxylamine | H2NOH |
| nitrosoamide | NONH2 |
| Renewable Energy to Fuels through Utilization of Energy-dense Liquids | REFUEL |
| Department of Energy | DOE |
| biocatalyzed by nitrate/nitrite reductase | bio-NRA |
| hydrogen evolution reaction | HER |
| adsorbed active hydrogen species | Had |
| ion chromatography | IC |
| ion selective electrode | ISE |
| hydrogen nuclear magnetic resonance spectroscopy | 1H NMR |
| single-atom catalyst | SAC |
| online differential electrochemical mass spectrometry | DEMS |
| X-ray photoelectron spectroscopy | XPS |
| X-ray adsorption spectroscopy | XAS |
| ultraviolet photoelectron spectroscopy | UPS |
| polyallylamine | PA |
References
- Fu, X.; Pedersen, J.B.; Zhou, Y.; Saccoccio, M.; Li, S.; Sažinas, R.; Li, K.; Andersen, S.Z.; Xu, A.; Deissler, N.H.; et al. Continuous-flow electrosynthesis of ammonia by nitrogen reduction and hydrogen oxidation. Science 2023, 379, 707–712. [Google Scholar] [CrossRef] [PubMed]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Salmon, N.; Bañares-Alcántara, R. Green ammonia as a spatial energy vector: A review. Sustain. Energy Fuels 2021, 5, 2814–2839. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Cherepanov, P.V.; Choi, J.; Suryanto, B.H.R.; Hodgetts, R.Y.; Bakker, J.M.; Ferrero Vallana, F.M.; Simonov, A.N. A Roadmap to the Ammonia Economy. Joule 2020, 4, 1186–1205. [Google Scholar] [CrossRef]
- Kandemir, T.; Schuster, M.E.; Senyshyn, A.; Behrens, M.; Schlögl, R. The Haber-Bosch process revisited: On the real structure and stability of “ammonia iron” under working conditions. Angew. Chem. Int. Ed. 2013, 52, 12723–12726. [Google Scholar] [CrossRef]
- Nishina, K. New ammonia demand: Ammonia fuel as a decarbonization tool and a new source of reactive nitrogen. Environ. Res. Lett. 2022, 17, 021003. [Google Scholar] [CrossRef]
- Van der Ham, C.J.; Koper, M.T.; Hetterscheid, D.G. Challenges in reduction of dinitrogen by proton and electron transfer. Chem. Soc. Rev. 2014, 43, 5183–5191. [Google Scholar] [CrossRef]
- Foster, S.L.; Bakovic, S.I.P.; Duda, R.D.; Maheshwari, S.; Milton, R.D.; Minteer, S.D.; Janik, M.J.; Renner, J.N.; Greenlee, L.F. Catalysts for nitrogen reduction to ammonia. Nat. Catal. 2018, 1, 490–500. [Google Scholar] [CrossRef]
- Chen, G.-F.; Cao, X.; Wu, S.; Zeng, X.; Ding, L.-X.; Zhu, M.; Wang, H. Ammonia Electrosynthesis with High Selectivity under Ambient Conditions via a Li+ Incorporation Strategy. J. Am. Chem. Soc. 2017, 139, 9771–9774. [Google Scholar] [CrossRef]
- DOE. Renewable Energy to Fuels through Utilization of Energy-Dense Liquides, Electrochemical Ammonia Conversion; DOE: Washington, DC, USA, 2020. [Google Scholar]
- Suryanto, B.H.R.; Du, H.-L.; Wang, D.; Chen, J.; Simonov, A.N.; MacFarlane, D.R. Challenges and prospects in the catalysis of electroreduction of nitrogen to ammonia. Nat. Catal. 2019, 2, 290–296. [Google Scholar] [CrossRef]
- Long, J.; Chen, S.; Zhang, Y.; Guo, C.; Fu, X.; Deng, D.; Xiao, J. Direct Electrochemical Ammonia Synthesis from Nitric Oxide. Angew. Chem. Int. Ed. 2020, 59, 9711–9718. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Priest, C.; Wang, G.; Wu, G. Restoring the Nitrogen Cycle by Electrochemical Reduction of Nitrate: Progress and Prospects. Small Methods 2020, 4, 2000672. [Google Scholar] [CrossRef]
- Menció, A.; Mas-Pla, J.; Otero, N.; Regàs, O.; Boy-Roura, M.; Puig, R.; Bach, J.; Domènech, C.; Zamorano, M.; Brusi, D.; et al. Nitrate pollution of groundwater; all right…, but nothing else? Sci. Total Environ. 2016, 539, 241–251. [Google Scholar] [CrossRef]
- Yao, F.; Jia, M.; Yang, Q.; Chen, F.; Zhong, Y.; Chen, S.; He, L.; Pi, Z.; Hou, K.; Wang, D.; et al. Highly selective electrochemical nitrate reduction using copper phosphide self-supported copper foam electrode: Performance, mechanism, and application. Water Res. 2021, 193, 116881. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Chen, Z.; Zhang, G.; Chen, W.; Peng, C.; Yang, X.; Zheng, L.; Li, Y.; Ren, X.; Cao, H.; et al. Elucidating the activity, mechanism and application of selective electrosynthesis of ammonia from nitrate on cobalt phosphide. Energy Environ. Sci. 2022, 15, 760–770. [Google Scholar] [CrossRef]
- Sundararajan, M.; Hillier, I.H.; Burton, N.A. Mechanism of Nitrite Reduction at T2Cu Centers: Electronic Structure Calculations of Catalysis by Copper Nitrite Reductase and by Synthetic Model Compounds. J. Chem. Phys. B 2007, 111, 5511–5517. [Google Scholar] [CrossRef]
- Min, B.; Gao, Q.; Yan, Z.; Han, X.; Hosmer, K.; Campbell, A.; Zhu, H. Powering the Remediation of the Nitrogen Cycle: Progress and Perspectives of Electrochemical Nitrate Reduction. Ind. Eng. Chem. Res. 2021, 60, 14635–14650. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Lanzarini-Lopes, M.; Hristovski, K.; Westerhoff, P. Electrocatalytic reduction of nitrate: Fundamentals to full-scale water treatment applications. Appl. Catal. B Environ. 2018, 236, 546–568. [Google Scholar] [CrossRef]
- Dima, G.E.; de Vooys, A.C.A.; Koper, M.T.M. Electrocatalytic reduction of nitrate at low concentration on coinage and transition-metal electrodes in acid solutions. J. Electroanal. Chem. 2003, 554–555, 15–23. [Google Scholar] [CrossRef]
- Katsounaros, I.; Kyriacou, G. Influence of the concentration and the nature of the supporting electrolyte on the electrochemical reduction of nitrate on tin cathode. Electrochim. Acta 2007, 52, 6412–6420. [Google Scholar] [CrossRef]
- Hristovski, K.D.; Markovski, J. Engineering metal (hydr)oxide sorbents for removal of arsenate and similar weak-acid oxyanion contaminants: A critical review with emphasis on factors governing sorption processes. Sci. Total Environ. 2017, 598, 258–271. [Google Scholar] [CrossRef] [PubMed]
- De Groot, M.T.; Koper, M.T.M. The influence of nitrate concentration and acidity on the electrocatalytic reduction of nitrate on platinum. J. Electroanal. Chem. 2004, 562, 81–94. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, C.; Yu, Y.; Lu, S.; Zhang, B. Recent advances in non-noble metal electrocatalysts for nitrate reduction. Chem. Eng. J. 2021, 403, 126269. [Google Scholar] [CrossRef]
- Montesinos, N.; Quici, N.; Destaillats, H.; Litter, M. Nitric oxide emission during the reductive heterogeneous photocatalysis of aqueous nitrate with TiO2. RSC Adv. 2015, 5, 85319–85322. [Google Scholar] [CrossRef]
- Tugaoen, H.O.N.; Garcia-Segura, S.; Hristovski, K.; Westerhoff, P. Challenges in photocatalytic reduction of nitrate as a water treatment technology. Sci. Total Environ. 2017, 599–600, 1524–1551. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.C.P.M.; Weber, M.; Nart, F.C. On the adsorption and reduction of NO3− ions at Au and Pt electrodes studied by in situ FTIR spectroscopy. J. Electroanal. Chem. 1996, 414, 163–170. [Google Scholar] [CrossRef]
- De, D.; Kalu, E.E.; Tarjan, P.P.; Englehardt, J.D. Kinetic Studies of the Electrochemical Treatment of Nitrate and Nitrite Ions on Iridium-Modified Carbon Fiber Electrodes. Chem. Eng. Technol. 2004, 27, 56–64. [Google Scholar] [CrossRef]
- Goldstein, S.; Behar, D.; Rajh, T.; Rabani, J. Nitrite Reduction to Nitrous Oxide and Ammonia by TiO2 Electrons in a Colloid Solution via Consecutive One-Electron Transfer Reactions. J. Phys. Chem. A 2016, 120, 2307–2312. [Google Scholar] [CrossRef]
- Su, J.F.; Ruzybayev, I.; Shah, I.; Huang, C.P. The electrochemical reduction of nitrate over micro-architectured metal electrodes with stainless steel scaffold. Appl. Catal. B Environ. 2016, 180, 199–209. [Google Scholar] [CrossRef]
- De Vooys, A.C.A.; Beltramo, G.L.; van Riet, B.; van Veen, J.A.R.; Koper, M.T.M. Mechanisms of electrochemical reduction and oxidation of nitric oxide. Electrochim. Acta 2004, 49, 1307–1314. [Google Scholar] [CrossRef]
- Yoshioka, T.; Iwase, K.; Nakanishi, S.; Hashimoto, K.; Kamiya, K. Electrocatalytic Reduction of Nitrate to Nitrous Oxide by a Copper-Modified Covalent Triazine Framework. J. Phys. Chem. C 2016, 120, 15729–15734. [Google Scholar] [CrossRef]
- Zheng, J.; Lu, T.; Cotton, T.M.; Chumanov, G. Photoinduced Electrochemical Reduction of Nitrite at an Electrochemically Roughened Silver Surface. J. Chem. Phys. B 1999, 103, 6567–6572. [Google Scholar] [CrossRef]
- Yang, J.; Duca, M.; Schouten, K.J.P.; Koper, M.T.M. Formation of volatile products during nitrate reduction on a Sn-modified Pt electrode in acid solution. J. Electroanal. Chem. 2011, 662, 87–92. [Google Scholar] [CrossRef]
- Duca, M.; Cucarella, M.O.; Rodriguez, P.; Koper, M.T.M. Direct Reduction of Nitrite to N2 on a Pt(100) Electrode in Alkaline Media. J. Am. Chem. Soc. 2010, 132, 18042–18044. [Google Scholar] [CrossRef]
- Duca, M.; Figueiredo, M.C.; Climent, V.; Rodriguez, P.; Feliu, J.M.; Koper, M.T.M. Selective Catalytic Reduction at Quasi-Perfect Pt(100) Domains: A Universal Low-Temperature Pathway from Nitrite to N2. J. Am. Chem. Soc. 2011, 133, 10928–10939. [Google Scholar] [CrossRef]
- Bartberger, M.D.; Liu, W.; Ford, E.; Miranda, K.M.; Switzer, C.; Fukuto, J.M.; Farmer, P.J.; Wink, D.A.; Houk, K.N. The reduction potential of nitric oxide (NO) and its importance to NO biochemistry. Proc. Natl. Acad. Sci. USA 2002, 99, 10958–10963. [Google Scholar] [CrossRef]
- Dutton, A.S.; Fukuto, J.M.; Houk, K.N. Theoretical Reduction Potentials for Nitrogen Oxides from CBS-QB3 Energetics and (C)PCM Solvation Calculations. Inorg. Chem. 2005, 44, 4024–4028. [Google Scholar] [CrossRef]
- Lacasa, E.; Cañizares, P.; Llanos, J.; Rodrigo, M.A. Effect of the cathode material on the removal of nitrates by electrolysis in non-chloride media. J. Hazard. Mater. 2012, 213–214, 478–484. [Google Scholar] [CrossRef]
- Guo, S.; Heck, K.; Kasiraju, S.; Qian, H.; Zhao, Z.; Grabow, L.C.; Miller, J.T.; Wong, M.S. Insights into Nitrate Reduction over Indium-Decorated Palladium Nanoparticle Catalysts. ACS Catal. 2018, 8, 503–515. [Google Scholar] [CrossRef]
- Chaplin, B.P.; Reinhard, M.; Schneider, W.F.; Schüth, C.; Shapley, J.R.; Strathmann, T.J.; Werth, C.J. Critical Review of Pd-Based Catalytic Treatment of Priority Contaminants in Water. Environ. Sci. Technol. 2012, 46, 3655–3670. [Google Scholar] [CrossRef]
- Taguchi, S.; Feliu, J.M. Electrochemical reduction of nitrate on Pt(S)[n(1 1 1) × (1 1 1)] electrodes in perchloric acid solution. Electrochim. Acta 2007, 52, 6023–6033. [Google Scholar] [CrossRef]
- Ghodbane, O.; Sarrazin, M.; Roué, L.; Bélanger, D. Electrochemical Reduction of Nitrate on Pyrolytic Graphite-Supported Cu and Pd–Cu Electrocatalysts. J. Electrochem. Soc. 2008, 155, 117–123. [Google Scholar] [CrossRef]
- Hu, T.; Wang, C.; Wang, M.; Li, C.M.; Guo, C. Theoretical Insights into Superior Nitrate Reduction to Ammonia Performance of Copper Catalysts. ACS Catal. 2021, 11, 14417–14427. [Google Scholar] [CrossRef]
- Andersen, S.Z.; Colic, V.; Yang, S.; Schwalbe, J.A.; Nielander, A.C.; McEnaney, J.M.; Enemark-Rasmussen, K.; Baker, J.G.; Singh, A.R.; Rohr, B.A.; et al. A rigorous electrochemical ammonia synthesis protocol with quantitative isotope measurements. Nature 2019, 570, 504–508. [Google Scholar] [CrossRef]
- Hodgetts, R.Y.; Kiryutin, A.S.; Nichols, P.; Du, H.-L.; Bakker, J.M.; Macfarlane, D.R.; Simonov, A.N. Refining Universal Procedures for Ammonium Quantification via Rapid 1H NMR Analysis for Dinitrogen Reduction Studies. ACS Energy Lett. 2020, 5, 736–741. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, R.; Bian, X.; Zhou, C.; Zhao, Y.; Zhang, S.; Wu, F.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; et al. Ammonia Detection Methods in Photocatalytic and Electrocatalytic Experiments: How to Improve the Reliability of NH3 Production Rates? Adv. Sci. 2019, 6, 1802109. [Google Scholar] [CrossRef]
- Zhou, L.; Boyd, C.E. Comparison of Nessler, phenate, salicylate and ion selective electrode procedures for determination of total ammonia nitrogen in aquaculture. Aquaculture 2016, 450, 187–193. [Google Scholar] [CrossRef]
- Zhu, Y.; Yuan, D.; Lin, H.; Zhou, T.J. Determination of Ammonium in Seawater by Purge-and-Trap and Flow Injection with Fluorescence Detection. Anal. Lett. 2015, 49, 665–675. [Google Scholar] [CrossRef]
- Thomas, D.H.; Rey, M.; Jackson, P.E. Determination of inorganic cations and ammonium in environmental waters by ion chromatography with a high-capacity cation-exchange column. J. Chromatogr. A 2002, 956, 181–186. [Google Scholar] [CrossRef]
- Leduy, A.; Samson, R. Testing of an ammonia ion selective electrode for ammonia nitrogen measurement in the methanogenic sludge. Biotechnol. Lett. 1982, 4, 303–306. [Google Scholar] [CrossRef]
- Liu, J.; Kelley, M.S.; Wu, W.; Banerjee, A.; Douvalis, A.P.; Wu, J.; Zhang, Y.; Schatz, G.C.; Kanatzidis, M.G. Nitrogenase-mimic iron-containing chalcogels for photochemical reduction of dinitrogen to ammonia. Proc. Natl. Acad. Sci. USA 2016, 113, 5530–5535. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhan, G.; Yang, J.; Quan, F.; Mao, C.; Liu, Y.; Wang, B.; Lei, F.; Li, L.; Chan, A.W.M.; et al. Efficient Ammonia Electrosynthesis from Nitrate on Strained Ruthenium Nanoclusters. J. Am. Chem. Soc. 2020, 142, 7036–7046. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Zhang, X.; Cai, W.; Zhao, H.; Zhang, Y.; Sun, Y.; Hu, Z.; Li, S.; Lai, J.; Wang, L. Facet-controlled palladium nanocrystalline for enhanced nitrate reduction towards ammonia. J. Colloid Interface Sci. 2021, 600, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Bagger, A.; Rossmeisl, J. Electrochemical Nitric Oxide Reduction on Metal Surfaces. Angew. Chem. Int. Ed. 2021, 60, 21966–21972. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Li, H.; Xiao, J. The progresses in electrochemical reverse artificial nitrogen cycle. Curr. Opin. Electrochem. 2023, 37, 101179. [Google Scholar] [CrossRef]
- Ko, B.H.; Hasa, B.; Shin, H.; Zhao, Y.; Jiao, F. Electrochemical Reduction of Gaseous Nitrogen Oxides on Transition Metals at Ambient Conditions. J. Am. Chem. Soc. 2022, 144, 1258–1266. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Corma, A. Metal Catalysts for Heterogeneous Catalysis: From Single Atoms to Nanoclusters and Nanoparticles. Chem. Rev. 2018, 118, 4981–5079. [Google Scholar] [CrossRef]
- Fu, X.; Zhao, X.; Hu, X.; He, K.; Yu, Y.; Li, T.; Tu, Q.; Qian, X.; Yue, Q.; Wasielewski, M.R.; et al. Alternative route for electrochemical ammonia synthesis by reduction of nitrate on copper nanosheets. Appl. Mater. Today 2020, 19, 100620. [Google Scholar] [CrossRef]
- Vogler, A. B.C. Gates, L.; Guczi, H. Knözinger (Eds.): Metal Clusters in Catalysis, Vol. 29 aus der Reihe: Studies in Surface Science. Elsevier, Amsterdam, Oxford, New York, Tokyo 1986. 648 Seiten, Preis: Dfl. 195. Ber. Bunsenges. Phys. Chem. 1987, 91, 767–768. [Google Scholar] [CrossRef]
- Yang, J.; Qi, H.; Li, A.; Liu, X.; Yang, X.; Zhang, S.; Zhao, Q.; Jiang, Q.; Su, Y.; Zhang, L.; et al. Potential-Driven Restructuring of Cu Single Atoms to Nanoparticles for Boosting the Electrochemical Reduction of Nitrate to Ammonia. J. Am. Chem. Soc. 2022, 144, 12062–12071. [Google Scholar] [CrossRef]
- Katsounaros, I.; Figueiredo, M.C.; Chen, X.; Calle-Vallejo, F.; Koper, M.T.M. Interconversions of nitrogen-containing species on Pt(100) and Pt(111) electrodes in acidic solutions containing nitrate. Electrochim. Acta 2018, 271, 77–83. [Google Scholar] [CrossRef]
- Pérez-Gallent, E.; Figueiredo, M.C.; Katsounaros, I.; Koper, M.T.M. Electrocatalytic reduction of Nitrate on Copper single crystals in acidic and alkaline solutions. Electrochim. Acta 2017, 227, 77–84. [Google Scholar] [CrossRef]
- Chen, L.-F.; Xie, A.-Y.; Lou, Y.-Y.; Tian, N.; Zhou, Z.-Y.; Sun, S.-G. Electrochemical synthesis of Tetrahexahedral Cu nanocrystals with high-index facets for efficient nitrate electroreduction. J. Electroanal. Chem. 2022, 907, 116022. [Google Scholar] [CrossRef]
- Hu, Q.; Qin, Y.; Wang, X.; Wang, Z.; Huang, X.; Zheng, H.; Gao, K.; Yang, H.; Zhang, P.; Shao, M.; et al. Reaction intermediate-mediated electrocatalyst synthesis favors specified facet and defect exposure for efficient nitrate–ammonia conversion. Energy Environ. Sci. 2021, 14, 4989–4997. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, M.; Ren, K.; Ren, T.; Liu, M.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Atomic defects in pothole-rich two-dimensional copper nanoplates triggering enhanced electrocatalytic selective nitrate-to-ammonia transformation. J. Mater. Chem. A 2021, 9, 16411–16417. [Google Scholar] [CrossRef]
- Sun, T.; Zhang, G.; Xu, D.; Lian, X.; Li, H.; Chen, W.; Su, C. Defect chemistry in 2D materials for electrocatalysis. Mater. Today Energy 2019, 12, 215–238. [Google Scholar] [CrossRef]
- Shi, H.; Li, C.; Wang, L.; Wang, W.; Meng, X. Selective reduction of nitrate into N2 by novel Z-scheme NH2-MIL-101(Fe)/BiVO4 heterojunction with enhanced photocatalytic activity. J. Hazard. Mater. 2022, 424, 127711. [Google Scholar] [CrossRef]
- Zhao, J.; Li, N.; Yu, R.; Zhao, Z.; Nan, J. Magnetic field enhanced denitrification in nitrate and ammonia contaminated water under 3D/2D Mn2O3/g-C3N4 photocatalysis. Chem. Eng. J. 2018, 349, 530–538. [Google Scholar] [CrossRef]
- Adamu, H.; McCue, A.J.; Taylor, R.S.F.; Manyar, H.G.; Anderson, J.A. Simultaneous photocatalytic removal of nitrate and oxalic acid over Cu2O/TiO2 and Cu2O/TiO2-AC composites. Appl. Catal. B Environ. 2017, 217, 181–191. [Google Scholar] [CrossRef]
- Kumar, A.; Lee, J.; Kim, M.G.; Debnath, B.; Liu, X.; Hwang, Y.; Wang, Y.; Shao, X.; Jadhav, A.R.; Liu, Y.; et al. Efficient Nitrate Conversion to Ammonia on f-Block Single-Atom/Metal Oxide Heterostructure via Local Electron-Deficiency Modulation. ACS Nano 2022, 16, 15297–15309. [Google Scholar] [CrossRef]
- Yu, T.; Liu, L.; Yang, F. Heterojunction between anodic TiO2/g-C3N4 and cathodic WO3/W nano-catalysts for coupled pollutant removal in a self-biased system. Chin. J. Catal. 2017, 38, 270–277. [Google Scholar] [CrossRef]
- Deng, Z.; Ma, C.; Fan, X.; Li, Z.; Luo, Y.; Sun, S.; Zheng, D.; Liu, Q.; Du, J.; Lu, Q.; et al. Construction of CoP/TiO2 nanoarray for enhanced electrochemical nitrate reduction to ammonia. Mater. Today Phys. 2022, 28, 100854. [Google Scholar] [CrossRef]
- Liu, S.; Cui, L.; Yin, S.; Ren, H.; Wang, Z.; Xu, Y.; Li, X.; Wang, L.; Wang, H. Heterointerface-triggered electronic structure reformation: Pd/CuO nano-olives motivate nitrite electroreduction to ammonia. Appl. Catal. B Environ. 2022, 319, 121876. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Jia, R.; Yu, Y.; Zhang, B. Unveiling the Activity Origin of a Copper-based Electrocatalyst for Selective Nitrate Reduction to Ammonia. Angew. Chem. Int. Ed. 2020, 59, 5350–5354. [Google Scholar] [CrossRef]
- Chen, G.-F.; Yuan, Y.; Jiang, H.; Ren, S.-Y.; Ding, L.-X.; Ma, L.; Wu, T.; Lu, J.; Wang, H. Electrochemical reduction of nitrate to ammonia via direct eight-electron transfer using a copper–molecular solid catalyst. Nat. Energy 2020, 5, 605–613. [Google Scholar] [CrossRef]
- Reyter, D.; Reyter, D.; Chamoulaud, G.; Chamoulaud, G.; Bélanger, D.; Roué, L. Electrocatalytic reduction of nitrate on copper electrodes prepared by high-energy ball milling. J. Electroanal. Chem. 2006, 596, 13–24. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Zhang, Z.; Mo, Z.; Wang, C.; Gao, S. Flower-like open-structured polycrystalline copper with synergistic multi-crystal plane for efficient electrocatalytic reduction of nitrate to ammonia. Nano Energy 2022, 97, 107124. [Google Scholar] [CrossRef]
- Yuan, J.; Xing, Z.; Tang, Y.; Liu, C. Tuning the Oxidation State of Cu Electrodes for Selective Electrosynthesis of Ammonia from Nitrate. ACS Appl. Mater. Interfaces 2021, 13, 52469–52478. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Jia, X.; He, Y.; Zhang, H.; Zhou, X.; Zhang, H.; Zhang, S.; Dong, Y.; Hu, X.; Kuklin, A.V.; et al. Two-dimensional BCN matrix inlaid with single-atom-Cu driven electrochemical nitrate reduction reaction to achieve sustainable industrial-grade production of ammonia. Appl. Mater. Today 2021, 25, 101206. [Google Scholar] [CrossRef]
- Qin, J.; Chen, L.; Wu, K.; Wang, X.; Zhao, Q.; Li, L.; Liu, B.; Ye, Z. Electrochemical Synthesis of Ammonium from Nitrates via Surface Engineering in Cu2O(100) Facets. ACS Appl. Energy Mater. 2022, 5, 71–76. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, A.; Wang, Z.; Huang, L.; Li, J.; Li, F.; Wicks, J.; Luo, M.; Nam, D.H.; Tan, C.S.; et al. Enhanced Nitrate-to-Ammonia Activity on Copper-Nickel Alloys via Tuning of Intermediate Adsorption. J. Am. Chem. Soc. 2020, 142, 5702–5708. [Google Scholar] [CrossRef]
- Ge, Z.-X.; Wang, T.-J.; Ding, Y.; Yin, S.-B.; Li, F.-M.; Chen, P.; Chen, Y. Interfacial Engineering Enhances the Electroactivity of Frame-Like Concave RhCu Bimetallic Nanocubes for Nitrate Reduction. Adv. Energy Mater. 2022, 12, 2103916. [Google Scholar] [CrossRef]
- Liu, J.-X.; Richards, D.; Singh, N.; Goldsmith, B.R. Activity and Selectivity Trends in Electrocatalytic Nitrate Reduction on Transition Metals. ACS Catal. 2019, 9, 7052–7064. [Google Scholar] [CrossRef]
- Barrabés, N.; Just, J.; Dafinov, A.; Medina, F.; Fierro, J.L.G.; Sueiras, J.E.; Salagre, P.; Cesteros, Y. Catalytic reduction of nitrate on Pt-Cu and Pd-Cu on active carbon using continuous reactor: The effect of copper nanoparticles. Appl. Catal. B Environ. 2006, 62, 77–85. [Google Scholar] [CrossRef]
- Zhang, Q.; Ding, L.; Cui, H.; Zhai, J.; Wei, Z.; Li, Q. Electrodeposition of Cu-Pd alloys onto electrophoretic deposited carbon nanotubes for nitrate electroreduction. Appl. Surf. Sci. 2014, 308, 113–120. [Google Scholar] [CrossRef]
- Lei, X.; Liu, F.; Li, M.; Ma, X.; Wang, X.; Zhang, H. Fabrication and characterization of a Cu-Pd-TNPs polymetallic nanoelectrode for electrochemically removing nitrate from groundwater. Chemosphere 2018, 212, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-J.; Wu, Z.-L.; Lin, C.-Y.; Huang, Y.-H.; Huang, C.-P. Manipulating the crystalline morphology and facet orientation of copper and copper-palladium nanocatalysts supported on stainless steel mesh with the aid of cationic surfactant to improve the electrochemical reduction of nitrate and N2 selectivity. Appl. Catal. B Environ. 2020, 273, 119053. [Google Scholar] [CrossRef]
- Kerkeni, S.; Lamy-Pitara, E.; Barbier, J. Copper–platinum catalysts prepared and characterized by electrochemical methods for the reduction of nitrate and nitrite. Catal. Today 2002, 75, 35–42. [Google Scholar] [CrossRef]
- Wang, J.; Teng, W.; Ling, L.; Fan, J.; Zhang, W.x.; Deng, Z.-l. Nanodenitrification with bimetallic nanoparticles confined in N-doped mesoporous carbon. Environ. Sci. Nano 2020, 7, 1496–1506. [Google Scholar] [CrossRef]
- Liu, H.; Lang, X.; Zhu, C.; Timoshenko, J.; Rüscher, M.; Bai, L.; Guijarro, N.; Yin, H.; Peng, Y.; Li, J.; et al. Efficient Electrochemical Nitrate Reduction to Ammonia with Copper-Supported Rhodium Cluster and Single-Atom Catalysts. Angew. Chem. Int. Ed. 2022, 61, e202202556. [Google Scholar] [CrossRef]
- He, W.; Zhang, J.; Dieckhofer, S.; Varhade, S.; Brix, A.C.; Lielpetere, A.; Seisel, S.; Junqueira, J.R.C.; Schuhmann, W. Splicing the active phases of copper/cobalt-based catalysts achieves high-rate tandem electroreduction of nitrate to ammonia. Nat. Commun. 2022, 13, 1129. [Google Scholar] [CrossRef]
- Mattarozzi, L.; Cattarin, S.; Comisso, N.; Gambirasi, A.; Guerriero, P.; Musiani, M.; Vázquez-Gómez, L.; Verlato, E. Hydrogen evolution assisted electrodeposition of porous Cu-Ni alloy electrodes and their use for nitrate reduction in alkali. Electrochim. Acta 2014, 140, 337–344. [Google Scholar] [CrossRef]
- Mattarozzi, L.; Cattarin, S.; Comisso, N.; Gerbasi, R.; Guerriero, P.; Musiani, M.; Vázquez-Gómez, L.; Verlato, E. Electrodeposition of Compact and Porous Cu-Zn Alloy Electrodes and Their Use in the Cathodic Reduction of Nitrate. J. Electrochem. Soc. 2015, 162, D236. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Wang, W.; Yin, L.; Crittenden, J.C. Electrocatalytic nitrate reduction to ammonia on defective Au1Cu (111) single-atom alloys. Appl. Catal. B Environ. 2022, 310, 121346. [Google Scholar] [CrossRef]
- Xu, Y.; Ren, K.; Ren, T.; Wang, M.; Liu, M.; Wang, Z.; Li, X.; Wang, L.; Wang, H. Cooperativity of Cu and Pd active sites in CuPd aerogels enhances nitrate electroreduction to ammonia. Chem. Commun. 2021, 57, 7525–7528. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Chen, Z.; Xiong, S.; Chen, J.; Wang, C.; Wang, R.; Kuwahara, Y.; Luo, J.; Yamashita, H.; Peng, Y.; et al. Alloying effect-induced electron polarization drives nitrate electroreduction to ammonia. Chem. Catal. 2021, 1, 1088–1103. [Google Scholar] [CrossRef]
- Fang, J.-Y.; Zheng, Q.-Z.; Lou, Y.-Y.; Zhao, K.-M.; Hu, S.-N.; Li, G.; Akdim, O.; Huang, X.-Y.; Sun, S.-G. Ampere-level current density ammonia electrochemical synthesis using CuCo nanosheets simulating nitrite reductase bifunctional nature. Nat. Commun. 2022, 13, 7899. [Google Scholar] [CrossRef] [PubMed]
- Butcher, D.P.; Gewirth, A.A. Nitrate reduction pathways on Cu single crystal surfaces: Effect of oxide and Cl−. Nano Energy 2016, 29, 457–465. [Google Scholar] [CrossRef]
- Bae, S.-E.; Stewart, K.L.; Gewirth, A.A. Nitrate Adsorption and Reduction on Cu(100) in Acidic Solution. J. Am. Chem. Soc. 2007, 129, 10171–10180. [Google Scholar] [CrossRef]
- Magnussen, O.M. Ordered Anion Adlayers on Metal Electrode Surfaces. Chem. Rev. 2002, 102, 679–726. [Google Scholar] [CrossRef]
- Kleinert, M.; Cuesta, A.; Kibler, L.A.; Kolb, D.M. In-situ observation of an ordered sulfate adlayer on Au(100) electrodes. Surf. Sci. 1999, 430, L521–L526. [Google Scholar] [CrossRef]
- Li, W.; Xiao, C.; Zhao, Y.; Zhao, Q.; Fan, R.; Xue, J. Electrochemical Reduction of High-Concentrated Nitrate Using Ti/TiO2 Nanotube Array Anode and Fe Cathode in Dual-Chamber Cell. Catal. Lett. 2016, 146, 2585–2595. [Google Scholar] [CrossRef]
- Zhang, C.; He, D.; Ma, J.; Waite, T.D. Active chlorine mediated ammonia oxidation revisited: Reaction mechanism, kinetic modelling and implications. Water Res. 2018, 145, 220–230. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).