Author Contributions
Conceptualization, S.H., Y.S. and J.W.; methodology, S.H., Y.S. and J.W.; investigation, Y.S. and D.Z.; validation, D.Z.; resources, J.W. and Q.W.; data curation, Y.S., S.H. and J.W.; writing—original draft preparation, Y.S., S.H. and J.W.; writing—review and editing, J.W., D.Z., Y.S., Q.W. and S.H.; visualization, Y.S. and S.H.; supervision, J.W. and Q.W.; project administration, J.W.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
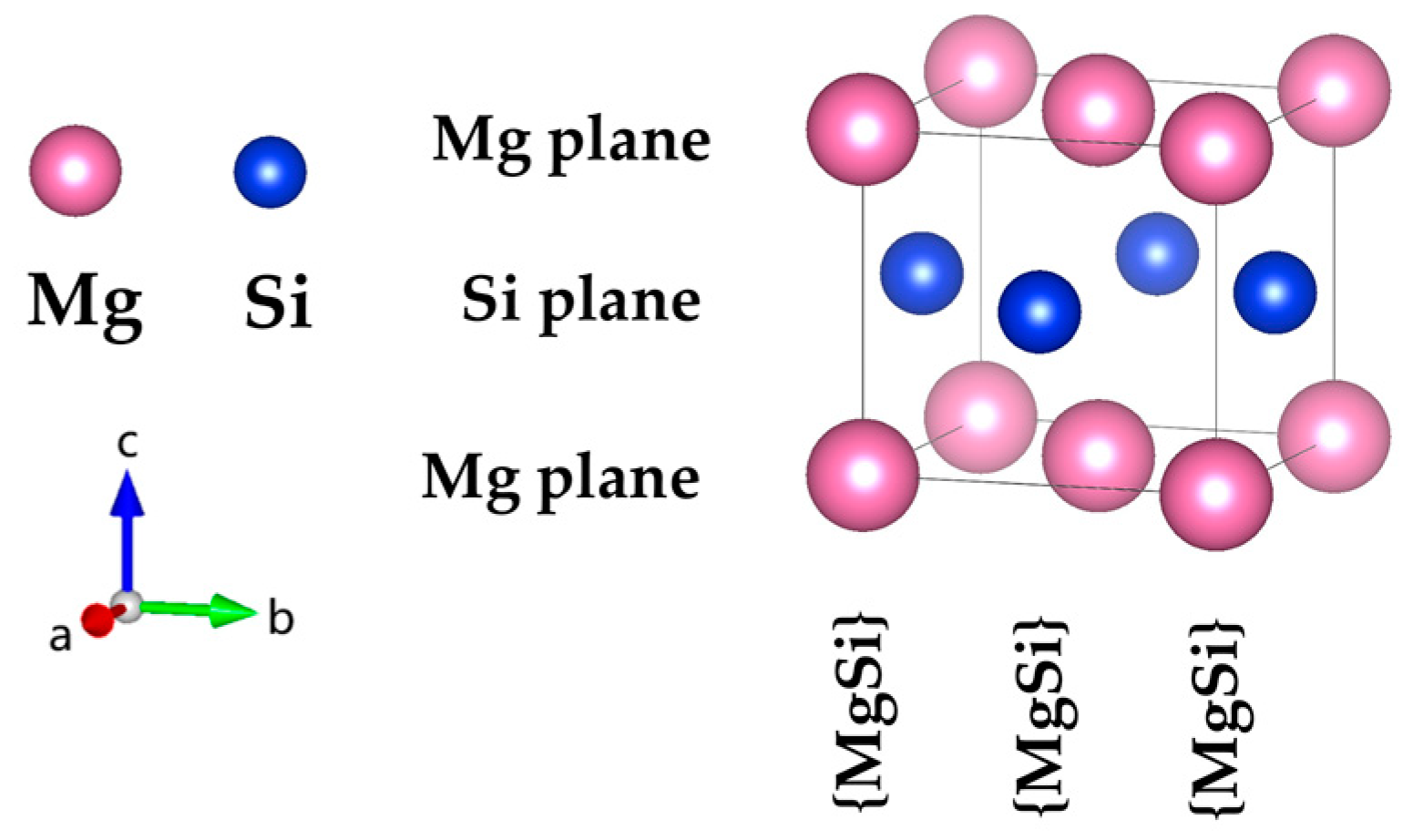
Figure 1.
The atomic structure model of the fcc-type Mg
1Si
1 precipitation in the GP zones of Al-Mg-Si reported by van Huis et al. [
28] and Matsuda et al. [
30]. (The variables a, b, and c represent the
x,
y, and
z axes, respectively, while the arrows indicate the positive directions.).
Figure 1.
The atomic structure model of the fcc-type Mg
1Si
1 precipitation in the GP zones of Al-Mg-Si reported by van Huis et al. [
28] and Matsuda et al. [
30]. (The variables a, b, and c represent the
x,
y, and
z axes, respectively, while the arrows indicate the positive directions.).
Figure 2.
Atomic models of GP zones in Al-Mg-Si alloys with different Mg/Si/Mg cell numbers m. (a) Mg2Si1Al1, (b) Mg2Si1Al3 and (c) Mg2Si1Al5 are one Mg/Si/Mg unit cell with different solute atomic concentrations, (d) Mg3Si2Al3, (e) Mg3Si2Al5, and (f) Mg3Si2Al7 are two Mg/Si/Mg unit cells with different solute atomic concentrations, m Mg/Si/Mg unit cells and so on, the pink plane represents the {MgSi} (100) atomic plane. (The variables a, b, and c represent the x, y, and z axes, respectively, while the arrows indicate the positive directions.).
Figure 2.
Atomic models of GP zones in Al-Mg-Si alloys with different Mg/Si/Mg cell numbers m. (a) Mg2Si1Al1, (b) Mg2Si1Al3 and (c) Mg2Si1Al5 are one Mg/Si/Mg unit cell with different solute atomic concentrations, (d) Mg3Si2Al3, (e) Mg3Si2Al5, and (f) Mg3Si2Al7 are two Mg/Si/Mg unit cells with different solute atomic concentrations, m Mg/Si/Mg unit cells and so on, the pink plane represents the {MgSi} (100) atomic plane. (The variables a, b, and c represent the x, y, and z axes, respectively, while the arrows indicate the positive directions.).
Figure 3.
Atomic models of GP zones with different {MgSi} (100) layer numbers n in Al-Mg-Si alloys. (a) {MgSi}1Al2, (b) {MgSi}1Al6, and (c) {MgSi}1Al10 are monolayer {MgSi} layers with different solute atomic concentrations (n = 1), (d) {MgSi}2Al12, (e) {MgSi}2Al8, and (f) {MgSi}2Al4 are bilayer {MgSi} layers with different solute atomic concentrations (n = 2), n layers {MgSi} and so on, the pink plane represents the {MgSi} (100) atomic plane. (The variables a, b, and c represent the x, y, and z axes, respectively, while the arrows indicate the positive directions.)
Figure 3.
Atomic models of GP zones with different {MgSi} (100) layer numbers n in Al-Mg-Si alloys. (a) {MgSi}1Al2, (b) {MgSi}1Al6, and (c) {MgSi}1Al10 are monolayer {MgSi} layers with different solute atomic concentrations (n = 1), (d) {MgSi}2Al12, (e) {MgSi}2Al8, and (f) {MgSi}2Al4 are bilayer {MgSi} layers with different solute atomic concentrations (n = 2), n layers {MgSi} and so on, the pink plane represents the {MgSi} (100) atomic plane. (The variables a, b, and c represent the x, y, and z axes, respectively, while the arrows indicate the positive directions.)
Figure 4.
(a) Atomic model for calculation of point-defect occupancy propensity. (b) Atomic model of interaction between vacancies within GP zones and point defects. Site 1, 2, 3, 4, and B indicates that the Cu atom/Vac incorporates in the Si layer, Mg layer, Al layer nearest to the GP zones, Al layer next nearest to the GP zones, and Al matrix away from the GP zones, respectively. (The variables a, b, and c represent the x, y, and z axes, respectively, while the arrows indicate the positive directions.)
Figure 4.
(a) Atomic model for calculation of point-defect occupancy propensity. (b) Atomic model of interaction between vacancies within GP zones and point defects. Site 1, 2, 3, 4, and B indicates that the Cu atom/Vac incorporates in the Si layer, Mg layer, Al layer nearest to the GP zones, Al layer next nearest to the GP zones, and Al matrix away from the GP zones, respectively. (The variables a, b, and c represent the x, y, and z axes, respectively, while the arrows indicate the positive directions.)
Figure 5.
The relationship between solute atom concentration and formation enthalpy in the atomic configuration of GP zones corresponding to different Mg/Si/Mg unit cell numbers m.
Figure 5.
The relationship between solute atom concentration and formation enthalpy in the atomic configuration of GP zones corresponding to different Mg/Si/Mg unit cell numbers m.
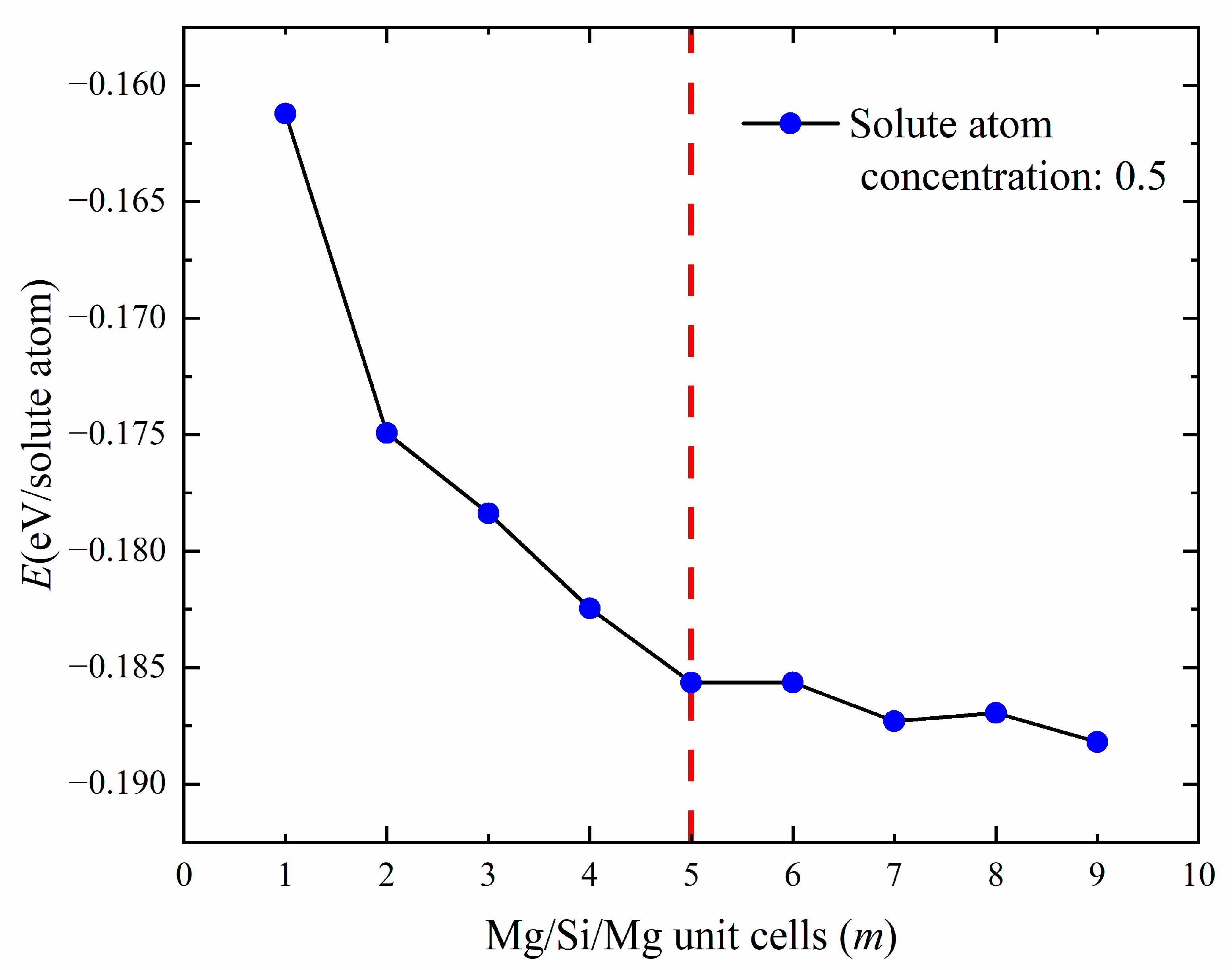
Figure 6.
The line graph of the relationship between the number of Mg/Si/Mg unit cells m and the formation enthalpy (eV/solute atom) when the solute atom concentration is 0.5.
Figure 6.
The line graph of the relationship between the number of Mg/Si/Mg unit cells m and the formation enthalpy (eV/solute atom) when the solute atom concentration is 0.5.
Figure 7.
The change rate of interplanar spacing along the (001) direction of GP zones in Al-Mg-Si alloys.
Figure 7.
The change rate of interplanar spacing along the (001) direction of GP zones in Al-Mg-Si alloys.
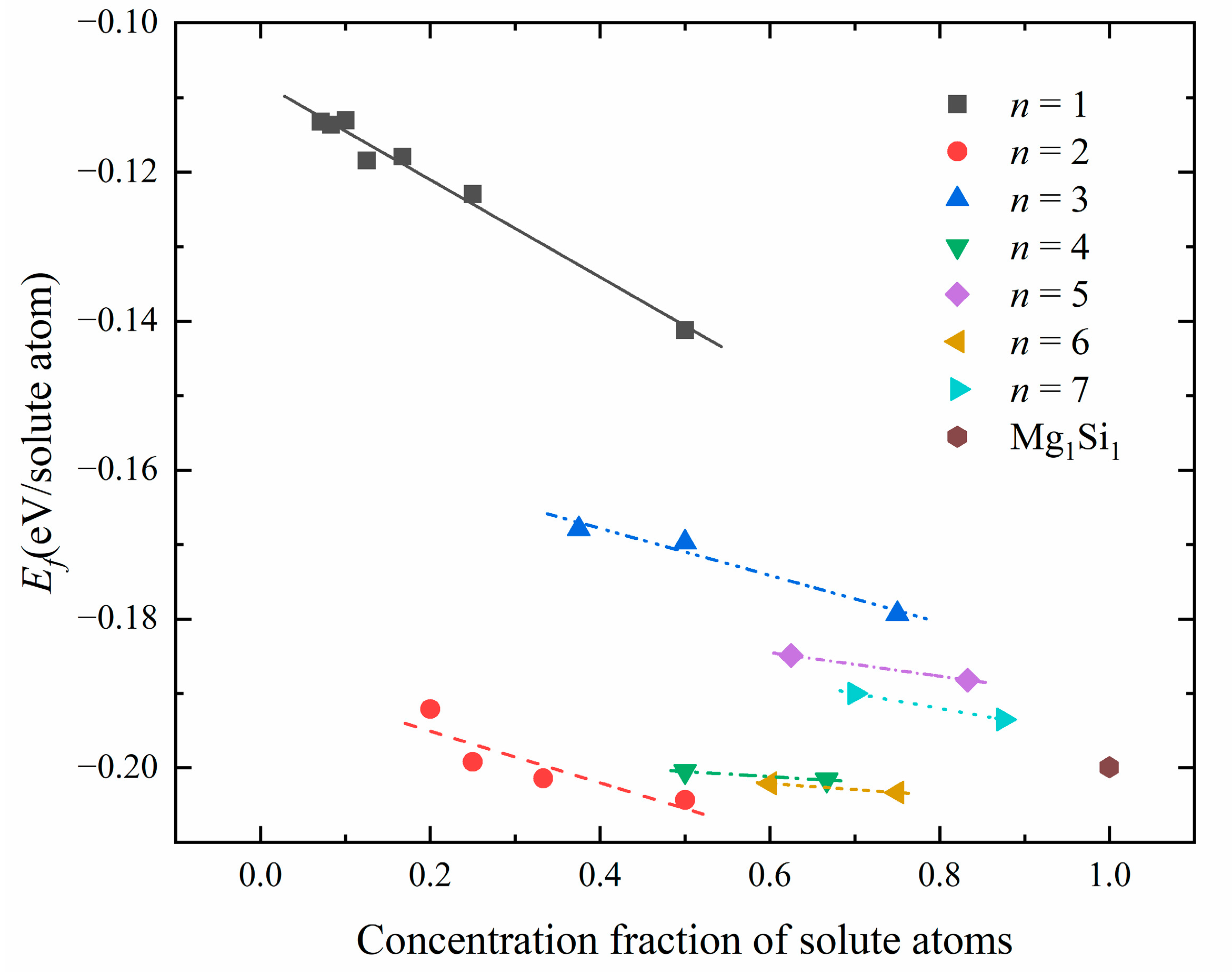
Figure 8.
The relationship between solute atom concentration and formation enthalpy in atomic configurations corresponding to different number n of {MgSi} layers.
Figure 8.
The relationship between solute atom concentration and formation enthalpy in atomic configurations corresponding to different number n of {MgSi} layers.
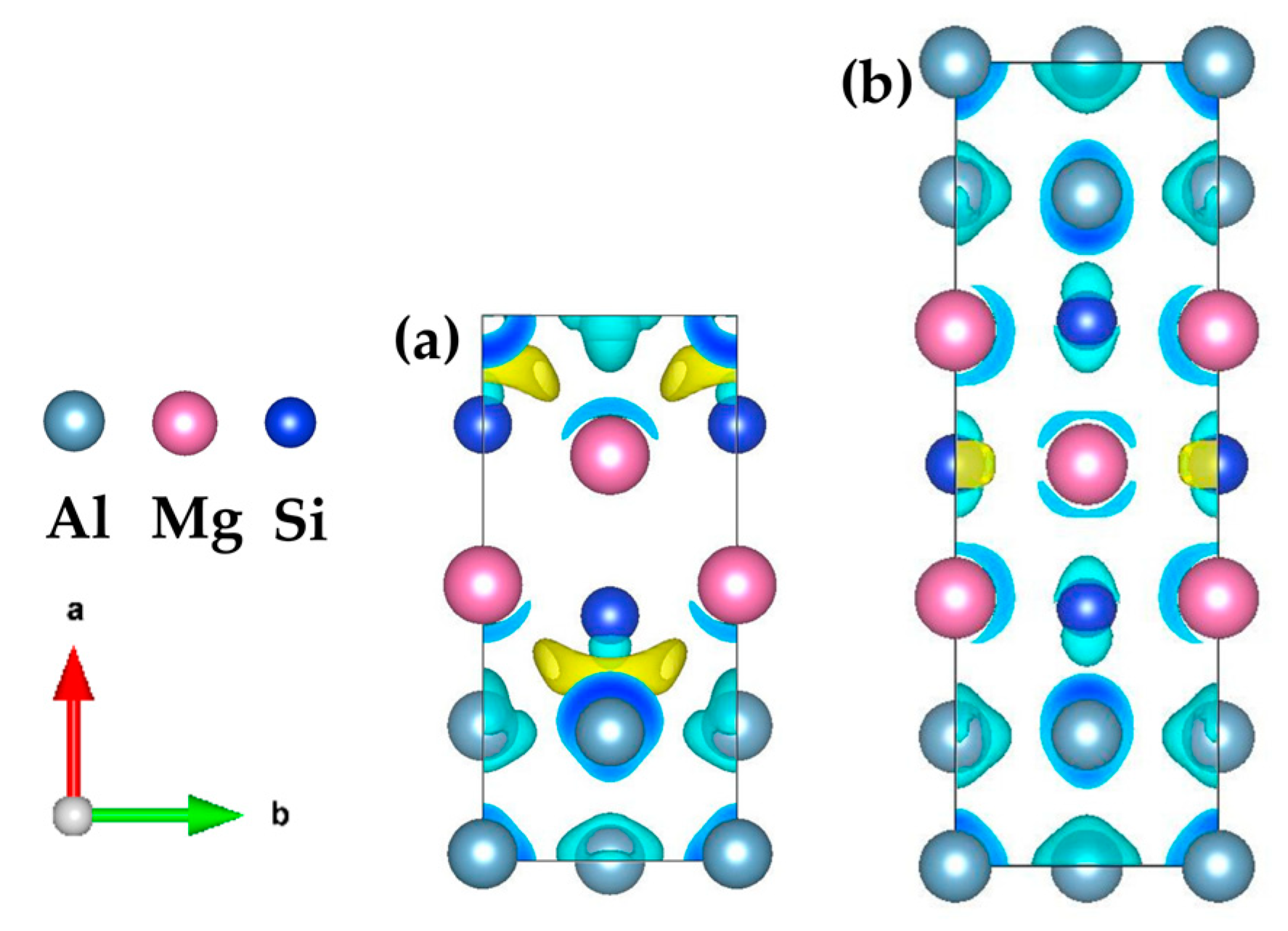
Figure 9.
Three-dimensional charge-density difference map of (a) {MgSi}2Al4 and (b) {MgSi}3Al6, with an isosurface value of 0.0055 e/Å3. (The variables a, b represent the x, y axes, respectively, while the arrows indicate the positive directions.)
Figure 9.
Three-dimensional charge-density difference map of (a) {MgSi}2Al4 and (b) {MgSi}3Al6, with an isosurface value of 0.0055 e/Å3. (The variables a, b represent the x, y axes, respectively, while the arrows indicate the positive directions.)
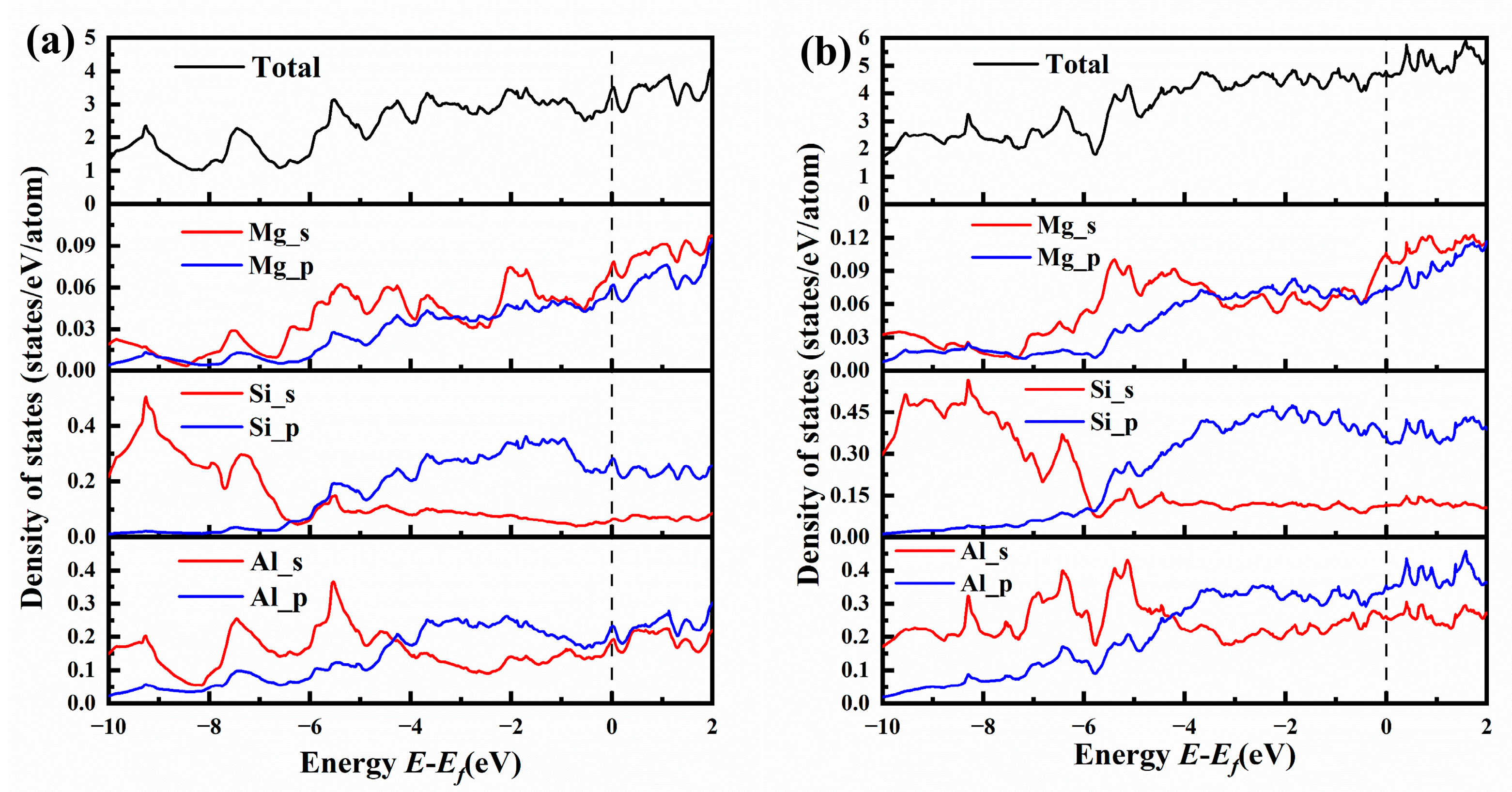
Figure 10.
The total and partial electronic density of states (TDOSs and PDOSs) for (a) {MgSi}2Al4 and (b) {MgSi}3Al6.
Figure 10.
The total and partial electronic density of states (TDOSs and PDOSs) for (a) {MgSi}2Al4 and (b) {MgSi}3Al6.
Figure 11.
The most energetically favorable {MgSi}2Al4 configuration in the calculated GP-zones configuration and the atom model of Cu atom replacing other atoms. (a) The {MgSi}2Al4 configuration, (b) the 2 × 1 × 3 supercell of {MgSi}2Al4, and (c) the atom model in which one Cu atom replaces Al, Mg, and Si atoms, respectively. (The variables a, b, and c represent the x, y, and z axes, respectively, while the arrows indicate the positive directions.)
Figure 11.
The most energetically favorable {MgSi}2Al4 configuration in the calculated GP-zones configuration and the atom model of Cu atom replacing other atoms. (a) The {MgSi}2Al4 configuration, (b) the 2 × 1 × 3 supercell of {MgSi}2Al4, and (c) the atom model in which one Cu atom replaces Al, Mg, and Si atoms, respectively. (The variables a, b, and c represent the x, y, and z axes, respectively, while the arrows indicate the positive directions.)
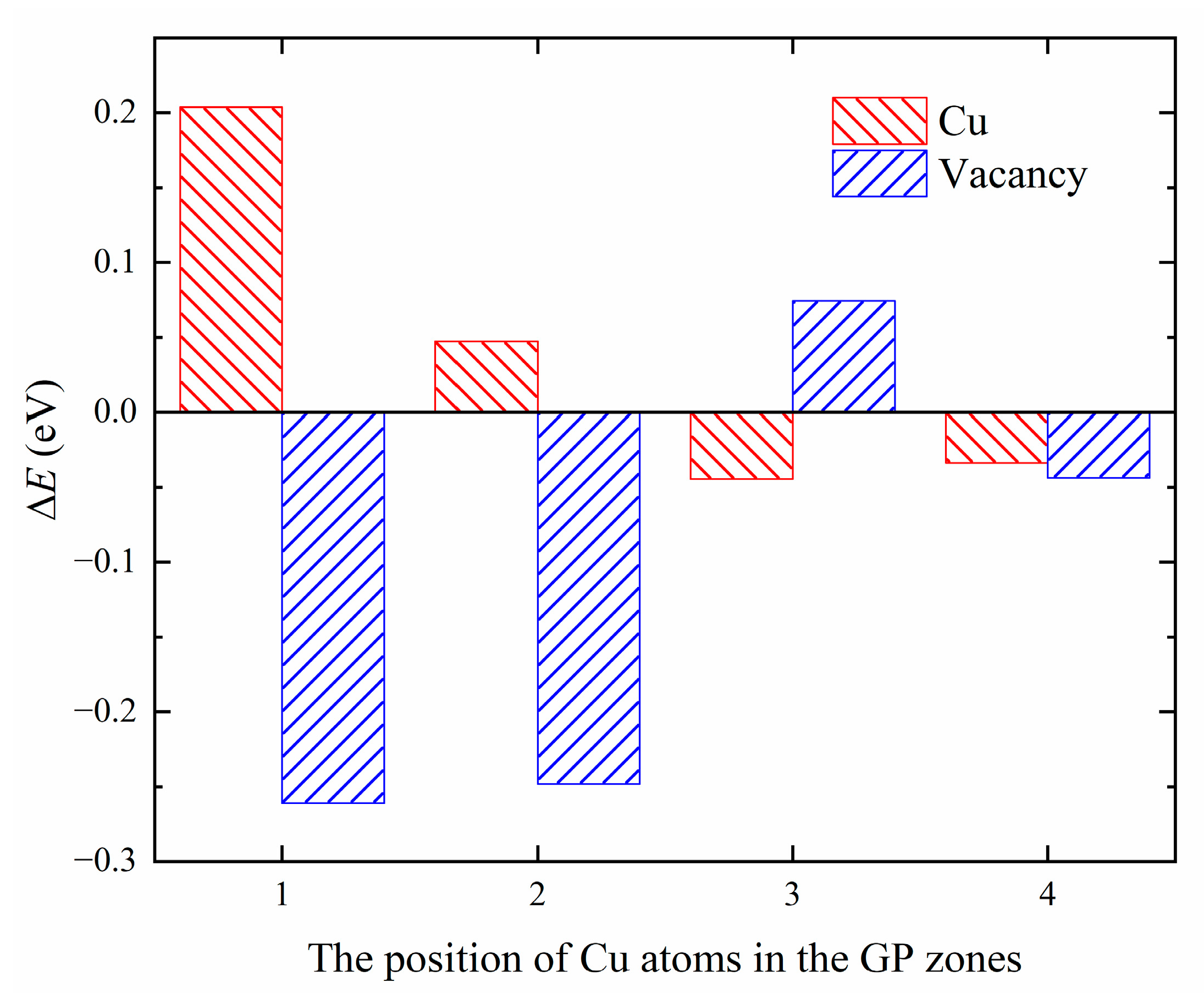
Figure 12.
The energy difference of Cu/Vac defects at different positions.
Figure 12.
The energy difference of Cu/Vac defects at different positions.
Table 1.
The number m of the Mg/Si/Mg unit cell, solute atom concentration, and formation enthalpy corresponding to different atomic configurations in GP zones.
Table 1.
The number m of the Mg/Si/Mg unit cell, solute atom concentration, and formation enthalpy corresponding to different atomic configurations in GP zones.
| Configuration | Mg/Si/Mg Unit Cell Number m | xsolute | E (eV/Solute Atom) |
|---|
| Mg1Si1 | — | 1.000 | −0.1999 |
| Mg2Si1Al1 | 1 | 0.750 | −0.1779 |
| Mg2Si1Al3 | 1 | 0.500 | −0.1612 |
| Mg2Si1Al5 | 1 | 0.375 | −0.1571 |
| Mg3Si2Al3 | 2 | 0.625 | −0.1767 |
| Mg3Si2Al5 | 2 | 0.500 | −0.1749 |
| Mg3Si2Al7 | 2 | 0.417 | −0.1707 |
| Mg4Si3Al1 | 3 | 0.875 | −0.1903 |
| Mg4Si3Al3 | 3 | 0.700 | −0.1840 |
| Mg4Si3Al5 | 3 | 0.583 | −0.1806 |
| Mg4Si3Al7 | 3 | 0.500 | −0.1784 |
| Mg5Si4Al1 | 4 | 0.900 | −0.1921 |
| Mg5Si4Al3 | 4 | 0.750 | −0.1868 |
| Mg5Si4Al5 | 4 | 0.649 | −0.1858 |
| Mg5Si4Al9 | 4 | 0.500 | −0.1825 |
| Mg6Si5Al1 | 5 | 0.917 | −0.1937 |
| Mg6Si5Al3 | 5 | 0.786 | −0.1901 |
| Mg6Si5Al5 | 5 | 0.688 | −0.1873 |
| Mg6Si5Al11 | 5 | 0.500 | −0.1856 |
| Mg7Si6Al1 | 6 | 0.929 | −0.1947 |
| Mg7Si6Al3 | 6 | 0.813 | −0.1912 |
| Mg7Si6Al13 | 6 | 0.500 | −0.1856 |
| Mg8Si7Al1 | 7 | 0.938 | −0.1955 |
| Mg8Si7Al15 | 7 | 0.500 | −0.1873 |
| Mg9Si8Al17 | 8 | 0.500 | −0.1870 |
| Mg10Si9Al19 | 9 | 0.500 | −0.1882 |
Table 2.
Changes of lattice constants before and after Mg6Si5Al11 atomic configuration relaxed in Al-Mg-Si alloys.
Table 2.
Changes of lattice constants before and after Mg6Si5Al11 atomic configuration relaxed in Al-Mg-Si alloys.
| Mg6Si5Al11 | a (Å) | b (Å) | c (Å) | V (Å3) |
|---|
| Initial | 4.043 | 4.043 | 44.473 | 726.949 |
| Relaxed | 4.141 | 4.141 | 43.917 | 752.998 |
| Rate of change | 2.42% | 2.42% | −1.25% | 3.58% |
Table 3.
Changes of lattice constants before and after GP zones relaxed in Al-Mg-Si alloys.
Table 3.
Changes of lattice constants before and after GP zones relaxed in Al-Mg-Si alloys.
| {MgSi} Atomic Layer | a (Å) | b (Å) | c (Å) | V (Å3) |
|---|
| Initial | 4.043 | 4.043 | 20.215 | 330.431 |
| Relaxed | 4.141 | 4.141 | 19.972 | 342.477 |
| Rate of change | 2.42% | 2.42% | −1.20% | 3.65% |
Table 4.
Lattice mismatch information along the (001) direction in the GP zones of Al-Mg-Si alloys.
Table 4.
Lattice mismatch information along the (001) direction in the GP zones of Al-Mg-Si alloys.
| The Number of (001) Plane | Variation of (001) Interplanar Spacing | Change Rate of (001) Interplanar Spacing | Atomic-Layer Displacement |
|---|
| 1 | −0.02176 | −1.08% | −1.08% |
| 2 | −0.01693 | −0.84% | −0.96% |
| 3 | −0.01561 | −0.77% | −0.90% |
| 4 | −0.02923 | −1.45% | −1.03% |
| 5 | −0.03801 | −1.88% | −1.20% |
| 6 | 0.16225 | 8.03% | 0.34% |
| 7 | −0.05865 | −2.90% | −0.13% |
| 8 | −0.07139 | −3.53% | −0.55% |
| 9 | −0.05996 | −2.97% | −0.82% |
| 10 | −0.07402 | −3.66% | −1.10% |
| 11 | −0.05469 | −2.71% | −1.25% |
Table 5.
The number n of {MgSi} layers, solute atom concentration, and formation enthalpy corresponding to different atomic configurations in GP zones.
Table 5.
The number n of {MgSi} layers, solute atom concentration, and formation enthalpy corresponding to different atomic configurations in GP zones.
| Configuration | Number of Layers (n) | xsolute | E (eV/Solute Atom) |
|---|
| {MgSi}Al2 | 1 | 0.500 | −0.1412 |
| {MgSi}Al6 | 1 | 0.250 | −0.1229 |
| {MgSi}Al10 | 1 | 0.167 | −0.1179 |
| {MgSi}Al14 | 1 | 0.125 | −0.1184 |
| {MgSi}Al18 | 1 | 0.100 | −0.1130 |
| {MgSi}Al22 | 1 | 0.083 | −0.1136 |
| {MgSi}Al26 | 1 | 0.071 | −0.1132 |
| {MgSi}2Al4 | 2 | 0.500 | −0.2043 |
| {MgSi}2Al8 | 2 | 0.333 | −0.2014 |
| {MgSi}2Al12 | 2 | 0.250 | −0.1992 |
| {MgSi}2Al16 | 2 | 0.200 | −0.1921 |
| {MgSi}3Al2 | 3 | 0.750 | −0.1793 |
| {MgSi}3Al6 | 3 | 0.500 | −0.1697 |
| {MgSi}3Al10 | 3 | 0.375 | −0.1679 |
| {MgSi}4Al4 | 4 | 0.667 | −0.2016 |
| {MgSi}4Al8 | 4 | 0.500 | −0.2005 |
| {MgSi}5Al2 | 5 | 0.833 | −0.1882 |
| {MgSi}5Al6 | 5 | 0.625 | −0.1849 |
| {MgSi}6Al4 | 6 | 0.750 | −0.2033 |
| {MgSi}6Al8 | 6 | 0.600 | −0.2021 |
| {MgSi}7Al2 | 7 | 0.875 | −0.1935 |
| {MgSi}7Al6 | 7 | 0.700 | −0.1900 |
Table 6.
The formation enthalpy of the configurations corresponding to the substitution of different atoms by Cu atoms in the GP zones in Al-Mg-Si(-Cu) alloys.
Table 6.
The formation enthalpy of the configurations corresponding to the substitution of different atoms by Cu atoms in the GP zones in Al-Mg-Si(-Cu) alloys.
| Configurations | Substitution Atoms | E (eV/Solute Atom) |
|---|
| {MgSi}2Al4 | — | −0.2043 |
| Mg6Si6Al11Cu | Al | −0.2054 |
| Mg5Si6Al12Cu | Mg | −0.1882 |
| Mg6Si5Al12Cu | Si | −0.1946 |
Table 7.
The interaction energy of Cu defects at different positions.
Table 7.
The interaction energy of Cu defects at different positions.
| Position i | Cu-Vac (eV/Solute Atom) |
|---|
| 1—inside Si layer | 0.2500 |
| 2—inside Mg layer | 0.0023 |
| 3—next-nearest layer | −0.0404 |
| 4—next-nearest layer | −0.0236 |