Effects of a Cellulose Aerogel Template on the Preparation and Adsorption Properties of Coal Gangue-Based Multistage Porous ZSM−5
Abstract
1. Introduction
2. Experiment Materials and Methods
2.1. Experiment Materials
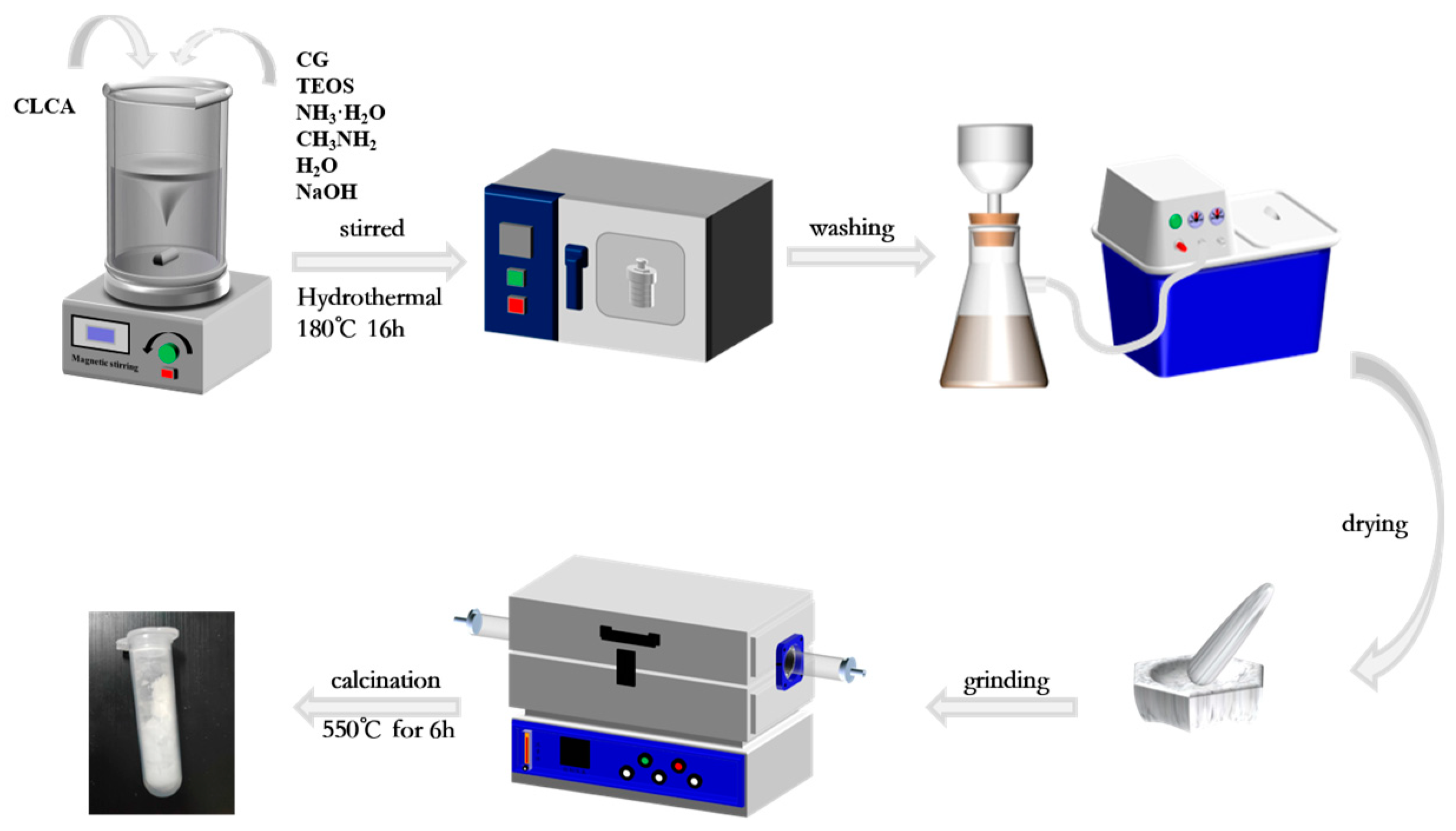
2.2. Synthesis of ZSM−5/CLCA
2.3. Characterization Method
2.4. Adsorption Experiment
3. Results and Discussion
3.1. Effects of Preparation Conditions on the Synthesis of ZSM−5/CLCA
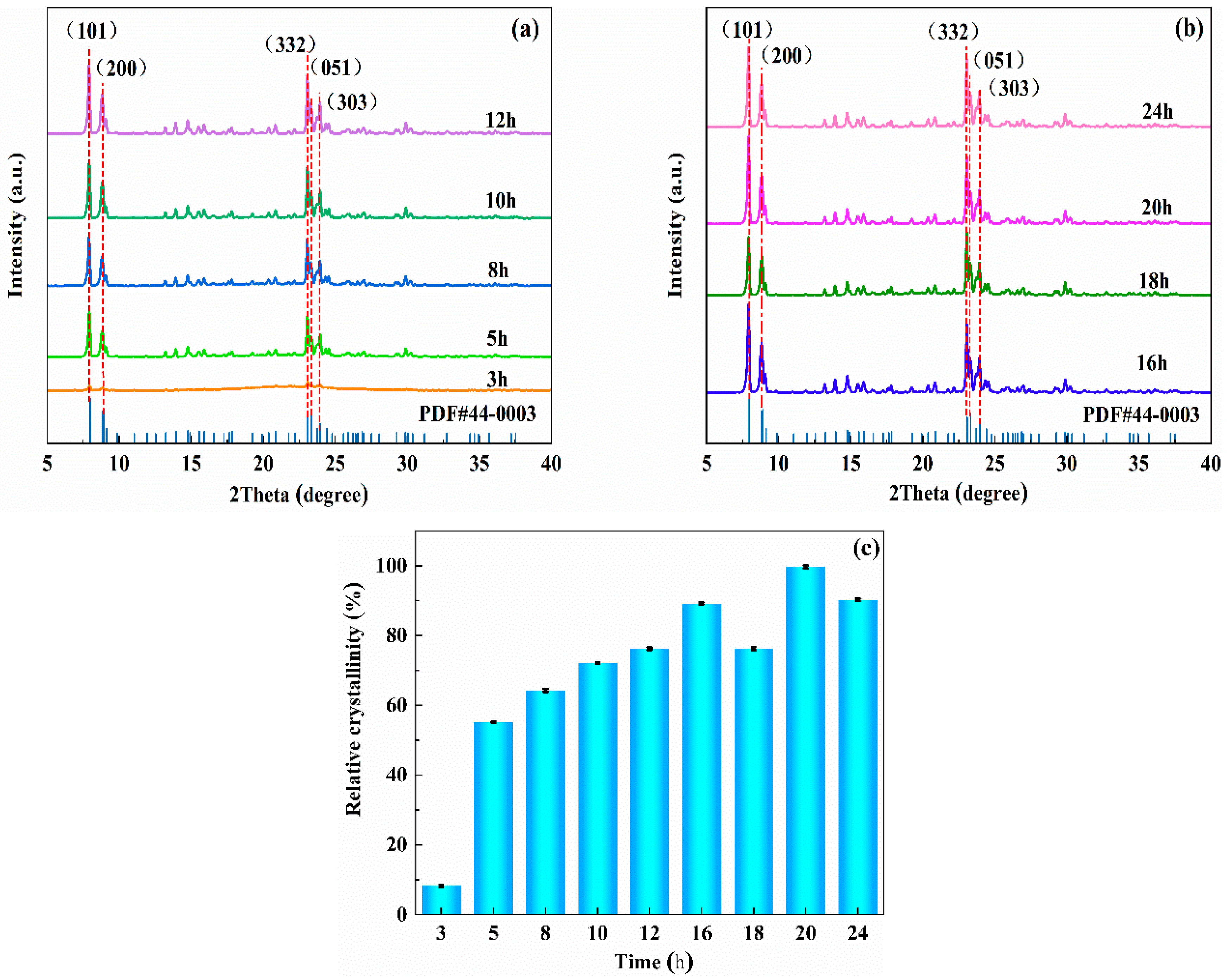
3.1.1. Effects of Crystallization Time on the Synthesis of ZSM−5/CLCA
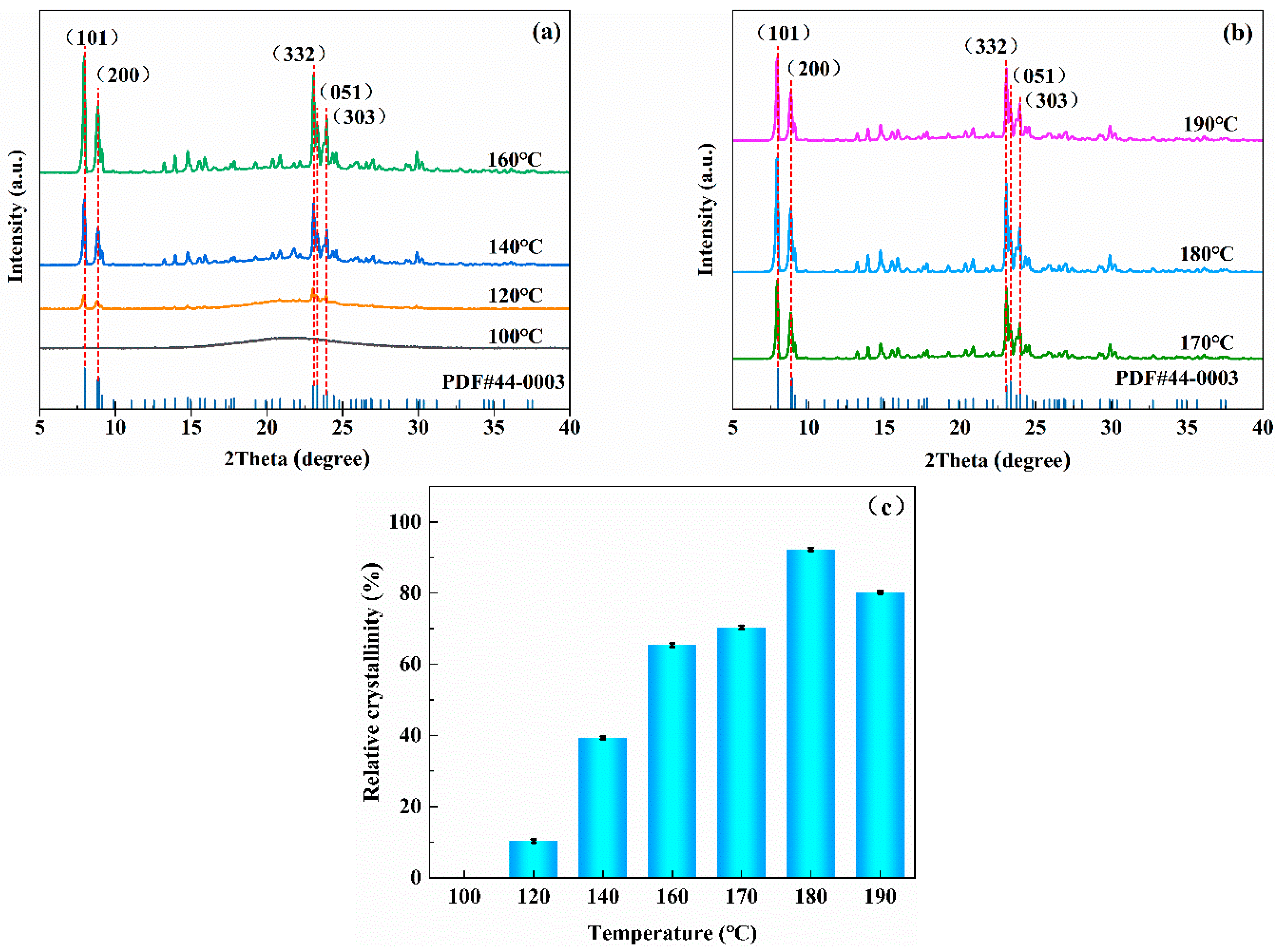
3.1.2. Effects of Crystallization Temperatures on the Synthesis of ZSM−5/CLCA
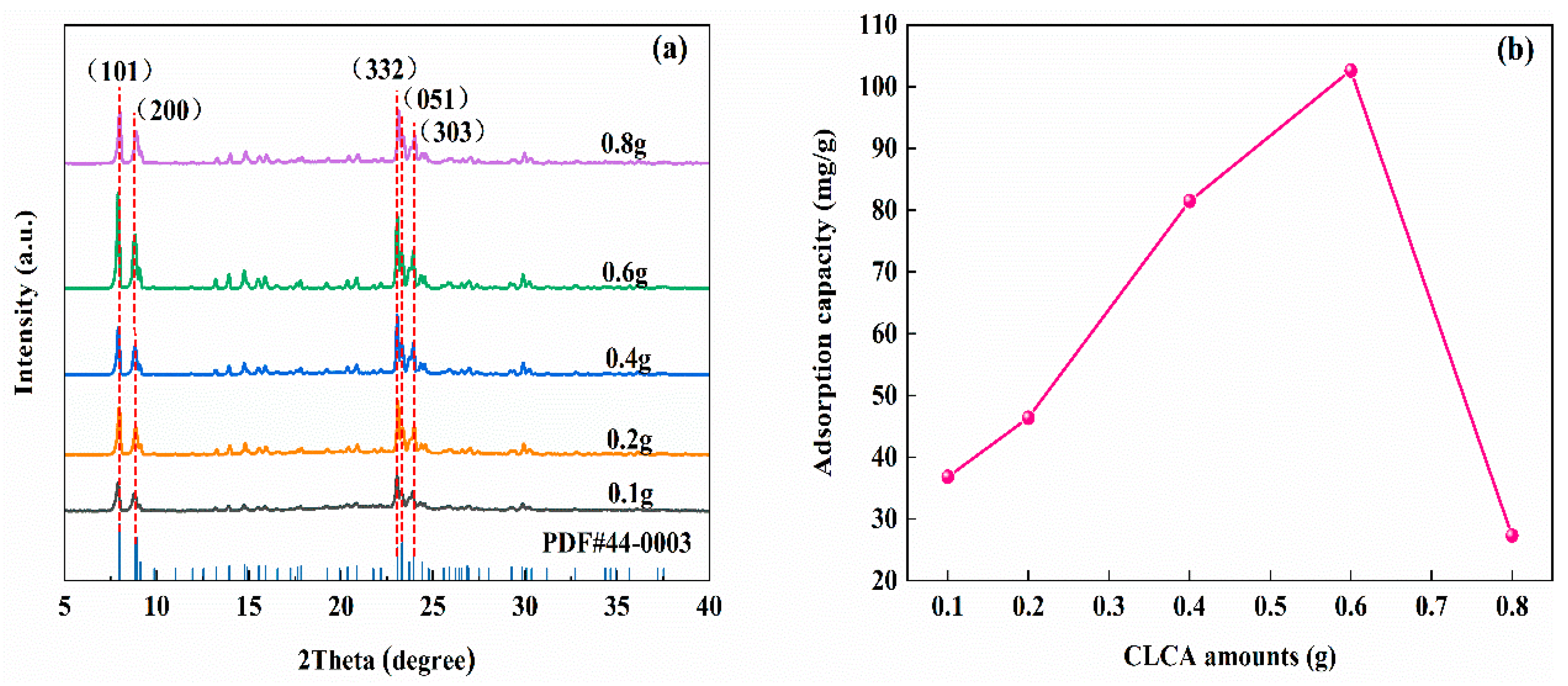
3.1.3. Effects of Cellulose Aerogel Addition on the Synthesis of ZSM−5/CLCA
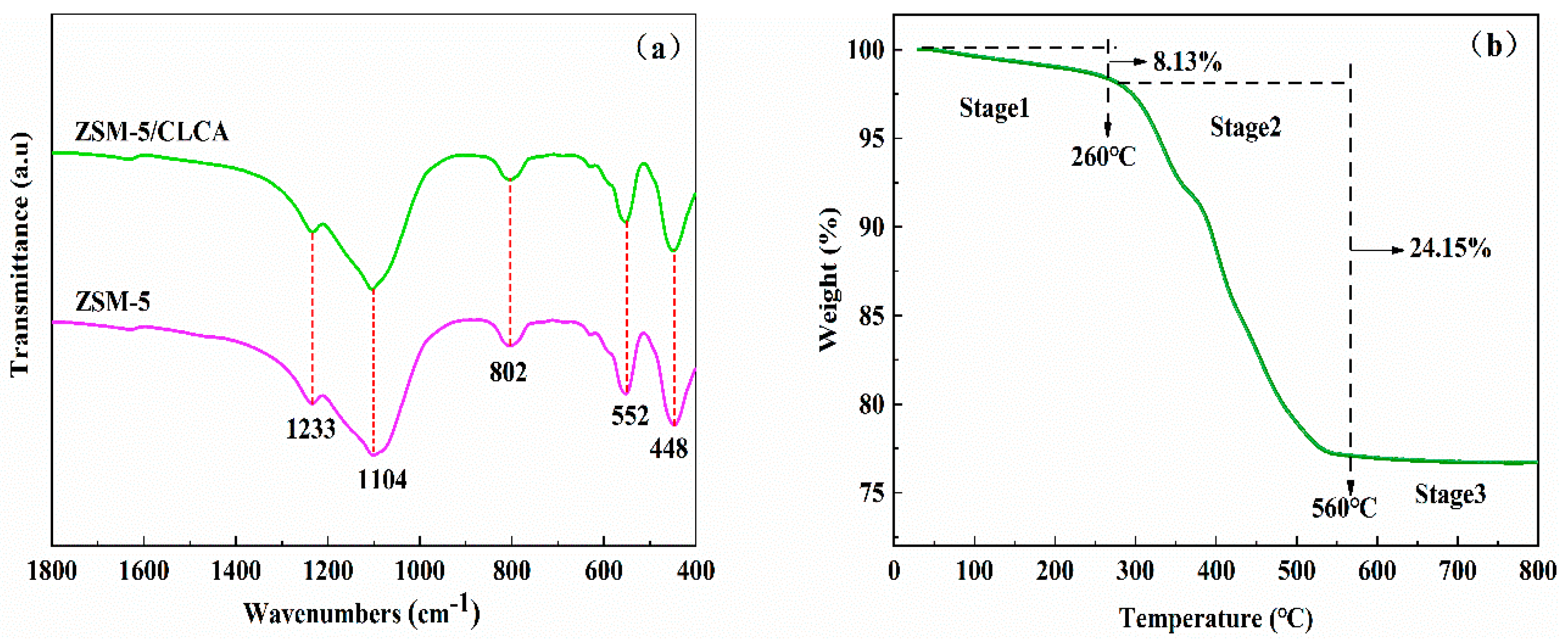
3.1.4. FT-IR and TG Analysis of ZSM−5/CLCA
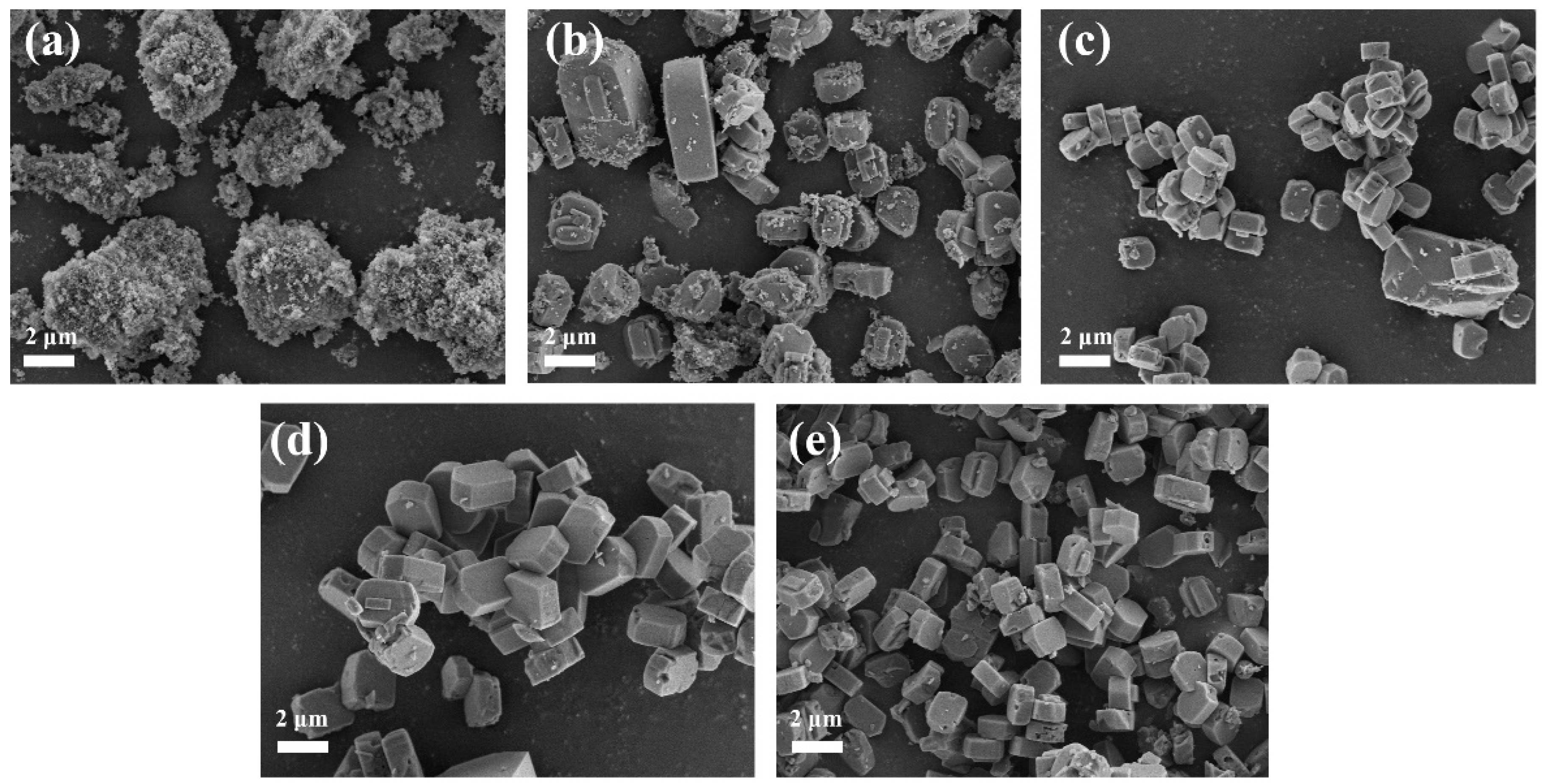
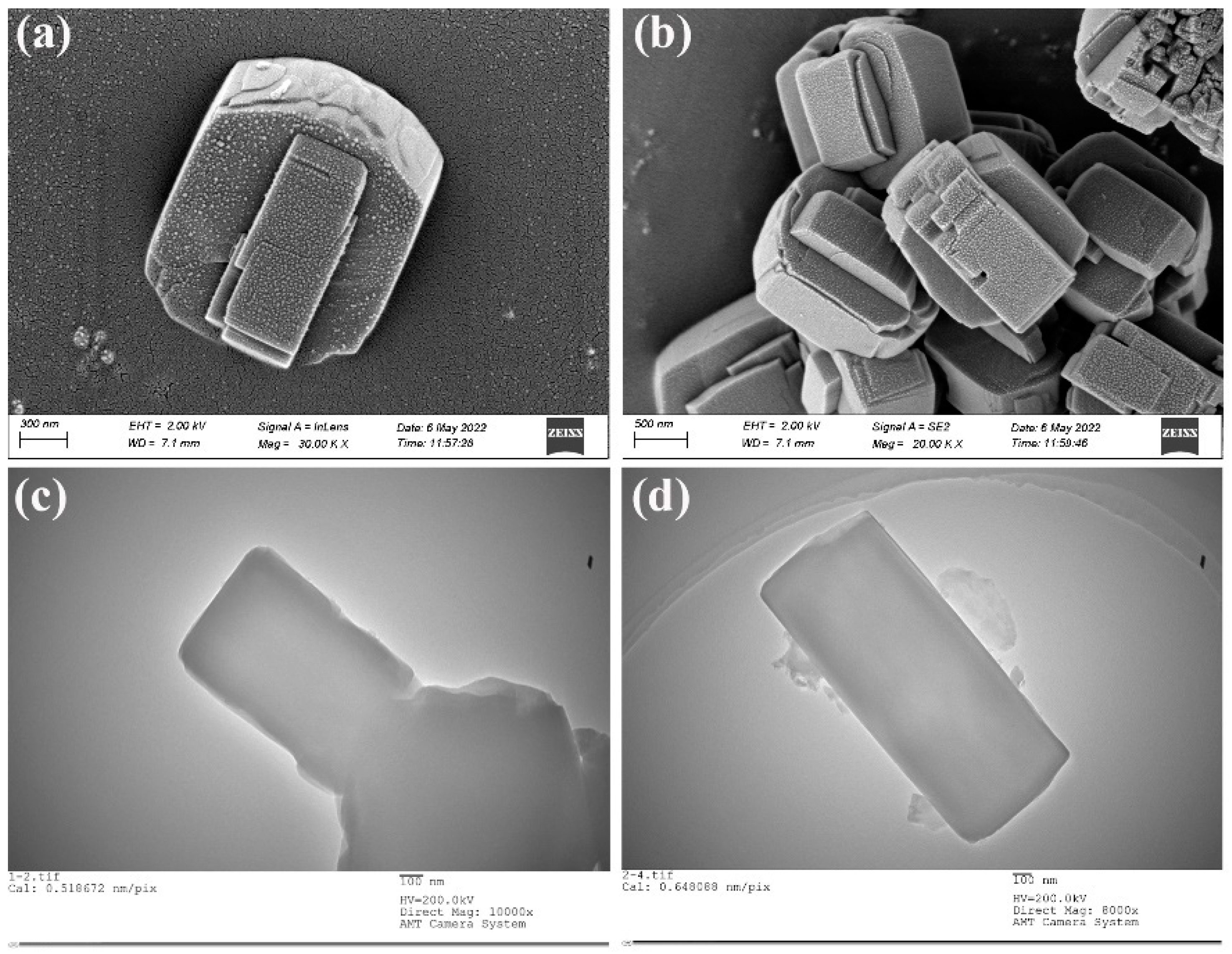
3.1.5. SEM and TEM Analysis of ZSM−5/CLCA
3.1.6. BET Analysis of ZSM−5/CLCA
3.2. Adsorption Performance Analysis
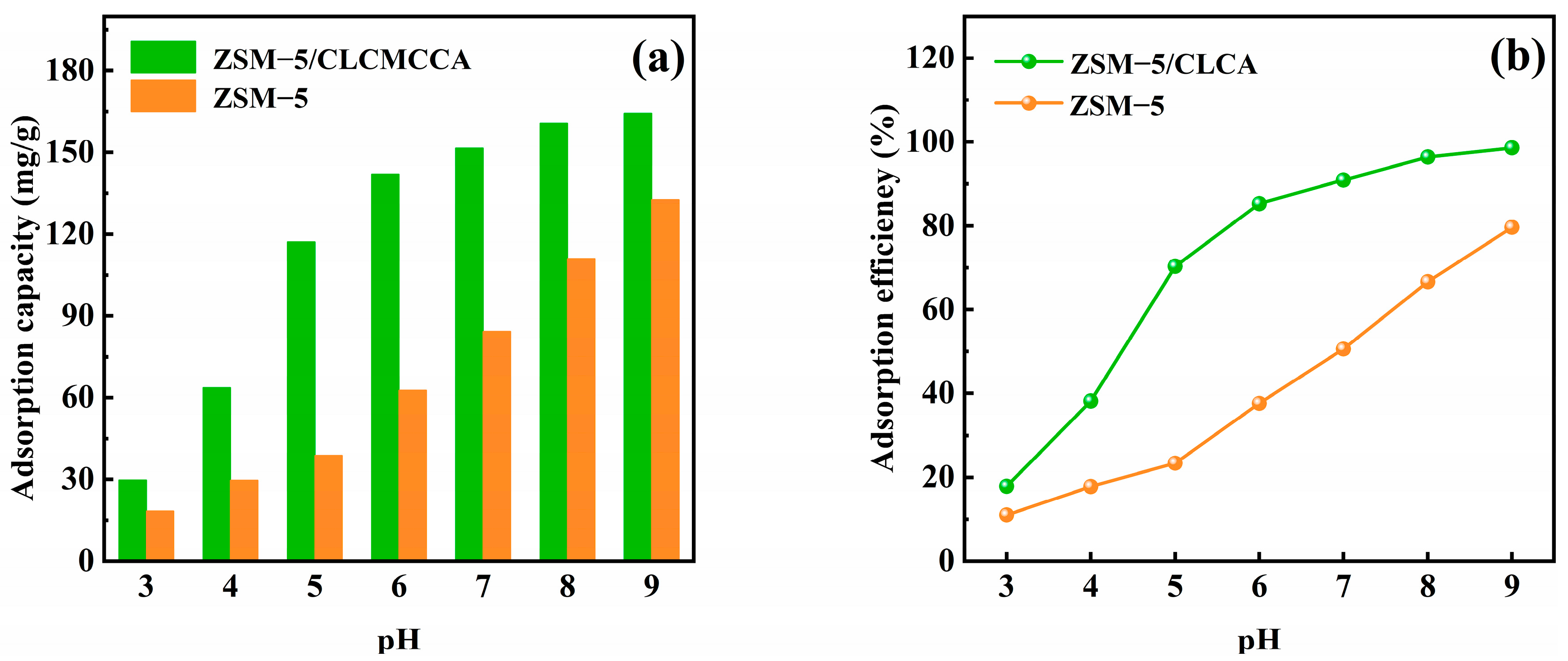
3.2.1. Effect of pH Value on Adsorption
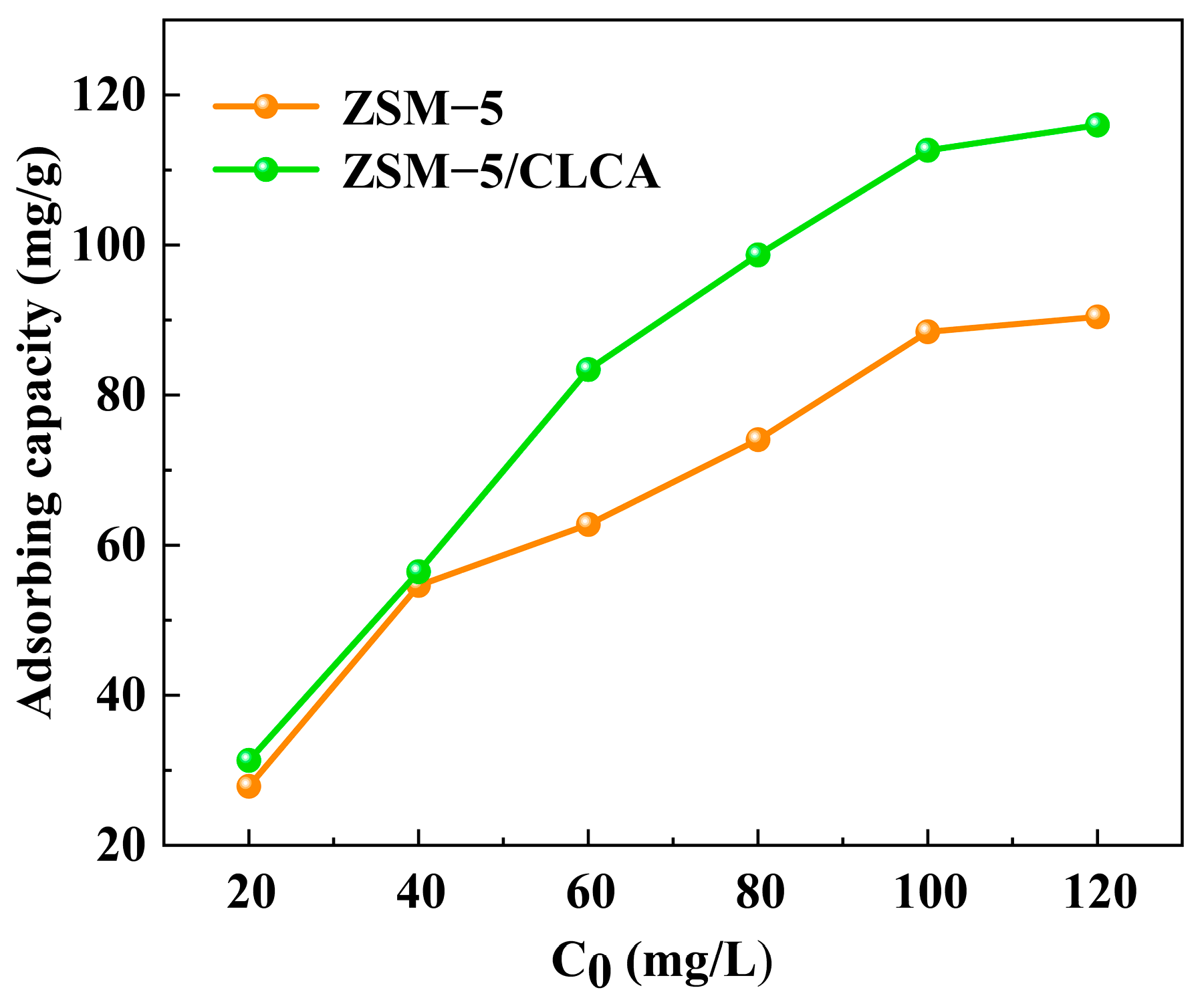
3.2.2. Effect of Different Initial Concentrations on Adsorption
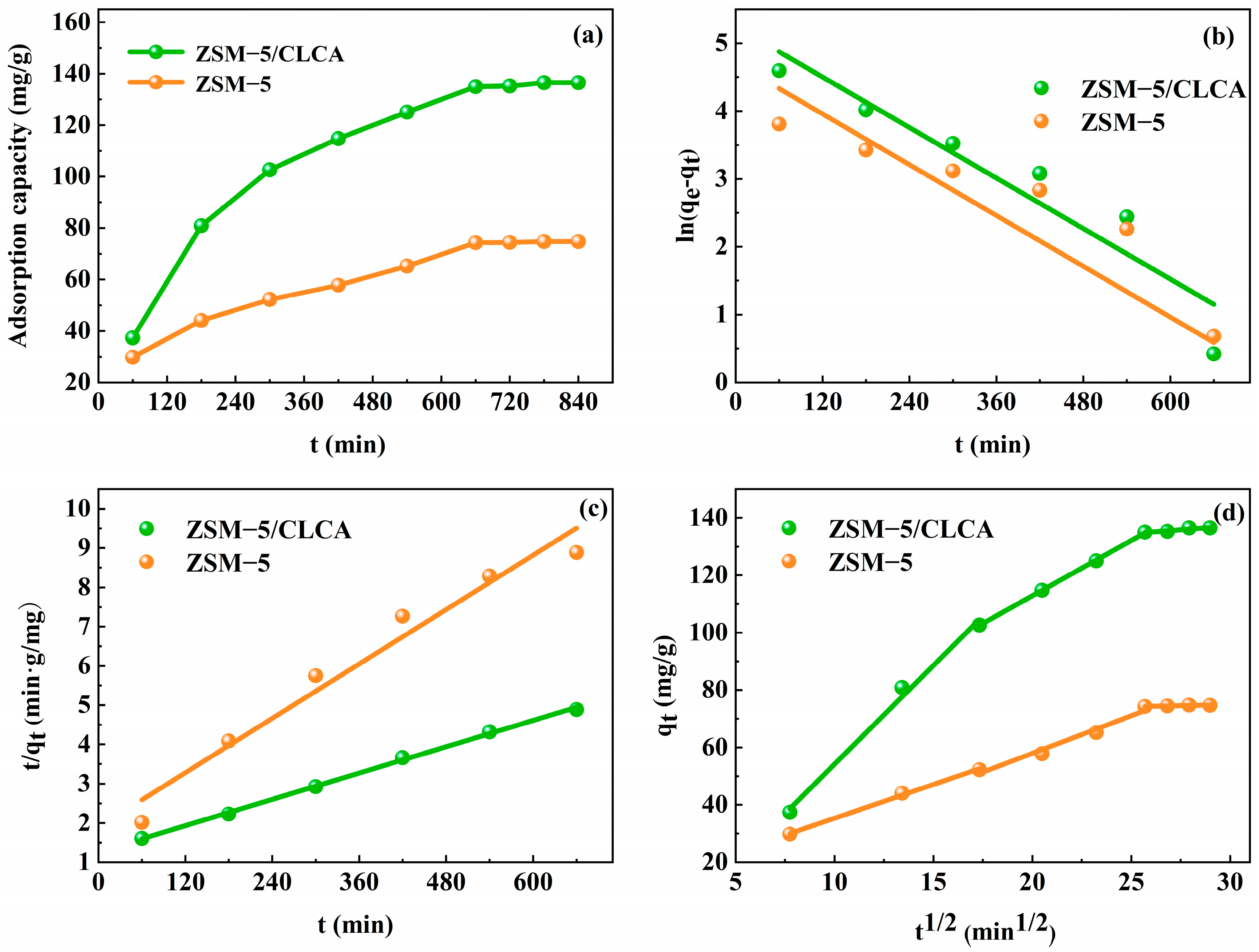
3.2.3. Adsorption Kinetic Model
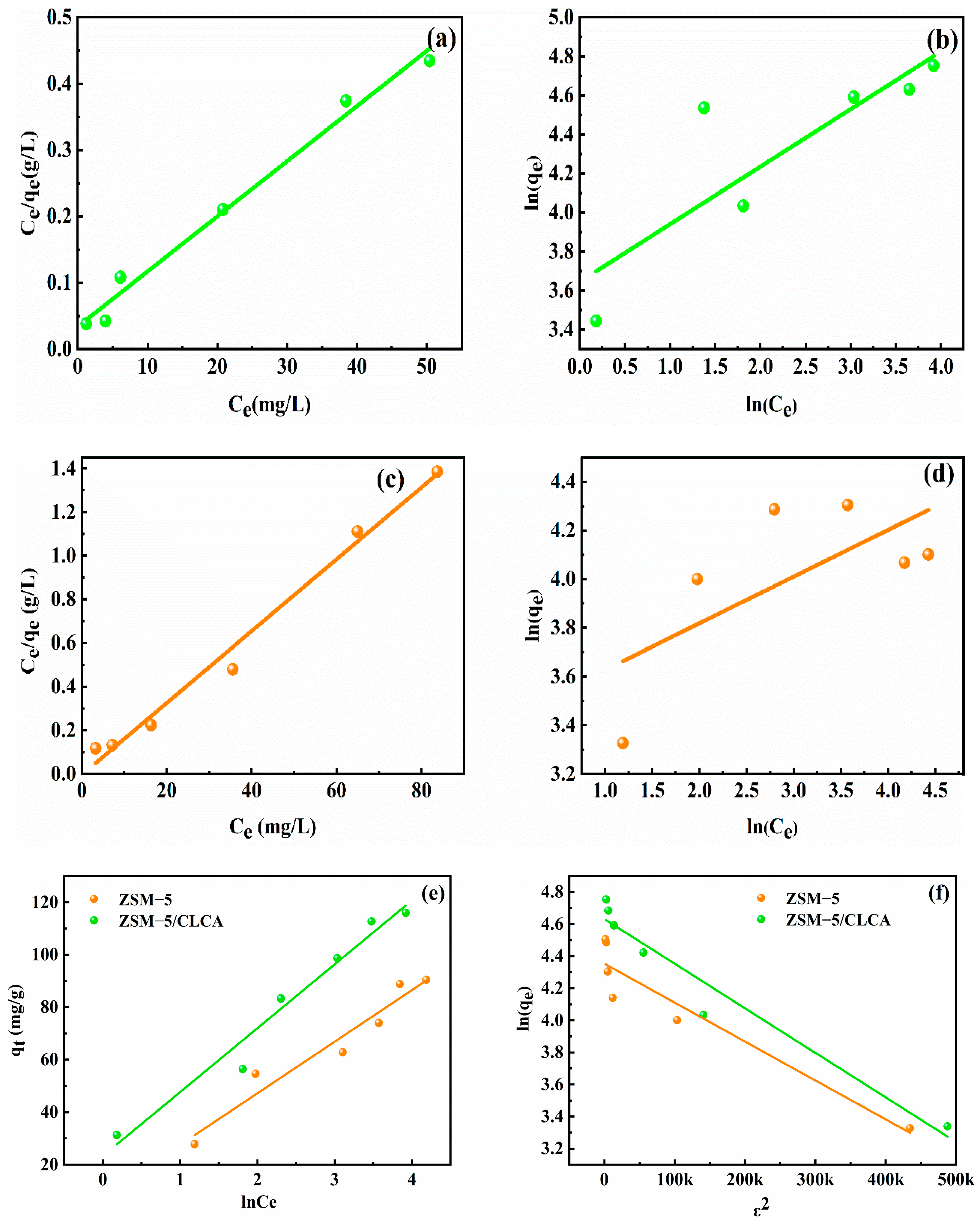
3.2.4. Isothermal Adsorption Model
3.2.5. Adsorption Thermodynamic Model
4. Conclusions
- (1)
- Crystallization time, crystallization temperature, and the additive amount of template significantly affect the structure and properties of the ZSM−5/CLCA molecular sieve. When the optimum preparation conditions are: the crystallization time is 16 h, the crystallization temperature is 180 °C, and the additive volume of CLCA is 0.6 g, the adsorption of MG could reach 136.5 mg/g, which was much higher than the adsorption amount of the ZSM−5 molecular sieve for MG.
- (2)
- The results of the adsorption experiments show that the adsorption of MG by ZSM−5/CLCA is a spontaneous heat absorption reaction, which is in accordance with the quasi-secondary kinetic equation and the Langmuir isothermal adsorption model. By calculating the Temkind isothermal adsorption model and the D-R isothermal adsorption model, it is clear that the adsorption process involves electrostatic interaction and chemisorption by ion exchange.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dhaouefi, Z.; Lahmar, A.; Khlifi, R.; Ben Toumia, I.; Elgueder, D.; Chekir-Ghedira, L. Evaluation of eventual toxicities of treated textile wastewater using anoxic-aerobic algal-bacterial photobioreactor. Environ. Geochem. Health 2022, 44, 4285–4297. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Li, L.; Xiao, Y.; Wang, Y.; Sun, K.; Wang, H.; Liu, L. Synthesis of mixed-ligand Cu–MOFs and their adsorption of malachite green. Rsc. Adv. 2017, 7, 30904–30910. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Muthamizh, S.; Sureshbabu, K.; Munusamy, S.; Padmanaban, A.; Kaaviya, A.; Nagarajan, R.; Stephen, A.; Narayanan, V. Photocatalytic properties of amine functionalized Bi2Sn2O7/rGO nanocomposites. J. Phys. Chem. Solids 2018, 118, 21–31. [Google Scholar] [CrossRef]
- Gnanamoorthy, G.; Karthikeyan, V.; Ali, D.; Kumar, G.; Yadav, V.K.; Narayanan, V. Global popularization of CuNiO2 and their rGO nanocomposite loveabled to the photocatalytic properties of methylene blue. Environ. Res. 2022, 204, 112338. [Google Scholar] [CrossRef]
- Bilgic, A.; Cimen, A.; Bastug, E.; Kursunlu, A.N. Fluorescent sporopollenin microcapsule modified by BODIPY for sensitive & selective recognition and efficient removal of Cu (II) from aqueous solution. Chem. Eng. Res. Des. 2022, 178, 61–72. [Google Scholar] [CrossRef]
- Qu, W.; Yuan, T.; Yin, G.; Xu, S.; Zhang, Q.; Su, H. Effect of properties of activated carbon on malachite green adsorption. Fuel 2019, 249, 45–53. [Google Scholar] [CrossRef]
- Yun, Y.; Gao, R.; Yue, H.; Liu, X.; Li, G.; Sang, N. Polycyclic aromatic hydrocarbon (PAH)-containing soils from coal gangue stacking areas contribute to epithelial to mesenchymal transition (EMT) modulation on cancer cell metastasis. Sci. Total Environ. 2017, 580, 632–640. [Google Scholar] [CrossRef]
- Gao, S.; Zhang, S.; Guo, L. Application of coal gangue as a coarse aggregate in green concrete production: A Review. Materials 2021, 14, 6803. [Google Scholar] [CrossRef]
- Wang, B.; Ma, Y.; Lee, X.; Wu, P.; Liu, F.; Zhang, X.; Li, L.; Chen, M. Environmental-friendly coal gangue-biochar composites reclaiming phosphate from water as a slow-release fertilizer. Sci. Total Environ. 2021, 758, 143664. [Google Scholar] [CrossRef]
- Yuan, X.; Wu, H.; Wang, P.; Xu, F.; Ding, S. Thermal activation of coal gangue with low Al/Si ratio as supplementary cementitious materials. Molecules 2022, 27, 7268. [Google Scholar] [CrossRef]
- Li, J.; Wang, J. Comprehensive utilization and environmental risks of coal gangue: A review. J. Clean. Prod. 2019, 239, 117946. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, J.; Xu, R. Needs and trends in rational synthesis of zeolitic materials. Chem. Soc. Rev. 2012, 41, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Dong, X.; Zhu, Z.; Dong, Y. Environment-oriented low-cost porous mullite ceramic membrane supports fabricated from coal gangue and bauxite. J. Hazard. Mater. 2014, 273, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, B.; Siedlecka, E. Removing heavy metals from wastewaters with use of shales accompanying the coal beds. J. Environ. Manag. 2015, 155, 58–66. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, Z.; Han, L.; Zhang, Y.; Li, L.; Zhu, S.; Li, Z.P.J.; Wang, D. Synthesis of coal-analcime composite from coal gangue and its adsorption performance on heavy metal ions. J. Hazard. Mater. 2022, 423, 127027. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, G.; Peng, S.; Zhou, C. Synthesis of calcium silicate hydrate from coal gangue for Cr(VI) and Cu(II) removal from aqueous solution. Molecules 2021, 26, 6192. [Google Scholar] [CrossRef]
- Kong, D.; Zhou, Z.; Song, S.; Jiang, R. Acid leaching extraction mechanism of aluminum and iron ions from coal gangue based on CaF2 assistance and process optimization. Materials 2023, 16, 499. [Google Scholar] [CrossRef]
- Liang, Z.; Gao, Q.; Wu, Z.; Gao, H. Removal and kinetics of cadmium and copper ion adsorption in aqueous solution by zeolite NaX synthesized from coal gangue. Environ. Sci. Pollut. Res. 2022, 29, 84651–84660. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, C.; Shen, H.; Du, J.; Li, Q.; Wu, W.; Guo, B.; Liu, G. Green synthesis of ceramsite from industrial wastes and its application in selective adsorption: Performance and mechanism. Environ. Res. 2022, 214, 113786. [Google Scholar] [CrossRef]
- Bu, N.; Liu, X.; Song, S.; Liu, J.; Yang, Q.; Li, R.; Zheng, F.; Yan, L.; Zhen, Q.; Zhang, J. Synthesis of NaY zeolite from coal gangue and its characterization for lead removal from aqueous solution. Adv. Powder Technol. 2020, 31, 2699–2710. [Google Scholar] [CrossRef]
- Ge, Q.; Moeen, M.; Tian, Q.; Xu, J.; Feng, K. Highly effective removal of Pb(2+) in aqueous solution by Na-X zeolite derived from coal gangue. Environ. Sci. Pollut. Res. 2020, 27, 7398–7408. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, B.; Zhang, X.; Lee, X.; Chen, M.; Feng, Q.; Chen, S. Insights into Cr(VI) removal mechanism in water by facile one-step pyrolysis prepared coal gangue-biochar composite. Chemosphere 2022, 299, 134334. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Sun, A.; Wu, X.; Hai, G.; Hu, J.; Li, T.; Li, G. Facile synthesis of hierarchical nanocrystalline ZSM−5 zeolite under mild conditions and its catalytic performance. J. Colloid Interface Sci. 2011, 361, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Morris, R.E.; James, S.L. Solventless synthesis of zeolites. Angew. Chem. Int. Ed. 2013, 52, 2163–2165. [Google Scholar] [CrossRef]
- Jia, Y.; Shi, Q.; Wang, J.; Ding, C.; Zhang, K. Synthesis, characterization, and catalytic application of hierarchical nano-ZSM−5 zeolite. RSC Adv. 2020, 10, 29618–29626. [Google Scholar] [CrossRef]
- Yuan, N.; Tan, K.; Zhang, X.; Zhao, A.; Guo, R. Synthesis and adsorption performance of ultra-low silica-to-alumina ratio and hierarchical porous ZSM−5 zeolites prepared from coal gasification fine slag. Chemosphere 2022, 303, 134839. [Google Scholar] [CrossRef]
- Gholipour, F.; Mofarahi, M. Adsorption equilibrium of methane and carbon dioxide on zeolite 13X: Experimental and thermodynamic modeling. J. Supercrit. Fluids 2016, 111, 47–54. [Google Scholar] [CrossRef]
- Srasri, K.; Thongroj, M.; Chaijiraaree, P.; Thiangtham, S.; Manuspiya, H.; Pisitsak, P.; Ummartyotin, S. Recovery potential of cellulose fiber from newspaper waste: An approach on magnetic cellulose aerogel for dye adsorption material. Int. J. Biol. Macromol. Struct. Funct. Interact. 2018, 119, 662–668. [Google Scholar] [CrossRef]
- Zhou, S.; Jin, X.; Hao, J.; Zhou, M.; Qiao, X.; Pang, X. Facile preparation of lignosulfonate-based superparamagnetic colloidal nanocrystal clusters (CNCs). Compos. Commun. 2021, 28, 100946. [Google Scholar] [CrossRef]
- Xia, J.; Liu, Z.; Chen, Y.; Cao, Y.; Wang, Z. Effect of lignin on the performance of biodegradable cellulose aerogels made from wheat straw pulp-LiCl/DMSO solution. Cellulose 2020, 27, 879–894. [Google Scholar] [CrossRef]
- Crini, G.; Peindy, H.; Gimbert, F.; Robert, C. Removal of C.I. Basic Green 4 (Malachite Green) from aqueous solutions by adsorption using cyclodextrin-based adsorbent: Kinetic and equilibrium studies. Sep. Purif. Technol. 2007, 53, 97–110. [Google Scholar] [CrossRef]
- Atirah Mat, S.S.; Syed Zuber, S.Z.H.; Enche Ab Rahim, S.K.; Ahmad Sohaimi, K.S.; Abdul Halim, N.A.; Zainudin, N.F.; Yusoff, N.A.; Rohaizad, N.M.; Ishak, N.H.; Anuar, A.; et al. Malachite green adsorption by spent coffee grounds. IOP Conf. Ser. Mater. Sci. Eng. 2018, 318, 012015. [Google Scholar] [CrossRef]
- Ozcan, A.S.; Erdem, B.; Ozcan, A. Adsorption of acid blue 193 from aqueous solutions onto Na-bentonite and DTMA-bentonite. J. Colloid Interface Sci. 2004, 280, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Mu’azu, N.D.; Al-Yahya, M.; Al-Haj-Ali, A.M.; Abdel-Magid, I.M. Specific energy consumption reduction during pulsed electrochemical oxidation of phenol using graphite electrodes. J. Environ. Chem. Eng. 2016, 4, 2477–2486. [Google Scholar] [CrossRef]
- Bahar, M.M.; Mahbub, K.R.; Naidu, R.; Megharaj, M. As(V) removal from aqueous solution using a low-cost adsorbent coir pith ash: Equilibrium and kinetic study. Environ. Technol. Innov. 2018, 9, 198–209. [Google Scholar] [CrossRef]
- Mohammadi, A.; Daemi, H.; Barikani, M. Fast removal of malachite green dye using novel superparamagnetic sodium alginate-coated Fe3O4 nanoparticles. Int. J. Biol. Macromol. 2014, 69, 447–455. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, C.; Guan, H.; Li, L.; Fan, L.; Wang, Y.; Liu, L.; Meng, Q.; Zhang, R. Magnetic metal organic frameworks (MOFs) composite for removal of lead and malachite green in wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 382–390. [Google Scholar] [CrossRef]
- Abate, G.Y.; Alene, A.N.; Habte, A.T.; Addis, Y.A. Adsorptive Removal of Basic Green Dye from Aqueous Solution Using Humic Acid Modified Magnetite Nanoparticles: Kinetics, Equilibrium and Thermodynamic Studies. J. Polym. Environ. 2020, 29, 967–984. [Google Scholar] [CrossRef]
- Lima, E.C.; Gomes, A.A.; Tran, H.N. Comparison of the nonlinear and linear forms of the van’t Hoff equation for calculation of adsorption thermodynamic parameters (∆S° and ∆H°). J. Mol. Liq. 2020, 311, 113315. [Google Scholar] [CrossRef]
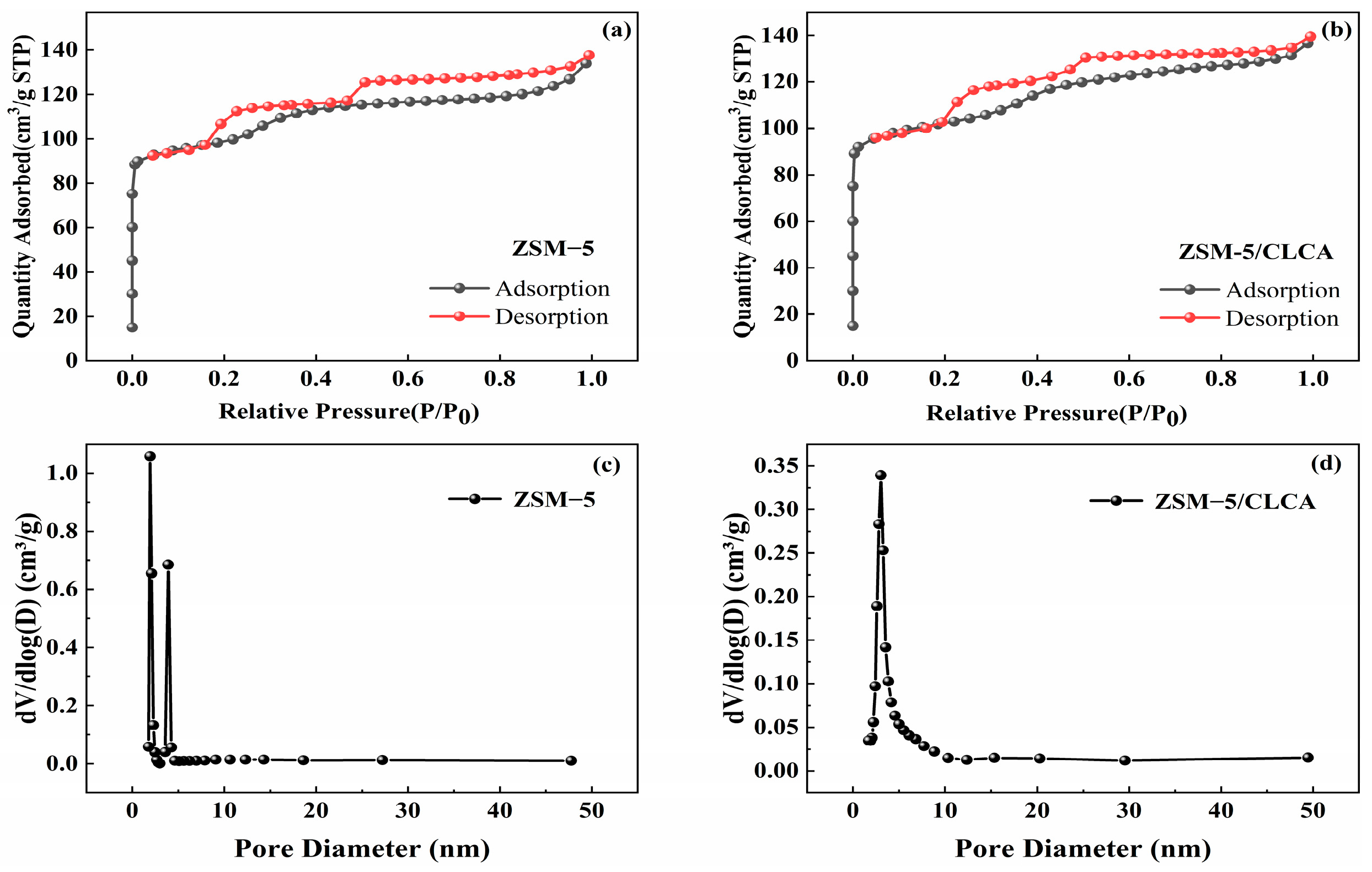
| Sample | SBET (m2⋅g−1) | Smic (m2⋅g−1) | Vtotal (cm3⋅g−1) | Vmic (cm3⋅g−1) |
|---|---|---|---|---|
| ZSM−5 | 385 | 266 | 0.1959 | 0.1023 |
| ZSM−5/CLCA | 398 | 312 | 0.2029 | 0.1210 |
| Sample | qe,exp (mg/g) | Pseudo-First-Order Dynamic Parameters | Pseudo-Second-Order Dynamic Parameters | ||||
|---|---|---|---|---|---|---|---|
| qe1 (mg/g) | k1 | R2 | qe2 (mg/g) | K2 | R2 | ||
| ZSM−5/CLCA | 136.50 | 189.98 | 6.21 × 10−3 | 0.8955 | 178.57 | 2 × 10−5 | 0.9987 |
| ZSM−5 | 74.78 | 110.73 | 6.24 × 10−3 | 0.7429 | 86.66 | 7 × 10−5 | 0.966 |
| Sample | The First Stage | The Second Stage | The Third Stage | |||
|---|---|---|---|---|---|---|
| kP1 | C1 | kP2 | C2 | kP3 | C3 | |
| ZSM−5/CLCA | 6.8786 | −14.6136 | 3.8504 | 35.8441 | 0.5302 | 121.2816 |
| ZSM−5 | 2.3556 | 11.7786 | 2.6181 | 5.5893 | 0.1501 | 70.4392 |
| Sample | Langmuir Isothermal Adsorption Parameters | Freundlich Isothermal Adsorption Parameters | ||||
|---|---|---|---|---|---|---|
| qm(mg/g) | KL | R2 | 1/n | KF | R2 | |
| ZSM−5/CLCA | 120.37 | 0.2450 | 0.9863 | 0.2949 | 38.2889 | 0.7392 |
| ZSM−5 | 60.75 | 0.3791 | 0.9871 | 0.1918 | 31.0441 | 0.4650 |
| Sample | Temkin Isotherm | D-R Isotherm | ||||
| KT | b | R2 | qm (mg/g) | E (KJ/mol) | R2 | |
| ZSM−5/CLCA | 0.9588 | 101.87 | 0.9680 | 102.65 | 37.2726 | 0.9510 |
| ZSM−5 | 0.4022 | 126.11 | 0.9441 | 77.75 | 34.8332 | 0.9064 |
| Adsorbent | qe (mg/g) | Reference |
|---|---|---|
| Alg-Fe3O4 | 47.84 | [36] |
| Cu-MOFs/Fe3O4 | 113.67 | [37] |
| HA-Fe3O4 | 79.30 | [38] |
| ZSM−5/CLCA | 120.37 | This work |
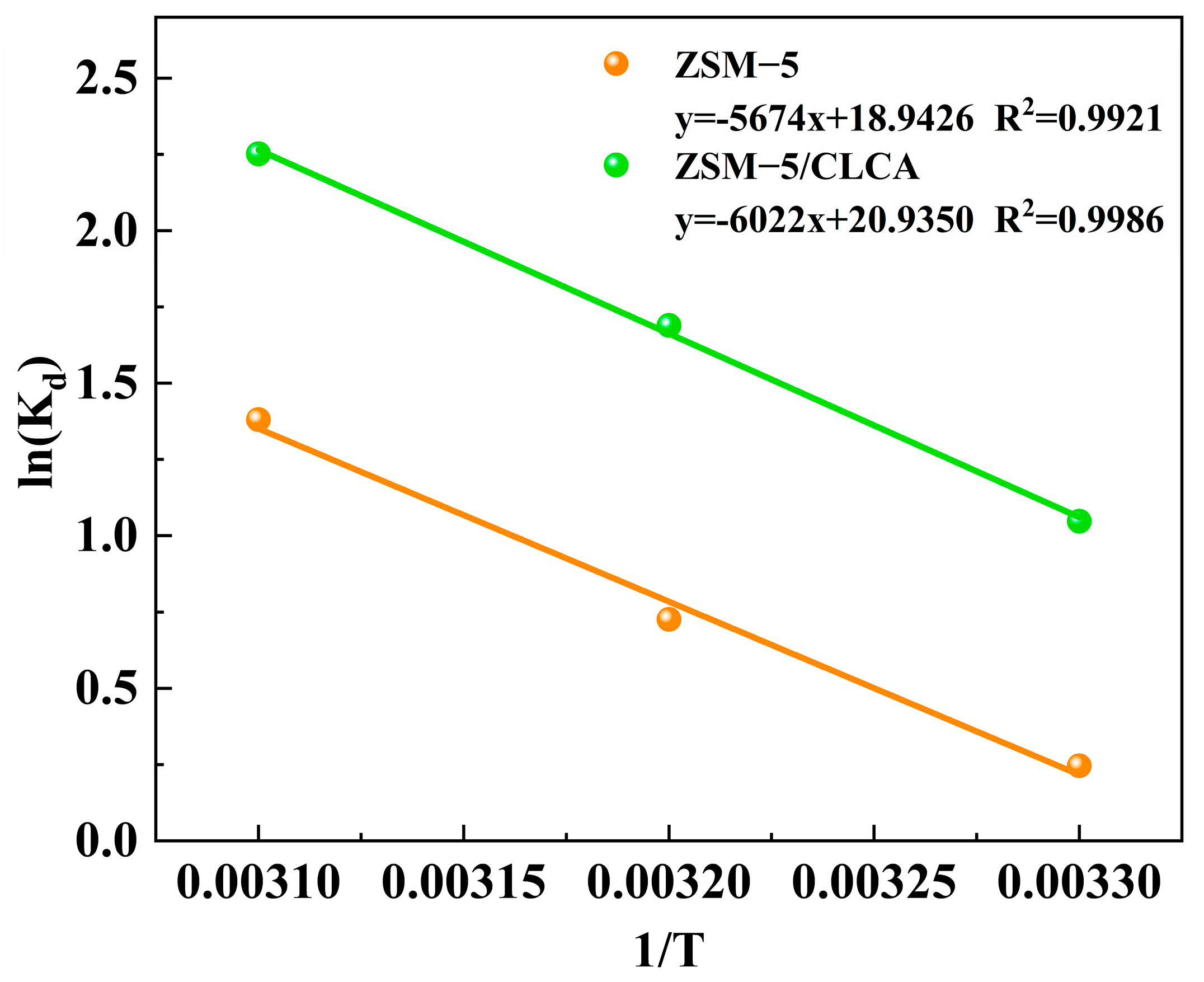
| Sample | T (K) | lnKd | ΔG° (kJ/mol) | ΔH° (kJ/mol) | ΔS° (J/mol/k) |
|---|---|---|---|---|---|
| ZSM−5/CLCA | 303 | 1.0475 | −2.6671 | 50.0711 | 174.0536 |
| 313 | 1.6894 | −4.4077 | |||
| 323 | 2.2520 | −6.1482 | |||
| ZSM−5 | 303 | 0.2460 | −0.5455 | 47.1736 | 157.4888 |
| 313 | 0.7258 | −2.1204 | |||
| 323 | 1.3809 | −3.6953 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, X.; Ding, C.; Yang, H.; Zhu, X. Effects of a Cellulose Aerogel Template on the Preparation and Adsorption Properties of Coal Gangue-Based Multistage Porous ZSM−5. Materials 2023, 16, 3896. https://doi.org/10.3390/ma16113896
Ma X, Ding C, Yang H, Zhu X. Effects of a Cellulose Aerogel Template on the Preparation and Adsorption Properties of Coal Gangue-Based Multistage Porous ZSM−5. Materials. 2023; 16(11):3896. https://doi.org/10.3390/ma16113896
Chicago/Turabian StyleMa, Xue, Chengli Ding, Hongsheng Yang, and Xiao Zhu. 2023. "Effects of a Cellulose Aerogel Template on the Preparation and Adsorption Properties of Coal Gangue-Based Multistage Porous ZSM−5" Materials 16, no. 11: 3896. https://doi.org/10.3390/ma16113896
APA StyleMa, X., Ding, C., Yang, H., & Zhu, X. (2023). Effects of a Cellulose Aerogel Template on the Preparation and Adsorption Properties of Coal Gangue-Based Multistage Porous ZSM−5. Materials, 16(11), 3896. https://doi.org/10.3390/ma16113896





