Preparation of One-Dimensional Polyaniline Nanotubes as Anticorrosion Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparations
2.3. Characterization
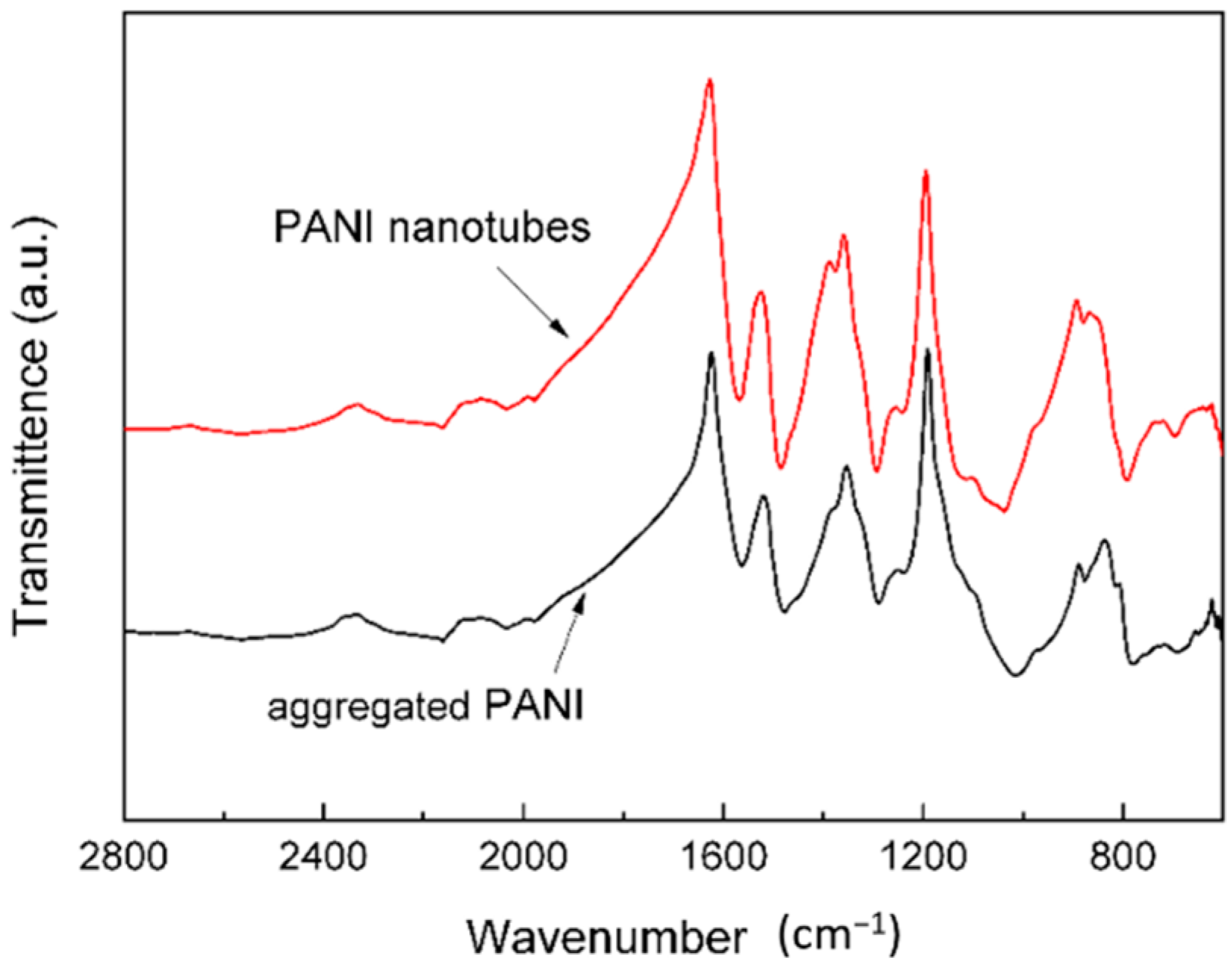
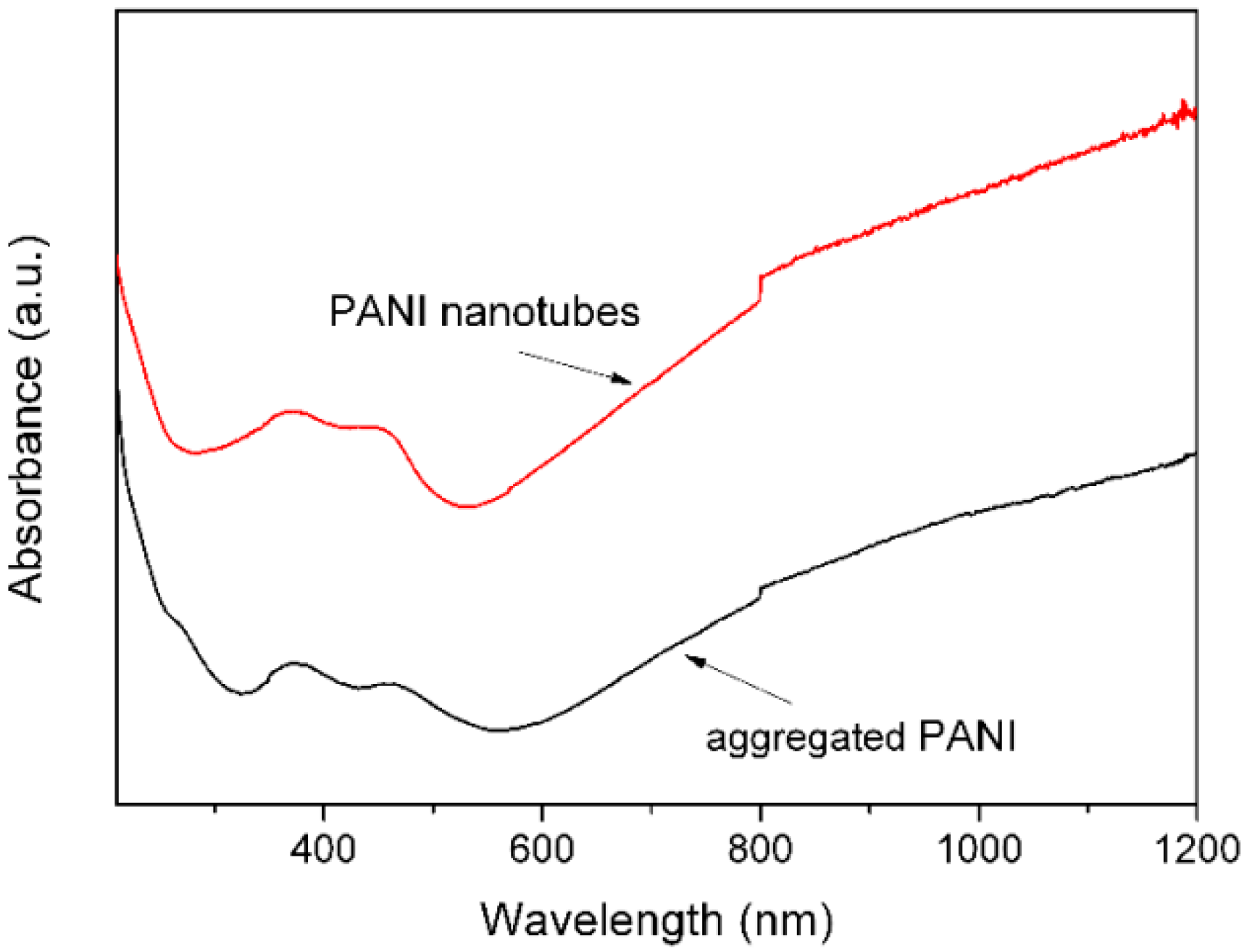
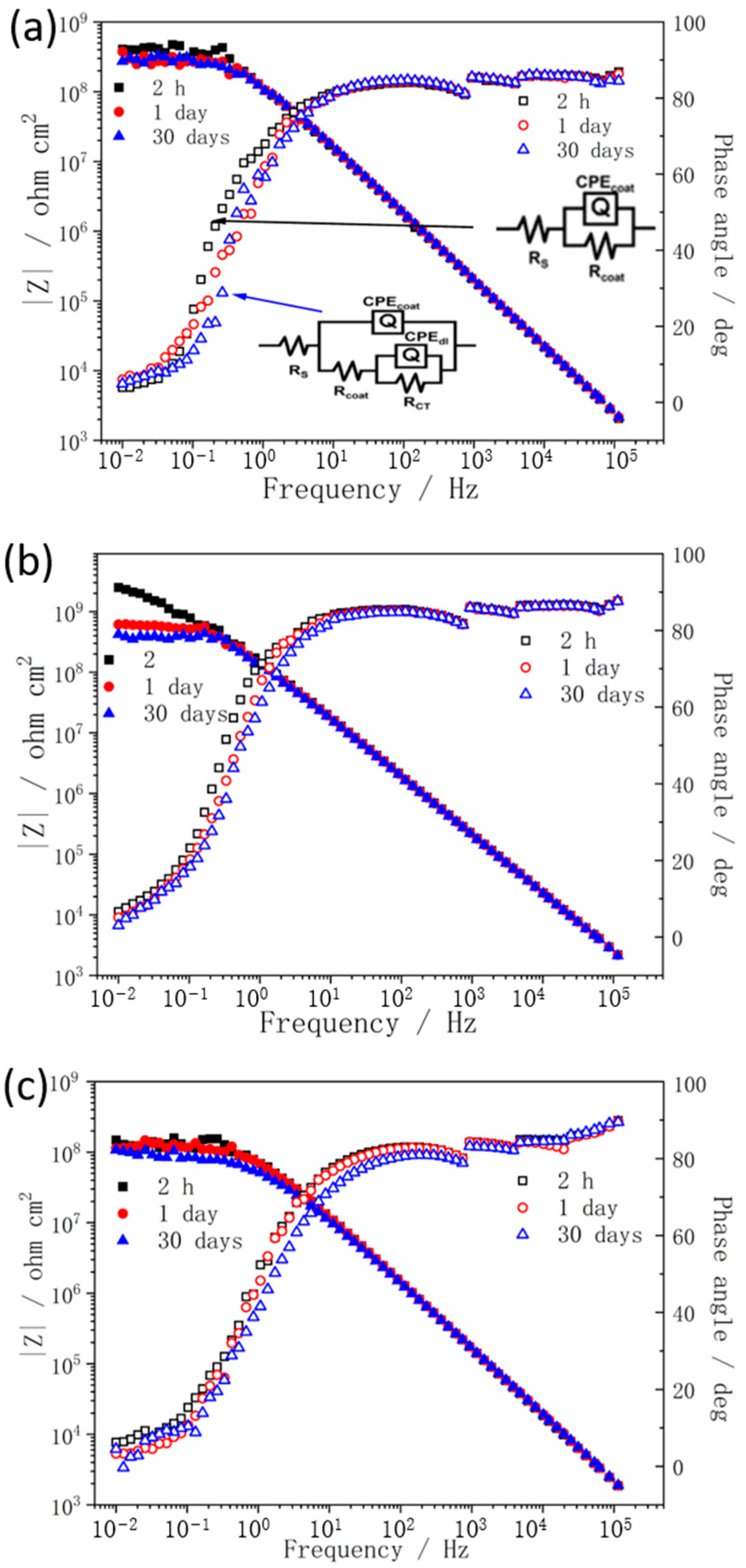
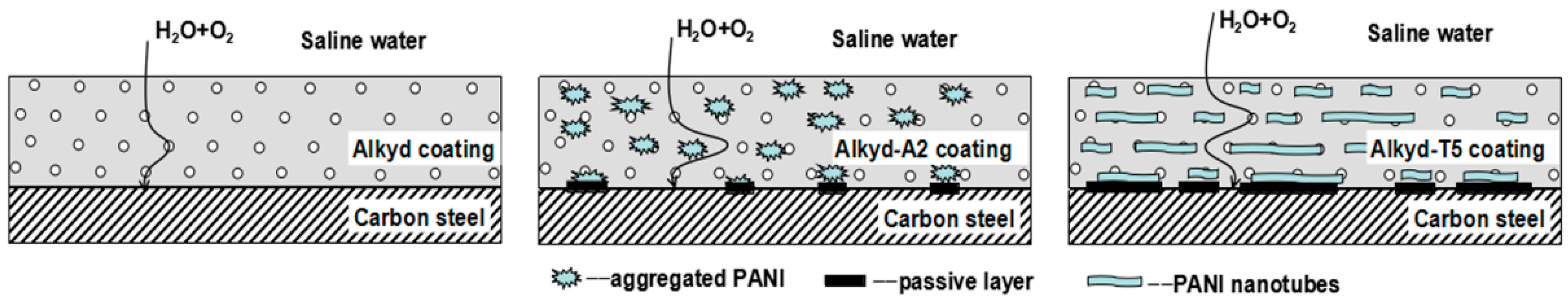
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hatchett, D.W.; Josowicz, M.; Janata, J. Comparison of chemically and electrochemically synthesized polyaniline films. J. Electrochem. Soc. 2019, 146, 4535–4538. [Google Scholar] [CrossRef]
- Viva, F.A.; Andrade, E.M.; Molina, F.V.; Florit, M.I. Electropolymerization of 2-methoxy aniline. Electrochemical and spectroscopical product characterization. J. Electrochem. Soc. 1999, 471, 180–189. [Google Scholar] [CrossRef]
- MacDiarmid, A.G. Nobel Lecture: “Synthetic metals”: A novel role for organic polymers. Rev. Mod. Phys. 2001, 73, 701–712. [Google Scholar] [CrossRef] [Green Version]
- Pud, A.; Ogurtsov, N.; Korzhenko, A.; Shapoval, G. Some aspects of preparation methods and properties of polyaniline blends and composites with organic polymers. Prog. Polym. Sci. 2003, 28, 1701–1753. [Google Scholar] [CrossRef]
- Sun, Q.; Park, M.-C.; Deng, Y. Dendritic superstructure formation of polyaniline prepared using a water-soluble polyelectrolyte copolymer as the support matrix. Mater. lett. 2007, 61, 3052–3055. [Google Scholar] [CrossRef]
- Hugotlegoff, A.; Bernard, M.C. Protonation and oxidation processes in polyaniline thin-films studied by optical multichannel analysis and in-situ raman-spectroscopy. Synth. Met. 1993, 60, 115–131. [Google Scholar] [CrossRef]
- Gospodinova, N.; Terlemezyan, L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Prog. Polym. Sci. 1998, 23, 1443–1484. [Google Scholar] [CrossRef]
- Neoh, K.G.; Kang, E.T.; Khor, S.H.; Tan, K.L. Stability studies of polyaniline. Polym. Degrad. Stab. 1990, 27, 107–117. [Google Scholar] [CrossRef]
- Amano, K.; Ishikawa, H.; Kobayashi, A.; Satoh, M.; Hasegawa, E. Thermal-stability of chemically synthesized polyaniline. Synth. Met. 1994, 62, 229–232. [Google Scholar] [CrossRef]
- Kraljić, M.; Mandić, Z.; Duić, L. Inhibition of steel corrosion by polyaniline coatings. Corros. Sci. 2003, 45, 181–198. [Google Scholar] [CrossRef]
- Deberry, D.W. Modification of the electrochemical and corrosion behavior of stainless-steels with an electroactive coating. J. Electrochem. Soc. 1985, 132, 1022–1026. [Google Scholar] [CrossRef]
- Bereket, G.; Hur, E.; Sahin, Y. Electrochemical synthesis and anti-corrosive properties of polyaniline, poly(2-anisidine), and poly(aniline-co-2-anisidine) films on stainless steel. Prog. Org. Coat. 2005, 54, 63–72. [Google Scholar] [CrossRef]
- Karpakam, V.; Kamaraj, K.; Sathiyanarayanan, S. Electrosynthesis of PANI-nano TiO2 composite coating on steel and its anti-corrosion performance. J. Electrochem. Soc. 2011, 158, C416. [Google Scholar] [CrossRef]
- Abu, Y.M.; Aoki, K. Corrosion protection by polyaniline-coated latex microspheres. J. Electrochem. Soc. 2005, 583, 133–139. [Google Scholar] [CrossRef]
- Yao, B.; Wang, G.C.; Ye, J.K.; Li, X.W. Corrosion inhibition of carbon steel by polyaniline nanofibers. Mater. Lett. 2008, 62, 1775–1778. [Google Scholar] [CrossRef]
- Yang, X.G.; Li, B.; Wang, H.Z.; Hou, B.R. Anticorrosion performance of polyaniline nanostructures on mild steel. Prog. Org. Coat. 2010, 69, 267–271. [Google Scholar] [CrossRef]
- Guo, R.; Wang, J.; Wang, H.H.; Fei, G.Q.; Wang, C.Y.; Sun, L.Y.; Wallace, G.G. Engineering the poly(vinyl alcohol)-polyaniline colloids for high-performance waterborne alkyd anticorrosion coating. Appl. Surf. Sci. 2019, 481, 960–971. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, J.; Nie, X.; Wei, D.; Zhang, N.; Peng, S.; Dong, X.; Li, Y.; Fang, P. One-step firing of carbon fiber and ceramic precursors for high performance electro-thermal composite: Influence of graphene coating. Mater. Des. 2020, 191. [Google Scholar] [CrossRef]
- Tian, Z.F.; Yu, H.J.; Wang, L.; Saleem, M.; Ren, F.J.; Ren, P.F.; Chen, Y.S.; Sun, R.L.; Sun, Y.B.; Huang, L. Recent progress in the preparation of polyaniline nanostructures and their applications in anticorrosive coatings. RSC Adv. 2014, 4, 28195–28208. [Google Scholar] [CrossRef]
- Schauer, T.; Joos, A.; Dulog, L.; Eisenbach, C.D. Protection of iron against corrosion with polyaniline primers. Prog. Org. Coat. 1998, 33, 20–27. [Google Scholar] [CrossRef]
- Olad, A.; Ramazani, Z. Preparation, characterization, and anticorrosive properties of polyaniline nanotubes. Int. J. Polym. Mater. 2012, 61, 949–962. [Google Scholar] [CrossRef]
- Huang, J.; Virji, S.; Weiller, B.H.; Kaner, R.B. Nanostructured polyaniline sensors. Chemistry 2004, 10, 1314–1319. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudian, M.R.; Alias, Y.; Basirun, W.J. Effect of narrow diameter polyaniline nanotubes and nanofibers in polyvinyl butyral coating on corrosion protective performance of mild steel. Prog. Org. Coat. 2012, 75, 301–308. [Google Scholar] [CrossRef]
- Trchova, M.; Sedenkova, I.; Konyushenko, E.N.; Stejskal, J.; Holler, P.; Ciric-Marjanovic, G. Evolution of polyaniline nanotubes: The oxidation of aniline in water. J. Phys. Chem. B 2006, 110, 9461–9468. [Google Scholar] [CrossRef]
- Konyushenko, E.N.; Stejskal, J.; Šeděnková, I.; Trchová, M.; Sapurina, I.; Cieslar, M.; Prokeš, J. Polyaniline nanotubes: Conditions of formation. Polym. Int. 2006, 55, 31–39. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Sonawane, S.H. New approach for simultaneous enhancement of anticorrosive and mechanical properties of coatings: Application of water repellent nano CaCO3–PANI emulsion nanocomposite in alkyd resin. Chem. Eng. J. 2010, 156, 177–183. [Google Scholar] [CrossRef]
- Wei, Z.X.; Zhang, Z.M.; Wan, M.X. Formation mechanism of self-assembled polyaniline micro/nanotubes. Langmuir 2002, 18, 917–921. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, M. Self-assembly of polyaniline—from nanotubes to hollow microspheres. Adv. Funct. Mater. 2003, 13, 815–820. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Trchová, M.; Konyushenko, E.N.; Holler, P. The genesis of polyaniline nanotubes. Polymer 2006, 47, 8253–8262. [Google Scholar] [CrossRef]
- Janosevic, A.; Ciric-Marjanovic, G.; Marjanovic, B.; Holler, P.; Trchova, M.; Stejskal, J. Synthesis and characterization of conducting polyaniline 5-sulfosalicylate nanotubes. Nanotechnology 2008, 19, 135606. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Trchova, M.; Konyushenko, E.N. Oxidation of aniline: Polyaniline granules, nanotubes, and oligoaniline microspheres. Macromolecules 2008, 41, 3530–3536. [Google Scholar] [CrossRef]
- Tran, H.D.; Li, D.; Kaner, R.B. One-dimensional conducting polymer nanostructures: Bulk synthesis and applications. Adv. Mater. 2009, 21, 1487–1499. [Google Scholar] [CrossRef]
- Li, G.C.; Pang, S.P.; Xie, G.W.; Wang, Z.B.; Peng, H.R.; Zhang, Z.K. Synthesis of radially aligned polyaniline dendrites. Polymer 2006, 47, 1456–1459. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Li, X.; Lu, D. Surface properties of polyaniline/nano-TiO2 composites. Appl. Surf. Sci. 2004, 229, 395–401. [Google Scholar] [CrossRef]
- Gai, L.; Du, G.; Zuo, Z.; Wang, Y.; Liu, D.; Liu, H. Controlled synthesis of hydrogen titanate−polyaniline composite nanowires and their resistance−temperature characteristics. J. Phys. Chem. C 2009, 113, 7610–7615. [Google Scholar] [CrossRef]
- Bian, C.; Yu, Y.; Xue, G. Synthesis of conducting polyaniline/TiO2 composite nanofibres by one-stepin situ polymerization method. J. Appl. Polym. Sci. 2007, 104, 21–26. [Google Scholar] [CrossRef]
- Alam, J.; Riaz, U.; Ahmad, S. High performance corrosion resistant polyaniline/alkyd ecofriendly coatings. Curr. Appl. Phys. 2009, 9, 80–86. [Google Scholar] [CrossRef]




| Electrodes | Icorr (μA cm−2) | Ecorr (V) | ba (mV/dec) | bc (mV/dec) |
|---|---|---|---|---|
| Uncoated CS | 9.514 | −0.541 | 85.5 | 95.1 |
| CS-A-PANI | 6.028 | −0.481 | 70.9 | 85.3 |
| CS-N-PANI | 2.253 | −0.345 | 66.5 | 71 |
| Samples | Rc (Ω · cm2) | CPEc−T (F · cm−2) | CPEc−P | |||
|---|---|---|---|---|---|---|
| Value | Error (%) | Value | Error (%) | Value | Error (%) | |
| Alkyd | 1.48 × 107 | 1.54 | 2.55 × 10−9 | 5.45 | 0.90 | 0.62 |
| Alkyd−A2 | 7.90 × 107 | 5.31 | 1.69 × 10−9 | 2.01 | 0.92 | 0.26 |
| Alkyd−A5 | 3.01 × 107 | 2.88 | 2.43 × 10−9 | 5.54 | 0.90 | 0.69 |
| Alkyd−T2 | 7.38 × 108 | 9.14 | 1.26 × 10−9 | 5.89 | 0.93 | 0.79 |
| Alkyd−T5 | 1.32 × 109 | 4.87 | 1.08 × 10−9 | 4.04 | 0.95 | 0.55 |
| Alkyd−T8 | 5.30 × 107 | 1.42 | 2.31 × 10−9 | 1.96 | 0.91 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, G.; Liu, F.; Hou, N.; Peng, S.; He, C.; Fang, P. Preparation of One-Dimensional Polyaniline Nanotubes as Anticorrosion Coatings. Materials 2022, 15, 3192. https://doi.org/10.3390/ma15093192
Yang G, Liu F, Hou N, Peng S, He C, Fang P. Preparation of One-Dimensional Polyaniline Nanotubes as Anticorrosion Coatings. Materials. 2022; 15(9):3192. https://doi.org/10.3390/ma15093192
Chicago/Turabian StyleYang, Guangyuan, Fuwei Liu, Ning Hou, Sanwen Peng, Chunqing He, and Pengfei Fang. 2022. "Preparation of One-Dimensional Polyaniline Nanotubes as Anticorrosion Coatings" Materials 15, no. 9: 3192. https://doi.org/10.3390/ma15093192
APA StyleYang, G., Liu, F., Hou, N., Peng, S., He, C., & Fang, P. (2022). Preparation of One-Dimensional Polyaniline Nanotubes as Anticorrosion Coatings. Materials, 15(9), 3192. https://doi.org/10.3390/ma15093192







