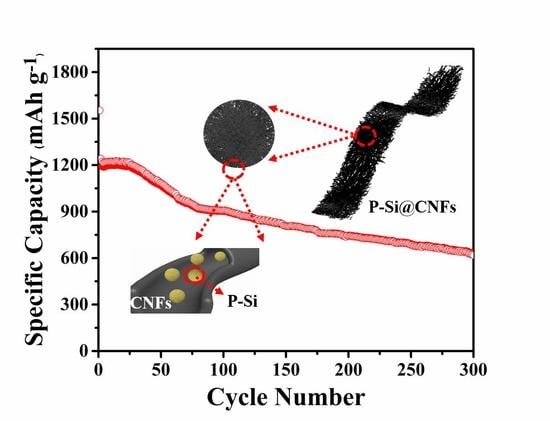
Flexible Porous Silicon/Carbon Fiber Anode for High−Performance Lithium−Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
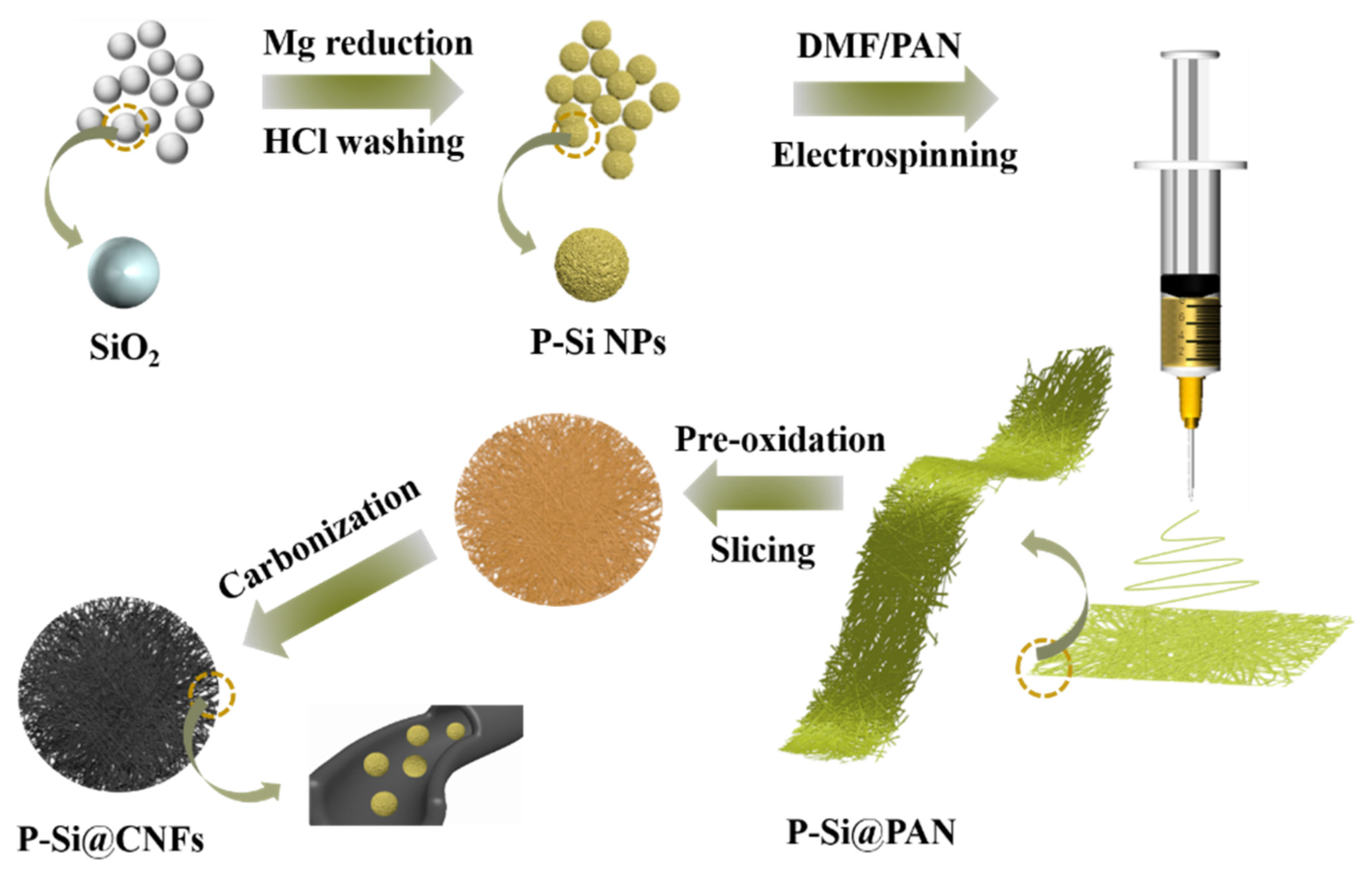
2.1. Synthesis of SiO2 Microspheres
2.2. Synthesis of P−Si NPs
2.3. Synthesis of P−Si@CNFs
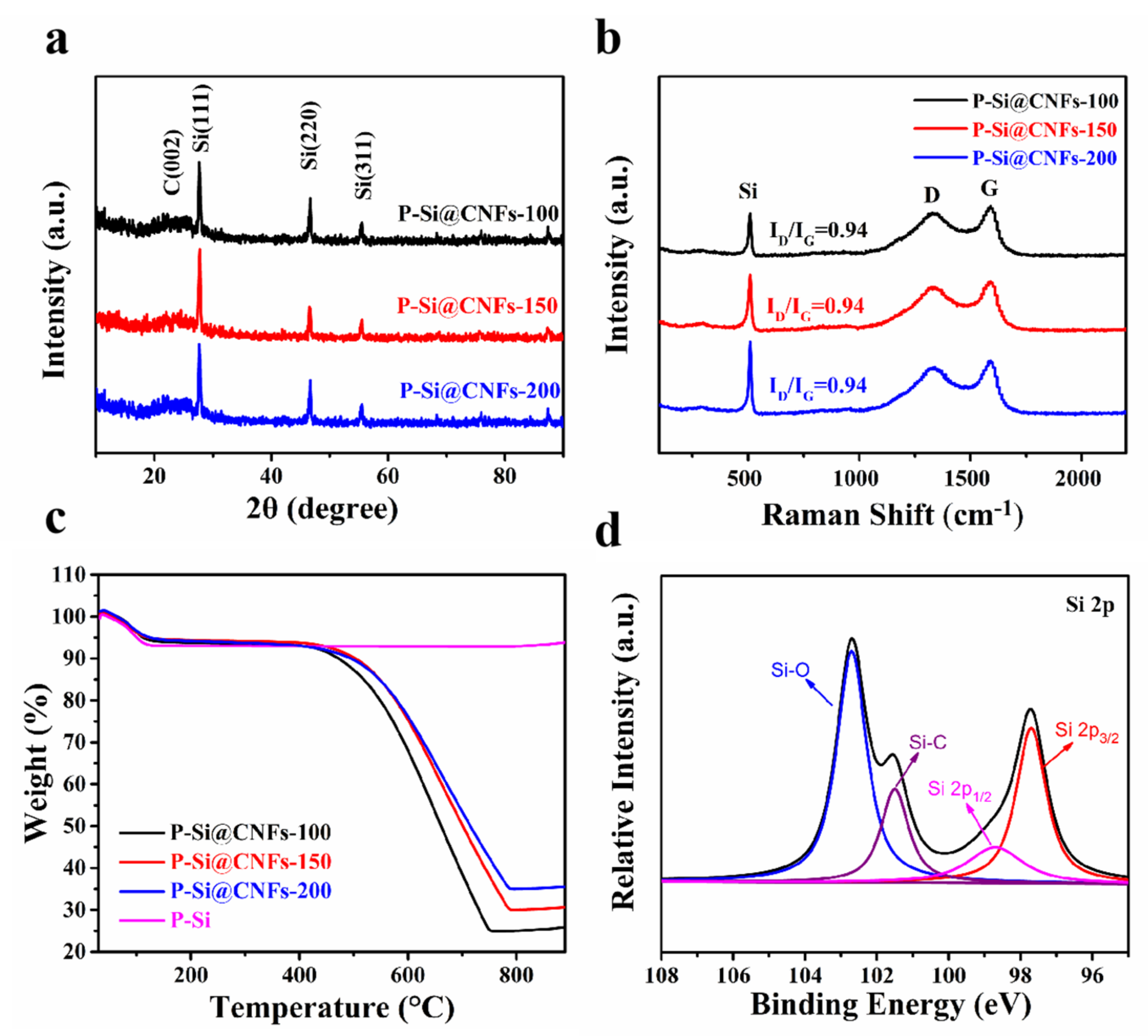
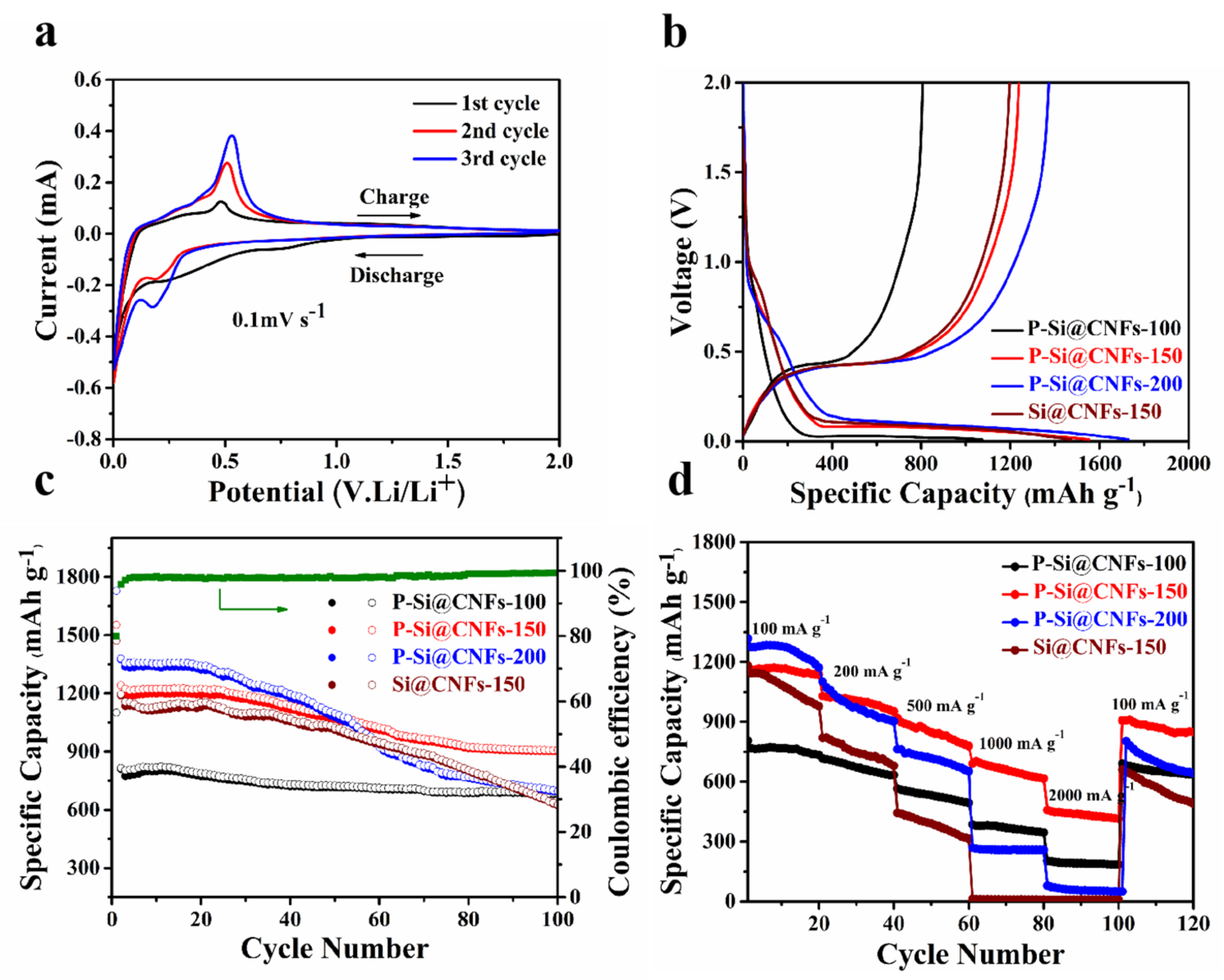
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almehmadi, F.A.; Alqaed, S.; Mustafa, J.; Jamil, B.; Sharifpur, M.; Cheraghian, G. Combining an active method and a passive method in cooling lithium−ion batteries and using the generated heat in heating a residential unit. J. Energy Storage 2022, 49, 104181. [Google Scholar] [CrossRef]
- Xu, Q.; Sun, J.-K.; Li, J.-Y.; Yin, Y.-X.; Guo, Y.-G. Scalable synthesis of spherical Si/C granules with 3D conducting networks as ultrahigh loading anodes in lithium−ion batteries. Energy Storage Mater. 2018, 12, 54–60. [Google Scholar] [CrossRef]
- Yan, M.-Y.; Li, G.; Zhang, J.; Tian, Y.-F.; Yin, Y.-X.; Zhang, C.-J.; Jiang, K.-C.; Xu, Q.; Li, H.-L.; Guo, Y.-G. Enabling SiOx/C Anode with High Initial Coulombic Efficiency through a Chemical Pre−Lithiation Strategy for High−Energy−Density Lithium−Ion Batteries. ACS Appl. Mater. Interfaces 2020, 12, 27202–27209. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.; Chae, S.; Ma, J.; Ko, M.; Cho, J. Fast−charging high−energy lithium−ion batteries via implantation of amorphous silicon nanolayer in edge−plane activated graphite anodes. Nat. Commun. 2017, 8, 812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Yang, D.; Li, J.; Su, F. Nanostructured Si−Based Anodes for Lithium−Ion Batteries. J. Nanosci. Nanotechnol. 2015, 15, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Alqaed, S.; Almehmadi, F.A.; Mustafa, J.; Husain, S.; Cheraghian, G. Effect of nano phase change materials on the cooling process of a triangular lithium battery pack. J. Energy Storage 2022, 51, 104326. [Google Scholar] [CrossRef]
- Xu, S.; Hou, X.; Wang, D.; Zuin, L.; Zhou, J.; Hou, Y.; Mann, M. Insights into the Effect of Heat Treatment and Carbon Coating on the Electrochemical Behaviors of SiO Anodes for Li−Ion Batteries. Adv. Energy Mater. 2022. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, H.; Wang, Y.; Xia, L.; Tan, Q.; Li, H.; Zhong, Z.; Su, F.; Zhao, X.S. Growth of silicon/carbon microrods on graphite microspheres as improved anodes for lithium−ion batteries. J. Mater. Chem. A 2013, 1, 4483–4489. [Google Scholar] [CrossRef]
- Wang, J.; Gao, C.; Yang, Z.; Zhang, M.; Li, Z.; Zhao, H. Carbon−coated mesoporous silicon shell−encapsulated silicon nano−grains for high performance lithium−ion batteries anode. Carbon 2022, 192, 277–284. [Google Scholar] [CrossRef]
- Zhang, Y.; Du, N.; Zhu, S.; Chen, Y.; Lin, Y.; Wu, S.; Yang, D. Porous silicon in carbon cages as high−performance lithium−ion battery anode Materials. Electrochim. Acta 2017, 252, 438–445. [Google Scholar] [CrossRef]
- Nie, P.; Le, Z.; Chen, G.; Liu, D.; Liu, X.; Wu, H.B.; Xu, P.; Li, X.; Liu, F.; Chang, L.; et al. Graphene Caging Silicon Particles for High−Performance Lithium−Ion Batteries. Small 2018, 14, 1800635. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Zhou, X.; Tang, J.; Ding, J.; Chen, S.; Zhang, J.; Hu, T.; Yang, X.-S.; Wang, X.; Yang, J. Boron−Doped Spherical Hollow−Porous Silicon Local Lattice Expansion toward a High−Performance Lithium−Ion−Battery Anode. Inorg. Chem. 2019, 58, 4592–4599. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xi, F.; Ma, Q.; Wan, X.; Li, S.; Ma, W.; Chen, X.; Chen, Z.; Deng, R.; Ji, J.; et al. A nanosilver−actuated high−performance porous silicon anode from recycling of silicon waste. Mater. Today Nano 2022, 17, 100162. [Google Scholar] [CrossRef]
- Guo, S.; Hu, X.; Hou, Y.; Wen, Z. Tunable Synthesis of Yolk–Shell Porous Silicon@Carbon for Optimizing Si/C−Based Anode of Lithium−Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 42084–42092. [Google Scholar] [CrossRef]
- Shao, F.; Li, H.; Yao, L.; Xu, S.; Li, G.; Li, B.; Zou, C.; Yang, Z.; Su, Y.; Hu, N.; et al. Binder−Free, Flexible, and Self−Standing Non−Woven Fabric Anodes Based on Graphene/Si Hybrid Fibers for High−Performance Li−Ion Batteries. ACS Appl. Mater. Interfaces 2021, 13, 27270–27277. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, X.; Li, J.; Liu, G.; Jia, D.; Ma, Z.; Zhang, L.; Peng, Z.; Zhu, X. High−performance, flexible, binder−free silicon–carbon anode for lithium storage applications. Electrochem. Commun. 2022, 137, 107257. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, Y.; Shao, J.; Shen, Z.; Chen, R.; Zhang, X.; He, X.; Song, Y.; Xing, X. Pyrolytic carbon−coated silicon/carbon nanofiber composite anodes for high−performance lithium−ion batteries. J. Power Sources 2015, 298, 130–137. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.-W.; Zhao, W.; Cui, Y. A pomegranate−inspired nanoscale design for large−volume−change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Ghanooni Ahmadabadi, V.; Shirvanimoghaddam, K.; Kerr, R.; Showkath, N.; Naebe, M. Structure−rate performance relationship in Si nanoparticles−carbon nanofiber composite as flexible anode for lithium−ion batteries. Electrochim. Acta 2020, 330, 135232. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; Al-Mamun, M.; Dou, Y.; Liu, P.; Su, D.; Wang, G.; Zhang, S.; Wang, D.; Zhao, H. A Hollow−Shell Structured V2O5 Electrode−Based Symmetric Full Li−Ion Battery with Highest Capacity. Adv. Energy Mater. 2019, 9, 1900909. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, Q.; Liao, X.; Dou, Y.; Liu, P.; Al-Mamun, M.; Wang, Y.; Zhang, S.; Zhao, S.; Wang, D.; et al. Scalable and controllable fabrication of CNTs improved yolk−shelled Si anodes with advanced in operando mechanical quantification. Energy Environ. Sci. 2021, 14, 3502–3509. [Google Scholar] [CrossRef]
- Mu, G.; Ding, Z.; Mu, D.; Wu, B.; Bi, J.; Zhang, L.; Yang, H.; Wu, H.; Wu, F. Hierarchical void structured Si/PANi/C hybrid anode material for high−performance lithium−ion batteries. Electrochim. Acta 2019, 300, 341–348. [Google Scholar] [CrossRef]
- Ji, L.; Zhang, X. Electrospun carbon nanofibers containing silicon particles as an energy−storage medium. Carbon 2009, 47, 3219–3226. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Y.; Jiang, B.; Zhao, Y.; Guo, X.; Ma, T. Hollow−structure engineering of a silicon–carbon anode for ultra−stable lithium−ion batteries. Dalton Trans. 2020, 49, 5669–5676. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Qu, S.; Zhao, P. A facile fabrication of micro/nano−sized silicon/carbon composite with a honeycomb structure as high−stability anodes for lithium−ion batteries. J. Electroanal. Chem. 2021, 884, 115074. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, Y.; Ren, Y.; Mu, T.; Yin, X.; Du, C.; Huo, H.; Cheng, X.; Zuo, P.; Yin, G. Engineering Molecular Polymerization for Template−Free SiOx/C Hollow Spheres as Ultrastable Anodes in Lithium−Ion Batteries. Adv. Funct. Mater. 2021, 31, 2101145. [Google Scholar] [CrossRef]
- He, Y.; Xiang, K.; Zhou, W.; Zhu, Y.; Chen, X.; Chen, H. Folded−hand silicon/carbon three−dimensional networks as a binder−free advanced anode for high−performance lithium−ion batteries. Chem. Eng. J. 2018, 353, 666–678. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Wang, Y.; Zhang, Z.; Kan, G.; Wang, C.; Zhong, Z.; Su, F. Graphitized porous carbon microspheres assembled with carbon black nanoparticles as improved anode materials in Li−ion batteries. J. Mater. Chem. A 2014, 2, 10161–10168. [Google Scholar] [CrossRef]
- Guan, P.; Li, J.; Lu, T.; Guan, T.; Ma, Z.; Peng, Z.; Zhu, X.; Zhang, L. Facile and Scalable Approach To Fabricate Granadilla−like Porous−Structured Silicon−Based Anode for Lithium Ion Batteries. ACS Appl. Mater. Interfaces 2018, 10, 34283–34290. [Google Scholar] [CrossRef]
- Jia, H.; Zheng, J.; Song, J.; Luo, L.; Yi, R.; Estevez, L.; Zhao, W.; Patel, R.; Li, X.; Zhang, J.-G. A novel approach to synthesize micrometer−sized porous silicon as a high performance anode for lithium−ion batteries. Nano Energy 2018, 50, 589–597. [Google Scholar] [CrossRef]
- Yoon, N.; Young, C.; Kang, D.; Park, H.; Lee, J.K. High−conversion reduction synthesis of porous silicon for advanced lithium battery anodes. Electrochim. Acta 2021, 391, 138967. [Google Scholar] [CrossRef]
- Cao, M.; Liao, F.; Wang, Q.; Luo, W.; Ma, Y.; Zheng, X.; Wang, Y.; Zhang, L. Rational design of ZnS/CoS heterostructures in three dimensional N−doped CNTs for superior lithium storage. J. Alloys Compd. 2021, 859, 157867. [Google Scholar] [CrossRef]
- Zhu, G.; Zhang, F.; Li, X.; Luo, W.; Li, L.; Zhang, H.; Wang, L.; Wang, Y.; Jiang, W.; Liu, H.K.; et al. Engineering the Distribution of Carbon in Silicon Oxide Nanospheres at the Atomic Level for Highly Stable Anodes. Angew. Chem. Int. Ed. 2019, 58, 6669–6673. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Zhu, X.; Li, X.; Jia, D.; Li, D.; Ma, Z.; Li, J. Flexible Porous Silicon/Carbon Fiber Anode for High−Performance Lithium−Ion Batteries. Materials 2022, 15, 3190. https://doi.org/10.3390/ma15093190
Liu G, Zhu X, Li X, Jia D, Li D, Ma Z, Li J. Flexible Porous Silicon/Carbon Fiber Anode for High−Performance Lithium−Ion Batteries. Materials. 2022; 15(9):3190. https://doi.org/10.3390/ma15093190
Chicago/Turabian StyleLiu, Gang, Xiaoyi Zhu, Xiaohua Li, Dongchen Jia, Dong Li, Zhaoli Ma, and Jianjiang Li. 2022. "Flexible Porous Silicon/Carbon Fiber Anode for High−Performance Lithium−Ion Batteries" Materials 15, no. 9: 3190. https://doi.org/10.3390/ma15093190
APA StyleLiu, G., Zhu, X., Li, X., Jia, D., Li, D., Ma, Z., & Li, J. (2022). Flexible Porous Silicon/Carbon Fiber Anode for High−Performance Lithium−Ion Batteries. Materials, 15(9), 3190. https://doi.org/10.3390/ma15093190