Study on the Surface of Cobalt-Chromium Dental Alloys and Their Behavior in Oral Cavity as Cast Materials
Abstract
:1. Introduction
1.1. Corrosion at the Surface of Co–Cr Alloys
1.2. Structure of Co–Cr Alloys
1.3. Oxidation of Co–Cr Alloys
2. Materials and Methods
2.1. Materials
- -
- Co59.5Cr31.5Mo5Si2Mn1.0 Super C (Dental Alloy Products) [35] alloy named: 0.
- -
- Co61Cr26Mo6W5Fe0.5Si1 Wirobond C alloy (Bego Gmbh) [36] alloy named: 5.
- -
- Co59Cr25Mo4W10Si1Mn0.8 alloy Heraenium P (Heraeus Kulzer) [37] alloy named: 10.
- -
- Co55.2Cr24W15Fe4Si1Mn0.8 alloy Heraenium Pw (Heraeus Kulzer) [38] alloy named: 15.
- -
- Co54.1Cr20W16.4Fe7.5Si1.5Mn0.3 Starloy Soft (Dentsply) [39] alloy named: 16.4.
2.2. Methods
2.2.1. Corrosion
Electrochemical Impedance Spectroscopy (EIS)
Open Circuit Potential
Linear Polarization Measurement
2.2.2. X-ray Diffractometry
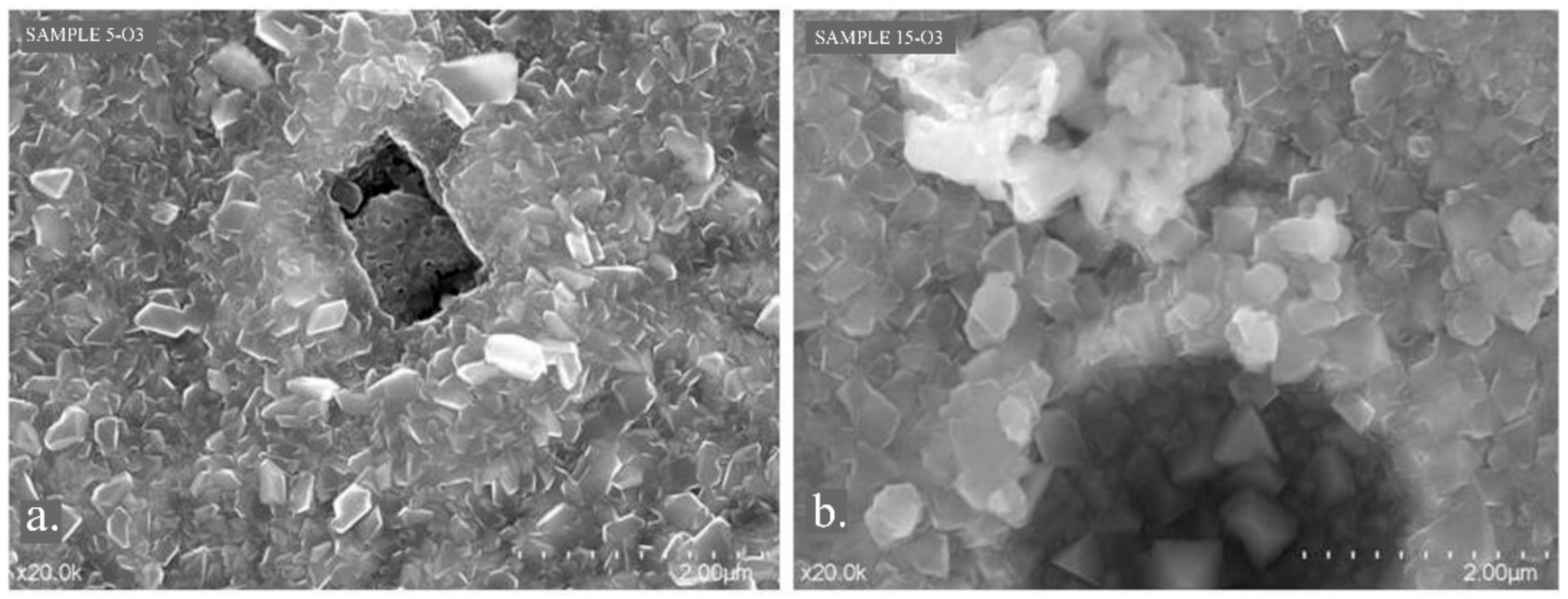
2.2.3. SEM—Scanning Electron Microscopy
3. Results
- -
- OTI1: vacuum treatment with high temperature oxidation of samples P (G2), followed by samples O1 (5-O1, 15-O1).
- -
- OTI2: high temperature oxidation vacuum heat treatment of O1 samples, followed by O2 samples (5-O2, 15-O2).
- -
- OC3: heat treatment in atmospheric air with continuous oxidation of O2 samples, followed by O3 samples (5-O3, 15-O3).
3.1. Corrosion
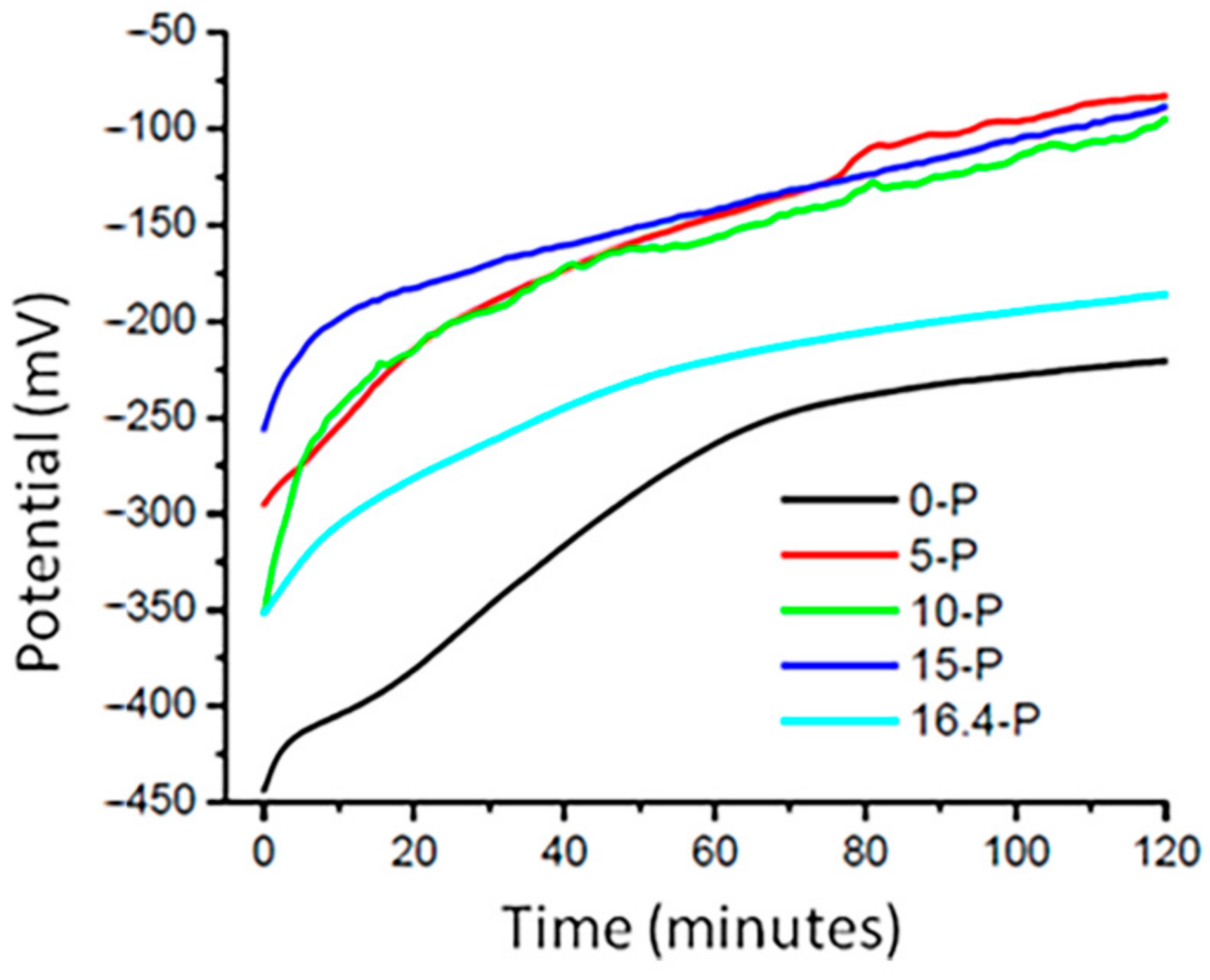
3.1.1. Open Circuit Potential (OCP) Measurement
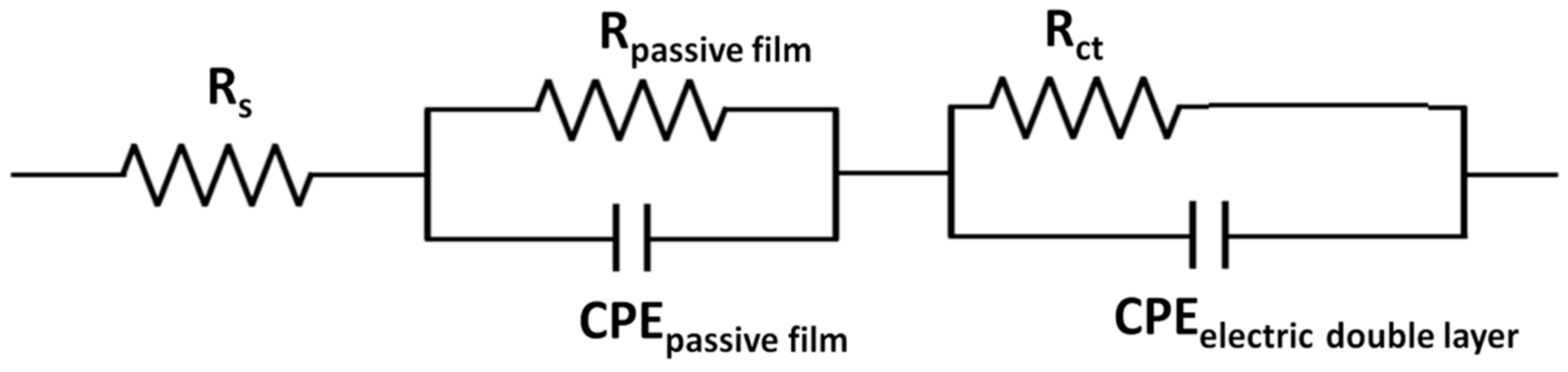
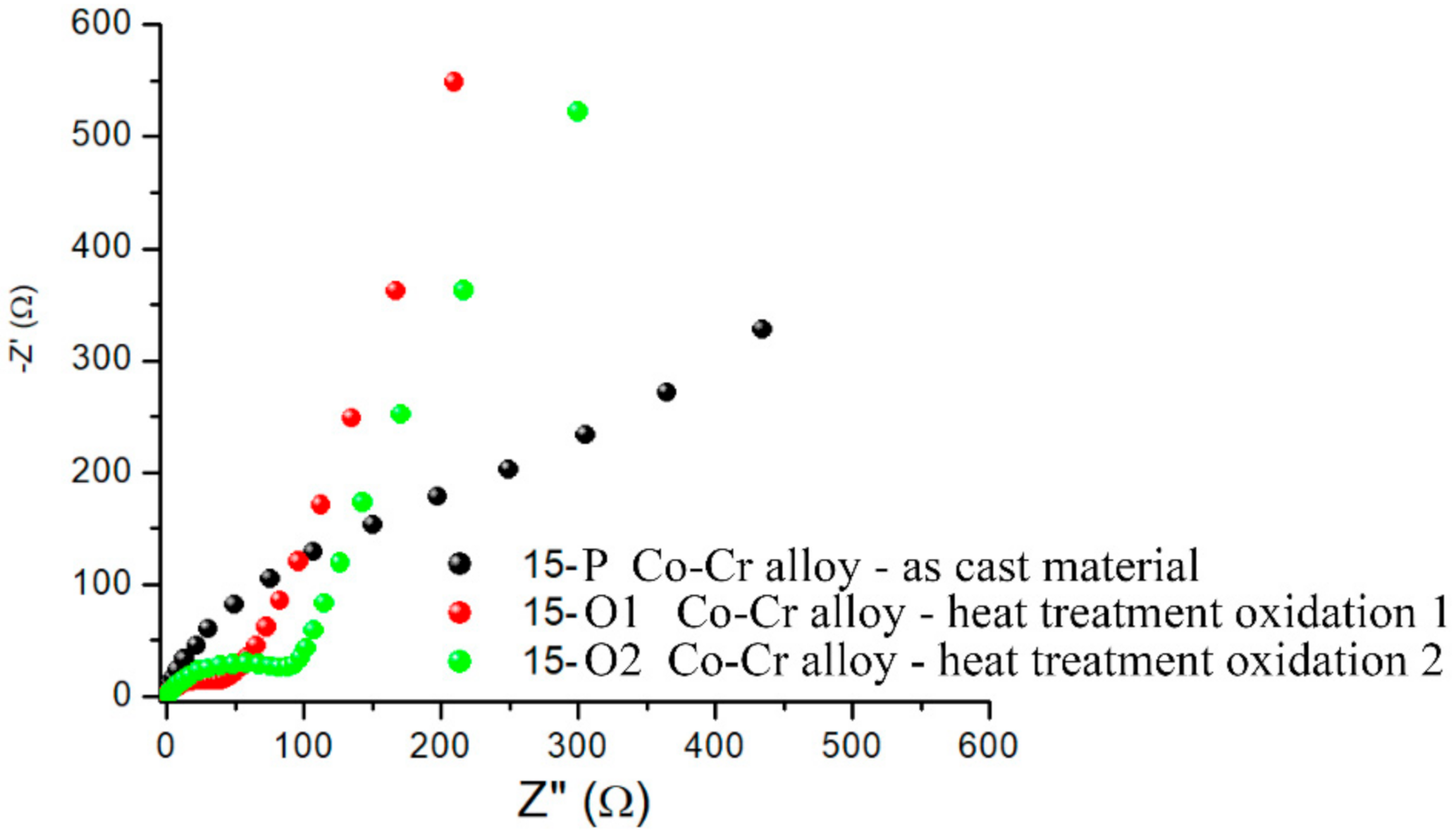
3.1.2. Electrochemical Impedance Spectroscopy (EIS)
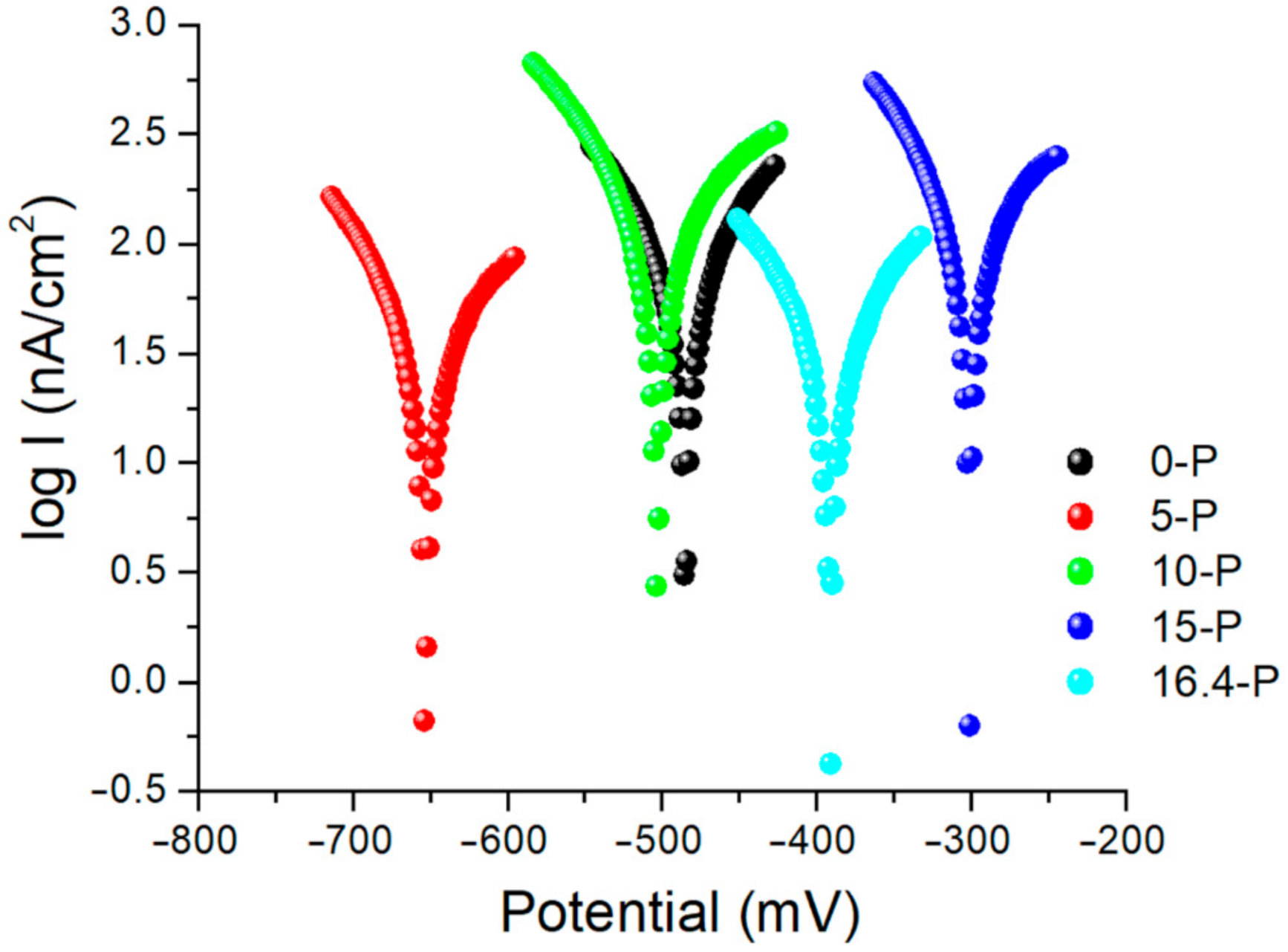
3.1.3. Linear Polarization Measurement
3.1.4. Electrochemical Impedance Studies to Characterize the Properties of the 15-P Alloy after Various Processing Steps
3.2. Scanning Electron Microscopy and EDS Spectrometry
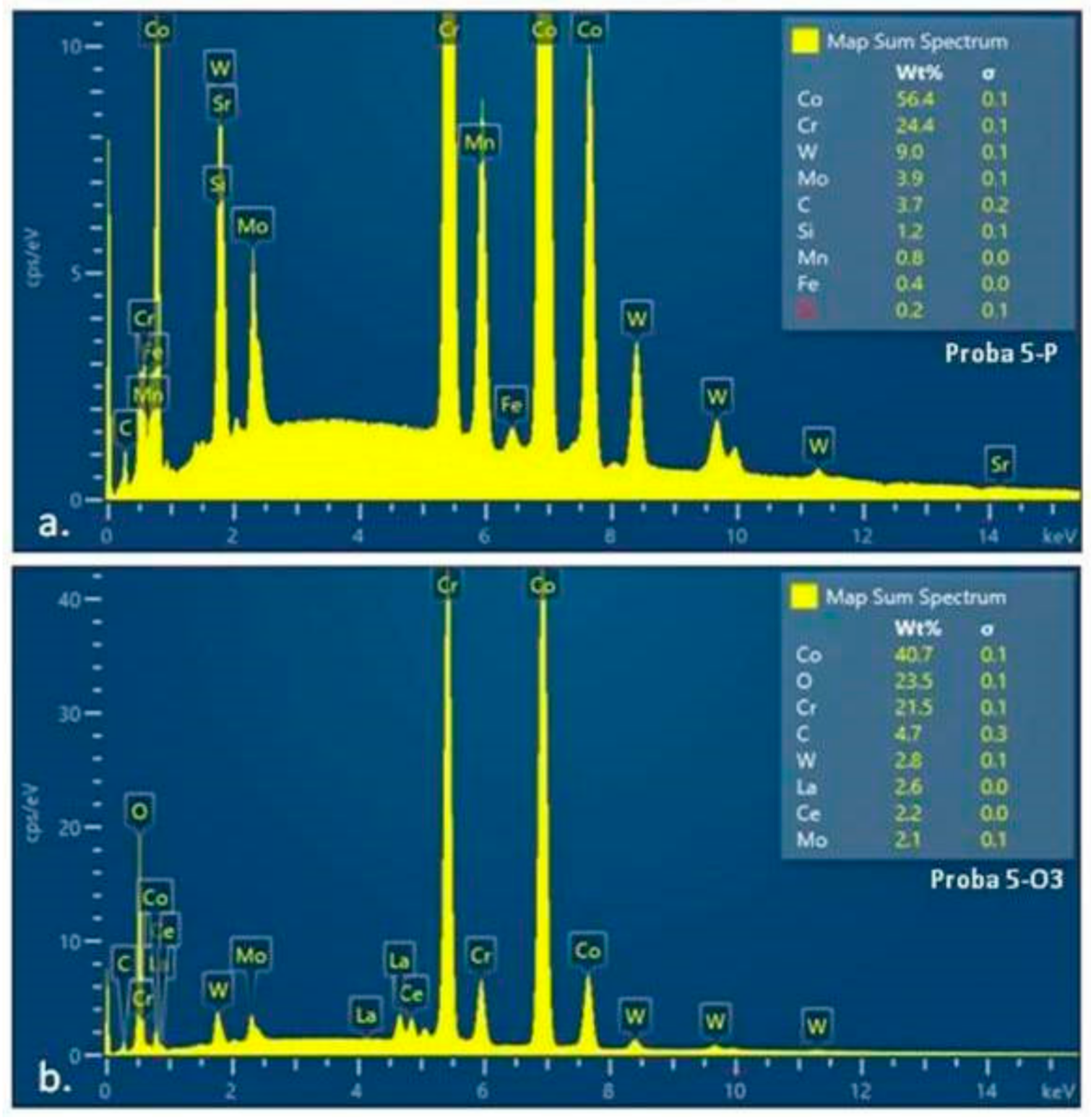
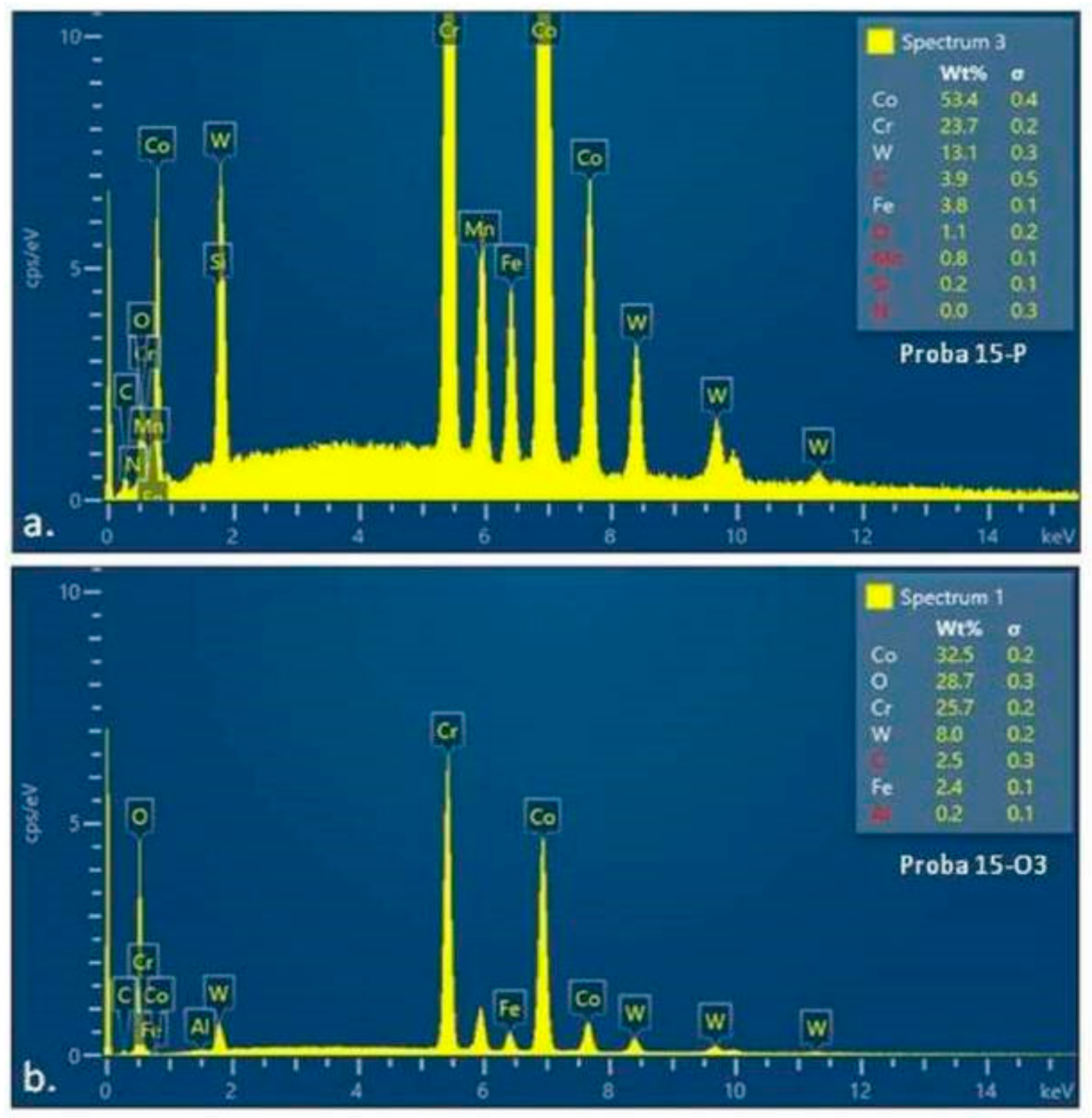
3.3. Energy-Dispersive X-ray Spectroscopy: EDS
3.4. X-ray Diffraction
- -
- Co; a = 3.54 Å;
- -
- Cr; a = 3.6 Å;
- -
- Fe; a = 3.64 Å;
- -
- Mo; a = 4.03 Å;
- -
- W; a = 4.06 Å;
- -
- Co; a = b = 2.507 Å, and c = 4.08 Å.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uriciuc, W.A. Research about Precision Casting of Removable-Fixed Ensemble Applied in Dental Prosthodontics. In International Conference on Advancements of Medicine and Health Care through Technology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 379–382. [Google Scholar]
- Iulian, A.; Cosmin, S.; Aurora, A. Adhesion aspects in biomaterials and medical devices. J. Adhes. Sci. Technol. 2016, 30, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Arnold, C.; Hey, J.; Schweyen, R.; Setz, J.M. Accuracy of CAD-CAM-fabricated removable partial dentures. J. Prosthet. Dent. 2018, 119, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, M.; Laptoiu, D.; Miculescu, F.; Antoniac, I.V. Metal Allergy and other Adverse Reactions in Patients with Total Hip Replacement. Adv. Mater. Res. 2015, 1114, 283–287. [Google Scholar] [CrossRef]
- Kim, H.R.; Jang, S.-H.; Kim, Y.K.; Son, J.S.; Min, B.K.; Kim, K.-H.; Kwon, T.-Y. Microstructures and Mechanical Properties of Co-Cr Dental Alloys Fabricated by Three CAD/CAM-Based Processing Techniques. Materials 2016, 9, 596. [Google Scholar] [CrossRef]
- Uriciuc, W.A.; Boșca, A.B.; Babtan, A.M.; Feurden, C.N.; Ionel, A.; Vermeșan, H.; Popa, C.O.; Ilea, A. Optimization of the Manufacturing Process by Molding Cobalt-Chrome Alloys in Assembled Dental Frameworks. Prosthesis 2021, 3, 245–260. [Google Scholar] [CrossRef]
- Uriciuc, W.A.; Vermesan, H.; Tiuc, A.E.; Ilea, A.; Bosca, A.B.; Popa, C.O. Casting over Metal Method Used in Manufacturing Hybrid Cobalt-Chromium Dental Prosthetic Frameworks Assembles. Materials 2021, 14, 539. [Google Scholar] [CrossRef]
- Petrut, A.; Ivanov, A.; Antoniac, I.; Miculescu, F.; Cosmeleata, G. Experimental researches about technological and microstructural aspects in the case of cobalt-chromium dental alloys. Eur. Cells Mater. 2007, 13, 28. [Google Scholar]
- Uriciuc, W.A.; Vermeșan, H.; Boșca, A.B.; Ilea, A. Interaction of Saliva with Cobalt-Chromium-Based Dental Alloys in Casted Prosthetic Pieces. Curr. Trends Biomedical. Eng. Biosci. 2018, 14, 555882. [Google Scholar] [CrossRef]
- Upadhyay, D.; Panchal, M.A.; Dubey, R.S.; Srivastava, V.K. Corrosion of alloys used in dentistry: A review. Mater. Sci. Eng. A 2006, 432, 1–11. [Google Scholar] [CrossRef]
- Wataha, J.C.; Schmalz, G. Dental Alloys. In Biocompatibility of Dental Materials; Springer: Berlin/Heidelberg, Germany, 2009; pp. 221–254. [Google Scholar] [CrossRef]
- Kassapidou, M.; Franke Stenport, V.; Hjalmarsson, L.; Johansson, C.B. Cobalt-chromium alloys in fixed prosthodontics in Sweden. Acta Biomater. Odontol. Scand. 2017, 3, 53–62. [Google Scholar] [CrossRef]
- Lucchetti, M.C.; Fratto, G.; Valeriani, F.; De Vittori, E.; Giampaoli, S.; Papetti, P.; Spica, V.R.; Manzon, L. Cobalt-chromium alloys in dentistry: An evaluation of metal ion release. J. Prosthet. Dent. 2015, 114, 602–608. [Google Scholar] [CrossRef] [PubMed]
- Puskar, T.; Jevremovic, D.; Williams, R.J.; Eggbeer, D.; Vukelic, D.; Budak, I. A Comparative Analysis of the Corrosive Effect of Artificial Saliva of Variable pH on DMLS and Cast Co-Cr-Mo Dental Alloy. Materials 2014, 7, 6486–6501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, R.W.-W.; Yang, C.-C.; Huang, C.-A.; Chen, Y.-S. Electrochemical corrosion studies on Co–Cr–Mo implant alloy in biological solutions. Mater. Chem. Phys. 2005, 93, 531–538. [Google Scholar] [CrossRef]
- Lee, S.-H.; Takahashi, E.; Nomura, N.; Chiba, A. Effect of Carbon Addition on Microstructure and Mechanical Properties of a Wrought Co–Cr–Mo Implant Alloy. Mater. Trans. 2006, 47, 287–290. [Google Scholar] [CrossRef] [Green Version]
- Alifui-Segbaya, F.; Foley, P.; Williams, R.J. The corrosive effects of artificial saliva on cast and rapid manufacture-produced cobalt chromium alloys. Rapid Prototyp. J. 2013, 19, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Surmann, H.; Huser, J. Automatic electropolishing of cobalt chromium dental cast alloys with a fuzzy logic controller. J. Comput. Chem. Eng. 1998, 22, 1099–1111. [Google Scholar] [CrossRef]
- Reimann, L.; Dobrzański, L.A. Influence of the casting temperature on dental Co-base alloys properties. Arch. Mater. Sci. Eng. 2013, 60, 5–12. [Google Scholar]
- O’Brien, J.O. Dental Materials and Their Selection, 3rd ed.; Quintessence Publishing Co, Inc.: Hanover Park, IL, USA, 2002; ISBN 0-86715-406-3. [Google Scholar]
- Miculescu, M.; Bane, M.; Miculescu, F.; Ciocan, L.T.; Preda, O.; Antoniac, I.V. A Study on Metal-Ceramic Interface on Metal Base Dental Alloys. Key Eng. Mater. 2015, 638, 14–19. [Google Scholar] [CrossRef]
- Ghiban, A.; Ghiban, B.; Borţun, C.M.; Ghiban, N.; Antoniac, I.V. Heat Treatments Influence on Corrosion Resistance of some New Dental Cobalt Alloys. Solid State Phenom. 2012, 188, 93–97. [Google Scholar] [CrossRef]
- Saldıvar, A.; López, H. Role of aging on the martensitic transformation in a cast cobalt alloy. Scr. Mater. 2001, 45, 427–433. [Google Scholar] [CrossRef]
- Lashgari, H.R.; Zangeneh, S.; Hasanabadi, F.; Saghafi, M. Microstructural evolution during isothermal aging and strain-induced transformation followed by isothermal aging in Co-Cr-Mo-C alloy: A comparative study. Mater. Sci. Eng. A 2010, 527, 4082–4091. [Google Scholar] [CrossRef]
- Mori, M.; Yamanaka, K.; Chiba, A. Phase decomposition in biomedical Co–29Cr–6Mo–0.2N alloy during isothermal heat treatment at 1073K. J. Alloy. Compd. 2014, 590, 411–416. [Google Scholar] [CrossRef]
- Lu, Y.; Wu, S.; Gan, Y.; Zhang, S.; Guo, S.; Lin, J.; Lin, J. Microstructure, mechanical property and metal release of As-SLM CoCrW alloy under different solution treatment conditions. J. Mech. Behav. Biomed. Mater. 2016, 55, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; López, H. Strain induced ε-martensite in a Co–Cr–Mo alloy: Grain size effects. Mater. Lett. 1999, 39, 244–248. [Google Scholar] [CrossRef]
- Savencu, C.E.; Porojan, L.; Bîrdeanu, M.; Boloș, A.; Porojan, S.; Antoniac, A.; Gradinaru, S. Surface Characteristics of Base Metal Dental Alloys Processed by Alternative Procedures. In Defect and Diffusion Forum; Trans Tech Publications Ltd: Geneva, Switzerland, 2017; Volume 376, pp. 1–11. [Google Scholar] [CrossRef]
- Wu, Y.; Moser, J.; Jameson, L.; Malone, W. The effect of oxidation heat treatment on porcelain bond strength in selected base metal alloys. J. Prosthet. Dent. 1991, 66, 439–444. [Google Scholar] [CrossRef]
- Dent, R.J.; Preston, J.D.; Moffa, J.P.; Caputo, A. Effect of oxidation on ceramometal bond strength. J. Prosthet. Dent. 1982, 47, 59–62. [Google Scholar] [CrossRef]
- Jones, D.W. Coatings of Ceramics on Metals. Ann. N. Y. Acad. Sci. 1988, 523, 19–37. [Google Scholar] [CrossRef]
- Park, S.-J.; Seo, S.-M.; Yoo, Y.-S.; Jeong, H.-W.; Jang, H. Effects of Cr, W, and Mo on the High Temperature Oxidation of Ni-Based Superalloys. Materials 2019, 12, 2934. [Google Scholar] [CrossRef] [Green Version]
- Park, W.U.; Park, H.G.; Hwang, K.H.; Zhao, J.; Lee, J.K. Interfacial Property of Dental Cobalt–Chromium Alloysand Their Bonding Strength with Porcelains. J. Nanosci. Nanotechnol. 2017, 17, 2585–2588. [Google Scholar] [CrossRef]
- Uriciuc, W.A.; Vermeşan, H.; Botean, A.I.; Nistor, L.; Catalin, P.O.P.A. Research on the surface of the dental alloys with cobalt-crom base. Acta Tech. Napoc.-Ser. Appl. Math. Mech. Eng. 2019, 62. Available online: https://atna-mam.utcluj.ro/index.php/Acta/article/view/1244 (accessed on 13 March 2022).
- Available online: http://www.alloysonline.com/DAP/html/ENGLISH/daprodlookup/ro_productlookup.html (accessed on 14 June 2021).
- Available online: https://www.bego.com/conventional-solutions/alloys/product/Category/list/category/23/ (accessed on 13 June 2021).
- Available online: https://www.kulzer.com/int2/en/products/heraenium-p-for-c-and-b.html (accessed on 13 June 2021).
- Available online: https://www.kulzer.com/en/en/products/heraenium-pw-for-c-and-b.html (accessed on 13 June 2021).
- Available online: https://www.dentsplysirona.com/content/dam/dentsply/pim/manufacturer/Prosthetics/Fixed/Alloys/CoCr_Alloys/StarLoy_C/PR%20StarLoy_EN.pdf (accessed on 14 June 2021).







| Alloys | Co [%] | Cr [%] | Mo [%] | W [%] | Fe [%] | Si [%] | C [%] | Mn [%] | N [%] | Ce [%] | Nb [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 59.5 | 31.5 | 5 | - | x | 2 | - | x | - | - | |
| 5 | 61 | 26 | 6 | 5 | 0.5 | 1 | 0.02 | - | - | 0.5 | 0.9 |
| 10 | 59 | 25 | 4 | 10 | - | 1 | - | 0.8 | 0.2 | - | - |
| 15 | 55.2 | 24 | - | 15 | 4 | 1 | - | 0.8 | x | - | - |
| 16.4 | 54.1 | 20 | - | 16.4 | 7.5 | 1.5 | - | 0.3 | - | - | 0.2 |
| Alloys | E0 (T = 0 min) (mV) | E120 (T = 120 min) (mV) | ΔE (mV) | RSD (%) |
|---|---|---|---|---|
| 0-P | −443.62 | −220.75 | 222.87 | 3.4 |
| 5-P | −295.31 | −83.12 | 212.19 | 2.7 |
| 10-P | −351.87 | −95.00 | 256.87 | 1.9 |
| 15-P | −256.37 | −88.56 | 167.81 | 2.1 |
| 16.4-P | −351.50 | −186.31 | 165.20 | 4.3 |
| Sample | RS [Ωcm2] | Rct [Ωcm2] | CPEelectric double layer [F/cm2] | CPEpassive film [F/cm2] |
|---|---|---|---|---|
| 0-P | 16,296 | 232,660 | 1.3204 × 10−5 | 0.88785 |
| 5-P | 15,437 | 537,080 | 1.4201 × 10−5 | 0.87963 |
| 10-P | 16,934 | 176,901 | 1.6782 × 10−5 | 0.87001 |
| 15-P | 14,553 | 146,120 | 1.6131 × 10−5 | 0.87704 |
| 16.4-P | 14,306 | 509,751 | 13987 × 10−5 | 0.83287 |
| Alloys | E(I = 0) [mV] | Icor [μA/cm2] | Rp [kohm/cm2] | βa [mV] | βc [mV] | Vcor [μm/an] |
|---|---|---|---|---|---|---|
| 0-P | −484.7 | 73.2902 | 243.23 | 112.4 | −103.2 | 1.0960 |
| 5-P | −652.6 | 35.4831 | 537.08 | 140.7 | −91.00 | 0.5308 |
| 10-P | −502.9 | 141.57 | 178.91 | 214.1 | −118.8 | 2.1170 |
| 15-P | −300.9 | 86.4078 | 161.62 | 113.2 | −71.8 | 1.2920 |
| 16.4-P | −391.0 | 35.7285 | 511.57 | 117.2 | −106.6 | 0.5344 |
| Elements | wt% | wt% Sigma |
|---|---|---|
| C | 4.74 | 0.26 |
| O | 23.46 | 0.13 |
| Cr | 21.48 | 0.08 |
| Co | 40.75 | 0.14 |
| Mo | 2.06 | 0.06 |
| La | 2.58 | 0.04 |
| Ce | 2.16 | 0.04 |
| W | 2.78 | 0.06 |
| Total: | 100.00 | |
| Elements | wt% | wt% Sigma |
|---|---|---|
| C | 2.70 | 0.15 |
| O | 27.36 | 0.12 |
| S | 0.02 | 0.02 |
| Cr | 19.50 | 0.07 |
| Fe | 2.84 | 0.03 |
| Co | 38.38 | 0.11 |
| W | 9.19 | 0.09 |
| Total: | 100.00 | |
| Sample 5 | 5-A | 5-P | 5-O1 | 5-O2 | 5-O3 |
| Crystallite size hexagonal Co (Å) | 153 | 128 | - | - | - |
| Crystallite size cubic Co (Å) | 287 | 266 | 456 | 500 | 556 |
| Sample 15 | 15-A | 15-P | 15-O1 | 15-O2 | 15-O3 |
| Crystallite size hexagonal Co (Å) | 142 | 114 | - | - | - |
| Crystallite size cubic Co (Å) | 210 | 190 | 406 | 412 | 442 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uriciuc, W.A.; Boșca, A.B.; Băbțan, A.-M.; Vermeșan, H.; Cristea, C.; Tertiș, M.; Pășcuță, P.; Borodi, G.; Suciu, M.; Barbu-Tudoran, L.; et al. Study on the Surface of Cobalt-Chromium Dental Alloys and Their Behavior in Oral Cavity as Cast Materials. Materials 2022, 15, 3052. https://doi.org/10.3390/ma15093052
Uriciuc WA, Boșca AB, Băbțan A-M, Vermeșan H, Cristea C, Tertiș M, Pășcuță P, Borodi G, Suciu M, Barbu-Tudoran L, et al. Study on the Surface of Cobalt-Chromium Dental Alloys and Their Behavior in Oral Cavity as Cast Materials. Materials. 2022; 15(9):3052. https://doi.org/10.3390/ma15093052
Chicago/Turabian StyleUriciuc, Willi Andrei, Adina Bianca Boșca, Anida-Maria Băbțan, Horațiu Vermeșan, Cecilia Cristea, Mihaela Tertiș, Petru Pășcuță, Gheorghe Borodi, Maria Suciu, Lucian Barbu-Tudoran, and et al. 2022. "Study on the Surface of Cobalt-Chromium Dental Alloys and Their Behavior in Oral Cavity as Cast Materials" Materials 15, no. 9: 3052. https://doi.org/10.3390/ma15093052
APA StyleUriciuc, W. A., Boșca, A. B., Băbțan, A.-M., Vermeșan, H., Cristea, C., Tertiș, M., Pășcuță, P., Borodi, G., Suciu, M., Barbu-Tudoran, L., Popa, C. O., & Ilea, A. (2022). Study on the Surface of Cobalt-Chromium Dental Alloys and Their Behavior in Oral Cavity as Cast Materials. Materials, 15(9), 3052. https://doi.org/10.3390/ma15093052














