Increasing Acid Concentration, Time and Using a Two-Part Silane Potentiates Bond Strength of Lithium Disilicate–Reinforced Glass Ceramic to Resin Composite: An Exploratory Laboratory Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
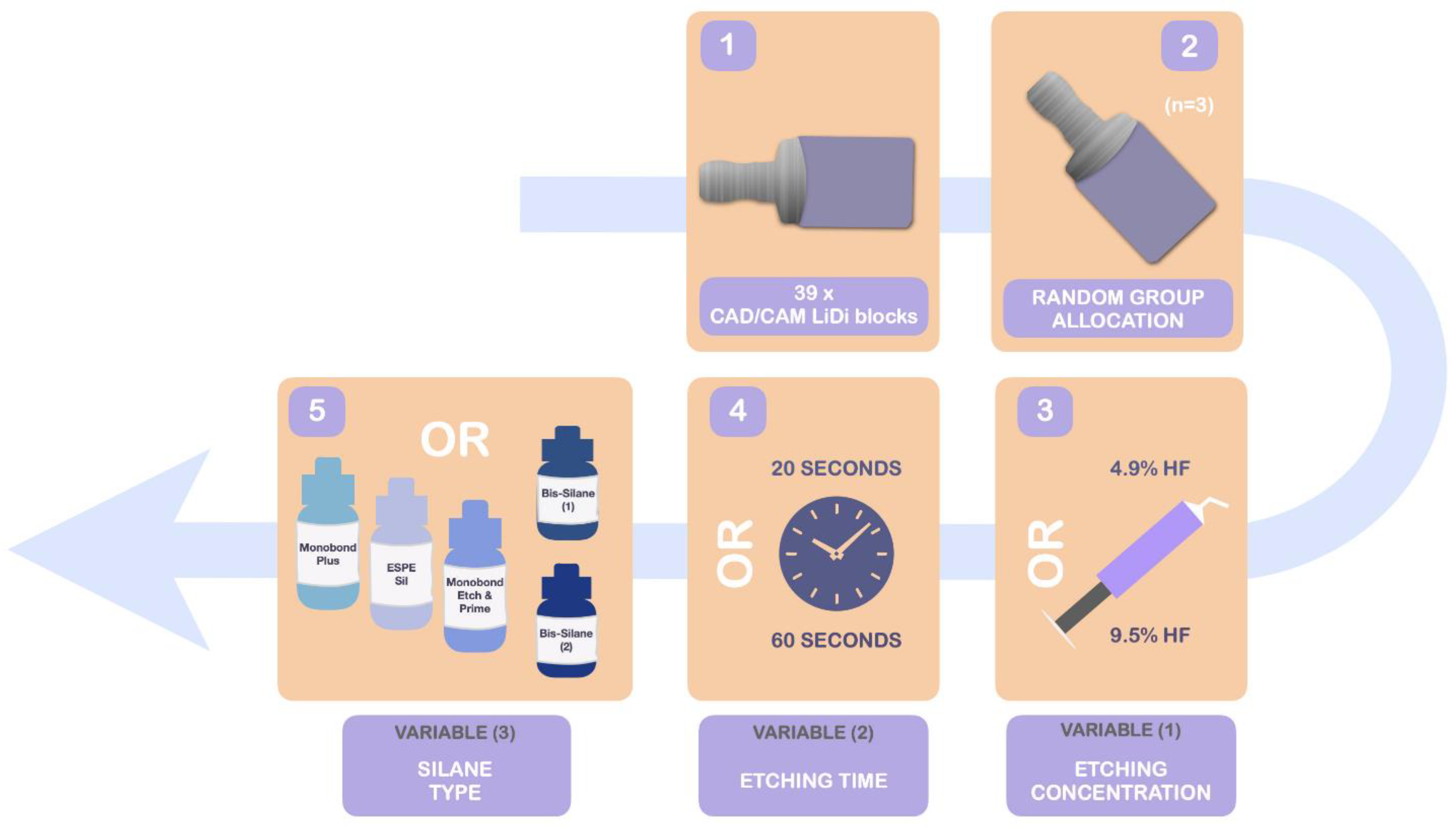
2.2. Sample Allocation and Preparation
2.3. Microtensile Bond Strength (μTBS) Test
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Özcan, M.; Dündar, M.; Erhan Çömlekoğlu, M. Adhesion concepts in dentistry: Tooth and material aspects. J. Adhes. Sci. Technol. 2012, 26, 2661–2681. [Google Scholar] [CrossRef] [Green Version]
- Giordano, R.; McLaren, E. Ceramics overview: Classification by microstructure and processing methods. Compend. Contin. Educ. Dent. 2010, 31, 18–30. [Google Scholar]
- Ho, G.W.; Matinlinna, J.P. Insights on Ceramics as Dental Materials. Part I: Ceramic Material Types in Dentistry. Silicon 2011, 3, 109–115. [Google Scholar] [CrossRef] [Green Version]
- Zarone, F.; Di Mauro, M.I.; Ausiello, P.; Ruggiero, G.; Sorrentino, R. Current status on lithium disilicate and zirconia: A narrative review. BMC Oral Health 2019, 19, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Baldissara, P.; Llukacej, A.; Ciocca, L.; Valandro, F.L.; Scotti, R. Translucency of zirconia copings made with different CAD/CAM systems. J. Prosthet. Dent. 2010, 104, 6–12. [Google Scholar] [CrossRef]
- Li, R.W.K.; Chow, T.W.; Matinlinna, J.P. Ceramic dental biomaterials and CAD/CAM technology: State of the art. J. Prosthodont. Res. 2014, 58, 208–216. [Google Scholar] [CrossRef] [Green Version]
- Fabian Fonzar, R.; Carrabba, M.; Sedda, M.; Ferrari, M.; Goracci, C.; Vichi, A. Flexural resistance of heat-pressed and CAD-CAM lithium disilicate with different translucencies. Dent. Mater. 2017, 33, 63–70. [Google Scholar] [CrossRef]
- Zandinejad, A.; Lin, W.S.; Atarodi, M.; Abdel-Azim, T.; Metz, M.J.; Morton, D. Digital Workflow for Virtually Designing and Milling Ceramic Lithium Disilicate Veneers: A Clinical Report. Oper. Dent. 2015, 40, 241–246. [Google Scholar] [CrossRef]
- Sailer, I.; Benic, G.I.; Fehmer, V.; Hämmerle, C.H.F.; Mühlemann, S. Randomized controlled within-subject evaluation of digital and conventional workflows for the fabrication of lithium disilicate single crowns. Part II: CAD-CAM versus conventional laboratory procedures. J. Prosthet. Dent. 2017, 118, 43–48. [Google Scholar] [CrossRef] [Green Version]
- Magne, P.; Razaghy, M.; Carvalho, M.A.; Soares, L.M. Luting of inlays, onlays, and overlays with preheated restorative composite resin does not prevent seating accuracy. Int. J. Esthet. Dent. 2018, 13, 318–332. [Google Scholar]
- Krummel, A.; Garling, A.; Sasse, M.; Kern, M. Influence of bonding surface and bonding methods on the fracture resistance and survival rate of full-coverage occlusal veneers made from lithium disilicate ceramic after cyclic loading. Dent. Mater. 2019, 35, 1351–1359. [Google Scholar] [CrossRef] [PubMed]
- Kalavacharla, V.K.; Lawson, N.C.; Ramp, L.C.; Burgess, J.O.; Kalavacharla, V.K.; Lawson, N.C.; Burgess, J.O. Influence of Etching Protocol and Silane Treatment with a Universal Adhesive on Lithium Disilicate Bond Strength. Oper. Dent. 2015, 40, 372–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruzi, G.; Carvalho, A.O.; Giannini, M.; Maia, H.P.; Magne, P. Post-etching cleaning influences the resin shear bond strength to CAD/CAM lithium-disilicate ceramics. Appl. Adhes. Sci. 2017, 5, 17. [Google Scholar] [CrossRef] [Green Version]
- Rita, A.; Reis, J.; Santos, I.C.; Delgado, A.H.S.; Rua, J.; Proença, L.; Mendes, J.J. Influence of silane type and application time on the bond strength to leucite reinforced ceramics. Ann. Med. 2021, 53, S50–S51. [Google Scholar] [CrossRef]
- Puppin-Rontani, J.; Sundfeld, D.; Costa, A.R.; Correr, A.B.; Puppin-Rontani, R.M.; Borges, G.A.; Sinhoreti, M.A.C.; Correr-Sobrinho, L. Effect of Hydrofluoric Acid Concentration and Etching Time on Bond Strength to Lithium Disilicate Glass Ceramic. Oper. Dent. 2017, 42, 606–615. [Google Scholar] [CrossRef]
- Prochnow, C.; Venturini, A.B.; Grasel, R.; Gundel, A.; Bottino, M.C.; Valandro, L.F. Adhesion to a Lithium Disilicate Glass Ceramic Etched with Hydrofluoric Acid at Distinct Concentrations. Braz. Dent. J. 2018, 29, 492–499. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Matinlinna, J.P. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent. Mater. 2012, 28, 467–477. [Google Scholar] [CrossRef]
- Matinlinna, J.P.; Lung, C.Y.K.; Tsoi, J.K.H. Silane adhesion mechanism in dental applications and surface treatments: A review. Dent. Mater. 2018, 34, 13–28. [Google Scholar] [CrossRef]
- Garfias, C.S.; De Goes, M.F. Dissolution Depth and Surface Morphological Alterations in Ultrathin Glass Ceramic Etched with Different Hydrofluoric Acid -etching Protocols. J. Adhes. Dent. 2021, 23, 579–587. [Google Scholar] [CrossRef]
- Lopes, G.; Perdigão, J.; Baptista, D.; Ballarin, A. Does a Self-Etching Ceramic Primer Improve Bonding to Lithium Disilicate Ceramics? Bond Strengths and FESEM Analyses. Oper. Dent. 2018, 44, 210–218. [Google Scholar] [CrossRef]
- Dimitriadi, M.; Zafiropoulou, M.; Zinelis, S.; Silikas, N.; Eliades, G. Silane reactivity and resin bond strength to lithium disilicate ceramic surfaces. Dent. Mater. 2019, 35, 1082–1094. [Google Scholar] [CrossRef] [PubMed]
- França, R.; Bebsh, M.; Haimeur, A.; Fernandes, A.C.; Sacher, E. Physicochemical surface characterizations of four dental CAD/CAM lithium disilicate-based glass ceramics on HF etching: An XPS study. Ceram. Int. 2020, 46, 1411–1418. [Google Scholar] [CrossRef]
- Cardenas, A.M.; Siqueira, F.; Hass, V.; Malaquias, P.; Gutierrez, M.F.; Reis, A.; Perdigão, J.; Loguercio, A.D. Effect of MDP-containing Silane and Adhesive Used Alone or in Combination on the Long-term Bond Strength and Chemical Interaction with Lithium Disilicate Ceramics. J. Adhes. Dent. 2017, 19, 203–212. [Google Scholar] [CrossRef]
- Maruo, Y.; Nishigawa, G.; Yoshihara, K.; Minagi, S.; Matsumoto, T.; Irie, M. Does 8-methacryloxyoctyl trimethoxy silane (8-MOTS) improve initial bond strength on lithium disilicate glass ceramic? Dent. Mater. 2017, 33, e95–e100. [Google Scholar] [CrossRef] [PubMed]
- Bortolotto, T.; Guillarme, D.; Gutemberg, D.; Veuthey, J.L.; Krejci, I. Composite resin vs resin cement for luting of indirect restorations: Comparison of solubility and shrinkage behavior. Dent. Mater. J. 2013, 32, 834–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopes, L.C.P.; Terada, R.S.S.; Tsuzuki, F.M.; Giannini, M.; Hirata, R. Heating and preheating of dental restorative materials—a systematic review. Clin. Oral Investig. 2020, 24, 4225–4235. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, S.; Breschi, L.; Özcan, M.; Pfefferkorn, F.; Ferrari, M.; Van Meerbeek, B. Academy of Dental Materials guidance on in vitro testing of dental composite bonding effectiveness to dentin/enamel using micro-tensile bond strength (μTBS) approach. Dent. Mater. 2017, 33, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Magne, P.; Bruzi, G.; Carvalho, A.; Enciso, R.; Giannini, M. Optimization of Heat-Dried Silane Application for CAD/CAM Ceramic Resin Bonding. Nanosci. Nanotechnol. 2018, 2. [Google Scholar] [CrossRef]
- Ramakrishnaiah, R.; Alkheraif, A.A.; Divakar, D.D.; Matinlinna, J.P.; Vallittu, P.K. The Effect of Hydrofluoric Acid Etching Duration on the Surface Micromorphology, Roughness, and Wettability of Dental Ceramics. Int. J. Mol. Sci. 2016, 17, 822. [Google Scholar] [CrossRef] [Green Version]
- Soares, C.J.; Soares, P.V.; Pereira, J.C.; Fonseca, R.B. Surface treatment protocols in the cementation process of ceramic and laboratory-processed composite restorations: A literature review. J. Esthet. Restor. Dent. 2005, 17, 224–235. [Google Scholar] [CrossRef]
- Chen, J.H.; Matsumura, H.; Atsuta, M. Effect of different etching periods on the bond strength of a composite resin to a machinable porcelain. J. Dent. 1998, 26, 53–58. [Google Scholar] [CrossRef]
- Menees, T.S.; Lawson, N.C.; Beck, P.R.; Burgess, J.O. Influence of particle abrasion or hydrofluoric acid etching on lithium disilicate flexural strength. J. Prosthet. Dent. 2014, 112, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Poulon-Quintin, A.; Ogden, E.; Large, A.; Vaudescal, M.; Labrugère, C.; Bartala, M.; Bertrand, C. Chemical surface modification of lithium disilicate needles of a silica-based ceramic after HF-etching and ultrasonic bath cleaning: Impact on the chemical bonding with silane. Dent. Mater. 2021, 37, 832–839. [Google Scholar] [CrossRef]
- Delgado, A.H.; Reis, J.; Rita, A.; Caetano Santos, I.; Rua, J.; Proença, L.; Mendes, J.J. Silane coupling agent type and application time influences the bond strength of resin-feldspathic CAD/CAM ceramics. Restor. Dent. Endod. 2019, 44, OP-21. [Google Scholar]
- Yu, H.; Yoshida, K.; Cheng, H.; Sawase, T. Bonding of different self-adhesive resins to high-strength composite resin block treated with surface conditioning. J. Prosthodont. Res. 2019, 63, 340–346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koko, M.; Takagaki, T.; Abdou, A.; Inokoshi, M.; Ikeda, M.; Wada, T.; Uo, M.; Nikaido, T.; Tagami, J. Effects of the ratio of silane to 10-methacryloyloxydecyl dihydrogenphosphate (MDP) in primer on bonding performance of silica-based and zirconia ceramics. J. Mech. Behav. Biomed. Mater. 2020, 112, 104026. [Google Scholar] [CrossRef]
- Bruzi, G.; Carvalho, A.O.; Giannini, M.; Maia, H.P.; Magne, P. Bonding of CAD/CAM lithium disilicate restorations with regular and flowable composite resin with and without wetting resin. Appl. Adhes. Sci. 2018, 6, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Dimitriadi, M.; Zinelis, S.; Zafiropoulou, M.; Silikas, N.; Eliades, G. Self-Etch Silane Primer: Reactivity and Bonding with a Lithium Disilicate Ceramic. Materials 2020, 13, 641. [Google Scholar] [CrossRef] [Green Version]
- El-Damanhoury, H.M.; Gaintantzopoulou, M.D. Self-etching ceramic primer versus hydrofluoric acid etching: Etching efficacy and bonding performance. J. Prosthodont. Res. 2018, 62, 75–83. [Google Scholar] [CrossRef]
- Swank, H.M.; Motyka, N.C.; Bailey, C.W.; Vandewalle, K.S. Bond strength of resin cement to ceramic with simplified primers and pretreatment solutions-PubMed. Gen. Dent. 2018, 66, 33–37. [Google Scholar]
- Lyann, S.K.; Takagaki, T.; Nikaido, T.; Uo, M.; Ikeda, M.; Sadr, A.; Tagami, J. Effect of Different Surface Treatments on the Tensile Bond Strength to Lithium Disilicate Glass Ceramics. J. Adhes. Dent. 2018, 20, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Wille, S.; Lehmann, F.; Kern, M. Durability of Resin Bonding to Lithium Disilicate and Zirconia Ceramic using a Self-etching Primer. J. Adhes. Dent. 2017, 19, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Dapieve, K.S.; Aragonez, G.C.; Prochnow, C.; Burgo, T.A.d.L.; Rippe, M.P.; Pereira, G.K.R.; Venturini, A.B.; Valandro, L.F. Different Etching Times of a One-step Ceramic Primer: Effect on the Resin Bond Strength Durability to a CAD/CAM Lithium-Disilicate Glass-Ceramic. J. Adhes. Dent. 2021, 23, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Güngör, M.B.; Nemli, S.K.; Bal, B.T.; Ünver, S.; Dogan, A. Effect of surface treatments on shear bond strength of resin composite bonded to CAD/CAM resin-ceramic hybrid materials. J. Adv. Prosthodont. 2016, 8, 259–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, C.; Yu, J.; Wang, Y.; Tang, C.; Huang, C. Acidic pH weakens the bonding effectiveness of silane contained in universal adhesives. Dent. Mater. 2018, 34, 809–818. [Google Scholar] [CrossRef]
- Zakir, M.; Tsoi, J.K.H.; Chu, C.H.; Lung, C.Y.K.; Matinlinna, J.P. Bonding Dissimilar Materials in Dentistry: A Critical Review. Rev. Adhes. Adhes. 2014, 2, 413–432. [Google Scholar] [CrossRef]
| Material | Type | Manufacturer | Batch No. | Composition |
|---|---|---|---|---|
| IPS Ceramic Etching Gel | Etchant | Ivoclar-Vivadent, Schaan, Liechtenstein | T29351 | Hydrofluoric acid 4.9% |
| Porcelain Etchant 9.5% | Etchant | Bisco Inc., Schaumburg, IL, USA | 1600002039 | Aqueous solution of hydrofluoric acid (9.5%), 50–70% polyacrylamidomethylpropane sulfonic acid sodium fluoride |
| Bis-Silane | Silane | Bisco, Schaumburg, IL, USA | 1600001184 1600001185 | Part A: >85% ethanol, 5–10% 3-(Trimethoxysilyl) propyl-2-methyl-2-propenoic acid Part B: 30–50% ethanol, 1–5% phosphoric acid |
| Monobond Plus | Silane | Ivoclar-Vivadent, Schaan, Liechtenstein | V21266 | 50–100% ethanol, disulfit methacrylate, ≤2.5% phosphoric acid dimethacrylate, ≤2.5% 3-trimethoxysilylpropyl methacrylate |
| ESPE Sil Silane Coupling Agent | Silane | 3M ESPE AG, Seefeld, Germany | 632307 | >97% ethanol, <3% 3-trimethoxysilylpropyl methacrylate, <2% methyl ethyl ketone |
| Monobond Etch & Prime | Silane | Ivoclar-Vivadent, Schaan, Liechtenstein | W05619 | 10–25% butanol, 2.5–10% tetrabutyl ammonium dihydrogen trifluoride, methacrylated phosphoric acid ester, <2.5% bis(triethoxysilyl) ethane |
| Optibond FL | Bonding agent | Kerr, CA, USA | 6158322 | Adhesive: Bis-GMA, HEMA, GDMA, CQ, ODMAB, fillers (~48%) |
| Resin Composite Enamel Plus HRi | Composite | Micerium S.p.A., Avegno, Ge, Italy | 2017000418 2016001162 2016008171 | UDMA, Bis-GMA, Butanediol, Dimethacrylate, Glass fillers, Zirconia oxide nanoparticles |
| Lithium disilicate ceramic IPS e.max CAD A3-HT/C14 | Ceramic | Ivoclar-Vivadent, Schaan, Liechtenstein | V49313 | SiO2, Li2O, K2O, MgO, Al2O3, P2O5, other oxides |
| Bis-Silane | Monobond Plus | ESPE Sil Silane CA | |||||
|---|---|---|---|---|---|---|---|
| HF Ac. Conc. (%) | Etching Time (s) | n | M ± SE | n | M ± SE | n | M ± SE |
| 9.5 | 20 | 66 | 7.0 ± 0.5 aA | 64 | 10.4 ± 0.8 aBC | 79 | 12.4 ± 0.9 aB |
| 60 | 76 | 16.6 ± 1.0 bA | 69 | 12.3 ± 0.7 aB | 78 | 12.4 ± 0.8 aB | |
| 4.9 | 20 | 74 | 8.5 ± 0.7 aA | 73 | 4.8 ± 0.4 bB | 53 | 0.6 ± 0.1 bC |
| 60 | 72 | 8.9 ± 0.7 aA | 71 | 6.8 ± 0.4 cAB | 80 | 5.5 ± 0.6 cB | |
| Source | Type III Sum of Squares | df | Mean Square | F | p |
|---|---|---|---|---|---|
| Model | 13,683.276 | 11 | 1243.934 | 35.380 | <0.001 |
| HF acid concentration | 7599.039 | 1 | 7599.039 | 216.129 | <0.001 |
| Etching time | 2017.635 | 1 | 2017.635 | 57.385 | <0.001 |
| Silane type | 956.592 | 2 | 478.296 | 13.604 | <0.001 |
| HF acid concentration × etching time | 112.829 | 1 | 112.829 | 3.209 | 0.074 |
| Etching time × silane type | 388.375 | 2 | 194.198 | 5.523 | 0.004 |
| HF acid concentration × silane type | 1405.889 | 2 | 702.944 | 19.993 | <0.001 |
| HF acid concentration × etching time × silane type | 1821.657 | 2 | 910.828 | 25.905 | <0.001 |
| Error | 29,604.431 | 842 | 35.160 | ||
| Total | 113,456.851 | 854 |
| Bis-Silane | Monobond Plus | ESPE Sil Silane Coupling Agent | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HF Ac. Conc. (%) | Etching Time (s) | A | M | C | PTF | A | M | C | PTF | A | M | C | PTF |
| 9.5 | 20 | 25.0 | 45.0 | 17.5 | 12.5 | 28.4 | 55.2 | 6.0 | 10.4 | 13.6 | 75.3 | 2.5 | 8.6 |
| 60 | 29.9 | 51.9 | 16.9 | 1.3 | 23.0 | 62.2 | 14.9 | 0.0 | 13.1 | 82.1 | 0.0 | 4.8 | |
| 4.9 | 20 | 51.3 | 29.5 | 1.3 | 17.9 | 22.2 | 54.3 | 0.0 | 23.5 | 46.6 | 1.4 | 0.0 | 52.1 |
| 60 | 38.5 | 43.6 | 1.3 | 16.7 | 22.2 | 67.9 | 1.2 | 8.6 | 59.0 | 10.8 | 1.2 | 28.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almiro, M.; Marinho, B.; Delgado, A.H.S.; Rua, J.; Monteiro, P.; Santos, I.C.; Proença, L.; Mendes, J.J.; Gresnigt, M.M.M. Increasing Acid Concentration, Time and Using a Two-Part Silane Potentiates Bond Strength of Lithium Disilicate–Reinforced Glass Ceramic to Resin Composite: An Exploratory Laboratory Study. Materials 2022, 15, 2045. https://doi.org/10.3390/ma15062045
Almiro M, Marinho B, Delgado AHS, Rua J, Monteiro P, Santos IC, Proença L, Mendes JJ, Gresnigt MMM. Increasing Acid Concentration, Time and Using a Two-Part Silane Potentiates Bond Strength of Lithium Disilicate–Reinforced Glass Ceramic to Resin Composite: An Exploratory Laboratory Study. Materials. 2022; 15(6):2045. https://doi.org/10.3390/ma15062045
Chicago/Turabian StyleAlmiro, Matilde, Beatriz Marinho, António H. S. Delgado, João Rua, Paulo Monteiro, Inês Caetano Santos, Luís Proença, José João Mendes, and Marco M. M. Gresnigt. 2022. "Increasing Acid Concentration, Time and Using a Two-Part Silane Potentiates Bond Strength of Lithium Disilicate–Reinforced Glass Ceramic to Resin Composite: An Exploratory Laboratory Study" Materials 15, no. 6: 2045. https://doi.org/10.3390/ma15062045
APA StyleAlmiro, M., Marinho, B., Delgado, A. H. S., Rua, J., Monteiro, P., Santos, I. C., Proença, L., Mendes, J. J., & Gresnigt, M. M. M. (2022). Increasing Acid Concentration, Time and Using a Two-Part Silane Potentiates Bond Strength of Lithium Disilicate–Reinforced Glass Ceramic to Resin Composite: An Exploratory Laboratory Study. Materials, 15(6), 2045. https://doi.org/10.3390/ma15062045









