Viscoelastic Properties of Fully Biomass-Based Transparent Plastic Comprising Cellulose Acetate and Citrate Ester
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Film Preparation
2.2. Measurements
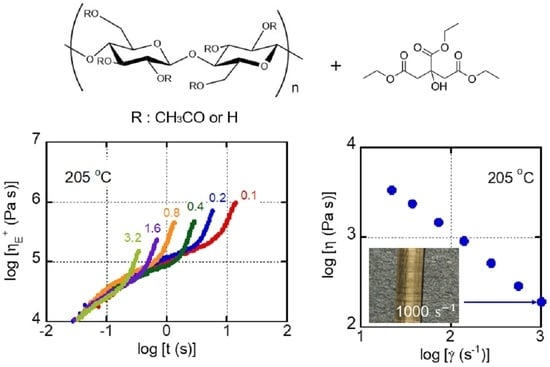
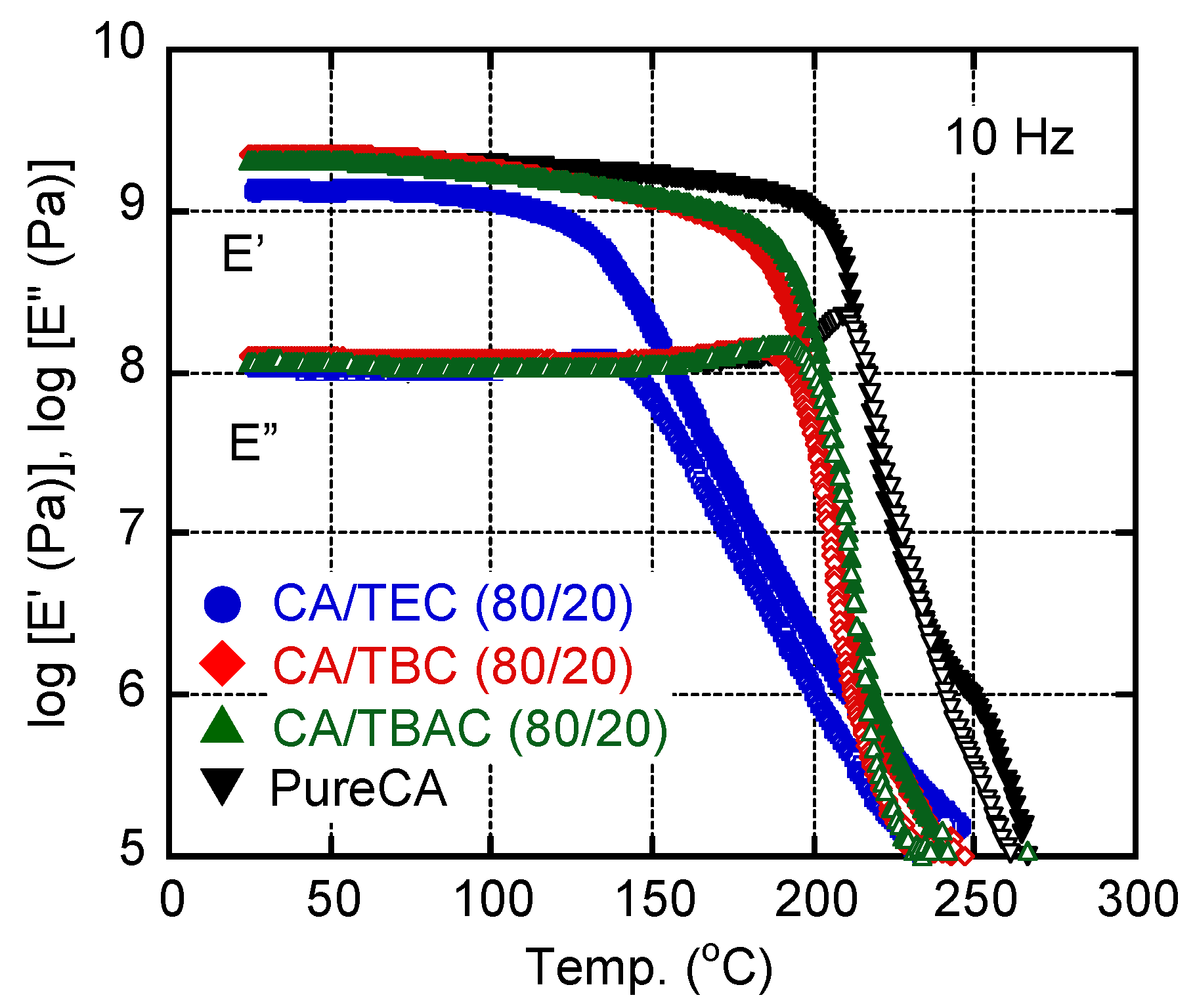
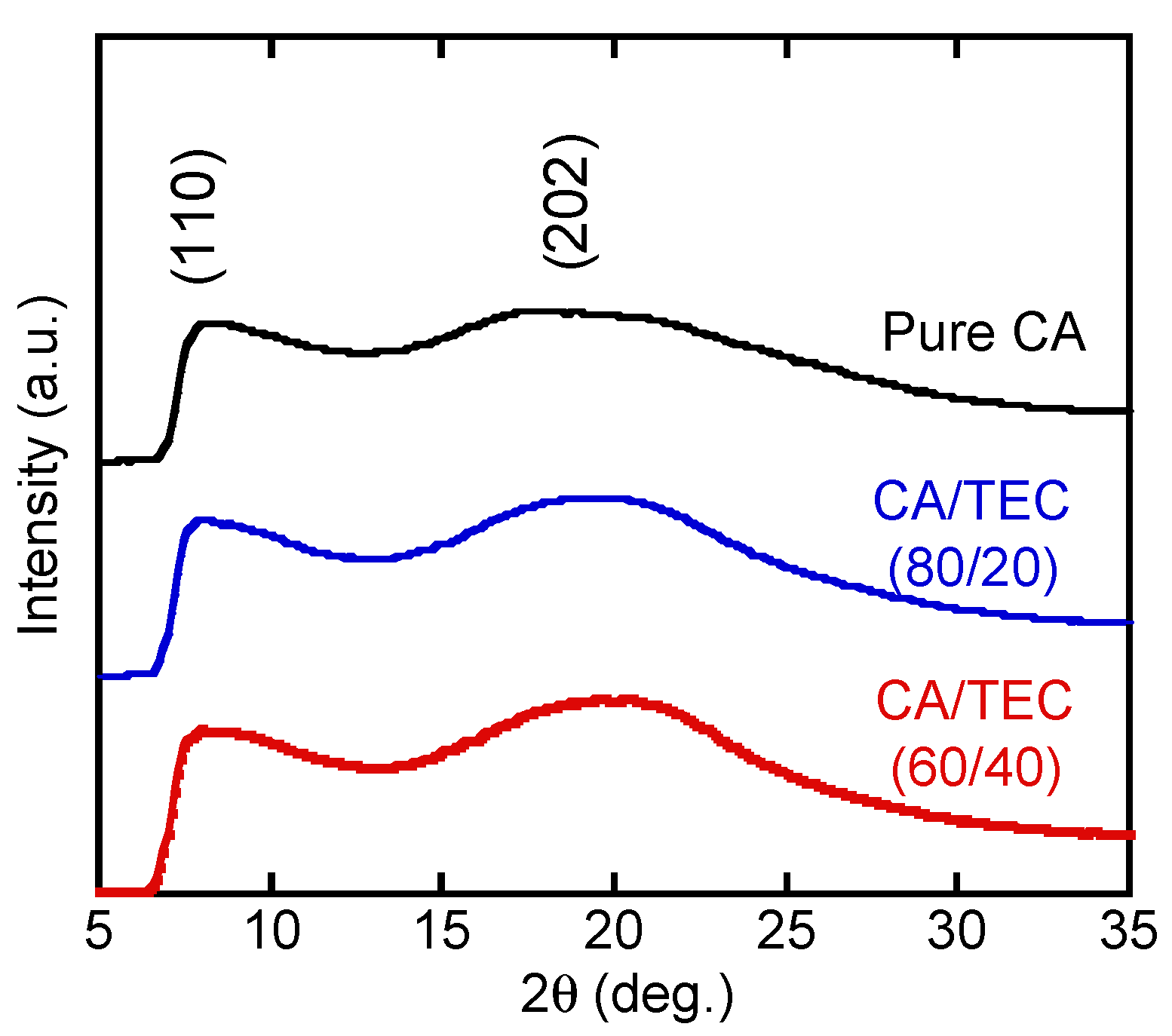
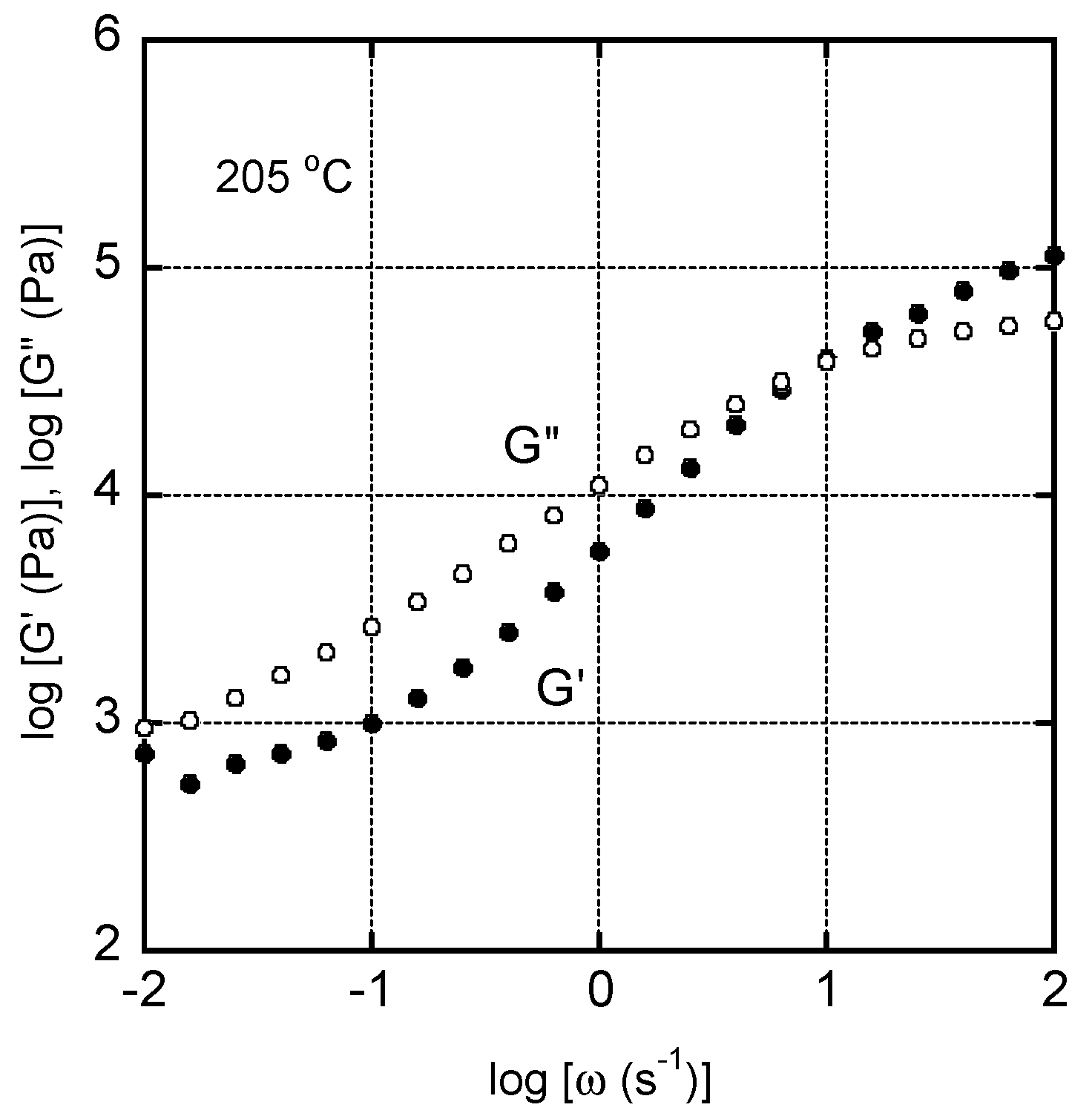
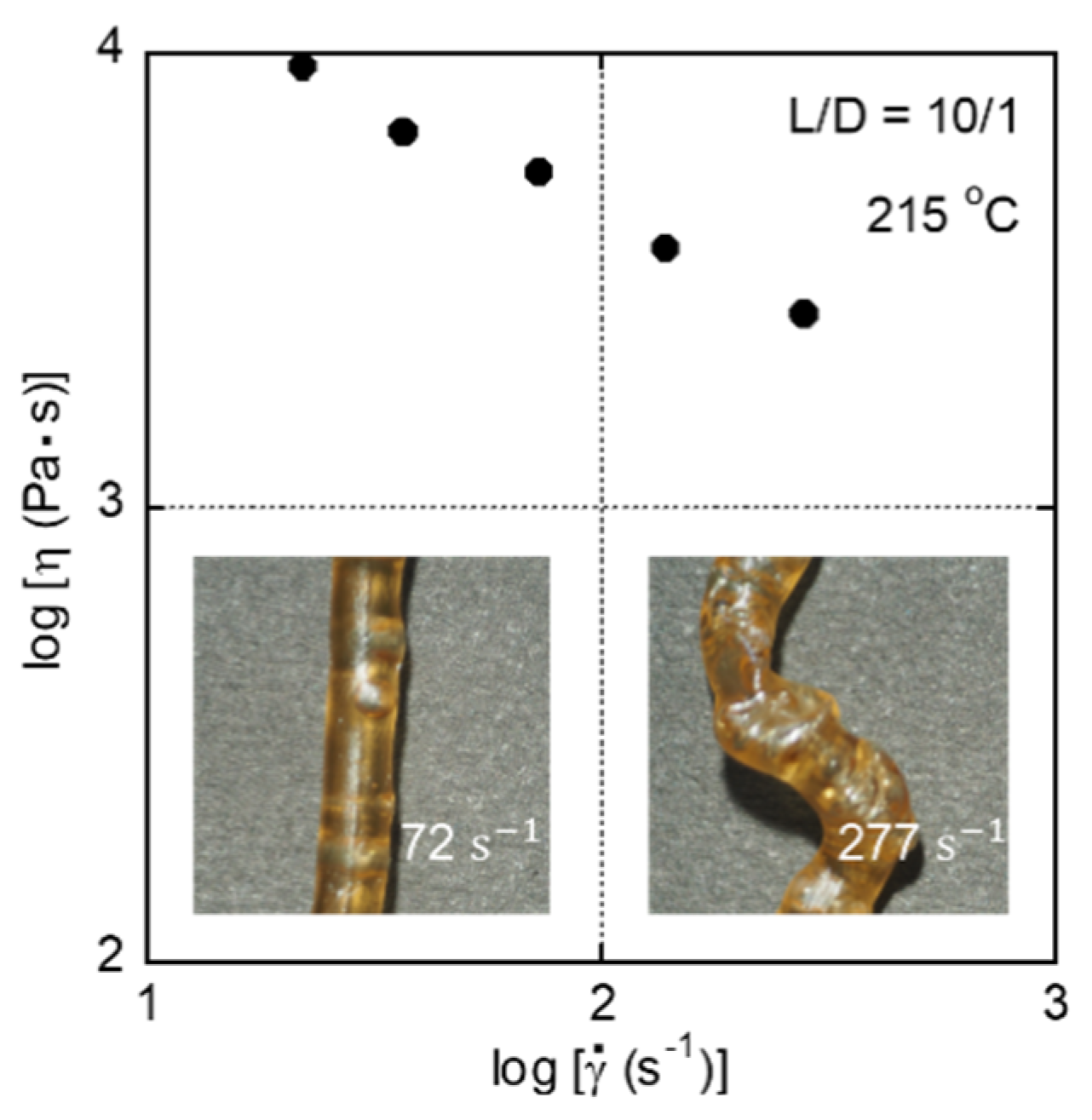
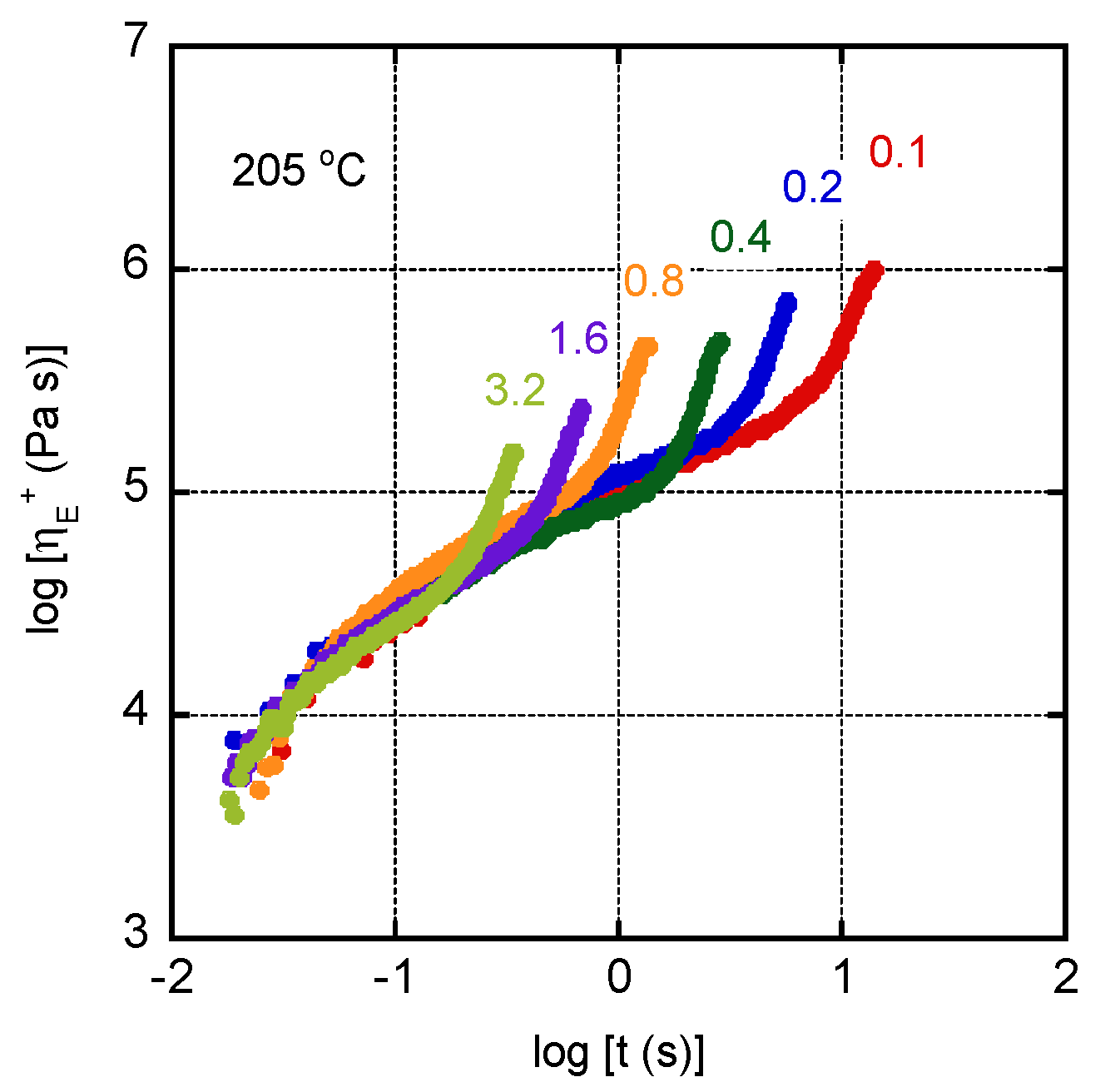
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marsh, J.T.; Wood, F.C. An Introduction to the Chemistry of Cellulose; Chapman & Hall: London, UK, 1942; Volume 150, p. 7. [Google Scholar]
- Müller, F.; Leuschke, C. Organic cellulose esters thermoplastic molding compounds. In Engineering Thermoplastics: Polycarbonates, Polyacetals, Polyesters, Cellulose Esters; Bottenbruch, L., Ed.; Hanser: Munich, Germany, 1996; pp. 385–442. [Google Scholar]
- Edgar, K.J.; Buchanan, C.M.; Debenham, J.S.; Rundquist, P.A.; Seiler, B.D.; Shelton, M.C.; Tindall, D. Advances in cellulose ester performance and application. Prog. Polym. Sci. 2001, 26, 1605–1688. [Google Scholar] [CrossRef]
- Kamide, K.; Saito, M. Thermal analysis of cellulose acetate solids with total degrees of substitution of 0.49, 1.75, 2.46, and 2.92. Polym. J. 1985, 17, 919–928. [Google Scholar] [CrossRef]
- Sata, M.; Murayama, M.; Shimamoto, S. Properties and applications of cellulose triacetate film. Macromol. Symp. 2004, 208, 323–333. [Google Scholar] [CrossRef]
- Songsurang, K.; Mohd Edeerozey, A.M.; Miyagawa, A.; Phulkerd, P.; Nobukawa, S.; Yamaguchi, M. Optical anisotropy in solution-cast film of cellulose triacetate. Cellulose 2013, 20, 83–96. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Okada, K.; Mohd Edeerozey, A.M.; Shiroyama, Y.; Iwasaki, T.; Okamoto, K. Extraordinary wavelength dispersion of orientation birefringence for cellulose esters. Macromolecules 2009, 42, 9034–9040. [Google Scholar] [CrossRef]
- Hatamoto, K.; Shimada, H.; Kondo, M.; Nobukawa, S.; Yamaguchi, M. Effect of acetyl substitution on the optical anisotropy of cellulose acetate films. Cellulose 2018, 25, 4453–4463. [Google Scholar] [CrossRef]
- Wang, B.; Chan, J.; Peng, H.; Gai, J.; Kang, J.; Cao, Y. Investigation on changes in the miscibility, morphology, rheology and mechanical behavior of melt processed cellulose acetate through adding polyethylene glycol as a plasticizer. J. Macromol. Sci. 2016, 55, 894–907. [Google Scholar] [CrossRef]
- Songsurang, K.; Shimada, H.; Nobukawa, S.; Yamaguchi, M. Control of three-dimensional refractive indices of uniaxially-stretched cellulose triacetate with low-molecular-weight compounds. Eur. Polym. J. 2014, 89, 105–112. [Google Scholar] [CrossRef]
- Park, H.M.; Misra, M.; Drzal, L.T.; Mohanty, A.K. “Green” Nanocomposites from cellulose acetate bioplastic and clay: Effect of eco-friendly triethyl citrate plasticizer. Biomacromolecules 2004, 5, 2281–2288. [Google Scholar] [CrossRef]
- Rynkowska, E.; Fatyeyeva, K.; Kujawa, J.; Dzieszkowski, K.; Wolan, A.; Kujawski, W. Plasticizer incorporation on the physicochemical and transport properties of cellulose acetate propionate-based membranes. Polymers 2018, 10, 86. [Google Scholar] [CrossRef]
- Rodriguez, F.J.; Abarca, R.L.; Bruna, J.E.; Moya, P.E.; Galotto, M.J.; Guarda, A.; Padula, M. Effect of organoclay and preparation method on properties of antimicrobial cellulose acetate films. Polym. Comp. 2019, 40, 2311–2319. [Google Scholar] [CrossRef]
- Ardila-Diaz, L.D.; de Oliveira, T.V.; Soares, N.F.F. Development and evaluation of the chromatic behavior of an intelligent packaging material based on cellulose acetate incorporated with polydiacetylene for an efficient packaging. Biosensors 2020, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, R.; Kabasci, S.; Heim, H. Thermal properties of plasticized cellulose acetate and its b-relaxation phenomenon. Polymers 2021, 13, 1356. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, S.C.; Silva, R.R.A.; de Oliveira, T.V.; Stringheta, P.C.; Pinto, M.R.M.R.; Soares, N.F.F. Glycerol and triethyl citrate plasticizer effects on molecular, thermal, mechanical, and barrier properties of cellulose acetate films. Food Biosci. 2021, 42, 101202. [Google Scholar] [CrossRef]
- Shimada, H.; Nobukawa, S.; Yamaguchi, M. Development of microporous structure and its application to optical film for cellulose triacetate containing diisodecyl adipate. Carbohydr. Polym. 2015, 120, 22–28. [Google Scholar] [CrossRef]
- De Gennes, P. Scaling Concepts in Polymer Physics; Cornell University Press: Ithaca, NY, USA, 1979. [Google Scholar]
- Bendaoud, A.; Chalamet, Y. Plasticizing effects of ionic liquid on cellulose acetate obtained by melt processing. Carbohydr. Polym. 2014, 108, 75–82. [Google Scholar] [CrossRef]
- Dreux, X.; Majeste, J.; Carrot, C.; Argoud, A.; Vergelati, C. Viscoelastic behaviour of cellulose acetate/triacetin blends by rheology in the melt state. Carbohydr. Polym. 2019, 222, 114973. [Google Scholar] [CrossRef]
- Sadayoshi, W.; Mitsuo, T.J. Acetylation behavior of cellulose. J. Soc. Chem. Ind. Japan 1968, 71, 1883–1891. [Google Scholar]
- Monisha, S.; Selvasekarapandian, S.; Mathavan, T.; Benial, A.M.F.; Manoharan, S.; Karthikeyan, S. Preparation and characterization of biopolymer electrolyte based on cellulose acetate for potential applications in energy storage devices. J. Mater. Sci. Mater. Electron. 2016, 27, 9314–9324. [Google Scholar] [CrossRef]
- Monisha, S.; Mathavan, T.; Selvasekarapandian, S.; Benial, A.M.F.; Peruma, M. Preparation and characterization of cellulose acetate and lithium nitrate for advanced electrochemical devices. Ionics 2017, 23, 2697–2706. [Google Scholar] [CrossRef]
- Rajesh, S.; Shobana, K.H.; Anithara, S.; Mohan, D.R. Preparation, morphology, performance, and hydrophilicity studies of poly(amide-imide) incorporated cellulose acetate ultrafiltration membranes. Ind. Eng. Chem. Res. 2011, 50, 5550–5564. [Google Scholar] [CrossRef]
- Sugiyama, J.; Horikawa, Y. The cellulose microfibril and its polymorphs. J. Wood Res. Soc. 2008, 54, 49–57. [Google Scholar] [CrossRef][Green Version]
- Okamoto, K.; Ichikawa, T.; Yokohama, T.; Yamaguchi, M. Miscibility, mechanical and thermal properties of poly(lactic acid)/polyester-diol blends. Eur. Polym. J. 2009, 45, 2304–2312. [Google Scholar] [CrossRef]
- Huang, T.; Miura, M.; Nobukawa, S.; Yamaguchi, M. Crystallization behavior and dynamic mechanical properties of poly(L-lactic acid) with poly(ethylene glycol) terminated by benzoate. J. Polym. Environ. 2014, 22, 183–189. [Google Scholar] [CrossRef]
- Kong, J.; Li, Y.; Bai, Y.; Li, Z.; Cao, Z.; Yu, Y.; Han, C.; Dong, L. High-performance biodegradable polylactide composites fabricated using a novel plasticizer and functionalized eggshell powder. Inter. J. Biol. Macromol. 2018, 112, 46–53. [Google Scholar] [CrossRef]
- Yamaguchi, M. Flow instability in capillary extrusion of plasticized poly(vinyl chloride). J. Appl. Polym. Sci. 2001, 82, 1277–1283. [Google Scholar] [CrossRef]
- Yamada, T.; Kida, T.; Yamaguchi, M. Effect of thermal history on the structure and mechanical properties of a thermoplastic polyester elastomer. Polymers 2022, 238, 124376. [Google Scholar] [CrossRef]
- Macosko, C.W. Rheology: Principles, Measurements, and Applications; Wiley: New York, NY, USA, 1994; pp. 244–247. [Google Scholar]
- Sako, T.; Date, J.; Hagi, M.; Hiraoka, T.; Matsuoka, S.; Yamaguchi, M. Anomalous viscosity decrease of polycarbonate by addition of polystyrene. Polymers 2019, 170, 135–141. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Takahashi, M. Rheological properties of low-density polyethylenes produced by tubular and vessel processes. Polymers 2001, 42, 8663–8670. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Wagner, M.H. Impact of processing history on rheological properties for branched polypropylene. Polymer 2006, 47, 3629–3635. [Google Scholar] [CrossRef]
- Meller, M.; Luciani, A.; Sarioglu, A.; Manson, J.E. Flow through a convergence. Part 1: Critical conditions for unstable flow. Polym. Eng. Sci. 2002, 42, 611–633. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Todd, D.B.; Gogos, C.G. Rheological properties of LDPE processed by conventional processing machines. Adv. Polym. Technol. 2003, 22, 179–187. [Google Scholar] [CrossRef]
- Liu, L.; Gong, D.; Bratasz, L.; Zhu, Z.; Wang, C. Degradation markers and plasticizer loss of cellulose acetate films during ageing. Polym. Degrad. Stab. 2019, 168, 108952. [Google Scholar] [CrossRef]
- Kouda, S. Prediction of processability at extrusion coating for low-density polyethylene. Polym. Eng. Sci. 2008, 48, 1094–1102. [Google Scholar] [CrossRef]
- Kugimoto, D.; Kouda, S.; Yamaguchi, M. Modification of poly(lactic acid) rheological properties using ethylene–vinyl acetate copolymer. J. Polym. Environ. 2021, 29, 121–129. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Suzuki, K. Enhanced strain hardening in elongational viscosity for HDPE/crosslinked HDPE blend. II. Processability of thermoforming. J. Appl. Polym. Sci. 2002, 86, 79–83. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Suzuki, K. Rheological properties and foam processability for blends of linear and crosslinked polyethylenes. J. Polym. Sci. B Polym. Phys. 2001, 39, 2159–2167. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, T.; Kida, T.; Yamaguchi, M. Viscoelastic Properties of Fully Biomass-Based Transparent Plastic Comprising Cellulose Acetate and Citrate Ester. Materials 2022, 15, 3038. https://doi.org/10.3390/ma15093038
Kimura T, Kida T, Yamaguchi M. Viscoelastic Properties of Fully Biomass-Based Transparent Plastic Comprising Cellulose Acetate and Citrate Ester. Materials. 2022; 15(9):3038. https://doi.org/10.3390/ma15093038
Chicago/Turabian StyleKimura, Takeyoshi, Takumitsu Kida, and Masayuki Yamaguchi. 2022. "Viscoelastic Properties of Fully Biomass-Based Transparent Plastic Comprising Cellulose Acetate and Citrate Ester" Materials 15, no. 9: 3038. https://doi.org/10.3390/ma15093038
APA StyleKimura, T., Kida, T., & Yamaguchi, M. (2022). Viscoelastic Properties of Fully Biomass-Based Transparent Plastic Comprising Cellulose Acetate and Citrate Ester. Materials, 15(9), 3038. https://doi.org/10.3390/ma15093038