Oxidation Mechanism of Core-Shell Structured Al@PVDF Powders Synthesized by Solvent/Non-Solvent Method
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Preparation of Al@PVDF Powders
2.3. Characterization
3. Results and Discussions
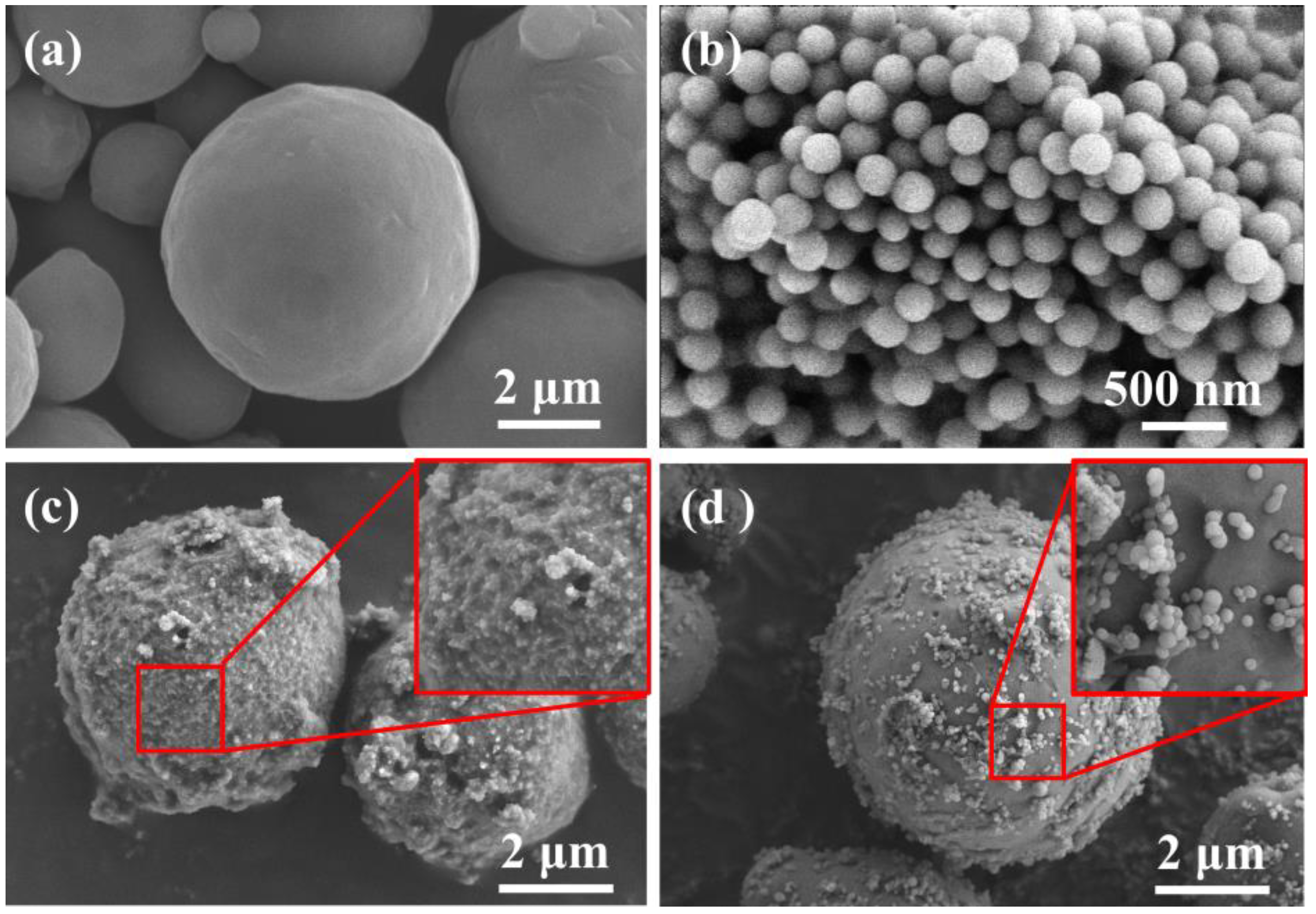
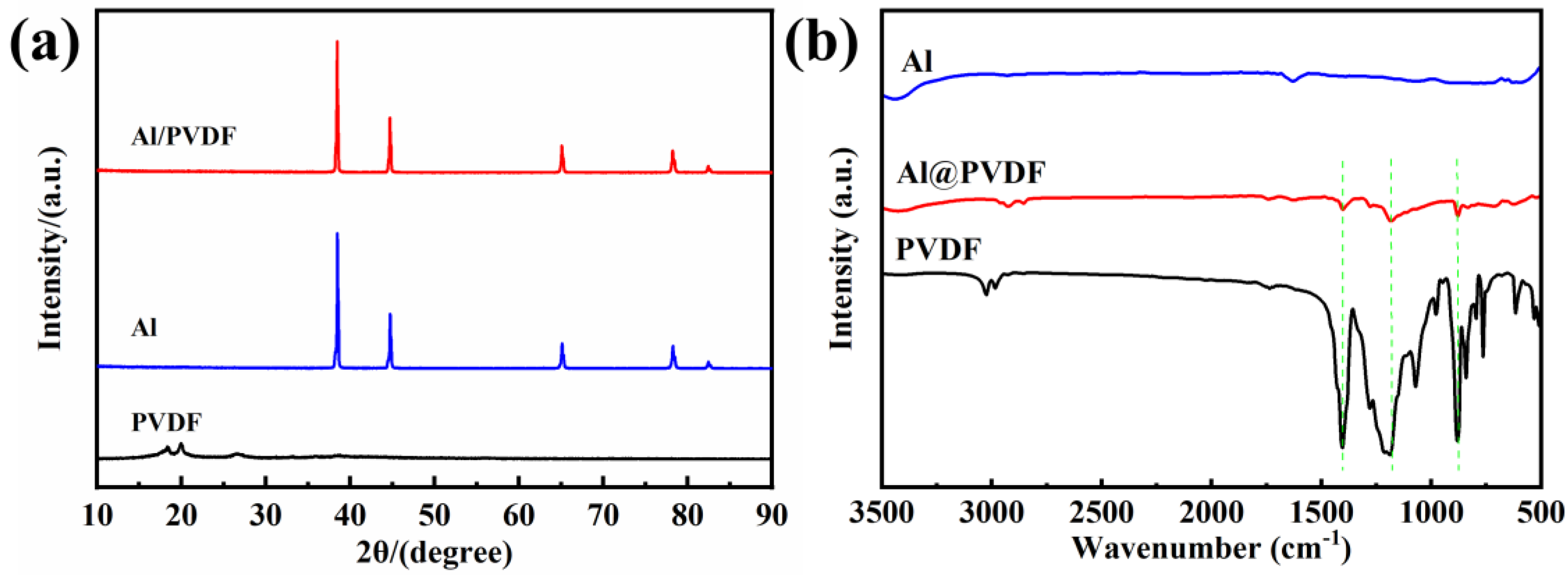
3.1. Morphology and Composition of Al@PVDF Powders
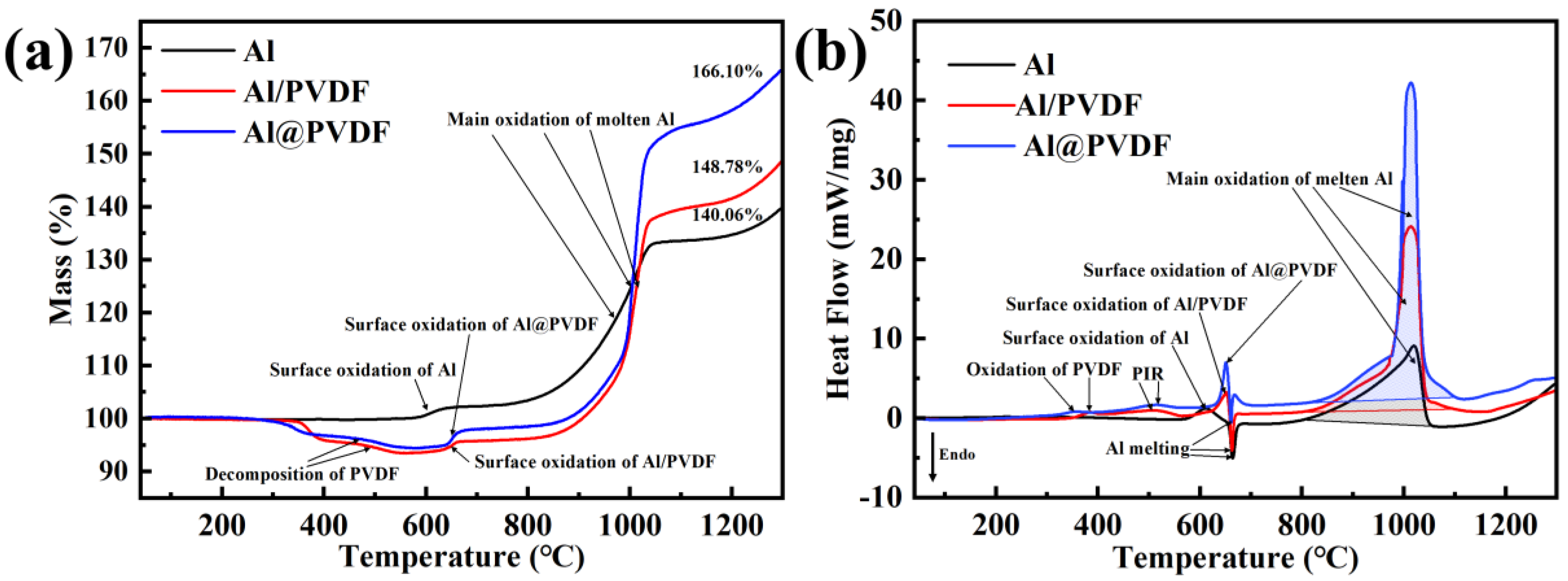
3.2. Thermal Properties of Al@PVDF Powders
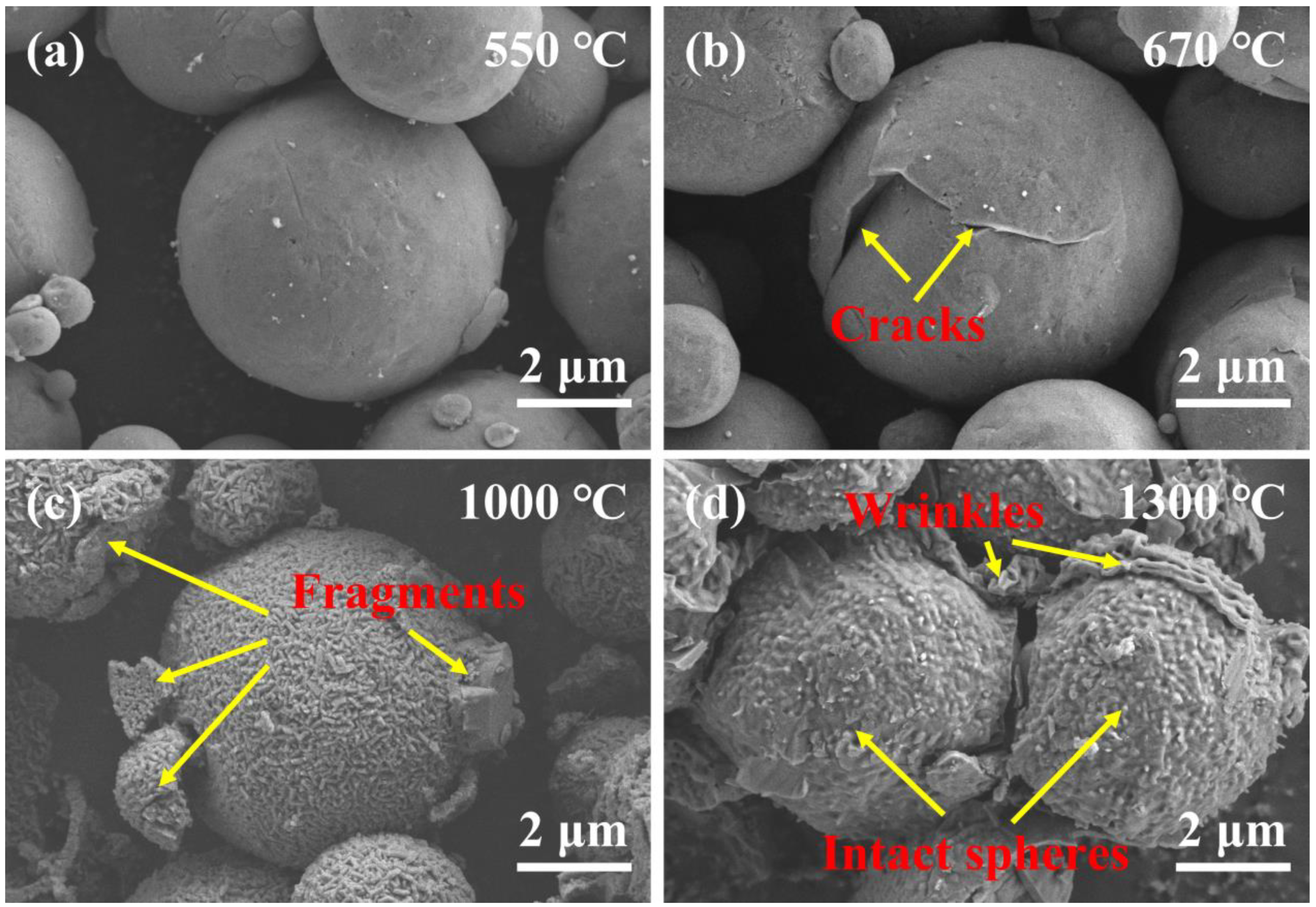
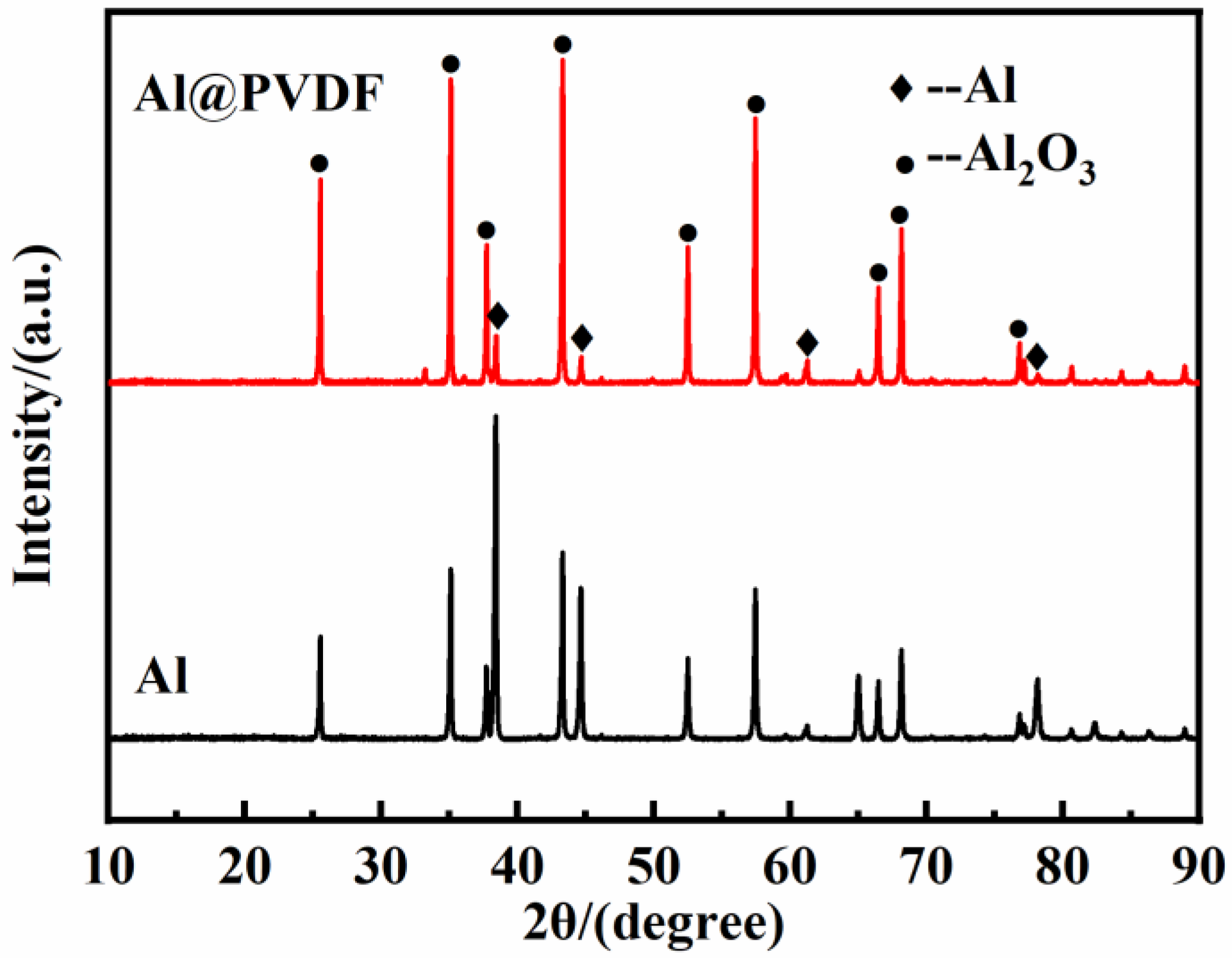
3.3. Oxidation Mechanism of Al@PVDF Powders
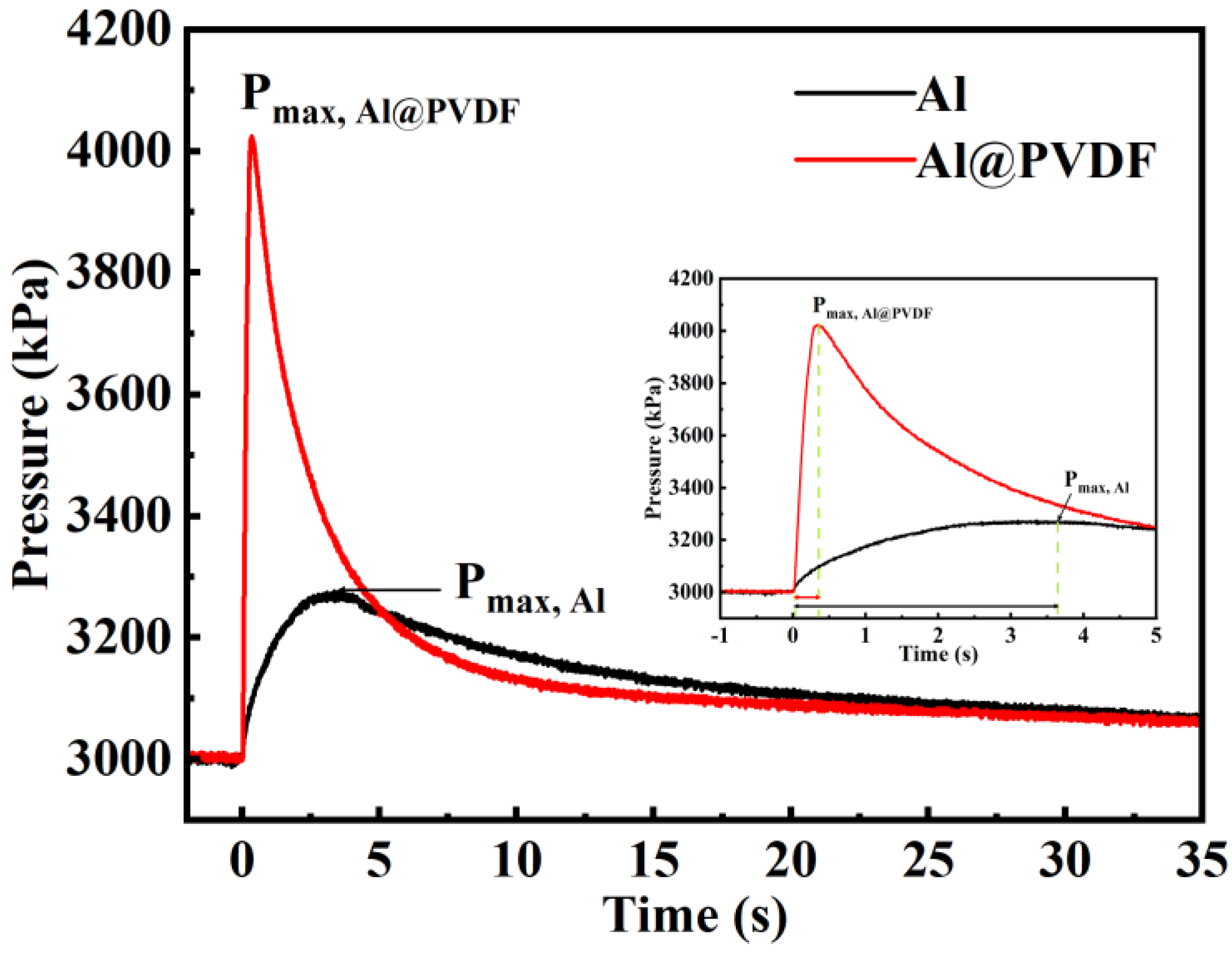
3.4. Pressure Output Capacity of Al@PVDF Powders
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ivanov, Y.; Osmonoliev, M.; Sedoi, V.; Arkhipov, V.A.; Bondarchuk, S.S.; Vorozhtsov, A.B.; Korotkikh, A.G.; Kuznetsov, V.T. Productions of ultra-fine powders and their use in high energetic compositions. Propellants Explos. Pyrotech. 2010, 28, 319–333. [Google Scholar] [CrossRef]
- Arkhipov, V.A.; Korotkikh, A.G. The influence of aluminum powder dispersity on composite solid propellants ignitability by laser radiation. Combust. Flame 2012, 159, 409–415. [Google Scholar] [CrossRef]
- He, W.; Liu, P.-J.; He, G.-Q.; Gozin, M.; Yan, Q.-L. Highly reactive metastable intermixed composites (MICs): Preparation and characterization. Adv. Mater. 2018, 30, e1706293. [Google Scholar] [CrossRef] [PubMed]
- Mccollum, J.; Pantoya, M.L.; Iacono, S.T. Activating aluminum reactivity with fluoropolymer coatings for improved energetic composite Combustion. ACS Appl. Mater. Interfaces 2015, 7, 18742. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Delisio, J.B.; Holdren, S.; Wu, T.; Yang, Y.; Hu, J.; Zachariah, M.R. Mesoporous silica spheres incorporated aluminum/poly (vinylidene fluoride) for enhanced burning propellants. Adv. Eng. Mater. 2018, 20, 1700547. [Google Scholar] [CrossRef]
- Nie, H.; Zhang, S.; Schoenitz, M.; Dreizin, E. Reaction interface between aluminum and water. Int. J. Hydrog. Energy 2013, 38, 11222–11232. [Google Scholar] [CrossRef]
- Kim, S.; Lim, J.; Lee, S.; Jeong, J.; Yoon, W. Study on the ignition mechanism of Ni-coated aluminum particles in air. Combust. Flame 2018, 198, 24–39. [Google Scholar] [CrossRef]
- Zhu, B.Z.; Li, F.; Sun, Y.L.; Wu, Y.X.; Wang, Q.C.; Wang, Q.; Han, W.K. Effects of different additives on the ignition and combustion characteristics of micrometer-sized aluminum powder in steam. Energy Fuels 2017, 31, 8674–8684. [Google Scholar] [CrossRef]
- Meziani, M.J.; Bunker, C.E.; Lu, F.; Li, H.; Wang, W.; Guliants, E.A.; Quinn, R.A.; Sun, Y.-P. Formation and properties of stabilized aluminum nanoparticles. ACS Appl. Mater. Interfaces 2009, 1, 703–709. [Google Scholar] [CrossRef]
- Guo, L.; Song, W.; Xie, C.; Zhang, X.; Hu, M. Characterization and thermal properties of carbon-coated aluminium nanopowders prepared by laser-induction complex heating in methane. Mater. Lett. 2007, 61, 3211–3214. [Google Scholar] [CrossRef]
- Vorozhtsov, A.B.; DeLuca, L.T.; Reina, A.; Lerner, M.I.; Rodkevich, N.G. Effects of HTPB-coating on nano-sized aluminum in solid rocket propellant performance. Sci. Technol. Energetic Mater. 2015, 76, 105–109. [Google Scholar]
- Liu, S.; Ye, M.; Han, A.; Chen, X. Preparation and characterization of energetic materials coated superfine aluminum particles. Appl. Surf. Sci. 2014, 288, 349–355. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, L.; Mao, Y.; Gong, F. An effective way to enhance energy output and combustion characteristics of Al/PTFE. Combust. Flame 2020, 214, 419–425. [Google Scholar] [CrossRef]
- Kim, D.W.; Kim, K.T.; Min, T.S.; Kim, S.H. Improved energetic-behaviors of spontaneously surface-mediated Al particles. Sci. Rep. 2017, 7, 4659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.T.; Kim, D.W.; Kim, S.H.; Kim, C.K.; Choi, Y.J. Synthesis and improved explosion behaviors of aluminum powders coated with nano-sized nickel film. Appl. Surf. Sci. 2017, 415, 104–108. [Google Scholar] [CrossRef]
- Cheng, Z.; Chu, X.; Yin, J.; Dai, B.; Zhao, W.; Jiang, Y.; Xu, J.; Zhong, H.; Zhao, P.; Zhang, L. Formation of composite fuels by coating aluminum powder with a cobalt nanocatalyst: Enhanced heat release and catalytic performance. Chem. Eng. J. 2020, 385, 123859. [Google Scholar] [CrossRef]
- Wang, H.; Rehwoldt, M.; Kline, D.J.; Wu, T.; Wang, P.; Zachariah, M.R. Comparison study of the ignition and combustion characteristics of directly-written Al/PVDF, Al/Viton and Al/THV composites. Combust. Flame 2019, 201, 181–186. [Google Scholar] [CrossRef]
- Huang, C.; Jian, G.; Delisio, J.B.; Wang, H.; Zachariah, M.R. Electrospray deposition of energetic polymer nanocomposites with high mass particle loadings: A prelude to 3D printing of rocket motors. Adv. Eng. Mater. 2015, 17, 95–101. [Google Scholar] [CrossRef]
- Wang, J.; Qiao, Z.; Yang, Y.; Shen, J.; Long, Z.; Li, Z.; Cui, X.; Yang, G. Core–Shell Al-Polytetrafluoroethylene (PTFE) Configurations to Enhance Reaction Kinetics and Energy Performance for Nanoenergetic Materials. Chem. A Eur. J. 2016, 22, 279–284. [Google Scholar] [CrossRef]
- DeLisio, J.B.; Hu, X.; Wu, T.; Egan, G.C.; Young, G.; Zachariah, M.R. Probing the reaction mechanism of aluminum/poly (vinylidene fluoride) composites. J. Phys. Chem. B 2016, 120, 5534–5542. [Google Scholar] [CrossRef]
- Padhye, R.; Aquin, A.; Tunega, D.; Pantoya, M.L. Fluorination of an Alumina Surface: Modeling Aluminum-Fluorine Reaction Mechanisms. ACS Appl. Mater. Interfaces 2017, 9, 24290–24297. [Google Scholar] [CrossRef]
- Fu, L.; Hashim, N.A.; Liu, Y.; Abed, M.R.M.; Li, K. Progress in the production and modification of PVDF membranes. J. Membrane Sci. 2011, 375, 1–27. [Google Scholar]
- Lyu, J.-Y.; Chen, S.; He, W.; Zhang, X.X.; Tang, D.Y.; Liu, P.J.; Yan, Q.L. Fabrication of high-performance graphene oxide doped PVDF/CuO/Al nanocomposites via electrospinning. Chem. Eng. J. 2019, 368, 129–137. [Google Scholar] [CrossRef]
- Wang, H.; Kline, D.; Rehwoldt, M.; Wu, T.; Zhao, W.; Wang, X.; Zachariah, M.R. Architecture can significantly alter the energy release rate from nanocomposite Energetics. ACS Appl. Polym. Mater. 2019, 1, 982–989. [Google Scholar] [CrossRef]
- Chen, S.; Yu, H.; Zhang, W.; Shen, R.; Guo, W.; DeLuca, L.T.; Wang, H.; Ye, Y. Sponge-like Al/PVDF films with laser sensitivity and high combustion performance prepared by rapid phase inversion. Chem. Eng. J. 2020, 396, 124962. [Google Scholar] [CrossRef]
- Ji, J.; Liang, L.; Xu, H.; Xiang, G.; Li, H.; Li, P.; Zhou, X.; Guo, X. Facile solvent evaporation synthesis of core-shell structured Al@PVDF nanoparticles with excellent corrosion resistance and combustion properties. Combust. Flame 2022, 238, 111925. [Google Scholar] [CrossRef]
- Ke, X.; Guo, S.; Zhang, G.; Zhou, X.; Xiao, L.; Hao, G.; Wang, N.; Jiang, W. Safe preparation, energetic performance and reaction mechanism of corrosion-resistant Al/PVDF nanocomposite films. J. Mater. Chem. A 2018, 6, 17713–17723. [Google Scholar] [CrossRef]
- Eslami, A.; Hosseini, S.G.; Shariaty, S.H.M. Stabilization of ammonium azide particles through its microencapsulation with some organic coating agents. Powder Technol. 2011, 208, 137–143. [Google Scholar] [CrossRef]
- Trunov, M.A.; Schoenitz, M.; Zhu, X.; Dreizin, E.L. Effect of polymorphic phase transformations in Al2O3 film on oxidation kinetics of aluminum powders. Combust. Flame 2005, 140, 310–318. [Google Scholar] [CrossRef]
- Hasani, S.; Panjepour, M.; Shamanian, M. The oxidation mechanism of pure aluminum powder particles. Oxid. Met. 2012, 78, 179–195. [Google Scholar] [CrossRef]
- Zhao, B.; Sun, S.; Luo, Y.; Cheng, Y. Fabrication of polytetrafluoroethylene coated micron aluminium with enhanced oxidation. Materials 2020, 13, 3384. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Guo, X.; Chen, L.; Huang, S.; Zhao, L.; Ji, J.; Zhou, X. Alcohol-thermal synthesis of approximately core-shell structured Al@CuO nanothermite with improved heat-release and combustion characteristics. Combust. Flame 2021, 228, 331–339. [Google Scholar] [CrossRef]
- Rufino, B.; Coulet, M.V.; Bouchet, R.; Isnard, O.; Denoyel, R. Structural changes and thermal properties of aluminium micro-and nano-powders. Acta Mater. 2010, 58, 4224–4232. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Qin, M.; Yi, Z.; Deng, H.; Wang, J.; Sun, Y.; Luo, G.; Shen, Q. Oxidation Mechanism of Core-Shell Structured Al@PVDF Powders Synthesized by Solvent/Non-Solvent Method. Materials 2022, 15, 3036. https://doi.org/10.3390/ma15093036
Wang C, Qin M, Yi Z, Deng H, Wang J, Sun Y, Luo G, Shen Q. Oxidation Mechanism of Core-Shell Structured Al@PVDF Powders Synthesized by Solvent/Non-Solvent Method. Materials. 2022; 15(9):3036. https://doi.org/10.3390/ma15093036
Chicago/Turabian StyleWang, Chuanbin, Mei Qin, Zhuoran Yi, Haoyuan Deng, Junjie Wang, Yi Sun, Guoqiang Luo, and Qiang Shen. 2022. "Oxidation Mechanism of Core-Shell Structured Al@PVDF Powders Synthesized by Solvent/Non-Solvent Method" Materials 15, no. 9: 3036. https://doi.org/10.3390/ma15093036
APA StyleWang, C., Qin, M., Yi, Z., Deng, H., Wang, J., Sun, Y., Luo, G., & Shen, Q. (2022). Oxidation Mechanism of Core-Shell Structured Al@PVDF Powders Synthesized by Solvent/Non-Solvent Method. Materials, 15(9), 3036. https://doi.org/10.3390/ma15093036





