Study on the Design, Preparation, and Performance Evaluation of Heat-Resistant Interlayer-Polyimide-Resin-Based Neutron-Shielding Materials
Abstract
:1. Introduction
2. Material and Method
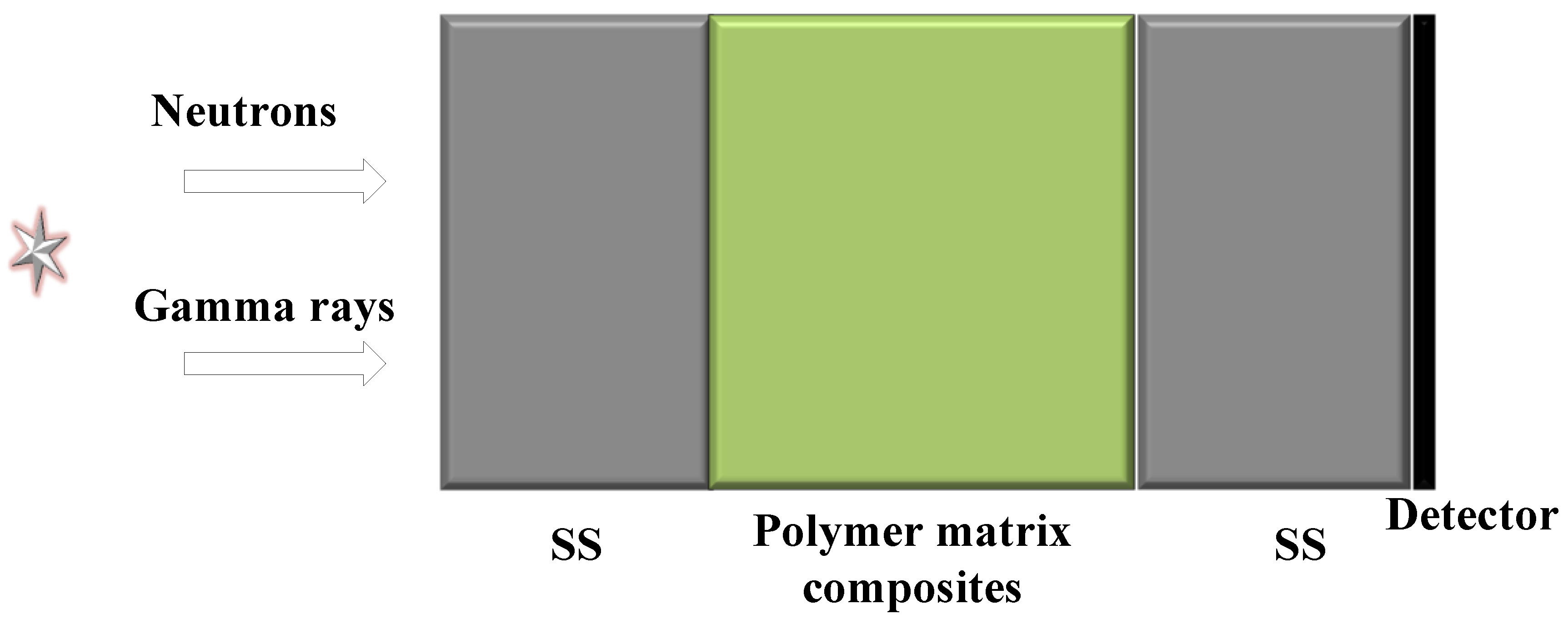
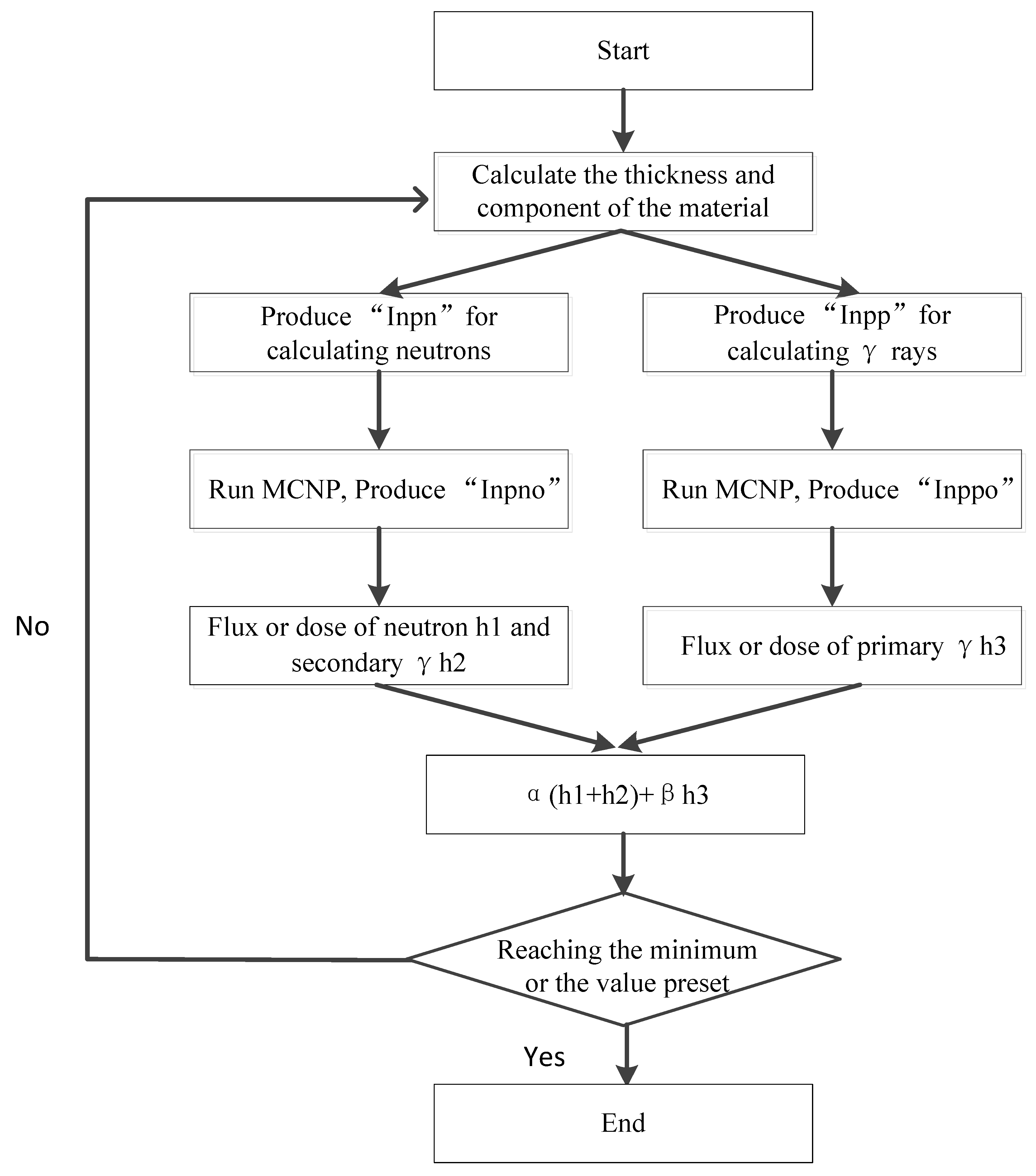
2.1. Optimal Design of the Materials
- (1)
- Input the parameters expressing the thickness of the shield and the components of the materials.
- (2)
- Produce the “inpn” file and “inpp” file for simulating the neutron and γ ray transmission in the material.
- (3)
- The “inpn” file and “inpp” file are calculated by MCNP, and the “outpn” file and “outpp” are produced.
- (4)
- Extract the data expressing the dose equivalent of neutron in the “outpn” file and the γ-rays in the “outpp” file.
- (5)
- The program stops when the fitness value is not changed or the iteration times reach to N0 (the generation number). If not, a new thickness and component will be produced and then the next calculation will start.
2.2. Preparation of Materials
3. Results and Discussion
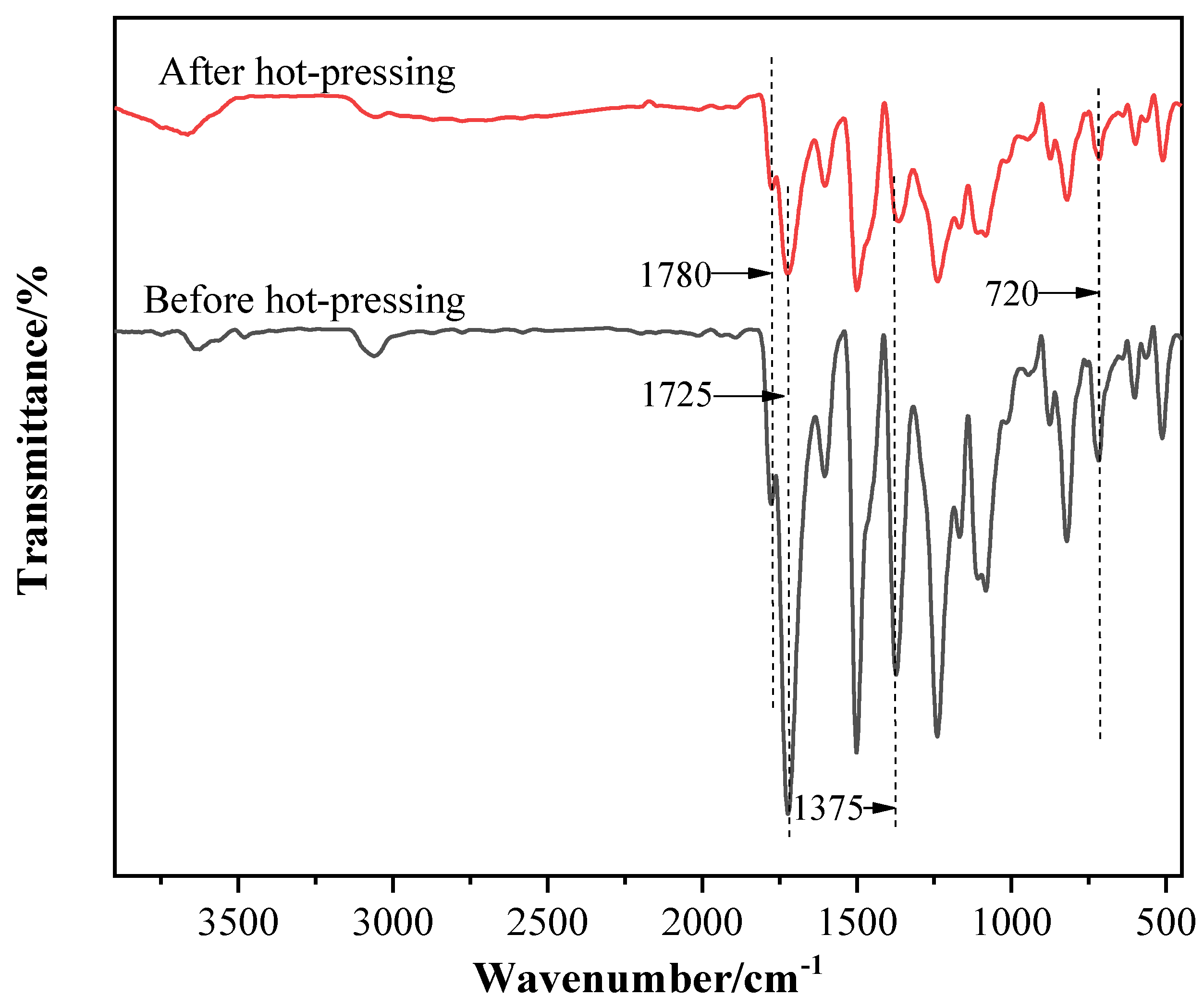
3.1. Analysis of Chemical Structure and Composition
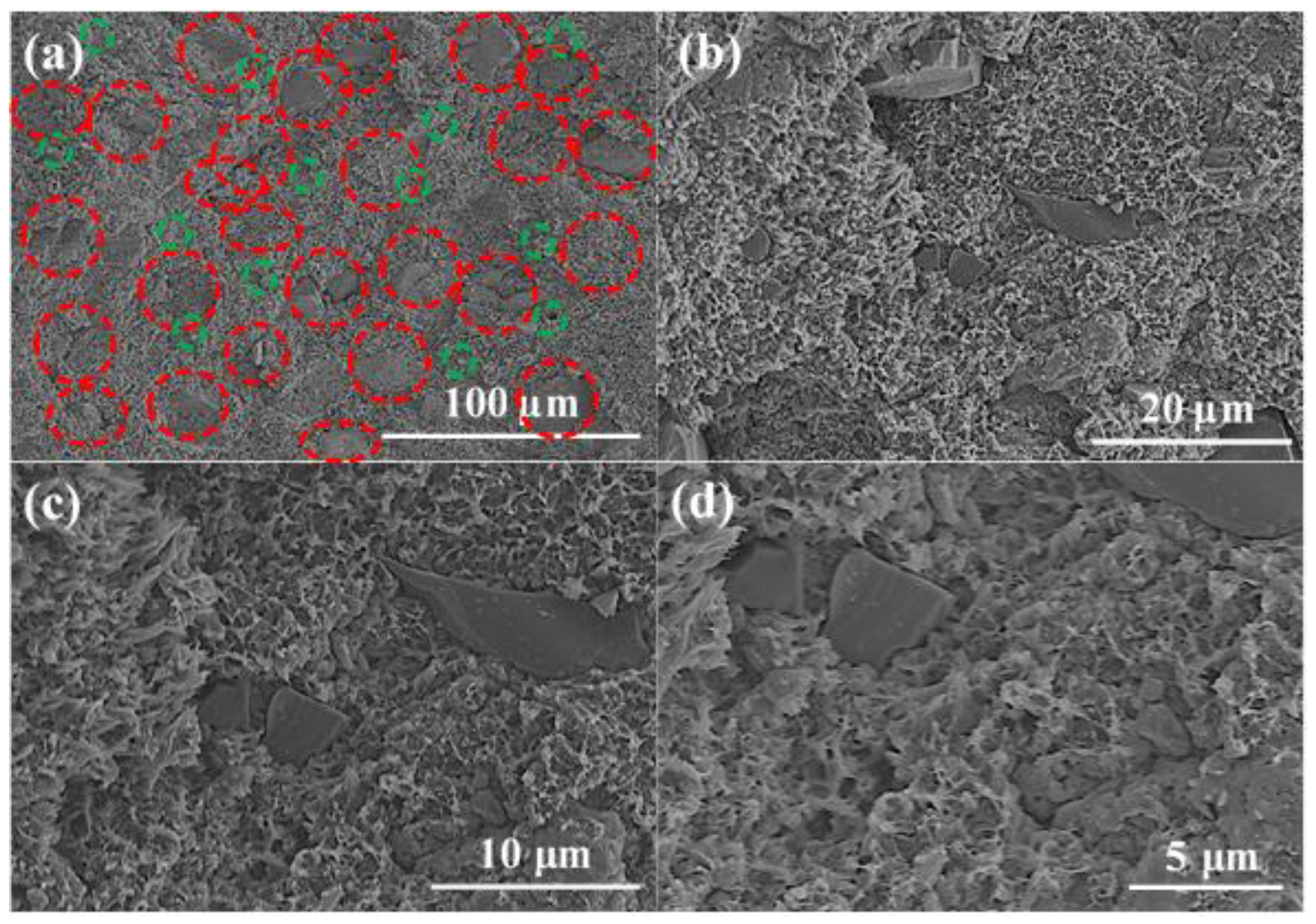
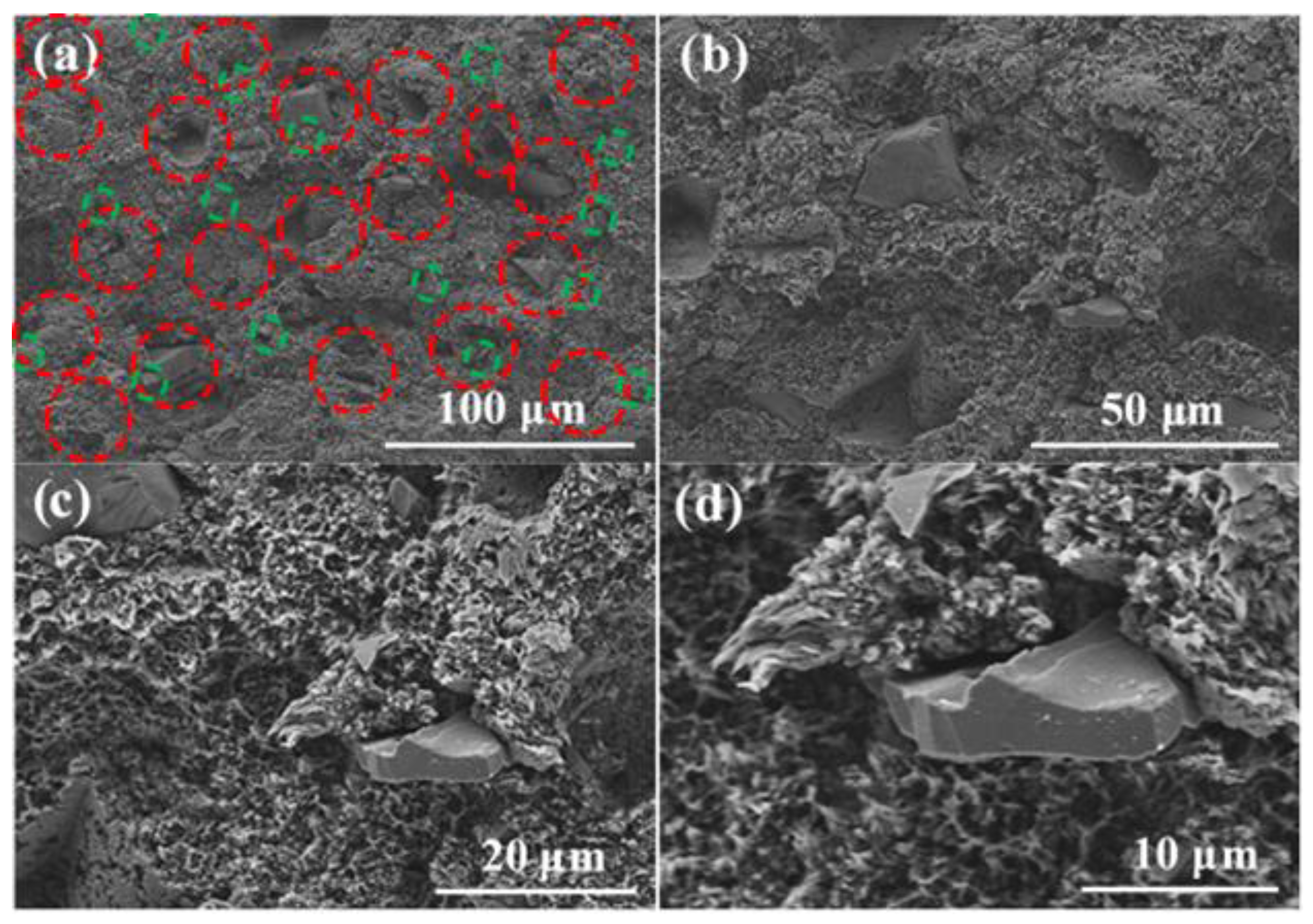
3.2. Microscopic Morphology Test and Analysis
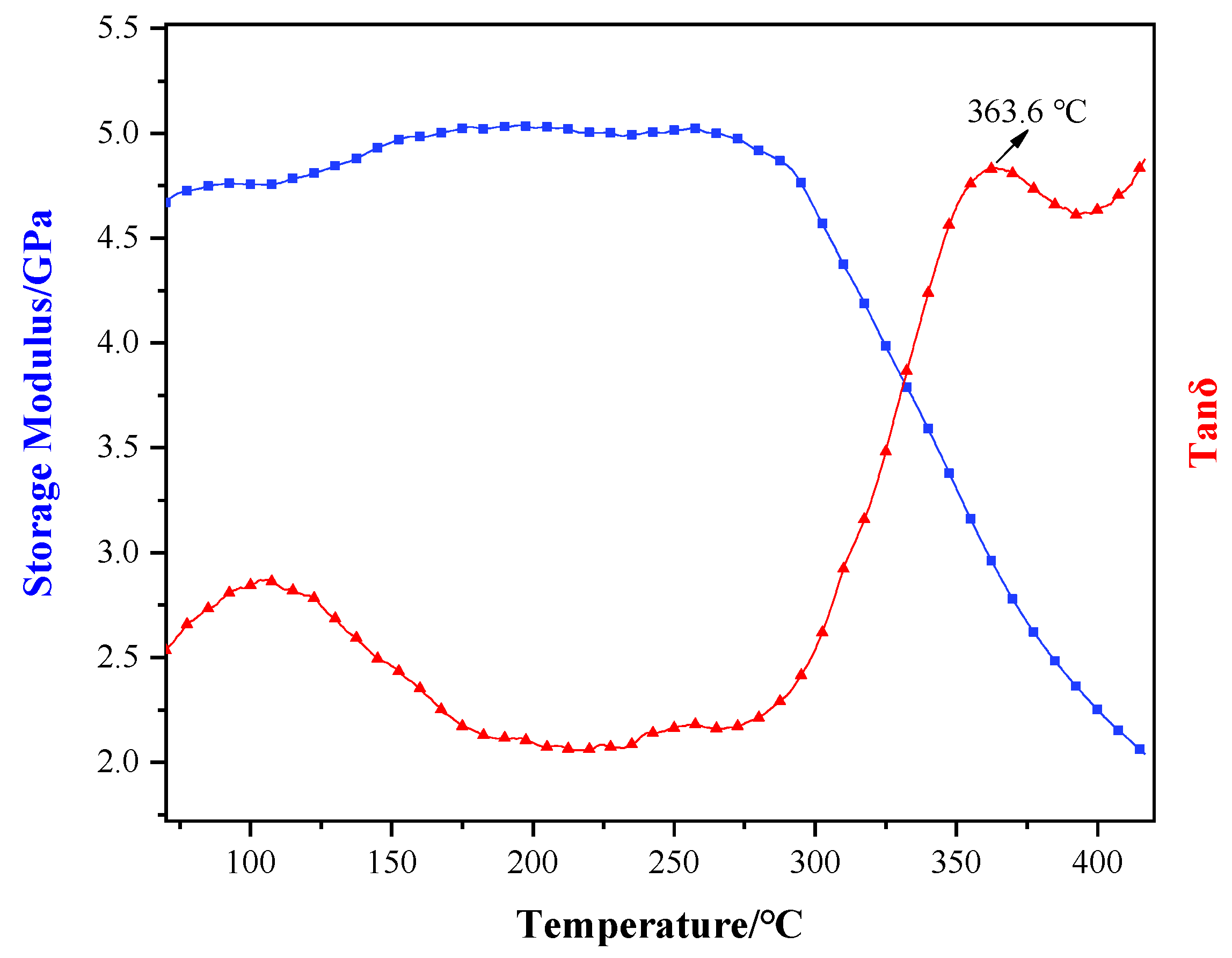
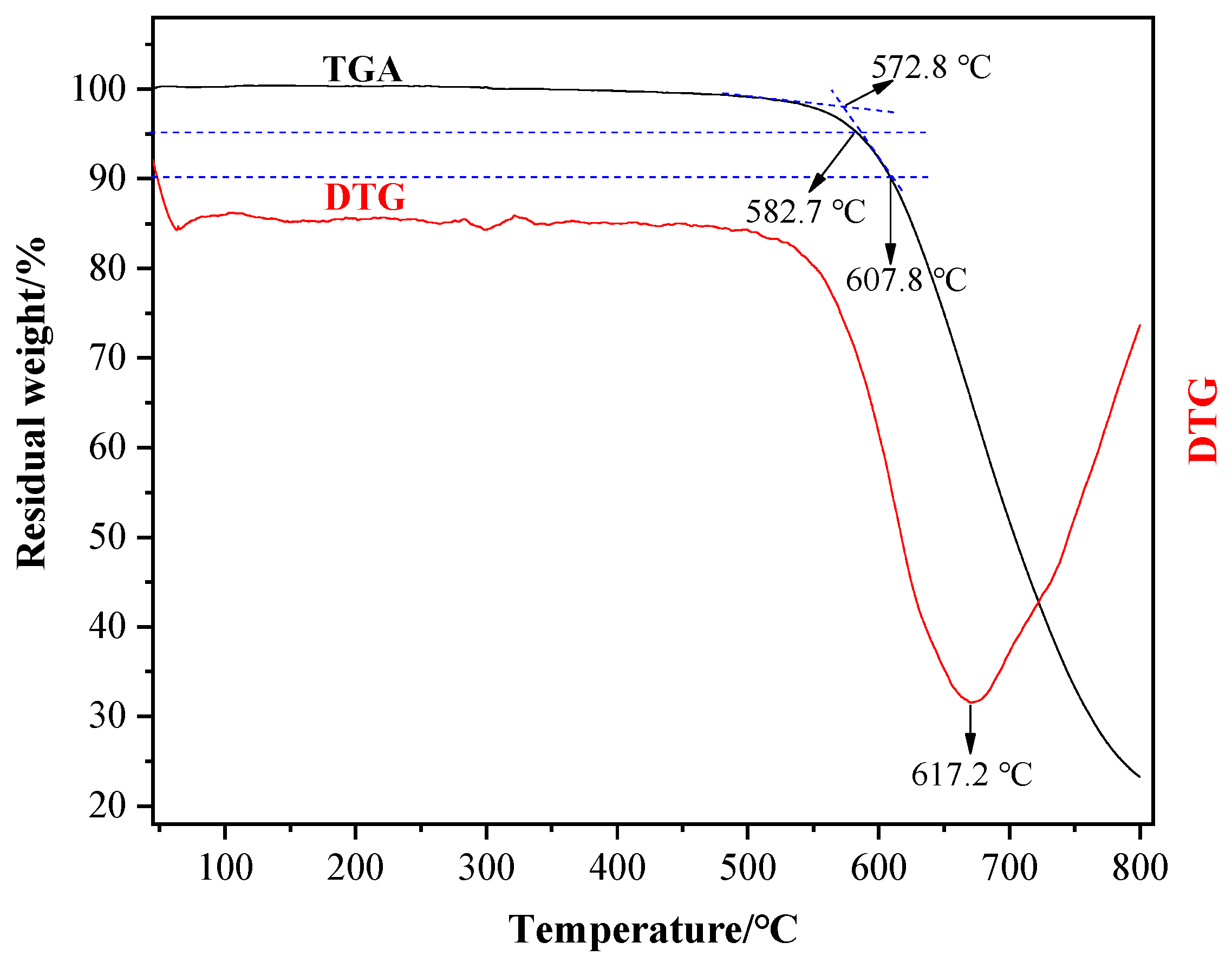
3.3. Thermal Properties Analysis
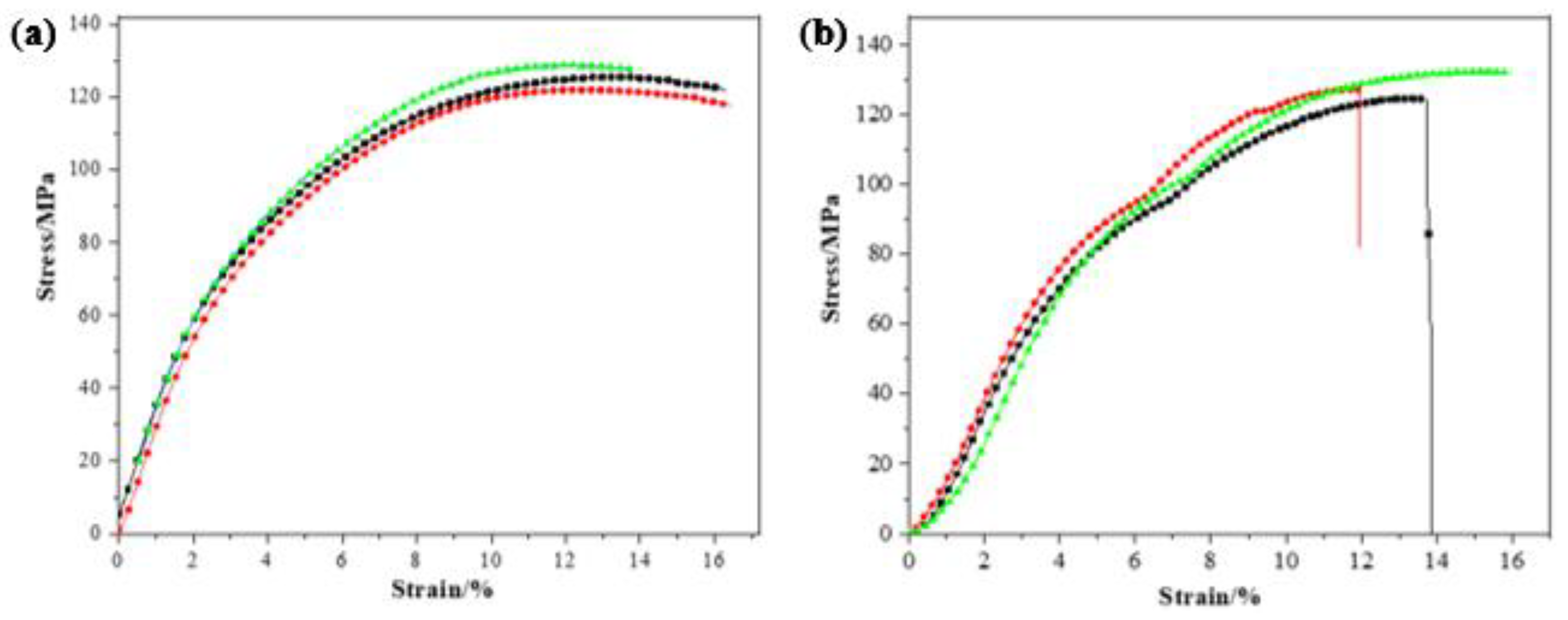
3.4. Mechanical Properties Analysis
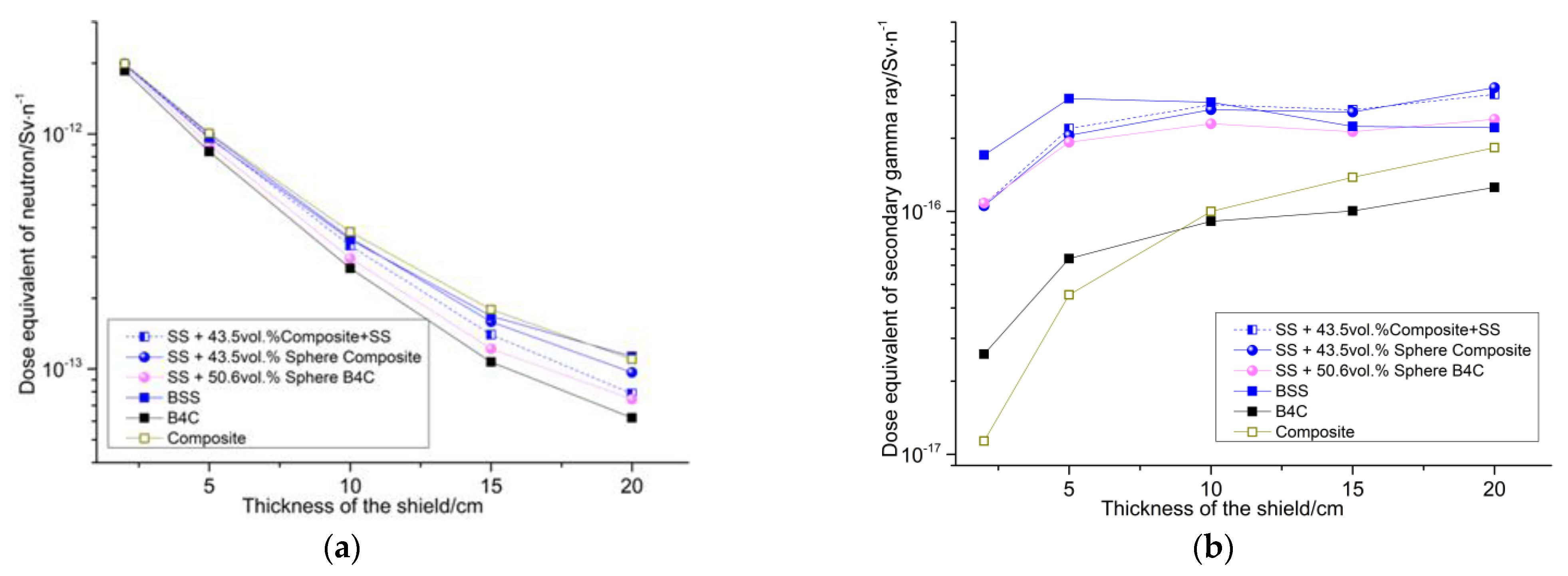
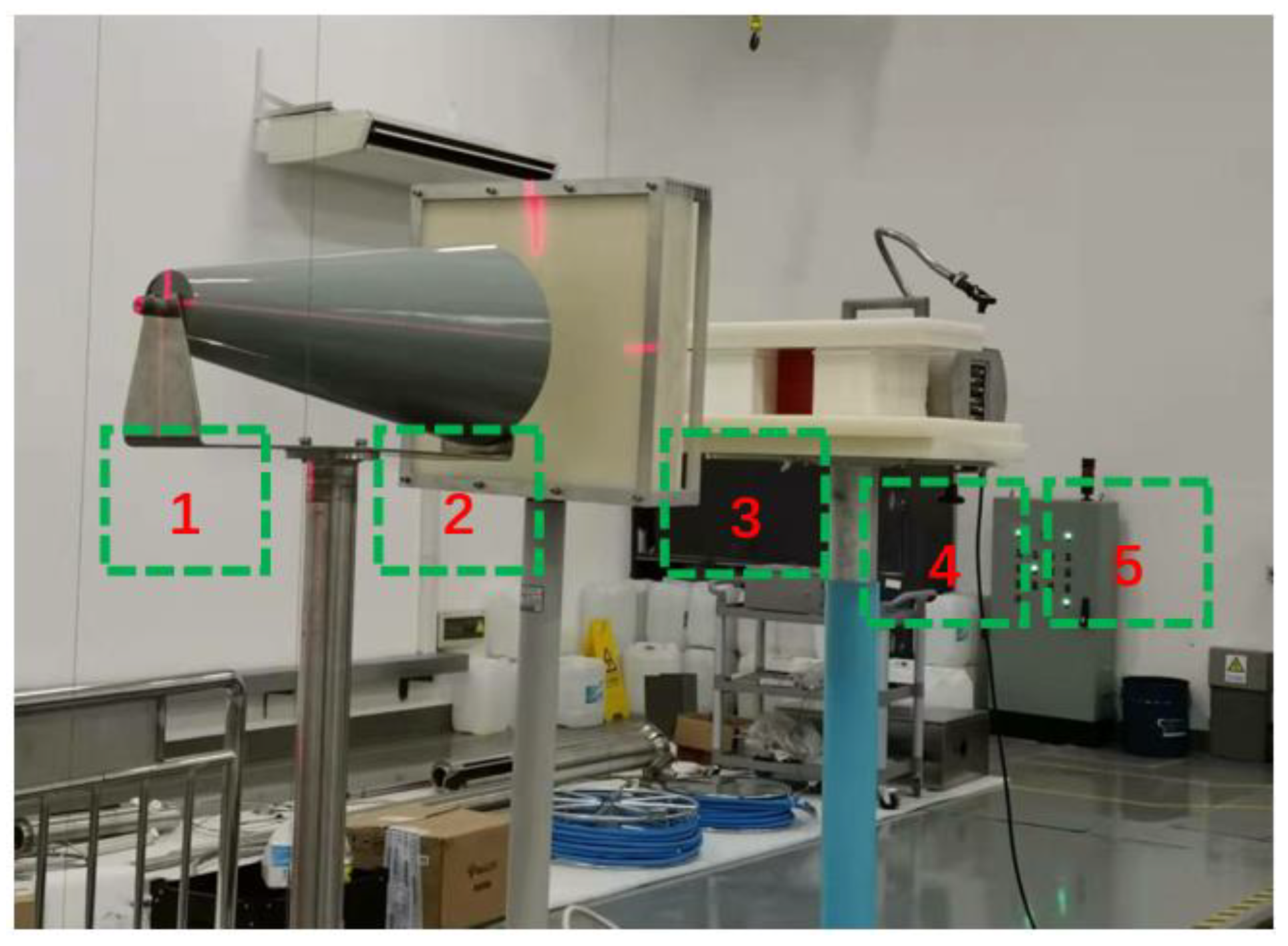
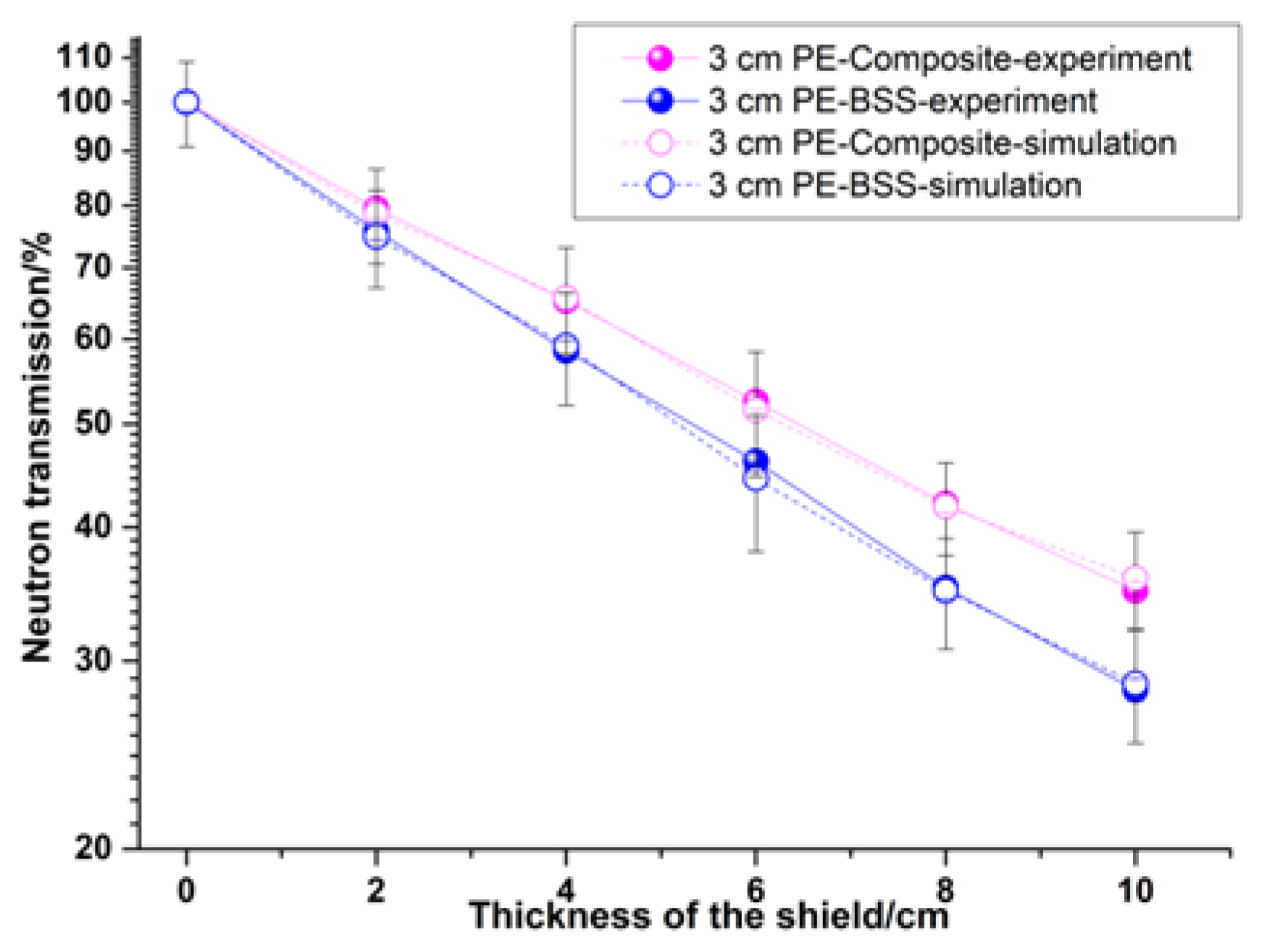
3.5. Shielding Performance Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sun, W.-Q.; Hu, G.; Yu, X.-H.; Shi, J.; Xu, H.; Wu, R.-J.; He, C.; Yi, Q.; Hu, H.-S. Study on a High-Boron-Content Stainless Steel Composite for Nuclear Radiation. Materials 2021, 7004, 7004. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Shi, G.; Hu, H.; Yang, Q.; Yu, B.; Sun, W. Development of gradient composite shielding material for shielding neutrons and gamma rays. Nucl. Eng. Technol. 2020, 52, 2387–2393. [Google Scholar] [CrossRef]
- Türkaslan, S.S.; Ugur, S.S.; Türkaslan, B.E.; Fantuzzi, N. Evaluating the X-ray-Shielding Performance of Graphene-Oxide- Coated Nanocomposite Fabric. Materials 2022, 15, 1441. [Google Scholar] [CrossRef] [PubMed]
- Al-Balushi, M.A.; Ahmed, N.M.; Zyoud, S.H.; Ali, M.K.M.; Akhdar, H.; Aldaghri, O.A.; Ibnaouf, K.H. Ionization Radiation Shielding Effectiveness of Lead Acetate, Lead Nitrate, and Bismuth Nitrate-Doped Zinc Oxide Nanorods Thin Films: A Comparative Evaluation. Materials 2022, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- ALMisned, G.; Zakaly, H.M.H.; Issa, S.A.M.; Ene, A.; Kilic, G.; Bawazeer, O.; Almatar, A.; Shamsi, D.; Rabaa, E.; Sideig, Z.; et al. Gamma-Ray Protection Properties of Bismuth-Silicate Glasses against Some Diagnostic Nuclear Medicine Radioisotopes: A Comprehensive Study. Materials 2021, 14, 6668. [Google Scholar] [CrossRef] [PubMed]
- Almurayshid, M.; Alssalim, Y.; Aksouh, F.; Almsalam, R.; ALQahtani, M.; Sayyed, M.I.; Almasoud, F. Development of New Lead-Free Composite Materials as Potential Radiation Shields. Materials 2021, 14, 4957. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Dong, L.-M.; Wang, C. Study on boron carbide/ultra high molecular weight polyethylene composites for neutron shielding. Mater. Eng. 2010, 2, 337–340. [Google Scholar]
- Harrison, C.E. High Density Polythene/Boron Containing Composites for Radiation Shielding Applications. Ph.D. Thesis, University of Kentucky, Lexington, KY, USA, 2008. [Google Scholar]
- Okuno, K. Neutron shielding material based on colemanite and epoxy resin. Radiat. Prot. Dosim. 2005, 115, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, A.M.; Anayama, Y.; Ohnishi, S.; Sakurai, S.; Kaminaga, A.; Okuno, K. Development of Flexible Neutron-Shielding Resin as an Additional Shielding Material. J. Nucl. Sci. Technol. 2011, 48, 585–590. [Google Scholar] [CrossRef]
- Kipcak, A.S.; Gurses, P.; Derun, E.M.; Tugrul, N. Characterization of boron carbide particles and its shielding behavior against neutron radiation. Energy Convers. Manag. 2013, 72, 39–44. [Google Scholar] [CrossRef]
- Sakuraia, Y.; Sasakib, A. Development of neutron shielding material using metathesis-polymer matrix. Nucl. Instrum. Methods Phys. Res. A 2004, 522, 455–461. [Google Scholar] [CrossRef]
- Dezfuli, S.M.; Sabzi, M. Deposition of ceramic nanocomposite coatings by electroplating process: A review of layer-deposition mechanisms and effective parameters on the formation of the coating. Ceram. Int. 2019, 45, 21835–21842. [Google Scholar] [CrossRef]
- Clad Aluminum Alloy Products and Methods of Making the Same. U.S. Patent 20,180,304,584, 4 August 2020.
- Saffari, H.; Shamanian, M.; Bahrami, A.; Szpunar, J.A. Effects of ERNiCr-3 butter layer on the microstructure and mechanical properties of API 5L X65/AISI304 dissimilar joint. J. Manuf. Process. 2020, 50, 305–318. [Google Scholar] [CrossRef]
- Hu, G.; Hu, H.; Sun, W.; Yan, Y.; Yan, M. Designing and preparing for a 288 °C heat resistant and inhibition of gas production resin matrix nuclear radiation shielding material. AIP Adv. 2019, 12, 125044-1–125044-7. [Google Scholar] [CrossRef] [Green Version]
- Ling, Y.; Zhanshan, Y.; Shuqin, Y.; Xiuhua, P.; Peng, P. Preparation of hydrogel containing gentamicin and its drug delivery in vitro. J. Radiat. Res. Radiat. Process. 2005, 23, 355–358. [Google Scholar]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002, 17, 13–36. [Google Scholar] [CrossRef]
- Abdulrahman, S.T.; Sabu, T.; Ahmad, Z. Micro and Nanostructured Composite Materials for Neutron Shielding Applications; Elsevier: Amsterdam, The Netherlands, 2020; ISBN 978-0-12-819459-1. [Google Scholar]
- Hu, G.; Hu, H.; Yang, Q.; Yu, B. Weiqiang Sun. Study on the design and experimental verification of multilayer radiation shield against mixed neutrons and γ-rays. Nucl. Eng. Technol. 2020, 52, 178–184. [Google Scholar] [CrossRef]


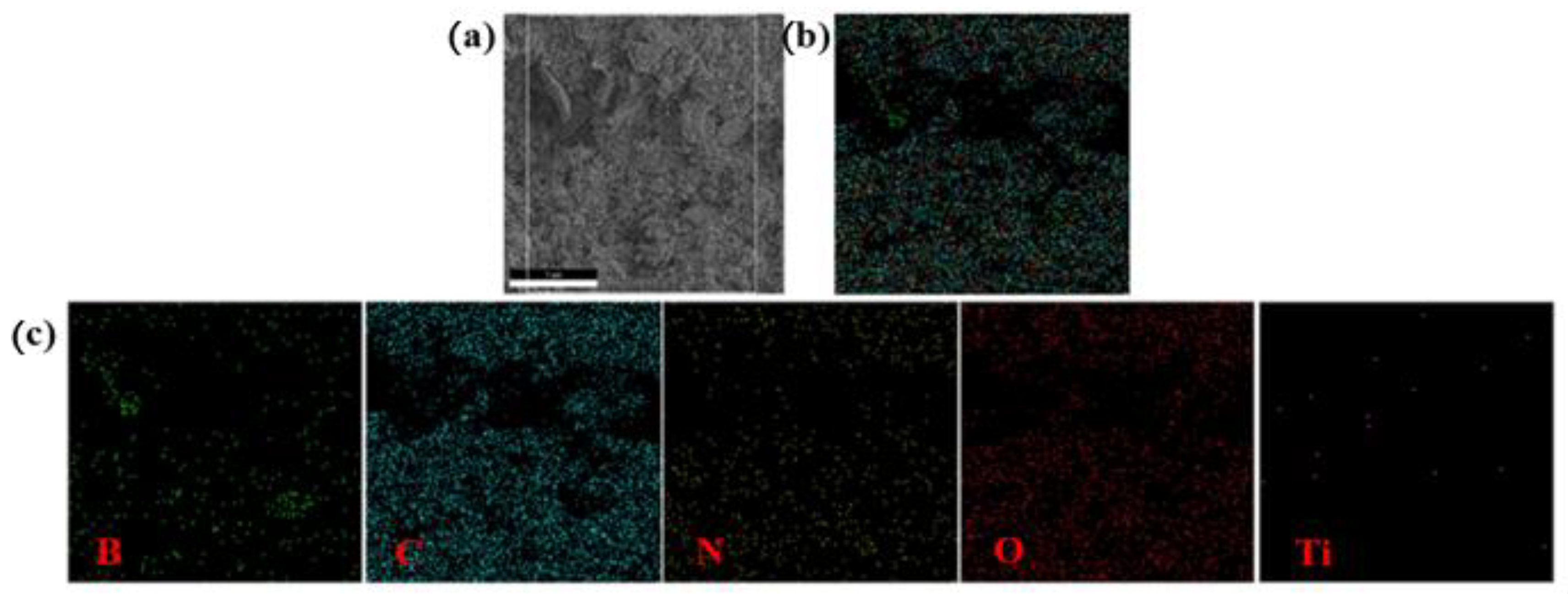
| Element | B | C | N | O | Ti |
|---|---|---|---|---|---|
| Measured value (wt%) | 7.38 | 62.21 | 10.90 | 18.48 | 1.03 |
| Theoretical value (wt%) | 7.82 | 62.30 | 9.54 | 16.84 | 1.80 |
| Test | Section Width (mm) | Section Thickness (mm) | Absorption Work (J) | Impact Resistance Strength (kJ/m2) | |
|---|---|---|---|---|---|
| Before Aging | 1 | 10.25 | 3.57 | 0.42 | 11.48 ± 0.57 |
| 2 | 10.24 | 3.34 | 0.41 | 11.99 ± 0.60 | |
| 3 | 10.22 | 3.64 | 0.44 | 11.83 ± 0.59 | |
| Average | - | - | - | 11.77 ± 0.59 | |
| After Aging | 1 | 10.16 | 3.37 | 0.40 | 11.68 ± 0.58 |
| 2 | 10.22 | 3.59 | 0.42 | 11.45 ± 0.57 | |
| 3 | 10.15 | 3.49 | 0.41 | 11.57 ± 0.58 | |
| Average | - | - | - | 11.57 ± 0.58 | |
| Material | Composite (10 cm) | BSS (10 cm) | ||
|---|---|---|---|---|
| - | Experiment | Simulation | Experiment | Simulation |
| Transmission (%) | 34.88 | 35.87 | 28.18 | 28.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Liu, D.; Sun, W.-Q.; Wu, R.-J.; Liao, W.; Li, X.-L.; Hu, G.; Hu, H.-S. Study on the Design, Preparation, and Performance Evaluation of Heat-Resistant Interlayer-Polyimide-Resin-Based Neutron-Shielding Materials. Materials 2022, 15, 2978. https://doi.org/10.3390/ma15092978
Xu H, Liu D, Sun W-Q, Wu R-J, Liao W, Li X-L, Hu G, Hu H-S. Study on the Design, Preparation, and Performance Evaluation of Heat-Resistant Interlayer-Polyimide-Resin-Based Neutron-Shielding Materials. Materials. 2022; 15(9):2978. https://doi.org/10.3390/ma15092978
Chicago/Turabian StyleXu, Hu, Dan Liu, Wei-Qiang Sun, Rong-Jun Wu, Wu Liao, Xiao-Ling Li, Guang Hu, and Hua-Si Hu. 2022. "Study on the Design, Preparation, and Performance Evaluation of Heat-Resistant Interlayer-Polyimide-Resin-Based Neutron-Shielding Materials" Materials 15, no. 9: 2978. https://doi.org/10.3390/ma15092978
APA StyleXu, H., Liu, D., Sun, W.-Q., Wu, R.-J., Liao, W., Li, X.-L., Hu, G., & Hu, H.-S. (2022). Study on the Design, Preparation, and Performance Evaluation of Heat-Resistant Interlayer-Polyimide-Resin-Based Neutron-Shielding Materials. Materials, 15(9), 2978. https://doi.org/10.3390/ma15092978





