Abstract
This paper presents studies on the possibility of utilization of technogenic waste from the metallurgical industry by the method of complex processing in order to reduce the anthropogenic load on the environment of the region with the example of the zinc silicate-magnetite-carbon system. The selected sample of clinker dump from welting was subjected to chemical and scanning electron microscopic analyses and thermodynamic modeling. Thermodynamic studies were carried out in the temperature range 1600–2200 K and pressure p = 0.1 MPa, modeling the process of electric melting of clinker from welting in an arc furnace using the software application Astra 4 developed at the Bauman Moscow State Technical University (Moscow, Russian Federation). As a result of the thermodynamic modeling, the optimal temperature range was established, which was 1800–1900 K. Thermodynamic studies established that it is possible to drive away zinc from the system under study by 99–100% in the entire temperature range under study. The maximum degree of silicon extraction (αSi) in the alloy is up to 69.44% at T = 1900 K, and the degree of iron extraction (αFe) in the alloy is up to 99.996%. In particular, it was determined and proved that clinker waste from welting can act as a secondary technogenic raw material when it is processed as a mono mixture to produce iron silicides with a silicon content of 18 to 28%.
1. Introduction
Industrial waste located in dumps and containing in its chemical composition heavy non-ferrous metals and various compounds of silicon, calcium, aluminum and iron has a negative impact on the environment from the point of view of ecology, in particular on public health, flora and fauna [1,2,3,4,5,6,7,8]. However, based on its chemical composition, it is valuable and can serve as a secondary technogenic raw material for various kinds of industries, for example in the metallurgical, construction, chemical and other industries [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53]. Thus, in Kazakhstan, in the process of extracting non-ferrous metals at a number of plants, since the 1920s and up to the present, in a number of areas, in particular, in the east Kazakhstan and Turkestan regions, there is a significant amount of clinker waste from the cultivation of various raw materials, which is now stored in dumps, occupying fertile lands and polluting the soil, surface and groundwater, the atmosphere, penetrating animal and human organisms through the migration of heavy metals along the food chain [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53].
According to various estimates, at present, in the village of Achisai (Turkestan region), in the dumps, there are 4.5–5.7 million tons of zinc industry waste formed over the years of the Achisai polymetallic combine, which contains, according to various data, at least 102 thousand tons of zinc (Zn), 16 thousand tons of lead (Pb), 410 thousand tons of silicon (Si), 1.1 million tons of iron (Fe) and about 780–815 thousand tons of carbon (C). Despite this, clinker from welting is now considered only as raw material to extract carbon and obtain an iron-containing magnetic concentrate with the extraction of up to 80% of iron into it. At the same time, silicon, calcium, aluminum and non-ferrous metals are not extracted and pass into the flotation tails of the non-magnetic fraction, which are recommended for use in the production of building materials [8,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53].
In different years, there have been many attempts to use clinker from welting. In particular, a study [54] showed the possibility of obtaining building materials and mineral wool from Achisai clinker, as well as the possibility of using it in road construction [55]. There is a well-known work on the processing of clinker welting slags from mine lead smelting by magnetic separation to obtain (25–30%) ferromagnetic concentrate with a content of 75–89% Fe and 1–1.5% Cu [56]. At the same time, the magnetic concentrate was used in the fusing of slag, in the charge of agglomeration of lead production, in the enrichment of oxidized copper ores (instead of cast iron shavings), and a coal concentrate with a content of 58%C was obtained from the non-magnetic fraction, which is recommended for use in welding by blowing into the furnace or granulating with recycled dust [57].
At the Elektrozink plant, an experiment was carried out on blowing clinker with compressed air (blowing off carbon) and feeding a coal–air mixture into the head of the Welz furnace. However, despite an increase in productivity (by 10%) and a decrease in coke consumption, the experiment was discontinued due to deterioration in the quality of Welz oxide due to contamination with ash and carbon [58].
The experience of processing rich clinker in Bulgaria is interesting [59]. Clinker containing (%): Cu—2.23; Zn—1.31; Pb—1.25; C—19.1; SiO2—20.0; S—4.47, as well as 200 g/t Ag and 12 g/t Au, is subjected to screening. Class +16 mm is shipped to copper smelters, the rest (−16 mm) is divided into heavy suspension, after which the heavy fraction is sent to the copper smelter, and the light fraction is used in Welz furnaces. At the same time, the extraction of copper into products for metallurgical processing is up to 93%.
For the processing of clinkers poor in precious metals, more complex technological schemes are used, with a combination of flotation and magnetic separation. Thus, foreign researchers were able to achieve the extraction of copper and gold into processed products up to 91.7% and silver—89.1%. At foreign enterprises, this makes it possible to obtain concentrates with a content of 1.5% Cu, 515–620 g/t Ag, with a content of Cu in the non-magnetic fraction of 0.05%, C—80% (Peru, La Oroya plant) or Cu—1.6%, Au—3.2 g/t and 544 g/t Ag (Japan, Aizu plant) [60,61].
For the extraction of non-ferrous metals from the clinkers of the UCCC and CHECZ in GINTSVETMET, a chloride-distilling method in a fluidized bed furnace has been developed [62]. The method was tested on a semi-industrial installation with an hourly capacity of 165 kg, for raw materials. The disadvantages of the method are the long duration of the process—5.5 h—a large consumption of concentrated CaCl2 solution—30% of the ore mass—as well as a relatively high residual content of Zn (0.5%) and Cu (0.25%) in the cinder.
The Kazakh Chemical Technological Institute (KazSSR) has developed a chloride method for processing UKCC clinkers in a tubular rotary kiln with a combination of chloride distillation of non-ferrous metals in the furnace [63]. Despite the fact that the economic effect of the developed method is USD ≈ 10 for each ton of clinker, the method cannot be considered rational, since it provides for the processing of the charge, in which the share of non-metallic components accounts for 55.9%.
In the 1990s, Yuzhpolymetal CJSC began work on the processing of Achisai clinker to obtain magnetic concentrate and coke, which never received a mass character, limiting itself to research experiments. However, the technological indicators of this process (including the extraction of non-ferrous metals) are not described in the special literature. None of the above methods have been implemented, and the clinker from the welting is not being disposed of at the moment and continues to pollute the environment of the region.
In the conducted experiments, using thermodynamic modeling based on the Astra 4 program, the possibility of complex utilization of clinker dump to reduce anthropogenic impact on the biosphere of the region was investigated using the example of the ZnO∙SiO2-FeO∙Fe2O3-C system with the production of iron silicides and zinc sublimates from it.
2. Materials and Methods
As the material under study, a sample taken from the clinker dump of zinc oxide ores was used. The studied technogenic waste in the form of clinker from welting was subjected to chemical [64], scanning electron microscopic [65] analyses and thermodynamic modeling of its processing by electric melting in an arc furnace to produce iron silicides and zinc-containing sublimates.
At the present stage, when solving many scientific and technical problems, the issues of studying high-temperature processes with physicochemical transformations, for example, combustion processes, play a significant role. The experimental methods of studying processes of this kind are usually expensive and often not feasible at all. Under these conditions, a computational experiment performed using a computer acquires special importance, which allows analyzing states and processes and drawing conclusions about the behavior of the objects under study based on model representations [66,67].
The main assumption, in this case, is the assumption of the existence of a local equilibrium in the system, which makes it possible to carry out calculations using the mathematical apparatus of equilibrium thermodynamics [66,67].
The main task of modeling thermodynamic equilibrium is to determine the phase and chemical composition, as well as the values of the thermodynamic parameters of the system under study [66,67].
Thermodynamic modeling of chemical and phase transformations in the system under study was carried out using the computer program Astra 4, which was developed by a group of scientists of the Bauman Moscow State Technical University and operates using the principle of maximum entropy [66,67].
The Astra software package is based on the principle of maximum entropy—a factor associated with the degree of ordering of the energy state of the microparticles that make up the working fluid. The laws of statistical physics allow us to find the number of discrete states that a specific (given) microstate implements. A comparison of this value with entropy allows us to establish that the latter is a measure of the probability of the state of the system. Therefore, maximum entropy corresponds to the equilibrium conditions of the considered set of particles of the working medium, i.e., the relationship between the probability of the state of the system and its entropy (S) allows us to formulate an extreme condition defining the state of the system by expressions:
where:
S = Smax Mi = const, Un—const, v = const,
Un is the total internal energy;
Mj is the mass of the working fluid;
v is the specific volume of the entire system.
The formulation of the thermodynamic modeling problem requires assigning two conditions for the equilibrium of the system under study with the environment. These conditions can be either numerical values of the thermodynamic characteristics of the equilibrium or functional relations between the parameters of this state. To describe the system itself as a material object, it is necessary to know only the content of the chemical elements forming it. Internal and interphase interactions are described by model thermodynamic relations, for the closure of which the properties of only individual substances—the equilibrium component—are used [66,67].
Due to its accessible formulation of the problem of various kinds of modeling, the developed Astra 4 complex promotes the use of the thermodynamic method for the study of various possibilities of the course of a wide variety of processes under conditions of various physicochemical states [66,67].
3. Results
In the course of the research, a chemical analysis of technogenic waste—clinker from welting—was carried out, the results of the study of which are given in Table 1.

Table 1.
Chemical composition of clinker blade from welting.
Having studied the results of the chemical analysis, they approximately coincide with previous studies of clinker’s chemical composition with predominance in iron composition [9,39,68].
Additionally, a sample of clinker from welting was analyzed on a scanning electron microscope of the brand JSM-6490l (Joel, manufactured in Japan) to obtain its elemental composition. The results of the studies are shown in Figure 1. From these results, it follows that the present waste in the form of clinker from welting in its composition contains elements such as zinc, lead, calcium, silicon, oxygen, iron, aluminum (which is also confirmed by the results of the chemical analysis carried out earlier) [9,39,68].
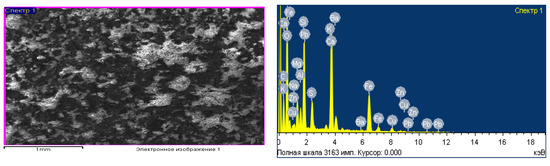
Figure 1.
Micrography (a) and elemental analysis (b) of metallurgical waste.
From various studies of scientists [9,39,58], it is known that clinkers from the welting of various kinds of materials contain Fe (iron) in the reduced state in the form of elemental (Fe) and oxide state (FeO, FeO∙Fe2O3), Zn (zinc) silicate form (ZnO∙SiO2), and carbon is present in the form of coke that did not have time to react in the welting of zinc oxide ores.
Based on the results of determining the chemical composition of the waste under study given in Table 1 and previously obtained data by various scientists [9,68,69,70], chemical and phase interaction was simulated in the temperature range 1600–2200 K with a pressure of p = 0.1 MPa in the heterogeneous system under study ZnO∙SiO2-FeO∙Fe2O3-C, where the interaction of magnetite (FeO∙Fe2O3) with zinc silicate (ZnO∙SiO2) and carbon (C), which are contained in the clinker from Waelz process (Figure 2, Figure 3 and Figure 4, and Table 2).
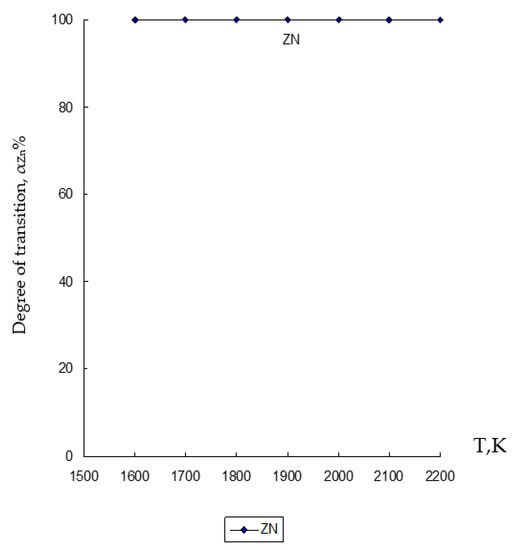
Figure 2.
Influence of temperature (T) on the degree of distribution (α) of Zn in the ZnO∙SiO2-FeO∙Fe2O3-C system.
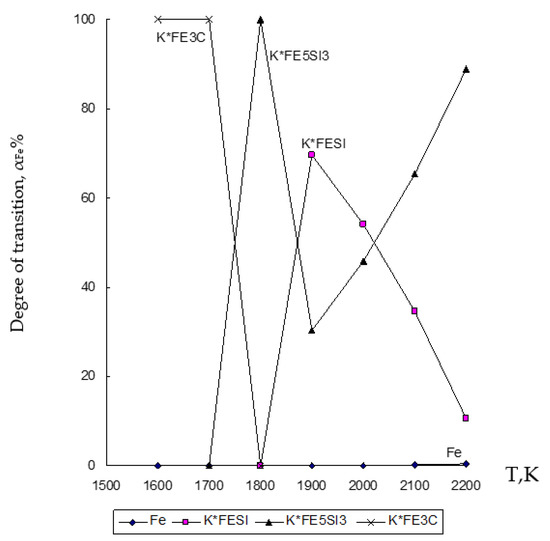
Figure 3.
Influence of temperature (T) on the degree of distribution (α) of Fe in the ZnO∙SiO2-FeO∙Fe2O3-C system.
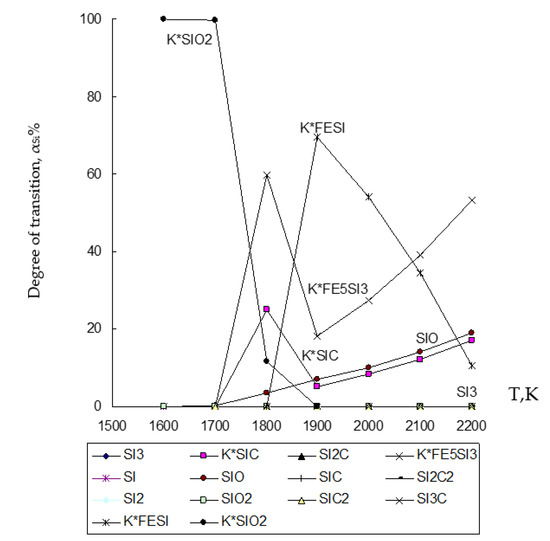
Figure 4.
Influence of temperature (T) on the degree of distribution (α) of Si in the ZnO∙SiO2-FeO∙Fe2O3-C system.

Table 2.
Influence of temperature (T) on the degree of distribution of (α) oxygen (O2) and carbon (C) in the ZnO∙SiO2-FeO∙Fe2O3-C system.
In studies on the interaction in the ZnO∙SiO2-FeO∙Fe2O3-C system, the following chemical equation was adopted as the basic one:
3ZnO∙SiO2 + FeO∙Fe2O3 + 13C = 3 Zn + 3FeSi + 13CO.
The results of modeling the chemical and phase interaction in the system ZnO∙SiO2-FeO∙Fe2O3-C are shown in Figure 2, Figure 3 and Figure 4 and in Table 2.
The effect of temperature on the distribution of zinc (Zn), iron (Fe), silicon (Si), carbon (C), oxygen (O2) in the ZnO∙SiO2-FeO∙Fe2O3-C system is characterized by the formation of seven elements and compounds: Zn, Fe, C, k*C (where k*- means condensed phase), Si, Si2, Si3, FeSi, Fe5Si3, k*Fe3C, k*SiC, CO and CO2.
4. Discussion
From the results obtained in the chemical and phase modeling carried out under the conditions of the ZnO∙SiO2-FeO∙Fe2O3-C system, it is clear that zinc (Zn) completely passes into the gas phase with a 100% degree of transition in the entire set temperature regime (Figure 2).
Figure 3 shows that in the ZnO∙SiO2-FeO∙Fe2O3-C system, iron (Fe) passes into an alloy with a transition degree of up to 99.996% for a compound formed as Fe5Si3 at 1800 K, and up to 69.58% for a compound formed as FeSi at 1900 K.
The degree of distribution of silicon (Si) in the ZnO∙SiO2-FeO∙Fe2O3-C system into iron-containing compounds of the condensed phase is shown in Figure 4. Figure 4 shows that the degree of Si transition to the alloy reaches up to 59.88% for the compound formed as Fe5Si3 at 1800 K, and up to 69.44% for the compound formed as FeSi at 1900 K.
Table 2 shows the results of the degree of distribution of (α) oxygen (O2) and carbon (C) in the ZnO∙SiO2-FeO∙Fe2O3-C system from temperature. Table 2 shows that oxygen is mainly distributed into compounds such as CO, CO2, k*SiO2. So, at T = 1900 K, oxygen is distributed into the following compounds: CO2 by 98.297%; SiO by 1.693%; CO by 0.01% and SiO2 by 0.00006%.
The degree of distribution of (α) carbon (C) in the ZnO∙SiO2-FeO∙Fe2O3-C system from temperature is mainly represented by such compounds as CO2 (from 54.025% to 98.721%), k*C (from 38.192% to 38.249%) and k*Fe3C by 7.714% (Table 2).
Based on the obtained results of the thermodynamic modeling, which are presented in Figure 2, Figure 3 and Figure 4, it follows that under the given temperature conditions in the conditions of the ZnO∙SiO2-FeO∙Fe2O3-C system, it is possible to almost completely extract Zn with its transfer to the gas phase with a transition degree (αZn) equal to 100%. Simultaneously with this simulation, the conversion of iron (10.6–99.996%) and silicon (10.7–69.44%) into an alloy with the possibility of forming iron silicide compounds with a predicted Si content within 18–28% (which, according to GOST 1415-93 (ISO 5445-80), can be identified as ferrosilicon grades FS18, FS20 and FS25) under optimum conditions (1900–2000 K) of the studied temperature regime. The obtained results of the study of the disposal of clinker waste in order to reduce the anthropogenic impact on the biosphere of the region with the possibility of obtaining ferroalloy and zinc sublimates are new and can complement the ongoing research in this direction [8,24,25,26,27,28,39,71,72,73,74,75,76].
5. Conclusions
Based on the obtained results of studies of the possibility of complex utilization by processing technogenic waste in the form of clinker in order to reduce the anthropogenic impact on the biosphere of the region, the following conclusions can be drawn using the example of the ZnO∙SiO2-FeO∙Fe2O3-C system with the production of ferroalloy and zinc sublimates:
- -
- from the clinker dump, it is possible to obtain a low-grade silicon-containing ferroalloy with a Si content in the range of 18–28% and Fe in the range of 73–82% and extract Zn into the gas phase in the range of 99–100% in the form of zinc sublimates in the optimal temperature range of 1800–1900 K;
- -
- zinc contained in the clinker can be driven into the gas phase by 100% with further capture as zinc sublimates;
- -
- technogenic waste—the clinker dump from rolling zinc oxide ores, according to its chemical and elemental compositions, can act as a secondary technogenic raw material for the metallurgical and chemical industries;
- -
- modeling of clinker utilization by electric melting in an arc furnace will contribute to its processing and, accordingly, reduce the anthropogenic impact of its dump on the biosphere of the region with a multiplicative socio-ecological and economic effect.
Author Contributions
Conceptualization, A.K. (Alexandr Kolesnikov) and O.K.; methodology, A.K. (Alexandr Kolesnikov) and A.U.; investigation, R.F., A.U., M.A., S.K., A.K. (Alexander Klyuev), I.V., S.S. and A.B.; data curation, A.K. (Alexandr Kolesnikov) and I.V.; writing—original draft preparation, A.K. (Alexandr Kolesnikov), S.S. and A.N.; writing—review and editing, O.K., A.N. and A.B.; supervision, A.K. (Alexander Klyuev) and S.K.; project administration, A.K. (Alexandr Kolesnikov), R.F. and M.A.; funding acquisition, A.K. (Alexandr Kolesnikov) and R.F. All authors have read and agreed to the published version of the manuscript.
Funding
The research is partially funded by the Ministry of Science and Higher Education of the Russian Federation under the strategic academic leadership program “Priority 2030” (Agreement 075-15-2021-1333 dated 30.09.2021).
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kenzhegaliev, N.A.; Umanets, V.N.; Bugayeva, G.G.; Zavalishin, V.S.; Kogut, A. Prospects for the Development of Technogenic Deposits in Kazakhstan. Available online: https://scholar.google.ru/citations?view_op=view_citation&hl=ru&user=c01tBw4AAAAJ&citation_for_view=c01tBw4AAAAJ:RYcK_YlVTxYC (accessed on 11 February 2022).
- Petrenko, E.S.; Vechkinzova, E.A.; Urazbekov, A.K. Context analysis and prospects of development of the mining and metallurgical industry of Kazakhstan. J. Int. Econ. Aff. 2019, 9, 2661–2676. [Google Scholar] [CrossRef]
- Khoroshavin, L.B.; Perepelitsyn, V.A.; Kochkin, D.K. Problems of technogenic resources. Refract. Ind. Ceram. 1998, 39, 366–368. [Google Scholar] [CrossRef]
- Satbaev, B.N.; Koketaev, A.I.; Aimbetova, O.; Shalabaev, N.T.; Satbaev, A.B. Environmental Technology for the Integrated Disposal of Man-Made Wastes of the Metallurgical Industry: Self-Curing, Chemically Resistant Refractory Mass1. Refract. Ind. Ceram. 2019, 60, 318–322. [Google Scholar] [CrossRef]
- Chukarina, Y.A.; Sopova, O.N.; Zueva, S.B.; Filimonova, O.N.; Veglio, F. Mathematical Simulation of Industrial Waste Processing. Young Sci. 2012, 2, 91–94. [Google Scholar]
- Kolesnikov, A.; Fediuk, R.; Kolesnikova, O.; Zhanikulov, N.; Zhakipbayev, B.; Kuraev, R.; Akhmetova, E.; Shal, A. Processing of Waste from Enrichment with the Production of Cement Clinker and the Extraction of Zinc. Materials 2022, 15, 324. [Google Scholar] [CrossRef]
- Baidya, R.; Ghosh, S.K.; Parlikar, U.V. Co-processing of Industrial Waste in Cement Kiln—A Robust System for Material and Energy Recovery. Procedia Environ. Sci. 2016, 31, 309–317. [Google Scholar] [CrossRef]
- Kapsalyamov, B.A. Possibility of joint manufacture of ferroalloys and nonferrous metals by an electrothermal method. Russ. Met. 2010, 2010, 1151–1155. [Google Scholar] [CrossRef]
- Kolesnikov, A.S.; Kenzhibaeva, G.S.; Botabaev, N.E.; Kutzhanova, A.N.; Iztleuov, G.M.; Suigenbaeva, A.Z.; Ashirbekov, K.A.; Kolesnikova, O.G. Thermodynamic Modeling of Chemical and Phase Transformations in a Waelz Process-Slag—Carbon System. Refract. Ind. Ceram. 2020, 61, 289–292. [Google Scholar] [CrossRef]
- Vasilieva, N.V.; Fedorova, E.R. Process control quality analysis. Tsvetnye Met. 2020, 2020, 70–76. [Google Scholar] [CrossRef]
- Ferreira, W.L.; Reis, É.L.; Lima, R.M.F. Incorporation of residues from the minero-metallurgical industry in the production of clay–lime brick. J. Clean. Prod. 2015, 87, 505–510. [Google Scholar] [CrossRef][Green Version]
- Kolesnikov, A.S.; Naraev, V.N.; Natorhin, M.I.; Saipov, A.A.; Kolesnikova, O.G. Review of the processing of minerals and technogenic sulfide raw material with the extraction of metals and recovering elemental sulfur by electrochemical methods. Rasayan J. Chem. 2020, 13, 2420–2428. [Google Scholar] [CrossRef]
- Peng, Z.; Gregurek, D.; Wenzl, C.; White, J.F. Slag Metallurgy and Metallurgical Waste Recycling. JOM 2016, 68, 2313–2315. [Google Scholar] [CrossRef]
- Efremova, S.V. Scientific and technical solutions to the problem of utilization of waste from plant- and mineral-based industries. Russ. J. Gen. Chem. 2012, 82, 963–968. [Google Scholar] [CrossRef]
- Mamyrbekova, A.; Mamitova, A.D.; Mamyrbekova, A. Electrochemical Behavior of Sulfur in Aqueous Alkaline Solutions. Russ. J. Phys. Chem. A 2018, 92, 582–586. [Google Scholar] [CrossRef]
- Abisheva, Z.S.; Bochevskaya, E.G.; Zagorodnyaya, A.N.; Shabanova, T.A.; Karshigina, Z.B. Technology of phosphorus slag processing for preparation of precipitated silica. Theor. Found. Chem. Eng. 2013, 47, 428–434. [Google Scholar] [CrossRef]
- Myrzabekov, B.E.; Bayeshov, A.B.; Makhanbetov, A.B.; Mishra, B.; Baigenzhenov, O.S. Dissolution of Platinum in Hydrochloric Acid Under Industrial-Scale Alternating Current Polarization. Metall. Mater. Trans. B 2018, 49, 23–27. [Google Scholar] [CrossRef]
- Medina, D.; Anderson, C.G. A Review of the Cyanidation Treatment of Copper-Gold Ores and Concentrates. Metals 2020, 10, 897. [Google Scholar] [CrossRef]
- Lis, T.; Nowacki, K.; Zelichowska, M.; Kania, H. Innovation in metallurgical waste management. Metalurgija 2015, 54, 283–285. [Google Scholar]
- Borisov, D.; Stefanov, B.; Stoyanov, S.K. An algorithm for metallurgical waste minimization. J. Chem. Technol. Metall. 2014, 49, 99–105. [Google Scholar]
- Uzhkenov, B.S. Mineral raw materials base of the Republic of Kazakhstan: Condition, prospects of exploration. Gorn. Zhurnal 2011, 9, 8–10. [Google Scholar]
- Rybak, J.; Kongar-syuryun, C.; Tyulyaeva, Y.; Khayrutdinov, A.M. Creation of Backfill Materials Based on Industrial Waste. Minerals 2021, 11, 739. [Google Scholar] [CrossRef]
- Kolesnikov, A.S.; Zhakipbaev, B.Y.; Zhanikulov, N.N.; Kolesnikova, O.G.; Akhmetova, K.; Kuraev, R.M.; Shal, A.L. Review of technogenic waste and methods of its processing for the purpose of complex utilization of tailings from the enrichment of non-ferrous metal ores as a component of the raw material mixture in the production of cement clinker. Rasayan J. Chem. 2021, 14, 997–1005. [Google Scholar] [CrossRef]
- Guan, J.; Wang, Y.; Cheng, L.; Xie, Y.; Zhang, L. Fabrication and characterization of Short silicon nitride fibers from direct nitridation of ferrosilicon in N2 atmosphere. Materials 2018, 11, 2003. [Google Scholar] [CrossRef] [PubMed]
- Janerka, K.; Kostrzewski, Ł.; Stawarz, M.; Jezierski, J. The importance of SiC in the process of melting ductile iron with a variable content of charge materials. Materials 2020, 13, 1231. [Google Scholar] [CrossRef] [PubMed]
- Ziatdinov, M.; Zhukov, A.; Promakhov, V. Combustion synthesis of composition ferroalloys. Materials 2018, 11, 2117. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Su, Y.; Xu, G.; Chen, Y.; You, G. Research Progress on Controlled Low-Strength Materials: Metallurgical Waste Slag as Cementitious Materials. Materials 2022, 15, 727. [Google Scholar] [CrossRef]
- Legemza, J.; Findorák, R.; Bul’ko, B.; Briančin, J. New approach in research of quartzes and quartzites for ferroalloys and silicon production. Metals 2021, 11, 670. [Google Scholar] [CrossRef]
- Małek, M.; Jackowski, M.; Łasica, W.; Dydek, K.; Boczkowska, A. An experimental study of possible post-war ferronickel slag waste disposal in szklary (Lower silesian, poland) as partial aggregate substitute in concrete: Characterization of physical, mechanical, and thermal properties. Materials 2021, 14, 2552. [Google Scholar] [CrossRef]
- Jordanov, N.B.; Georgiev, I.; Karamanov, A. Sintered glass-ceramics, self-glazed materials and foams from metallurgical waste slag. Materials 2021, 14, 2263. [Google Scholar] [CrossRef]
- Mróz, J.; Konstanciak, A.; Warzecha, M.; Więcek, M.; Hutny, A.M. Research on reduction of selected iron-bearing waste materials. Materials 2021, 14, 1914. [Google Scholar] [CrossRef]
- Terrones-Saeta, J.M.; Suárez-Macías, J.; Moreno-López, E.R.; Corpas-Iglesias, F.A. Determination of the chemical, physical and mechanical characteristics of electric arc furnace slags and environmental evaluation of the process for their utilization as an aggregate in bituminous mixtures. Materials 2021, 14, 782. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, I.; Piccolo, F.; Traven, K.; Češnovar, M.; Ducman, V.; Leonelli, C. Alkali activation of metallurgical slags: Reactivity, chemical behavior, and environmental assessment. Materials 2021, 14, 639. [Google Scholar] [CrossRef] [PubMed]
- Famielec, S. Chromium concentrate recovery from solid tannery waste in a thermal process. Materials 2020, 13, 1533. [Google Scholar] [CrossRef] [PubMed]
- Pizoń, J.; Gołaszewski, J.; Alwaeli, M.; Szwan, P. Properties of concrete with recycled concrete aggregate containing metallurgical sludge waste. Materials 2020, 13, 1448. [Google Scholar] [CrossRef]
- Liu, M.; Ma, G.; Zhang, X.; Liu, J.; Wang, Q. Preparation of black ceramic tiles using waste copper slag and stainless steel slag of electric arc furnace. Materials 2020, 13, 776. [Google Scholar] [CrossRef]
- Fechet, R.; Zlagnean, M.; Moanta, A.; Ciobanu, L. Mining Wastes—Sampling, processing and using in manufacture portland cement. Rom. J. Miner. Depos. 2010, 84, 67–70. [Google Scholar]
- Taimasov, B.T.; Sarsenbayev, B.K.; Khudyakova, T.M.; Kolesnikov, A.S.; Zhanikulov, N.N. Development and testing of low-energy-intensive technology of receiving sulphate-resistant and road portlandcement. Eurasian Chem. J. 2017, 19, 347–355. [Google Scholar] [CrossRef]
- Kolesnikov, A.S. Kinetic investigations into the distillation of nonferrous metals during complex processing of waste of metallurgical industry. Russ. J. Non-Ferr. Met. 2015, 56, 1–5. [Google Scholar] [CrossRef]
- Satbaev, B.; Yefremova, S.; Zharmenov, A.; Kablanbekov, A.; Yermishin, S.; Shalabaev, N.; Satbaev, A.; Khen, V. Rice husk research: From environmental pollutant to a promising source of organo-mineral raw materials. Materials 2021, 14, 4119. [Google Scholar] [CrossRef]
- Nadirov, K.S.; Zhantasov, M.K.; Sakybayev, B.A.; Orynbasarov, A.K.; Bimbetova, G.Z.; Sadyrbayeva, A.S.; Kolesnikov, A.S.; Ashirbayev, H.A.; Zhantasova, D.M.; Tuleuov, A.M. The study of the gossypol resin impact on adhesive properties of the intermediate layer of the pipeline three-layer rust protection coating. Int. J. Adhes. Adhes. 2017, 78, 195–199. [Google Scholar] [CrossRef]
- Khudyakova, T.M.; Kolesnikov, A.S.; Zhakipbaev, B.E.; Kenzhibaeva, G.S.; Kutzhanova, A.N.; Iztleuov, G.M.; Zhanikulov, N.N.; Kolesnikova, O.G.; Mynbaeva, E. Optimization of Raw Material Mixes in Studying Mixed Cements and Their Physicomechnical Properties. Refract. Ind. Ceram. 2019, 60, 76–81. [Google Scholar] [CrossRef]
- Koteleva, N.; Kuznetsov, V.; Vasilyeva, N. A simulator for educating the digital technologies skills in industry. Part one. dynamic simulation of technological processes. Appl. Sci. 2021, 11, 10885. [Google Scholar] [CrossRef]
- Stoianova, A.; Vasilyeva, N. Production Process Data as a Tool for Digital Transformation of Metallurgical Companies. In Proceedings of the XIV International Scientific Conference “INTERAGROMASH 2021”, Rostov-on-Don, Russia, 24–26 February 2021; Springer: Cham, Switzerland, 2021; Volume 246, pp. 780–787. [Google Scholar] [CrossRef]
- Boikov, A.V.; Savelev, R.V.; Payor, V.A.; Potapov, A.V. Evaluation of bulk material behavior control method in technological units using dem. Part 2. CIS Iron Steel Rev. 2020, 20, 3–6. [Google Scholar] [CrossRef]
- De Azevedo, A.R.G.; Klyuev, S.; Marvila, M.T.; Vatin, N.; Alfimova, N.; de Lima, T.E.S.; Fediuk, R.; Olisov, A. Investigation of the Potential Use of Curauá Fiber for Reinforcing Mortars. Fibers 2020, 8, 69. [Google Scholar] [CrossRef]
- Chernysheva, N.; Lesovik, V.; Fediuk, R.; Vatin, N. Improvement of performances of the gypsum-cement fiber reinforced composite (GCFRC). Materials 2020, 13, 3847. [Google Scholar] [CrossRef]
- Tolstoy, A.; Lesovik, V.; Fediuk, R.; Amran, M.; Gunasekaran, M.; Vatin, N.; Vasilev, Y. Production of Greener High-Strength Concrete Using Russian Quartz Sandstone Mine Waste Aggregates. Materials 2020, 13, 5575. [Google Scholar] [CrossRef]
- Fediuk, R.; Mosaberpanah, M.A.; Lesovik, V. Development of fiber reinforced self-compacting concrete (FRSCC): Towards an efficient utilization of quaternary composite binders and fibers. Adv. Concr. Constr. 2020, 9, 387–395. [Google Scholar]
- Volodchenko, A.A.; Lesovik, V.S.; Cherepanova, I.A.; Volodchenko, A.N.; Zagorodnjuk, L.H.; Elistratkin, M.Y. Peculiarities of non-autoclaved lime wall materials production using clays. IOP Conf. Ser. Mater. Sci. Eng. 2018, 327, 022021. [Google Scholar] [CrossRef]
- Utelbaeva, A.B.; Ermakhanov, M.N.; Zhanabai, N.Z.; Utelbaev, B.T.; Mel’Deshov, A.A. Hydrogenation of benzene in the presence of ruthenium on a modified montmorillonite support. Russ. J. Phys. Chem. A 2013, 87, 1478–1481. [Google Scholar] [CrossRef]
- Volokitina, I.E.; Volokitin, A.V. Evolution of the Microstructure and Mechanical Properties of Copper during the Pressing–Drawing Process. Phys. Met. Met. 2018, 119, 917–921. [Google Scholar] [CrossRef]
- Volokitina, I.E.; Kurapov, G.G. Effect of Initial Structural State on Formation of Structure and Mechanical Properties of Steels Under ECAP. Met. Sci. Heat Treat. 2018, 59, 786–792. [Google Scholar] [CrossRef]
- Pestunova, N.P.; Ognev, Y.G. Physico-chemical research in metallurgy of lead and zinc. Sci. Work. 1980, 36, 37–42. [Google Scholar]
- Abdeev, M.A.; Yusupova, A.I.; Piskunov, V.M.; Kolesnikov, A.V. Extraction of Valuable Components from Dump Products of Heavy Non-Ferrous Metals; Tsvetmetinformation: Moscow, Russia, 1980; pp. 1–48. [Google Scholar]
- Topchaev, V.P.; Khodov, N.V.; Davidson, A.N.; Eputaev, G.A. The use of clinker coke to intensify the welting process. Non-Ferr. Met. 1972, 1, 23–24. [Google Scholar]
- Kolesnikov, A.V.; Pusko, A.G.; Divak, A.A. The effect of calcium and magnesium compounds on zinc distillation in the production of zinc whitewash. Non-Ferr. Met. 1977, 6, 15–17. [Google Scholar]
- Snurnikov, A.P. Complex Use of Mineral Resources in Non-Ferrous Metallurgy; Metallurgy: Moscow, Russia, 1965; pp. 1–358. [Google Scholar]
- Mitrofanov, S.I.; Meshchaninova, V.I. Combined Processes of Processing of Non-Ferrous Metal Ores; Subsoil: Moscow, Russia, 1998; pp. 1–230. [Google Scholar]
- Fetterolf, L.D. Electric melting of zinc clinker on mirror cast iron at the New Jersey Zinc plant. In Proceedings of the 28th Conference on Electric Melting, New York, NY, USA, 10–14 February 1970; Volume 2, pp. 409–422. [Google Scholar]
- Kasivadi, M.; Kimarlin, G. Arme World Symposium on Zinc Mining and Metallurgy Jead; American Institute of Mining, Metallurgical and Petroleum Engineers: New York, NY, USA, 1970; Volume 2, pp. 430–442. [Google Scholar]
- Sanakulov, K.S.; Khasanov, A.S. Processing of Copper Production; Fan Publication: Tashkent, Uzbekistan, 2007; pp. 1–255. [Google Scholar]
- Ospanov, S.S. Chloride Technology of Processing of Lead-Zinc Industrial Products and Hard-to-Enrich Ores: Abstract; Candidate of Technical Sciences: Alma-Ata, Kazakhstan, 1985; pp. 1–29. [Google Scholar]
- Karpov, Y.A.; Baranovskaya, V.B. Issues of standardization of the methods of chemical analysis in metallurgy. Ind. Lab. Diagn. Mater. 2019, 85, 5–14. [Google Scholar] [CrossRef]
- Lifshin, E.; Morris, W.G.; Bolon, R.B. The Scanning Electron Microscope and its Applications in Metallurgy. JOM 2017, 21, 43–50. [Google Scholar] [CrossRef]
- Trusov, B.G. Code system for simulation of phase and chemical equilibriums at higher temperatures. Eng. J. Sci. Innov. 2012, 1, 240–249. [Google Scholar]
- Trusov, B.G. Simulation of Kinetics of Chemical Conversions: Thermodynamic Approach. Her. Bauman Mosc. State Tech. Univ. Nat. Sci. 2005, 3, 26–38. [Google Scholar]
- Grudinsky, P.I.; Zinoveev, D.V.; Dyubanov, V.G.; Kozlov, P.A. State of the Art and Prospect for Recycling of Waelz Slag from Electric Arc Furnace Dust Processing. Inorg. Mater. Appl. Res. 2019, 10, 1220–1226. [Google Scholar] [CrossRef]
- Vogelbacher, M.; Keller, S.; Zehm, W.; Matthes, J. Advanced Methods for Kiln-Shell Monitoring to Optimize the Waelz Process for Zinc Recycling. Processes 2021, 9, 1062. [Google Scholar] [CrossRef]
- Panshin, A.M.; Shakirzyanov, R.M.; Izbrekht, P.A.; Zatonskiy, A.V. Basic ways of improvement of zinc production at JSC “Chelyabinsk Zinc Plant”. Tsvetnye Met. 2015, 5, 19–21. [Google Scholar] [CrossRef]
- Sariev, O.; Kim, S.; Zhumagaliev, Y.; Kelamanov, B.; Sultanov, M.; Nurgali, N. Viscosity and crystallization temperature of ferroalloy slags from Kazakhstan ore. Metalurgija 2020, 59, 525–528. [Google Scholar]
- Zhuniskaliyev, T.; Nurumgaliyev, A.; Zayakin, O.; Mukhambetgaliyev, Y.; Kuatbay, Y.; Mukhambetkaliyev, A. Investigation and comparison of the softening temperature of manganese ores used for the production of complex ligatures based on Fe-Si-Mn-Al. Metalurgija 2020, 59, 521–524. [Google Scholar]
- Kuatbay, Y.; Nurumgaliyev, A.; Shabanov, Y.; Zayakin, O.; Gabdullin, S.; Zhuniskaliyev, T. Melting of high-carbon ferrochrome using coal of the saryadyr deposit. Metalurgija 2022, 61, 367–370. [Google Scholar]
- Sariev, O.; Kelamanov, B.; Zhumagaliyev, Y.; Kim, S.; Abdirashit, A.; Almagambetov, M. Remelting the high-carbon ferrochrome dust in a direct current arc furnace (DCF). Metalurgija 2020, 59, 533–536. [Google Scholar]
- Klyuev, S.V.; Khezhev, T.A.; Pukharenko, Y.V.; Klyuev, A.V. Fibers and their properties for concrete reinforcement. Mater. Sci. Forum 2018, 945, 125–130. [Google Scholar] [CrossRef]
- Begich, Y.E.; Klyuev, S.V.; Jos, V.A.; Cherkashin, A.V. Fine-grained concrete with various types of fibers. Mag. Civ. Eng. 2020, 97, 9702. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).