Nonlinear Optical Limiting and Radiation Shielding Characteristics of Sm2O3 Doped Cadmium Sodium Lithium Borate Glasses
Abstract
:1. Introduction
2. Experimental Procedures
3. Results and Discussion
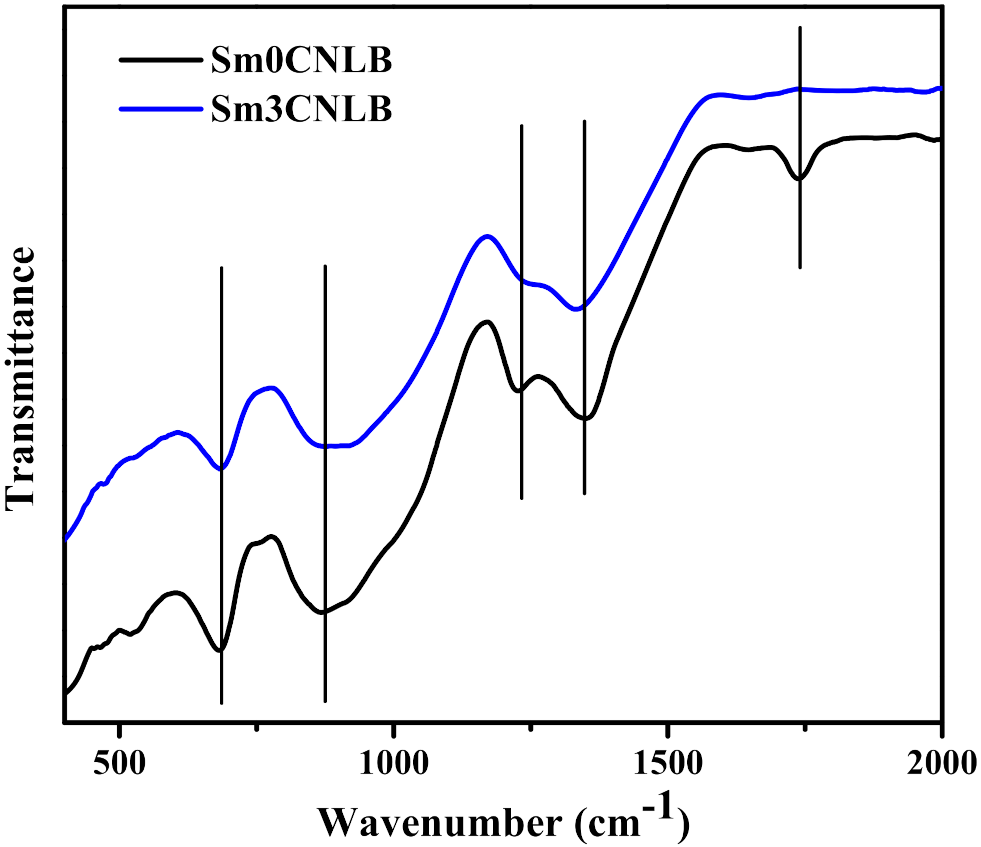
3.1. Structural Properties of SmxCNLB Glasses
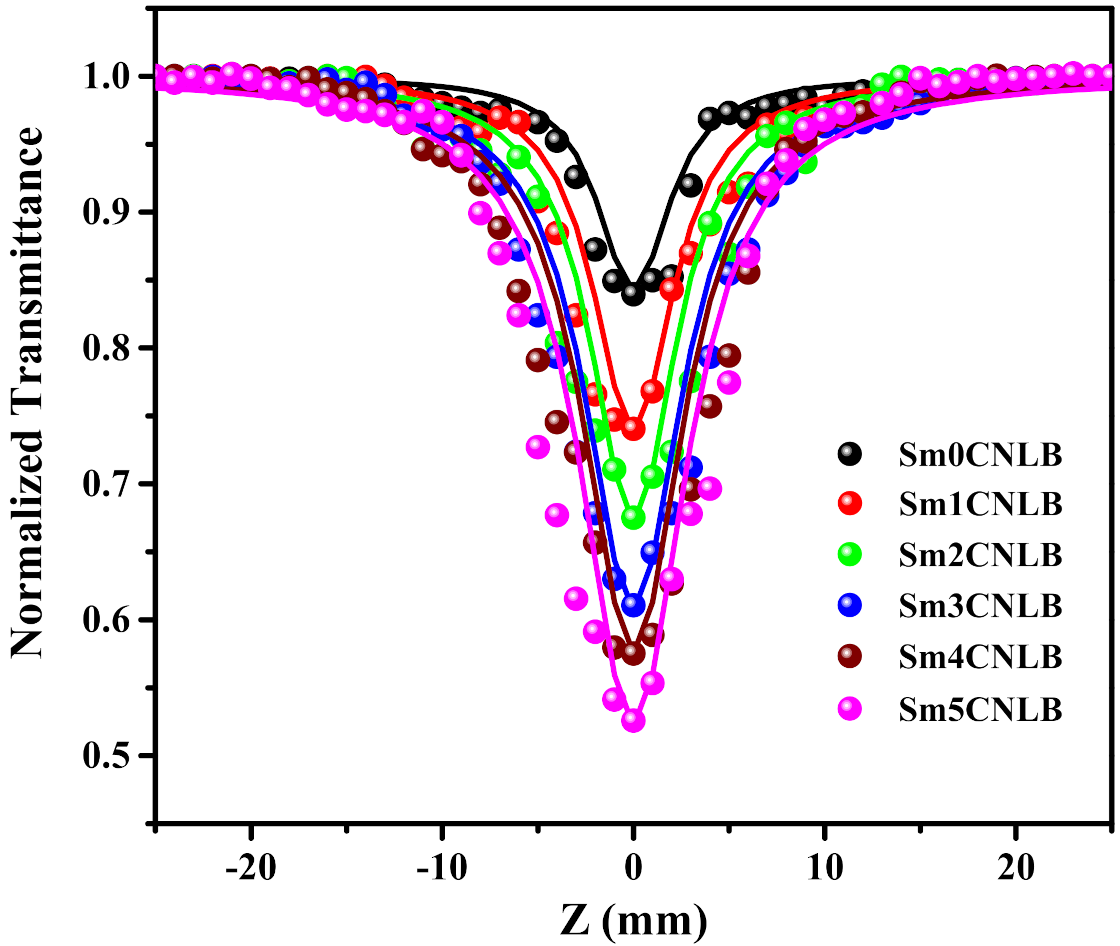
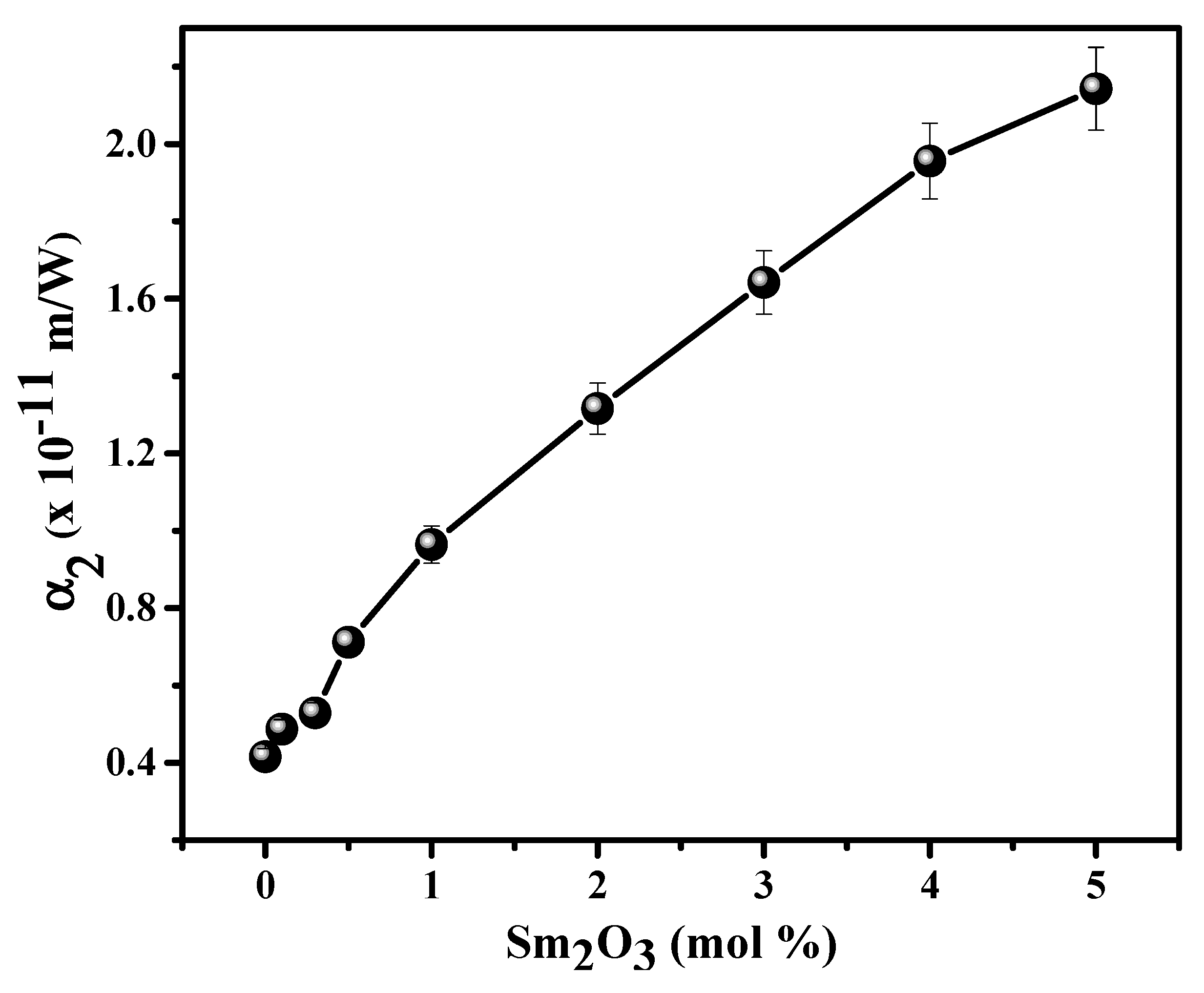
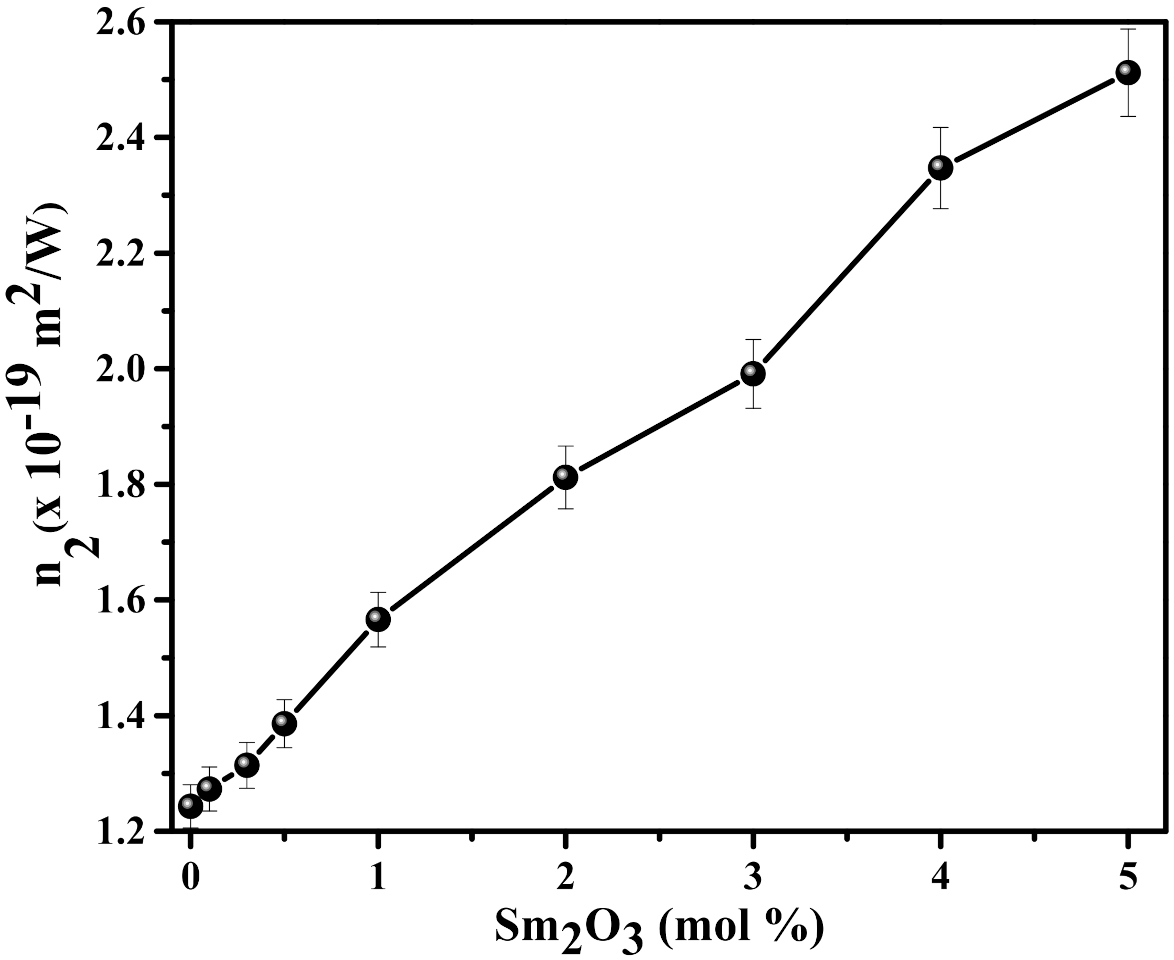
3.2. NLO and OL Characteristics of SmxCNLB Glasses
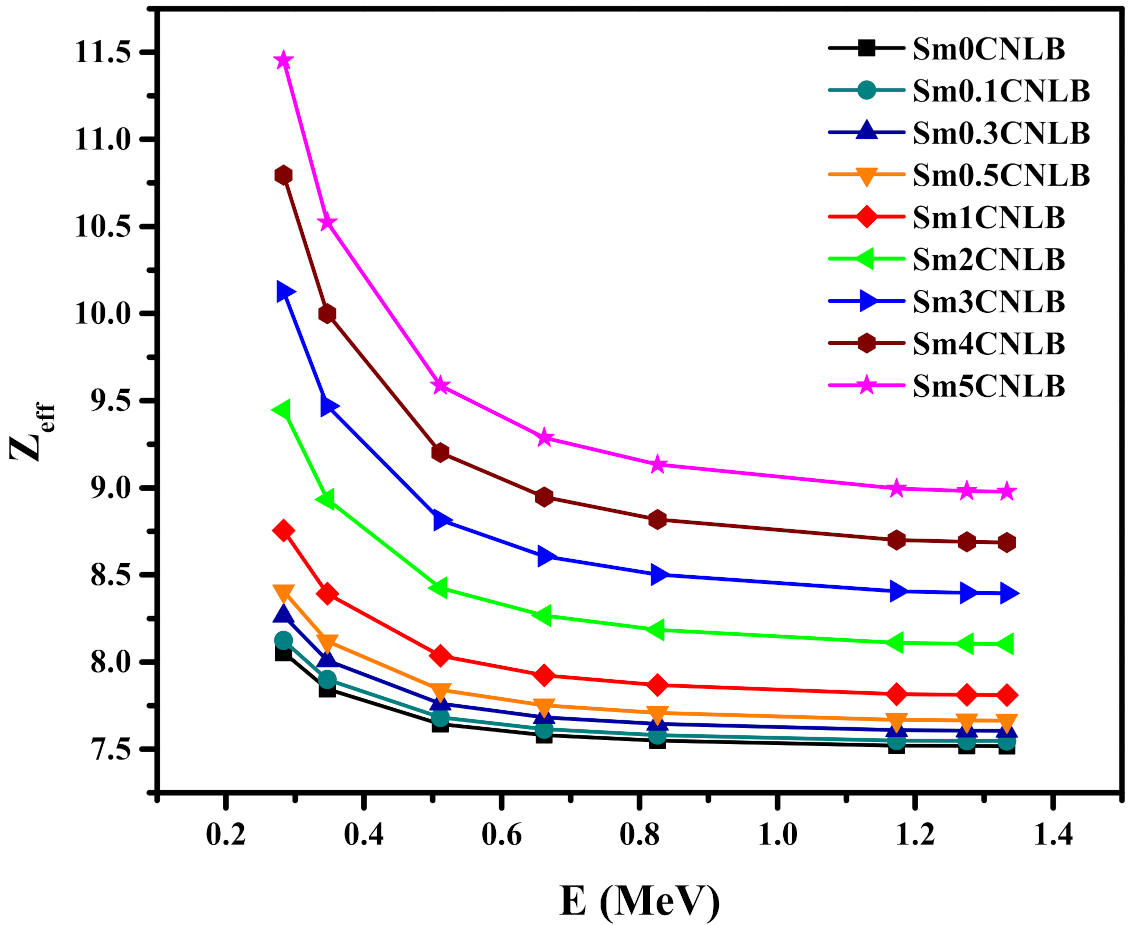
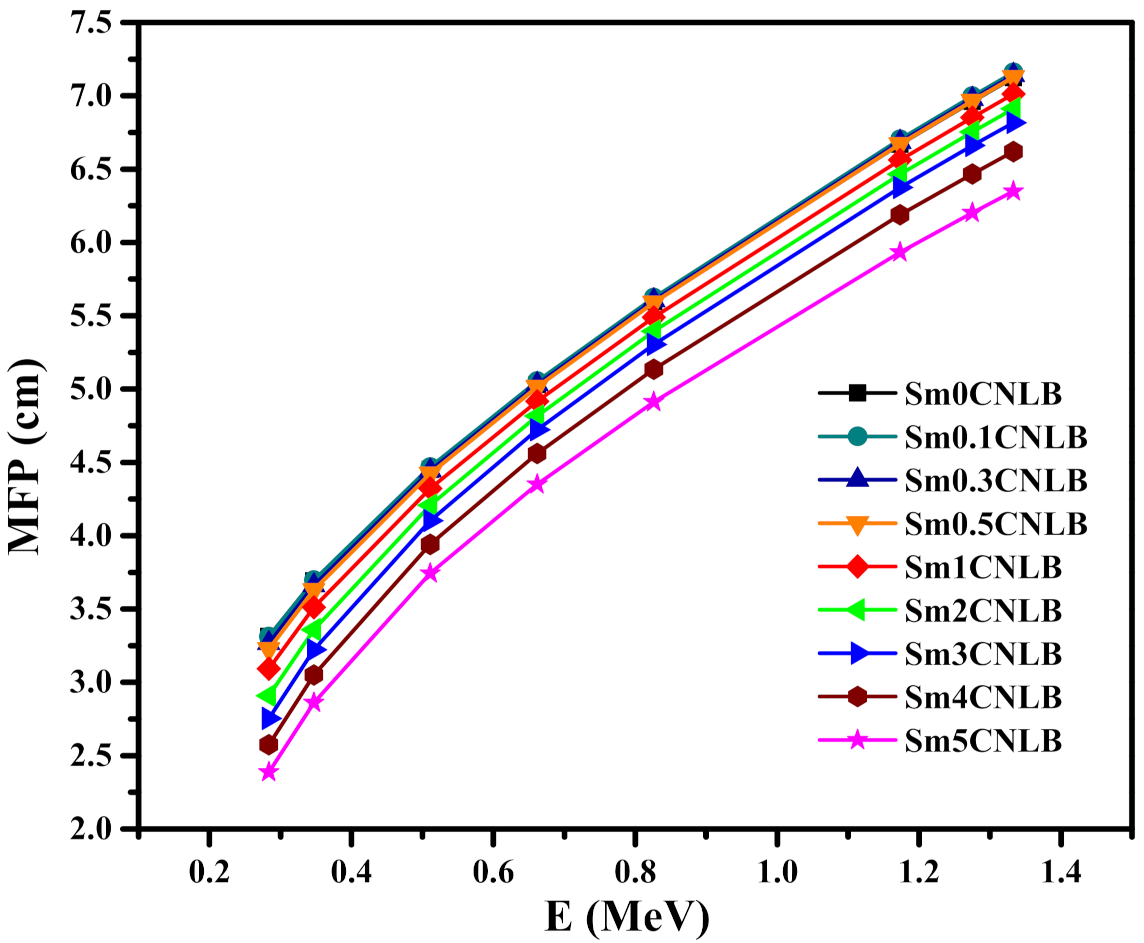
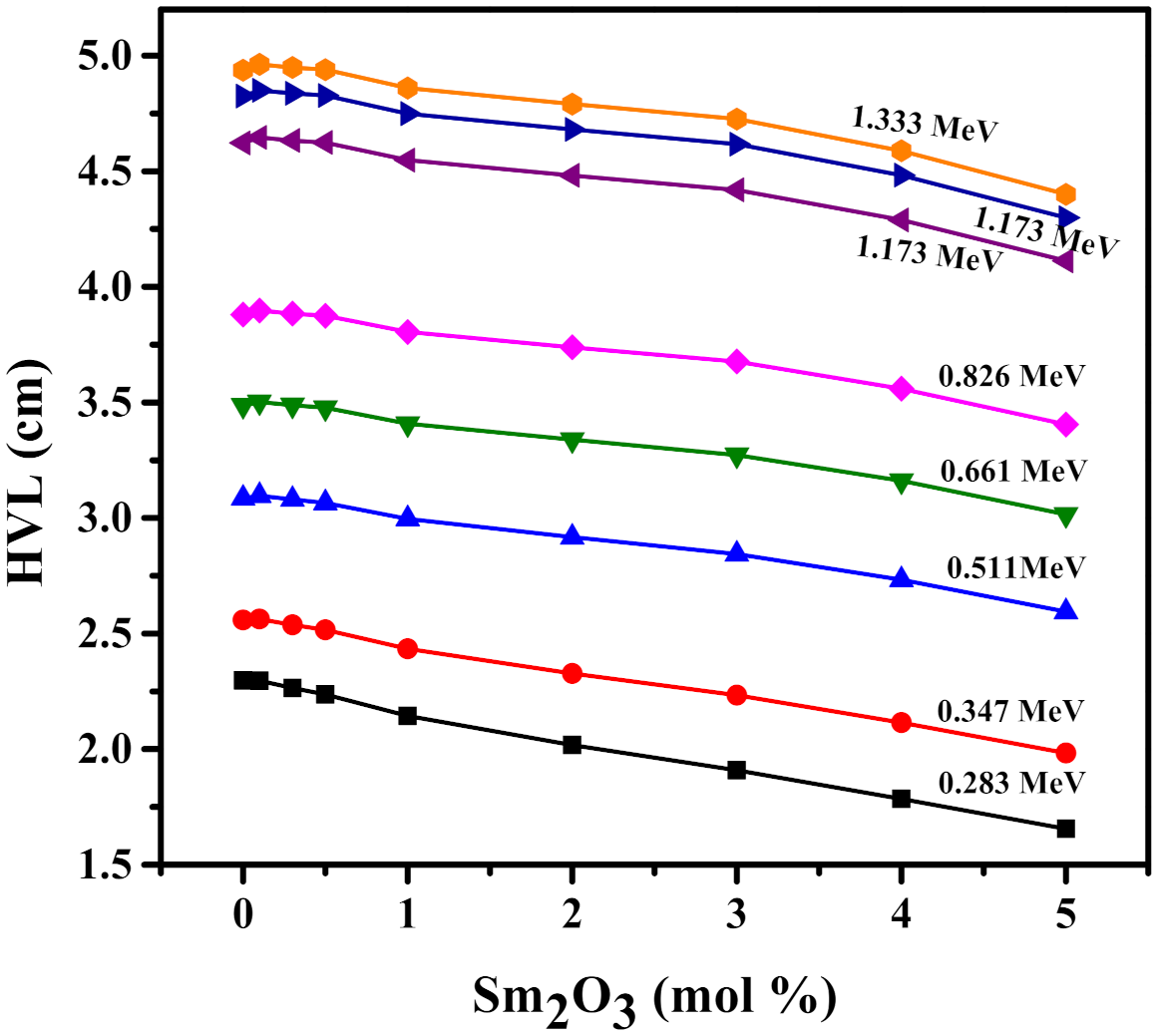
3.3. Radiation Shielding Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poirier, G.; de Araújo, C.B.; Messaddeq, Y.; Ribeiro, S.J.L.; Poulain, M. Tungstate fluorophosphate glasses as optical limiters. Appl. Phys. 2002, 91, 10221. [Google Scholar] [CrossRef] [Green Version]
- BNgoy, P.; May, A.K.; Mack, J.; Nyokong, T. Optical Limiting and Femtosecond Pump-Probe Transient Absorbance Properties of a 3,5-distyrylBODIPY Dye. Front. Chem. 2019, 7, 740. [Google Scholar]
- Vishnumurthy, K.A.; Sunitha, M.S.; Adhikari, A.V. New optical limiting polymeric materials with different π-electron conjugation bridge structures: Synthesis and characterization. Eur. Polym. J. 2012, 48, 1575–1585. [Google Scholar] [CrossRef]
- Maciel, G.S.; Rakov, N.; de Araújo, C.B.; Lipovskii, A.A.; Tagantsev, D.K. Optical limiting behavior of a glass–ceramic containing sodium niobate crystallites. Appl. Phys. Lett. 2001, 79, 584. [Google Scholar] [CrossRef]
- Shi, Z.; Dong, N.; Zhang, D.; Jiang, X.; Du, G.; Lv, S.; Chen, J.; Wang, J.; Zhou, S. Transparent niobate glass–ceramics for optical limiting. J. Am. Ceram. Soc. 2019, 102, 3965–3971. [Google Scholar] [CrossRef]
- Rao, M.V.; Kumar, V.V.R.K.; Shihab, N.K.; Rao, D.N. Third order nonlinear and optical limiting properties of alkaline bismuth borate glasses. Opt. Laser Technol. 2018, 107, 110–115. [Google Scholar] [CrossRef]
- Hegde, V.; Kamath, S.D.; Kebaili, I.; Sayyed, M.I.; Sathish, K.N.; Viswanath, C.S.D.; Pramod, A.G.; Ramesh, P.; Keshavamurthy, K.; Devarajulu, G.; et al. Photoluminescence, nonlinear optical and gamma radiation shielding properties of high concentration of Eu2O3 doped heavy metal borate glasses. Optik 2022, 251, 168433. [Google Scholar] [CrossRef]
- Sun, W.; Byeon, C.C.; Lawson, C.M.; Gray, G.M.; Wang, D. Third-order nonlinear optical properties of an expanded porphyrin cadmium complex. Appl. Phys. Lett. 2000, 77, 1759. [Google Scholar] [CrossRef]
- Sun, X.; Yu, R.Q.; Xu, G.Q.; Hor, T.S.A.; Ji, W. Broadband optical limiting with multiwalled carbon nanotubes. Appl. Phys. Lett. 1998, 73, 3632. [Google Scholar] [CrossRef]
- Almeida, J.M.P.; da Silva, D.S.; Kassab, L.R.P.; Zilio, S.C.; Mendonça, C.R.; de Boni, L. Ultrafast third-order optical nonlinearities of heavy metal oxide glasses containing gold nanoparticles. Opt. Mater. 2014, 36, 829–832. [Google Scholar] [CrossRef]
- Qiao, B.; Chen, F.; Nie, Q.; Dai, S.; Zhang, X.; Huang, Y. Improved nonlinear optical properties of chalcogenide glasses in Ge-Sn-Se ternary system by thermal treatment. Opt. Mater. Express 2016, 6, 1644–1652. [Google Scholar]
- Thangaraj, M.; Vinitha, G.; Girisun, T.C.S.; Anandan, P.; Ravi, G. Third order nonlinear optical properties and optical limiting behavior of alkali metal complexes of p-nitrophenol. Opt. Laser Technol. 2015, 73, 130–134. [Google Scholar] [CrossRef]
- Xue, D.; Betzler, K.; Hesse, H.; Lammers, D. Nonlinear optical properties of borate crystals. Solid State Commun. 2000, 114, 21–25. [Google Scholar] [CrossRef]
- Chen, C.; Li, R. The anionic group theory of the non-linear optical effect and its applications in the development of new high-quality NLO crystals in the borate series. Int. Rev. Phys. Chem. 1988, 8, 65–91. [Google Scholar] [CrossRef]
- Santos, S.N.C.; Paula, K.T.; Almeida, J.M.P.; Hernandes, A.C.; Mendonça, C.R. Effect of Tb3+/Yb3+ in the nonlinear refractive spectrum of CaLiBO glasses. J. Non. Cryst. Solids 2019, 524, 119637. [Google Scholar] [CrossRef]
- Nanda, K.; Kundu, R.S.; Sharma, S.; Mohan, D.; Punia, R.; Kishore, N. Study of vibrational spectroscopy, linear and non-linear optical properties of Sm3+ ions doped BaO–ZnO–B2O3 glasses. Solid State Sci. 2015, 45, 15–22. [Google Scholar] [CrossRef]
- Nanda, K.; Kundu, R.S.; Punia, R.; Mohan, D.; Kishore, N. Resonant and Non-resonant Nonlinear Optical Properties of Er3+ modified BaO–ZnO–B2O3 Glasses at 532 and 1550 nm. J. Non. Cryst. Solids 2020, 541, 120155. [Google Scholar] [CrossRef]
- Jagannath, G.; Eraiah, B.; Gaddam, A.; Fernandes, H.; Brazete, D.; Jayanthi, K.; Krishnakanth, K.N.; Rao, S.V.; Ferreira, J.M.F.; Annapurna, K.; et al. Structural and Femtosecond Third-Order Nonlinear Optical Properties of Sodium Borate Oxide Glasses: Effect of Antimony. J. Phys. Chem. C 2019, 123, 5591–5602. [Google Scholar] [CrossRef] [Green Version]
- Almeida, J.M.P.; de Boni, L.; Hernandes, A.C.; Mendonça, C.R. Third-order nonlinear spectra and optical limiting of lead oxifluoroborate glasses. Opt. Express 2011, 19, 17220. [Google Scholar] [CrossRef]
- Ramesh, P.; Hegde, V.; Pramod, A.G.; Eraiah, B.; Rao, S.V.; Shisina, S.; Das, S.; Agarkov, D.A.; Eliseeva, G.M.; Jagannath, G.; et al. Effect of Eu3+ in tuning the ultrafast third-order optical nonlinearity in heavy metal borate glasses. Opt. Mater. 2020, 108, 110051. [Google Scholar] [CrossRef]
- Jagannath, G.; Gaddam, A.; Rao, S.V.; Agarkov, D.A.; Korableva, G.M.; Ghosh, M.; Dey, K.K.; Ferreira, J.M.F.; Allu, A.R. Tunable femtosecond nonlinear absorption and optical limiting thresholds of La2O3–B2O3 glasses by controlling the borate structural units. Scr. Mater. 2022, 211, 114530. [Google Scholar] [CrossRef]
- Keshavamurthy, K.; Swetha, B.N.; Sathish, K.N.; Pramod, A.G.; Kebaili, I.; Sayyed, M.I.; Itigi, S.; Ramesh, P.; Hegde, V.; Murthy, N.L.; et al. Near-infrared nonlinear optical characteristics of silver nanoparticles embedded borate glasses activated with Sm3+ ions: Effect of heat treatment. Infrared Phys. Technol. 2021, 119, 103959. [Google Scholar] [CrossRef]
- Keshavamurthy, K.; Swetha, B.N.; Al-Harbi, F.F.; Jagannath, G.; Almuqrin, A.H.; Sayyed, M.I.; Ahmed, S.B.; Pramod, A.G.; Itigi, S.; Ramesh, P.; et al. Improved near–infrared nonlinear optical properties of Sm3+ containing borate glasses: Effect of silver nanoparticles concentration. Opt. Mater. 2021, 122, 111804. [Google Scholar] [CrossRef]
- Yasmin, S.; Barua, B.S.; Khandaker, M.U.; Chowdhury, F.U.Z.; Rashid, M.A.; Bradley, D.A.; Olatunji, M.A.; Kamal, M. Studies of ionizing radiation shielding effectiveness of silica-based commercial glasses used in Bangladeshi dwellings. Res. Phys. 2018, 9, 541–549. [Google Scholar] [CrossRef]
- Yasmin, S.; Rozaila, Z.S.; Khandaker, M.U.; Barua, B.S.; Chowdhury, F.U.Z.; Rashid, M.A.; Bradley, D.A. The radiation shielding offered by the commercial glass installed in Bangladeshi dwellings. Eff. Def. Solids 2018, 173, 657–672. [Google Scholar] [CrossRef]
- Kamislioglu, M. Research on the effects of bismuth borate glass system on nuclear radiation shielding parameters. Res. Phys. 2021, 22, 103844. [Google Scholar] [CrossRef]
- Kaewjaeng, S.; Kothan, S.; Chaiphaksa, W.; Chanthima, N.; Rajaramakrishna, R.; Kim, H.J.; Kaewkhao, J. High transparency La2O3–CaO–B2O3–SiO2 glass for diagnosis x-rays shielding material application. Radiat. Phys. Chem. 2019, 160, 41–47. [Google Scholar] [CrossRef]
- Kavaz, E.; Ekinci, N.; Tekin, H.O.; Sayyed, M.I.; Aygün, B.; Perişanoğlu, U. Estimation of gamma radiation shielding qualification of newly developed glasses by using WinXCOM and MCNPX code. Prog. Nucl. Energy 2019, 115, 12–20. [Google Scholar] [CrossRef]
- Rosso, M.; Blasi, G.; Gherlone, E.; Rosso, R. Effect of Granulocyte-Macrophage Colony-Stimulating Factor on Prevention of Mucositis in Head and Neck Cancer Patients Treated with Chemo-Radiotherapy. J. Chemother. 1997, 9, 382–385. [Google Scholar] [CrossRef]
- Lucchese, A.; Matarese, G.; Manuelli, M.; Ciuffreda, C.; Bassani, L.; Isola, G.; Cordasco, G.; Gherlone, E. Reliability and efficacy of palifermin in prevention and management of oral mucositis in patients with acute lymphoblastic leukemia: A randomized, double-blind controlled clinical trial. Minerva Stomatol. 2016, 65, 43–50. [Google Scholar]
- Hivrekar, M.M.; Bhoyar, D.N.; Mande, V.K.; Dhole, V.V.; Solunke, M.B.; Jadhav, K.M. Effect of RE (Nd3+, Sm3+) oxide on structural, optical properties of Na2O–Li2O–ZnO–B2O3 glass system. AIP Conf. Proc. 2018, 1953, 090074. [Google Scholar]
- Sheik-Bahae, M.; Said, A.A.; Wei, T.H.; Hagan, D.J.; van Stryland, E.W. Sensitive measurement of optical nonlinearities using a single beam. IEEE J. Quantum Electron. 1990, 26, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Limkitjaroenporn, P.; Kaewkhao, J.; Limsuwan, P.; Chewpraditkul, W. Physical, optical, structural and gamma-ray shielding properties of lead sodium borate glasses. J. Phys. Chem. Solids 2011, 72, 245–251. [Google Scholar] [CrossRef]
- Kashif, I.; El-Maboud, A.A.; El-Said, R.; Sakr, E.M.; Soliman, A.A. The role of lead oxide on structural and physical properties of lithium diborate glasses. J. Alloys Compd. 2012, 539, 124–128. [Google Scholar] [CrossRef]
- Kamitsos, E.I.; Patsis, A.P.; Karakassides, M.A.; Chryssikos, G.D. Infrared reflectance spectra of lithium borate glasses. J. Non. Cryst. Solids 1990, 126, 52–67. [Google Scholar] [CrossRef]
- Cheng, Y.; Xiao, H.; Guo, W.; Guo, W. Structure and Crystallization Kinetics of Bi2O3–B2O3 Glasses. Thermochim. Acta 2006, 444, 173–178. [Google Scholar] [CrossRef]
- Saddeek, Y.B.; Gaafar, M.S. Physical and structural properties of some bismuth borate glasses. Mater. Chem. Phys. 2009, 115, 280–286. [Google Scholar] [CrossRef]
- Ramesh, P.; Hegde, V.; Keshavamurthy, K.; Pramod, A.G.; Jagannath, G.; Aloraini, D.A.; Almuqrin, A.H.; Sayyed, M.I.; Harisha, K.S.; Khan, S.; et al. Influence of gamma irradiation on photoluminescence and nonlinear optical properties of Eu3+ activated heavy metal borate glasses. Opt. Mater. 2021, 116, 111102. [Google Scholar] [CrossRef]
- Jagannath, G.; Sayyed, M.I.; Alhuthali, A.M.S. Nanosecond nonlinear optical, optical limiting and gamma radiation shielding attributes of Eu3+ ions doped heavy metal borate glasses. Ceram. Int. 2021, 47, 14330–14340. [Google Scholar] [CrossRef]
- Jagannath, G.; Pramod, A.G.; Keshavamurthy, K.; Swetha, B.N.; Eraiah, B.; Rajaramakrishna, R.; Ramesh, P.; Vinod, H.; Prashantha, S.C.; Alhuthali, A.M.S.; et al. Nonlinear optical, optical limiting and radiation shielding features of Eu3+ activated borate glasses. Optik 2021, 232, 166563. [Google Scholar]
- Ramesh, P.; Jagannath, G.; Eraiah, B.; Rao, S.V.; Kokila, M.K. Influence of PbO on Nonlinear Optical Properties of Eu3+ Doped La2O3–PbO–B2O3 Glasses; AIP Publishing: College Park, MD, USA, 2020; p. 80048. [Google Scholar]
- Zhao, X.; Wang, X.; Lin, H.; Wang, Z. Electronic polarizability and optical basicity of lanthanide oxides. Phys. B Condens. Matter 2007, 392, 132–136. [Google Scholar] [CrossRef]
- Shanmugavelu, B.; Kumar, V.V.R.K.; Kuladeep, R.; Rao, D.N. Third order nonlinear optical properties of bismuth zinc borate glasses. J. Appl. Phys. 2013, 114, 243103. [Google Scholar] [CrossRef]
- Şakar, E.; Özpolat, Ö.F.; Alım, B.; Sayyed, M.I.; Kurudirek, M. Phy-X / PSD: Development of a user friendly online software for calculation of parameters relevant to radiation shielding and dosimetry. Radiat. Phys. Chem. 2020, 166, 108496. [Google Scholar] [CrossRef]
- Sayyed, M.I.; Almuqrin, A.H.; Kumar, A.; Jecong, J.F.M.; Akkurt, I. Optical, mechanical properties of TeO2–CdO–PbO–B2O3 glass systems and radiation shielding investigation using EPICS2017 library. Optik 2021, 242, 167342. [Google Scholar] [CrossRef]



| Glass Compositions | Excitation Wavelength and Other Details | α2 (×10−11 m/W) | n2 (×10−18 m2/W) | Reference |
|---|---|---|---|---|
| xSm2O3–(100–x)[0.1BaO–0.4ZnO–0.5B2O3], x = 0 mol % xSm2O3–(100–x)[0.1BaO–0.4ZnO–0.5B2O3], x = 0.5 mol % xSm2O3–(100–x)[0.1BaO–0.4ZnO–0.5B2O3], x = 1.0 mol % xSm2O3–(100–x)[0.1BaO–0.4ZnO–0.5B2O3], x = 1.5 mol % xSm2O3–(100–x)[0.1BaO–0.4ZnO–0.5B2O3], x = 2 mol % | 532 nm (5 ns 10 Hz) | 0.572 1.128 1.507 1.928 2.746 | 0.108 0.771 0.899 1.022 1.299 | [17] |
| xEr2O3–(100–x)[0.1BaO–0.4ZnO–0.5B2O3] with x = 0.5 mol % xEr2O3–(100–x)[0.1BaO–0.4ZnO–0.5B2O3] with x = 1 mol % xEr2O3–(100–x)[0.1BaO–0.4ZnO–0.5B2O3] with x = 1.5 mol % xEr2O3–(100–x)[0.1BaO–0.4ZnO–0.5B2O3] with x = 2 mol % | 532 nm (5 ns 10 Hz) | 0.359 0.476 0.574 0.797 | 0.902 1.085 1.124 1.431 | [10] |
| 20Na2O–40Bi2O3–(40–x)B2O3–xEu2O3with x = 0 mol % 20Na2O–40Bi2O3–(40–x)B2O3–xEu2O3with x = 0.5 mol % 20Na2O–40Bi2O3–(40–x)B2O3–xEu2O3with x = 1 mol % 20Na2O–40Bi2O3–(40–x)B2O3–xEu2O3with x = 1.5 mol % 20Na2O–40Bi2O3–(40–x)B2O3–xEu2O3with x = 1.5 mol % | 532 nm (5 ns 1 Hz) | 1.55 1.74 1.98 2.16 2.37 | 1.61 1.94 2.26 2.74 3.28 | [15] |
| 10Sb2O3–20Na2O–(70–x)B2O3–xEu2O3with x = 0 mol % 10Sb2O3–20Na2O–(70–x)B2O3–xEu2O3with x = 0.5 mol % 10Sb2O3–20Na2O–(70–x)B2O3–xEu2O3with x = 1 mol % 10Sb2O3–20Na2O–(70–x)B2O3–xEu2O3with x = 1.5 mol % 10Sb2O3–20Na2O–(70–x)B2O3–xEu2O3with x = 2 mol % | 532 nm (5 ns 1 Hz) | 0.921 1.132 1.532 2.141 2.824 | 0.426 1.491 2.344 2.712 2.915 | [37] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almuqrin, A.H.; Gangareddy, J.; Hivrekar, M.M.; Pramod, A.G.; Sayyed, M.I.; Keshavamurthy, K.; Fatima, N.; Jadhav, K.M. Nonlinear Optical Limiting and Radiation Shielding Characteristics of Sm2O3 Doped Cadmium Sodium Lithium Borate Glasses. Materials 2022, 15, 2330. https://doi.org/10.3390/ma15062330
Almuqrin AH, Gangareddy J, Hivrekar MM, Pramod AG, Sayyed MI, Keshavamurthy K, Fatima N, Jadhav KM. Nonlinear Optical Limiting and Radiation Shielding Characteristics of Sm2O3 Doped Cadmium Sodium Lithium Borate Glasses. Materials. 2022; 15(6):2330. https://doi.org/10.3390/ma15062330
Chicago/Turabian StyleAlmuqrin, Aljawhara H., Jagannath Gangareddy, Mahesh M. Hivrekar, A. G. Pramod, M. I. Sayyed, K. Keshavamurthy, Naseem Fatima, and K. M. Jadhav. 2022. "Nonlinear Optical Limiting and Radiation Shielding Characteristics of Sm2O3 Doped Cadmium Sodium Lithium Borate Glasses" Materials 15, no. 6: 2330. https://doi.org/10.3390/ma15062330
APA StyleAlmuqrin, A. H., Gangareddy, J., Hivrekar, M. M., Pramod, A. G., Sayyed, M. I., Keshavamurthy, K., Fatima, N., & Jadhav, K. M. (2022). Nonlinear Optical Limiting and Radiation Shielding Characteristics of Sm2O3 Doped Cadmium Sodium Lithium Borate Glasses. Materials, 15(6), 2330. https://doi.org/10.3390/ma15062330







