Biocompatible Materials for Orbital Wall Reconstruction—An Overview
Abstract
1. Introduction
1.1. Causes of Orbital Fracture
1.2. Types of Orbital Fractures
1.3. Management of Orbital Fracture
2. Biomaterials for Reconstruction
2.1. Bone
2.2. Cartilage
2.3. Alloplastic Materials
2.3.1. Metals
2.3.2. Resorbable Osteosynthesis Materials
2.3.3. Material Combinations
2.3.4. Patient-Specific Implants
2.3.5. Resorbable Sheeting
3. Recent Discoveries
4. Surface Modifications of Metallic Biomaterials, Ceramics, and Polymers Using Additive Manufacturing
5. Prospects and Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shumway, C.L.; Mollag, M.; Wade, M. Anatomy, Head and Neck, Orbit Bones; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Gart, M.S.; Gosain, A.K. Evidence-based medicine: Orbital floor fractures. Plast. Reconstr. Surg. 2014, 134, 1345–1355. [Google Scholar] [CrossRef] [PubMed]
- Turvey, T.A.; Golden, B.A. Orbital anatomy for the surgeon. Oral Maxillofac. Surg. Clin. N. Am. 2012, 24, 525–536. [Google Scholar] [CrossRef]
- Boyette, J.R.; Pemberton, J.D.; Bonilla-Velez, J. Management of orbital fractures: Challenges and solutions. Clin. Ophthalmol. 2015, 9, 2127–2137. [Google Scholar] [CrossRef] [PubMed]
- Grob, S.; Yonkers, M.; Tao, J. Orbital fracture repair. Semin. Plast. Surg. 2017, 31, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Homer, N.; Huggins, A.; Durairaj, V.D. Contemporary management of orbital blowout fractures. Curr. Opin. Otolaryngol. Head Neck Surg. 2019, 27, 310–316. [Google Scholar] [CrossRef]
- El-Hadad, C.; Deschênes, J.; Arthurs, B. Orbital floor fracture. Can. Med. Assoc. J. 2021, 193, E289. [Google Scholar] [CrossRef] [PubMed]
- Lozada, K.N.; Cleveland, P.W.; Smith, J.E. Orbital trauma. Semin. Plast. Surg. 2019, 33, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.M.; Glava, I.P. Orbital fractures: A review. Clin. Ophthalmol. 2011, 5, 95–100. [Google Scholar] [CrossRef]
- Koenen, L.; Waseem, M. Orbital Floor Fracture; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- El-Mahallawy, Y.A.; Al-Mahalawy, H.A. Evaluation of orbital volume after orbitozygomatic complex fractures fixation: A radiographical study. J. Oral Biol. Craniofacial Res. 2020, 10, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Essig, H.; Dressel, L.; Rana, M.; Rana, M.; Kokemueller, H.; Ruecker, M.; Gellrich, N.C. Precision of posttraumatic primary orbital reconstruction using individually bent titanium mesh with and without navigation: A retrospective study. Head Face Med. 2013, 9, 18. [Google Scholar] [CrossRef]
- Kanno, T.; Sukegawa, S.; Karino, M.; Furuki, Y. Navigation-assisted orbital trauma reconstruction using a bioactive osteoconductive/bioresorbable u-HA/PLLA System. J. Maxillofac. Oral Surg. 2019, 18, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Clauser, L.; Galiè, M.; Pagliaro, F.; Tieghi, R. Posttraumatic enophthalmos: Etiology, principles of reconstruction, and correction. J. Craniofac. Surg. 2008, 19, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Caldarelli, C.; Benech, R.; Iaquinta, C. Superior orbital fissure syndrome in lateral orbital wall fracture: Management and classification update. Craniomaxillofac. Trauma Reconstr. 2016, 9, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Cho, R.I.; Davies, B.W. Combined orbital floor and medial wall fractures involving the inferomedial strut: Repair technique and case series using preshaped porous polyethylene/titanium implants. Craniomaxillofac. Trauma Reconstr. 2013, 6, 161–170. [Google Scholar] [CrossRef]
- Bregman, J.A.; Vakharia, K.T.; Idowu, O.O.; Vagefi, M.R.; Grumbine, F.L. Outpatient surgical management of orbital blowout fractures. Craniomaxillofac. Trauma Reconstr. 2019, 12, 205–210. [Google Scholar] [CrossRef]
- Hsu, C.-K.; Hsieh, M.-W.; Chang, H.-C.; Tai, M.-C.; Chien, K.-H. Anatomic factors predicting postoperative strabismus in orbital wall fracture repair. Sci. Rep. 2019, 9, 14785. [Google Scholar] [CrossRef] [PubMed]
- Jamari, J.; Ammarullah, M.I.; Saad, A.P.; Syahrom, A.; Uddin, M.; van der Heide, E.; Basri, H. The Effect of bottom profile dimples on the femoral head on wear in metal-on-metal total hip arthroplasty. J. Funct. Biomater. 2021, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca stress simulation of metal-on-metal total hip arthroplasty during normal walking activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef]
- Balaji, S.M.; Balaji, P. Surgical correction of diplopia in orbital fracture: Influence of material and design. Ann. Maxillofac. Surg. 2019, 9, 129–134. [Google Scholar] [CrossRef]
- Aldekhayel, S.; Aljaaly, H.; Fouda-Neel, O.; Shararah, A.-W.; Zaid, W.S.; Gilardino, M. Evolving trends in the management of orbital floor fractures. J. Craniofac. Surg. 2014, 25, 258–261. [Google Scholar] [CrossRef]
- Chowdhury, K.; Krause, G.E. Selection of materials for orbital floor reconstruction. Arch. Otolaryngol. Head Neck Surg. 1998, 124, 1398–1401. [Google Scholar] [CrossRef] [PubMed]
- Neumann, A.; Kevenhoerster, K. Biomaterials for craniofacial reconstruction. GMS Curr. Top. Otorhinolaryngol. Head Neck Surg. 2009, 8, Doc08. [Google Scholar] [CrossRef] [PubMed]
- Thrivikraman, G.; Athirasala, A.; Twohig, C.; Boda, S.K.; Bertassoni, L.E. Biomaterials for craniofacial bone regeneration. Dent. Clin. N. Am. 2017, 61, 835–856. [Google Scholar] [CrossRef] [PubMed]
- Baino, F. Biomaterials and implants for orbital floor repair. Acta Biomater. 2011, 7, 3248–3266. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.-X.; Sun, X.-M.; Zhang, Y.-G.; Zhang, Y. Materials to facilitate orbital reconstruction and soft tissue filling in posttraumatic orbital deformaties. Plast. Aesthetic Res. 2016, 3, 86. [Google Scholar] [CrossRef]
- Tessier, P.; Kawamoto, H.; Matthews, D.; Posnick, J.; Raulo, Y.; Tulasne, J.F.; Wolfe, S.A. Autogenous bone grafts and bone substitutes—Tools and techniques: I. A 20,000-case experience in maxillofacial and craniofacial surgery. Plast. Reconstr. Surg. 2005, 116, 6S–24S. [Google Scholar] [CrossRef] [PubMed]
- Jeyaraj, P. Split Calvarial Grafting for Closure of Large Cranial Defects: The Ideal Option? J. Maxillofac. Oral Surg. 2019, 18, 518–530. [Google Scholar] [CrossRef] [PubMed]
- Koryczan, P.; Zapała, J.; Gontarz, M.; Wyszyńska-Pawelec, G. Comparison of the results of the treatment of enophthalmos in orbital blowout fracture in children/adolescents and adults. Dent. Med. Probl. 2021, 58, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, M.; Matsuzawa, Y.; Mori, H.; Matsunaga, K.; Kamiishi, H. Orbital wall reconstruction with bone grafts from the outer cortex of the mandible. J. Cranio-Maxillofac. Surg. 2004, 32, 374–380. [Google Scholar] [CrossRef]
- Al-Anezi, M.; Mahran, H.; Alomaym, M.; Albati, S.; Alharbi, M. Role of titanium mesh as a reconstruction material for orbital floor defects in cases of orbital blowout trauma. OHDM 2018, 17, 1–5. [Google Scholar]
- Rallis, G.; Mourouzis, C.; Papakosta, V.; Papanastasiou, G.; Zachariades, N. Reasons for miniplate removal following maxillofacial trauma: A 4-year study. J. Cranio-Maxillo-Facial Surg. 2006, 34, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Tuan, R.S.; Chen, A.F.; Klatt, B.A. Cartilage regeneration. J. Am. Acad. Orthop. Surg. 2013, 21, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Harish, S. Autogenous grafts for orbital floor reconstruction: A review. Int. J. Oral Craniofacial Sci. 2017, 3, 046–052. [Google Scholar] [CrossRef]
- Bayat, M.; Momen-Heravi, F.; Khalilzadeh, O.; Mirhosseni, Z.; Sadeghi-Tari, A. Comparison of conchal cartilage graft with nasal septal cartilage graft for reconstruction of orbital floor blowout fractures. Br. J. Oral Maxillofac. Surg. 2010, 48, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhang, Z.; Tang, W.; Li, Z.; Deng, Y. Bioresorbable Material in secondary orbital reconstruction surgery. J. Ophthalmol. 2019, 2019, 8715314. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E., 3rd; Messo, E. Use of nonresorbable alloplastic implants for internal orbital reconstruction. J. Oral Maxillofac. Surg. 2004, 62, 873–881. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef]
- Dorri, M.; Oliver, R. WITHDRAWN: Resorbable versus titanium plates for facial fractures. Cochrane Database Syst. Rev. 2018, 5, CD007158. [Google Scholar] [CrossRef]
- On, S.W.; Cho, S.W.; Byun, S.H.; Yang, B.E. Bioabsorbable osteofixation materials for maxillofacial bone surgery: A review on polymers and magnesium-based materials. Biomedicines 2020, 8, 300. [Google Scholar] [CrossRef]
- Katou, F.; Andoh, N.; Motegi, K.; Nagura, H. Immuno-inflammatory responses in the tissue adjacent to titanium miniplates used in the treatment of mandibular fractures. J. Cranio-Maxillofac. Surg. 1996, 24, 155–162. [Google Scholar] [CrossRef]
- Matusiewicz, H. Potential release of in vivo trace metals from metallic medical implants in the human body: From ions to nanoparticles—A systematic analytical review. Acta Biomater. 2014, 10, 2379–2403. [Google Scholar] [CrossRef] [PubMed]
- Blessing, N.W.; Rong, A.J.; Tse, B.C.; Erickson, B.P.; Lee, B.W.; Johnson, T.E. Orbital bony reconstruction with presized and precontoured porous polyethylene-titanium implants. Ophthal. Plast. Reconstr. Surg. 2021, 37, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Briguglio, F.; Falcomatà, D.; Marconcini, S.; Fiorillo, L.; Briguglio, R.; Farronato, D. The Use of titanium mesh in guided bone regeneration: A systematic review. Int. J. Dent. 2019, 2019, 9065423. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, C.; Bianchi, A.; Mazzoni, S.; Cipriani, R.; Campobassi, A. Oromandibular reconstruction using a fibula osteocutaneous free flap: Four different “Preplating” techniques. Plast. Reconstr. Surg. 2006, 118, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Su, Y.X.; Yan, Y.; Choi, W.S.; Yang, W.F.; Zhang, C.; Chen, X.; Curtin, J.P.; Ouyang, J.; Zhang, B. A Systematic approach for making 3D-printed patient-specific implants for craniomaxillofacial reconstruction. Engineering 2020, 6, 1291–1301. [Google Scholar] [CrossRef]
- Martola, M.; Lindqvist, C.; Hänninen, H.; Al-Sukhun, J. Fracture of titanium plates used for mandibular reconstruction following ablative tumor surgery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 80B, 345–352. [Google Scholar] [CrossRef]
- Katakura, A.; Shibahara, T.; Noma, H.; Yoshinari, M. Material analysis of AO plate fracture cases. J. Oral Maxillofac. Surg. 2004, 62, 348–352. [Google Scholar] [CrossRef]
- Wang, Y.-T.; Huang, S.-F.; Fang, Y.-T.; Huang, S.-C.; Cheng, H.-F.; Chen, C.-H.; Wang, P.-F.; Lin, C.-L. Anatomical Thin titanium mesh plate structural optimization for zygomatic-maxillary complex fracture under fatigue testing. Biomed Res. Int. 2018, 2018, 9398647. [Google Scholar] [CrossRef]
- Purnell, C.A.; Vaca, E.E.; Ellis, M.F. Orbital Fracture reconstruction using prebent, anatomic titanium plates: Technical tips to avoid complications. J. Craniofac. Surg. 2018, 29, e515–e517. [Google Scholar] [CrossRef]
- Tarsitano, A.; Badiali, G.; Pizzigallo, A.; Marchetti, C. Orbital reconstruction: Patient-specific orbital floor reconstruction using a mirroring technique and a customized titanium mesh. J. Craniofac. Surg. 2016, 27, 1822–1825. [Google Scholar] [CrossRef]
- Schumann, P.; Lindhorst, D.; Wagner, M.E.H.; Schramm, A.; Gellrich, N.-C.; Rücker, M. Perspectives on resorbable osteosynthesis materials in craniomaxillofacial surgery. Pathobiology 2013, 80, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Young, S.M.; Sundar, G.; Lim, T.-C.; Lang, S.S.; Thomas, G.; Amrith, S. Use of bioresorbable implants for orbital fracture reconstruction. Br. J. Ophthalmol. 2017, 101, 1080–1085. [Google Scholar] [CrossRef]
- Lee, D.J.; Kwon, J.; Kim, Y.-I.; Kwon, Y.H.; Min, S.; Shin, H.W. Coating Medpor(®) Implant with Tissue-Engineered Elastic Cartilage. J. Funct. Biomater. 2020, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M.; Park, S.H.; Lee, J.S.; Kim, H.D.; Hwang, M.K.; Kim, M.W. Improvement of Infraorbital Rim contour Using Medpor. Arch. Craniofacial Surg. 2016, 17, 77–81. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lin, I.-C.; Liao, S.-L.; Lin, L.L.K. Porous polyethylene implants in orbital floor reconstruction. J. Formos. Med. Assoc. 2007, 106, 51–57. [Google Scholar] [CrossRef]
- Enislidis, G. Treatment of orbital fractures: The case for treatment with resorbable materials. J. Oral Maxillofac. Surg. 2004, 62, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non-resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ren, Z.; Xu, Y.; Pang, S.; Zhao, X.; Zhao, Y. Biodegradable magnesium alloys developed as bone repair materials: A review. Scanning 2018, 2018, 9216314. [Google Scholar] [CrossRef]
- Garibaldi, D.C.; Iliff, N.T.; Grant, M.P.; Merbs, S.L. Use of porous polyethylene with embedded titanium in orbital reconstruction: A review of 106 patients. Ophthal. Plast. Reconstr. Surg. 2007, 23, 439–444. [Google Scholar] [CrossRef]
- Lieger, O.; Richards, R.; Liu, M.; Lloyd, T. Computer-assisted design and manufacture of implants in the late reconstruction of extensive orbital fractures. Arch. Facial Plast. Surg. 2010, 12, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-B.; Mao, C.; Liu, X.-J.; Guo, C.-B.; Yu, G.-Y.; Peng, X. Outcomes of orbital floor reconstruction after extensive maxillectomy using the computer-assisted fabricated individual titanium mesh technique. J. Oral Maxillofac. Surg. 2015, 73, 2065.e1–2065.e15. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.S.; Jeong, W.S.; Chang, T.J.; Koh, K.S.; Choi, J.-W. Customized orbital wall reconstruction using three-dimensionally printed rapid prototype model in patients with orbital wall fracture. J. Craniofac. Surg. 2016, 27, 2020–2024. [Google Scholar] [CrossRef] [PubMed]
- Raisian, S.; Fallahi, H.R.; Khiabani, K.S.; Heidarizadeh, M.; Azdoo, S. Customized titanium mesh based on the 3D printed model vs. manual intraoperative bending of titanium mesh for reconstructing of orbital bone fracture: A randomized clinical trial. Rev. Recent Clin. Trials 2017, 12, 154–158. [Google Scholar] [CrossRef]
- Stoor, P.; Suomalainen, A.; Lindqvist, C.; Mesimäki, K.; Danielsson, D.; Westermark, A.; Kontio, R.K. Rapid prototyped patient specific implants for reconstruction of orbital wall defects. J. Cranio-Maxillofac. Surg. 2014, 42, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Mommaerts, M.Y.; Büttner, M.; Vercruysse, H.J.; Wauters, L.; Beerens, M. Orbital Wall Reconstruction with two-piece puzzle 3D printed implants: Technical note. Craniomaxillofac. Trauma Reconstr. 2016, 9, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Rotaru, H.; Stan, H.; Florian, I.S.; Schumacher, R.; Park, Y.-T.; Kim, S.-G.; Chezan, H.; Balc, N.; Baciut, M. Cranioplasty with custom-made implants: Analyzing the cases of 10 patients. J. Oral Maxillofac. Surg. 2012, 70, e169–e176. [Google Scholar] [CrossRef] [PubMed]
- Wilde, F.; Kasper, R.; Sakkas, A.; Pietzka, S.; Winter, K.; Schramm, A.; Mascha, F. Biomechanical in-vitro study concerning the stability of customized CAD/CAM mandibular reconstruction plates. Comparison of additively and subtractively manufactured as well as hand-bended plates. Int. J. Oral Maxillofac. Surg. 2019, 48, 150–151. [Google Scholar] [CrossRef]
- Strong, E.B.; Fuller, S.C.; Wiley, D.F.; Zumbansen, J.; Wilson, M.D.; Metzger, M.C. Preformed vs intraoperative bending of titanium mesh for orbital reconstruction. Otolaryngol. Head Neck Surg. 2013, 149, 60–66. [Google Scholar] [CrossRef]
- Smeets, M.; Snel, R.; Sun, Y.; Dormaar, T.; Politis, C. Late reconstruction of extensive orbital floor fracture with a patient-specific implant in a bombing victim. J. Korean Assoc. Oral Maxillofac. Surg. 2020, 46, 353–357. [Google Scholar] [CrossRef]
- Zimmerer, R.M.; Ellis, E., 3rd; Aniceto, G.S.; Schramm, A.; Wagner, M.E.H.; Grant, M.P.; Cornelius, C.-P.; Strong, E.B.; Rana, M.; Chye, L.T.; et al. A prospective multicenter study to compare the precision of posttraumatic internal orbital reconstruction with standard preformed and individualized orbital implants. J. Cranio-Maxillofac. Surg. 2016, 44, 1485–1497. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Chen, H.; Sun, Y.-J.; Wang, B.-F.; Che, L.; Liu, S.-Y.; Li, G.-Y. Clinical effects of 3-D printing-assisted personalized reconstructive surgery for blowout orbital fractures. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2051–2057. [Google Scholar] [CrossRef] [PubMed]
- Goranova, K.L.; Kattenhøj Sloth Overgaard, A.K.; Gitsov, I. Hydroxyapatite-poly(d,l-lactide) Nanografts. synthesis and characterization as bone cement additives. Molecules 2021, 26, 424. [Google Scholar] [CrossRef] [PubMed]
- Gradinaru, S.; Popescu, L.M.; Piticescu, R.M.; Zurac, S.; Ciuluvica, R.; Burlacu, A.; Tutuianu, R.; Valsan, S.-N.; Motoc, A.M.; Voinea, L.M. Repair of the orbital wall fractures in rabbit animal model using nanostructured hydroxyapatite-based implant. Nanomaterials 2016, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Archer, E.; Torretti, M.; Madbouly, S. Biodegradable polycaprolactone (PCL) based polymer and composites. Phys. Sci. Rev. 2021. [Google Scholar] [CrossRef]
- Teo, L.; Teoh, S.H.; Liu, Y.; Lim, L.; Tan, B.; Schantz, J.-T.; Seah, L.L. A novel bioresorbable implant for repair of orbital floor fractures. Orbit 2015, 34, 192–200. [Google Scholar] [CrossRef]
- Lee, H.B.H.; Nunery, W.R. Orbital adherence syndrome secondary to titanium implant material. Ophthal. Plast. Reconstr. Surg. 2009, 25, 33–36. [Google Scholar] [CrossRef]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J.; Symes, R.; Tieppo, M.; Collins, C.; Cao, A.; et al. Metallic biomaterials: Current challenges and opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef]
- Pagel, M.; Beck-Sickinger, A.G. Multifunctional biomaterial coatings: Synthetic challenges and biological activity. Biol. Chem. 2017, 398, 3–22. [Google Scholar] [CrossRef]
- Bose, S.; Robertson, S.F.; Bandyopadhyay, A. Surface modification of biomaterials and biomedical devices using additive manufacturing. Acta Biomater. 2018, 66, 6–22. [Google Scholar] [CrossRef] [PubMed]
- Bonda, D.J.; Manjila, S.; Selman, W.R.; Dean, D. The recent revolution in the design and manufacture of cranial implants: Modern advancements and future directions. Neurosurgery 2015, 77, 814–824; discussion 824. [Google Scholar] [CrossRef]
- Nyberg, E.L.; Farris, A.L.; Hung, B.P.; Dias, M.; Garcia, J.R.; Dorafshar, A.H.; Grayson, W.L. 3D-printing technologies for craniofacial rehabilitation, reconstruction, and regeneration. Ann. Biomed. Eng. 2017, 45, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Verret, D.J.; Ducic, Y.; Oxford, L.; Smith, J. Hydroxyapatite cement in craniofacial reconstruction. Otolaryngol. Head Neck Surg. 2005, 133, 897–899. [Google Scholar] [CrossRef] [PubMed]
- Popescu, L.M.; Piticescu, R.M.; Antonelli, A.; Rusti, C.F.; Carboni, E.; Sfara, C.; Magnani, M.; Badilita, V.; Vasile, E.; Trusca, R.; et al. Recent advances in synthesis, characterization of hydroxyapatite/polyurethane composites and study of their biocompatible properties. J. Mater. Sci. Mater. Med. 2013, 24, 2491–2503. [Google Scholar] [CrossRef] [PubMed]
- Matic, D.B.; Manson, P.N. Biomechanical analysis of hydroxyapatite cement cranioplasty. J. Craniofac. Surg. 2004, 15, 413–415. [Google Scholar] [CrossRef] [PubMed]
- Ikbal, H.; Chattopadhyay, P.; Jayanth Perumal, S. Biomaterials for Orbital Reconstruction. Saudi J. Oral Dent. Res. 2021, 6, 581–591. [Google Scholar] [CrossRef]
- Zemba, M.; Stamate, A.-C.; Tataru, C.P.; Branisteanu, D.C.; Balta, F. Conjunctival flap surgery in the management of ocular surface disease (Review). Exp. Ther. Med. 2020, 20, 3412–3416. [Google Scholar] [CrossRef]
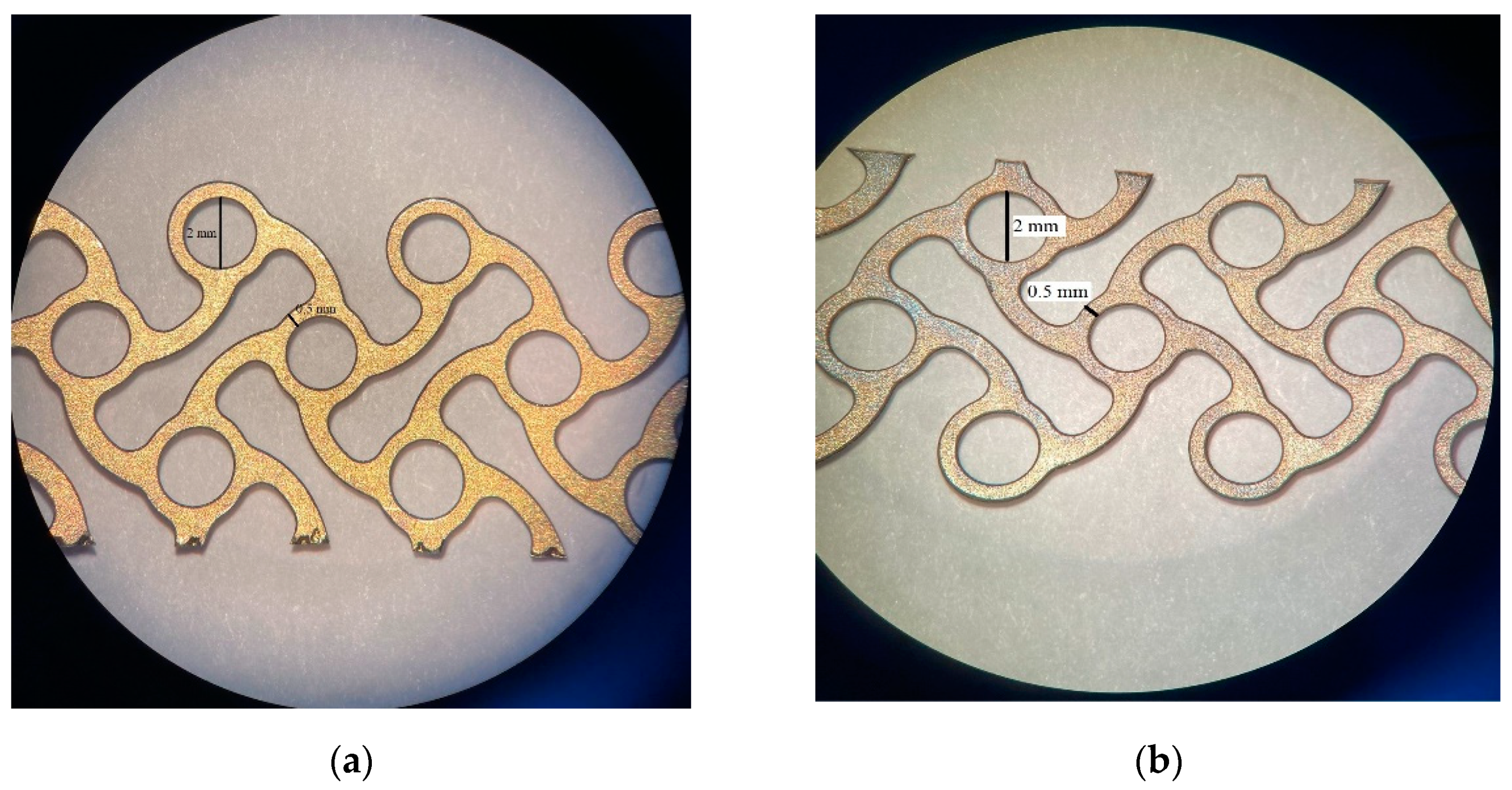
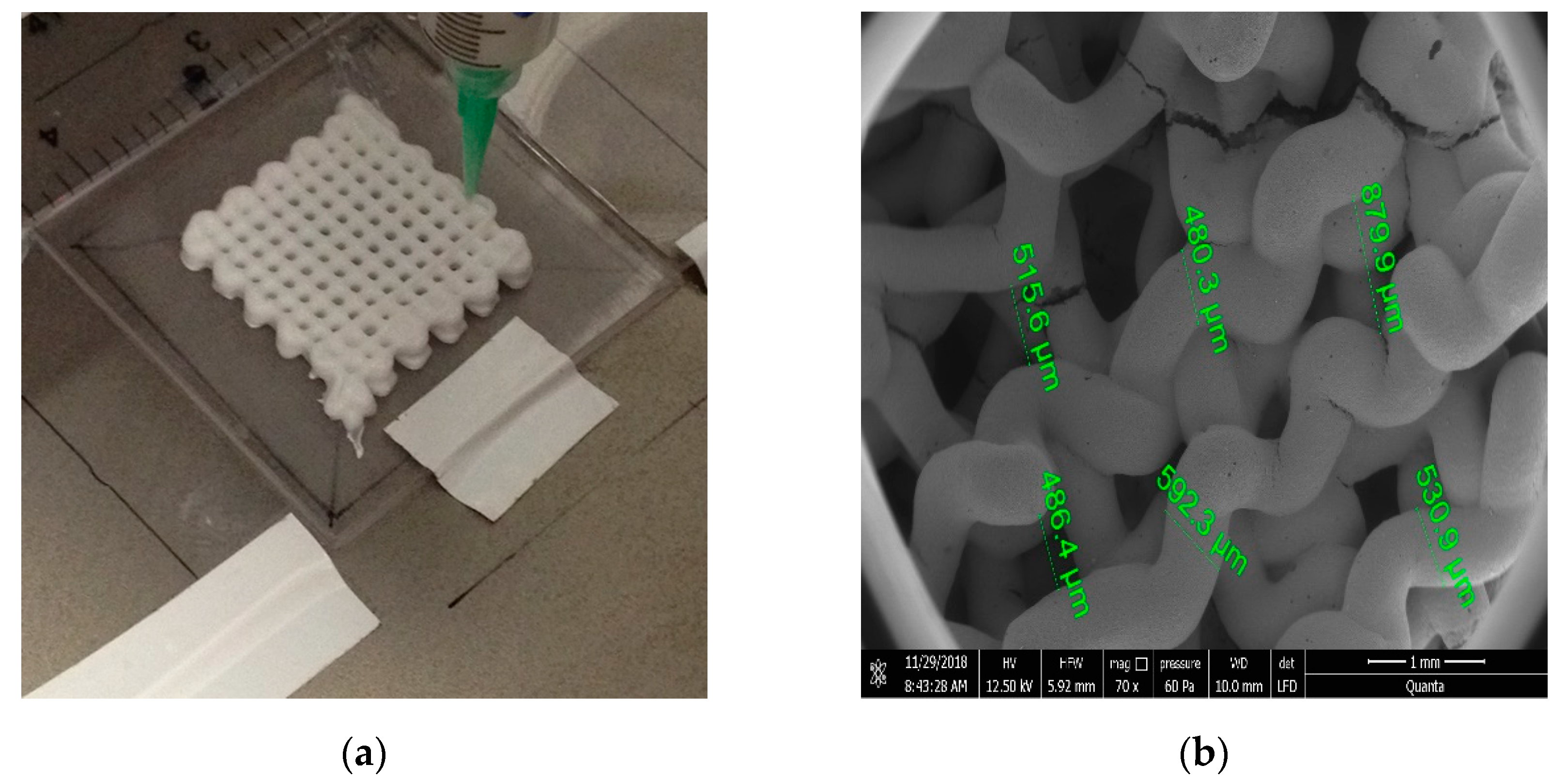

| Implant Material | Indication | |
|---|---|---|
| Autograft | Bone | Pediatric fractures (<7 years of age) |
| Cartilage | Small orbital fractures | |
| Alloplast | Resorbable sheeting | Pediatric fractures |
| Porous polyethylene | Defects with solid edges | |
| Titanium mesh | Large defect of the orbital floor Small gaps with stable lateral and medial borders | |
| Patient-specific implant | Complex and extensive orbital defects |
| Biological Factors | Effect |
|---|---|
| Transforming growth factor β 1 (TGF-β1) | × regulates bone remodeling |
| Bone morphogenetic protein-2 (BMP-2) | × strong osteoinductive effect |
| Vascular endothelial growth factors (VEGFs) | × stimulates the formation of new blood vessels × used for vascular materials as well as for bone regeneration |
| Fibroblast growth factors (bFGF or FGF-2) | × pro-angiogenic role × increases cell proliferation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vasile, V.A.; Istrate, S.; Iancu, R.C.; Piticescu, R.M.; Cursaru, L.M.; Schmetterer, L.; Garhöfer, G.; Cherecheanu, A.P. Biocompatible Materials for Orbital Wall Reconstruction—An Overview. Materials 2022, 15, 2183. https://doi.org/10.3390/ma15062183
Vasile VA, Istrate S, Iancu RC, Piticescu RM, Cursaru LM, Schmetterer L, Garhöfer G, Cherecheanu AP. Biocompatible Materials for Orbital Wall Reconstruction—An Overview. Materials. 2022; 15(6):2183. https://doi.org/10.3390/ma15062183
Chicago/Turabian StyleVasile, Victor A., Sinziana Istrate, Raluca C. Iancu, Roxana M. Piticescu, Laura M. Cursaru, Leopold Schmetterer, Gerhard Garhöfer, and Alina Popa Cherecheanu. 2022. "Biocompatible Materials for Orbital Wall Reconstruction—An Overview" Materials 15, no. 6: 2183. https://doi.org/10.3390/ma15062183
APA StyleVasile, V. A., Istrate, S., Iancu, R. C., Piticescu, R. M., Cursaru, L. M., Schmetterer, L., Garhöfer, G., & Cherecheanu, A. P. (2022). Biocompatible Materials for Orbital Wall Reconstruction—An Overview. Materials, 15(6), 2183. https://doi.org/10.3390/ma15062183








