Covariates Relating to Implant Failure and Marginal Bone Loss of a Novel Triangular Neck-Implant Placed by Post-Graduate Students: A 1-Year Prospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patient Selection—Inclusion/Exclusion Criteria
2.3. Surgical Procedure
2.4. Study Variables and Measurements
2.4.1. Radiographic Assessment
2.4.2. Clinical Assessment
2.5. Statistical Methods
3. Results
3.1. Implant Survival
3.2. Marginal Bone Loss and Implant Success
4. Discussion
5. Conclusions
- The survival and success rates of the V3 triangular-neck implants placed and by inexperienced post-graduate students at 1-year follow-up were 97.5% and 96.7%, respectively.
- No contributing factors were identified regarding implant failure; however, a relevant association was found involving patients with smoking habits, where implant failure was five times higher (OR = 5.31, p = 0.09) in smokers.
- The mean MBL was 0.51 ± 0.44 mm, and the inexperience of the rehabilitation team did not contribute to additional bone loss.
- Gender was the single covariate to significantly impact the MBL.
- Timing of implant placement, delayed vs. immediate, displayed a tendency to affect it, although not significantly.
- BoP at the buccal sites of the implants was the only predictive factor of bone loss.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- den Hartog, L.; Raghoebar, G.M.; Slater, J.J.; Stellingsma, K.; Vissink, A.; Meijer, H.J. Single-tooth implants with different neck designs: A randomized clinical trial evaluating the aesthetic outcome. Clin. Implant Dent. Relat. Res. 2013, 15, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Ragucci, G.M.; Giralt-Hernando, M.; Méndez-Manjón, I.; Cantó-Navés, O.; Hernández-Alfaro, F. Factors Affecting Implant Failure and Marginal Bone Loss of Implants Placed by Post-Graduate Students: A 1-Year Prospective Cohort Study. Materials 2020, 13, 4511. [Google Scholar] [CrossRef] [PubMed]
- Supplement, D.; D’Avenia, F.; Del Fabbro, M.; Karanxha, L.; Weinstein, T.; Corbella, S.; Fumagalli, D.; Francetti, L.; Taschieri, S. Hard and soft tissue changes in the rehabilitation of the anterior maxilla with triangular shape neck implants: A retrospective clinical study with a one-year follow up. J. Biol. Regul. Homeost. Agents 2020, 33, 13–21. [Google Scholar]
- Linkevicius, T.; Linkevicius, R.; Gineviciute, E.; Alkimavicius, J.; Mazeikiene, A.; Linkeviciene, L. The influence of new immediate tissue level abutment on crestal bone stability of subcrestally placed implants: A 1-year randomized controlled clinical trial. Clin. Implant Dent. Relat. Res. 2021, 23, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Sinibaldi, R.; Conti, A.; Sinjari, B.; Spadone, S.; Pecci, R.; Palombo, M.; Komlev, V.; Ortore, M.; Tromba, G.; Capuani, S.; et al. Multimodal-3D imaging based on μMRI and μCT techniques bridges the gap with histology in visualization of the bone regeneration process. J. Tissue Eng. Regen. Med. 2017, 12, 750–761. [Google Scholar] [CrossRef] [PubMed]
- Li Manni, L.; Lecloux, G.; Rompen, E.; Aouini, W.; Shapira, L.; Lambert, F. Clinical and radiographic assessment of circular versus triangular cross-section neck Implants in the posterior maxilla: A 1-year randomized controlled trial. Clin. Oral Implant. Res. 2020, 31, 814–824. [Google Scholar] [CrossRef]
- Pimenta, J.; Szmukler-Moncler, S.; Raigrodski, A.J. Physical characterization of 3 implant systems made of distinct materials with distinct surfaces. J. Prosthet. Dent. 2021. [Google Scholar] [CrossRef]
- Linkevicius, T.; Linkevicius, R.; Alkimavicius, J.; Linkeviciene, L.; Andrijauskas, P.; Puisys, A. Influence of titanium base, lithium disilicate restoration and vertical soft tissue thickness on bone stability around triangular-shaped implants: A prospective clinical trial. Clin. Oral Implant. Res. 2018, 29, 716–724. [Google Scholar] [CrossRef]
- Steiner, C.; Karl, M.; Grobecker-Karl, T. Insertion and Loading Characteristics of Three Different Bone-Level Implants. Int. J. Oral Maxillofac. Implant. 2020, 35, 560–565. [Google Scholar] [CrossRef]
- Lee, D.-W.; Choi, Y.-S.; Park, K.H.; Kim, C.-S.; Moon, I.-S. Effect of microthread on the maintenance of marginal bone level: A 3-year prospective study. Clin. Oral Implant. Res. 2007, 18, 465–470. [Google Scholar] [CrossRef]
- Shin, Y.-K.; Han, C.-H.; Heo, S.-J.; Kim, S.; Chun, H.-J. Radiographic evaluation of marginal bone level around implants with different neck designs after 1 year. Int. J. Oral Maxillofac. Implant. 2006, 21, 789–794. [Google Scholar]
- Eshkol-Yogev, I.; Tandlich, M.; Shapira, L. Effect of implant neck design on primary and secondary implant stability in the posterior maxilla: A prospective randomized controlled study. Clin. Oral Implant. Res. 2019, 30, 1220–1228. [Google Scholar] [CrossRef] [PubMed]
- Szmukler-Moncler, S.; Troiano, M.; Kotsakis, G.A. Exclusion from oral environment enables bony integration of subcrestal implant-abutment connection. Clin. Oral Implant. Res. 2021, 32, S22–S77. [Google Scholar]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implant. 1986, 1, 11–25. [Google Scholar]
- Buser, D.; Weber, H.-P.; Lang, N.P. Tissue integration of non-submerged implants. l-year results of a prospective study with 100 ITI hollow-cylinder and hollow-screw implants. Clin. Oral Implant. Res. 1990, 1, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Beschnidt, S.M.; Cacaci, C.; Dedeoglu, K.; Hildebrand, D.; Hulla, H.; Iglhaut, G.; Krennmair, G.; Schlee, M.; Sipos, P.; Stricker, A.; et al. Implant success and survival rates in daily dental practice: 5-year results of a non-interventional study using CAMLOG SCREW-LINE implants with or without platform-switching abutments. Int. J. Implant Dent. 2018, 4, 33. [Google Scholar] [CrossRef]
- Jung, R.E.; Zembic, A.; Pjetursson, B.E.; Zwahlen, M.; Thoma, D. Systematic review of the survival rate and the incidence of biological, technical, and aesthetic complications of single crowns on implants reported in longitudinal studies with a mean follow-up of 5 years. Clin. Oral Implant. Res. 2012, 23 (Suppl. 6), 2–21. [Google Scholar] [CrossRef]
- Pjetursson, B.E.; Thoma, D.; Jung, R.; Zwahlen, M.; Zembic, A. A systematic review of the survival and complication rates of implant-supported fixed dental prostheses (FDPs) after a mean observation period of at least 5 years. Clin. Oral Implant. Res. 2012, 23 (Suppl. 6), 22–38. [Google Scholar] [CrossRef]
- Preiskel, H.W.; Tsolka, P. Treatment outcomes in implant therapy: The influence of surgical and prosthodontic experience. Int. J. Prosthodont. 1995, 8, 273–279. [Google Scholar]
- Sendyk, D.I.; Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A.; Deboni, M.C. Does surgical experience influence implant survival rate? A systematic review and meta-analysis. Int. J. Prosthodont. 2017, 30, 341–347. [Google Scholar] [CrossRef]
- Suarez, F.; Chan, H.-L.; Monje, A.; Galindo-Moreno, P.; Wang, H.-L. Effect of the timing of restoration on implant marginal bone loss: A systematic review. J. Periodontol. 2013, 84, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, J.; Machtei, E.E.; Frankental, S.; Gabay, E.; Mayer, Y.; Joseph, L.; Cohen, O. Clinical and patient-related outcomes of a tapered implant system with switched platform conical abutments: A private practice field trial. J. Oral Implant. 2018, 44, 326–329. [Google Scholar] [CrossRef] [PubMed]
- Javed, F.; Romanos, G.E. The role of primary stability for successful immediate loading of dental implants. A literature review. J. Dent. 2010, 38, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Dard, M.; Kuehne, S.; Obrecht, M.; Grandin, M.; Helfenstein, J.; Pippenger, B. Integrative performance analysis of a novel bone level tapered implant. Adv. Dent. Res. 2016, 28, 28–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testori, T.; Del Fabbro, M.; Szmukler-Moncler, S.; Francetti, L.; Weinstein, R.L. Immediate occlusal loading of Osseotite implants in the completely edentulous mandible. Int. J. Oral Maxillofac. Implant. 2003, 18, 544–551. [Google Scholar]
- Beer, A.; Gahleitner, A.; Holm, A.; Tschabitscher, M.; Homolka, P. Correlation of insertion torques with bone mineral density from dental quantitative CT in the mandible. Clin. Oral Implant. Res. 2003, 14, 616–620. [Google Scholar] [CrossRef]
- Alsaadi, G.; Quirynen, M.; Komárek, A.; Van Steenberghe, D. Impact of local and systemic factors on the incidence of late oral implant loss. Clin. Oral Implant. Res. 2008, 19, 670–676. [Google Scholar]
- Kolte, A.P.; Bawankar, P.V.; Kolte, R.A.; Shrirao, T. Peri-implant tissue stability in premolar and molar sites: A retrospective clinical and radiographic analysis. Quintessence Int. 2021, 52, 584–595. [Google Scholar]
- Hamudi, N.; Barnea, E.; Weinberg, E.; Laviv, A.; Mijiritsky, E.; Matalon, S.; Chaushu, L.; Kolerman, R. The association of the one-abutment at one-time concept with marginal bone loss around the SLA and platform switch and conical abutment implants. J. Clin. Med. 2021, 11, 74. [Google Scholar] [CrossRef]
- Bressan EGrusovin, M.G.; D’Avenia, F.; Neumann, K.; Sbricoli, L.; Luongo, G.; Esposito, M. The influence of repeated abutment changes on peri-implant tissue stability: 3-year post-loading results from a multicenter randomized controlled trial. Eur. J. Oral Implantol. 2017, 10, 373–390. [Google Scholar]
- Sinjari, B.; D’Addazio, G.; Traini, T.; Varvara, G.; Scarano, A.; Murmura, G.; Caputi, S. A 10-year retrospective comparative human study on screw-retained versus cemented dental implant abutments. J. Biol. Regul. Homeost. Agents 2019, 33, 787–797. [Google Scholar]
- Linkevicius, T.; Apse, P.; Grybauskas, S.; Puisys, A. The influence of soft tissue thickness on crestal bone changes around implants: A 1-year prospective controlled clinical trial. Int. J. Oral Maxillofac. Implant. 2009, 24, 712–719. [Google Scholar]
- Linkevicius, T.; Puisys, A.; Steigmann, M.; Vindasiute, E.; Linkeviciene, L. Influence of vertical soft tissue thickness on crestal bone changes around implants with platform switching: A comparative clinical study. Clin. Implant Dent. Relat. Res. 2015, 17, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Camacho-Alonso, F.; Tallarico, M.; Meloni, S.; Xhanari, E.; Penarrocha-Oltra, D. Mucosa thickness and peri-implant crestal bone stability: A clinical and histologic prospective cohort Trial. Int. J. Oral Maxillofac. Implant. 2017, 32, 675–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saglanmak, A.; Gultekin, A.; Cinar, C.; Szmukler-Moncler, S.; Karabuda, C. Effect of soft tissue thickness on crestal bone loss of early loaded implants with platform switching: 1- and 5-year data. Quintessence Int. 2021, 426–433. [Google Scholar]
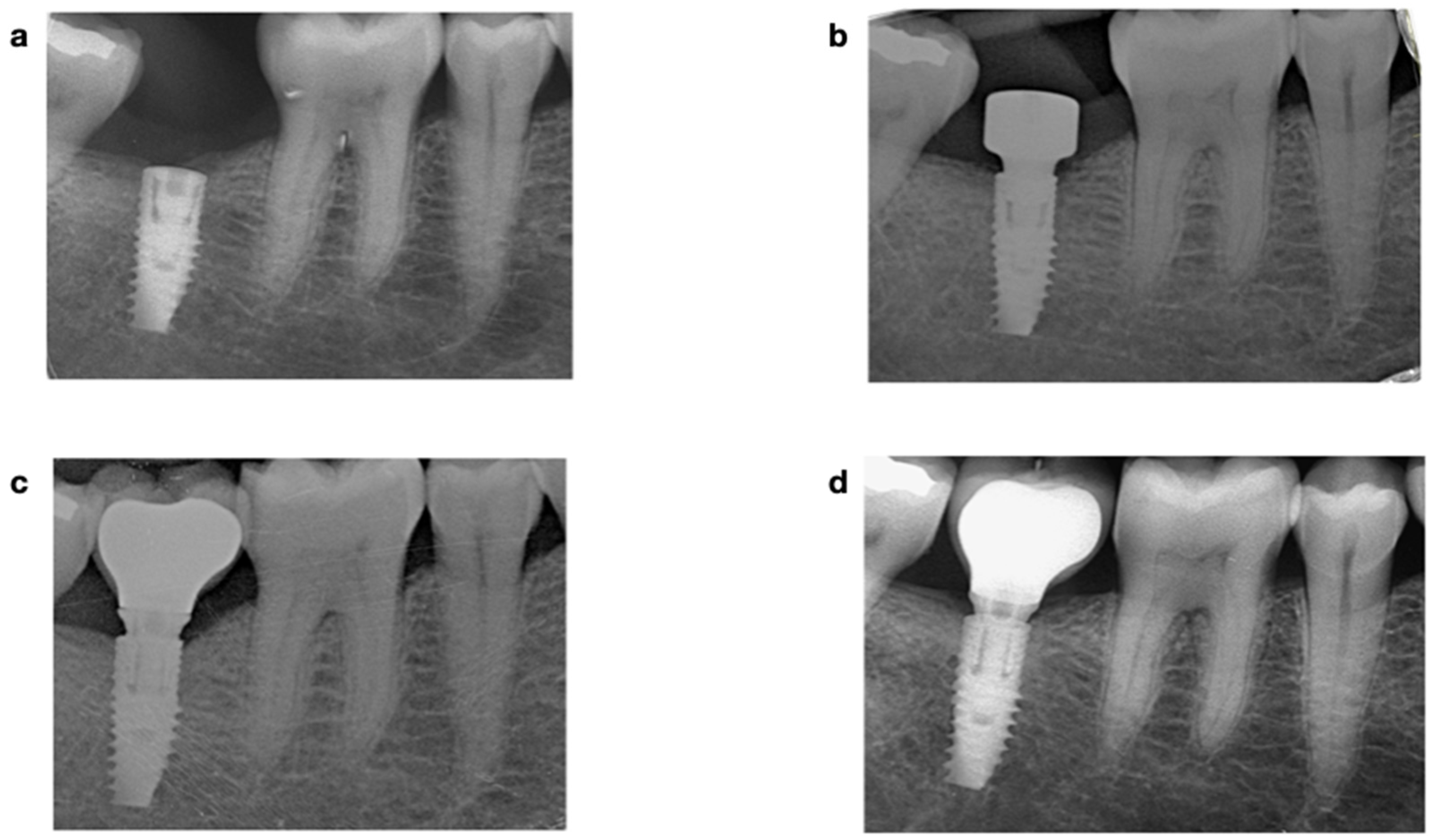
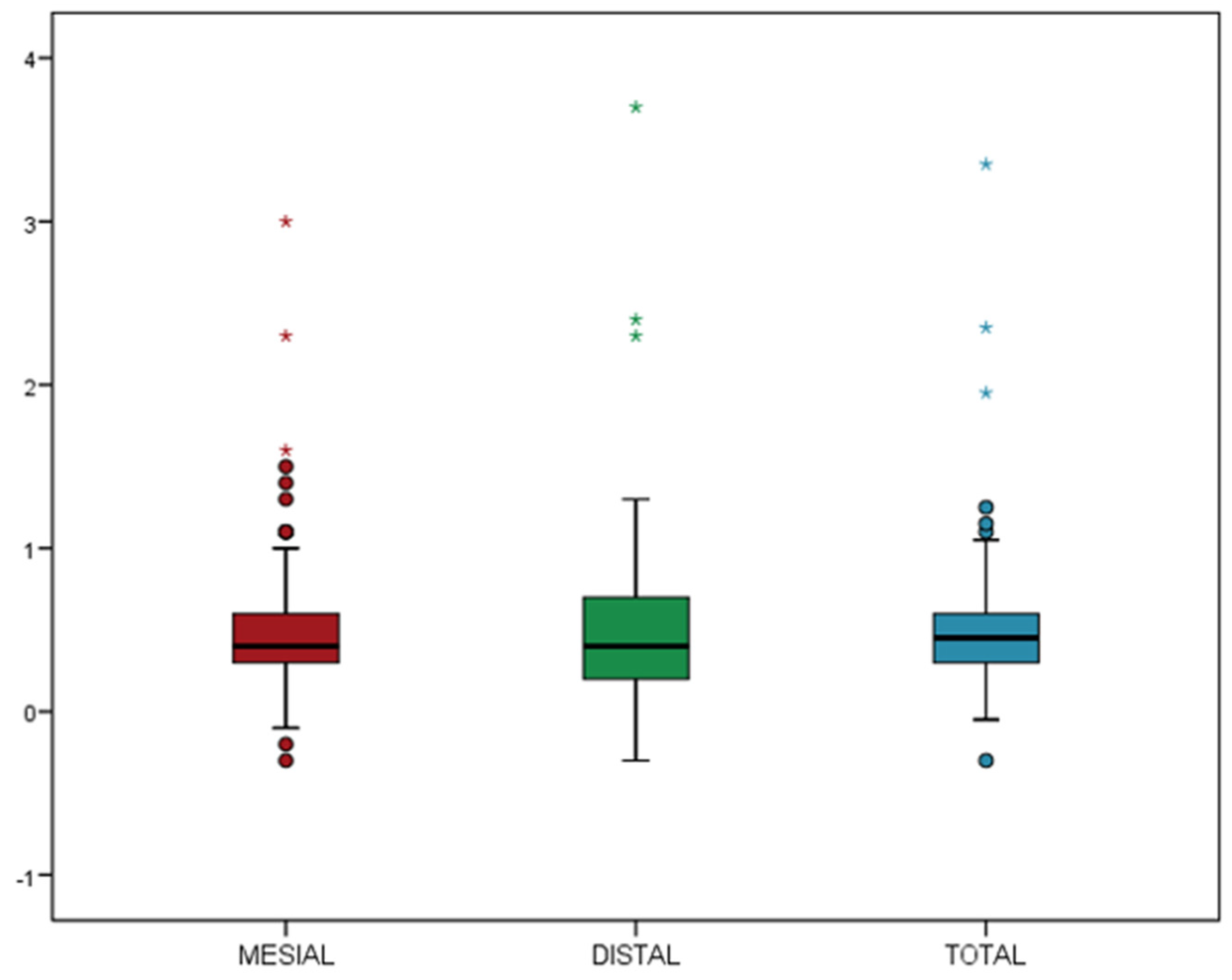
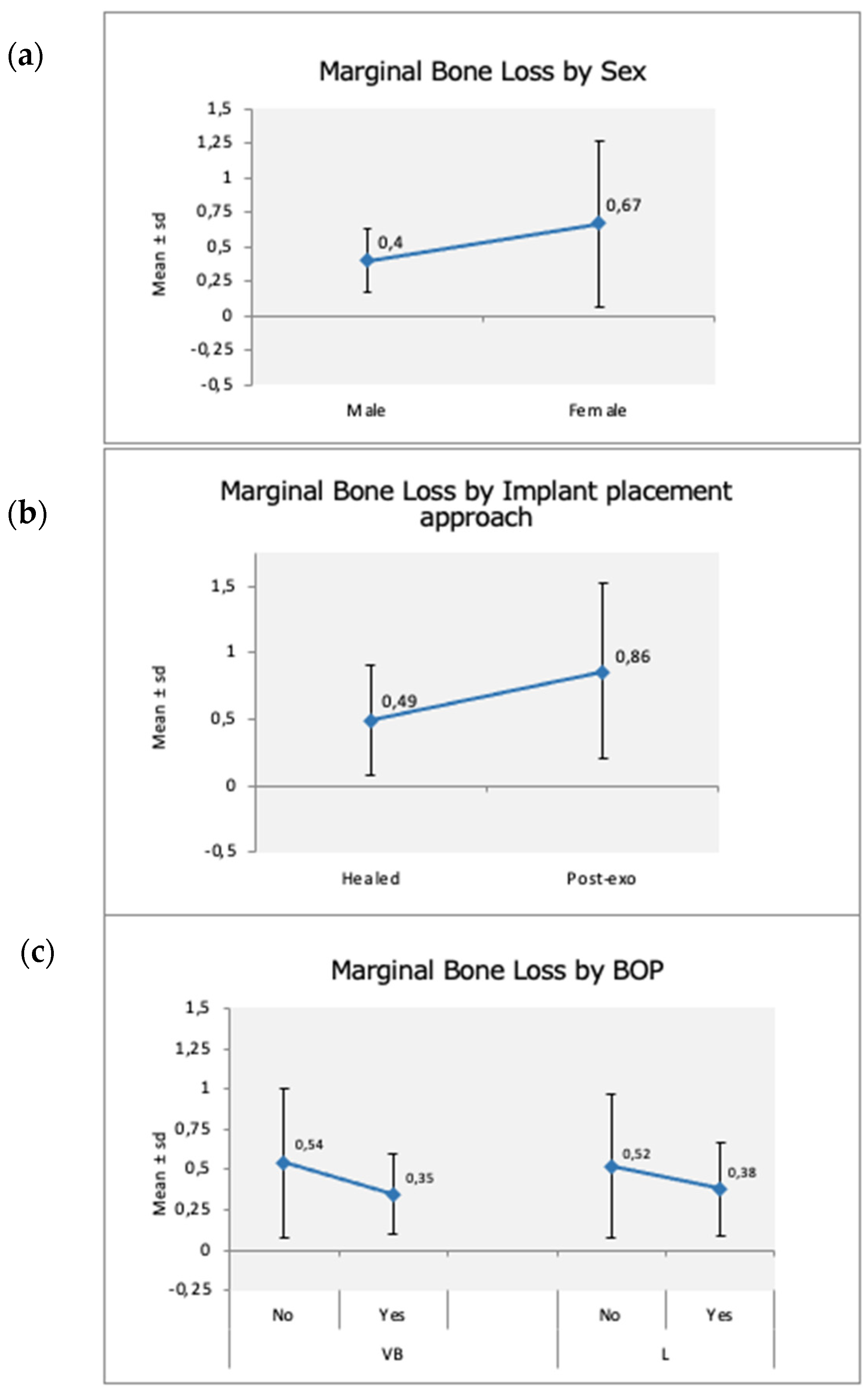
| Demographic Variables | Implant Variables | Surgical Variables | Prosthetic Variables |
|---|---|---|---|
| Age | Diameter | Corono-apical implant depth | Screw-retained |
| Gender | Length | Bone/sinus grafting | Cemented |
| Smoking | Local site | Healing protocol | Crown-implant ratio |
| Periodontal disease | Jaw | Insertion torque | |
| Controlled diabetes | Abutment height | ||
| Oral hygiene | Soft tissue thickness | ||
| Bone quality | Phenotype | ||
| Probing depth | |||
| Keratinized mucosa | |||
| Bleeding on probing |
| (a) | |||||
| Ø 3.3 mm | Ø 3.9 mm | Ø 4.3 mm | Ø 5 mm | p | |
| 29.50% | 28.80% | 26.60% | 29.00% | ||
| Mean | 40.0 ± 6.1 | 37.3 ± 12.2 | 36.40 ± 10.0 | 37.5 ± 10.1 | >0.05 |
| Median | 40 | 40 | 35 | 37.5 | |
| (b) | |||||
| 8 mm | 10 mm | 11.5 mm | 13 mm | ||
| Mean | 36.1 ± 11.8 | 40.5 ± 9.6 | 34.8 ± 11.1 | 35.0 ± 7.8 | |
| Median | 40 | 40 | 35 | 35 | |
| Implant Failure | Category * | OR | CI 95% | p-Value |
|---|---|---|---|---|
| Sex | Male (n = 25) | 1 | - | - |
| Female (n = 22) | 2.78 | 0.26–29.3 | 0.396 | |
| Smoking habits | No (n = 11) | 1 | - | - |
| Yes (n = 36) | 5.31 | 0.48–58.3 | 0.172 | |
| Controlled Diabetes | No (n = 40) | - | - | 1.000 |
| Yes (n = 7) | - | - | ||
| History of Periodontitis | No (n = 26) | - | - | 0.244 |
| Yes (n = 21) | - | - | ||
| Sector | Anterior (n = 41) | 1 | - | - |
| Posterior (n = 79) | 1.04 | 0.09–11.8 | 0.975 | |
| Jaw | Maxilla (n = 66) | 1 | - | - |
| Mandible (n = 54) | 2.50 | 0.21–29.7 | 0.468 | |
| Implant diameter (mm) | 0.91 | 0.58–1.41 | 0.659 | |
| Implant length (mm) | 0.94 | 0.69–1.29 | 0.691 | |
| Surgical protocol | 1 stage (n = 46) | - | - | 0.522 |
| 2 stages (n = 73) | - | - |
| MBL vs. Independent Variables | Category | Beta | CI 95% | p-Value |
|---|---|---|---|---|
| Sex | Male (0.40 ± 0.23) | 0 | ||
| Female (0.67 ± 0.60) | 0.27 | 0.04–0.48 | 0.020 * | |
| Smoking habits | No (0.48 ± 0.36) | 0 | ||
| Yes (0.60 ± 0.61) | 0.12 | −0.17–0.40 | 0.430 | |
| Controlled Diabetes | No (0.49 ± 0.43) | 0 | ||
| Yes (0.62 ± 0.53) | 0.12 | −0.18–0.42 | 0.424 | |
| History of Periodontitis | No (0.55 ± 0.56) | 0 | ||
| Yes (0.47 ± 0.26) | −0.08 | −0.27–0.12 | 0.428 | |
| Sector of the jaw | Anterior (0.57 ± 0.61) | 0 | ||
| Posterior (0.48 ± 0.33) | −0.09 | −0.28–0.09 | 0.332 | |
| Jaw | Maxilla (0.57 ± 0.55) | 0 | ||
| Mandible (0.44 ± 0.25) | −0.13 | −0.31–0.06 | 0.185 | |
| Implant diameter (mm) | (0.51 ± 0.44) | −0.05 | −0.14–0.04 | 0.294 |
| Implant length (mm) | (0.51 ± 0.44) | 0.03 | −0.01–0.06 | 0.151 |
| Surgical protocol | 1 stage (0.51 ± 0.40) | 0 | ||
| 2 stages (0.52 ± 0.47) | 0.01 | −0.17–0.18 | 0.960 | |
| Implant site | Healed ridge (delayed) (0.49 ± 0.42) | 0 | ||
| post-exo (immediate) (0.86 ± 0.66) | 0.37 | −0.07–0.81 | 0.095 | |
| Bone grafting | No (0.49 ± 0.46) | 0 | ||
| Yes (0.63 ± 0.35) | 0.13 | −0.08–0.35 | 0.212 | |
| Implant depth at placement | (0.51 ± 0.44) | −0.19 | −0.50–0.12 | 0.227 |
| Torque (Ncm) | (0.48 ± 0.35) | 0.00 | −0.01–0.01 | 0.232 |
| Parameters | Category * | Beta | CI 95% | p-Value |
|---|---|---|---|---|
| BoP (Buccal) | No (0.54 ± 0.46) | 0 | ||
| Yes (0.35 ± 0.25) | −0.19 | −0.36–−0.02 | 0.030 * | |
| BoP (lingual/palatal) | No (0.52 ± 0.45) | 0 | ||
| Yes (0.38 ± 0.29) | −0.14 | −0.38–0.10 | 0.250 | |
| PD total | (0.51 ± 0.44) | −0.03 | −0.15–0.10 | 0.682 |
| Plaque (Buccal) | No (0.51 ± 0.43) | 0 | ||
| Yes (0.5 ± 0.55) | 0.04 | −0.27–0.36 | 0.775 | |
| Plaque (lingual/palatal) | No (0.51 ± 0.43) | 0 | ||
| Yes (0.50 ± 0.55) | −0.01 | −0.31–0.29 | 0.948 | |
| KT | −0.03 | −0.12–0.05 | 0.399 | |
| KT groups | <2 mm (0.56 ± 0.44) | 0 | ||
| >2 mm (0.50 ± 0.45) | −0.06 | −0.27–0.15 | 0.594 | |
| Ti-base | No (0.47 ± 0.34) | 0 | ||
| Yes (0.54 ± 0.50) | 0.07 | −0.05–0.19 | 0.240 | |
| Multi-unit | No (0.54 ± 0.47) | 0 | ||
| Yes (0.46 ± 0.37) | −0.09 | −0.21–0.04 | 0.175 | |
| Soft tissue phenotype | Thin (0.51 ± 0.49) | 0 | ||
| Thick (0.55 ± 0.44) | 0.01 | −0.25–0.26 | 0.965 | |
| Gingival Thickness | (0.80 ± 1.04) | −0.04 | −0.25–0.16 | 0.689 |
| Bone Quality | (0.51 ± 0.44) | 0.16 | −0.05–0.37 | 0.130 |
| Papilla index (mesial) | (0.52 ± 0.46) | 0.908 | ||
| 0 (0.51 ± 0.38) | 0 | |||
| 1 (0.52 ± 0.51) | 0.01 | −0.36–0.37 | 0.973 | |
| 2 (0.51 ± 0.39) | −0.01 | −0.38–0.36 | 0.964 | |
| Papilla index (distal) | (0.52 ± 0.46) | 0.684 | ||
| 0 (0.54 ± 0.60) | 0 | |||
| 1 (0.52 ± 0.40) | −0.02 | −0.28–0.24 | 0.894 | |
| 2 (0.45 ± 0.28) | −0.08 | −0.37–0.20 | 0.572 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giralt-Hernando, M.; Ragucci, G.M.; Cantó-Naves, O.; Valls-Ontañón, A.; Hernández-Alfaro, F. Covariates Relating to Implant Failure and Marginal Bone Loss of a Novel Triangular Neck-Implant Placed by Post-Graduate Students: A 1-Year Prospective Cohort Study. Materials 2022, 15, 1987. https://doi.org/10.3390/ma15061987
Giralt-Hernando M, Ragucci GM, Cantó-Naves O, Valls-Ontañón A, Hernández-Alfaro F. Covariates Relating to Implant Failure and Marginal Bone Loss of a Novel Triangular Neck-Implant Placed by Post-Graduate Students: A 1-Year Prospective Cohort Study. Materials. 2022; 15(6):1987. https://doi.org/10.3390/ma15061987
Chicago/Turabian StyleGiralt-Hernando, Maria, Gian Maria Ragucci, Oriol Cantó-Naves, Adaia Valls-Ontañón, and Federico Hernández-Alfaro. 2022. "Covariates Relating to Implant Failure and Marginal Bone Loss of a Novel Triangular Neck-Implant Placed by Post-Graduate Students: A 1-Year Prospective Cohort Study" Materials 15, no. 6: 1987. https://doi.org/10.3390/ma15061987
APA StyleGiralt-Hernando, M., Ragucci, G. M., Cantó-Naves, O., Valls-Ontañón, A., & Hernández-Alfaro, F. (2022). Covariates Relating to Implant Failure and Marginal Bone Loss of a Novel Triangular Neck-Implant Placed by Post-Graduate Students: A 1-Year Prospective Cohort Study. Materials, 15(6), 1987. https://doi.org/10.3390/ma15061987







