Molybdenum Oxide Nanoparticle Aggregates Grown by Chemical Vapor Transport
Abstract
:1. Introduction
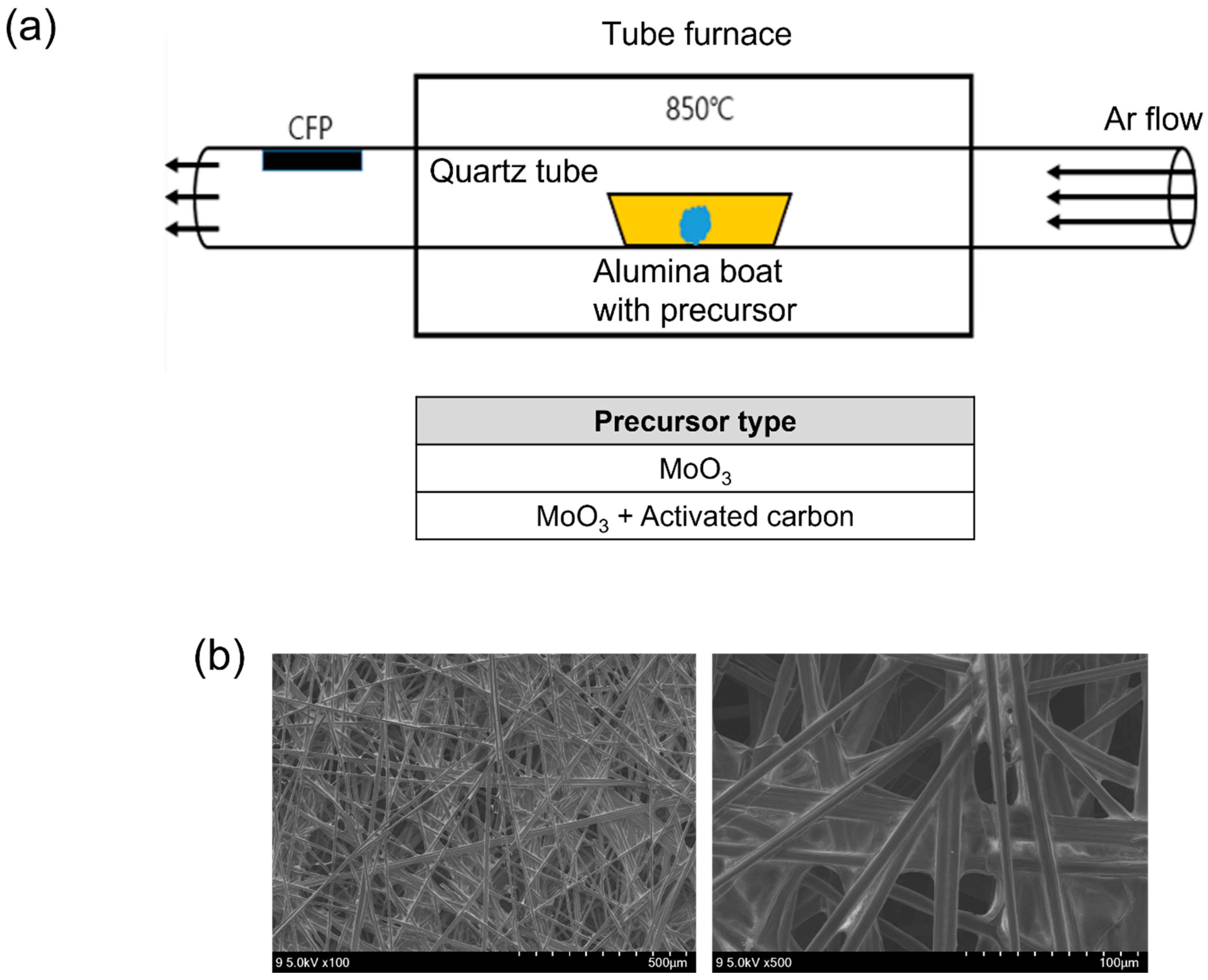
2. Experimental
2.1. Deposition of Molybdenum Oxide Nanoparticle Aggregates
2.2. Structural Characterization
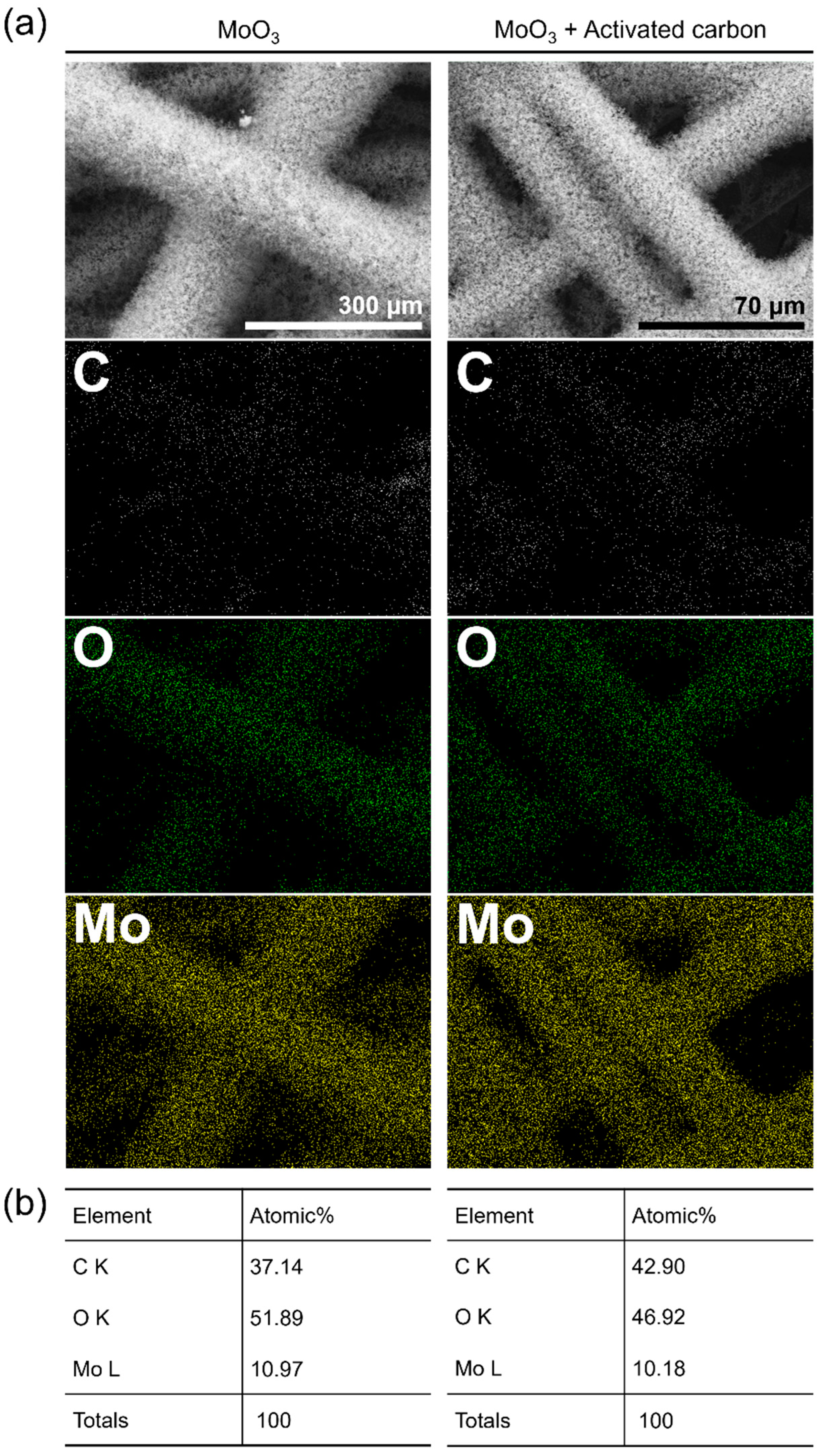
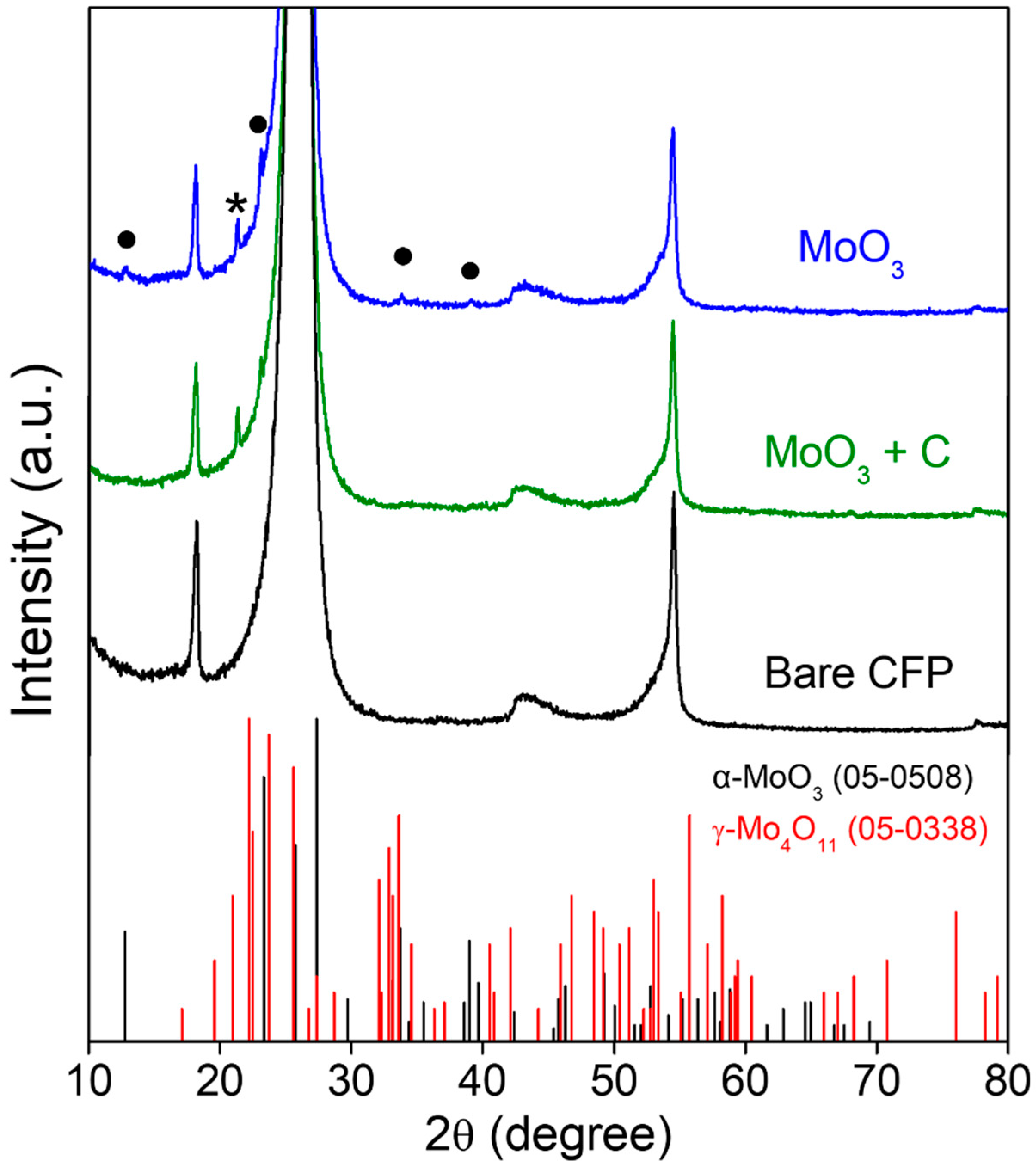
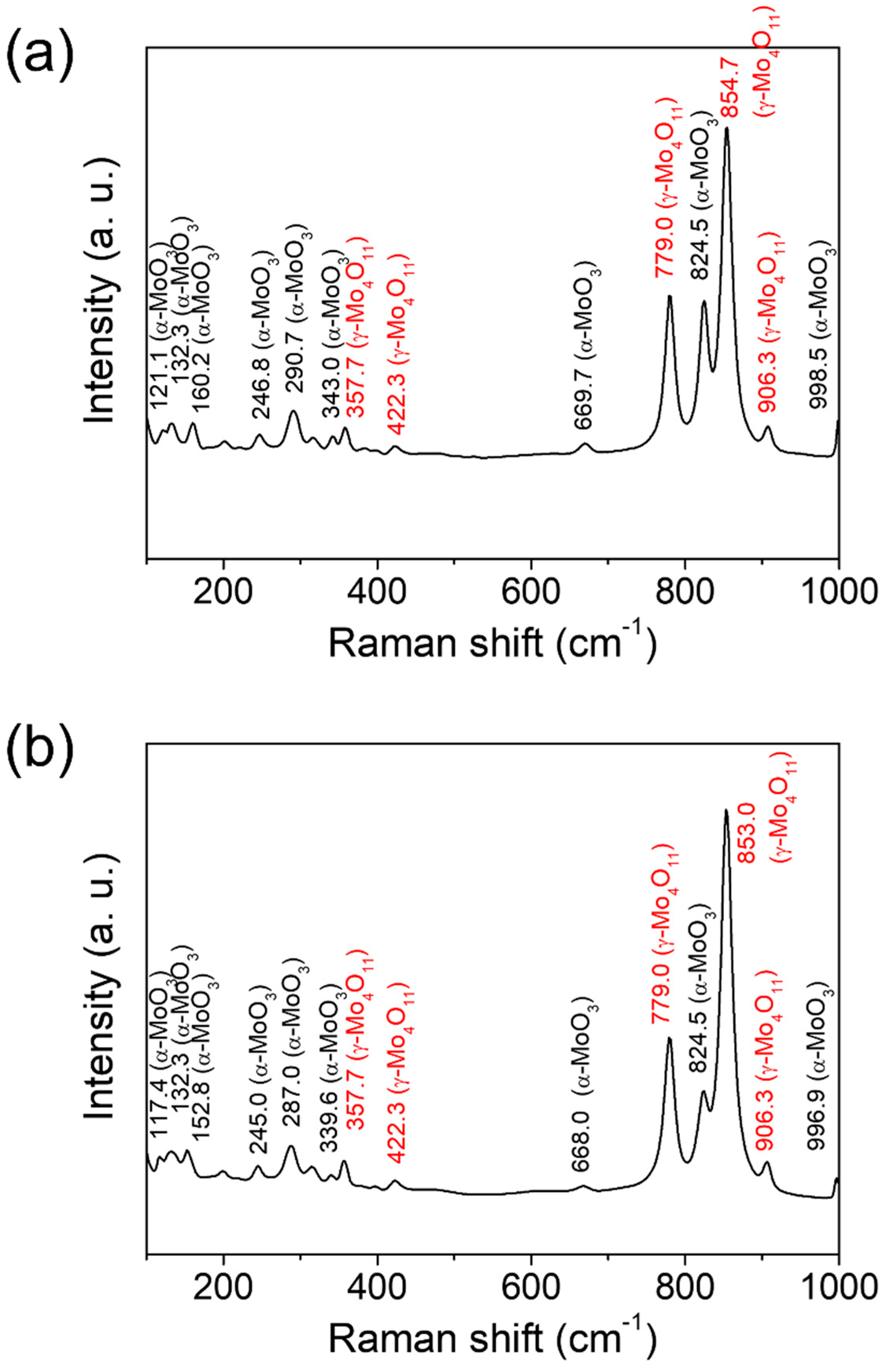
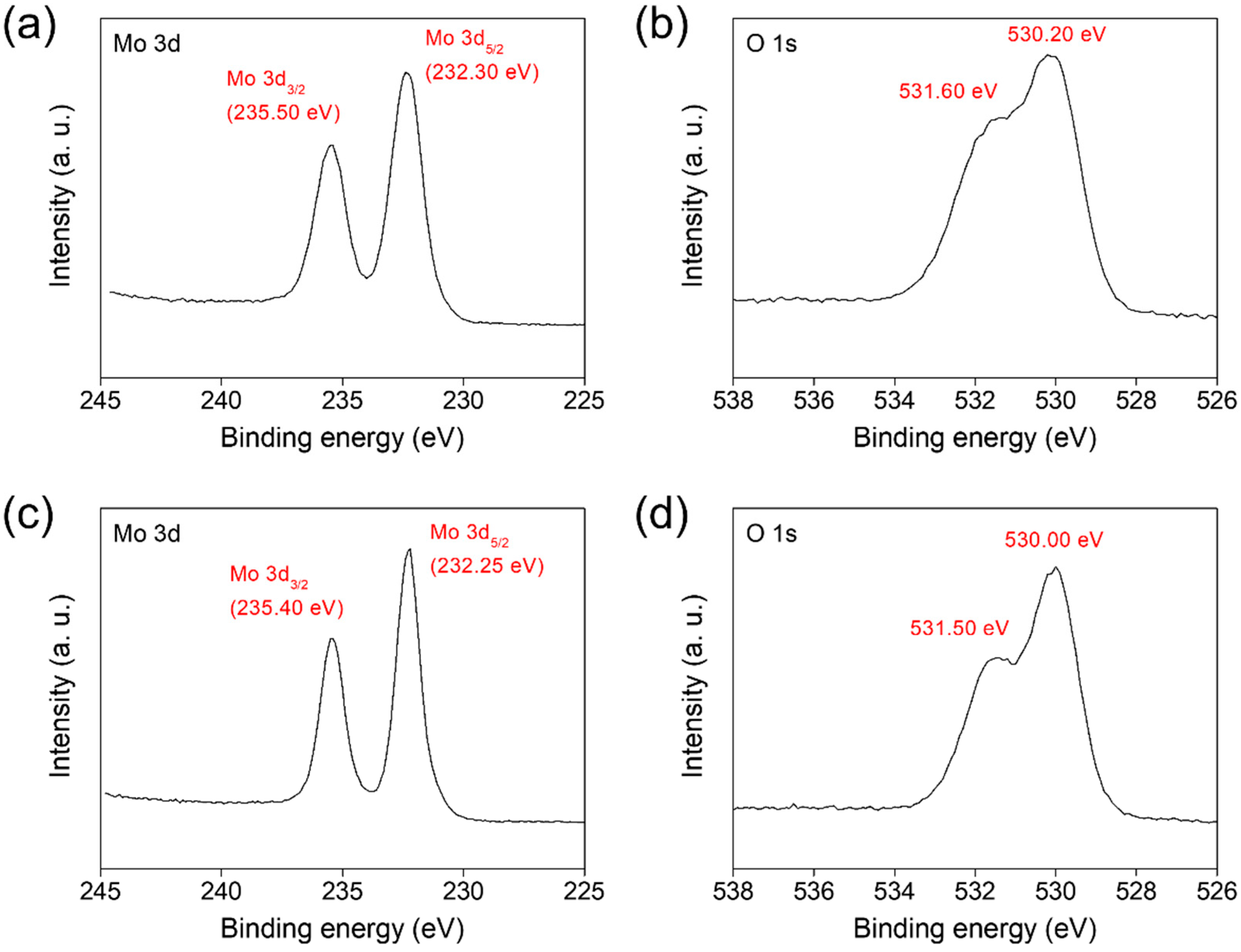
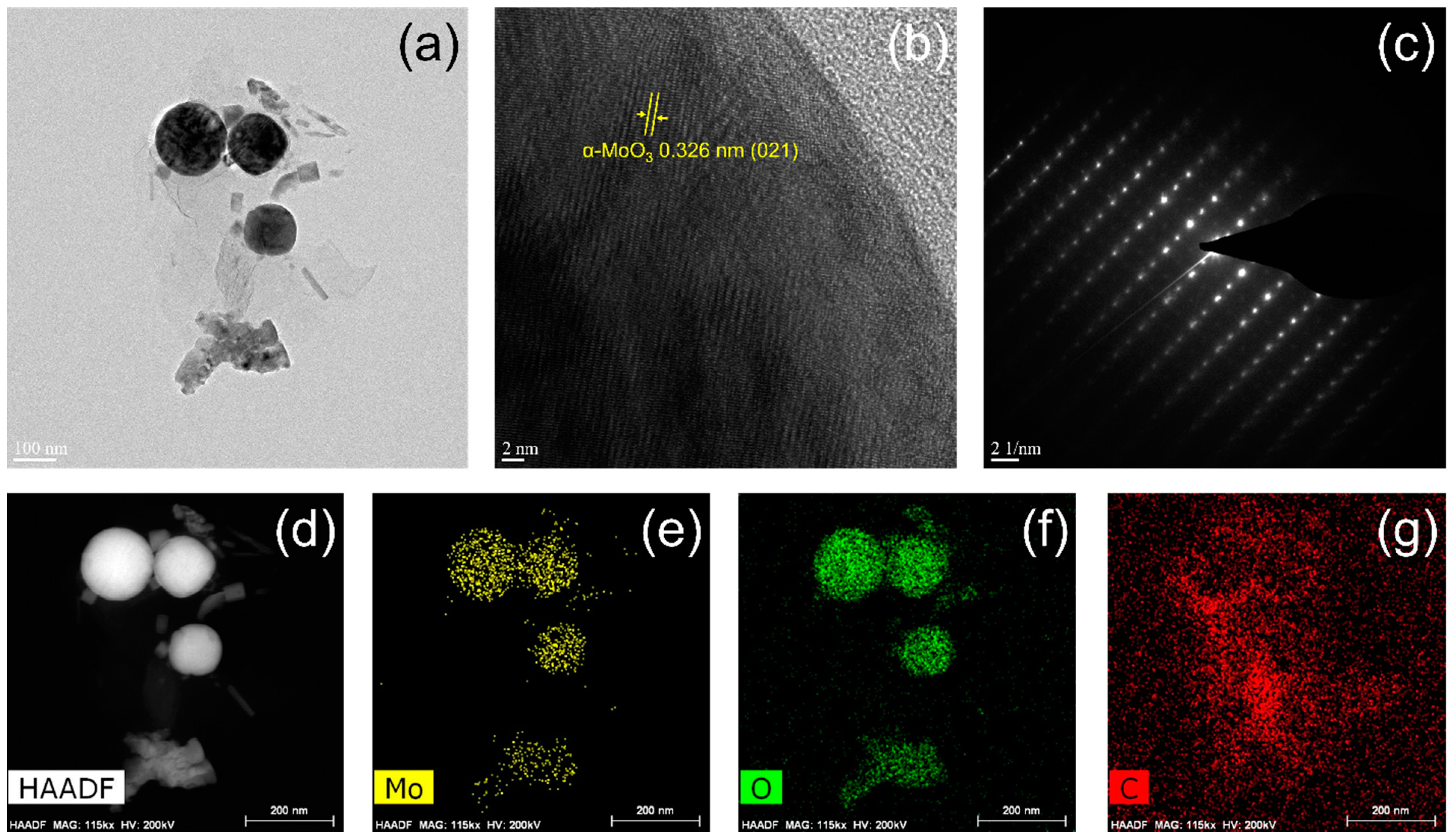
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novoselov, K.S.; Mishchenko, A.; Carvalho, A.; Neto, A.H.C. 2D materials and van der Waals heterostructures. Science 2016, 353, aac9439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mas-Ballesté, R.; Gómez-Navarro, C.; Gómez-Herrero, J.; Zamora, F. 2D materials: To graphene and beyond. Nanoscale 2011, 3, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sakthivel, T.; Seal, S. Recent development in 2D materials beyond graphene. Prog. Mater. Sci. 2015, 73, 44–126. [Google Scholar] [CrossRef]
- Miró, P.; Audiffred, M.; Heine, T. An atlas of two-dimensional materials. Chem. Soc. Rev. 2014, 43, 6537–6554. [Google Scholar] [CrossRef] [PubMed]
- Schaibley, J.R.; Yu, H.; Clark, G.; Rivera, P.; Ross, J.S.; Seyler, K.L.; Yao, W.; Xu, X. Valleytronics in 2D materials. Nat. Rev. Mater. 2016, 1, 16055. [Google Scholar] [CrossRef]
- Jin, H.; Guo, C.; Liu, X.; Liu, J.; Vasileff, A.; Jiao, Y.; Zheng, Y.; Qiao, S.-Z. Emerging two-dimensional nanomaterials for electrocatalysis. Chem. Rev. 2018, 118, 6337–6408. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Jiang, C.; Wei, S.-H. Gas sensing in 2D materials. Appl. Phys. Rev. 2017, 4, 021304. [Google Scholar] [CrossRef]
- Choi, Y.-H.; Lee, J.; Parija, A.; Cho, J.; Verkhoturov, S.V.; Al-Hashimi, M.; Fang, L.; Banerjee, S. An in Situ Sulfidation Approach for the Integration of MoS2 Nanosheets on Carbon Fiber Paper and the Modulation of Its Electrocatalytic Activity by Interfacing with nC60. ACS Catal. 2016, 6, 6246–6254. [Google Scholar] [CrossRef]
- Wang, X.; Feng, H.; Wu, Y.; Jiao, L. Controlled Synthesis of Highly Crystalline MoS2 Flakes by Chemical Vapor Deposition. J. Am. Chem. Soc. 2013, 135, 5304–5307. [Google Scholar] [CrossRef]
- Wang, S.; Rong, Y.; Fan, Y.; Pacios, M.; Bhaskaran, H.; He, K.; Warner, J.H. Shape Evolution of Monolayer MoS2 Crystals Grown by Chemical Vapor Deposition. Chem. Mater. 2014, 26, 6371–6379. [Google Scholar] [CrossRef]
- de Castro, I.A.; Datta, R.S.; Ou, J.Z.; Castellanos-Gomez, A.; Sriram, S.; Daeneke, T.; Kalantar-Zadeh, K. Molybdenum Oxides—From Fundamentals to Functionality. Adv. Mater. 2017, 29, 1701619. [Google Scholar] [CrossRef] [PubMed]
- Balendhran, S.; Deng, J.; Ou, J.Z.; Walia, S.; Scott, J.; Tang, J.; Wang, K.L.; Field, M.R.; Russo, S.; Zhuiykov, S.; et al. Enhanced charge carrier mobility in two-dimensional high dielectric molybdenum oxide. Adv. Mater. 2013, 25, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Mai, L.; Yang, F.; Zhao, Y.; Xu, X.; Xu, L.; Hu, B.; Luo, Y.; Liu, H. Molybdenum oxide nanowires: Synthesis & properties. Mater. Today 2011, 14, 346–353. [Google Scholar]
- Van Pham, D.; Patil, R.; Yang, C.-C.; Yeh, W.-C.; Liou, Y.; Ma, Y.-R. Impact of the crystal phase and 3d-valence conversion on the capacitive performance of one-dimensional MoO2, MoO3, and Magnéli-phase Mo4O11 nanorod-based pseudocapacitors. Nano Energy 2018, 47, 105–114. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, L.; Yuan, P.; Zou, J.; Wu, Y.; Yu, C. α-MoO3 nanobelts: A high performance cathode material for lithium ion batteries. J. Phys. Chem. C 2010, 114, 21868–21872. [Google Scholar] [CrossRef]
- Lalik, E.; David, W.I.F.; Barnes, P.; Turner, J.F.C. Mechanisms of reduction of MoO3 to MoO2 reconciled? J. Phys. Chem. B 2001, 105, 9153–9156. [Google Scholar] [CrossRef]
- Van Pham, D.; Patil, R.A.; Lin, J.-H.; Lai, C.-C.; Liou, Y.; Ma, Y.-R. Doping-free bandgap tuning in one-dimensional Magnéli-phase nanorods of Mo4O11. Nanoscale 2016, 8, 5559–5566. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.K.; Khalid, N. Surfactant-assisted synthesis of MoO3 nanorods and its application in photocatalytic degradation of different dyes in aqueous environment. J. Mol. Liq. 2021, 346, 117871. [Google Scholar] [CrossRef]
- Niu, Y.; Su, H.; Li, X.; Li, J.; Qi, Y. Synthesis of porous α-MoO3 microspheres as electrode materials for supercapacitors. J. Alloys Compd. 2022, 898, 162863. [Google Scholar] [CrossRef]
- Nagyné-Kovács, T.; Studnicka, L.; Lukács, I.E.; László, K.; Pasierb, P.; Szilágyi, I.M.; Pokol, G. Hydrothermal Synthesis and Gas Sensing of Monoclinic MoO3 Nanosheets. Nanomaterials 2020, 10, 891. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, I.; Ahmad, K.S. Modified sol gel synthesis of MoO3 NPs using organic template: Synthesis, characterization and electrochemical investigations. J. Sol-Gel Sci. Technol. 2021, 97, 178–190. [Google Scholar] [CrossRef]
- Patil, D.; Patil, V.; Patil, S.; Dongale, T.; Desai, N.; Patil, P.; Mane, R.; Bhosale, P.; Kadam, P.; Khot, K.V. Facile synthesis of MoO3 nanoplates based NO2 gas sensor: Ultra-selective and sensitive. Chem. Phys. Lett. 2021, 782, 139025. [Google Scholar] [CrossRef]
- Yang, G.; Feng, F.; Luo, Y.; Qin, J.; Yuan, F.; Wang, S.; Luo, S.; Ma, J. Facile synthesis of MoO3 nanodots self-assembled into hollow mesoporous silica: Enhancing efficient oxidative desulfurization and investigating reaction mechanism. J. Environ. Chem. Eng. 2021, 9, 106309. [Google Scholar] [CrossRef]
- Arya, N.; Avasthi, P.; Halder, A.; Balakrishnan, V. Nickel decorated MoO3 single crystal microflakes with multi-site functionality for enhanced hydrogen evolution reaction. Int. J. Hydrog. Energy 2021, 46, 1945–1954. [Google Scholar] [CrossRef]
- Guo, Y.; Kang, L.; Song, P.; Zeng, Q.; Tang, B.; Yang, J.; Wu, Y.; Tian, D.; Xu, M.; Zhao, W.; et al. MoO3–MoS2 vertical heterostructures synthesized via one-step CVD process for optoelectronics. 2D Mater. 2021, 8, 035036. [Google Scholar] [CrossRef]
- Chot, C.Y.; Chong, M.N.; Soh, A.K.; Tan, K.W.; Ocon, J.D.; Saint, C. Facile synthesis and characterisation of functional MoO3 photoanode with self-photorechargeability. J. Alloys Compd. 2020, 838, 155624. [Google Scholar] [CrossRef]
- Choi, M.; Kang, S.K.; Kim, S.; Kim, S.J.; An, G.H.; Shin, S.J.; Kim, D.; Rahman, I.N.; Bang, J.; Kim, K.; et al. Mo nitrides and carbonitrides via metallic phase transition of MoO3 films using ammonium salt precursors in chemical vapor deposition. Curr. Appl. Phys. 2021, 34, 1–6. [Google Scholar] [CrossRef]
- Cuando-Espitia, N.; Redenius, J.; Mensink, K.; Camacho-López, M.; Camacho-López, S.; Aguilar, G. Influence of oxygen pressure on the fs laser-induced oxidation of molybdenum thin films. Opt. Mater. Express 2018, 8, 581–596. [Google Scholar] [CrossRef]
- Windom, B.C.; Sawyer, W.G.; Hahn, D. A Raman Spectroscopic Study of MoS2 and MoO3: Applications to Tribological Systems. Tribol. Lett. 2011, 42, 301–310. [Google Scholar] [CrossRef]
- Mihalev, M.; Hardalov, C.; Christov, C.; Michailov, M.; Ranguelov, B.; Leiste, H. Structural and adhesional properties of thin MoO3 films prepared by laser coating. J. Physics Conf. Ser. 2014, 514, 0120222. [Google Scholar] [CrossRef] [Green Version]
- Morales-Luna, M.; Tomás, S.A.; Arvizu, M.; Pérez-González, M.; Campos-Gonzalez, E. The evolution of the Mo5+ oxidation state in the thermochromic effect of MoO3 thin films deposited by rf magnetron sputtering. J. Alloys Compd. 2017, 722, 938–945. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology (NIST). NIST X-ray Photoelectron Spectroscopy Database. Available online: http://srdata.nist.gov/xps (accessed on 1 February 2022).



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-H. Molybdenum Oxide Nanoparticle Aggregates Grown by Chemical Vapor Transport. Materials 2022, 15, 2182. https://doi.org/10.3390/ma15062182
Choi Y-H. Molybdenum Oxide Nanoparticle Aggregates Grown by Chemical Vapor Transport. Materials. 2022; 15(6):2182. https://doi.org/10.3390/ma15062182
Chicago/Turabian StyleChoi, Yun-Hyuk. 2022. "Molybdenum Oxide Nanoparticle Aggregates Grown by Chemical Vapor Transport" Materials 15, no. 6: 2182. https://doi.org/10.3390/ma15062182
APA StyleChoi, Y.-H. (2022). Molybdenum Oxide Nanoparticle Aggregates Grown by Chemical Vapor Transport. Materials, 15(6), 2182. https://doi.org/10.3390/ma15062182





