The Adjunctive Use of Leucocyte- and Platelet-Rich Fibrin in Periodontal Endosseous and Furcation Defects: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
- ✓ “Is the addition of PRF to other surgical techniques beneficial?”;
- ✓ “Is the addition of PRF to other regenerative techniques, such as osseous grafts, GTR or EMD, beneficial?”.
2. Materials and Methods
2.1. Protocol
2.2. Eligibility Criteria
2.2.1. Types of Participants
2.2.2. Types of Interventions
2.2.3. Type of Comparison
2.2.4. Type of Outcome Measures
2.2.5. Types of Studies
2.3. Search Strategy
- PubMed (searched 16 June 2021) (Listing S1);
- Scopus (searched 16 June 2021) (Listing S2);
- Cochrane Library (searched 16 June 2021) (Listing S3);
- Lilacs (searched 16 June 2021) (Listing S4);
- Grey Literature Report (searched 16 June 2021) (Listing S5).
2.4. Selection Process
2.5. Data Synthesis
2.6. Risk of Bias Assessment
2.7. Data Analysis
3. Results
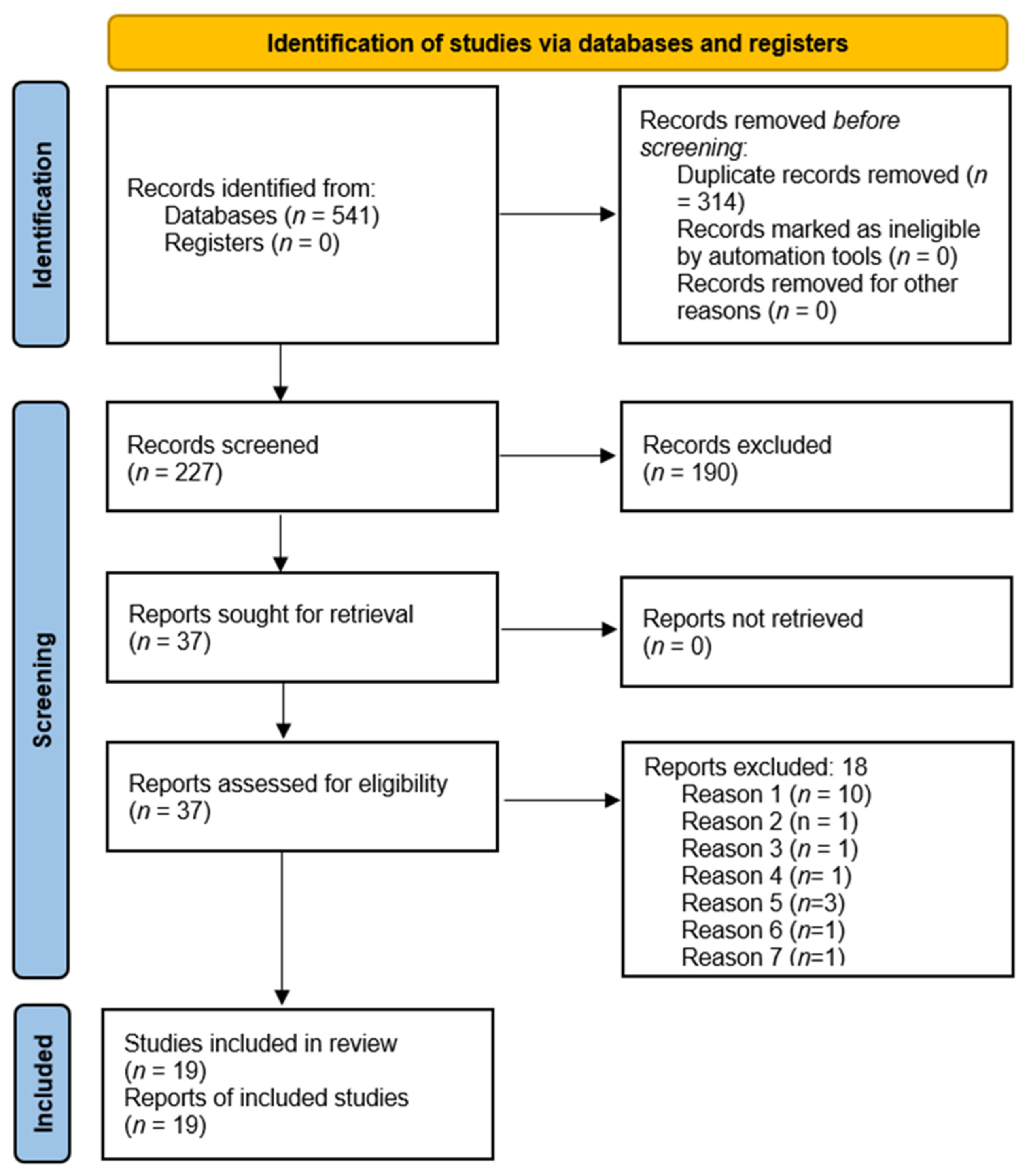
3.1. Study Selection
3.2. Study Characteristics
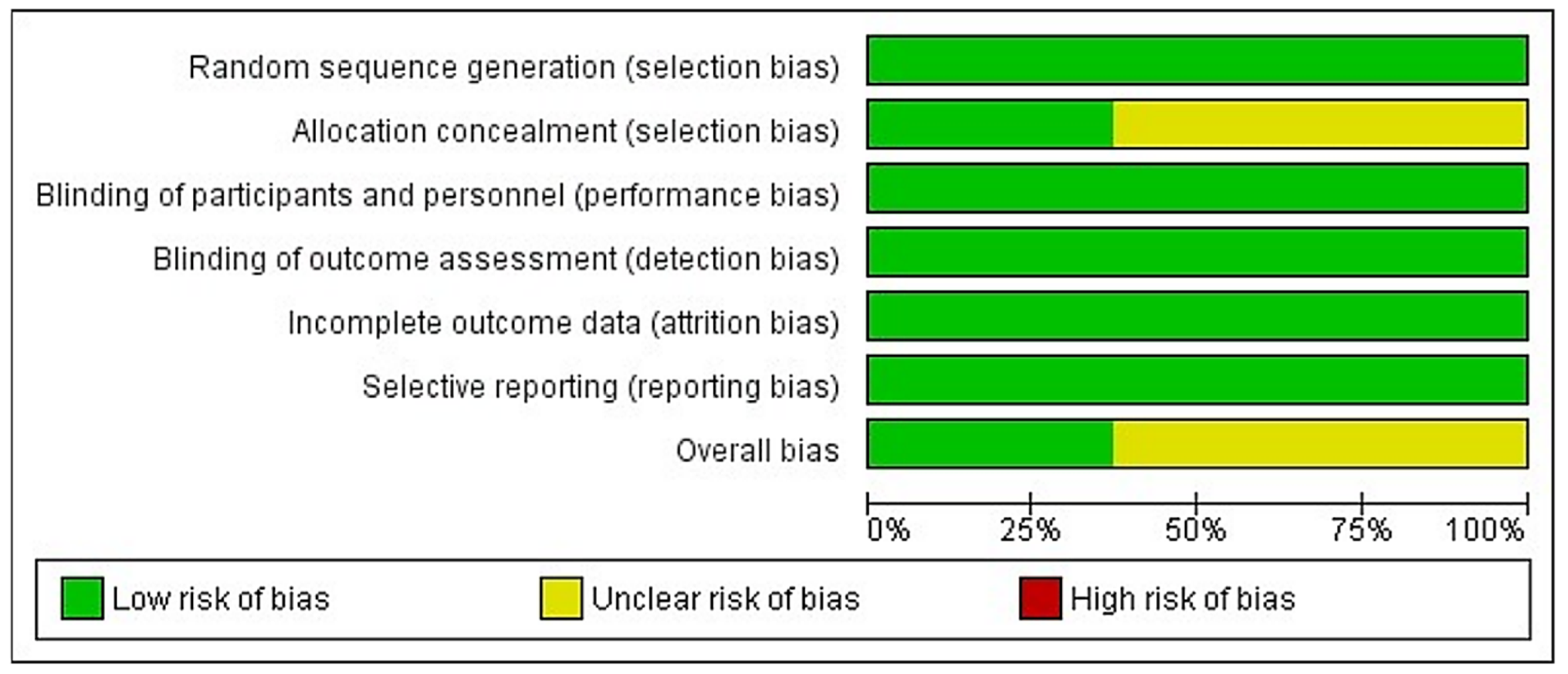
3.3. Risk of Bias in Individual Studies
3.4. Synthesis of Results
- For endosseous defects:
- °
- (L-PRF + OFD) vs. OFD alone;
- °
- (L-PRF + OG) vs. OG alone;
- °
- (L-PRF + EMD) vs. EMD alone;
- °
- (L-PRF + GTR) vs. GTR alone.
- For furcation defects:
- °
- ° (L-PRF + OFD) vs. OFD alone;
- °
- ° (L-PRF + OG) vs. OG alone.
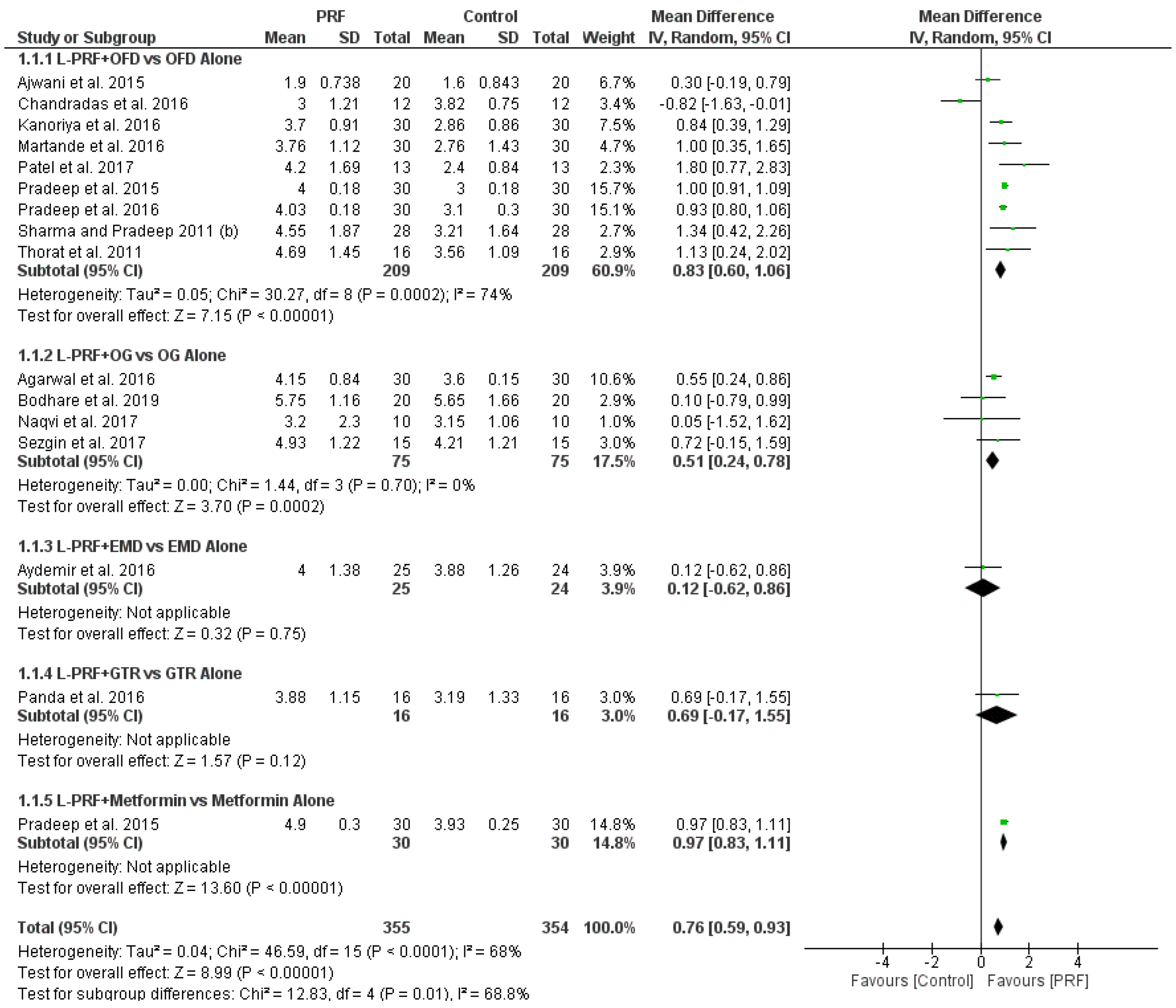
3.4.1. (L-PRF + OFD) vs. OFD Alone in Endosseous Defects
3.4.2. (L-PRF + OG) vs. OG Alone in Endosseous Defects
3.4.3. (L-PRF + EMD) vs. EMD Alone in Endosseous Defects
3.4.4. (L-PRF + GTR) vs. GTR Alone in Endosseous Defects
3.4.5. (L-PRF + Metformin) vs. Metformin Alone in Endosseous Defects
3.4.6. (L-PRF + OFD) vs. OFD Alone in Furcation Defects
3.4.7. (L-PRF + OG) vs. OG Alone in Furcation Defects
4. Discussion
4.1. Meta-Analysis
4.1.1. (L-PRF + OFD) vs. OFD Alone in Endosseous Defects
4.1.2. (L-PRF + OG) vs. OG Alone in Endosseous Defects
4.2. Additional RCTs Evaluating L-PRF in Endosseous Defects
4.2.1. (L-PRF + EMD) vs. EMD Alone in Endosseous Defects
4.2.2. (L-PRF + GTR) vs. GTR Alone in Endosseous Defects
4.2.3. (L-PRF + Metformin) vs. Metformin Alone in Endosseous Defects
4.3. Furcation Defects
4.3.1. (L-PRF+ OFD) vs. OFD Alone in Furcation Defects
4.3.2. (L-PRF+ OG) vs. OG Alone in Furcation Defects
4.4. Secondary Outcomes
4.4.1. Gingival Margin Level (GML) Change
4.4.2. Percentage Defect Fill (%DF)
4.4.3. Uneventful Wound Healing
4.5. Future Research Directions
5. Conclusions
- For two- and/or three-wall endosseous defects and for class II furcation defects of systemically healthy non-smoking periodontitis patients, using L-PRF following OFD is a treatment option;
- The adjunctive use of L-PRF to OFD in two- and/or three-wall endosseous defects of systemically healthy non-smoking periodontitis patients is significantly beneficial for PPD reduction, CAL gain and DD reduction, as compared with OFD alone;
- The adjunctive use of L-PRF to OG in two- and/or three-wall endosseous defects of systemically healthy non-smoking chronic periodontitis patients is significantly beneficial for PPD reduction, CAL gain and DD reduction, as compared with OG alone.
- It seems that the addition of L-PRF to OFD in endosseous defects is more justified for the radiographic improvement expected to be achieved, than for the clinical one;
- For endosseous defects, the adjunctive use of L-PRF to GTR and EMD, as compared without L-PRF, has not been sufficiently documented;
- For endosseous defects, the adjunctive use of L-PRF to small biomolecules, such as metformin, has not been sufficiently documented;
- The adjunctive use of L-PRF to OFD in class II furcation defects of systemically healthy non-smoking periodontitis patients, seems to be significantly beneficial for PPD reduction, horizontal and vertical CAL gain and DD reduction, as compared with OFD alone;
- The adjunctive use of L-PRF to OG in class II furcation defects of systemically healthy non-smoking periodontitis patients, seems to be significantly beneficial for PPD reduction and CAL gain, as compared with OG alone;
- For furcation defects, the adjunctive use of L-PRF to GTR and EMD, as compared without L-PRF, has not been documented at all;
- For endosseous defects, further research is required on the adjunctive use of L-PRF to GTR and EMD, as compared without L-PRF;
- For furcation defects, further research is required on the adjunctive use of L-PRF to conventional regenerative techniques, as compared without L-PRF.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, H.-L.; Greenwell, H.; Fiorellini, J.; Giannobile, W.; Offenbacher, S.; Salkin, L.; Townsend, C.; Sheridan, P.; Genco, R.J.; Research, S.; et al. Periodontal Regeneration. J. Periodontol. 2005, 76, 1601–1622. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Nikolidakis, D.; Nikou, G.; Ivanovic, A.; Chapple, I.L.C.; Stavropoulos, A. Biomaterials for Promoting Periodontal Regeneration in Human Intrabony Defects: A Systematic Review. Periodontology 2015, 68, 182–216. [Google Scholar] [CrossRef] [PubMed]
- Nibali, L.; Koidou, V.P.; Nieri, M.; Barbato, L.; Pagliaro, U.; Cairo, F. Regenerative Surgery versus Access Flap for the Treatment of Intra-Bony Periodontal Defects: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2020, 47 (Suppl. 2), 320–351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onicas, M.I.; Narita, L.E.; Mester, A.; Onisor, F.; Mancini, L. Platelet-Rich Fibrin: A Viable Therapy for Endodontic-Periodontal Lesions? A Preliminary Assessment. Appl. Sci. 2021, 11, 7081. [Google Scholar] [CrossRef]
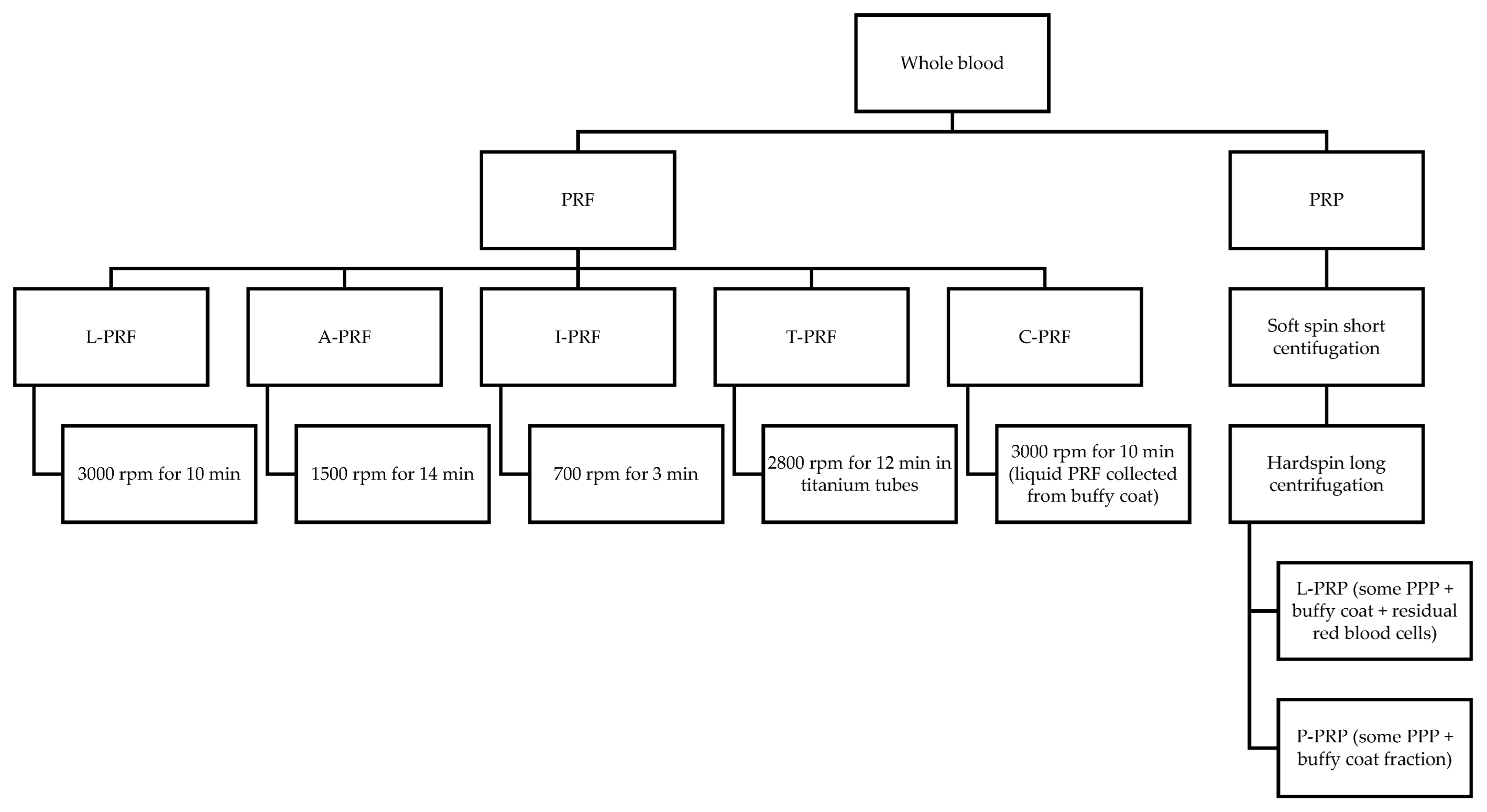
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of Platelet Concentrates: From Pure Platelet-Rich Plasma (P-PRP) to Leucocyte- and Platelet-Rich Fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Les, C.; Sader, R.; Kirkpatrick, C.J.; Choukroun, J. Advanced Platelet-Rich Fibrin: A New Concept for Cell-Based Tissue Engineering by Means of Inflammatory Cells. J. Oral Implantol. 2014, 40, 679–689. [Google Scholar] [CrossRef]
- Tunali, M.; Özdemir, H.; Küçükodaci, Z.; Akman, S.; Yaprak, E.; Toker, H.; Firatli, E. A Novel Platelet Concentrate: Titanium-Prepared Platelet-Rich Fibrin. BioMed Res. Int. 2014, 2014, 209548. [Google Scholar] [CrossRef]
- Miron, R.J.; Fujioka-Kobayashi, M.; Hernandez, M.; Kandalam, U.; Zhang, Y.; Ghanaati, S.; Choukroun, J. Injectable Platelet Rich Fibrin (i-PRF): Opportunities in Regenerative Dentistry? Clin. Oral Investig. 2017, 21, 2619–2627. [Google Scholar] [CrossRef]
- Miron, R.J.; Chai, J.; Zhang, P.; Li, Y.; Wang, Y.; de Almeida Barros Mourão, C.F.; Sculean, A.; Fujioka Kobayashi, M.; Zhang, Y. A Novel Method for Harvesting Concentrated Platelet-Rich Fibrin (C-PRF) with a 10-Fold Increase in Platelet and Leukocyte Yields. Clin. Oral Investig. 2020, 24, 2819–2828. [Google Scholar] [CrossRef]
- Choukroun, J.; Abba, F.; Schoeffler, C.; Vervelle, A. Une Opportunite En Paro-Implantologie: Le PRF. Implantodontie 2001, 42, 55–62. [Google Scholar]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part III: Leucocyte Activation: A New Feature for Platelet Concentrates? Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e51–e55. [Google Scholar] [CrossRef] [PubMed]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Gogly, B. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part II: Platelet-Related Biologic Features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, e45–e50. [Google Scholar] [CrossRef] [PubMed]
- Khorshidi, H.; Raoofi, S.; Bagheri, R.; Banihashemi, H. Comparison of the Mechanical Properties of Early Leukocyte- and Platelet-Rich Fibrin versus PRGF/Endoret Membranes. Int. J. Dent. 2016, 2016, 1849207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dohan Ehrenfest, D.M.; Pinto, N.R.; Pereda, A.; Jiménez, P.; del Corso, M.; Kang, B.S.; Nally, M.; Lanata, N.; Wang, H.L.; Quirynen, M. The Impact of the Centrifuge Characteristics and Centrifugation Protocols on the Cells, Growth Factors, and Fibrin Architecture of a Leukocyte- and Platelet-Rich Fibrin (L-PRF) Clot and Membrane. Platelets 2018, 29, 171–184. [Google Scholar] [CrossRef]
- Kobayashi, E.; Flückiger, L.; Fujioka-Kobayashi, M.; Sawada, K.; Sculean, A.; Schaller, B.; Miron, R.J. Comparative Release of Growth Factors from PRP, PRF, and Advanced-PRF. Clin. Oral Investig. 2016, 20, 2353–2360. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; de Peppo, G.M.; Doglioli, P.; Sammartino, G. Slow Release of Growth Factors and Thrombospondin-1 in Choukroun’s Platelet-Rich Fibrin (PRF): A Gold Standard to Achieve for All Surgical Platelet Concentrates Technologies. Growth Factors 2009, 27, 63–69. [Google Scholar] [CrossRef]
- Bhattacharya, H.; Gummaluri, S.; Astekar, M.; Gummaluri, R. Novel Method of Determining the Periodontal Regenerative Capacity of T-PRF and L-PRF: An Immunohistochemical Study. Dent. Med. Probl. 2020, 57, 137–144. [Google Scholar] [CrossRef]
- Zhang, J.; Yin, C.; Zhao, Q.; Zhao, Z.; Wang, J.; Miron, R.J.; Zhang, Y. Anti-inflammation Effects of Injectable Platelet-rich Fibrin via Macrophages and Dendritic Cells. J. Biomed. Mater. Res. Part A 2020, 108, 61–68. [Google Scholar] [CrossRef]
- Wang, Z.; Mudalal, M.; Sun, Y.; Liu, Y.; Wang, J.; Wang, Y.; Sun, X.; Zhou, Y. The Effects of Leukocyte-Platelet Rich Fibrin (L-PRF) on Suppression of the Expressions of the Pro-Inflammatory Cytokines, and Proliferation of Schwann Cell, and Neurotrophic Factors. Sci. Rep. 2020, 10, 2421. [Google Scholar] [CrossRef]
- Fujioka-Kobayashi, M.; Katagiri, H.; Kono, M.; Schaller, B.; Zhang, Y.; Sculean, A.; Miron, R.J. Improved Growth Factor Delivery and Cellular Activity Using Concentrated Platelet-Rich Fibrin (C-PRF) When Compared with Traditional Injectable (i-PRF) Protocols. Clin. Oral Investig. 2020, 24, 4373–4383. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Starting a review. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 8–11. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Considering bias and conflicts of interest among the included studies. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley: Hoboken, NJ, USA, 2019; pp. 177–199. [Google Scholar] [CrossRef]
- Lekovic, V.; Milinkovic, I.; Aleksic, Z.; Jankovic, S.; Stankovic, P.; Kenney, E.B.; Camargo, P.M. Platelet-Rich Fibrin and Bovine Porous Bone Mineral vs. Platelet-Rich Fibrin in the Treatment of Intrabony Periodontal Defects. J. Periodontal Res. 2012, 47, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.R.; Rao, N.S.; Agarwal, E.; Bajaj, P.; Kumari, M.; Naik, S.B. Comparative Evaluation of Autologous Platelet-Rich Fibrin and Platelet-Rich Plasma in the Treatment of 3-Wall Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2012, 83, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, P.; Pradeep, A.R.; Agarwal, E.; Rao, N.S.; Naik, S.B.; Priyanka, N.; Kalra, N. Comparative Evaluation of Autologous Platelet-Rich Fibrin and Platelet-Rich Plasma in the Treatment of Mandibular Degree II Furcation Defects: A Randomized Controlled Clinical Trial. J. Periodontal Res. 2013, 48, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Elgendy, E.A.; Shady, T.E.A. Clinical and Radiographic Evaluation of Nanocrystalline Hydroxyapatite with or without Platelet-Rich Fibrin Membrane in the Treatment of Periodontal Intrabony Defects. J. Indian Soc. Periodontol. 2015, 19, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Patel, J.; Dave, D.; Shah, S. Comparative Evaluation of Platelet-Rich Fibrin with Demineralized Freeze-Dried Bone Allograft in Periodontal Infrabony Defects: A Randomized Controlled Clinical Study. J. Indian Soc. Periodontol. 2015, 19, 56–60. [Google Scholar] [CrossRef]
- Siddiqui, Z.R.; Jhingran, R.; Bains, V.K.; Srivastava, R.; Madan, R.; Rizvi, I. Comparative Evaluation of Platelet-Rich Fibrin versus Beta-Tri-Calcium Phosphate in the Treatment of Grade II Mandibular Furcation Defects Using Cone-Beam Computed Tomography. Eur. J. Dent. 2016, 10, 496–506. [Google Scholar] [CrossRef] [Green Version]
- Qiao, J.; Duan, J.; Zhang, Y.; Chu, Y.; Sun, C. The Effect of Concentrated Growth Factors in the Treatment of Periodontal Intrabony Defects. Future Sci. OA 2016, 2, 4. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, I.; Chandran, S.; Nadig, P. Comparative Evaluation of the Efficacy of Platelet-Rich Fibrin and Calcium Phosphosilicate Putty Alone and in Combination in the Treatment of Intrabony Defects: A Randomized Clinical and Radiographic Study. Contemp. Clin. Dent. 2017, 8, 205. [Google Scholar] [CrossRef]
- Bajaj, P.; Agarwal, E.; Rao, N.S.; Naik, S.B.; Pradeep, A.R.; Kalra, N.; Priyanka, N.; Kumari, M. Autologous Platelet-Rich Fibrin in the Treatment of 3-Wall Intrabony Defects in Aggressive Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1186–1191. [Google Scholar] [CrossRef]
- Chatterjee, A.; Pradeep, A.R.; Garg, V.; Yajamanya, S.; Ali, M.M.; Priya, V.S. Treatment of Periodontal Intrabony Defects Using Autologous Platelet-Rich Fibrin and Titanium Platelet-Rich Fibrin: A Randomized, Clinical, Comparative Study. J. Investig. Clin. Dent. 2017, 8, e12231. [Google Scholar] [CrossRef] [PubMed]
- Lohi, H.S.; Nayak, D.G.; Uppoor, A.S. Comparative Evaluation of the Efficacy of Bioactive Ceramic Composite Granules Alone and in Combination with Platelet Rich Fibrin in the Treatment of Mandibular Class II Furcation Defects: A Clinical and Radiographic Study. J. Clin. Diagn. Res. JCDR 2017, 11, ZC76–ZC80. [Google Scholar] [CrossRef] [PubMed]
- Pradeep, A.R.; Bajaj, P.; Rao, N.S.; Agarwal, E.; Naik, S.B. Platelet-Rich Fibrin Combined with a Porous Hydroxyapatite Graft for the Treatment of 3-Wall Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 1288–1296. [Google Scholar] [CrossRef] [PubMed]
- Betancourt, P.; Elgueta, R.; Fuentes, R. Treatment of Endo-Periodontal Lesion Using Leukocyte-Platelet-Rich Fibrin. A Case Report. Colomb. Med. (Cali Colomb.) 2017, 48, 204–207. [Google Scholar] [CrossRef] [Green Version]
- Cieplik, F.; Tabenski, L.; Hiller, K.-A.; Schmalz, G.; Buchalla, W.; Christgau, M. Influence of Autogenous Platelet Concentrate on Combined GTR/Graft Therapy in Intra-Bony Defects: A 13-Year Follow-up of a Randomized Controlled Clinical Split-Mouth Study. J. Clin. Periodontol. 2018, 45, 382–391. [Google Scholar] [CrossRef] [PubMed]
- Wanikar, I.; Rathod, S.; Kolte, A.P. Clinico-Radiographic Evaluation of 1% Alendronate Gel as an Adjunct and Smart Blood Derivative Platelet Rich Fibrin in Grade II Furcation Defects. J. Periodontol. 2019, 90, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Pardo-Zamora, G.; Moreno-Rodríguez, J.A.; Ortiz-Ruíz, A.J. Non-Incised Papilla Surgical Approach and Leukocyte Platelet-Rich Fibrin in Periodontal Reconstruction of Deep Intrabony Defects: A Case Series. Int. J. Environ. Res. Public Health 2021, 18, 2465. [Google Scholar] [CrossRef]
- Lei, L.; Yu, Y.; Ke, T.; Sun, W.; Chen, L. The Application of Three-Dimensional Printing Model and Platelet-Rich Fibrin Technology in Guided Tissue Regeneration Surgery for Severe Bone Defects. J. Oral Implantol. 2019, 45, 35–43. [Google Scholar] [CrossRef]
- Gummaluri, S.S.; Bhattacharya, H.S.; Astekar, M.; Cheruvu, S. Evaluation of Titanium-Prepared Platelet-Rich Fibrin and Leucocyte Platelet-Rich Fibrin in the Treatment of Intra-Bony Defects: A Randomized Clinical Trial. J. Dent. Res. Dent. Clin. Dent. Prospect. 2020, 14, 83–91. [Google Scholar] [CrossRef]
- Sharma, A.; Pradeep, A.R. Treatment of 3-Wall Intrabony Defects in Patients with Chronic Periodontitis with Autologous Platelet-Rich Fibrin: A Randomized Controlled Clinical Trial. J. Periodontol. 2011, 82, 1705–1712. [Google Scholar] [CrossRef]
- Thorat, M.; Pradeep, A.R.; Pallavi, B. Clinical Effect of Autologous Platelet-Rich Fibrin in the Treatment of Intra-Bony Defects: A Controlled Clinical Trial. J. Clin. Periodontol. 2011, 38, 925–932. [Google Scholar] [CrossRef]
- Ajwani, H.; Shetty, S.; Gopalakrishnan, D.; Kathariya, R.; Kulloli, A.; Dolas, R.S.; Pradeep, A.R. Comparative Evaluation of Platelet-Rich Fibrin Biomaterial and Open Flap Debridement in the Treatment of Two and Three Wall Intrabony Defects. J. Int. Oral Health JIOH 2015, 7, 32–37. [Google Scholar] [PubMed]
- Pradeep, A.R.; Nagpal, K.; Karvekar, S.; Patnaik, K.; Naik, S.B.; Guruprasad, C.N. Platelet-Rich Fibrin with 1% Metformin for the Treatment of Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2015, 86, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Kanoriya, D.; Pradeep, A.R.; Singhal, S.; Garg, V.; Guruprasad, C.N. Synergistic Approach Using Platelet-Rich Fibrin and 1% Alendronate for Intrabony Defect Treatment in Chronic Periodontitis: A Randomized Clinical Trial. J. Periodontol. 2016, 87, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Chandradas, N.; Ravindra, S.; Rangaraju, V.; Jain, S.; Dasappa, S. Efficacy of Platelet Rich Fibrin in the Treatment of Human Intrabony Defects with or without Bone Graft: A Randomized Controlled Trial. J. Int. Soc. Prev. Community Dent. 2016, 6, S153–S159. [Google Scholar] [CrossRef] [Green Version]
- Martande, S.S.; Kumari, M.; Pradeep, A.R.; Singh, S.P.; Suke, D.K.; Guruprasad, C.N. Platelet-Rich Fibrin Combined with 1.2% Atorvastatin for Treatment of Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2016, 87, 1039–1046. [Google Scholar] [CrossRef]
- Pradeep, A.R.; Garg, V.; Kanoriya, D.; Singhal, S. Platelet-Rich Fibrin with 1.2% Rosuvastatin for Treatment of Intrabony Defects in Chronic Periodontitis: A Randomized Controlled Clinical Trial. J. Periodontol. 2016, 87, 1468–1473. [Google Scholar] [CrossRef]
- Patel, G.K.; Gaekwad, S.S.; Gujjari, S.K.; Veerendra Kumar, S.C. Platelet-Rich Fibrin in Regeneration of Intrabony Defects: A Randomized Controlled Trial. J. Periodontol. 2017, 88, 1192–1199. [Google Scholar] [CrossRef]
- Agarwal, A.; Gupta, N.D.; Jain, A. Platelet Rich Fibrin Combined with Decalcified Freeze-Dried Bone Allograft for the Treatment of Human Intrabony Periodontal Defects: A Randomized Split Mouth Clinical Trail. Acta Odontol. Scand. 2016, 74, 36–43. [Google Scholar] [CrossRef]
- Naqvi, A.; Gopalakrishnan, D.; Bhasin, M.T.; Sharma, N.; Haider, K.; Martande, S. Comparative Evaluation of Bioactive Glass Putty and Platelet Rich Fibrin in the Treatment of Human Periodontal Intrabony Defects: A Randomized Control Trial. J. Clin. Diagn. Res. 2017, 11, 9–13. [Google Scholar] [CrossRef]
- Sezgin, Y.; Uraz, A.; Taner, I.L.; Çulhaoğlu, R. Effects of Platelet-Rich Fibrin on Healing of Intra-Bony Defects Treated with Anorganic Bovine Bone Mineral. Braz. Oral Res. 2017, 31, e15. [Google Scholar] [CrossRef] [Green Version]
- Bodhare, G.H.; Kolte, A.P.; Kolte, R.A.; Shirke, P.Y. Clinical and Radiographic Evaluation and Comparison of Bioactive Bone Alloplast Morsels When Used Alone and in Combination with Platelet-rich Fibrin in the Treatment of Periodontal Intrabony Defects—A Randomized Controlled Trial. J. Periodontol. 2019, 90, 584–594. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.; Sankari, M.; Satpathy, A.; Jayakumar, D.; Mozzati, M.; Mortellaro, C.; Gallesio, G.; Taschieri, S.; del Fabbro, M. Adjunctive Effect of Autologus Platelet-Rich Fibrin to Barrier Membrane in the Treatment of Periodontal Intrabony Defects. J. Craniofacial Surg. 2016, 27, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Aydemir Turkal, H.; Demirer, S.; Dolgun, A.; Keceli, H.G. Evaluation of the Adjunctive Effect of Platelet-Rich Fibrin to Enamel Matrix Derivative in the Treatment of Intrabony Defects. Six-Month Results of a Randomized, Split-Mouth, Controlled Clinical Study. J. Clin. Periodontol. 2016, 43, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Pradeep, A.R. Autologous Platelet-Rich Fibrin in the Treatment of Mandibular Degree II Furcation Defects: A Randomized Clinical Trial. J. Periodontol. 2011, 82, 1396–1403. [Google Scholar] [CrossRef]
- Kanoriya, D.; Pradeep, A.R.; Garg, V.; Singhal, S. Mandibular Degree II Furcation Defects Treatment with Platelet-Rich Fibrin and 1% Alendronate Gel Combination: A Randomized Controlled Clinical Trial. J. Periodontol. 2017, 88, 250–258. [Google Scholar] [CrossRef]
- Basireddy, A.; Prathypaty, S.K.; Yendluri, D.B.; Potharaju, S.P. Demineralized Freeze-Dried Bone Allograft with or without Platelet-Rich Fibrin in the Treatment of Mandibular Degree II Furcation Defects: A Clinical and Cone Beam Computed Tomography Study. J. Indian Soc. Periodontol. 2019, 23, 242–248. [Google Scholar] [CrossRef]
- Dambhare, A.; Bhongade, M.L.; Dhadse, P.V.; Sehdev, B.; Ganji, K.K.; Thakare, K.; Hiroshi, M.; Sugita, Y.; Maeda, H.; Alam, M.K. A Randomized Controlled Clinical Study of Autologous Platelet Rich Fibrin (PRF) in Combination with HA and Beta-TCP or HA and Beta-TCP Alone for Treatment of Furcation Defects. J. Hard Tissue Biol. 2019, 28, 185–190. [Google Scholar] [CrossRef] [Green Version]
- Del Fabbro, M.; Panda, S.; Jayakumar, N.D.; Sankari, M.; Varghese, S.; Ramamoorthi, S.; Ceci, C.; Ceresoli, V.; Taschieri, S. Autologous Platelet Concentrates for Treatment of Periodontal Defects. Cochrane Database Syst. Rev. 2014, 12, 1–20. [Google Scholar] [CrossRef]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative Potential of Leucocyte- and Platelet-Rich Fibrin. Part A: Intra-Bony Defects, Furcation Defects and Periodontal Plastic Surgery. A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2017, 44, 67–82. [Google Scholar] [CrossRef]
- Li, A.; Yang, H.; Zhang, J.; Chen, S.; Wang, H.; Gao, Y. Additive Effectiveness of Autologous Platelet-Rich Fibrin in the Treatment of Intrabony Defects. Medicine 2019, 98, e14759. [Google Scholar] [CrossRef]
- Miron, R.J.; Moraschini, V.; Fujioka-Kobayashi, M.; Zhang, Y.; Kawase, T.; Cosgarea, R.; Jepsen, S.; Bishara, M.; Canullo, L.; Shirakata, Y.; et al. Use of Platelet-Rich Fibrin for the Treatment of Periodontal Intrabony Defects: A Systematic Review and Meta-Analysis. Clin. Oral Investig. 2021, 25, 2461–2478. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ding, Y.; Cheng, G.; Meng, S. Use of Platelet-Rich Fibrin in the Treatment of Periodontal Intrabony Defects: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2021, 2021, 6669168. [Google Scholar] [CrossRef] [PubMed]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative Potential of Leucocyte- and Platelet-Rich Fibrin. Part B: Sinus Floor Elevation, Alveolar Ridge Preservation and Implant Therapy. A Systematic Review. J. Clin. Periodontol. 2017, 44, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, S.; Karanxha, L.; Goker, F.; Satpathy, A.; Taschieri, S.; Francetti, L.; Das, A.C.; Kumar, M.; Panda, S.; Del Fabbro, M. Autologous Platelet Concentrates in Treatment of Furcation Defects—A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarallo, F.; Mancini, L.; Pitzurra, L.; Bizzarro, S.; Tepedino, M.; Marchetti, E. Use of Platelet-Rich Fibrin in the Treatment of Grade 2 Furcation Defects: Systematic Review and Meta-Analysis. J. Clin. Med. 2020, 9, 2104. [Google Scholar] [CrossRef]
- Ustaoğlu, G.; Aydin, Z.U.; Özelçi, F. Comparison of GTR, T-PRF and Open-Flap Debridement in the Treatment of Intrabony Defects with Endo-Perio Lesions: A Randomized Controlled Trial. Med. Oral. Patol. Oral. Cir. Buca 2020, 25, e117–e123. [Google Scholar] [CrossRef]
- Smaïl-Faugeron, V.; Fron-Chabouis, H.; Courson, F.; Durieux, P. Comparison of Intervention Effects in Split-Mouth and Parallel-Arm Randomized Controlled Trials: A Meta-Epidemiological Study. BMC Med. Res. Methodol. 2014, 14, 64. [Google Scholar] [CrossRef] [Green Version]
- Lesaffre, E.; Philstrom, B.; Needleman, I.; Worthington, H. The Design and Analysis of Split-Mouth Studies: What Statisticians and Clinicians Should Know. Stat. Med. 2009, 28, 3470–3482. [Google Scholar] [CrossRef]
- Hujoel, P.P.; DeRouen, T.A. Validity Issues in Split-Mouth Trials. J. Clin. Periodontol. 1992, 19, 625–627. [Google Scholar] [CrossRef]
- Kotsakis, G.A.; Javed, F.; Hinrichs, J.E.; Karoussis, I.K.; Romanos, G.E. Impact of Cigarette Smoking on Clinical Outcomes of Periodontal Flap Surgical Procedures: A Systematic Review and Meta-Analysis. J. Periodontol. 2015, 86, 254–263. [Google Scholar] [CrossRef]
- Reynolds, M.A.; Kao, R.T.; Camargo, P.M.; Caton, J.G.; Clem, D.S.; Fiorellini, J.P.; Geisinger, M.L.; Mills, M.P.; Nares, S.; Nevins, M.L. Periodontal Regeneration–Intrabony Defects: A Consensus Report From the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S105–S107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yilmaz, D.; Dogan, N.; Ozkan, A.; Sencimen, M.; Ora, B.E.; Mutlu, I. Effect of Platelet Rich Fibrin and Beta Tricalcium Phosphate on Bone Healing. A Histological Study in Pigs. Acta Cir. Bras. 2014, 29, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rezuc, A.; Saavedra, C.; Maass, R.; Poblete, C.; Nappe, C. Histological Comparison of DBBM and Platelet Rich Fibrin for Guided Bone Regeneration in a Rabbit Model. J. Oral Biol. Craniofacial Res. 2020, 10, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.-O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Dohan, D.M. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part V: Histologic Evaluations of PRF Effects on Bone Allograft Maturation in Sinus Lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, 299–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Tangl, S.; Huber, C.D.; Lin, Y.; Qiu, L.; Rausch-Fan, X. Effects of Choukroun’s Platelet-Rich Fibrin on Bone Regeneration in Combination with Deproteinized Bovine Bone Mineral in Maxillary Sinus Augmentation: A Histological and Histomorphometric Study. J. Cranio-Maxillofac. Surg. 2012, 40, 321–328. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Cassetta, M.; Pacifici, A.; Stefanelli, L.V.; Scacco, S.; Dipalma, G.; Pacifici, L.; Inchingolo, F. Platelet Rich Fibrin (P.R.F.) in Reconstructive Surgery of Atrophied Maxillary Bones: Clinical and Histological Evaluations. Int. J. Med. Sci. 2012, 9, 872–880. [Google Scholar] [CrossRef]
- Bolukbasi, N.; Ersanlı, S.; Keklikoglu, N.; Basegmez, C.; Ozdemir, T. Sinus Augmentation with Platelet-Rich Fibrin in Combination with Bovine Bone Graft Versus Bovine Bone Graft in Combination with Collagen Membrane. J. Oral Implantol. 2015, 41, 586–595. [Google Scholar] [CrossRef]
- Cömert Kılıç, S.; Güngörmüş, M.; Parlak, S.N. Histologic and Histomorphometric Assessment of Sinus-Floor Augmentation with Beta-Tricalcium Phosphate Alone or in Combination with Pure-Platelet-Rich Plasma or Platelet-Rich Fibrin: A Randomized Clinical Trial. Clin. Implant Dent. Relat. Res. 2017, 19, 959–967. [Google Scholar] [CrossRef]
- Nizam, N.; Eren, G.; Akcalı, A.; Donos, N. Maxillary Sinus Augmentation with Leukocyte and Platelet-Rich Fibrin and Deproteinized Bovine Bone Mineral: A Split-Mouth Histological and Histomorphometric Study. Clin. Oral Implant. Res. 2018, 29, 67–75. [Google Scholar] [CrossRef]
- Pichotano, E.C.; Molon, R.S.; Souza, R.V.; Austin, R.S.; Marcantonio, E.; Zandim-Barcelos, D.L. Evaluation of L-PRF Combined with Deproteinized Bovine Bone Mineral for Early Implant Placement after Maxillary Sinus Augmentation: A Randomized Clinical Trial. Clin. Implant Dent. Relat. Res. 2019, 21, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Pepelassi, E.; Kaddas, C. The Use of Platelet-Rich Fibrin in Maxillary Sinus Augmentation: A Review of the Literature. J. Dent. Oral Disord. 2020, 6, 1143. [Google Scholar]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.J.; Mouhyi, J.; Dohan, D.M. Platelet-Rich Fibrin (PRF): A Second-Generation Platelet Concentrate. Part IV: Clinical Effects on Tissue Healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2006, 101, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Aricioglu, C.; Dolanmaz, D.; Esen, A.; Isik, K.; Avunduk, M.C. Histological Evaluation of Effectiveness of Platelet-Rich Fibrin on Healing of Sinus Membrane Perforations: A Preclinical Animal Study. J. Cranio-Maxillofac. Surg. 2017, 45, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.L.; et al. Use of Platelet-Rich Fibrin in Regenerative Dentistry: A Systematic Review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Liu, K.; Huang, Z.; Chen, Z.; Han, B.; Ouyang, X. Treatment of Periodontal Intrabony Defects Using Bovine Porous Bone Mineral and Guided Tissue Regeneration with/without Platelet-rich Fibrin: A Randomized Controlled Clinical Trial. J. Periodontol. 2021, 92, 1546–1553. [Google Scholar] [CrossRef]
 : Low Risk.
: Low Risk.  : Unclear Risk.
: Unclear Risk.
| Authors | Year | Reason of Exclusion |
|---|---|---|
| Lekovic et al. [23] | 2012 | No control group (Reason 2) |
| Pradeep et al. [24] | 2012 | Non-independence analysis unit (Reason 3) |
| Bajaj et al. [25] | 2013 | Mixed design clinical trial (Reason 1) |
| Elgendy et al. [26] | 2015 | Smokers included (Reason 4) |
| Shah et al. [27] | 2015 | Mixed design clinical trial (Reason 1) |
| Siddiqui et al. [28] | 2016 | Mixed design clinical trial (Reason 1) |
| Qiao et al. [29] | 2016 | Mixed design clinical trial (Reason 1) |
| Agarwal et al. [30] | 2017 | Mixed design clinical trial (Reason 1) |
| Bajaj et al. [31] | 2017 | Mixed design clinical trial (Reason 1) |
| Chatterjee et al. [32] | 2017 | Mixed design clinical trial (Reason 1) |
| Lohi et al. [33] | 2017 | Mixed design clinical trial (Reason 1) |
| Pradeep et al. [34] | 2017 | Mixed design clinical trial (Reason 1) |
| Betancourt et al. [35] | 2017 | Case report (Reason 5) |
| Cieplik et al. [36] | 2018 | Incomplete data (Reason 6) |
| Wanikar et al. [37] | 2019 | PRF was not used as an adjunct (Reason 7) |
| Pardo-Zamora et al. [38] | 2019 | Case series (Reason 5) |
| Lei et al. [39] | 2019 | Case report (Reason 5) |
| Gummaluri et al. [40] | 2020 | Mixed design clinical trial (Reason 1) |
| Endosseous Defects | |||||
|---|---|---|---|---|---|
| (L-PRF + OFD) vs. OFD Alone | |||||
| Author Year | Study Design Time | Population Characteristics | Interventions Groups | Parameters Evaluated | Outcomes (Test vs. Control Group) |
| Sharma and Pradeep 2011 (b) [41] | Parallel Time: 9 mo Ptis: Chronic 3 w | Smokers: Excluded Age: 35.34 ± 6.45 years Gender: 18 F/24 M Teeth treated: 69 n randomized (participants/teeth): 42/69 n evaluated (participants/teeth): 35/56 | (L-PRF + OFD) vs. OFD Test: L-PRF + OFD (n = 28) Control: OFD (n = 28) L-PRF preparation: 3000 rpm × 10 min Clots, membrane | Clinical: PPD, CAL, GML Radiographic: DD | PPD reduction: (4.55 ± 1.87 mm) vs. (3.21 ± 1.64 mm) (p = 0.006) CAL gain: (3.331 ± 1.76 mm) vs. (2.77 ± 1.44 mm) (p = 0.2143) GML change: (−0.10 ± 0.08 mm) vs. (0.67 ± 0.46 mm) (p < 0.001) DD reduction: (2.50 ± 0.78 mm) vs. (0.09 ± 0.11 mm) (p < 0.001) DF: (48.26 ± 5.72%) vs. (1.80 ± 1.56%) (p < 0.001) |
| Thorat et al., 2011 [42] | Parallel Time: 9 mo Ptis: Chronic 2–3 w | Smokers: Excluded Age: 31.12 ± 2.06 years Gender: 18 F/22 M Teeth treated: 40 n randomized (participants/teeth): 40/40 n evaluated (participants/teeth): 32/32 | (L-PRF + OFD) vs. OFD Test: L-PRF + OFD (n = 16) Control: OFD (n = 16) L-PRF preparation: 400 g × 12 min Clots, membrane | Clinical: PPD, CAL, GML Radiographic: DD | PPD reduction: (4.69 ± 1.45 mm) vs. (3.56 ± 1.09 mm) (p < 0.05) CA L gain: (4.13 ± 1.63 mm) vs. (2.13 ± 1.71 mm) (p < 0.05) GML change: (−0.31 ± 0.95 mm) vs. (−1.31 ± 1.01 mm) (p < 0.05) DD reduction: (2.12 ± 0.69 mm) vs. (1.24 ± 0.69 mm) (p < 0.05) |
| Ajwani et al., 2015 [43] | Split-mouth Time: 9 mo Ptis: NR 2–3 w | Smokers: Excluded Mean: 30.5 years Gender: 10 F/10 M Teeth treated: 40 n randomized (participants/teeth): 20/40 n evaluated (participants/teeth): 20/40 | (L-PRF + OFD) vs. OFD Test: L-PRF + OFD (n = 20) Control: OFD (n = 20) L-PRF preparation: 3000 rpm × 10 min Clots, membrane | Clinical: PPD, RAL, GML Radiographic: CEJ-BOD, AC-BOD, CEJ-AC | PPD reduction: (1.90 ± 0.738 mm) vs. (1.60 ± 0.843 mm) (p = 0.408) RAL gain: (1.80 ± 0.632 mm) vs. (1.30 ± 0.675 mm) (p = 0.105) GML change: (−0.30 ± 0.483 mm) vs. (−0.30 ± 0.675 mm) (p = 0.08) CEJ-BOD change: (2.60 ± 1.101 mm) vs. (1.30 ± 0.422 mm) (p = 0.003) AC-BOD change: (1.45 ± 0.497 mm) vs. (0.80 ± 0.350 mm) (p = 0.003) CEJ-AC change: (1.20 ± 1.006 mm) vs. (0.50 ± 0.471 mm) (p = 0.062) |
| Pradeep et al., 2015 [44] | Parallel Time: 9 mo Ptis: Chronic 3 w | Smokers: Excluded Age: 41 years Gender: 68 F/68 M Teeth treated: 64 (126 for all 4 groups) n randomized (participants/teeth): 64/64 (126/126 for all 4 groups) n evaluated (participants/teeth): 60/60 (120/120 for all 4 groups) | (L-PRF + OFD) vs. OFD Test 1: L-PRF + OFD (n = 30) Control: OFD (n = 30) L-PRF preparation: 3000 rpm × 10 min Clots, membrane Group 3 and 4 not included | Clinical: PPD, RAL, GML Radiographic: DD | PPD reduction: (4.00 ± 0.18 mm) vs. (3.00 ± 0.18) (p < 0.001) RAL gain: (4.03 ± 0.18 mm) vs. (2.96 ± 0.18 mm) (p < 0.001) GML change: (0.27 ± 0.007 mm) vs. (−0.06 ± 0.04 mm) (p < 0.001) DD reduction: (2.53 ± 0.30 mm) vs. (0.49 ± 0.27 mm) (p < 0.001) % DD reduction: (48.00 ± 0.0029%) vs. (9.14 ± 0.004%) (p < 0.001) |
| Smokers: Excluded Age: 41 years Gender: 68 F/68 M Teeth treated: 62 (126 for all 4 groups) n randomized (participants/teeth): 62/62 (126/126 for all 4 groups) n evaluated (participants/teeth): 60/60 (120/120 for all 4 groups) | (L-PRF + 1% MF + OFD) vs. (1%MF + OFD) Test 2: L-PRF + 1% MF + OFD (n = 30) Control: 1%MF + OFD (n = 30) L-PRF preparation: 3000 rpm × 10 min Clots, membrane Group 1 and 2 not included | Clinical: PPD, RAL, GML Radiographic: DD | PPD reduction: (4.90 ± 0.30 mm) vs. (3.93 ± 0.25) (p = 0.084) RAL gain: (4.90 ± 0.30 mm) vs. (3.93 ± 0.25 mm) (p = 0.079) GML change: (0.33 ± 0.07 mm) vs. (0.27 ± 0.05 mm) (p = 0.420) DD reduction: (2.77 ± 0.30 mm) vs.(2.56 ± 0.28 mm) (p < 0.05) % DD reduction: (52.65 ± 0.031%) vs. (48.69 ± 0.026%) (p < 0.05) | ||
| Kanoriya et al., 2016 [45] | Parallel Time: 9 mo Ptis: Chronic 3 w | Smokers: Excluded Age: 39 years Gender: 55 F/53 M Teeth treated: 64 (96 for all 3 groups) n randomized (participants/teeth): 64/64 (96/96 for all 3 groups) n evaluated (participants/teeth): 60/60 (90/90 for all 3 groups) | (L-PRF + OFD) vs. OFD Test group: L-PRF + OFD (n = 30) Control group: OFD alone (n = 30) L-PRF preparation: 3000 rpm × 10 min Clots, membrane Group 3 not included | Clinical: PPD, CAL, GML Radiographic: DD | PPD reduction: (3.70 ± 0.91 mm) vs. (2.86 ± 0.68 mm) (p < 0.05) CAL gain: (4.20 ± 0.66 mm) vs. (3.03 ± 0.18 mm) (p < 0.05) GML change: (0.24 ± 0.056 mm) vs. (−0.06 ± 0.07) (p < 0.05) DD reduction: (2.42 ± 0.21 mm) vs. (0.38 ± 0.26 mm) (p < 0.01) DF: (46.00 ± 1.89%) vs. (7.33 ± 4.86%) (p < 0.01) |
| Chandradas et al., 2016 [46] | Parallel Time: 9 mo Ptis: Chronic 2–3 w | Smokers: Excluded Age range: 35–50 years Gender: 18 F/18 M Teeth treated: 24 (36 for all 3 groups) n randomized (participants/teeth): 24/24 (36/36 for all 3 groups) n evaluated (participants/teeth): 24/24 (36/36 for all 3 groups) | (L-PRF + OFD) vs. OFD Test: L-PRF + OFD (n = 12) Control: OFD alone (n = 12) L-PRF preparation: 3000 rpm × 12 min Membrane Group 2 not included | Clinical: PPD, RAL, GR Radiographic: DD | PPD reduction: (3.00 ± 1.21 mm) vs. (3.82 ± 0.75 mm) (p = 0.109) RAL gain: (3.27 ± 0.65 mm) vs. (2.25 ± 0.62 mm) (p = 0.003) GML change: (−0.18 ± 0.40 mm) vs. (−1.33 ± 0.78 mm) (p = 0.002) DD reduction: (2.30 ± 0.83 mm) vs. (1.22 ± 0.62 mm) (p = 0.001) |
| Martande et al., 2016 [47] | Parallel Time: 9 mo Ptis: Chronic 3 w | Smokers: Excluded Mean age at baseline: 37.6 years Gender: 48 F/48 M Teeth treated: 64 (96 for all 3 groups) n randomized (participants/teeth): 64/64 (96/96 for all 3 groups) n evaluated (participants/teeth): 60/60 (90/90 for all 3 groups) | Comparison: L-PRF + OFD vs. OFD alone Test group: L-PRF + OFD (n = 30) Control group: OFD alone (n = 30) L-PRF preparation: 3000 rpm × 12–14 min Clots, membrane Group 3 not included | Clinical: PD, CAL, GML Radiographic: DD | PPD reduction: (3.76 ± 1.12 mm) vs. (2.76 ± 1.43 mm) (p = 0.01) CA gain L: (3.40 ± 1.13 mm) vs. (2.50 ± 1.33 mm) (p = 0.03) GML change: (0.22 ± 0.10 mm) vs. (0.06 ± 0.02 mm) (p < 0.001) DD reduction: (2.46 ± 0.33 mm) vs. (0.27 ± 0.19 mm) (p < 0.001) DF: (47.91 ± 4.79%) vs. (5.54 ± 1.71%) (p < 0.001) |
| Pradeep et al., 2016 [48] | Parallel Time: 9 mo Ptis: Chronic 2–3 w | Smokers: Excluded Age: 35 years Gender: 45 F/45 M Teeth treated: 60 (90 for all 3 groups) n randomized (participants/teeth): 60/60 (90/90 for all 3 groups) n evaluated (participants/teeth): 60/60 (90/90 for all 3 groups) | (L-PRF + OFD) vs. OFD Test: L-PRF + OFD (n = 30) Control: OFD (n = 30) L-PRF preparation: 3000 rpm × 10 min Membrane | Clinical: PPD, CAL Radiographic: DD | PPD reduction: (4.03 ± 0.18 mm) vs. (3.10 ± 0.30 mm) (p < 0.001) CAL gain: (3.30 ± 0.65 mm) vs. (2.47 ± 0.77 mm) (p < 0.001) DD reduction: (3.17 ± 0.65 mm) vs. (1.43 ± 0.50 mm) (p < 0.001) |
| Patel et al., 2017 [49] | Split-mouth Time:12 mo Ptis: Chronic 2–3 w | Smokers: Excluded Age: 44 ± 9 years Gender: 9 F/4 M Teeth treated: 26 n randomized (participants/teeth): 13/26 n evaluated (participants/teeth): 13/26 | (L-PRF + OFD) vs. OFD Test: L-PRF + OFD (n = 13) Control: OFD (n = 13) L-PRF preparation: 3000 rpm × 10 min Membrane | Clinical: PPD, CAL Radiographic: DF | PPD reduction: (4.20 ± 1.69 mm) vs. (2.40 ± 0.84 mm) (p = 0.001) CAL gain: (3.70 ± 0.67 mm) vs. (2.10 ± 0.74 mm) (p = 0.001) DF: (45.18 ± 7.57%) vs. (21.6 ± 9.3%) (p = 0.001) |
| (L-PRF + OG) vs. OG Alone | |||||
| Agarwal et al., 2016 [50] | Split-mouth Time:12 mo Ptis: Chronic 2–3 w | Smokers: Excluded Age: 52 ± 7 years Gender: 14 F/18 M Teeth treated: 64 n randomized (participants/teeth): 32/64 n evaluated (participants/teeth): 30/60 | (L-PRF + DFDBA) vs. (DFDBA + saline) Test: (L-PRF + DFDBA) (n = 30) Control: (DFDBA + saline) (n = 30) L-PRF preparation: 400 g × 12 min Clots, membrane | Clinical: PPD, CAL, REC Radiographic: CEJ-AC, AC-BOD, CEJ-BOD | PPD reduction: (4.15 ± 0.84 mm) vs. (3.60 ± 0.15 mm) (p < 0.05) CAL gain: (3.73 ± 0.74 mm) vs. (2.61 ± 0.68 mm) (p < 0.001) CEJ-AC change: (−0.23 ± 0.25 mm) vs. (−0.26 ± 0.25 mm) (p = 0.613) AC-BOD change: (3.73 ± 0.63 mm) vs. (2.75 ± 0.57) (p < 0.001) CEJ-BOD change: (3.50 ± 0.67 mm) vs. (2.49 ± 0.64 mm) (p < 0.001) |
| Naqvi et al., 2017 [51] | Split-mouth Time: 9 mo Ptis: Chronic 2–3 w | Smokers: Excluded Age: 20–50 years Gender: 3 F/7 M Teeth treated: 20 n randomized (participants/teeth): 10/20 n evaluated (participants/teeth): 10/20 | (L-PRF + BGP) vs. BGP Test: (L-PRF + BGP) (n = 10) Control: BGP (n = 10) L-PRF preparation: 3000 rpm × 10 min Membrane | Clinical: PPD, CAL Radiographic: DD | PPD reduction: (3.20 ± 2.30 mm) vs. (3.15 ± 1.06 mm) (p = 0.117) CAL gain: (4.10 ± 1.73 mm) vs. (3.15 ± 1.06 mm) (p = 0.155) DD reduction: (7.10 ± 1.37 mm) vs. (5.70 ± 1.64 mm) (p = 0.043) |
| Sezgin et al., 2017 [52] | Split-mouth Time: 6 mo Ptis: Chronic 2–3 w | Smokers: Excluded Age: 38–61 years Gender: 7 F/8 M Teeth treated: 30 n randomized (participants/teeth): 21/42 n evaluated (participants/teeth): 5/30 | (L-PRF + ABBM) vs. ABBM Test: (L-PRF + ABBM) (n = 15) Control: ABBM (n = 15) L-PRF preparation: 2700 rpm × 12 min Clots, membrane | Clinical: PPD, CAL, GR Radiographic: DD, vertical bone loss, defect angle | PPD reduction: (4.93 ± 1.22 mm) vs. (4.21 ± 1.21 mm) (p > 0.05) CAL gain: (4.47 ± 1.60 mm) vs. (3.27 ± 1.34 mm) (p < 0.05) GR: (0.46 ± 0.83 mm) vs. (0.94 ± 0.70 mm) (p > 0.05) DD reduction: (2.55 ± 1.15 mm) vs. (1.98 ± 0.80 mm) (p > 0.05) |
| Bodhare et al., 2019 [53] | Split-mouth Time: 6 mo Ptis: Chronic 2–3 w | Smokers: Excluded Age: 35.9 years Gender: 9 F/11 M Teeth treated: 40 n randomized (participants/teeth): 20/40 n evaluated (participants/teeth): 20/40 | (L-PRF + BG + OFD) vs. (BG + OFD) Test: (L-PRF + BG + OFD) (n = 20) Control: (BG + OFD) (n = 20) L-PRF preparation: 3000 rpm × 10 min Clots, membrane | Clinical: PPD, CAL, GML Radiographic: CEJ-BOD, CEJ-AC, AC-BOD, defect width (mesio-distal, bucco-lingual) | PPD reduction: (5.75 ± 1.16 mm) vs. (5.65 ± 1.66 mm) (p = 0.82) CAL gain: (5.05 ± 1.09 mm) vs. (4.20 ± 1.70 mm) (p = 0.02) GML change: (−0.80 ± 0.61 mm) vs. (−1.95 ± 1.09 mm) (p < 0.001) CEJ-BOD change: (3.30 ± 1.10 mm) vs. (2.49 ± 0.99 mm) (p = 0.02) AC-BOD change: (3.51 ± 1.71 mm) vs. (2.56 ± 0.94 mm) (p = 0.0077) CEJ-AC change: (0.13 ± 0.22 mm) vs. (0.33 ± 0.37 mm) (p = 0.2705) Defect width: Mesio-distal: (0.70 ± 0.68 mm) vs. (0.45 ± 0.18 mm) (p = 0.0047) Bucco-lingual: (1.60 ± 0.27 mm) vs. (1.33 ± 0.44 mm) (p = 0.00319) |
| (L-PRF + GTR) vs. GTR Alone | |||||
| Panda et al., 2016 [54] | Split-mouth Time: 9 mo Ptis: Chronic 3 w | Smokers: Excluded Age: 38.12 ± 2.06 years Gender: 8 F/10 M Teeth treated: 36 n randomized (participants/teeth): 18/36 n evaluated (participants/teeth): 6/32 | (L-PRF + GTR) vs. GTR Test: (L-PRF + GTR) (n = 16) Control: GTR (n = 16) L-PRF preparation: 3000 rpm × 10 min | Clinical: PPD, CAL, GML Radiographic: DD | PPD: (3.88 ± 1.15 mm) vs. (3.19 ± 1.33 mm) (p = 0.13) CAL gain: (4.44 ± 1.50 mm) vs. (3.38 ± 1.45 mm) (p = 0.05) GML change: (1.00 ± 0.67 mm) vs. (0.29 ± 0.49 mm) (p = 0.03) DD reduction: (2.10 ± 0.64 mm) vs. (0.80 ± 0.28 mm) (p < 0.001) |
| (L-PRF + EMD) vs. EMD Alone | |||||
| Aydemir et al., 2016 [55] | Split-mouth Time: 6 mo Ptis: Chronic 1-,2-,3-w | Smokers: Excluded Age: 38.53 ± 9.24 years Gender: 14 F/14 M Teeth treated: 56 n randomized (participants/teeth): 28/56 n evaluated (participants/teeth): 25/49 | (L-PRF + EMD) vs. EMD Test: (L-PRF + EMD) Control: EMD L-PRF preparation: 400 g × 10 min Membrane | Clinical: PPD, CAL. GR Radiographic: DD, CEJ-BOD, defect width, defect angle | PPD reduction: (4.00 ± 1.38 mm) vs. (3.88 ± 1.26 mm) (p = 0.746) CAL gain: (3.42 ± 1.28 mm) vs. (3.29 ± 1.30 mm) (p = 0.718) GR: (0.71 ± 0.86 mm) vs. (0.58 ± 0.78 mm) (p = 0.574) DD reduction: (1.17 ± 0.86 mm) vs. (1.19 ± 1.25 mm) (p = 0.937) |
| Furcation Defects | |||||
| (L-PRF + OFD) vs. OFD Alone | |||||
| Sharma and Pradeep 2011 (a) [56] | Split-mouth Time: 9 mo Ptis: NR Class II | Smokers: excluded Age: 34.2 years Gender: 8 F/10 M Teeth treated: 36 n randomized (participants/teeth): 8/36 n evaluated (participants/teeth): 8/36 | (OFD + L-PRF) vs. OFD Test: (OFD + L-PRF) (n = 36) Control: OFD (n = 36) L-PRF preparation: 3000 rpm × 10 min Clots, membrane | Clinical: PPD, RVCAL, RHCAL, GML Radiographic: DD, DF | PPD reduction: (4.056 ± 0.416 mm) vs. (2.889 ± 0.676 mm) (p < 0.001) RVCAL gain: (2.333 ± 0.485 mm) vs. (1.278 ± 0.461 mm) (p < 0.001) RHCAL gain: (2.667 ± 0.594 mm) vs. (1.889 ± 0.758) (p = 0.002) GM Lchange: (0.344 ± 0.086 mm) vs. (0.756 ± 0.115 mm) (p < 0.001) DD reduction: (2.006 ± 0.163 mm) vs. (0.622 ± 0.216) (p < 0.001) DF: (50.8 ± 6.24%) vs. (16.7 ± 6.42%) (p < 0.001) |
| Kanoriya et al., 2017 [57] | Parallel Time: 9 mo Ptis: Chronic Class II | Smokers: Excluded Age: 39.45 ± 5.20 years (control), 38.30 ± 5.35 years (test) Gender: 36 F/36 M Teeth treated: 52 (78 for all 3 groups) n randomized (participants/teeth): 52/52 (78/78 for all 3 groups) n evaluated (participants/teeth): 47/47 (72/72 for all 3 groups) | (L-PRF + OFD) vs. OFD Test: (L-PRF + OFD) (n = 23) Control: OFD (n = 24) L-PRF preparation: 3000 rpm × 10 min Clots, membrane Group 3 not included | Clinical: PPD, RVAL, RVHL Radiographic: DD | PPD reduction: (3.69 ± 0.76 mm) vs. (2.41 ± 0.77 mm) (p < 0.001) RVAL gain: (3.39 ± 0.49 mm) vs. (2.33 ± 0.48 mm) (p < 0.001) RVHL gain: (2.86 ± 0.062 mm) vs. (2.04 ± 0.35 mm) (p < 0.001) DD reduction: (2.59 ± 0.32 mm) vs. (0.52 ± 0.19 mm) (p < 0.001) |
| (L-PRF + OG) vs. OG Alone | |||||
| Basireddy et al., 2019 [58] | Split-mouth Time: 9 mo Ptis: Chronic Class II | Smokers: Excluded Age range: 30–50 years Gender: NR Teeth treated: 110 n randomized (participants/teeth): 14/28 n evaluated (participants/teeth): 14/28 | (L-PRF + DFDBA + OFD) vs. (DFDBA + OFD) Test: (L-PRF + DFDBA + OFD) (n = 14) Control: (DFDBA + OFD) (n = 14) L-PRF preparation: 3000 rpm × 10 min Membrane | Clinical: PD, RVCAL, RHCAL, GML Radiographic (CBCT): VDD, HDD | PD reduction: (2.50 ± 0.519 mm) vs. (2.336 ± 0.497 mm) (p > 0.05) RVCAL gain: (2.36 ± 0.497 mm) vs. (1.79 ± 0.802 mm) (p > 0.05) RHCAL gain: (4.57 ± 1.697 mm) vs. (1.50 ± 1.092 mm) (p < 0.001) GML change: (−0.21 ± 0.426 mm) vs. (−0.79 ± 0.579 mm) (p < 0.05) VDD reduction: (46.36 ± 22.7%) vs. (42.36 ± 21.35%) (p > 0.05) HDD reduction: (38.20 ± 12.57%) vs. (37.99 ± 13.56%) (p > 0.05) |
| Dambhare et al., 2019 [59] | Parallel Time:12 mo Ptis: Chronic Class II | Smokers: Excluded Age: 40 ± 4.29 years Gender: NR Teeth treated: 24 n randomized (participants/teeth): 24/24 n evaluated (participants/teeth): 24/24 | (L-PRF + HA + β-TCP + OFD) vs. (HA + β-TCP + OFD) Test: (L-PRF + HA + β-TCP + OFD) (n = 12) Control: (HA + β-TCP + OFD) (n = 12) L-PRF preparation: 400 g × 12 min Clots, membrane | Clinical: PPD, CAL, REC | PPD reduction: (2.00 ± 0.73 mm) vs. (0.50 ± 0.52 mm) (p < 0.05) CAL gain: (3.33 ± 0.83 mm) vs. (2.00 ± 0.85 mm) (p < 0.05) REC: (1.0 ± 1.12 mm) vs. (1.34 ± 1.07 mm) (p > 0.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pepelassi, E.; Deligianni, M. The Adjunctive Use of Leucocyte- and Platelet-Rich Fibrin in Periodontal Endosseous and Furcation Defects: A Systematic Review and Meta-Analysis. Materials 2022, 15, 2088. https://doi.org/10.3390/ma15062088
Pepelassi E, Deligianni M. The Adjunctive Use of Leucocyte- and Platelet-Rich Fibrin in Periodontal Endosseous and Furcation Defects: A Systematic Review and Meta-Analysis. Materials. 2022; 15(6):2088. https://doi.org/10.3390/ma15062088
Chicago/Turabian StylePepelassi, Eudoxie, and Maria Deligianni. 2022. "The Adjunctive Use of Leucocyte- and Platelet-Rich Fibrin in Periodontal Endosseous and Furcation Defects: A Systematic Review and Meta-Analysis" Materials 15, no. 6: 2088. https://doi.org/10.3390/ma15062088
APA StylePepelassi, E., & Deligianni, M. (2022). The Adjunctive Use of Leucocyte- and Platelet-Rich Fibrin in Periodontal Endosseous and Furcation Defects: A Systematic Review and Meta-Analysis. Materials, 15(6), 2088. https://doi.org/10.3390/ma15062088






