Structure and Properties of Bioactive Glass-Modified Calcium Phosphate/Calcium Sulfate Biphasic Porous Self-Curing Bone Repair Materials and Preliminary Research on Their Osteogenic Effect
Abstract
1. Introduction
2. Materials and Methods
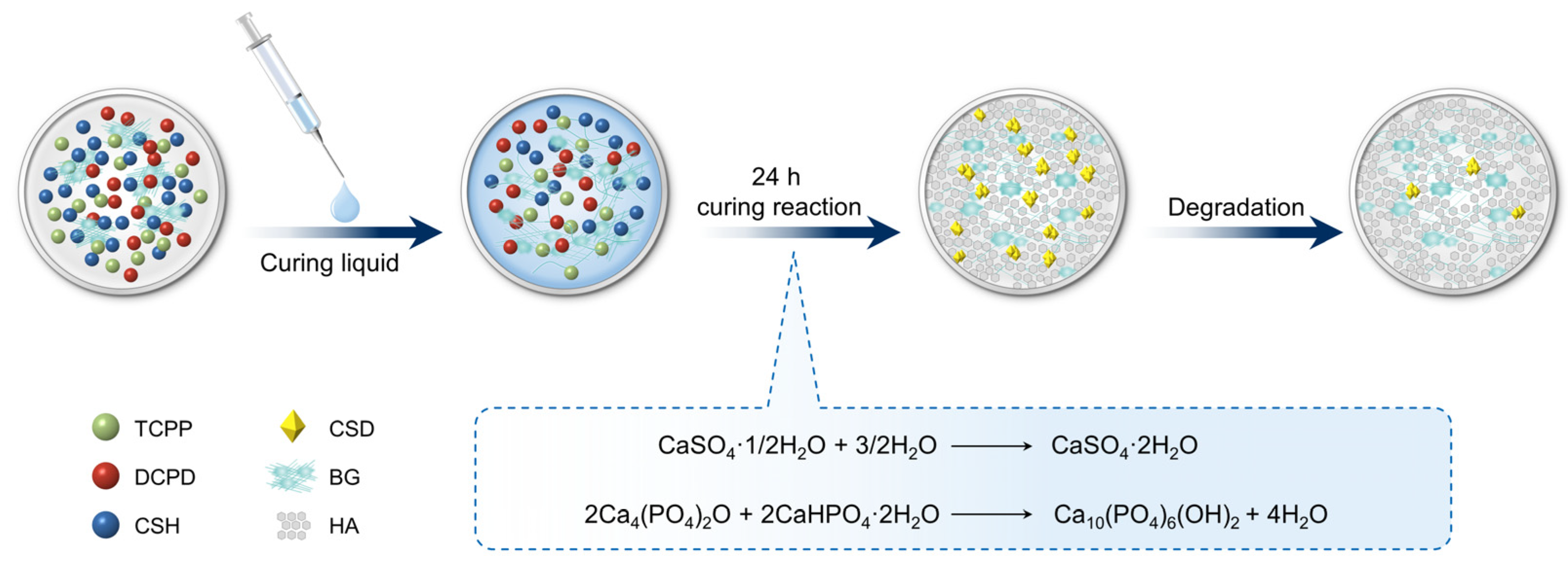
2.1. Material Preparation and Porous Structure Formation
2.2. Characterization of The Materials
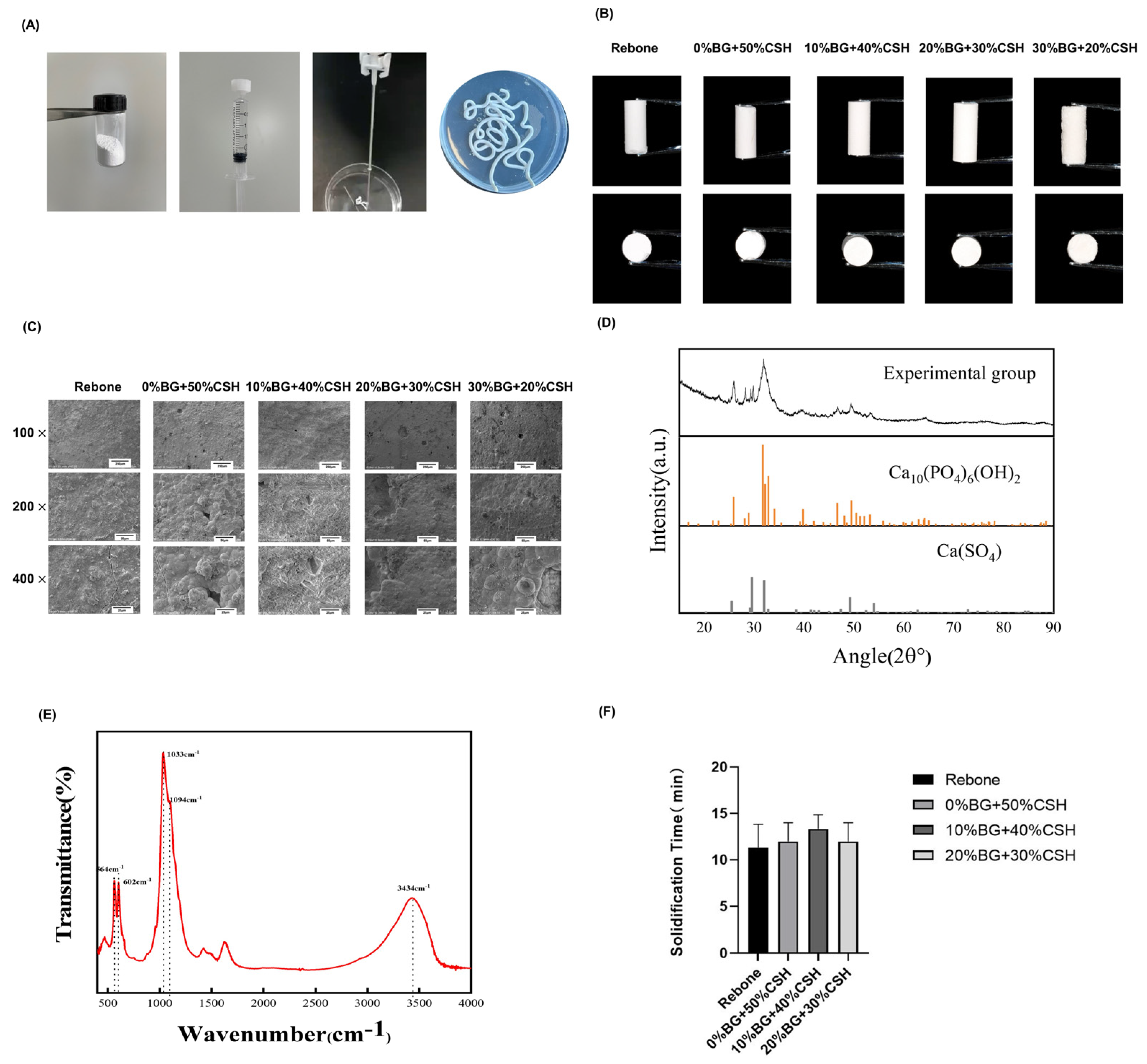
2.2.1. Appearance and Surface Morphology Observation
2.2.2. X-ray Diffractometer Analysis
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
2.2.4. Solidification Time
2.2.5. Injection Performance
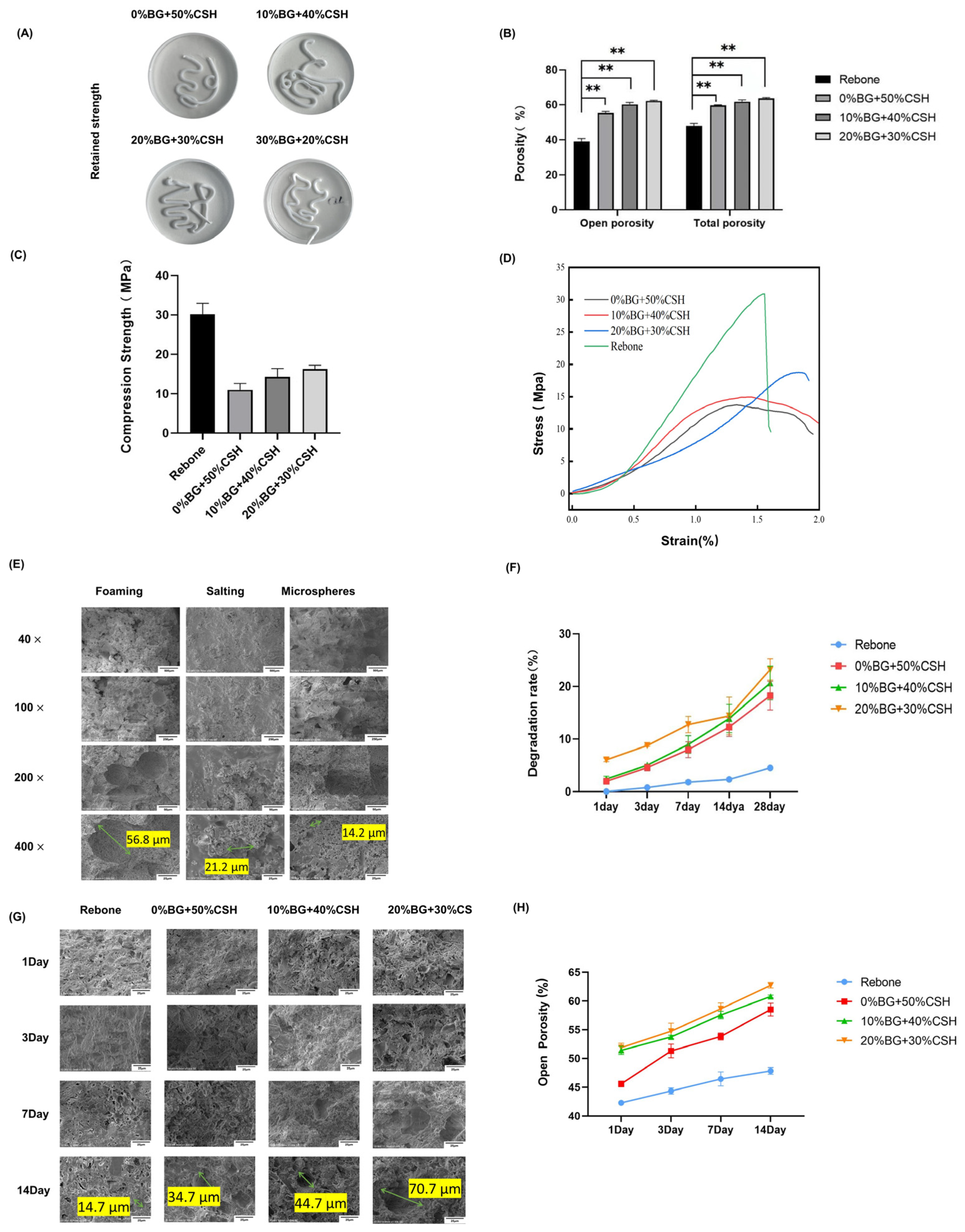
2.2.6. Anti-Collapse Performance
2.2.7. Porosity/Water Absorption
2.2.8. Compressive Strength Test
2.2.9. Material Degradation Performance
2.2.10. Microstructure after Degradation
2.3. Biosafety and Osteogenesis In Vitro Testing
2.3.1. Preparation of the Leachate and Total Elemental Analysis
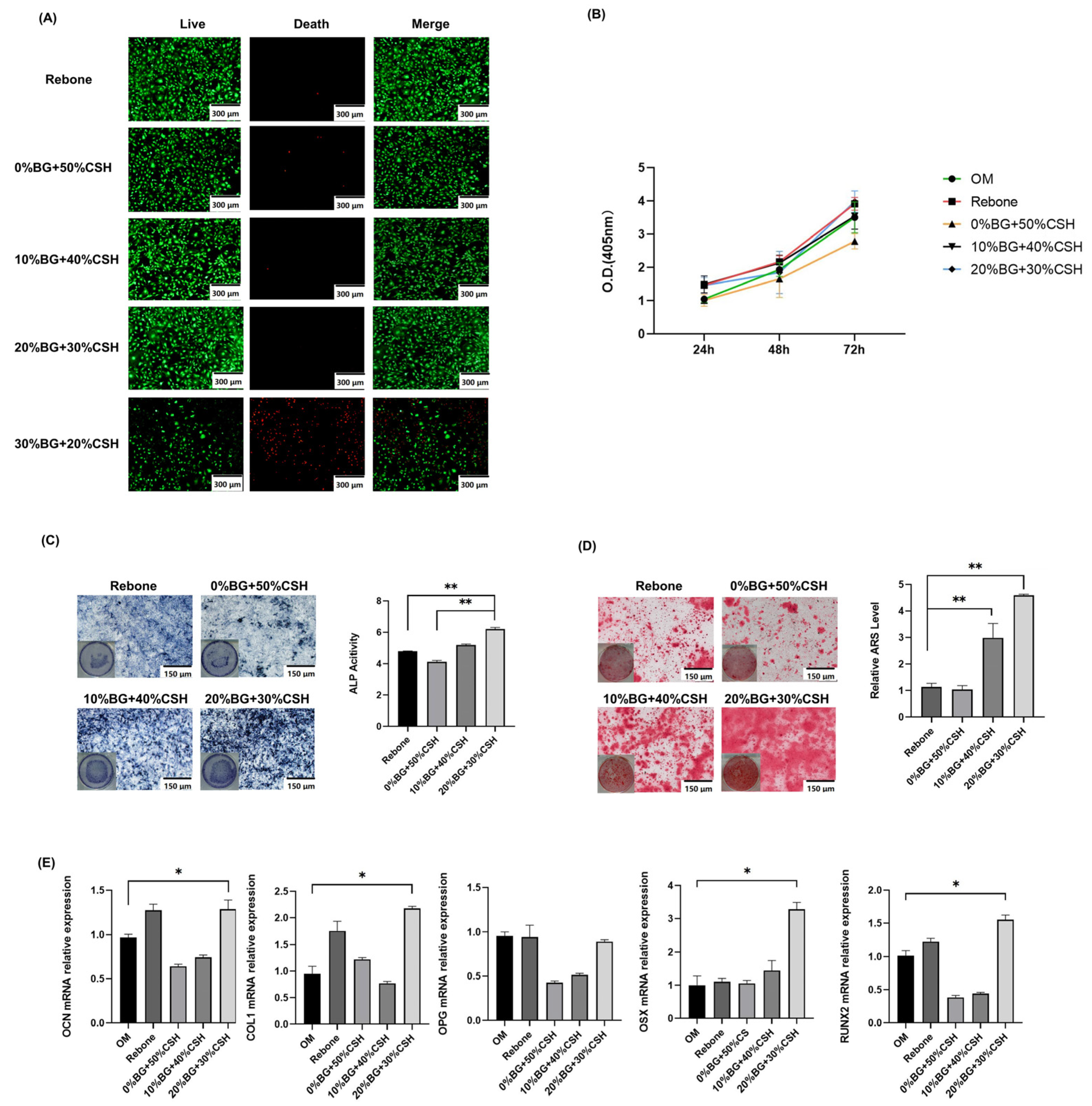
2.3.2. CCK-8 Proliferation Assay
2.3.3. Live/Dead Cell Staining
2.3.4. Alkaline Phosphatase (ALP) and Alizarin Red S (ARS) Staining
2.3.5. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT PCR) Assay
2.3.6. Statistical Analysis
3. Results
3.1. Material Synthesis and Characterization
3.2. Anti-Collapsibility, Porosity, and Degradation
3.3. Material Biosafety Performance Testing
3.4. Osteogenic Effect Detection
3.5. Detection of Osteogenic Gene Expression In Vitro
4. Discussion
- (1)
- Good biocompatibility to avoid immune reactions in the implanted area;
- (2)
- Good biodegradability to promote bone remodeling;
- (3)
- Porosity for cell adhesion and nutrient transport;
- (4)
- Moderate mechanical strength to support defect remodeling;
- (5)
- Bone conductivity and osteoinduction.
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McAllister, B.S.; Haghighat, K. Bone augmentation techniques. J. Periodontol. 2007, 78, 377–396. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kar, A.K.; Kaja, A.; Lim, E.J.; Choi, W.; Son, W.S.; Oh, J.-K.; Sakong, S.; Cho, J.-W. More weighted cancellous bone can be harvested from the proximal tibia with less donor site pain than anterior iliac crest corticocancellous bone harvesting: Retrospective review. J. Orthop. Surg. Res. 2021, 16, 220. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhou, L.; Lin, J.; Chen, J.; Huang, W.; Chen, Y. Immediate implant placement in anterior teeth with grafting material of autogenous tooth bone vs xenogenic bone. BMC Oral Health 2019, 19, 266. [Google Scholar] [CrossRef] [PubMed]
- Moore, W.R.; Graves, S.E.; Bain, G.I. Synthetic bone graft substitutes. ANZ J. Surg. 2001, 71, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Fairbairn, P.; Leventis, M.; Mangham, C.; Horowitz, R. Alveolar Ridge Preservation Using a Novel Synthetic Grafting Material: A Case with Two-Year Follow-Up. Case Rep. Dent. 2018, 2018, 6412806. [Google Scholar] [CrossRef]
- Jambhekar, S.; Kernen, F.; Bidra, A.S. Clinical and histologic outcomes of socket grafting after flapless tooth extraction: A systematic review of randomized controlled clinical trials. J. Prosthet. Dent. 2015, 113, 371–382. [Google Scholar] [CrossRef]
- Cheng, K.; Zhu, W.; Weng, X.; Zhang, L.; Liu, Y.; Han, C.; Xia, W. Injectable tricalcium phosphate/calcium sulfate granule enhances bone repair by reversible setting reaction. Biochem. Biophys. Res. Commun. 2021, 557, 151–158. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Liu, X.; Zhang, L.-P.; Ai, H.-J.; Cui, F.-Z. Improvement on the Performance of Bone Regeneration of Calcium Sulfate Hemihydrate by Adding Mineralized Collagen. Tissue Eng. Part A 2010, 16, 2075–2084. [Google Scholar] [CrossRef]
- Huhtinen, R.; Sandeman, S.; Rose, S.; Fok, E.; Howell, C.; Fröberg, L.; Moritz, N.; Hupa, L.; Lloyd, A. Examining porous bio-active glass as a potential osteo-odonto-keratoprosthetic skirt material. J. Mater. Sci. Mater. Med. 2013, 24, 1217–1227. [Google Scholar] [CrossRef]
- Mahdavi, F.S.; Salehi, A.; Seyedjafari, E.; Mohammadi-Sangcheshmeh, A.; Ardeshirylajimi, A. Bioactive glass ceramic nanoparticles-coated poly(l-lactic acid) scaffold improved osteogenic differentiation of adipose stem cells in equine. Tissue Cell 2017, 49, 565–572. [Google Scholar] [CrossRef]
- Vazquez, D.; Takagi, S.; Frukhtbeyn, S.; Chow, L.C. Effects of addition of mannitol crystals on the porosity and dissolution rates of a calcium phosphate cement. J. Res. Natl. Inst. Stand. Technol. 2010, 115, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Burguera, E.F.; Carey, L.E. Strong, macroporous, and in situ-setting calcium phosphate cement-layered structures. Biomaterials 2007, 28, 3786–3796. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Schek, R.M.; Mistry, A.S.; Shi, X.; Mikos, A.G.; Krebsbach, P.H.; Hollister, S.J. Functional Bone Engineering Using ex Vivo Gene Therapy and Topology-Optimized, Biodegradable Polymer Composite Scaffolds. Tissue Eng. 2005, 11, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lim, J.; Teoh, S.-H. Review: Development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol. Adv. 2013, 31, 688–705. [Google Scholar] [CrossRef] [PubMed]
- Komath, M.; Varma, H.K. Development of a fully injectable calcium phosphate cement for orthopedic and dental applications. Bull. Mater. Sci. 2003, 26, 415–422. [Google Scholar] [CrossRef]
- Ricci, J.L.; Weiner, M.J.; Mamidwar, S.; Alexander, H. Calcium Sulphate: Bioceramics and Their Clinical Application; Woodhead Publishing: Sawston, UK, 2008; p. 310. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, T.; Wang, Y.; Wang, Z.; Zhang, P.; Liu, J. Environmental pH-controlled loading and release of protein on mesoporous hydroxyapatite nanoparticles for bone tissue engineering. Mater. Sci. Eng. C 2015, 46, 158–165. [Google Scholar] [CrossRef]
- Chen, H.; Shen, M.; Shen, J.; Li, Y.; Wang, R.; Ye, M.; Li, J.; Zhong, C.; Bao, Z.; Yang, X.; et al. A new injectable quick hardening anti-collapse bone cement allows for improving biodegradation and bone repair. Biomater. Adv. 2022, 141, 213098. [Google Scholar] [CrossRef]
- Tadic, D.; Beckmann, F.; Schwarz, K.; Epple, M. A novel method to produce hydroxyapatite objects with interconnecting porosity that avoids sintering. Biomaterials 2004, 25, 3335–3340. [Google Scholar] [CrossRef]
- Arabnejad, S.; Johnston, R.B.; Pura, J.A.; Singh, B.; Tanzer, M.; Pasini, D. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints. Acta Biomater. 2016, 30, 345–356. [Google Scholar] [CrossRef]
- Yang, D.A.; Yang, Z.; Hu, W.; Li, Y.Y.; Wang, H. Synthesis of Calcium Phosphate/Gypsum Composite Powders. Key Eng. Mater. 2007, 280–283, 1559–1562. [Google Scholar]
- Mao, K.; Yang, Y.; Li, J.; Hao, L.; Tang, P.; Wang, Z.; Wen, N.; Du, M.; Wang, J.; Wang, Y. Investigation of the histology and interfacial bonding between carbonated hydroxyapatite cement and bone. Biomed. Mater. 2009, 4, 045003. [Google Scholar] [CrossRef] [PubMed]
- Tee, B.C.; Desai, K.G.H.; Kennedy, K.S.; Sonnichsen, B.; Kim, D.-G.; Fields, H.W.; Mallery, S.R.; Schwendeman, S.P.; Sun, Z. Reconstructing jaw defects with MSCs and PLGA-encapsulated growth factors. Am. J. Transl. Res. 2016, 8, 2693–2704. [Google Scholar] [PubMed]
- Ren, L.; Zhang, Z.; Deng, C.; Zhang, N.; Li, D. Antibacterial and pro-osteogenic effects of β-Defensin-2-loaded mesoporous bioglass. Dent. Mater. J. 2021, 40, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Menezes, J.; Bonardi, J.; Griza, G.; Okamoto, R.; Hochuli-Vieira, E. Comparative study of volumetric changes and trabecular microarchitecture in human maxillary sinus bone augmentation with bioactive glass and autogenous bone graft: A prospective and randomized assessment. Int. J. Oral Maxillofac. Surg. 2018, 47, 665–671. [Google Scholar] [CrossRef]
- Zheng, A.; Wang, X.; Xin, X.; Peng, L.; Su, T.; Cao, L.; Jiang, X. Promoting lacunar bone regeneration with an injectable hydrogel adaptive to the microenvironment. Bioact. Mater. 2022, 21, 403–421. [Google Scholar] [CrossRef]
- Krishnan, V.; Lakshmi, T. Bioglass: A novel biocompatible innovation. J. Adv. Pharm. Technol. Res. 2013, 4, 78–83. [Google Scholar] [CrossRef]
| Group | Component | |
|---|---|---|
| 1 | Control | Rebone (Commercial CP) |
| 2 | 0% BG | 0% BG + 50% CSH + 50% CP |
| 3 | 10% BG | 10% BG + 40% CSH + 50% CP |
| 4 | 20% BG | 20% BG + 30%CSH + 50%CP |
| 5 | 30% BG | 30% BG + 20% CSH + 50% CP |
| Gene | Forward | Reverse | Species |
|---|---|---|---|
| Gapdh | CGACAGTCAGCCGCATCTT | CCAATACGACCAAATCCGTTG | Mouse |
| Runx2 | GATGACACTGCCACCTCTGAC | GGGATGAAATGCTTGGGAAC | Mouse |
| Col1 | GATGGAGAACTGTCATGGGAAC | GGAGGATCATAGTGAGAGCGG | Mouse |
| Ocn | AGCAAAGGTGCAGCCTTTGT | GCGCCTGGGTCTCTTCACT | Mouse |
| Osx | CCTCCTCAGCTCACCTTCTC | GTTGGGAGCCCAAATAGAAA | Mouse |
| Opg | ACCCAGAAACTGGTCATCAGC | CTGCAATACACACACTCATCACT | Mouse |
| B | Ca | Mg | P | Si | Sr | |
|---|---|---|---|---|---|---|
| Rebone | 0.57 | 39 | 7.05 | 42 | 8.07 | 0.04 |
| 0% BG | 0.31 | 52 | 15.15 | 28 | 0.59 | 0.02 |
| 10% BG | 4.04 | 557 | 102.6 | 2.21 | 18.73 | 0.73 |
| 20% BG | 3.54 | 368 | 64.74 | 1.02 | 47.61 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, T.; Song, D.; Hu, S.; Li, X.; Li, M.; Wang, L.; Feng, H. Structure and Properties of Bioactive Glass-Modified Calcium Phosphate/Calcium Sulfate Biphasic Porous Self-Curing Bone Repair Materials and Preliminary Research on Their Osteogenic Effect. Materials 2022, 15, 7898. https://doi.org/10.3390/ma15227898
Tan T, Song D, Hu S, Li X, Li M, Wang L, Feng H. Structure and Properties of Bioactive Glass-Modified Calcium Phosphate/Calcium Sulfate Biphasic Porous Self-Curing Bone Repair Materials and Preliminary Research on Their Osteogenic Effect. Materials. 2022; 15(22):7898. https://doi.org/10.3390/ma15227898
Chicago/Turabian StyleTan, Tao, Danyang Song, Suning Hu, Xiangrui Li, Mei Li, Lei Wang, and Hailan Feng. 2022. "Structure and Properties of Bioactive Glass-Modified Calcium Phosphate/Calcium Sulfate Biphasic Porous Self-Curing Bone Repair Materials and Preliminary Research on Their Osteogenic Effect" Materials 15, no. 22: 7898. https://doi.org/10.3390/ma15227898
APA StyleTan, T., Song, D., Hu, S., Li, X., Li, M., Wang, L., & Feng, H. (2022). Structure and Properties of Bioactive Glass-Modified Calcium Phosphate/Calcium Sulfate Biphasic Porous Self-Curing Bone Repair Materials and Preliminary Research on Their Osteogenic Effect. Materials, 15(22), 7898. https://doi.org/10.3390/ma15227898







