Additive Manufacturing of AlSi10Mg and Ti6Al4V Lightweight Alloys via Laser Powder Bed Fusion: A Review of Heat Treatments Effects
Abstract
:| Table of Contents | ||
| Abstract | 1 | |
| 1. | Introduction | 2 |
| 2. | Laser-Pwoder Bed Fusion (L-PBF) Process | 3 |
| Process Parameters | 6 | |
| 3. | L-PBFed AlSi10Mg: Microstructure | 12 |
| 3.1. | As-Built Microstructure | 12 |
| 3.2. | Heat-Treated Microstructure | 18 |
| 4. | L-PBFed AlSi10Mg: Mechanical Properties | 22 |
| 5. | L-PBFed AlSi10Mg: The corrosion Resistance | 30 |
| 6. | L-PBFed Ti6Al4V: Microstructure | 33 |
| 6.1. | As-Built Microstructure | 33 |
| 6.2. | Heat-Treated Microstructure | 42 |
| Heat Treatments Effects on α′, α and β Phases | 51 | |
| 7. | L-PBFed Ti6Al4V: Mechanical Properties | 56 |
| 8. | L-PBFed Ti6Al4V: The Corrosion Resistance | 67 |
| 9. | Conclusions | 67 |
| 9.1 | Microstructure and Corrosion Resistance of AlSi10Mg | 68 |
| 9.2 | Mechanical Properties of AlSi10Mg | 68 |
| 9.3 | Microstructure and Corrosion Resistance of Ti6Al4V | 68 |
| 9.4 | Mechanical Properties of Ti6Al4V | 69 |
| 10. | Future Trends and Prospective | 69 |
| 11. | Acronyms | 70 |
| References | 71 | |
1. Introduction
2. Laser-Powder Bed Fusion (L-PBF) Process
2.1. Process Parameters
3. L-PBFed AlSi10Mg: Microstructure
3.1. As-Built Microstructure
3.2. Heat-Treated Microstructure
4. L-PBFed AlSi10Mg: Mechanical Properties
5. L-PBFed AlSi10Mg: The Corrosion Resistance
- The amount of the Si rejected from the α-Al;
- The formation of the ε-Mg2Si precipitates that increase the galvanic couple.
6. L-PBFed Ti6Al4V: Microstructure
6.1. As-Built Microstructure
6.2. Heat-Treated Microstructure
- low-SSTR (Solid Solution Temperature Region): Tdess < T ≤ 800 °C
- medium-SSTR: 800 < T ≤ 900 °C (~T0)
- high-SSTR: 900 < T ≤
Heat Treatment Effects on α′, α and β Phases
- direct cylinderization (Figure 53a): initiates at the edge of the α platelet where the different curvature allows for the mass transfer to the flat part of the same platelet. The subsequent formation of these ridges can induce a cylindrical morphology if the ridges join together.
- thermal grooving and boundary splitting (Figure 53c): induce α globularization due to the initial groove’s formation into triple junction generated by sub-grain boundaries into α lamellae with α/β interface, and due to the sequent Al and V diffusion that break the lamella with the β-phase formation.
- termination migration consists of the mass transfer from the curved surface of the lamella to the flat lamella.
7. L-PBFed Ti6Al4V: Mechanical Properties
| Process Parameters | ED (J/mm3) | Directions | Microstructure | E (GPa) | UTS (MPa) | YS (MPa) | A (%) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P (W) | v (mm/s) | h (μm) | t (μm) | BP (°C) | ||||||||
| -- | -- | -- | -- | -- | -- | -- | -- | -- | 895 | 825 | 10 | [318] |
| -- | -- | -- | -- | -- | -- | H(xy) | Fully α′ martensite | -- | 1274 ± 26 | 1047 ± 23 | 10 ± 1 | [305] |
| V | -- | 1219 ± 32 | 1043 ± 18 | 12 ± 1 | ||||||||
| 200 | -- | 150 | 150 | -- | -- | -- | Fully α′ martensite | -- | 1191 ± 6 | 970 ± 6 | 5.4 ± 1.4 | [265] |
| 200 | -- | 80 | 50 | 100 | -- | -- | Fully α′ martensite | 114 ± 5 | 1123 | 1139 | 6.0 | [226] |
| 370 | -- | Fully α′ martensite with increased laths 1 | 1234 | 1159 | 9.5 | |||||||
| 470 | -- | α′ → α decomposition | 1232 | 1173 | 9.7 | |||||||
| 570 | -- | Colonies α + β | 1233 | 1176 | 10.0 | |||||||
| 200 | -- | 80 | 50 | 670 | -- | Colonies α + β + β nano-particles + α globularization | 1201 | 1174 | 2.58 | [226] | ||
| 770 | -- | Increase the effects obtained at 670 °C | 207 2 | 748 2 | -- | -- | ||||||
| -- | -- | -- | -- | 37 | -- | -- | Fully α′ martensite | -- | 1236 ± 27 | 1181 ± 34 | 8.7 ± 0.5 | [285] |
| 1000 | 1400 | 230 | 100 | 70 | 38.3 | H | Fully α′ martensite (< 1μm) | -- | 1204 ± 27 | 1052 ± 16 | 2.1 ± 0.3 | [288] |
| V | -- | 1075 ± 80 | 958 ± 73 | 1.8 ± 0.2 | ||||||||
| 800 | 1925 | 190 | 50 | 43.7 | H | Fully α′ martensite (1–2 μm) | -- | 1176 ± 8 | 1024 ± 6 | 2.5 ± 0.4 | ||
| V | -- | 1086 ± 6 | 933 ± 7 | 2.5 ± 0.3 | ||||||||
| 350 | 770 | 180 | 50 | 50.5 | H | -- | 1251 ± 114 | 1067 ± 27 | 3.4 ± 1.3 | |||
| V | -- | 1191 ± 19 | 1065 ± 27 | 2.4 ± 1.2 | ||||||||
| 170 | 1250 | 100 | 30 | -- | 45.33 | -- | Fully α′ martensite | 109 ± 4 | 1218 ± 2 | 1015 ± 10 | 5.9 ± 1.0 | [271] |
| 375 | 1029 | 120 | 60 | -- | 50.62 | V | Ultrafine α + β | -- | ~1240 | 1106 ± 6 | 11.4 ± 0.4 | [221] 3 |
| 280 | 1200 | 140 | 30 | 100 | 55.56 | -- | Fully α′ martensite | 1206 ± 23 | 1041 ± 23 | 9.6 ± 0.4 | [288] | |
| 175 | 710 | 120 | 30 | -- | 68.47 | V | Fully α′ martensite | -- | ~1160 | -- | ~9 | [221] 3 |
| 175 | 710 | 120 | 30 | -- | 68.47 | H | Fully α′ martensite | -- | 1321 ± 6 | 1166 ± 6 | 2.0 ± 0.7 | [319] |
| 250 | 1600 | 60 | 30 | -- | 86.81 | -- | Fully acicular α′ martensite | -- | 1267 ± 5 | 1110 ± 9 | 7.3 ± 1.1 | [32] |
| 157 | 225 | 100 | 50 | -- | 139.56 | H(xz) | Fully α′ martensite | 115 ± 6 | 1143 ± 6 | 978 ± 5 | 11.8 ± 0.5 | [59] |
| H(xy) | 113 ± 5 | 1199 ± 49 | 1075 ± 25 | 7.6 ± 0.5 | ||||||||
| V | 119 ± 7 | 1117 ± 3 | 967 ± 10 | 8.9 ± 0.4 | ||||||||
8. L-PBFed Ti6Al4V: The Corrosion Resistance
9. Conclusions
9.1. Microstructure and Corrosion Resistance of AlSi10Mg
- The as-built microstructure is formed by a Si-eutectic network containing the α-Al matrix with Si particles, β-Al5FeSi intermetallics, GP zones and finely dispersed ε″/ε′ precipitates depending on the process parameters and/or BP temperatures.
- Increasing the BP temperature leads to increased stress relaxation, the number of precipitates and their size. Such effects decrease with increasing distance from the pre-heated BP plate.
- DA and SR: precipitation phenomena induced on as-built sample manufactured on a cold BP and Si-eutectic network destruction at T > 200 °C with Si particle coarsening.
- SHT (+AA): Si particles coarsen with total Si-eutectic network destruction and microstructure recrystallization. This induces precipitation phenomena and β-Al5FeSi formation. T6 can increase the vol% of pores, unlike the HIP HT.
- The corrosion resistance, which is characterized by an anisotropic mechanism in full and quasi-cellular structures, decreases from the as-built samples to the SR and T6 heat-treated samples due to both variations in the Si eutectic and precipitation phenomena.
9.2. Mechanical Properties of AlSi10Mg
- 6.
- Due to the strengthening phenomena conferred by this microstructure, the mechanical behavior is strongly anisotropic, exhibiting high tensile strength (UTS > ~400 MPa, YS > ~240 MPa) and low elongation (A < ~9%).
- 7.
- The post-HT tensile strength generally decreases while the ductility increases with increasing heat treatment temperatures. By increasing the DA temperatures above 255 °C, initiation of Si-eutectic network destruction induces a decrease in the tensile strength (UTS = ~340 MPa, YS = ~200 MPa) but also an increase in elongation with values higher than 10%. In this scenario, the anisotropic mechanical performance can be eliminated after the SR at 300 °C × 2 h.
- 8.
- The ductility can reach 23–29% after T6 and HIP HTs. Firstly, the subsequent AA to complete the T6 heat treatment recovers the tensile strength due to precipitation of the ε-Mg2Si (UTS = 230–330 MPa, YS = 180–280 MPa). Secondly, the tensile strength can be increased with the T6 rather than a DA after the HIP heat treatments.
- 9.
- In as-built samples and DA samples at low temperatures, the fracture mechanisms are dominated by both the laser scan tracks and MP boundaries, where the crack generally propagates (inter-layer and inter-track fractures). In other cases, the crack also propagates inside the center of the MP, generating a trans-track fracture. In relation to the SR at 300 °C and, therefore, after the T6 and HIP heat treatments, the fracture mechanisms are dominated by the Si-eutectic particles which generate the voids at their interface with the α-Al matrix. In this case, cracks can be deflected by the laser scan tracks and MP boundaries remaining after the heat treatment at high temperatures.
9.3. Microstructure and Corrosion Resistance of Ti6Al4V
- 10.
- The as-built microstructure is formed by columnar β-grains arranged along the directions of the heat flux. In relation to the BP temperature, process parameters and the distance from the BP plane, columnar grains can contain a fully α′ microstructure (primary, secondary, tertiary and quartic α′-martensite’s), a mixture of α′ and α + β lamellar phase, or a fully ultrafine α + β microstructure.
- 11.
- Corrosion resistance is influenced by the β grain boundary density and is characterized by anisotropic behavior, increasing from the as-built state to samples formed by an α + β microstructure due to the higher number of nucleation sites where the passivation layer can form.
- 12.
- The heat-treated microstructure is progressively more influenced by heat treatment temperatures as the latter increase. Starting from ~400 °C, the α′ → α decomposition takes place inducing the diffusion of the Al and V, which cause β precipitation. At around 704–705 °C, the α′ → α + β transformation occurs, increasing drastically after the 800 °C up to the β-transus. Above the critical temperature, and into high-SSTR, the tensile strength can be recovered with appropriate cooling methods, remembering that the α-phase progresses towards globularization. These effects become progressively more important above the β-transus, where the recrystallization process from columnar to equiaxed grains also begins, depending on the time that the material is held at this temperature.
9.4. Mechanical Properties of Ti6Al4V
- 13.
- The UTS, YS (higher than 1.1 GPa and 950 MPa, respectively) and elongation (A < 9%) do not exhibit great differences between as-built samples formed by α′ martensite or ultrafine α + β microstructure.
- 14.
- The SR and ANN heat treatments performed in low-SSTR, which are not affected by the different cooling rate (WQ, AC, FC), induce slight coarsening effects that reflect on the tensile strength: UTS > 1 GPa, YS > 900 MPa and A < 10%. Between the medium- and high-SSTRs, coarsening effects increase and the α′ → α + β is completed. In addition, the cooling method becomes significant, reaching the T0. The UTS and YS decrease up to ~970 and ~900 MPa, respectively, after the ANN HT at 850 °C × 5 h (FC).
- 15.
- The ductility also increases up to 14%, and the tensile strengths decrease at values that can also satisfy the ASTM F2924-12 standard specification after HTs performed into high-SSTR.
- 16.
- The balance between the tensile strengths and elongation can be obtained with a bimodal and/or a trimodal microstructure after an appropriate combination of different ANN heat treatments below and/or above the β-transus.
- 17.
- The fracture mechanisms of the as-built and unrecrystallized samples are related, firstly, to the load conditions and columnar β-grain directions and, secondly, to the α-phase crystallographic orientations. This damaging behavior, together with the presence of LOF pores in the xy plane, induces significant anisotropic mechanical properties that can be reduced with the HIP heat treatment. In this case, the as-built samples transform their structure into α + β Widmanstätten, where the β-phase between two adjacent α lamellae modifies the fracture mechanism. Focusing on bimodal and/or trimodal microstructures, crack propagation follows the α-phase, and is finally characterized by a tortuous path due to the presence of the β-grains boundaries and globular αp phase. The greatest effects are induced when this globular phase is located at β-grains boundary.
10. Future Trends and Prospective
11. Acronyms
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, J.H.; Yahata, B.D.; Hundley, J.M.; Mayer, J.A.; Schaedler, T.A.; Pollock, T.M. 3D printing of high-strength aluminium alloys. Nature 2017, 549, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.C.; Boyer, R.B. Opportunities and issues in the application of titanium alloys for aerospace components. Metals 2020, 10, 705. [Google Scholar] [CrossRef]
- Vanmuster, L.; D’Haeyer, C.; Coucke, P.; Braem, A.; Van Hooreweder, B. Mechanical behavior of Ti6Al4V produced by laser bed fusion with engineered open porosity for dental applications. J. Mech. Behav. Biomed. Mater. 2022, 126, 104974. [Google Scholar] [CrossRef]
- Bertam, M.; Buxmann, K.; Furrer, P. Analysis of greenhouse gas emissions related to aluminium transport applications. Int. J. Life Cycle Assess. 2009, 14, 62–69. [Google Scholar] [CrossRef]
- Rugg, D.; Dixon, M.; Burrows, J. High-temperature application of titanium alloys in gas turbine. Material life cycle opportunities and threats—An industrial perspective. Mater. High Temp. 2016, 33, 536–541. [Google Scholar] [CrossRef]
- EOS GmBH—Electro Optical Systems. Material Data Sheet: EOS Aluminium AlSi10Mg. München. Available online: www.eos.info (accessed on 6 December 2021).
- Aboulkhair, N.T.; Tuck, C.; Ashcroft, I.; Maskery, I.; Everitt, N.M. On the precipitation hardening of selective laser melted AlSi10Mg. Metall. Mater. Trans. A 2015, 46, 3337–3341. [Google Scholar] [CrossRef]
- Hartlieb, M. Aluminium alloys for structural die casting. Die Cast. Eng. 2013, 57, 40–43. [Google Scholar]
- Ghio, E.; Cerri, E. Work hardening in heat-treated AlSi10Mg alloy manufactured by selective laser melting: Effect of Layer Thickness and Hatch Spacing. Materials 2021, 14, 4901. [Google Scholar] [CrossRef]
- Kempen, K.; Thijs, L.; Van Humbeeck, J.; Kruth, J.-P. Mechanical properties of ALSi10Mg produced by selective laser melting. Phys. Procedia 2012, 39, 439–446. [Google Scholar] [CrossRef] [Green Version]
- Alghamdi, F.; Haghshenas, M. Microstructural and small-scale characterization of additive manufactured AlSi10Mg alloy. SN Appl. Sci. 2019, 1, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Schmid-Fetzer, R.S. Phase-diagram: The Beginning of Wisdom. J. Phase Equilibria Diff. 2014, 35, 735–760. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.V.; Rometsch, P.; Davies, C.H.J.; Huang, A.; Wu, X. Effect of heat treatment on the microstructure and anisotropy in mechanical properties of A357 alloy produced by selective laser melting. Mater. Des. 2018, 154, 275–290. [Google Scholar] [CrossRef]
- Cerri, E.; Ghio, E.; Bolelli, G. Effect of the distance from BP and post-heat treatment of AlSi10Mg manufactured by single- and multi-laser selective laser melting. JMEP 2021, 30, 4981–4992. [Google Scholar] [CrossRef]
- Caceres, C.H.; Davidson, C.J.; Griffiths, J.R.; Hogan, L.M.; Wang, Q.C. Hypoeutectic Al-Si-Mg foundry alloys. Metall. Mater. Trans. A 1999, 30, 2611–2618. [Google Scholar] [CrossRef]
- Wang, Y.; Liao, H.; Wu, Y.; Yang, J. Effect of Si content on microstructure and mechanical properties of Al-Si-Mg alloys. Mater. Des. 2014, 53, 634–638. [Google Scholar] [CrossRef]
- Donachie, M.J. Titanium: A Technical Guide, 2nd ed.; ASM International: Materials Park, OH, USA, 2000. [Google Scholar]
- Ducato, A.; Fratini, L.; La Cascia, M.; Mazzola, G. An automated visual inspection system for the classification of the phasaes of Ti6Al4V titanium alloy. In Proceedings of the Computers Analysis of Images and Patterns, York, UK, 27–29 August 2013; Springer: Berlin/Heidelberg, Germany, 2013; pp. 362–369. [Google Scholar] [CrossRef] [Green Version]
- Bower, K.; Murray, S.; Reinhart, A.; Nieto, A. Corrosion resistance of selective laser melted Ti-6Al-4V alloy in salt for environment. Res. Mater. 2020, 8, 100122. [Google Scholar] [CrossRef]
- Murr, L.E.; Quinones, S.A.; Gaytan, S.M.; Lopez, M.I.; Rodela, A.; Martinez, E.Y.; Hernandez, D.M.; Martinez, E.; Medina, F.; Wicker, R.B. Microstructure and mechanical properties of Ti-6Al-4V produced by rapid-layer manufacturing, for biomedical applications. J. Mech. Behav. Biomed. Mater. 2009, 2, 20–32. [Google Scholar] [CrossRef]
- Mistry, S.; Roy, S.; Maitra, N.J.; Roy, R.; Datta, S.; Chandra, A.; Sarkak, S. Safety and efficacy of additive and subtractive surface modification of Ti6Al4V endossesous implant in goat bone. J. Mech. Behav. Biomed. Mater. 2016, 57, 69–87. [Google Scholar] [CrossRef]
- Śliwa, R.E.; Bernaczek, J.; Budzik, G. The application of direct metal laser sintering (DMLS) of titanium alloy powder in fabricating components of aircraft structures. Key Eng. Mater. 2016, 687, 199–205. [Google Scholar] [CrossRef]
- Sibisi, N.P.; Popoola, A.P.I.; Arthur, N.K.K.; Pityana, S.L. Review on direct metal laser deposition manufacturing technology for Ti6Al4V alloy. J. Adv. Manuf. Tachnol. 2020, 107, 1163–1178. [Google Scholar] [CrossRef]
- Seshacharyulu, T.; Medeiros, S.C.; Morgan, J.T.; Malas, J.C.; Frazier, W.G.; Pradas, Y.V.R.K. Hot deformation and microstructural damage mechanisms in extra-low interstitial (ELI) grade Ti-6Al-4V. Mater. Sci. Eng. A 2020, 279, 289–299. [Google Scholar] [CrossRef]
- Chandra, T.; Wanderka, N.; Reimers, W.; Ionescu, M. Study of alpha-beta transformation in Ti-6Al-4V ELI, Mechanical and Microstructural characteristics. Mater. Sci. Forum 2010, 638–642, 712–717. [Google Scholar] [CrossRef]
- Lee, H.-S.; Yoon, J.-H.; Park, C.H.; Ko, Y.G.; Shin, D.H.; Lee, C.S. A study of diffusion bonding of superplastic Ti-6Al-4V ELI grade. J. Mater. Process. Technol. 2007, 187–188, 526–529. [Google Scholar] [CrossRef]
- Chang, J.Z.-C.; Tsai, P.-I.; Kuo, M.Y.-P.; Sun, J.-S.; Chen, S.-Y.; Shen, H.-H. Augmentation of DMLS biomimetic dental implants with weight bearing strut to balance of biological and mechanical demands: From Bench to Animal. Materials 2019, 12, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaikh, A.; Kumar, S.; Dawari, A.; Kirwai, S.; Patil, A.; Singh, R. Effect of temperature and cooling rates of α + β morphology of Ti-6Al-4V alloy. Procedia Struct. Integr. 2019, 14, 782–789. [Google Scholar] [CrossRef]
- Zhong, C.; Liu, J.; Zhao, T.; Schopphoven, T.; Fu, J.; Gasser, A.; Schleifenbaum, J.H. Laser metal deposition of Ti-6Al-4V—A brief review. Appl. Sci. 2019, 10, 764. [Google Scholar] [CrossRef] [Green Version]
- Gallaraga, H.; Warren, R.J.; Lados, D.A.; Dehoff, R.R.; Kirka, M.M.; Naudwana, P. Effects of heat treatments on microstructure and properties of Ti-6Al-4V ELI alloy fabricated by electron beam melting (EBM). Mater. Sci. Eng. A 2017, 685, 417–428. [Google Scholar] [CrossRef] [Green Version]
- Shao, H.; Zhao, Y.; Ge, P.; Zeng, W. Influence of cooling rate and aging on the lamellar microstrucutre and fractography of TC21 titanium alloy. Metallogr. Microstruct. Anal. 2013, 2, 35–41. [Google Scholar] [CrossRef] [Green Version]
- Vrancken, B.; Thijs, L.; Kruth, J.-P.; Van Humbeeck, J. Heat treatment of Ti6Al4V produced by selective laser melting: MicroStrucutre and Mechanical Properties. J. Alloys Compd. 2012, 541, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Riddle, M.; Graziano, D.; Warren, J.; Das, S.; Nimbalkar, S.; Cresko, J.; Masanet, E. Energy and emissions saving potential of additive manufacturing: The Case of Lightweight Aircraft Components. J. Chem. Prod. 2016, 135, 1559–1570. [Google Scholar] [CrossRef] [Green Version]
- Duda, T.; Raghanav, L.V. 3D metal printing technology: The Need to Re-invent Design Practice. AI Soc. 2018, 33, 241–252. [Google Scholar] [CrossRef]
- ASTM 52900:2015(E); Standard Terminology for Additive Manufacturing–General Principles–Terminology. ASTM: West Conshohocken, PA, USA, 2000.
- Khorasani, M.; Ghasemi, A.H.; Rolfe, B.; Gibson, I. Additive manufacturing a powerful tool for the aerospace industry. Rapid Prototyp. J. 2022, 28, 87–100. [Google Scholar] [CrossRef]
- Giganto, S.; Martínez-Pellitero, S.; Cuesta, E.; Zapico, P.; Barreiro, J. Proposal of design rules for improving the accuracy of selective laser melting (SLM) manufacturing using benchmarks parts. Rapid Prototyp. J. 2022. [Google Scholar] [CrossRef]
- Zhou, Y.; Abbara, M.E.; Fiang, D.; Azizi, A.; Poliks, M.D.; Ning, F. High-cycle fatigue properties of curved-surface AlS10Mg parts fabricated by powder bed fusion additive manufacturing. Rapid Prototyp. J. 2022. [Google Scholar] [CrossRef]
- Babuska, T.F.; Krick, B.A.; Susan, D.F.; Kustas, A.B. Comparison of powder bed fusion and direct energy deposition for tailoring mechanical properties of traditionally brittle alloys. Manuf. Lett. 2021, 28, 30–34. [Google Scholar] [CrossRef]
- Teixeira, Ó.; Silva, F.J.G.; Ferreira, L.P.; Atzeni, E. A review of heat treatments on Improving the quality and residual stresses of the Ti-6Al-4V parts produced by Additive Manufacturing. Materials 2020, 10, 1006. [Google Scholar] [CrossRef]
- Dutta, B.; Froes, F.H.S. The additive manufacturing (AM) of titanium alloys. Met. Powder Rep. 2017, 72, 96–106. [Google Scholar] [CrossRef]
- Arias-Gonzáles, F.; Rodríguez-Contreras, A.; Punset, M.; Manero, J.M.; Barro, Ó.; Fernández-Arias, M.; Lusquiños, F.; Javier Gil, F.; Pou, J. In-Situ laser direct energy deposition of biomedical Ti-Nb and Ti-Zr-Nb alloys from elemental powders. Materials 2021, 11, 1205. [Google Scholar] [CrossRef]
- Lee, H.; Lim, C.H.J.; Low, M.J.; Tham, N.; Murukeshan, V.M.; Kim, Y.-J. Lasers in additive manufacturing: A Review. Int. J. Precis. Eng. Manuf.-Green Technol. 2017, 4, 307–322. [Google Scholar] [CrossRef]
- Sames, W.J.; List, F.A.; Pannala, S.; Dehoff, R.R.; Babu, S.S. The metallurgy and processing science of metal additive manufacturing. Int. Mater. Rev. 2016, 61, 315–360. [Google Scholar] [CrossRef]
- Galba, M.J.; Reischle, M. Additive manufacturing of metals using powder-based technology. In Additive Manufacturing, 1st ed.; Bandyopadhyay, A., Bose, S., Eds.; Taylor & Francis Group: Boca Raton, FL, USA, 2016; Volume 4, p. 46. [Google Scholar] [CrossRef]
- Yang, L.; Hsu, K.; Baughman, B.; Godfrey, D.; Medina, F.; Menon, M.; Wiener, S. Additive Manufacturing of Metals: The Technology, Materials, Design and Production; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Kruth, J.-P.; Kumar, S. Statistical analysis of experimental parameters in selective laser sintering. Adv. Eng. Mater. 2005, 7, 750–755. [Google Scholar] [CrossRef]
- Tang, M.; Pistorius, P.C.; Benth, J.L. Prediction of a lack-of-fusion porosity for powder bed fusion. Add. Manuf. 2017, 14, 39–48. [Google Scholar] [CrossRef]
- Letenneur, M.; Kreitcberg, A.; Brailovski, V. Optimization of laser powder bed fusion processing using a combination of melt pool modeling and design of experiment approaches: Density Control. Manuf. Mater. Process. 2019, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Bai, S.; Perevoshchikova, N.; Sha, Y.; Wu, X. The effects of selective laser melting process parameters on relative density of the AlSi10Mg parts suitable procedures of the archimedes method. Appl. Sci. 2019, 9, 583. [Google Scholar] [CrossRef] [Green Version]
- Aboulkhair, N.T.; Everitt, N.M.; Ashcroft, I.; Tuck, C. Reducing porosity in AlSi10Mg parts processed by selective laser melting. Add. Manuf. 2014, 1–4, 77–86. [Google Scholar] [CrossRef]
- Majeed, A.; Zhang, Y.; Lv, J.; Peng, T.; Atta, Z.; Ahmed, A. Investigation of T4 and T6 heat treatment influences on relative density and porosity of AlSi10Mg alloy components manufactured by SLM. Comput. Ind. Eng. 2020, 139, 106194. [Google Scholar] [CrossRef]
- Girelli, L.; Tocci, M.; Gelfi, M.; Pola, A. Study of heat treatment parameters for additively manufactured AlSi10Mg in comparison with corresponding cast alloy. Mater. Sci. Eng. A 2019, 739, 317–328. [Google Scholar] [CrossRef]
- Yang, T.; Liu, T.; Liao, W.; MacDonald, E.; Wei, H.; Zhang, C.; Chen, X.; Zhang, K. Laser powder bed fusion of AlSi10Mg: Influence of Energy Intensities on Spatter and Porosity Evolution, Microstructure and Mechanical Properties. J. Alloys Compd. 2020, 849, 156300. [Google Scholar] [CrossRef]
- Riener, K.; Oswald, S.; Winkler, M.; Leichtfried, G.J. Influence of storage conditions and reconditioning of AlSi10Mg powder on the quality of parts produced by laser powder bed fusion (LPBF). Add. Manuf. 2021, 39, 101896. [Google Scholar] [CrossRef]
- Hastie, J.C.; Kartal, M.E.; Carte, L.N.; Attalah, M.M.; Mulvihillm, D.M. Classifying shape on internal pores within AlSi10Mg alloy manufactured by laser powder bed fusion using 3d X-Ray microcomputed tomography: Influence of process parameters and heat treatment. Mater. Charact. 2020, 163, 110225. [Google Scholar] [CrossRef]
- Wycisk, E.; Emmelmann, C.; Siddique, S.; Walther, F. High cycle fatigue (HCF) performance of Ti-6Al-4V alloy processed by selective laser melting. Adv. Mater. Res. 2013, 816–817, 134–139. [Google Scholar] [CrossRef]
- Edwards, P.; Ramulu, M. Fatigue performance evaluation of selective laser melted Ti-6Al-4V. Mater. Sci. Eng. A 2014, 598, 327–337. [Google Scholar] [CrossRef]
- Simonelli, M.; Tse, Y.Y.; Tuck, C. Effect of the build orientation on the mechanical properties and fracture modes of SLM Ti-6Al-4V. Mater. Sci. Eng. A 2014, 616, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Khorasani, A.; Gibson, I.; Goldberg, M.; Littlefair, G. On the role of different annealing heat treatments on mechanical properties and microstructure of selective laser melted and conventional wrought Ti-6Al-4V. Rapid. Prototyp. J. 2017, 23, 295–304. [Google Scholar] [CrossRef]
- Hu, Y.N.; Wu, S.C.; Withers, P.J.; Zhang, J.; Bao, H.Y.X.; Fu, Y.N.; Kang, G.Z. The effect of manufacturing defects of the fatigue life of selective laser melted Ti-6Al-4V structures. Mater. Des. 2020, 192, 108708. [Google Scholar] [CrossRef]
- Pal, S.; Lojen, G.; Hudak, R.; Rajtukova, V.; Brajlih, T.; Kokol, V.; Drstvenšek, I. As-fabricated surface morphologies of Ti-6Al-4V samples fabricated by different laser processing parameters in selective laser melting. Add. Manuf. 2020, 33, 101147. [Google Scholar] [CrossRef]
- Xiao, Z.; Chen, C.; Xhu, H.; Hu, Z.; Nagarajan, B.; Guo, L.; Zeng, X. Study of residual stress in selective laser melting of Ti6Al4V. Mater. Des. 2020, 193, 108846. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, Y.; Wang, D.; Xiao, Z.; Song, C.; Weng, C. Effect of heat treatment on the microstructure and mechanical properties of Ti6Al4V gradient structures manufactured by selective laser melting. Mater. Sci. Eng. A 2018, 736, 288–297. [Google Scholar] [CrossRef]
- Yan, X.; Li, Q.; Yin, S.; Chen, Z.; Jenkins, R.; Chen, C.; Wang, J.; Ma, W.; Bolot, R.; Lupoi, R.; et al. Mechanical and in vitro study of anisotropic Ti6Al4V lattice structure fabricated using selective lase melting. J. Alloys Compd. 2019, 782, 209–223. [Google Scholar] [CrossRef]
- Xiong, Y.-Z.; Gao, R.-N.; Zhang, H.; Dong, L.-L.; Li, J.-T.; Li, X. Rationally designed functionally graded porous Ti6Al4V scaffolds with high strength and toughness built via selective laser melting for load-bearing orthopedic applications. J. Mech. Behav. Biomed. Mater. 2020, 104, 103673. [Google Scholar] [CrossRef]
- Bartolomeu, F.; Costa, M.M.; Alves, N.; Miranda, G.; Silva, F.S. Selective laser melting of Ti6Al4V sub-millimetric cellular structures: Prediction of Dimensional Deviations and Mechanical Performance. J. Mech. Behav. Biomed. Mater. 2021, 113, 104123. [Google Scholar] [CrossRef] [PubMed]
- Olakanmi, E.O.; Cockrane, R.; Dalgarno, K. A review of selective laser sintering/melting (SLS/SLM) of aluminium powders: Processing, Microstructures and Properties. Prog. Mater. Sci. 2015, 74, 401–477. [Google Scholar] [CrossRef]
- Metel, A.S.; Stebulyanin, M.M.; Fedorov, V.S.; Okunkova, A.A. Powder density distribution for laser additive manufacturing (SLM): Potential, Fundamentals and Advanced Applications. Technologies 2019, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Coeck, S.; Bisht, M.; Plas, J.; Verbist, F. Prediction of lack of fusion porosity in selective laser melting based on MP monitoring data. Add. Manuf. 2019, 25, 347–356. [Google Scholar] [CrossRef]
- Xu, Z.W.; Wang, Q.; Wang, X.S.; Tan, C.H.; Guo, M.H.; Gao, P.B. High fatigue performance of AlSi10mG alloy produced by selective laser melting. Mech. Mater. 2020, 148, 103499. [Google Scholar] [CrossRef]
- Li, P.-H.; Guo, W.-G.; Huang, W.-D.; Su, Y.; Liu, X.; Yuan, X.-B. Thermomechanical response of 3D laser-deposited Ti6Al4V ally over a wide range of strain rates and temperatures. Mater. Sci. Eng. A 2015, 647, 34–42. [Google Scholar] [CrossRef]
- Bayat, M.; Thanki, A.; Mohanty, S.; Witvrouw, A.; Yang, S.; Thorborg, J.; Tiedje, J.N.; Hattel, J.H. Keyhole-induced porosities in laser-powder bed fusion (L-PBF) of Ti6Al4V: High-Fidelity Modeling and Experimental Validation. Add. Manuf. 2019, 30, 100835. [Google Scholar] [CrossRef]
- Wirth, F.; Frauchiger, A.; Gutknecht, K.; Cloots, M. Influence of the inert gas flow on the laser powder bed fusion (LPBF) process. In Industrializing Additive Manufacturing; Mebolt, M., Khahn, C.A., Eds.; Springer: Cham, Switzerland, 2020; pp. 192–204. [Google Scholar] [CrossRef]
- Weingarten, C.; Buchbinder, D.; Pirch, N.; Meiners, W.; Wissenbach, K.; Propawane, R. Formation and reduction of hydrogen during selective laser melting of AlS10Mg. J. Mater. Process. Technol. 2015, 221, 112–120. [Google Scholar] [CrossRef]
- Emminghaus, N.; Paul, J.; Hoff, C.; Hermsford, J.; Kairle, S. Development of an empirical process model for adjusted porosity in laser-based powder bed fusion Ti-6Al-4V. Int. J. Adv. Manuf. Technol. 2022, 118, 1239–1254. [Google Scholar] [CrossRef]
- Shiomi, M.; Osakada, K.; Nakamura, K.; Yamashita, T.; Abe, F. Residual stress within metallic model made by selective laser melting process. CIRP Ann. 2004, 53, 195–198. [Google Scholar] [CrossRef]
- Yu, G.; Gu, D.; Dai, D.; Xia, M.; Ma, C.; Chang, K. Influence of processing parameters on laser penetration depth and melting/re-melting densification during selective laser melting of aluminum alloy. Appl. Phys. A 2016, 122, 122–134. [Google Scholar] [CrossRef]
- Yu, W.; Sing, S.L.; Chua, C.K.; Tan, X. Influence od re-melting on surface roughness and porosity of AlSi10Mg parts fabricated by selective laser melting. J. Alloys Compd. 2019, 792, 574–581. [Google Scholar] [CrossRef]
- Maconachie, T.; Leary, M.; Zhang, J.; Medvedev, A.; Sarker, A.; Ruan, D.; Lu, G.; Faruque, O.; Brandt, M. Effect of build orientation on the quasi-static and dynamic response of SLM AlSi10Mg. Mater. Sci. Eng. A 2020, 788, 139445. [Google Scholar] [CrossRef]
- Murchio, S.; Dallago, M.; Zanini, F.; Carmignato, S.; Zappini, G.; Berto, F.; Maniglio, D.; Benedetti, M. Additively manufactured Ti-6Al-4V thin structures via laser powder bed fusion: Effect of Building Orientation on Geometrical Accuracy and Mechanical Properties. J. Mech. Behav. Biomed. Mater. 2021, 119, 104495. [Google Scholar] [CrossRef] [PubMed]
- Jucther, V.; Scharowsky, T.; Singer, R.F.; Körner, C. Processing window and evaporation phenomena for Ti-6Al-4V produced by selective electron beam melting. Acta Mater. 2014, 76, 252–258. [Google Scholar] [CrossRef] [Green Version]
- King, W.E.; Barth, H.D.; Castillo, U.M.; Gallegos, G.F.; Gibbs, J.W.; Hahn, D.E.; Kamath, C.; Rubenchik, A.M. Observation of keyhole-mode laser melting in laser powder-bed fusion additive manufacturing. J. Mater. Process. Technol. 2014, 214, 2915–2925. [Google Scholar] [CrossRef]
- Martin, A.A.; Calta, N.P.; Khairallah, S.A.; Wnag, J.; Depond, P.J.; Fong, A.Y.; Thampy, V.; Guss, G.M.; Kiss, A.M.; Stone, K.H.; et al. Dynamics of pore formation during laser powder bed fusion additive manufacturing. Nat. Commun. 2019, 10, 1987–1997. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Dai, S.; Liao, H.; Zhu, H. Pores and the formation mechanisms of SLMed AlSi10Mg. Rapid Prototyp. J. 2020, 26, 1657–1664. [Google Scholar] [CrossRef]
- Strano, G.; Hao, L.; Everson, R.M.; Evans, K.E. A new approach to the design and optimization of support structures in additive manufacturing. Int. J. Adv. Manuf. Technol. 2013, 66, 1247–1254. [Google Scholar] [CrossRef]
- Macías, J.G.S.; Douillard, T.; Zhao, L.; Maire, E.; Pyka, G.; Simar, A. Influence on microstructure, strength and ductility of BP temperature during laser powder bed fusion of AlSi10Mg. Acta Mater. 2020, 201, 231–243. [Google Scholar] [CrossRef]
- Oliviera, J.P.; Lalonde, A.D.; Ma, J. Processing parameters in laser powder bed fusion metal additive manufacturing. Mater. Des. 2020, 193, 108762. [Google Scholar] [CrossRef]
- Agarwala, M.; Bourell, D.; Beaman, J.; Marcus, H.; Barlow, J. Direct selective laser sintering of metals. Rapid Prototyp. J. 1995, 1, 26–36. [Google Scholar] [CrossRef]
- Sun, S.; Brandt, M.; Easton, M. Powder bed fusion processes: An Overview. In Additive Manufacturing: Materials, Design, Technologies and Applications; Brandt, M., Deans, M., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 55–77. [Google Scholar] [CrossRef]
- Liu, S.; Guo, H. Balling behavior of stainless steel and nickel powder during selective laser melting process. Int. J. Adv. Manuf. Syst. 2020, 13, 3632–3653. [Google Scholar] [CrossRef]
- Yadroitsev, I.; Gusarov, A.; Yadroitsava, I.; Smurov, I. Single track formation in selective laser melting of metal powders. J. Mater. Process. Technol. 2010, 210, 1624–1631. [Google Scholar] [CrossRef]
- Zhou, L.; Wen, S.F.; Song, B.; Zhou, X.; Teng, Q.S.; Wei, Q.; Shi, Y.S. A novel titanium alloy manufactured by selective laser melting microstructure, high temperature oxidation. Mater. Des. 2016, 89, 1199–1204. [Google Scholar] [CrossRef]
- Zhang, B.; Li, Y.; Bai, Q. Defect formation mechanisms in selective laser melting: A review. Chin. J. Mech. Eng. 2017, 30, 515–527. [Google Scholar] [CrossRef] [Green Version]
- Gouveia, R.M.; Silva, F.J.G.; Atzeni, E.; Sormaz, D.; Alves, J.L.; Pereira, A.B. Effect of scan strategies and use of support structures on surface quality and hardness of L-PBF AlSi10Mg parts. Materials 2020, 13, 2248. [Google Scholar] [CrossRef]
- Zafer, Y.E.; Goel, S.; Ganvir, A.; Jansson, A.; Joshi, S. Encapsulation of Electron Beam Melting produced alloy 718 to reduce surface connected defects by Hot Isostatic Pressing. Materials 2020, 13, 1226. [Google Scholar] [CrossRef] [Green Version]
- Dass, A.; Moridi, A. State of the art in direct energy deposition: From Additive Manufacturing to Materials Design. Coatings 2019, 9, 418. [Google Scholar] [CrossRef] [Green Version]
- Darvish, K.; Chen, Z.W.; Pasang, T. Reducing lack of fusion during selective laser melting of CoCrMo alloy. Effect of laser power on geometrical features of tracks. Mater. Des. 2016, 112, 357–366. [Google Scholar] [CrossRef]
- Pantawane, M.V.; Ho, Y.H.; Joshi, S.S.; Dahotre, N.B. Computational assessment of thermokinetics and associated microstructural evolution in laser powder bed fusion manufacturing of Ti-6Al-4V alloy. Sci. Rep. 2020, 10, 7579. [Google Scholar] [CrossRef] [PubMed]
- Mukherejee, T.; Wei, H.L.; De, A.; DebRoy, T. Heat and fluid flow in additive manufacturing—Part II: Powder Bed Fusion of Stainless Steel, and Titanium, Nickel and Aluminium Base Alloys. Compt. Mater. Sci. 2018, 150, 369–380. [Google Scholar] [CrossRef]
- Tiryakioğlu, M. The effect of hydrogen on pore formation in aluminium alloy castings: Myth versus reality. Metals 2020, 10, 368. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Long, M.; Liu, P.; Chen, D.; Chen, H.; Gui, L.; Liu, T.; Yu, S. Effects of partition coefficients, diffusion coefficients, and solidification paths on microsegregations in Fe-based multinary alloy. Metall. Mater. Trans. B 2017, 48, 2504–2515. [Google Scholar] [CrossRef]
- Gong, H.; Rafi, K.; Gu, H.; Janaki Ram, G.D.; Starr, T.; Stucker, B. Influence of defects on mechanical properties if Ti-6Al-4V components produced by selective laser melting and electron beam melting. Mater. Des. 2015, 86, 545–554. [Google Scholar] [CrossRef]
- Laursen, C.M.; DeJong, S.A.; Dickens, S.M.; Exil, A.N.; Susan, D.F.; Carroll, J.D. Relationship between ductility and the porosity of additively manufactured AlSi10Mg. Mater. Sci. Eng. A 2020, 795, 139922. [Google Scholar] [CrossRef]
- Plessis, A.D.; Yelamanchi, B.; Fischer, C.; Miller, J.; Beamer, C.; Rogers, K.; Cortes, P.; Els, J.; MacDonald, E. Productivity enhancement of laser powder bed fusion using compensated shelled geometries and hot isostatic pressing. Adv. Inf. Manuf. Eng. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Masuo, H.; Tanaka, Y.; Morokoshi, S.; Yagura, H.; Uchida, T.; Yamamoto, Y.; Murakami, Y. Influence of defects, surface roughness and HIP on fatigue strength of Ti-6Al-4V manufactured by additive manufacturing. Int. J. Fatigue 2018, 117, 163–179. [Google Scholar] [CrossRef]
- Ertuğrul, O.; Öter, Z.Ç.; Yilmaz, H.S.; Şahin, E.; Coşkun, M.; Tarakçi, G.; Koç, E. Effect of HIP process and subsequent heat treatment on microstructure and mechanical properties of direct metal laser sintered AlSi10Mg alloy. Rapid Prototyp. J. 2020, 26, 1421–1434. [Google Scholar] [CrossRef]
- Schneller, W.; Leitner, M.; Springer, S.; Grün, F.; Taschaner, M. Effect of HIP treatment on microstructure and fatigue strength of selectively laser melted AlSi10Mg. Manuf. Mater. Process. 2019, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Romero, C.; Yang, F.; Bolzoni, L. Fatigue and fracture properties of Ti alloys from powder-based processes—A review. Int. J. Fatigue 2018, 117, 407–419. [Google Scholar] [CrossRef]
- Leuders, S.; Lieneke, T.; Lammers, S.; Tröster, T.; Niendorf, T. On the fatigue properties of metals manufactured by selective laser melting—The role of ductility. J. Mater. Res. 2014, 19, 1911–1919. [Google Scholar] [CrossRef]
- Mahmud, A.; Huynh, T.; Zhou, L.; Hyer, H.; Mehta, A.; Imholte, D.D.; Woolsthenhulme, N.E.; Wachs, D.M.; Sohn. Mechanical behavior assessment of Ti-6Al-4V ELI alloy produced by laser powder bed fusion. Metals 2021, 11, 1671. [Google Scholar] [CrossRef]
- Kruth, J.P.; Froyen, L.; Van Vaerenbergh, J.; Mercelis, P.; Romboust, M.; Lauwers, B. Selective laser melting of iron-based powder. J. Mater. Process. Technol. 2014, 149, 616–622. [Google Scholar] [CrossRef]
- Masmoudi, A.; Bolot, R.; Coddet, C. Investigation of the powder laser-atmosphere interaction zone during the selective laser melting process. J. Mater. Process. Technol. 2015, 225, 122–132. [Google Scholar] [CrossRef]
- Santecchia, E.; Spigarelli, S.; Cabibbo, M. Material reuse in laser powder bed fusion: Side Effects of the Laser—Metal Powder Interaction. Metals 2020, 10, 341. [Google Scholar] [CrossRef] [Green Version]
- Pauzon, C.; Forêt, P.; Hryha, E.; Arunprasad, T.; Nyborg, L. Argon-helium mixtures as laser-powder bed fusion atmospheres: Towards Increased Build Rate of Ti-6Al-4V. J. Mater. Process. Technol. 2020, 279, 116555. [Google Scholar] [CrossRef]
- Huang, H.-Y. Effects of shielding gas composition and activating flux of GTAW weldments. Mater. Des. 2009, 30, 2404–2409. [Google Scholar] [CrossRef]
- Anwar, A.B.; Pham, Q.-C. Selective laser melting of AlSi10Mg: Effects of Scan Direction, Part Placement and Inert Gas Flow Velocity on Tensile Strength. J. Mater. Process. Technol. 2017, 240, 388–396. [Google Scholar] [CrossRef]
- Vastola, G.; Pei, Q.-X.; Zhang, Y.W. Predictive model for porosity in powder-bed fusion additive manufacturing at high beam energy regime. Add. Manuf. 2018, 22, 817–822. [Google Scholar] [CrossRef]
- Li, B.-Q.; Li, Z.; Bai, P.; Li, B.; Kuai, Z. Research on surface roughness of AlSi10Mg parts fabricated by laser powder bed fusion. Metals 2018, 8, 524. [Google Scholar] [CrossRef] [Green Version]
- Buchbinder, D.; Schleifenbaum, H.; Heidrich, S.; Meiners, W.; Bültmann, J. High power selective laser melting (HP SLM) of aluminium parts. Phys. Procedia. 2011, 12, 271–278. [Google Scholar] [CrossRef]
- Ansari, M.J.; Nsguyen, D.S.; Park, H.S. Investigation of SLM process in terms of temperature distribution and melting pool size: Modeling and Experimental Approaches. Materials 2019, 12, 1272. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Sun, S.; Brandt, M.; Yan, W. Experimental investigation and 3D finite element prediction of the heat affected zone during laser assisted machining of Ti6Al4V alloy. J. Mater. Process. Techonol. 2010, 210, 2215–2222. [Google Scholar] [CrossRef]
- Du, Y.; You, X.; Qiao, F.; Guo, L.; Liu, Z. A model for prediction the temperature field during selective laser melting. Results Phys. 2019, 12, 52–60. [Google Scholar] [CrossRef]
- Schwerz, C.; Nyborg, L. Linking in situ melt pool monitoring to melt size distributions and internal flaws in laser powder bed fusion. Metals 2021, 11, 1856. [Google Scholar] [CrossRef]
- Bhavar, V.; Kattire, P.; Patil, V.M.; Khot, S.M.; Gujar, K.; Singh, R.R. A review of powder bed technology of metal additive manufacturing. In Additive Manufacturing Handbook; Taylor & Francis Group: Abingdon, UK, 2017. [Google Scholar] [CrossRef]
- Concept Laser Website. Available online: www.conceptlaser.de (accessed on 11 December 2021).
- Fiegl, T.; Franke, M.; Körner, C. Impact of build envelope on the properties of additive manufactured parts from AlSi10Mg. Opt. Laser. Technol. 2019, 111, 51–57. [Google Scholar] [CrossRef]
- Shi, X.; Ma, S.; Liu, C.; Chen, C.; Wu, Q.; Chen, X.; Lu, J. Performance of high layer thickness in selective laser melting of Ti6Al4V. Materials 2016, 9, 975. [Google Scholar] [CrossRef] [Green Version]
- Bremer, S.; Meiner, W.; Diatlov, A. Selective laser melting—A manufacturing technology for the future? Laser Technik J. 2012, 9, 975–990. [Google Scholar] [CrossRef]
- Yu, T.; Hyer, H.; Sohn, Y.; Bai, Y.; Wu, D. Structure-property relationship in high strength and lightweight AlSi10Mg microlattice fabricated be selective laser melting. Mater. Des. 2019, 182, 108062. [Google Scholar] [CrossRef]
- Su, X.; Yang, Y. Research on tack overlapping during selective laser melting of powders. J. Mater. Process. Technol. 2012, 212, 2074–2079. [Google Scholar] [CrossRef]
- Zhang, C.; Zhu, H.; Hu, Z.; Zhang, L.; Zeng, X. A comparative study on single-laser and multi-laser selective laser melting AlSi10Mg: Defects, Microstructure and Mechanical Properties. Mater. Sci. Eng. A 2019, 746, 416–423. [Google Scholar] [CrossRef]
- Takata, N.; Kodaira, H.; Sekizawa, K.; Kobashi, M.; Suzuki, A. Change in microstructure of selectively laser melted AlSi10Mg alloy with heat treatments. Mater. Sci. Eng. A 2017, 704, 218–228. [Google Scholar] [CrossRef]
- Lingda, X.; Gouli, Z.; Gaoyang, M.; Chunming, W.; Ping, J. A phase-field simulation of columnar-to-equiaxed transition in the entire laser welding MP. J. Alloys Compd. 2021, 858, 157669. [Google Scholar] [CrossRef]
- Takata, N.; Nishida, R.; Suzuki, A.; Kobashi, M.; Kato, M. Crystallographic features of microstructure in maraging steel fabricated by selective laser melting. Metals 2018, 8, 440. [Google Scholar] [CrossRef] [Green Version]
- Hadadzadeh, A.; Amirkhiz, B.S.; Li, J.; Mohammadi, M. Columnar to equiaxed transition during direct laser metal sintering of AlSi10Mg alloy: Effect of Building Direction. Add. Manuf. 2018, 23, 121–131. [Google Scholar] [CrossRef]
- Paul, M.J.; Liu, Q.; Best, J.P.; Li, X.; Kruzic, J.J.; Ranamurty, U.; Gludovatz, B. Fracture resistance of AlSi10Mg fabricated by laser powder bed fusion. Acta Mater. 2021, 211, 116869. [Google Scholar] [CrossRef]
- Casati, R.; Nasab, M.H.; Coduri, M.; Tirelli, V.; Vedani, M. Effect of platform pre-heating and thermal treatments strategy on properties of AlSi10Mg alloy processed by selective laser melting. Metals 2018, 8, 954. [Google Scholar] [CrossRef] [Green Version]
- Van Cauwenbergh, P.; Samaee, V.; Thijs, L.; Nejezchlebová, J.; Sedlák, P.; Iveković, A.; Schyvers, D.; Van Hooreweder, B.; Vanmeensel, K. Unravelling the multi-scale structure-property relationship of laser powder bed fusion processed and heat-treated AlSi10Mg. Sci. Rep. 2021, 11, 6423. [Google Scholar] [CrossRef]
- Mtyjia, H.; Giessen, B.C.; Grant, N.J. The effect of cooling rate on the dendrite pacing on splat-cooled aluminium alloys. J. Int. Mater. 1968, 96, 30–32. [Google Scholar]
- Li, Y.; Gu, D. Parametric analysis of thermal behavior during selective laser melting additve manufacturing of aluminum alloy powder. Mater. Des. 2014, 63, 857–867. [Google Scholar] [CrossRef]
- Delahaye, J.; Tchuindjang, J.T.; Lecomte-Beckers, J.; Rigo, O.; Habreken, A.M.; Mertens, A. Influences of Si particles on fracture mechanisms of AlSi10Mg parts processed by selective lase melted. Acta Mater. 2019, 175, 160–170. [Google Scholar] [CrossRef]
- Padovano, E.; Badini, C.; Pantarelli, A.; Gili, F.; D’Aiuto, F. A comparative study of the effects of thermal treatments on AlSi10Mg produced by laser powder bed fusion. J. Alloys Compd. 2020, 831, 154822. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, X.; Ma, F.; Jiang, J.; Yang, W. Research on deposited tracks and microstructure of AlSi10Mg alloy produced by selective laser melting. Appl. Phys. A 2020, 126, 643–653. [Google Scholar] [CrossRef]
- Xiong, Z.H.; Liu, S.L.; Shi, Y.; Yang, Y.F.; Misra, R.D.K. Role of melt pool boundary condition in determining the mechanical properties of selective laser melting AlSi10Mg alloy. Mater. Sci. Eng. A 2019, 740–741, 148–156. [Google Scholar] [CrossRef]
- Bai, P.; Hou, P.; Kang, T.; Zhao, Z.; Du, W.; Liang, M.; Li, Y.; Liao, H.; Liu, Y. Failure analysis of the tree column structures type AlSi10Mg alloy branches manufactured by selective laser melting. Materials 2020, 13, 3969. [Google Scholar] [CrossRef]
- Zhou, L.; Mehta, A.; Schulz, E.; McWilliams, B.; Cho, K.; Sohn, Y. Microstructure, precipitates and hardness of selectively laser melted AlSi10Mg alloy before and after heat treatment. Mater. Charact. 2018, 143, 5–17. [Google Scholar] [CrossRef]
- Maamoun, A.H.; Elbestawi, M.; Dosbaeva, G.K.; Veldhius, S.C. Thermal post processing of AlSi10Mg parts produces by Selective Laser Melting using recycled powder. Addit. Manuf. 2018, 21, 234–247. [Google Scholar] [CrossRef]
- Yan, Q.; Song, B.; Shi, Y. Co MParative study of performance co MParision of AlSi10Mg alloy prepared by selective laser melting and casting. J. Mater. Sci. Technol. 2020, 41, 199–208. [Google Scholar] [CrossRef]
- Edwards, G.A.; Stiller, K.; Dunlop, G.L.; Couper, M.J. The precipitation sequence in Al-Si-Mg alloys. Acta Mater. 1998, 46, 3893–3904. [Google Scholar] [CrossRef]
- Dolon, W.T. Precipitation of aluminium in the silicon phase contained in W319 and A356 aluminium alloys. Metall. Mater. Trans. A 2003, 34, 523–529. [Google Scholar] [CrossRef]
- Li, Z.; Kuai, Z.; Bai, P.; Nie, Y.; Fu, G.; Liu, W.; Yang, S. Microstructure and tensile properties of AlSi10Mg alloy manufactured by multi-laser beam selective laser melting (SLM). Metals 2019, 9, 1337. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.; Yang, Y.; Xiao, Z.; Zhang, M.; Wang, D. Process optimization and mechanical properties evolution of AlSi10Mg0.75 by selective laser melting. Mater. Des. 2018, 140, 257–266. [Google Scholar] [CrossRef]
- Mathe, N.R.; Tshabalala, L.C. The validation of the microstructural evolution of selective laser-melted AlSi10Mg on the in-house built machine: Energy Density Studies. Prog. Addit. Manuf. 2019, 4, 431–442. [Google Scholar] [CrossRef]
- Fiocchi, J.; Tuissi, A.; Bassani, O.; Biffi, C.A. Low temperature annealing dedicated to AlSi10Mg selective laser melting products. J. Alloys Compd. 2017, 695, 3402–3409. [Google Scholar] [CrossRef]
- Tonelli, L.; Liverani, E.; Morri, A.; Ceschini, L. Role of direct aging and solution hea treatment on hardness, microstructure and residual stress of the A357 (AlSi17Mg0.6) alloy produced by powder bed fusion. Metall. Mater. Trans. 2021, 52, 2484–2496. [Google Scholar] [CrossRef]
- Baek, M.-S.; Kreethi, R.; Park, T.-H.; Sohn, Y.; Lee, K.-A. Influence of heat treatment on the high-cycle fatigue properties and fatigue damage mechanism of selective laser melted AlSi10Mg alloy. Mater. Sci. Eng. A 2021, 819, 141486. [Google Scholar] [CrossRef]
- Park, T.-A.; Baek, M.-S.; Hyer, H.; Sohn, Y.; Lee, K.-A. Effect of direct aging on microstructure and tensile properties of AlSi10Mg alloy manufactured by selective laser melting. Mater. Charact. 2021, 176, 111113. [Google Scholar] [CrossRef]
- Kleiner, S.; Zürcher, J.; Bauer, O.; Margraf, P. Heat treatment response of selectively laser melted AlSi10Mg. J. Heat Treat. Mater. 2020, 75, 327–341. [Google Scholar] [CrossRef]
- Mfusi, B.J.; Mathe, N.R.; Tshabalala, L.C.; Poopola, P.A. The effects of stress relief on the mechanical and fatigue properties of additively manufactured AlSi10Mg parts. Metals 2019, 9, 1216. [Google Scholar] [CrossRef] [Green Version]
- Marola, S.; Bosia, S.; Veltro, A.; Fiore, G.; Manfredi, D.; Lombardi, M.; Amato, G.; Barrico, M.; Battezzati, L. Residual stresses in additively manufactured AlSi10Mg: Raman spectroscopy and X-Ray diffraction analysis. Mater. Des. 2021, 202, 109550. [Google Scholar] [CrossRef]
- Bartlett, J.L.; Croom, B.P.; Burdick, J.; Henkel, D.; Li, X. Revealing mechanisms of residual stress development in additive manufacturing via digital image correlation. Addit. Manuf. 2018, 22, 1–12. [Google Scholar] [CrossRef]
- Paoletti, C.; Cerri, E.; Ghio, E.; Santecchia, E.; Cabibbo, M.; Spigarelli, S. Effect of low-temperature annealing of an AlSI10MG alloy produced by additive manufacturing: Experiments and Modelling. Metals 2021, 11, 179. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, L.; Feng, Z.; Zhang, W. Effects of heat treatments on microstructure and mechanical properties of SLMed Sc-Modified AlSi10Mg alloy. Prog. Nat. Sci. Mater. 2021, 31, 714–721. [Google Scholar] [CrossRef]
- Merino, J.; Ruvalcaba, B.; Varela, J.; Arrieta, E.; Murr, L.E.; Wicker, R.B.; Benedict, M.; Medina, F. Multiple, comparative heat treatment and aging schedules for controlling the microstructures and mechanical properties of laser powder bed fusion fabricated AlSi10Mg alloy. J. Mater. Res. Technol. 2021, 13, 669–685. [Google Scholar] [CrossRef]
- ASTM F3318-18; Standard for Additive Manufacturing—Finished Part Properties—Laser Beam. ASTM: West Conshohocken, PA, USA, 2018.
- Hafenstein, S.; Hitzler, L.; Sert, E.; Öchsner, A.; Merkel, M.; Werner, E. Hot isostatic pressing of aluminium-silicon alloys fabricated by laser powder-bed fusion. Technologies 2020, 8, 48. [Google Scholar] [CrossRef]
- Hirata, T.; Kimura, T.; Nakamoto, T. Effects of hot isostatic pressing and internal porosity on the performance of selective laser melted AlSi10Mg alloys. Mater. Sci. Eng. A 2020, 772, 138713. [Google Scholar] [CrossRef]
- Fusanová, M.; Dvorský, D.; Michalcová, A.; Vojtĕch, D. Changes in the microstructure and mechanical properties of additively manufactured AlSi10Mg alloy after exposure to elevated temperatures. Mater. Charact. 2018, 137, 119–126. [Google Scholar] [CrossRef]
- Ji, Y.; Dong, C.; Kong, D.; Li, X. Design materials based on simulation results of silicon induced segregation at AlSi10Mg interface fabricated by selective laser melting. J. Mater. Sci. Technol. 2020, 46, 145–155. [Google Scholar] [CrossRef]
- Li, W.; Li, S.; Liu, J.; Zhang, A.; Zhou, Y.; Wei, Q.; Yan, C.; Shi, Y. Effect of heat treatment on AlSI10Mg alloy fabricated by selective laser melting: Microstructure Evolution, Mechanical Properties and Fracture Mechanism. Mater. Sci. Eng. A 2016, 663, 116–125. [Google Scholar] [CrossRef]
- Wei, P.; Chen, Z.; Zhang, S.; Fang, X.; Lu, B.; Zhang, L.; Wei, Z. Effect of T6 heat treatment on the surface tribological and corrosion properties of AlSi10Mg samples produced by selective laser melting. Mater. Charact. 2021, 171, 110769. [Google Scholar] [CrossRef]
- Liu, M.; Takata, N.; Suzuki, A.; Kobashi, M. Effect of heat treatment on gradient microstructure of AlSi10Mg lattice structure manufactured by laser powder bed fusion. Materials 2020, 13, 2487. [Google Scholar] [CrossRef] [PubMed]
- Iturrioz, A.; Gil, E.; Petite, M.; Garciandia, F.; Mancisidor, A.M.; San Sebastian, M. Selective laser melting of Alsi10Mg alloy: Influence of Heat Treatment Condition on Mechanical Properties and Microstructure. Weld World 2018, 62, 885–892. [Google Scholar] [CrossRef]
- Chen, R.; Xu, Q.; Jia, Z.; Liu, B. Precipitation behavior and hardening effects of Si-containing dispersoids in Al-Si-Mg alloy during SHT. Mater. Des. 2016, 90, 1059–1068. [Google Scholar] [CrossRef]
- Li, Z.; Li, Z.; Tan, Z.; Xiong, D.-B.; Guo, Q. Stress relaxation and the cellular structure-dependence of plastic deformation in additively manufactured AlSi10Mg alloys. Int. J. Plast. 2020, 127, 102640. [Google Scholar] [CrossRef]
- Ben, D.D.; Ma, Y.R.; Yang, H.J.; Meng, L.X.; Shao, X.H.; Liu, H.Q.; Wang, S.G.; Duan, Q.Q.; Zhang, Z.F. Heterogeneous microstructure and voids dependence of tensile deformation in a selective laser melted AlSi10Mg alloy. Mater. Sci. Eng. A 2020, 798, 140109. [Google Scholar] [CrossRef]
- Bharath, C.; Shamanth, V.; Hemath, K. Studies on mechanical behaviour of AlSi10Mg alloy produced by selective laser melting and A360 alloy by die casting. Mater. Today Proc. 2021, 45, 78–81. [Google Scholar] [CrossRef]
- Zhao, L.; Macías, J.G.S.; Ding, L.; Idrissi, H.; Simar, A. Damage mechanisms in selective laser melted AlSi10Mg under as built and different post-process conditions. Mater. Sci. Eng. A 2019, 764, 138210. [Google Scholar] [CrossRef]
- Bagherifard, S.; Beretta, N.; Monti, S.; Riccio, M.; Bandini, M.; Guagliano, M. On the fatigue stresses enhancement of additive manufactured AlSi10Mg parts by mechanical and thermal post-processing. Mater. Des. 2018, 145, 28–41. [Google Scholar] [CrossRef]
- Ngnekon, J.N.D.; Nadot, Y.; Henaff, G.; Nicolai, J.; Kan, W.H.; Cairney, J.M.; Ridosz, L. Fatigue properties of AlSi10Mg produced by additive manufacturing. Int. J. Fatigue 2019, 119, 160–172. [Google Scholar] [CrossRef]
- Hadadzadeh, A.; Baxter, C.; Amirkhiz, B.S.; Mohammadi, M. Strengthening mechanisms in direct metal laser sintered AlSi10Mg: Comparison between Virgin and Recycled Powders. Add. Manuf. 2018, 23, 108–120. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, L.; Zhang, B.; Chen, Y.Z.; Liu, F. Microstructural evolution and strengthening mechanism of an Al-Si-Mg alloy processed by high-pressure torsion with different heat treatments. Mater. Sci. Eng. A 2020, 794, 139932. [Google Scholar] [CrossRef]
- Ryen, Ø.; Holmedal, B.; Nijs, O.; Nes, E.; Sjölander, E.; Holmedd, B.; Ekstrom, H. Strengthening mechanism in solid solution aluminum alloy. Metall. Mater. Trans. A 2006, 37, 1999–2006. [Google Scholar] [CrossRef]
- Dieter, G.E. Mechanical Metallurgy, 1st ed.; Mc-GrawHill: New York, NY, USA, 1986; pp. 212–220. [Google Scholar]
- Chen, B.; Moo, S.K.; Yao, X.; Bi, G.; Shen, J.; Umeda, J.; Kondoh, K. Strength and strain hardening of selective laser melted AlSi10Mg alloy. Scr. Mater. 2017, 141, 45–49. [Google Scholar] [CrossRef]
- Rodriguez, P. Sixty years dislocations. Bull. Mater. Sci. 1996, 19, 857–872. [Google Scholar] [CrossRef] [Green Version]
- Starink, M.J.; Wang, S.C. A model for the yield strength of overaged Al-Zn-Mg-Cu alloys. Acta Mater. 2003, 51, 5131–5150. [Google Scholar] [CrossRef] [Green Version]
- Girelli, L.; Giovagnoli, M.; Tocci, M.; Pola, A.; Fortini, A.; Merlin, M.; La Vecchia, G.M. Evaluation of impact behaviour of AlSi10Mg alloy produced using laser additive manufacturing. Mater. Sci. Eng. A 2019, 748, 38–51. [Google Scholar] [CrossRef]
- Roth, C.C.; Tancogne-Dejen, T.; Mohr, D. Plasticity and fracture of cast and SLM AlSi10Mg: High-throughput Testing and Modelling. Add. Manuf. 2021, 43, 101998. [Google Scholar] [CrossRef]
- Zhou, S.; Su, Y.; Gu, R.; Wang, Z.; Zhou, Y.; Ma, Q.; Yan, M. Impacts of defocusing amount and molten pool boundaries on mechanical properties and microstructure of selective laser melted AlSi10Mg. Materials 2019, 12, 73. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.W. Precipitation Hardening, 2nd ed.; Butterwirth-Heinemann: Oxford, UK; Boston, MA, USA, 1998. [Google Scholar]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Manfredi, D.; Fino, P.; Biamino, S.; Badini, C. Evaluation of corrosion resistance of Al-Si10Mg alloy obtained by means of direct metal laser sintering. J. Mater. Process. Technol. 2016, 231, 326–335. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T.; Pellegrini, S.; Ambrosio, E.P.; Calignano, F.; Manfredi, S.; Pavese, M.; Fino, P. Effect of heat treatment on corrosion resistance of DMLS AlSi10Mg alloy. Electrochem. Acta. 2016, 206, 346–355. [Google Scholar] [CrossRef]
- Cabrini, M.; Calignano, F.; Fino, P.; Lorenzo, S.; Lorusso, M.; Manfredi, D.; Testa, C.; Pastore, T. Corrosion behavior of heat-treated AlSi10Mg manufactured by laser powder bed fusion. Materials 2018, 11, 1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafieazad, M.; Mohammadi, M.; Nasiri, A.M. Microstructure and early stage corrosion performance of heat treated direct metal laser sintered AlSi10Mg. Add. Manuf. 2019, 28, 107–119. [Google Scholar] [CrossRef]
- Revilla, R.I.; Liang, J.; Godet, S.; De Graeve, I. Local corrosion behavior of additive manufactured AlSiMg alloy assessed by SEM and SKPFM. J. Electrochem. Soc. 2017, 164, C27–C35. [Google Scholar] [CrossRef]
- Fathi, P.; Mohammadi, M.; Duour, X.; Nasiri, A.M. A comparative study on corrosion and microstructure of direct laser sintered AlSi10Mg_200C and die cast A360.1 aluminium. J. Mater. Process. Technol. 2018, 259, 1–14. [Google Scholar] [CrossRef]
- Leon, A.; Shirizly, A.; Aghion, E. Corrosion behavior of AlSi10Mg alloy produced by additive manufacturing (AM) vs. its counterpart gravity cast alloy. Metals 2016, 6, 148. [Google Scholar] [CrossRef]
- Leon, A.; Aghion, E. Effect of surface roughness on corrosion fatigue performance of AlSi10Mg alloy produced by selective laser melting (SLM). Mater. Charact. 2017, 131, 188–194. [Google Scholar] [CrossRef]
- Revilla, R.I.; Verkens, D.; Rubben, T.; De Raeve, I. Corrosion and corrosion protection of additively manufactured aluminium alloys—A critical review. Materials 2020, 13, 4804. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, F.; Pastore, T.; Testa, C.; Manfredi, D.; Lorusso, M.; Calignano, F.; Pavese, M.; Andreatta, F. Corrosion behavior of AlSi10Mg alloy produced by laser powder bed fusion under chloride exposure. Corros. Sci. 2019, 152, 101–108. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, S.; Pastore, T. Corrosion behavior of aluminium-silicon alloys obtained by Direct Metal Laser Sintering. In Proceedings of the EUROCORR 2017—The Annual Congress of the European Federation of Corrosion, 20th International Corrosion Congress and Process Safety Congress, Prague, Czech Republic, 3–7 September 2017. [Google Scholar]
- Revilla, R.I.; De Graeve, I. Influence of Si content on the microstructure and corrosion behavior of additive manufactured Al-Si alloys. J. Electrochem. Soc. 2018, 165, C926–C932. [Google Scholar] [CrossRef]
- Cabrini, M.; Lorenzi, F.; Pastore, T.; Pellegrini, S.; Pavese, M.; Fino, P.; Ambrosio, E.P.; Calignano, F.; Manfredi, D. Corrosion resistance of direct metal lase sintering AlSi10Mg alloy. Suf. Interface Anal. 2016, 48, 818–826. [Google Scholar] [CrossRef]
- Rubben, T.; Revilla, R.I.; De Graeve, I. Influence of heat treatments on the corrosion mechanism of additive manufactured AlSi10Mg. Corros. Sci. 2019, 147, 406–415. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, J.; Gu, X.; Dai, N.; Qin, P.; Zhang, L.-C. Distinction of corrosion resistance of selective laser melted Al-12Si alloy on different planes. J. Alloys Compd. 2018, 747, 648–658. [Google Scholar] [CrossRef]
- Zeng, F.; Wei, Z.; Li, J.-F.; Li, C.-X.; Tan, X.; Zhang, Z.; Zheng, Z. Corrosion mechanism associated with Mg2Si and Si particles in Al-Si-Mg alloys. Trans. Nonferrous Mater. Soc. China 2011, 21, 2559–2567. [Google Scholar] [CrossRef]
- Gupta, R.K.; Sukiman, N.L.; Fleming, K.M.; Gibson, M.A.; Birbilis, N. Electrochemical behavior and localized corrosion associated with Mg2Si particles in Al and Mg alloys. ECS Electrochem. Lett. 2012, 1, C1–C3. [Google Scholar] [CrossRef]
- Ahlatci, H. Production and corrosion behaviours of the Al-12Si-XMg alloys containing in situ Mg2Si particles. J. Alloys Compd. 2010, 503, 122–126. [Google Scholar] [CrossRef]
- Sui, Q.; Li, P.; Wang, K.; Yin, X.; Liu, L.; Zhang, Y.; Zhang, Q.; Wang, S.; Wong, L. Effect of build orientation on the corrosion behavior and mechanical properties of selective laser melted Ti-6Al-4V. Metals 2019, 9, 976. [Google Scholar] [CrossRef] [Green Version]
- Lekoadi, P.; Tlotleng, M.; Annan, K.; Maledi, N.; Masina, B. Evolution of heat treatment parameters on microstructure and hardness properties of high-speed selective laser melted Ti6AL4V. Metals 2021, 11, 255. [Google Scholar] [CrossRef]
- He, J.; Li, D.; Jiong, W.; Ke, L.; Qin, G.; Ye, Y.; Qin, Q.; Qiu, D. The martensitic transformation and mechanical properties of Ti6Al4V prepared via selective laser melted. Materials 2019, 12, 321. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, G.; Sun, Q.; Li, H.; Sun, J.; Wang, X. Understanding the effect of scanning strategies on the microstructure and crystallography texture of Ti-6Al-4V alloy manufactured via laser powder bed fusion. J. Mater. Process. Technol. 2022, 299, 117366. [Google Scholar] [CrossRef]
- Lui, E.W.; Xu, W.; Pateros, A.; Qian, M.; Brandt, M. New development in selective laser melting of Ti-6Al-4V: A Wider Processing window for the achievement of fully lamellar α + β microstructures. JOM 2017, 69, 2679–2683. [Google Scholar] [CrossRef] [Green Version]
- Karami, K.; Blok, A.; Weber, L.; Ahmadi, S.M.; Petrov, R.; Nikolic, K.; Borisov, E.V.; Leeflang, S.; Ayas, C.; Zadpoor, A.A.; et al. Continuous and pulsed selective laser melting of Ti6Al4V lattice structures: Effect of Post-Processing on Microstructural Anisotropy and Fatigue Behavior. Add. Manuf. 2020, 36, 101433. [Google Scholar] [CrossRef]
- Cepeda-Jiménez, C.M.; Potenza, F.; Magalini, E.; Luchin, V.; Molinari, A.; Pérez-Prado, M.T. Effect of energy density on the microstructure and texture evolution of Ti-6Al-4V manufactured by laser powder bed fusion. Mater. Charact. 2020, 163, 110238. [Google Scholar] [CrossRef]
- Simonelli, M.; Tse, Y.Y.; Tuck, C. On the texture formation of the selective laser melted Ti-6Al-4V. Metall. Mater. Trans. 2014, 45, 2863–2872. [Google Scholar] [CrossRef]
- Kumar, P.; Prakash, O.; Ramamurty, U. Micro- and macro-structures and their influence on mechanical properties of selectively laser melted Ti-6Al-4V. Acta Mater. 2018, 154, 246–260. [Google Scholar] [CrossRef]
- Dilip, J.J.S.; Zhang, S.; Teng, C.; Zeng, K.; Robinson, C.; Pal, D.; Stucker, B. Influence of process parameters on the evolution of MP, porosity, and microstructures in Ti-6Al-4V alloy parts fabricated by selective laser melting. Prog. Add. Manuf. 2017, 2, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Brandt, M.; Sun, S.; Elambasseril, J.; Liu, Q.; Latham, K.; Xia, K.; Qian, M. Additive manufacturing of strong and ductile Ti-6Al-4V by selective laser melting via in situ martensite decomposition. Acta Mater. 2015, 85, 74–84. [Google Scholar] [CrossRef]
- Barriobero-Vila, P.; Gussone, J.; Haubrich, J.; Sandlöbes, S.; Da Silva, J.C.; Cloetens, P.; Schell, N.; Requena, G. Inducing stable α + β microstructures during selective laser melting of Ti-6Al-4V used intensified intrinsic heat treatment. Materials 2017, 10, 268. [Google Scholar] [CrossRef] [PubMed]
- Leuders, S.; Thöne, M.; Riemer, A.; Niendorf, T.; Tröster, T.; Richard, H.A.; Maier, H.J. On the mechanical behavior of titanium alloy Ti6Al4V manufactured by selective laser melting: Fatigue Resistance and Crack Growth Performance. Int. J. Fatigue 2013, 48, 300–307. [Google Scholar] [CrossRef]
- Bai, H.; Deng, H.; Chen, L.; Liu, X.; Qin, X.; Zhang, D.; Liu, T.; Cui, X. Effect of heat treatment on the microstructure and mechanical properties of selective laser-melted Ti64 and Ti-5Al-5Mo-5V-1Cr-1Fe. Metals 2021, 11, 534. [Google Scholar] [CrossRef]
- Brown, D.W.; Anghel, V.; Balogh, L.; Clausen, B.; Johson, J.S.; Martinez, R.M.; Pagan, D.C.; Rafailov, G.; Ravkov, L.; Strantza, M.; et al. Evolution of the microstructure of laser powder bed fusion Ti-6Al-4V during post-build heat treatment. Metall. Mater. Trans. A 2021, 52, 5165–5181. [Google Scholar] [CrossRef]
- Ali, H.; Ma, L.; Ghadbeigi, H.; Mumtaz, K. In situ residual stress reduction martensitic decomposition and mechanical properties enhancement through high temperature powder bed pre-heating of selective laser melted Ti6Al4V. Mater. Sci. Eng. A 2017, 695, 211–220. [Google Scholar] [CrossRef]
- Kaschel, F.R.; Vijayaraghavan, R.K.; Shmeliov, A.; McCarthy, E.K.; Canavan, M.; McNally, P.J.; Dowling, D.P.; Nicolosi, V.; Celikin, M. Mechanism of stress relaxation and phase transformation in additively manufactured Ti-6Al-4V via in-situ high temperature XRD and TEM analyses. Acta Mater. 2020, 188, 720–732. [Google Scholar] [CrossRef]
- Xing, L.-L.; Zhang, W.-J.; Zhao, C.-C.; Gao, W.-Q.; Shen, Z.-J.; Liu, W. Influence of powder bed temperature on the microstructure and mechanical properties of Ti-6Al-4V alloys fabricated via laser powder bed fusion. Materials 2021, 14, 2278. [Google Scholar] [CrossRef] [PubMed]
- Sallica-Leva, E.; Caram, R.; Jardini, A.L.; Fogagnolo, J.B. Ductility improvement due to martensite’ decomposition in porous Ti-6Al-4V parts produced by selective laser melting for orthopedic implants. J. Mech. Behav. Biomed. Mater. 2016, 54, 149–158. [Google Scholar] [CrossRef]
- Cao, S.; Chu, R.; Zhou, X.; Yang, K.; Jia, Q.; Lim, C.V.S.; Huang, A.; Wu, X. Role of martensite decomposition in tensile properties of selective laser melted Ti-6Al-4V. J. Alloys Compd. 2018, 744, 357–363. [Google Scholar] [CrossRef]
- Malý, M.; Höller, C.; Skolan, M.; Meier, B.; Koutný, D.; Pichler, R.; Sommitsch, C.; Paloušek, D. Effect of process parameters and high temperature pre heating on residual stress and relative density of Ti6Al4V processed by selective laser melting. Materials 2019, 12, 930. [Google Scholar] [CrossRef] [Green Version]
- Antonysamy, A.A.; Meyer, J.; Prangnell, P.B. Effect of build geometry on the β-grains structure and texture in additive manufacture of Ti-6Al-4V by selective electron beam melting. Mater. Charct. 2019, 84, 153–168. [Google Scholar] [CrossRef]
- Wu, S.Q.; Lu, Y.J.; Gan, Y.L.; Huang, T.T.; Zhao, C.Q.; Lin, J.J.; Guo, S.; Lin, J.X. Microstructural evolution and microhardness of a selective-laser-melted Ti-6Al-4V alloy after post heat treatment. J. Alloys Compd. 2016, 672, 643–652. [Google Scholar] [CrossRef]
- Wang, T.; Zhu, Y.Y.; Zhang, S.Q.; Tang, H.B.; Wang, H.M. Grain morphology evolution behaviour of titanium alloy components during laser melting deposition additive manufacturing. J. Alloys Compd. 2015, 632, 505–513. [Google Scholar] [CrossRef]
- Yang, J.; Yu, H.; Wang, Z.; Zeng, X. Effect of crystallographic orientation on mechanical anisotropy of selective laser melted Ti-6Al-4V alloy. Mater. Charact. 2017, 127, 137–145. [Google Scholar] [CrossRef]
- Yang, X.; Barrett, R.A.; Tong, M.; Harrison, N.M.; Leen, S.B. Towards a process-structure model for Ti-6Al-4V during additive manufacturing. J. Manuf. Proc. 2021, 61, 428–439. [Google Scholar] [CrossRef]
- DebRoy, T.; Wei, H.; Zuback, J.S.; Mukherjee, T.; Elmer, J.W.; Milewski, J.O.; Beese, A.M.; De Willson-Heid, A.; Zhang, W. Additive manufacturing of metallic components-process, structure and properties. Prog. Mater. Sci. 2018, 92, 112–124. [Google Scholar] [CrossRef]
- Saboori, A.; Gallo, D.; Biamino, S.; Fino, P.; Lombardi, M. An overview of additive manufacturing of titanium components by direct energy deposition: Microstructure and mechanical properties. App. Sci. 2017, 7, 883. [Google Scholar] [CrossRef] [Green Version]
- Fu, H.Z.; Lin, L. Progress of directional solidification in processing of advanced materials. Mater. Sci. Forum 2005, 475–479, 607–612. [Google Scholar] [CrossRef]
- Bontha, S.; Klingbeil, N.W.; Kobrin, P.A.; Fraser, H.L. Thermal process maps for predicting solidification microstructure in laser fabrication on thin-wall structures. J. Mater. Process. Technol. 2006, 178, 135–142. [Google Scholar] [CrossRef]
- Boyer, R.; Welsch, G.; Colling, E.W. Materials Properties: Handbook; ASM International: Novelty, OH, USA, 1994. [Google Scholar]
- Davis, W.J.; Chen, H.; Nomoto, K.; Wang, H.; Babu, S.; Priming, S.; Liao, X.; Brenn, A.; Ringer, S.P. Phase transformation pathways in Ti-6Al-4V manufactured via electron beam powder bed fusion. Acta Mater. 2021, 215, 117131. [Google Scholar] [CrossRef]
- Motyka, M.; Kubiak, K.; Sianiawski, J.; Ziaja, W. Phase transformation and characterization of α + β titanium alloys. In Comprehensive Materials Processing; Elsevier: Amsterdam, The Netherlands, 2014; pp. 7–36. [Google Scholar] [CrossRef]
- Yang, J.; Yu, H.; Yin, J.; Gao, M.; Wang, Z.; Zeng, X. Formation and control of martensite in Ti-6Al-4V alloy produced by selective laser melting. Mater. Des. 2016, 108, 308–318. [Google Scholar] [CrossRef]
- Haar, G.M.T.; Becker, T.H. Selective laser melting produced Ti-6Al-4V: Post-Process Heat Treatment to Achieve Superior Tensile Properties. Materials 2018, 11, 146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karimi, J.; Xie, M.S.; Wang, Z.; Prashanth, K.G. Influence of substructures on the selective laser melted Ti-6Al-4V alloy as a function of laser re-melting. J. Mater. Process. Mater. 2021, 68, 1387–1394. [Google Scholar] [CrossRef]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Van Humbeeck, J.; Kruth, J.-P. A study of the microstructural evolution during selective laser melting of Ti-6Al-4V. Acta Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Welsch, G.; Bunk, W. Deformation modes of the α-phase of Ti-6Al-4V as a function of oxygen concentration and aging temperature. Metall. Trans. A 1982, 13, 889–899. [Google Scholar] [CrossRef]
- Gehlin, P.C. The Crystallographic Structure of Ti3Al; Jaffe, R.I., Ed.; Permogon Press: New York, NY, USA, 1970. [Google Scholar]
- Williams, J.C.; Sommer, A.W.; Tung, P.P. The influence of oxygen concentration internal stress and dislocations arrangements in α titanium. Metall. Mater. Trans. B 1972, 3, 2979–2984. [Google Scholar] [CrossRef]
- Dear, F.F.; Konts, P.; Gault, B.; Ilavsky, J.; Rugg, D.; Dye, D. Mechanisms of Ti3Al precipitation in hpc α-Ti. Acta Mater. 2021, 212, 116811. [Google Scholar] [CrossRef]
- Pushilina, N.; Panin, A.; Syrtanov, M.; Kashkarov, E.; Kudiiarov, V.; Perevalova, O.; Laptev, R.; Lider, A.; Koptyug. Hydrogen-induced phase transformation and microstructure evolution for Ti-6Al-4V parts produced by electron beam melting. Metals 2018, 8, 301. [Google Scholar] [CrossRef] [Green Version]
- Leyens, C.; Peters, M. Application of titanium and titanium alloys. In Titanium Alloy, 1st ed.; Moiseyev, V.N., Ed.; Taylor & Francis Group: Abingdon, UK, 2005; p. 12. [Google Scholar] [CrossRef]
- Grabovetskaya, G.P.; Helnikova, E.N.; Kolobov, Y.R.; Chernov, I.P.; Naidenkin, E.V.; Nikitenkov, N.N.; Mishin, I.P. Evolution of the structural and phase states of Ti-6Al-4V alloy in forming submicroscopy crystalline structure with use of temporary hydrogenation. Russ. Phys. J. 2006, 49, 442–447. [Google Scholar] [CrossRef]
- Zhu, T.; Li, M. Lattice variations of Ti-6Al-4V alloy with hydrogen content. Mater. Charact. 2011, 62, 724–729. [Google Scholar] [CrossRef]
- Yu, J.; Rombouts, M.; Maes, G.; Motamans, F. Materials properties of Ti6Al4V parts produced by laser metal deposition. Phys. Procedia 2012, 39, 416–424. [Google Scholar] [CrossRef] [Green Version]
- Takase, A.; Ishimoto, T.; Morita, N.; Ikeo, N.; Nakano, T. Comparison of phase characteristics and residual stress in Ti-6Al-4V alloy manufactured by laser powder bed fusion (L-PBF) and electron beam powder bed fusion (EB-PBF) techniques. Crystals 2021, 11, 796. [Google Scholar] [CrossRef]
- Huang, S.; Sing, S.L.; de Looze, G.; Wilson, R.; Yeong, W.Y. Laser Powder bed fusion of titanium-tantalium alloys: Compositions and Designs for Biomedical Applications. J. Mech. Behav. Biomed. Mater. 2020, 108, 103775. [Google Scholar] [CrossRef]
- Xie, Z.; Dai, Y.; Ou, X.; Ni, S.; Song, M. Effects of selective laser melting build orientations on the microstructure and tensile performance of Ti-6Al-4V alloy. Mater. Sci. Eng. A 2020, 776, 139001. [Google Scholar] [CrossRef]
- Flißner-Rieger, C.; Tunes, M.A.; Gammer, C.; Jörg, T.; Pfeifer, T.; Musi, M.; Mendez-Martin, F.; Clemens, H. On the existence of orthorhombic martensite in a near-α titanium base alloy used for additive manufacturing. J. Alloys Compd. 2022, 897, 163155. [Google Scholar] [CrossRef]
- Katantseva, N.; Krakhmalev, P.; Thuvander, M.; Yadroitsev, I.; Vinogradova, N.; Ezhov, I. Martensitic transformation in Ti-6Al-4V (ELI) alloy manufactured by 3D printing. Mater. Charact. 2018, 146, 101–112. [Google Scholar] [CrossRef]
- Requena, G.; Cloetens, P.; Altendorfer, W.; Poletti, C.; Tolnai, D.; Warchomicka, F.; Degischer, H.P. Sub-micrometer synchrotron tomography of multiphase metals using Kirkpatrick-baez optics. Scripta Mater. 2009, 61, 760–763. [Google Scholar] [CrossRef]
- Li, C.; Li, G.; Yang, Y.; Varlioglu, M.; Yang, L. α″ martensitic twinning in α + β Ti-3.5Al-4.5Mo titanium alloy. J. Metall. 2011, 2011, 924032. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Yang, S.; Jiang, H.; Shi, Z.; Yang, M.; Wang, C. The clarification of the α″ phase precipitate from β phase in Ti-15Mn alloy by mismatch theory. Mater. Letter. 2017, 202, 138–141. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Yang, X.; Zhang, R.; Shen, Z.; Feng, Q. Specific heat treatment of selective laser melted Ti-6Al-4V for biomedical applications. Front. Mater. Sci. 2015, 9, 373–381. [Google Scholar] [CrossRef]
- Yadroitsev, I.; Yadroitsava, I.; Plessis, A.D.; MacDonald, E. Fundamentals of Laser Powder Bed Fusion of Metals, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 245–276. [Google Scholar]
- Cotulo, A.; Elangeswaran, C.; de Formanoir, C.; Muralidharan, G.K.; Van Hooreweder, B. Effect of heat treatments on fatigue properties of Ti-6Al-4V and 316L produced by laser powder bed fusion in as-built surface condition. In TMS 2019 148th Annual Meeting & Exhibition Supplemental Proceedings; Springer: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Eshawish, N.; Malinov, S.; Sha, W.; Walls, P. Microstructure and mechanical properties of Ti-6Al-4V manufactured by selective laser melting after stress relieving, hot isostatic pressing treatment, and post-heat treatment. J. Mater. Eng. Perform. 2021, 30, 5290–5296. [Google Scholar] [CrossRef]
- ASTM F3301-18a; Additive Manufacturing—Post Processing Methods—Standard Specification for Thermal Post-Processing Metals Part Made via Powder Bed Fusion. ASTM International: West Conshohocken, PA, USA, 2018.
- Bartsch, K.; Herzog, D.; Bossen, B.; Emmelmann, C. Material modelling of Ti-6Al-4V processed by laser powder bed fusion for application in macro-scale process simulation. Mater. Sci. Eng. A 2021, 814, 141237. [Google Scholar] [CrossRef]
- Longhitano, G.A.; Larosa, M.A.; Jardini, A.L.; de Carvalho Zavaglia, C.A.; Filipini Ierardi, M. Correlation between microstructure and mechanical properties under tensile and compression test heat-treated Ti-6Al-4V ELI alloy produced by AM for biomedical application. J. Mater. Process. Technol. 2018, 252, 202–210. [Google Scholar] [CrossRef]
- Meyer, L.W.; Krüger, L.; Sommer, K.; Halle, T.; Hockanf, M. Dynamic strength and failure behavior of titanium alloy TI-6Al-4V for a variation of heat treatments. Mech. Time-Depend. Mater. 2008, 12, 237–247. [Google Scholar] [CrossRef]
- Lin, Y.C.; Tang, Y.; Jiang, Y.-C.; Chen, J.; Wang, D.; He, D.-G. Precipitation of secondary phase transformation behavior of a solution-treated Ti-6Al-4V alloy during high-temperature aging. Adv. Eng. Mater. 2020, 22, 1901436. [Google Scholar] [CrossRef]
- Liu, C.; Yu, L.; Zhang, A.; Tian, X.; Liu, D.; Ma, S. Beta heat treatment of laser melting deposited high strength near β titanium alloy. Mater. Sci. Eng. A 2016, 673, 185–192. [Google Scholar] [CrossRef]
- Kent, D.; Wang, G.; Wang, W.; Dargusch, M.S. Influence of ageing temperature and heating rate on the properties and microstructure β Ti alloys, Ti-6Cr-5Mo-4Al. Mater. Sci. Eng. A 2012, 531, 98–106. [Google Scholar] [CrossRef]
- Muhammad, M.; Pegeus, J.W.; Shamsei, N.; Haghshenas, M. Effect of heat treatments on microstructure/small-scale properties of additive manufactured Ti-6Al-4V. Int. J. Adv. Manuf. 2019, 103, 4161–4172. [Google Scholar] [CrossRef]
- Jamshidi, P.; Aristizabal, H.; Kong, W.; Villapun, V.; Cox, S.C.; Grover, L.M.; Attallah, M.M. Selective laser melting of Ti-6Al-4V: The impact Post-Processing on the Tensile, Fatigue and Biological Properties for Medical Implant Applications. Materials 2020, 13, 2813. [Google Scholar] [CrossRef]
- Cox, S.C.; Jamshidi, P.; Eisenstein, N.M.; Webber, M.A.; Burton, H.; Moakes, R.J.; Addison, O.; Attallah, M.; Shepherd, D.E.; Grover, L.M. Surface finish has a critical influence of biofilm formation and mammalian cell attachment to additively manufactured prosthetics. ACS Biomater. Sci. Eng. 2017, 3, 1616–1626. [Google Scholar] [CrossRef]
- Chafino, J.A.; Yamanaka, K.; Mercier, F.; Rivory, P.; Balvoy, S.; Hartmann, D.J.; Chiba, A.; Fabregue, D. The influence of temperature during water-quench rapid heat treatment on the microstructure, mechanical properties and biocompatibility of Ti-6Al-4V ELI alloy. J. Mech. Behav. Biomed. Mater. 2019, 96, 144–151. [Google Scholar] [CrossRef]
- SAE AMS-H-81200C-2010; Heat Treatment of Titanium and Alloys. SAE International Group: Warrendale, PA, USA, 2010.
- Ji, Y.; Heo, T.W.; Zhang, F.; Chen, L.Q. Theoretical assessment on the phase transformation kinetic pathways of multi-component Ti alloys: Application to Ti-6Al-4V. J. Phase Equilibria Diffus. 2016, 37, 53–64. [Google Scholar] [CrossRef]
- Lu, S.L.; Qian, M.; Tang, H.P.; Yan, M.; Wang, J.; StJohn, D.H. Massive transformation in Ti-6Al-4V additively manufactured by selective electron beam melting. Acta Mater. 2016, 104, 303–311. [Google Scholar] [CrossRef]
- ASTM F1580-12; Standard Specification for Titanium and Titanium-6 Aluminium-4 Vanadium Alloy Powders for Coatings of Surgical Implants. ASTM International: West Conshohocken, PA, USA, 2012.
- Malinov, S.; Sha, W.; Guo, Z.; Tang, C.C.; Long, A.E. Synchrotron X-Ray diffraction study of the phase transformation in titanium alloys. Mater. Charact. 2002, 48, 279–295. [Google Scholar] [CrossRef] [Green Version]
- Etesami, S.A.; Fotovvati, B.; Asadi, E. Heat treatment of Ti-6Al-4V alloy manufactured by laser-based powder-bed fusion: Process, Microstructures, and Mechanical Properties Correlations. J. Alloys Compd. 2021, 895, 162618. [Google Scholar] [CrossRef]
- Losertová, M.; Kubeš, V. Microstructural and mechanical properties of selective laser melted Ti6Al4V alloy. IOP Conf. Ser. Mater. Sci. Eng. 2017, 266, 012009. [Google Scholar] [CrossRef]
- Li, C.-L.; Hing, J.-K.; Narayana, P.L.; Choi, S.-W.; Lee, S.W.; Park, C.H.; Yeom, J.T.; Mei, Q. Realizing superior ductility of selective laser melted Ti-6Al-4V through a multi-step heat treatment. Mater. Sci. Eng. A 2021, 799, 140367. [Google Scholar] [CrossRef]
- Cao, S.; Chen, Z.; Lim, C.V.S.; Yang, K.; Jia, Q.; Jarvis, T.; Tomus, D.; Wu, X. Defect, microstructure, and mechanical property of Ti-6Al-4V alloy fabricated by high-power selective laser melting. JOM 2017, 69, 2684–2693. [Google Scholar] [CrossRef]
- Cao, S.; Zou, Y.; Voon Samuel Lin, C.; Wu, X. Review of laser powder bed fusion (LPBF) fabricated Ti-6Al-4V: Process, Post-Process Treatment, Microstructure, and Property. Light Adv. Manuf. 2021, 2, 313–332. [Google Scholar] [CrossRef]
- Haubrick, J.; Gussone, J.; Barriero-Vila, P.; Kürnsteiner, P.; Jägle, E.A.; Raabe, D.; Schell, N.; Requena, G. The role of lattice defects, element portioning and intrinsic heat effects on the microstructure in selective laser melted Ti-6Al-4V. Acta Mater. 2019, 167, 136–148. [Google Scholar] [CrossRef]
- Callegari, B.; Marçola, J.V.; Aristizabal, K.; Soldera, F.A.; Müklich, F.; Pinto, H.C. Effect of microstructure on Ti3Al precipitation during ageing of Ti-6Al-4V alloy. MATEC Conf. 2020, 321, 12014. [Google Scholar] [CrossRef]
- Lütjering, G. Influence of processing on microstructure and mechanical properties of (α + β) titanium alloys. Mater. Sci. Eng. A 1998, 243, 32–45. [Google Scholar] [CrossRef]
- Schuster, J.C.; Palm, M. Reassessment of the binary aluminium-titanium phase diagram. J. Phase Equilibria Diffus. 2006, 27, 255–277. [Google Scholar] [CrossRef]
- Bagot, P.A.J.; Radecka, A.; Magyar, A.P.; Gong, Y.; Bell, D.C.; Smith, G.D.W.; Moody, M.P.; Dye, D.; Rugg, D. The effect of oxidation on the subsurface microstructure of a Ti-6Al-4V alloy. Scripta Mater. 2018, 148, 24–28. [Google Scholar] [CrossRef]
- Sun, W.; Ma, Y.E.; Zhang, W.; Qian, X.; Huang, W.; Wang, Z. Effects of build orientation on mechanical performance of laser powder bed fusion additively manufactured Ti6Al4V under different loadings. Adv. Eng. Mater. 2021, 23, 2100611. [Google Scholar] [CrossRef]
- Moridi, A.; Demir, A.G.; Caprio, L.; Hart, A.J.; Previtali, B.; Colosimo, B.M. Deformation and failure mechanisms of Ti-6Al-4V as built by selective laser melting. Mater. Sci. Eng. A 2019, 768, 138456. [Google Scholar] [CrossRef]
- Rafi, H.K.; Starr, T.L.; Stucker, B.E. A comparison of the tensile, fatigue, and fracture behavior of Ti-6Al-4V and 15-5PH stainless steel parts made by selective laser melting. Int. J. Adv. Manuf. Technol. 2013, 69, 1299–1309. [Google Scholar] [CrossRef]
- Vilardell, A.M.; Fredrksson, G.; Yadroitsev, I.; Krakhmalev, P. Fracture mechanisms in the as-built and stress-relieved laser powder bed fusion Ti-6Al-4V ELI alloy. Opt. Laser Technol. 2019, 109, 608–615. [Google Scholar] [CrossRef]
- Benzing, J.; Hrabe, N.; Quinn, T.; White, R.; Rentz, R.; Ahlfors, M. Hot isostatic pressing (HIP) to achieve isotropic microstructure and retain as-built strength in an additive manufacturing titanium alloy (Ti-6Al-4V). Mater. Lett. 2019, 257, 126690. [Google Scholar] [CrossRef]
- Li, P.; Warner, D.H.; Pegeus, J.W.; Roach, M.D.; Shamsaei, N.; Phan, H. Investigation of the mechanisms by which hot isostatic pressing improves the fatigue performance of powder bed fused Ti-6Al-4V. Int. J. Fatigue 2019, 120, 342–352. [Google Scholar] [CrossRef] [PubMed]
- Wycisk, E.; Siddique, S.; Hergoz, D.; Walther, F.; Emmelmann, C. Fatigue performance of laser additive manufactured TI-6Al-4V in very high cycle fatigue regime up to 109 cycles. Front. Mater. 2015, 2, 72. [Google Scholar] [CrossRef]
- Hergoz, D.; Bartsch, K.; Bossen, B. Productivity optimization of laser powder bed fusion by hot isostatic pressing. Add. Manuf. 2020, 36, 101494. [Google Scholar] [CrossRef]
- Kasperovich, G.; Hausmann, J. Improvement of fatigue resistance and ductility of Ti6Al4V processed by selective laser melting. J. Mater. Proc. Technol. 2015, 220, 202–214. [Google Scholar] [CrossRef]
- Chong, Y.; Bhattacharjee, T.; Tian, Y.; Shibata, A.; Tsuji, N. Deformation mechanism of bimodal microstructure in Ti-6Al-4V alloy: The Effects on Interfacial Annealing Temperature and Constituent Hardness. J. Mater. Res. Technol. 2021, 71, 138–151. [Google Scholar] [CrossRef]
- Sabban, R.; Bahl, S.; Chatterjee, K.; Suwas, S. Globularization using heat treatment in additively manufactured Ti-6Al-4V for high strength and toughness. Acta Mater. 2019, 162, 239–254. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, J.; Tan, H.; Zhang, G.; Lin, X.; Huang, W. Achieving superior ductility for laser solid formed extra low interstitial Ti-6Al-4V titanium alloy through equiaxial alpha microstructure. Scripta Mater. 2018, 146, 187–191. [Google Scholar] [CrossRef]
- Mierzejewska, Z.A. Effect of laser energy density, internal porosity and heat treatment on mechanical behavior of biomedical Ti6Al4V alloy obtained with DMLS technology. Materials 2019, 12, 2331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swarnakar, A.K.; Van der Biest, O.; Baufeld, B. Thermal expansion and lattice parameters of shaped metal deposited Ti-6Al-4V. J. Alloys Compd. 2011, 509, 2723–2728. [Google Scholar] [CrossRef]
- Slater, J.C. Atomic radii in crystals. J. Chem. Phys. 1964, 41, 3199–3205. [Google Scholar] [CrossRef]
- Tsai, M.-T.; Chen, Y.-W.; Chao, C.-Y.; Jang, J.S.C.; Tsai, C.-C.; Su, Y.-L.; Kuo, C.-N. Heat treatment effects on mechanical properties and microstructure evolution of Ti-6Al-4V alloy fabricated by laser powder bed fusion. J. Alloys Compd. 2020, 816, 152615. [Google Scholar] [CrossRef]
- Gallaraga, H.; Lados, D.A.; Dehoff, R.R.; Kirka, M.M.; Nandwana, P. Effects of the microstructure and porosity on properties of Ti-6Al-4V ELI alloy fabricated by electron beam melting (EBM). Add. Manuf. 2016, 10, 47–57. [Google Scholar] [CrossRef] [Green Version]
- Lifshtz, M.; Slyozov, V.V. The kinetics of precipitation from supersaturated solid solutions. J. Phys. Chem. Solids 1961, 19, 35–50. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, K.; Yang, Y.; Wang, H.; Zhu, Y.; Huang, A. Grain boundary α-phase precipitation and coarsening: Comparing Laser Powder Bed Fusion with As-cast Ti-6Al-4V. Scripta Mater. 2022, 207, 114261. [Google Scholar] [CrossRef]
- Cao, S.; Hu, Q.; Huang, A.; Chen, Z.; Sun, M.; Zhang, J.; Fu, C.; Jia, Q.; Lim, V.C.S.; Boyer, R.R.; et al. Static coarsening behavior of lamellar microstructure in selective laser melted Ti-6Al-4V. J. Mater. Sci. Technol. 2019, 35, 1578–1586. [Google Scholar] [CrossRef]
- Stefansonn, N.; Semiatin, S.L. Mechanisms of globularization of Ti-6Al-4V during static heat treatment. Metall. Mater. Trans. A 2003, 34, 691–698. [Google Scholar] [CrossRef]
- Roy, S.; Suwas, S. Orientation dependent spheroidization response and macro-zone formation during sub β-transus processing of Ti-6Al-4V alloy. Acta Mater. 2017, 134, 283–301. [Google Scholar] [CrossRef]
- Sharma, G.; Ramanujan, R.V.; Tiwari, G.P. Instability mechanisms in lamellar microstructures. Acta Mater. 2000, 48, 875–889. [Google Scholar] [CrossRef]
- ASTM F2924-12; Standard Specification for Additive Manufacturing Titanium-6 Aluminium-4 Vanadium with Powder Bed Fusion. ASM International: West Conshohocken, PA, USA, 2013.
- Mertens, A.; Reginster, A.; Paydas, H.; Contrepois, Q.; Dormol, T.; Lemaire, O.; Lecomte-Beckers, J. Mechanical properties of alloy Ti-6Al-4V and of stainless steel 316L processed by selective laser melting: Influence of Out-of-Equilibrium Microstructure. Powder Metall. 2014, 57, 184–190. [Google Scholar] [CrossRef]
- Carroll, B.E.; Palmer, T.A.; Beese, A.M. Anisotropy tensile behavior of Ti-6Al-4V components fabricated with direct energy deposition additive manufacturing. Acta Mater. 2015, 87, 309–320. [Google Scholar] [CrossRef]
- Wilson-Heid, A.E.; Wang, Z.; McCornac, B.; Beese, A.M. Quantitative relationship between anisotropic strain to failure and grain morphology in additively manufactured Ti-6Al-4V. Mater. Sci. Eng. A 2017, 706, 287–294. [Google Scholar] [CrossRef]
- Ronnenberg, I.; Davies, C.M.; Hooper, P.A. Revealing relationships between porosity, microstructure and mechanical properties of laser powder bed fusion 316L stainless steel through heat treatment. Mater. Des. 2020, 189, 108481. [Google Scholar] [CrossRef]
- Cain, V.; Thijs, L.; Van Humbeeck, B.; Van Hooreweder, B.; Knutsen, R. Crack propagation and fracture toughness of Ti6Al4V alloy produced by selective laser melting. Add. Manuf. 2015, 5, 68–76. [Google Scholar] [CrossRef]
- Zafari, A.; Xia, K. High ductility in a fully martensitic microstructure: A Paradox in a Ti alloy Produced by Selective Laser Melting. Mater. Res. Lett. 2018, 6, 627–633. [Google Scholar] [CrossRef]
- Vilaro, T.; Colin, C.; Bartout, J.D. As-fabricated and heat-treated microstructures of the Ti-6Al-4V alloy processed by selective laser melting. Metall. Mater. Trans. A 2011, 42, 3190–3199. [Google Scholar] [CrossRef]
- Muiruri, A.; Maringa, M.; du Preez, W. Validation of a microstructure-based model for prediction the high strain rate flow properties of various forms of additively manufactured Ti6Al4V (ELI) alloy. Metals 2011, 11, 1628. [Google Scholar] [CrossRef]
- Galindo-Fernàndez, M.A.; Mumtaz, K.; Rivera-Díaz-del-Castillo, P.E.J.; Galindo-Nova, E.I.; Ghadbeigi, H. A microstructure sensitive model for deformation of Ti-6Al-4V describing cast-and-wrought and additive manufacturing morphologies. Materials Des. 2018, 160, 350. [Google Scholar] [CrossRef]
- Todaro, C.J.; Easton, M.A.; Qiu, D.; Zhang, S.; Bermingham, M.J.; Lui, E.W.; Brandt, M.; StJohn, D.H.; Qian, M. Grain structure control during metal 3D printing by high-intensity ultrasound. Nat. Commun. 2020, 11, 142–151. [Google Scholar] [CrossRef]
- Qian, M.; Xu, W.; Brandt, M.; Tang, H.P. Additive manufacturing and post processing of Ti-6Al-4V for superior mechanical properties. MRS Bull. 2016, 41, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Akram, J.; Pal, D.; Stucker, B. Establishing flow stress and elongation relationship as a function of microstructural features of Ti6Al4V alloy processed using SLM. Designs 2019, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Waheed, S.; Balint, D.S.; Dunne, F.P.E. Slip transfer across phase boundaries in dual phase titanium alloys and the effect on strain rate sensitivity. Int. J. Plast. 2018, 104, 23–38. [Google Scholar] [CrossRef]
- Kohn, D.H.; Ducheyene, P. Tensile and fatigue strength of hydrogen-treated Ti6Al4V alloy. J. Mater. Sci. 1991, 26, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Fan, Y.; Sun, Q.; Zhang, G. Improvement of the crack propagation resistance in an α + β titanium alloy with a trimodal microstructure. Metals 2020, 10, 1058. [Google Scholar] [CrossRef]
- Hosseini, R.; Morakabarti, M.; Abbasi, S.M.; Hajari, A. Development of a trimodal microstructure with superior combined strength ductility and creep-rupture properties in a near α titanium alloy. Mater. Sci. Eng. A 2017, 696, 155–165. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Zou, Z.; Shi, Y.; Zhang, Y. The microstructure and tensile properties of additively manufactured Ti-6Al-2Zr-1Mo-1W with a trimodal microstructure obtained by multiple annealing heat treatment. Mater. Sci. Eng. A 2022, 831, 142241. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Zhang, H.-Y.; Zheng, C.; Yang, H.-Y.; Qin, P.; Zhao, C.; Lu, S.; Liang, S.-X.; Chai, L.; Zhang, L.-C. Corrosion behavior and characteristics of passive films of laser powder bed fusion produced Ti-6AlV in dynamic Hank’s solution. Mater. Des. 2021, 208, 109907. [Google Scholar] [CrossRef]
- Chin, T.-M.; Mahmudi, M.; Dai, W.; Elevany, A.; Liang, M.; Castaneda, M. Corrosion assessment of Ti6Al-4V fabricated using laser powder-bed fusion additive manufacturing. Electrochem. Acta 2018, 279, 143–151. [Google Scholar] [CrossRef]
- Zhao, B.; Wang, H.; Qiao, N.; Wang, C.; Hu, M. Corrosion resistance characteristics of a Ti-6Al-4V alloy scaffold that is fabricated by electron beam melting and selective laser for implantation in vivo. Mater. Sci. Eng. C 2017, 70, 832–841. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Zhang, J.; Chen, Y.; Zhang, L.-C. Heat treatment degrading the corrosion resistance of selective laser melted Ti-6Al-4V alloy. J. Electrochem. Cos. 2017, 164, C428–C434. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, L.-C.-; Zhang, J.; Chen, Q.; Wu, M. Corrosion behavior of selective laser melted Ti-6Al-4V alloy in NaCl solution. Corros. Soc. 2016, 102, 484–489. [Google Scholar] [CrossRef]
- Chandramohan, P.; Bhero, S.; Obadele, B.A.; Olubambi, B.A. Laser additive manufactured Ti-6Al-4V alloys: Tribology and Corrosion Studies. Int. J. Adv. Manuf. Technol. 2017, 92, 3051–3061. [Google Scholar] [CrossRef]
- Pazhanivel, B.; Sathiya, P.; Sozhan, G. Ultra-fine bimodal (α + β) microstructure induced mechanical strength and corrosion resistance of Ti-6Al-4V alloy produced via laser powder bed fusion process. Opt. Laser Technol. 2020, 125, 106017. [Google Scholar] [CrossRef]
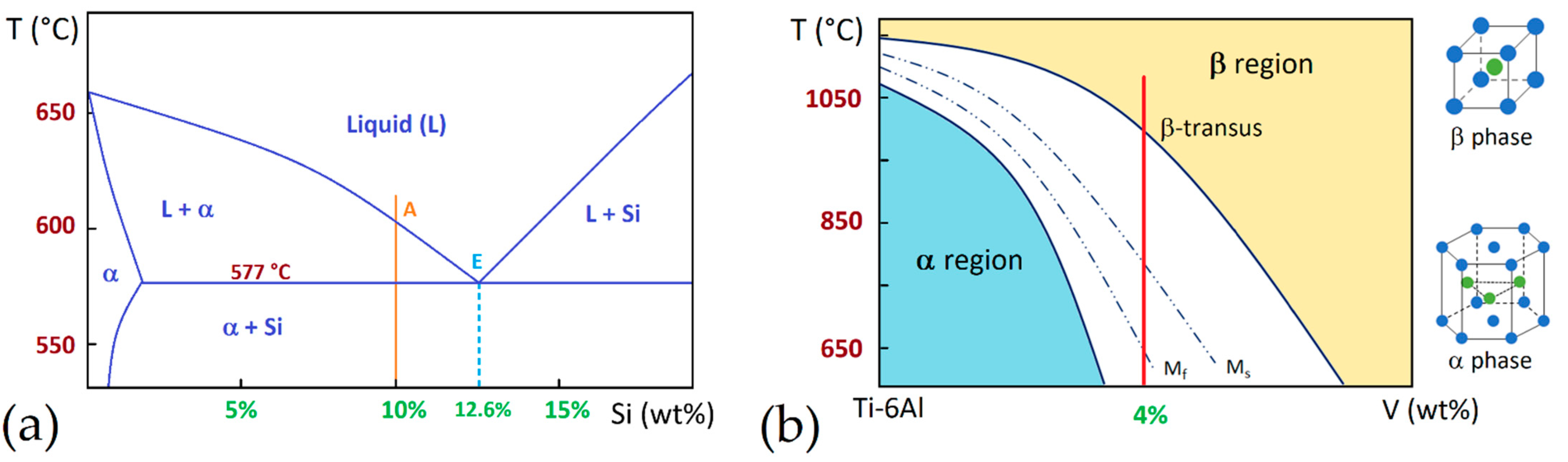

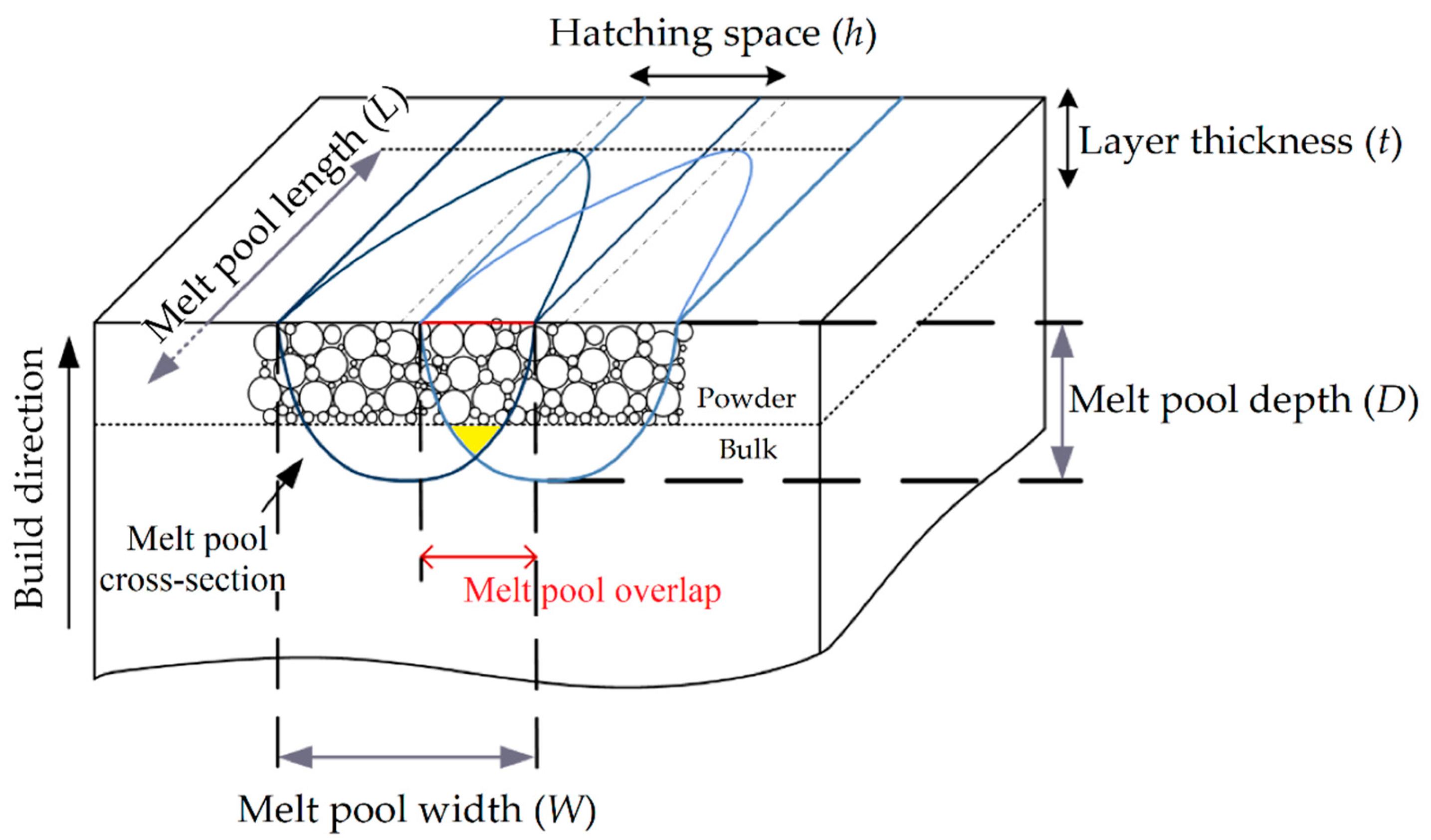

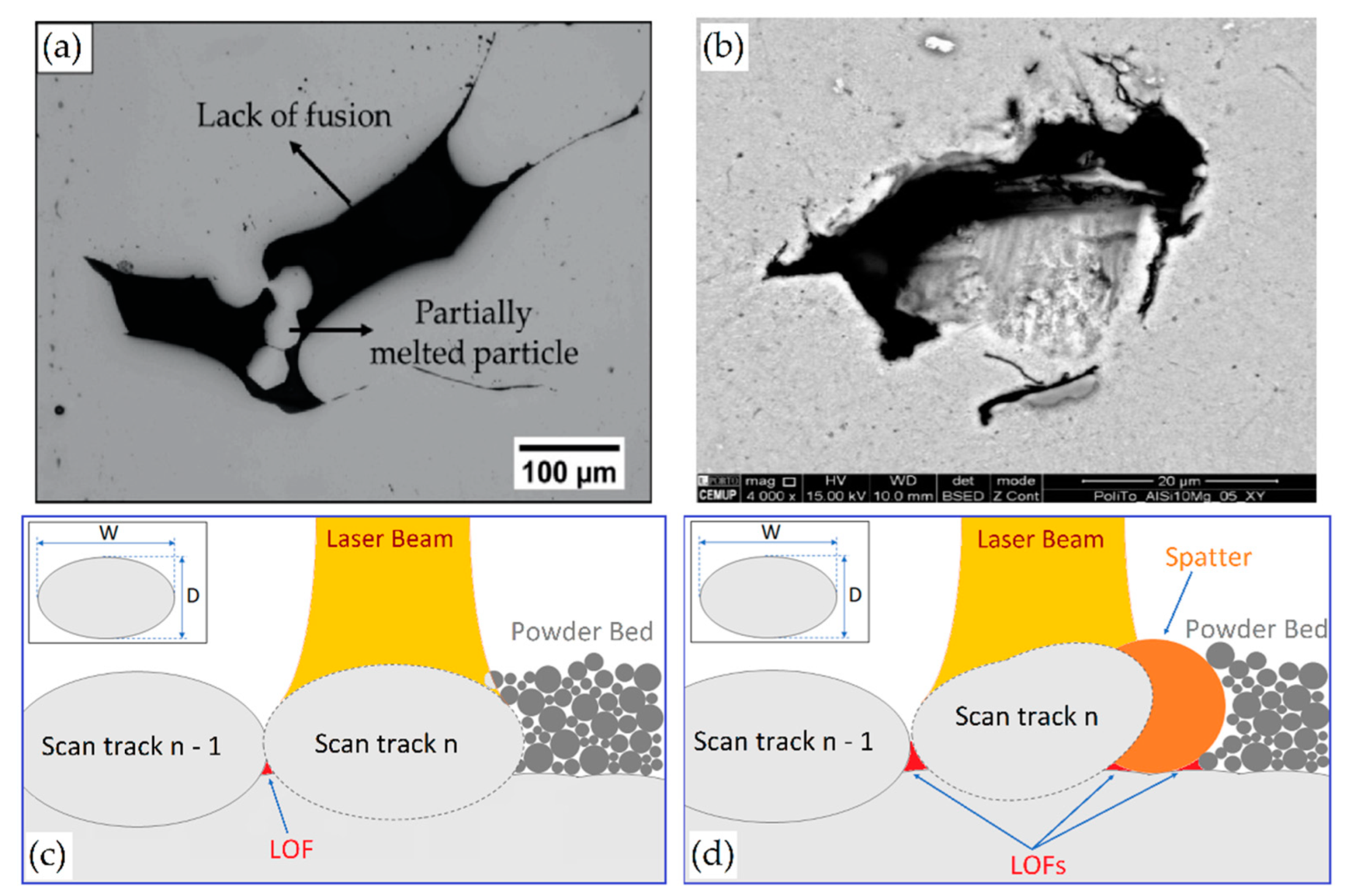
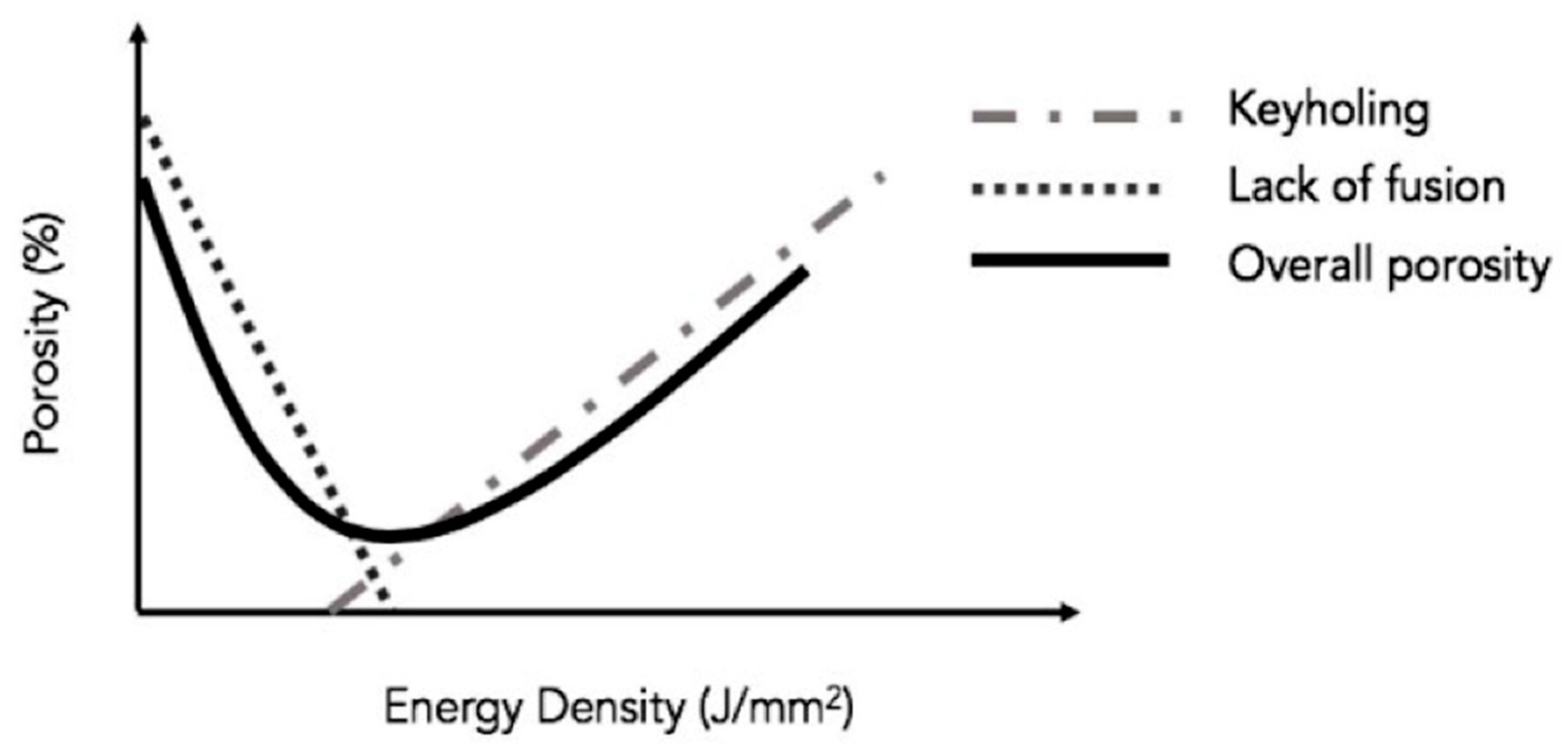


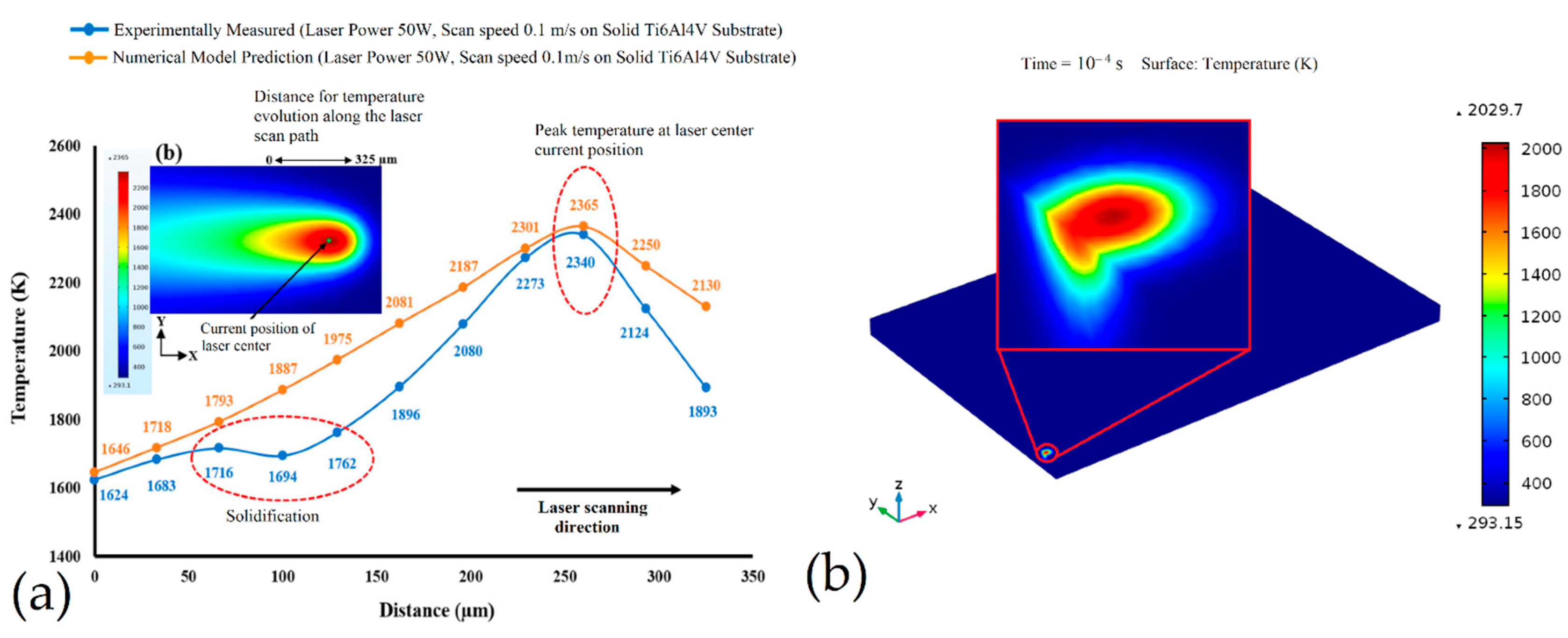
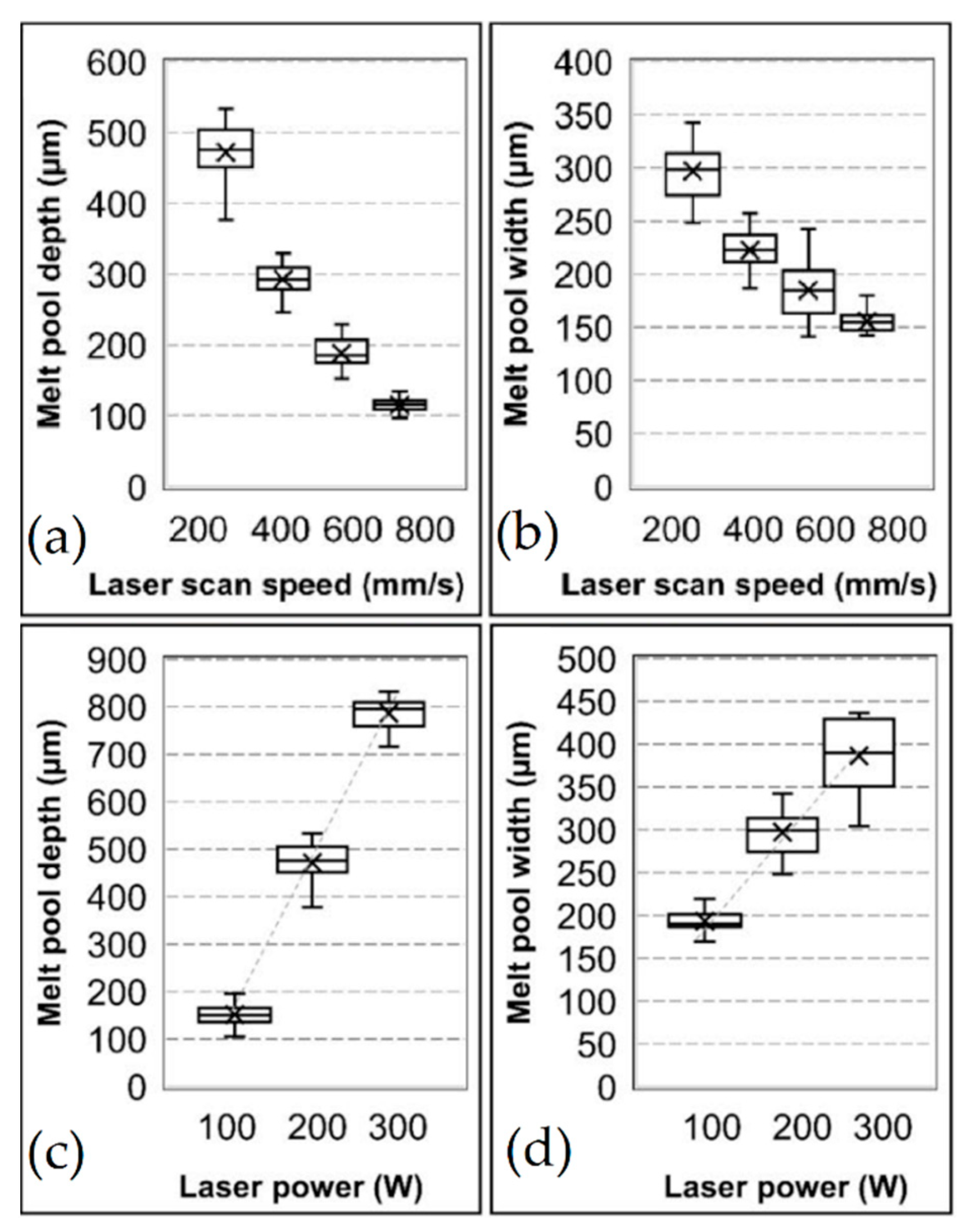
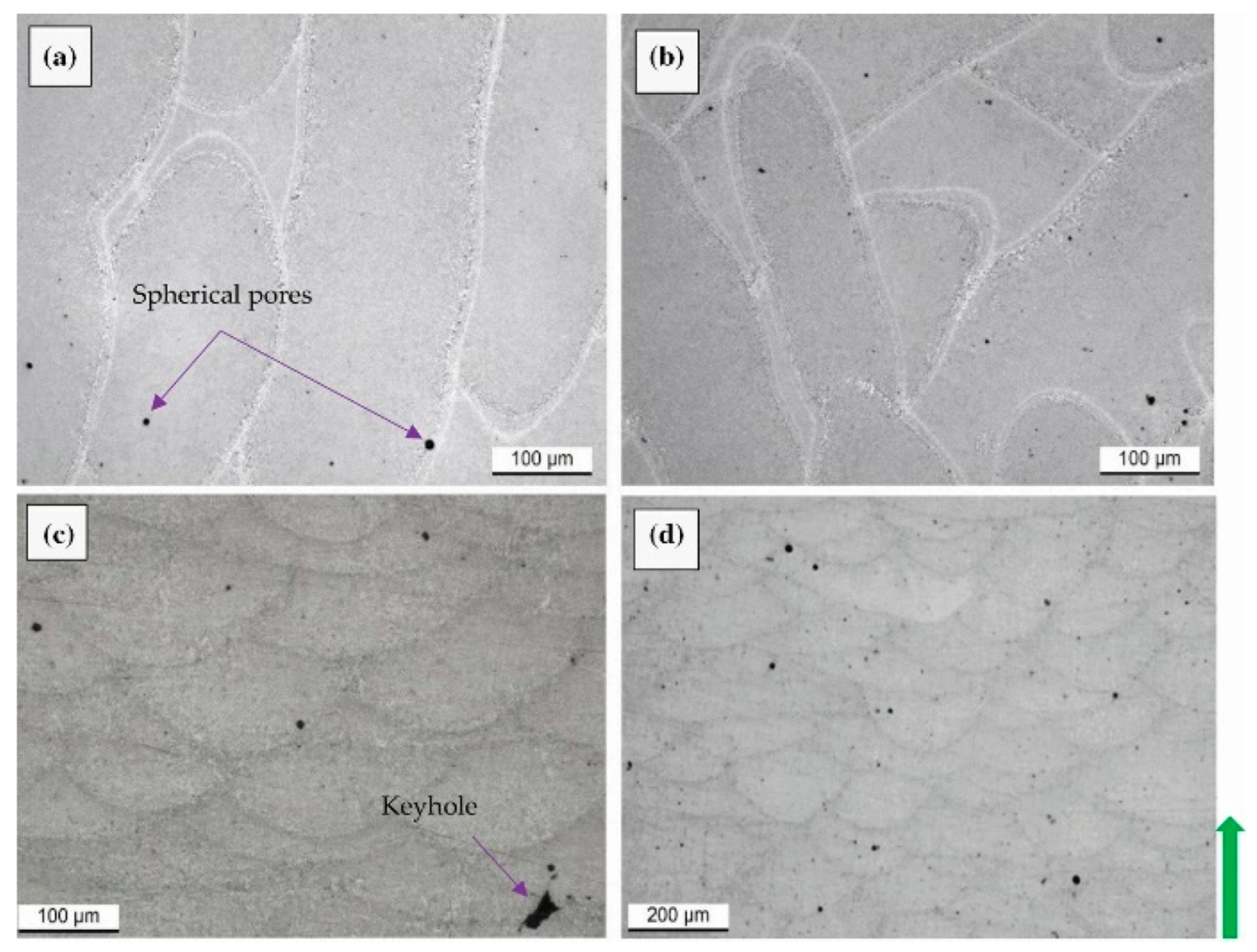
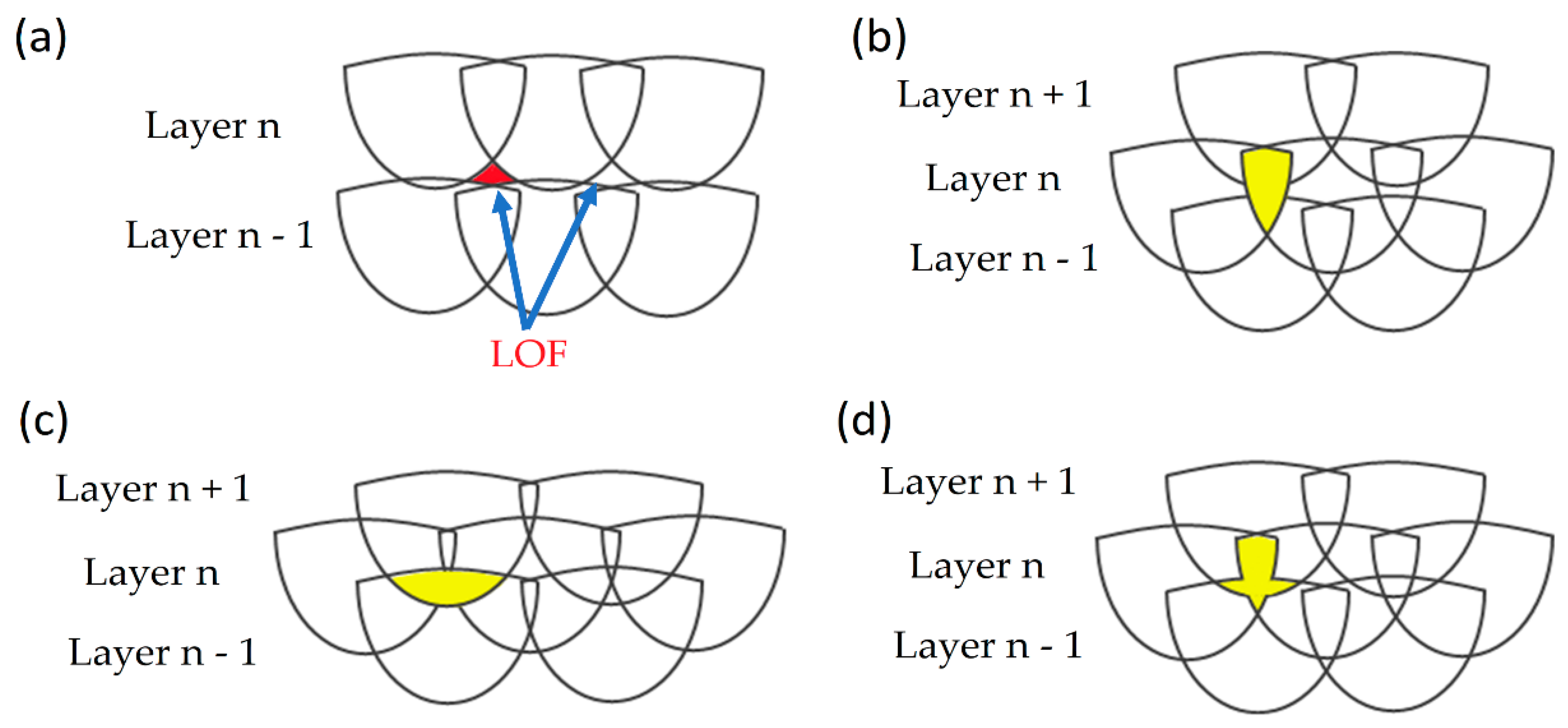
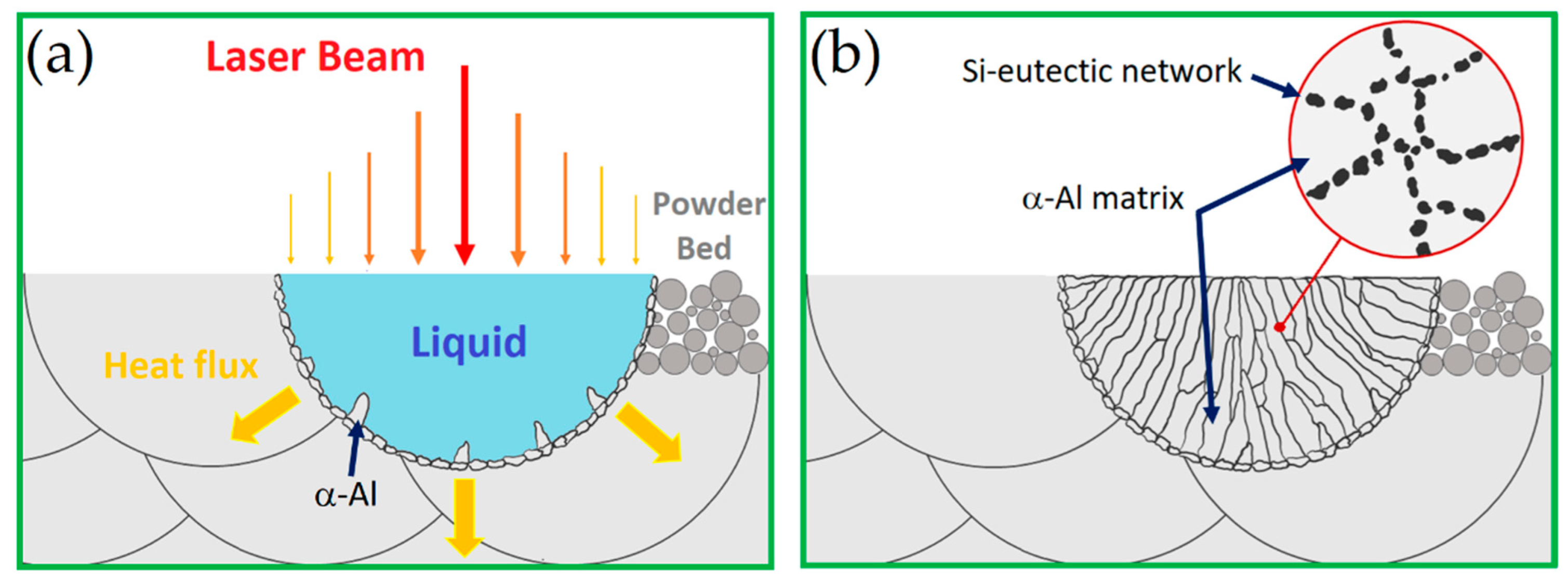
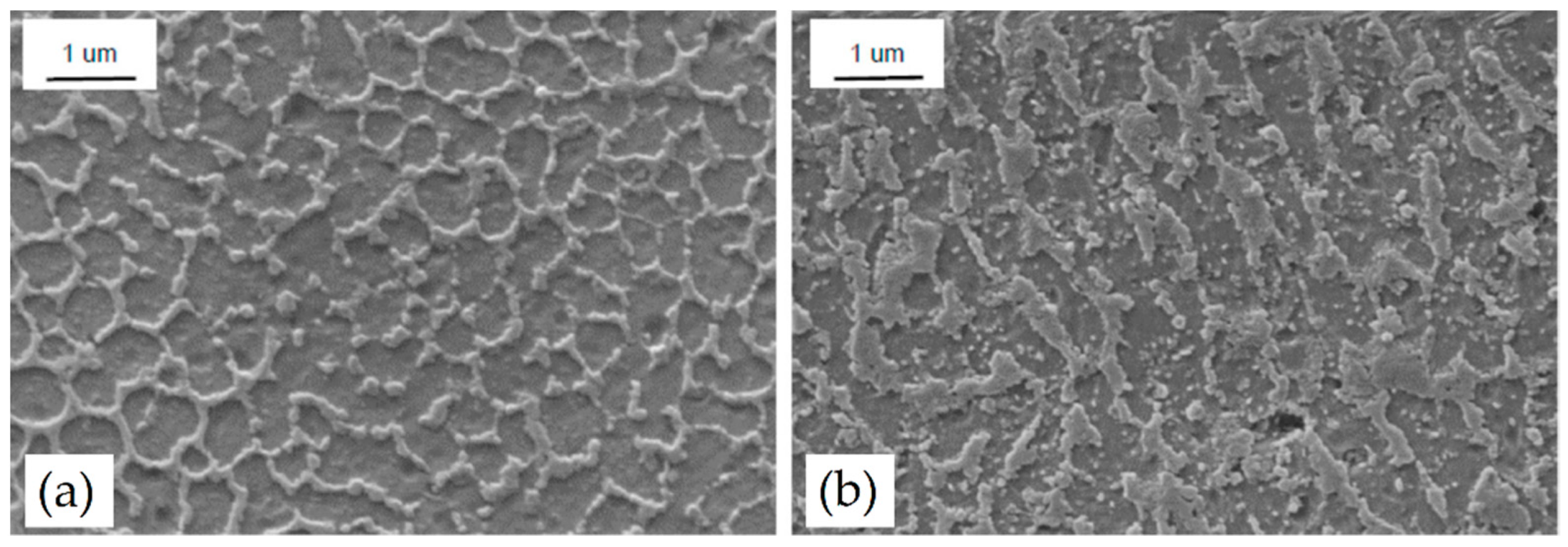
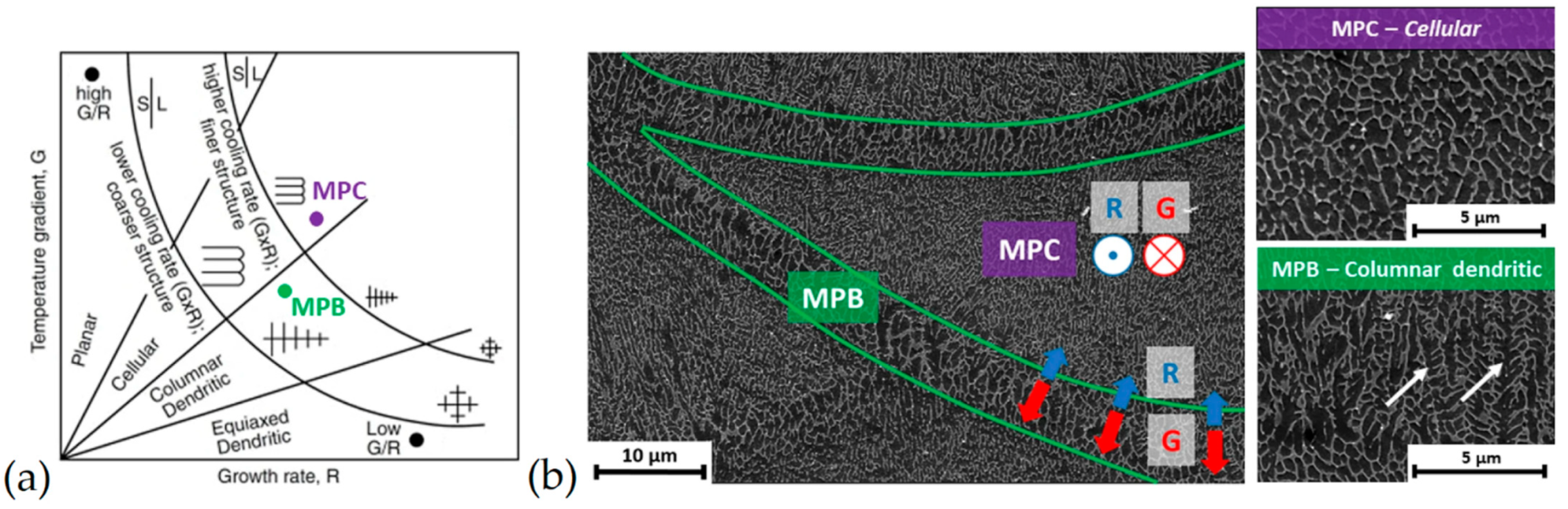
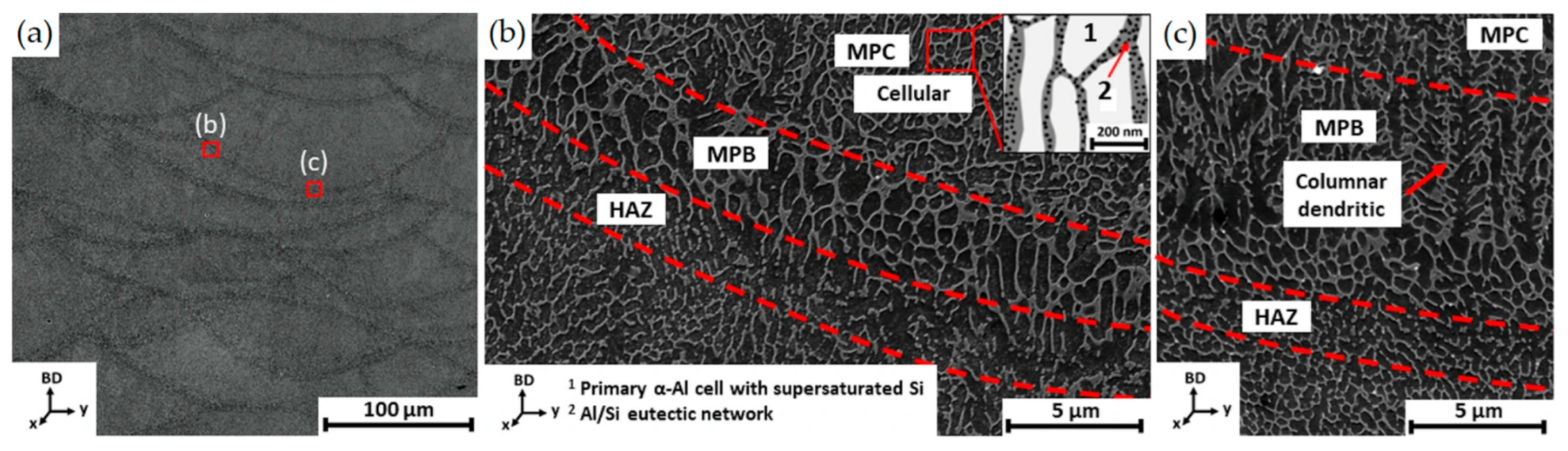
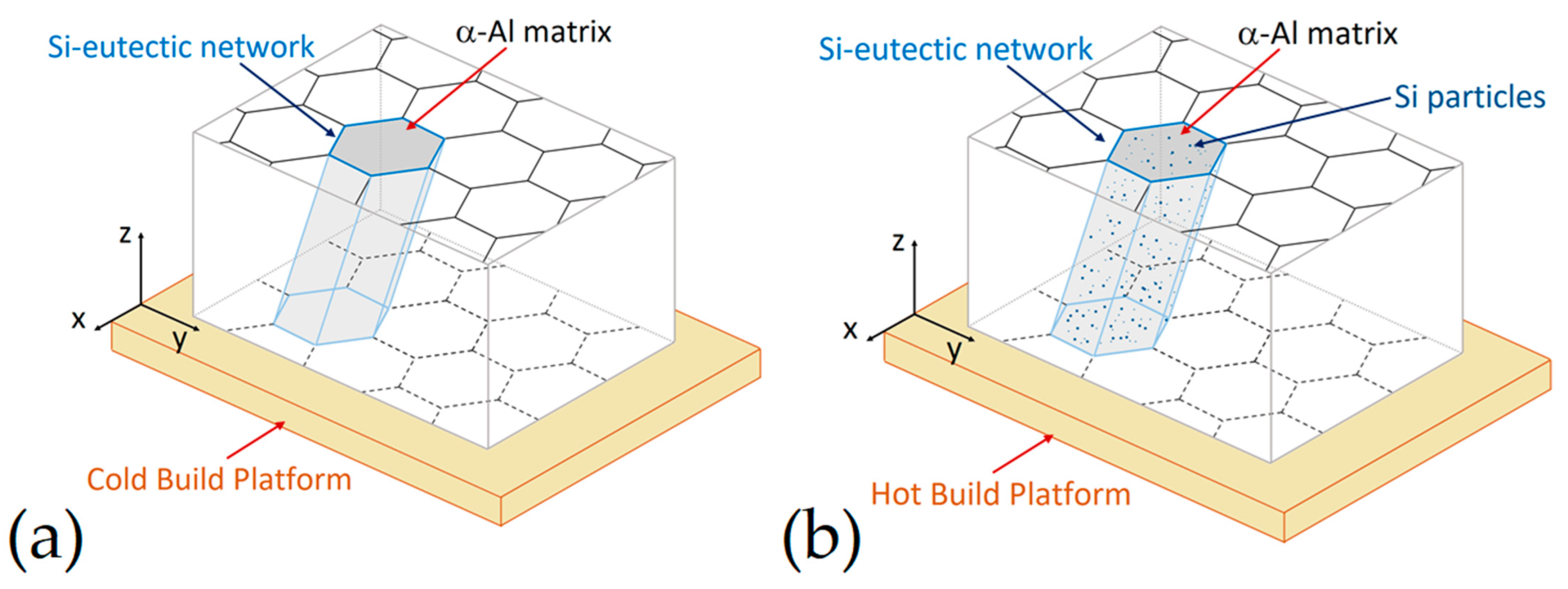
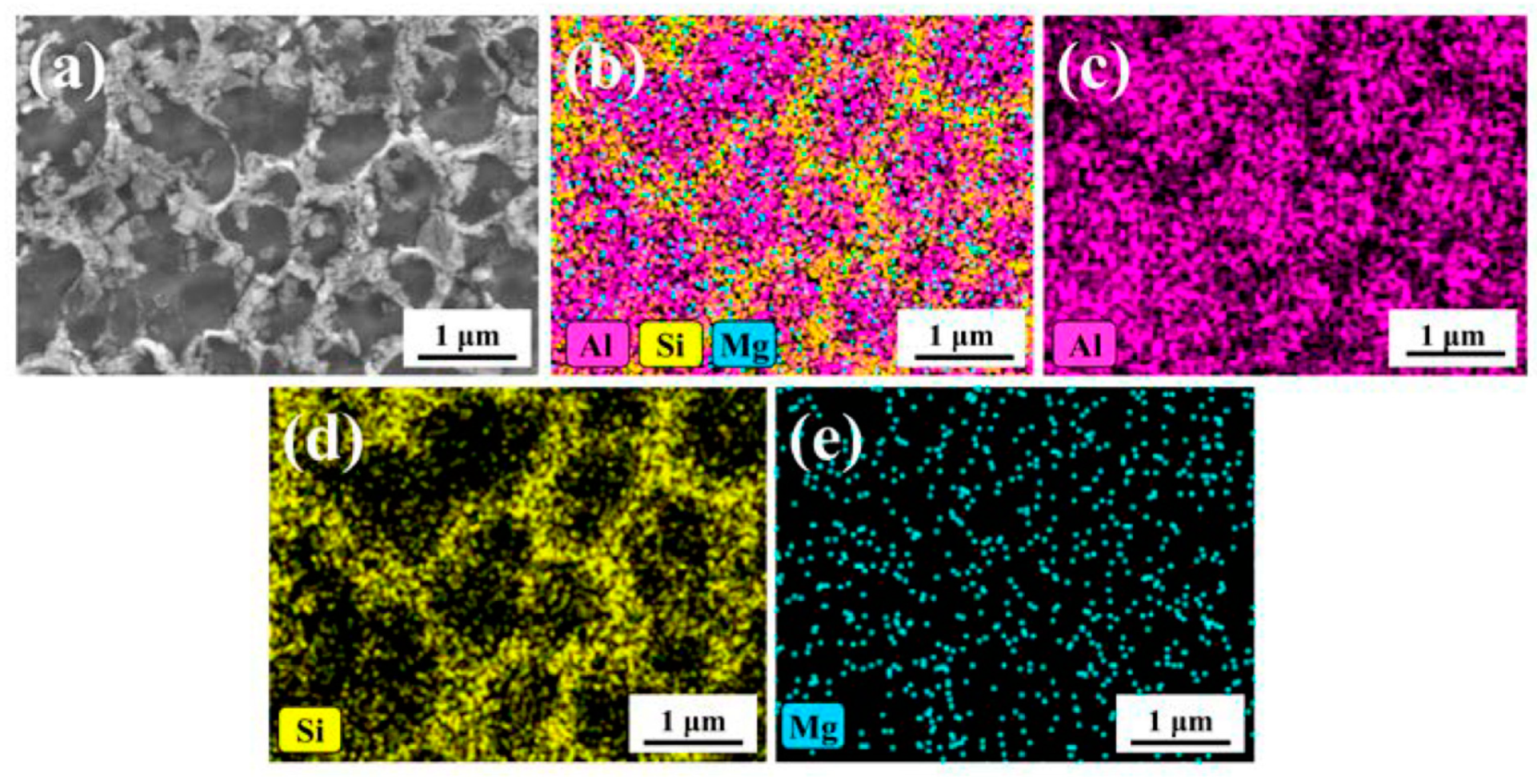
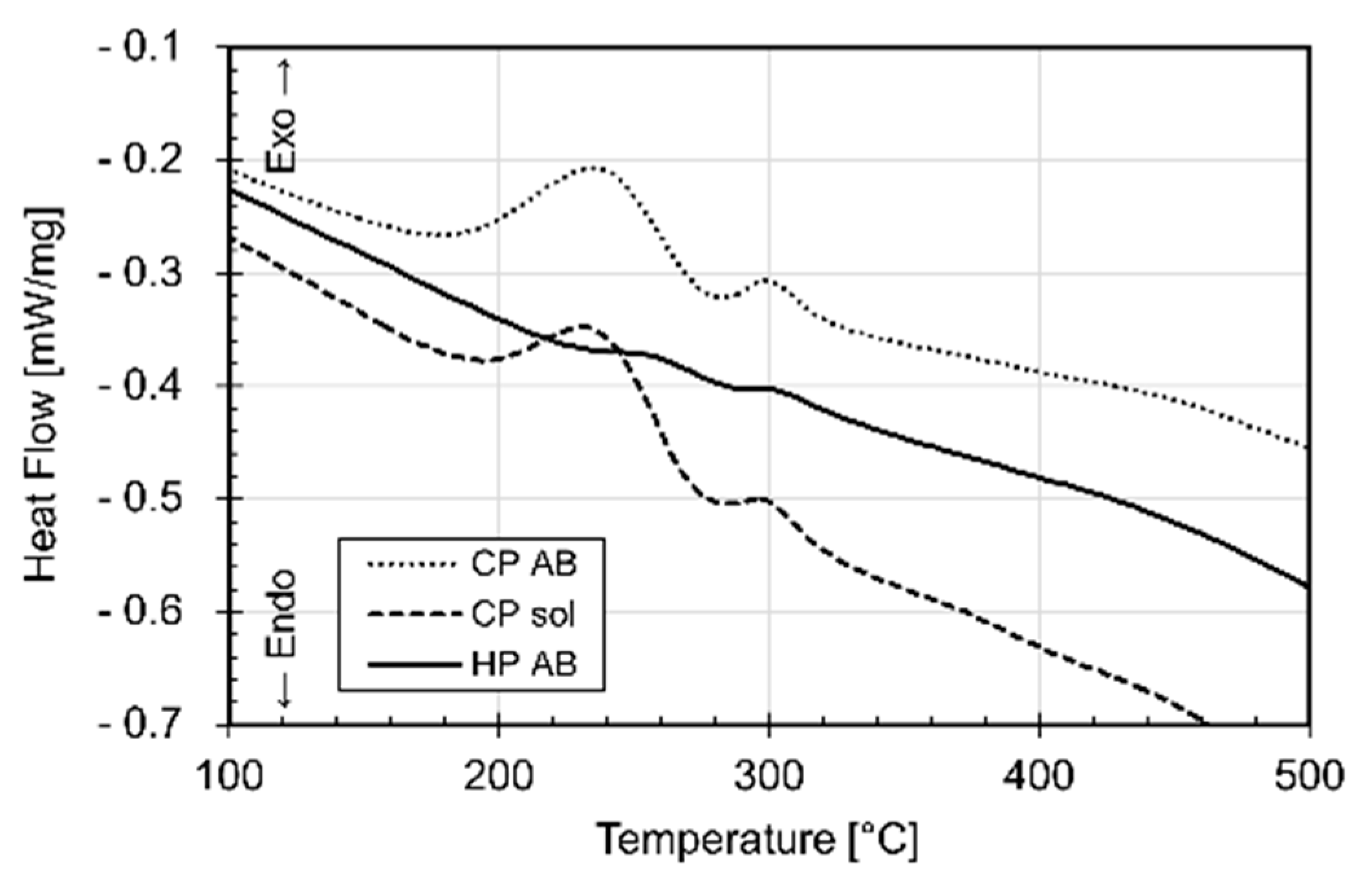


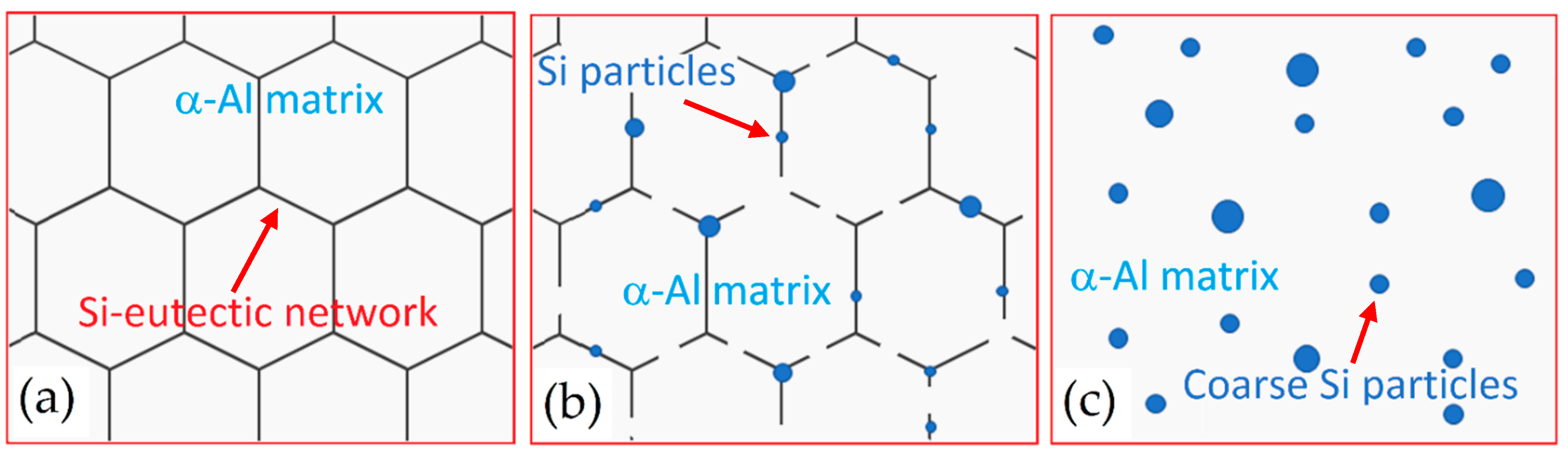
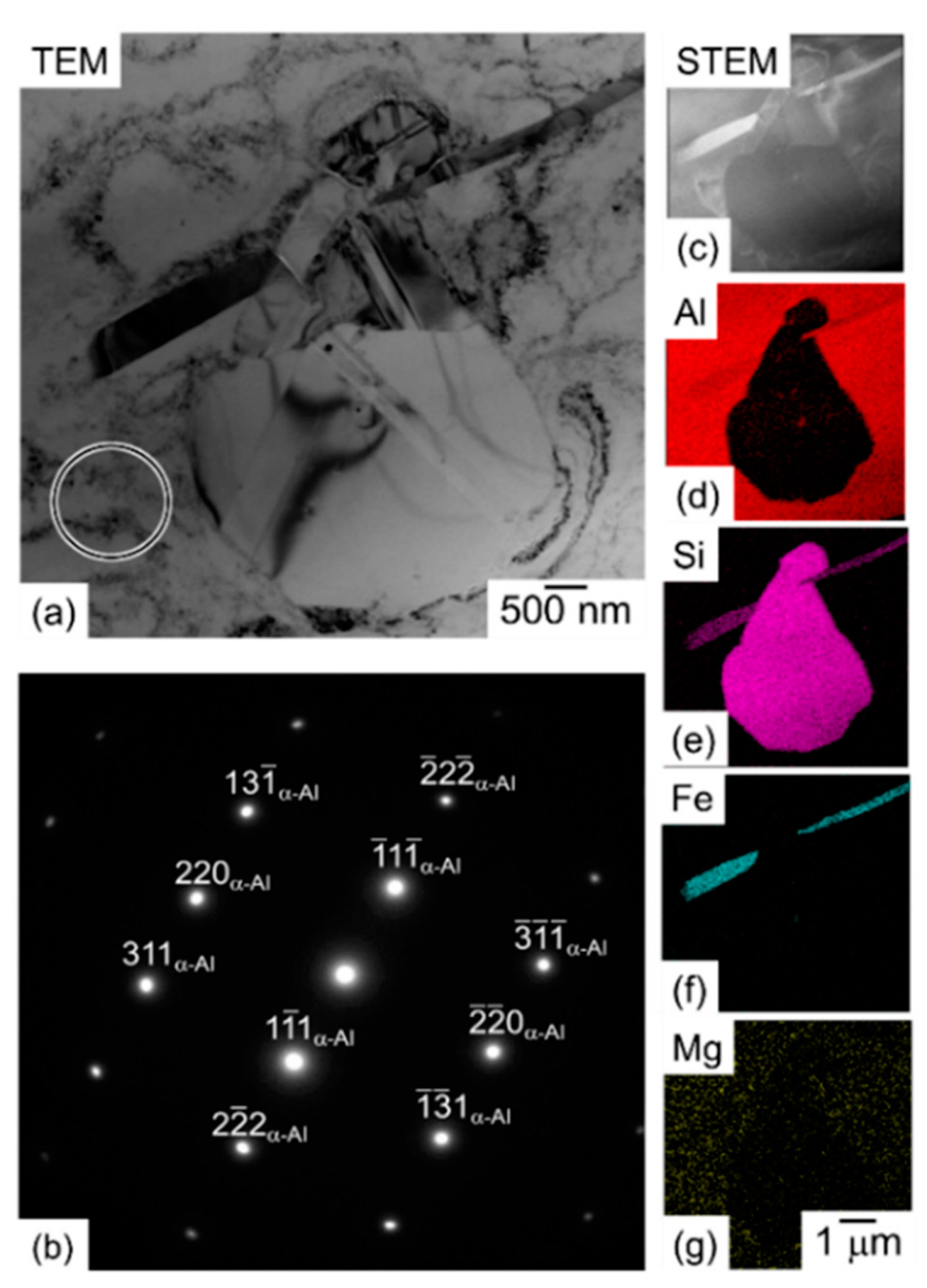

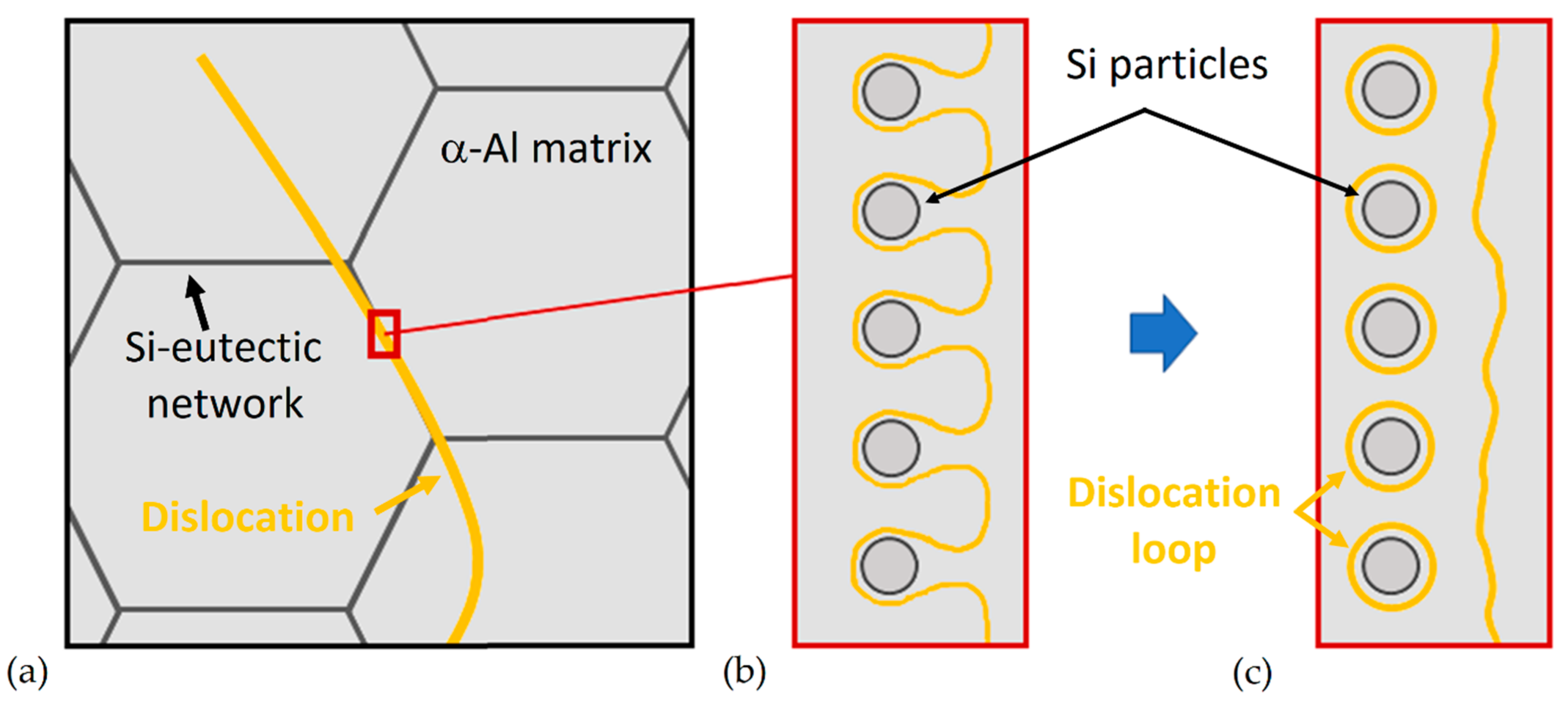
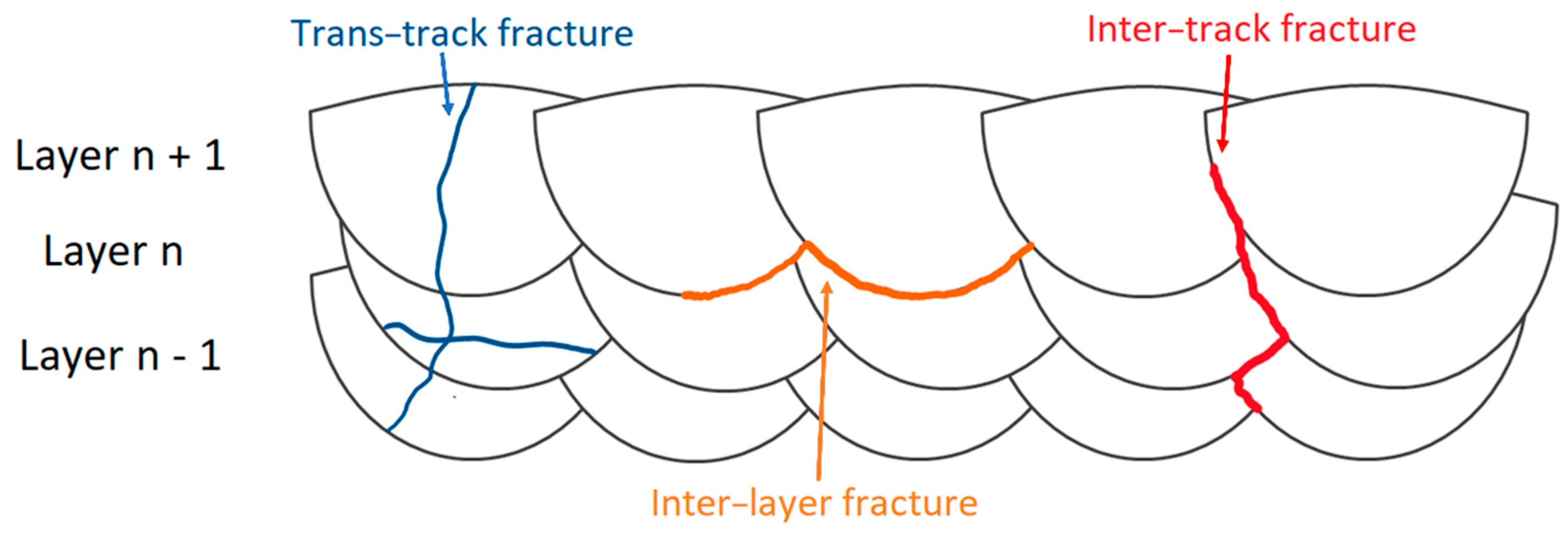
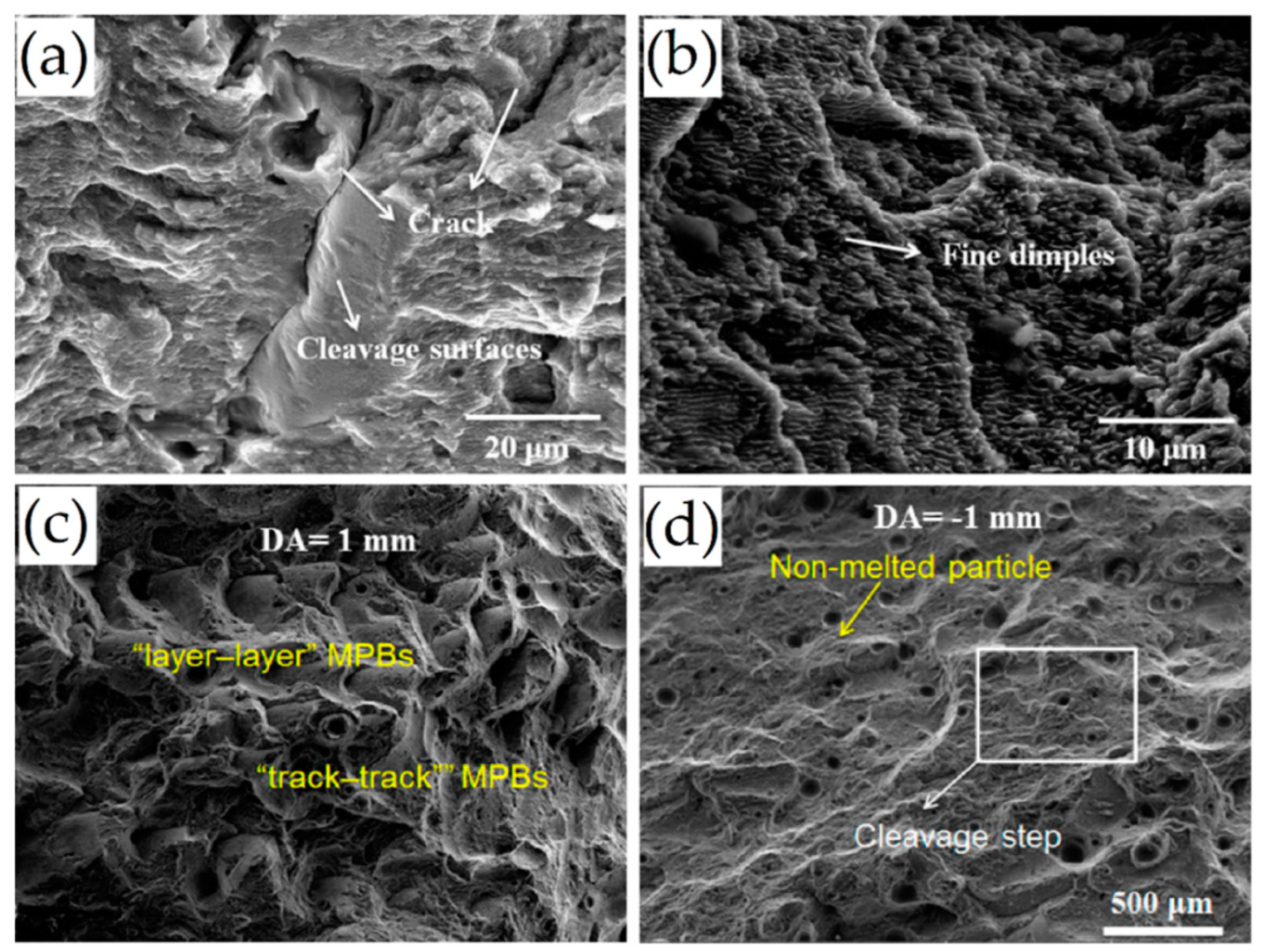
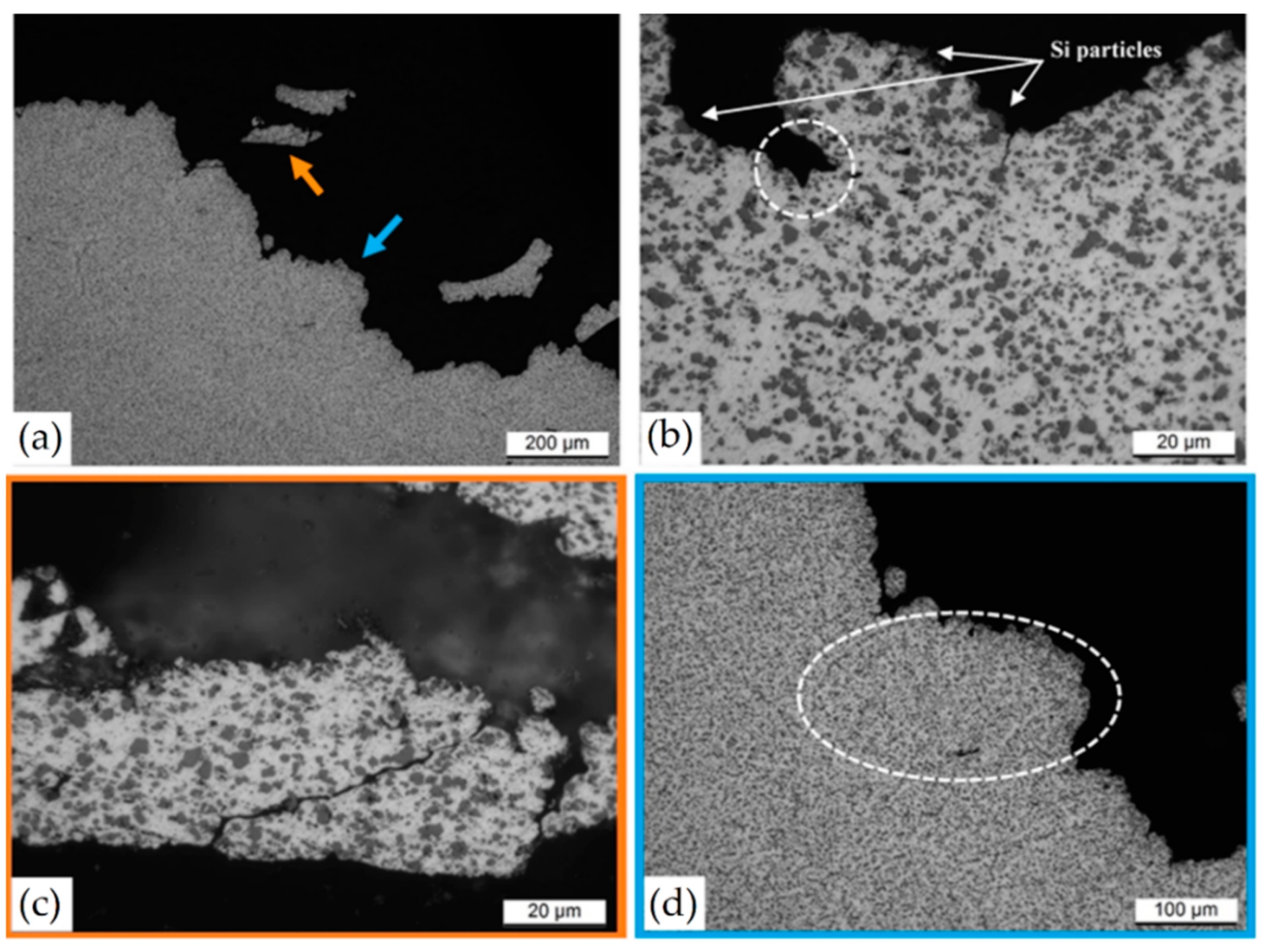

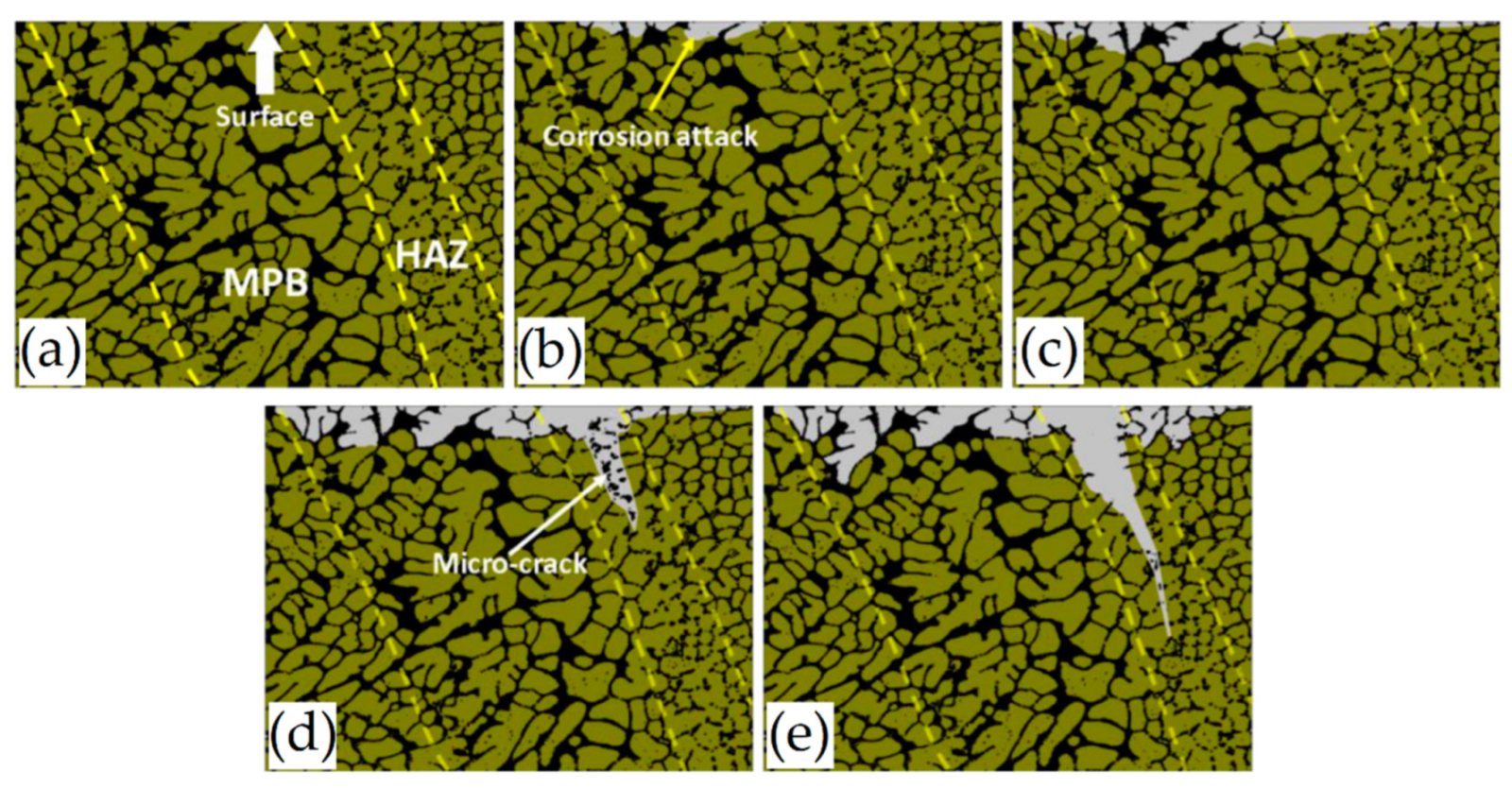

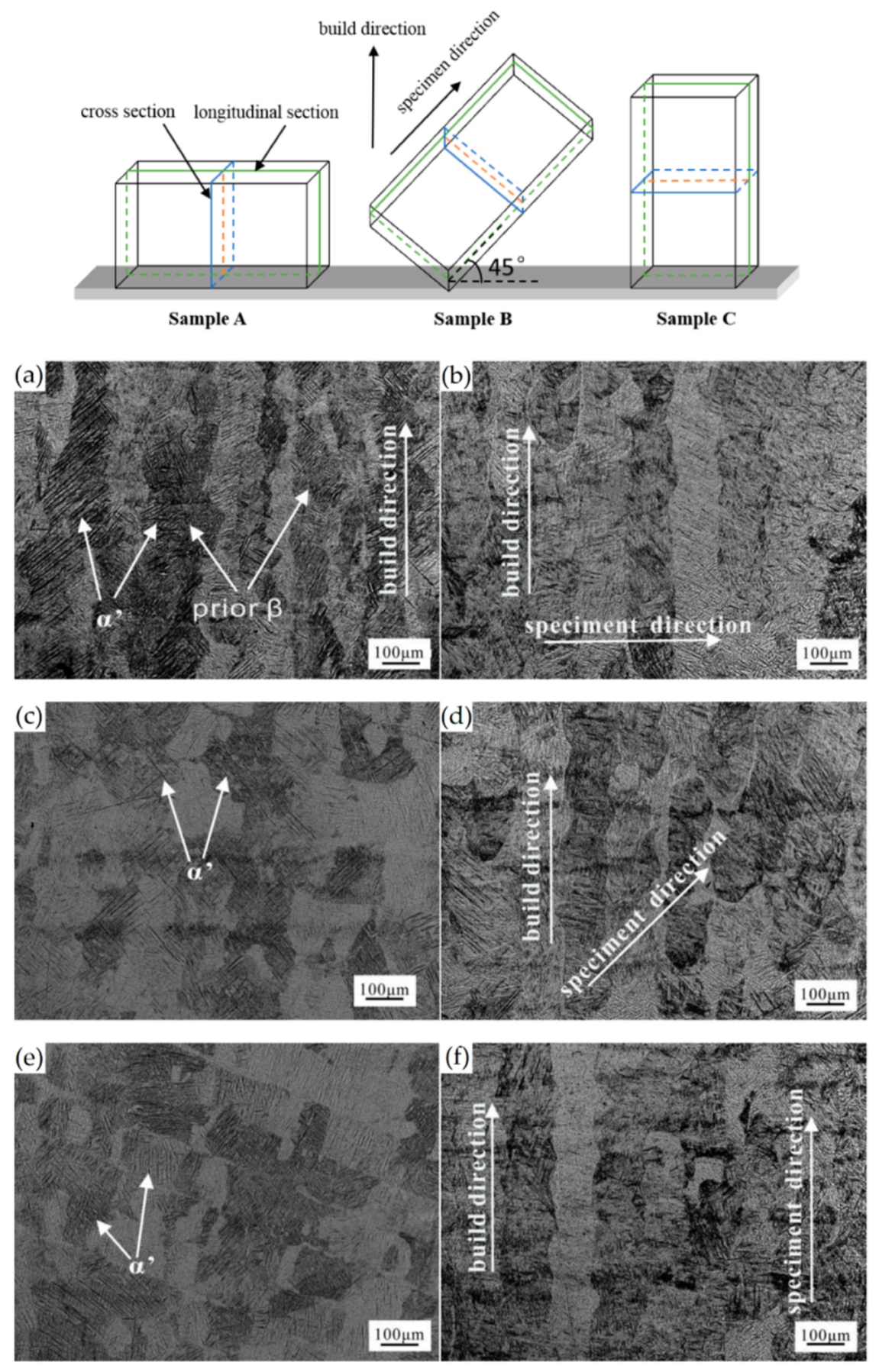

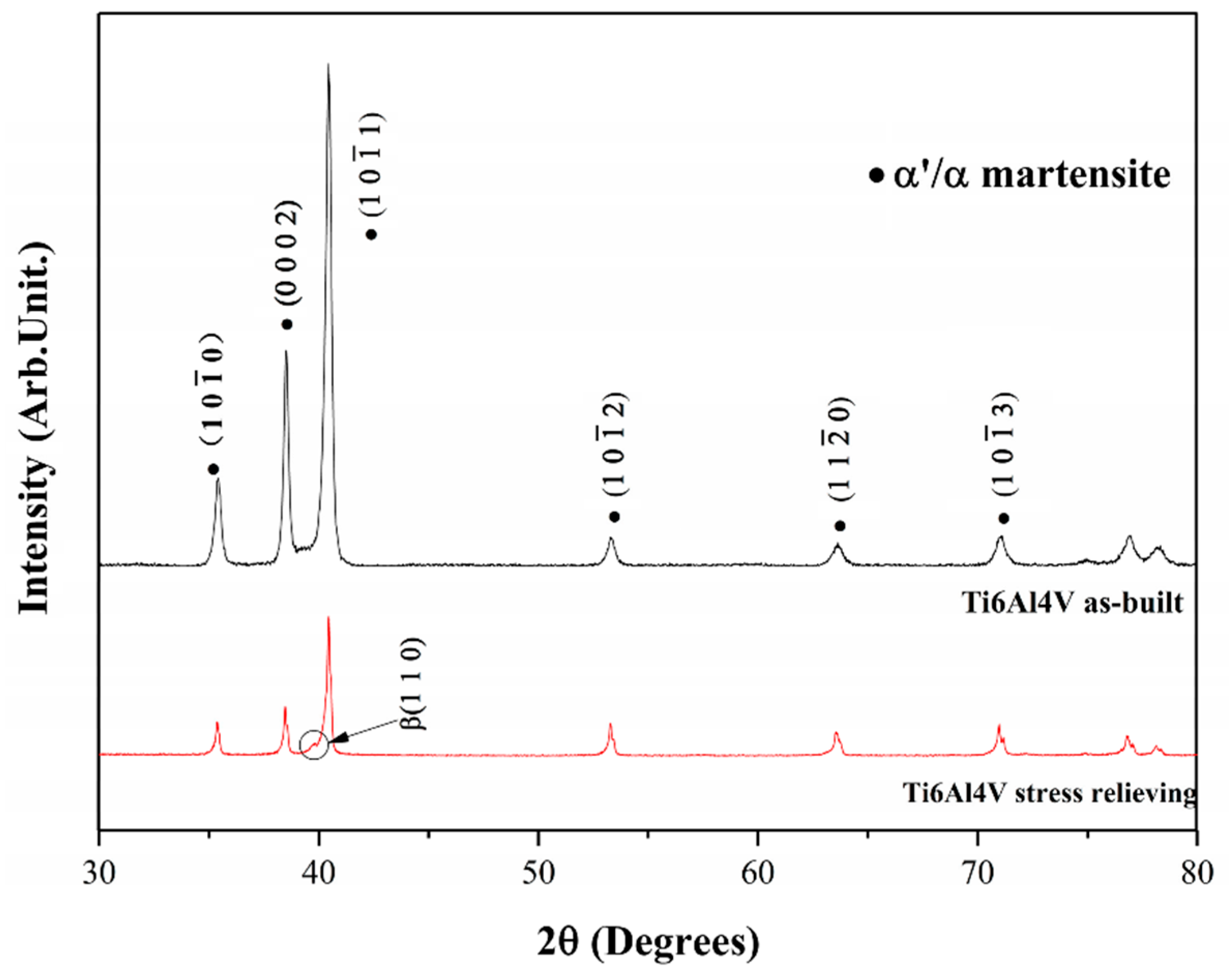

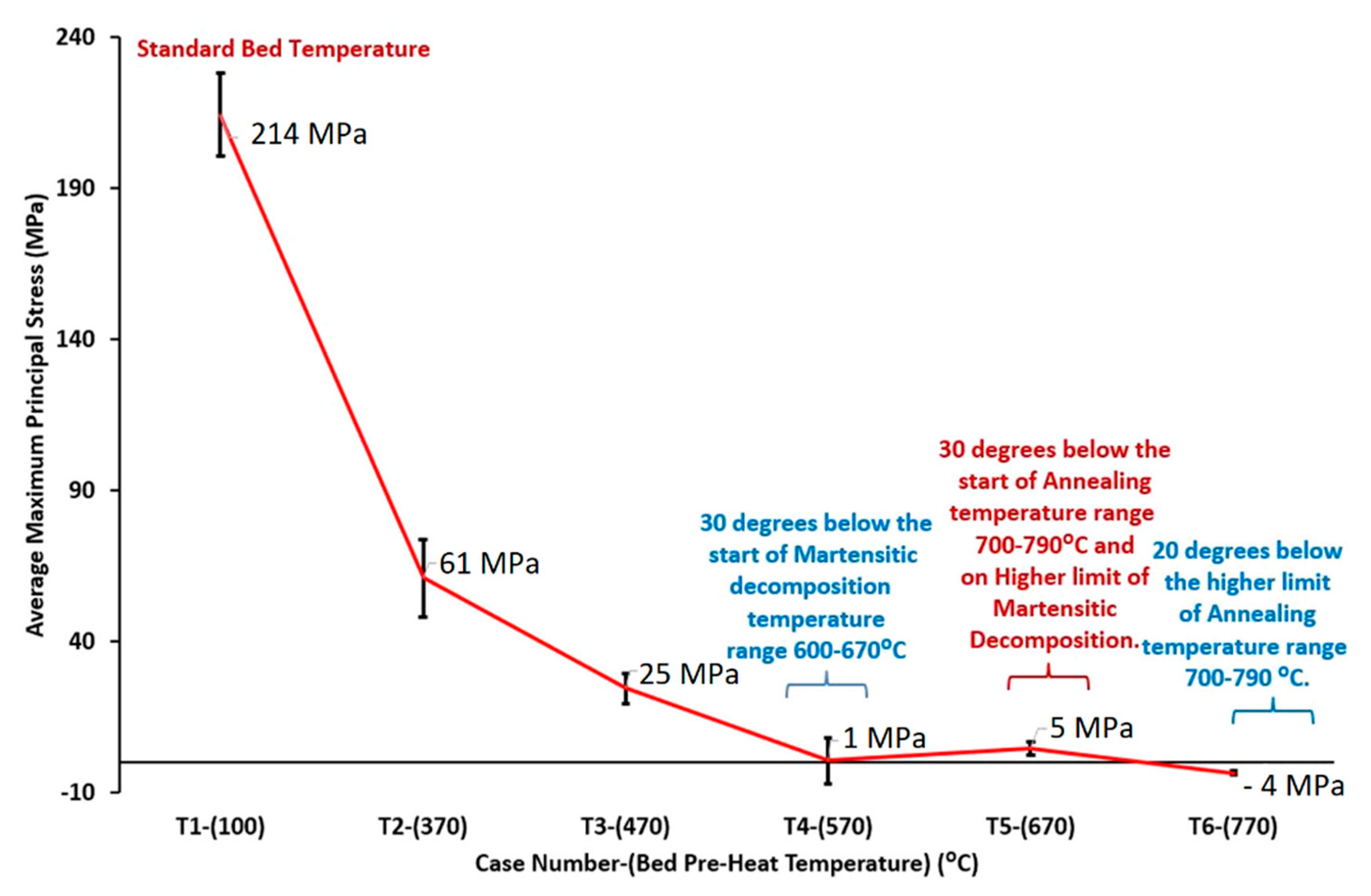
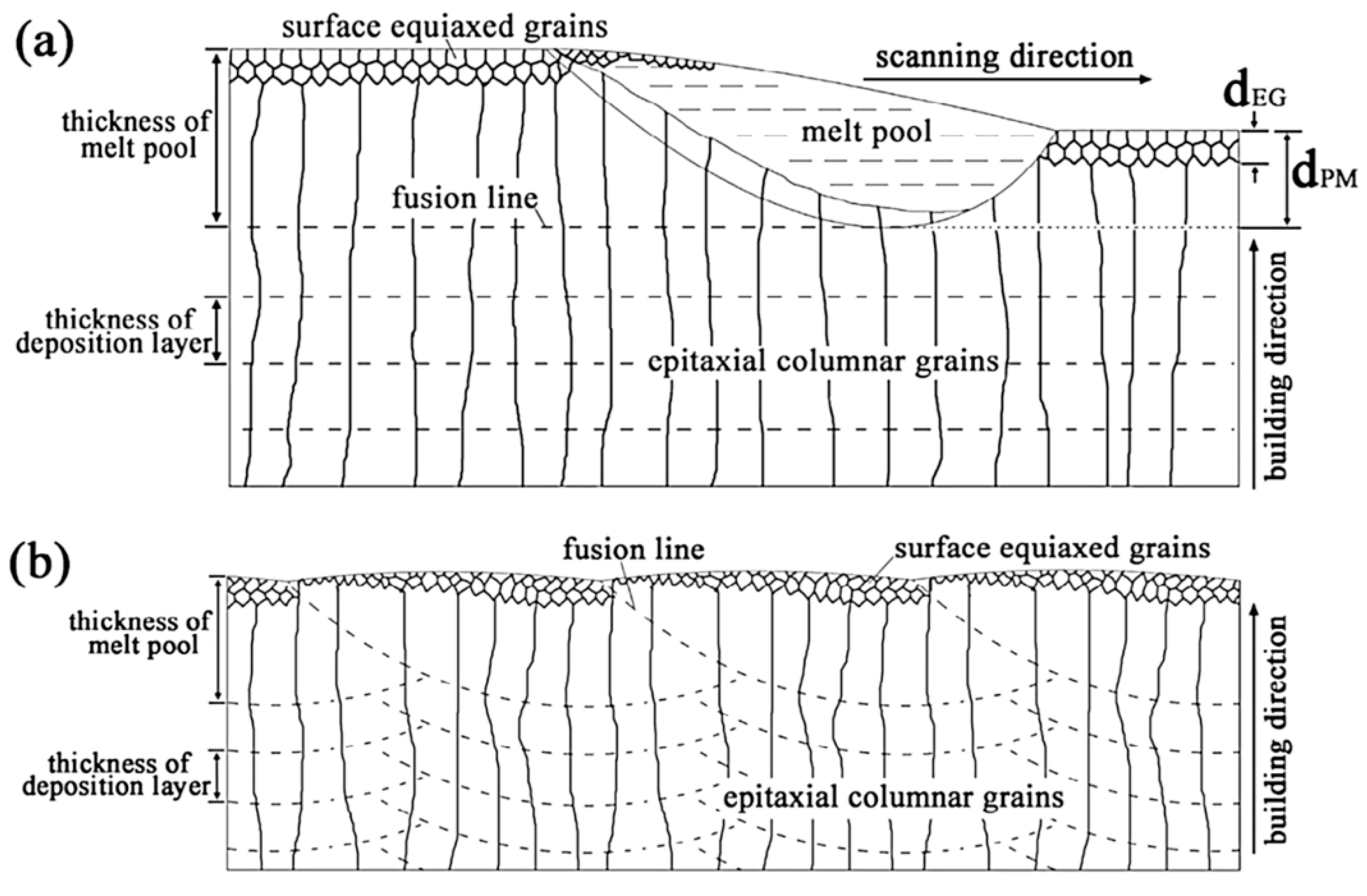
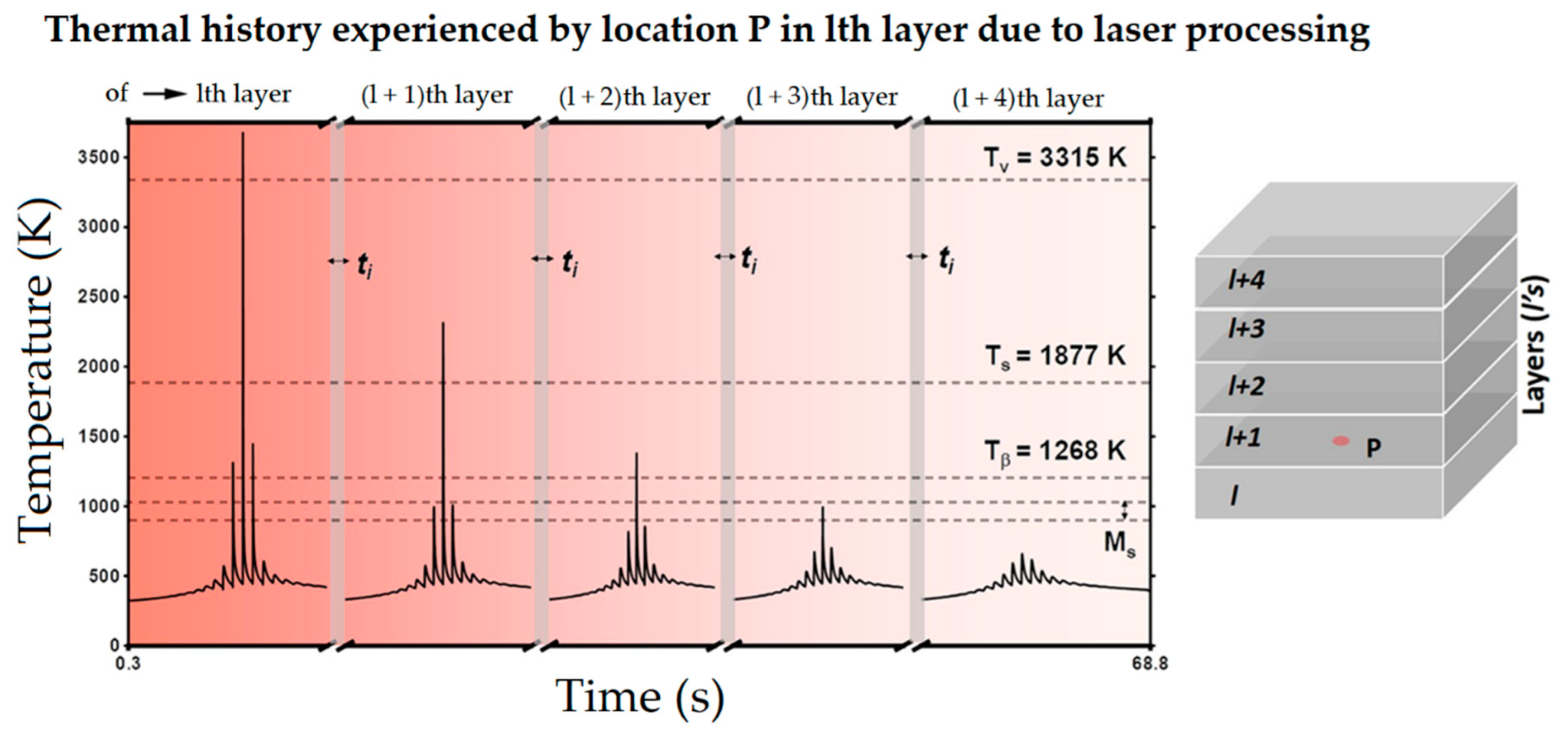
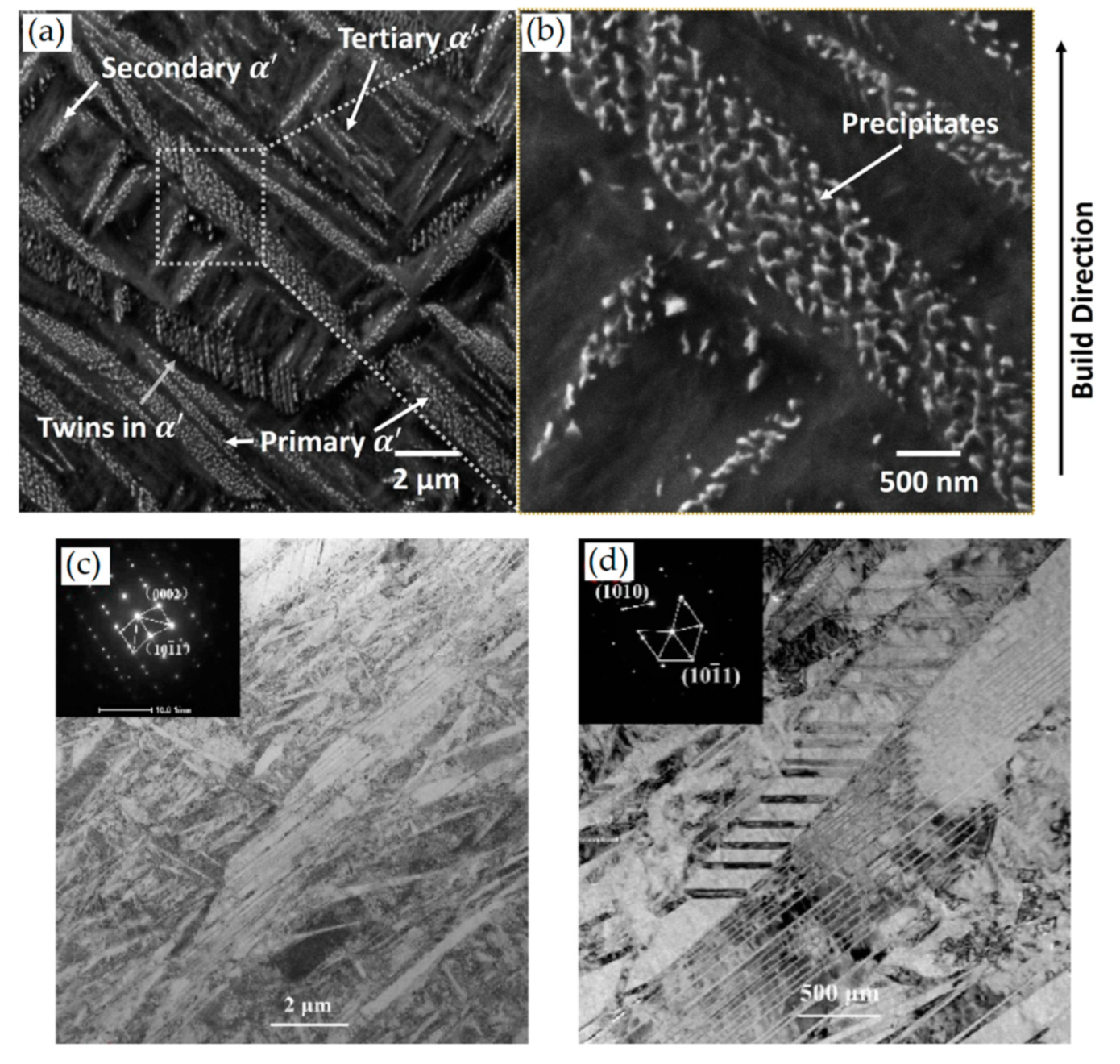
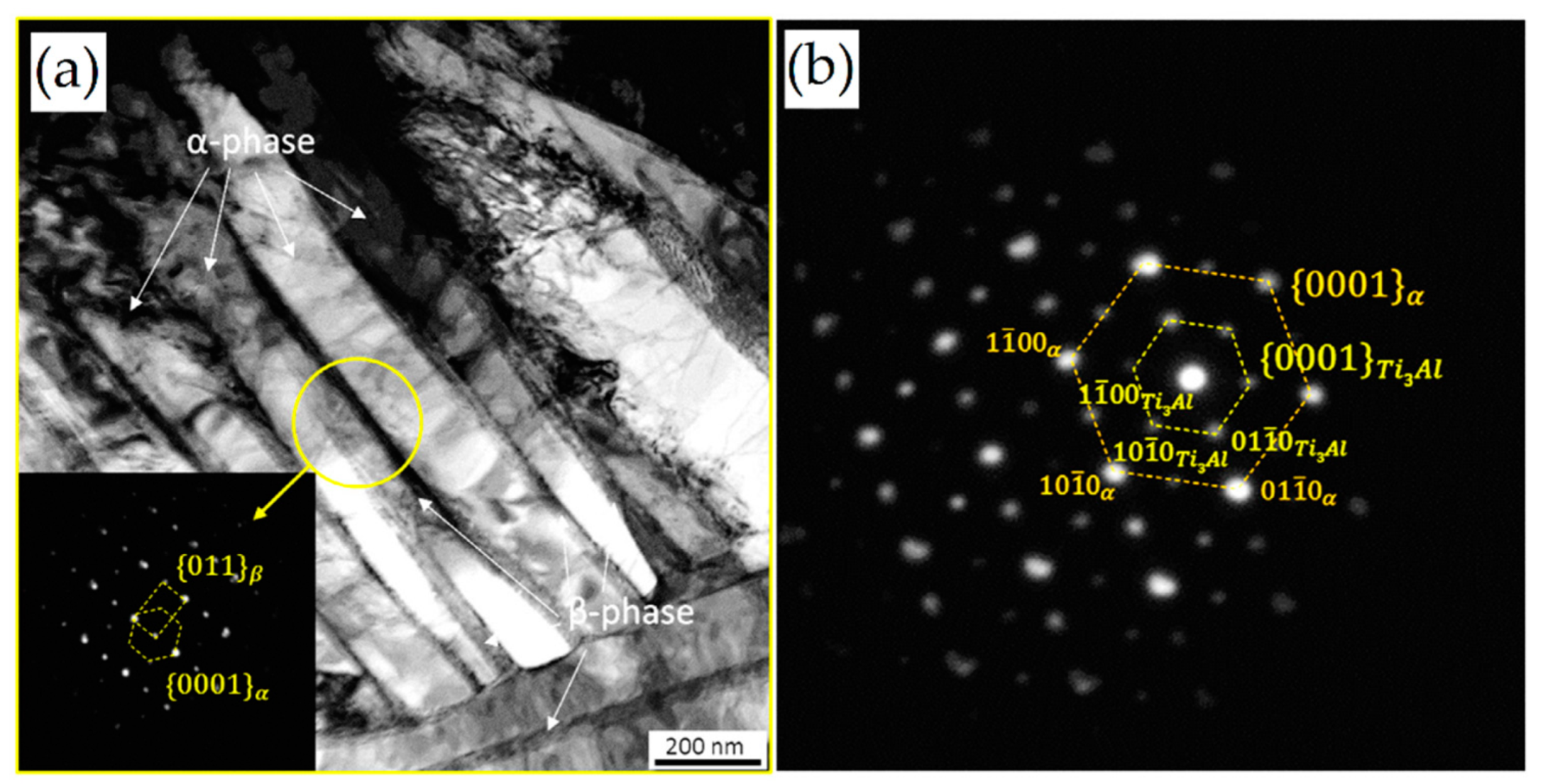
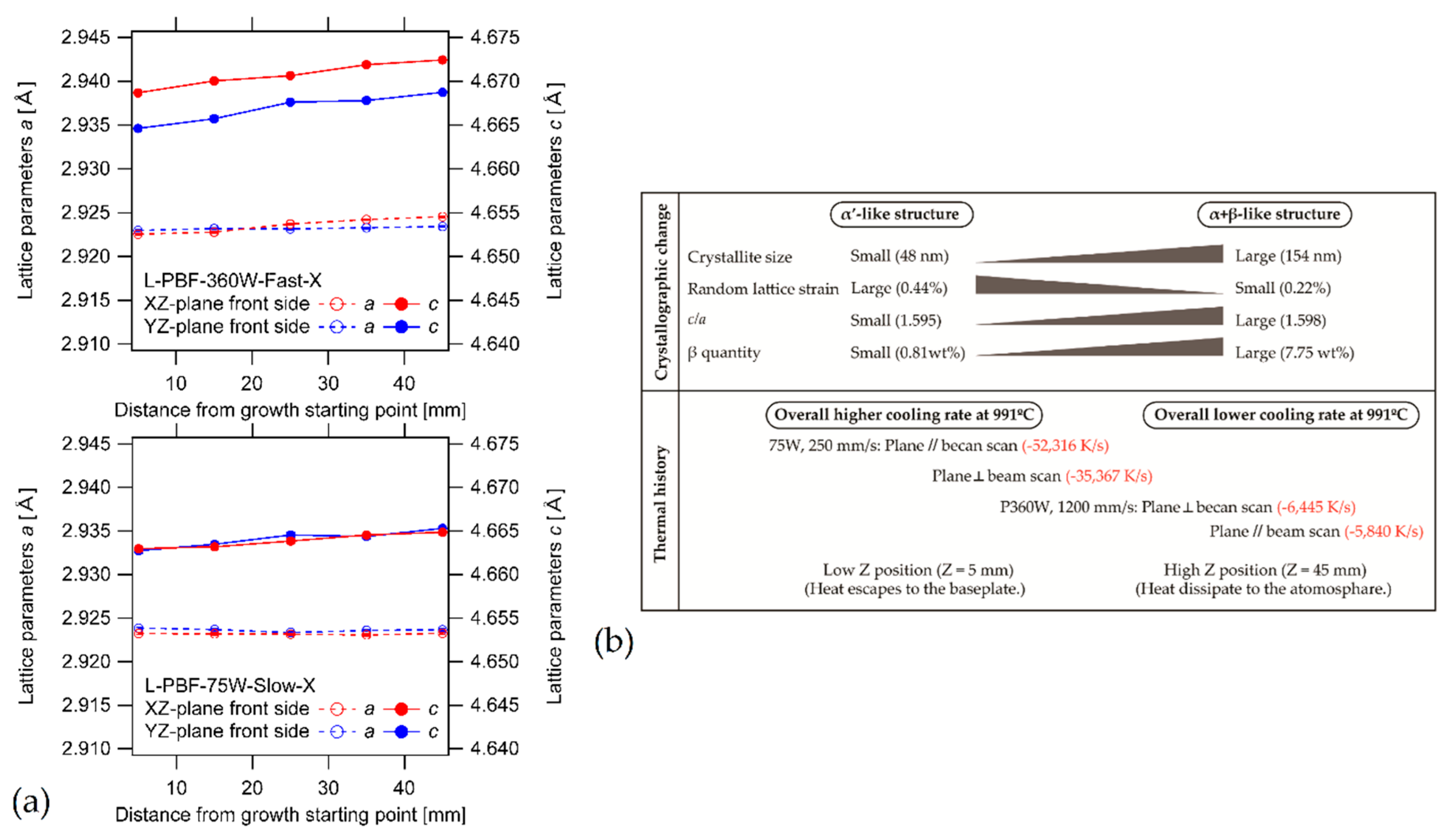
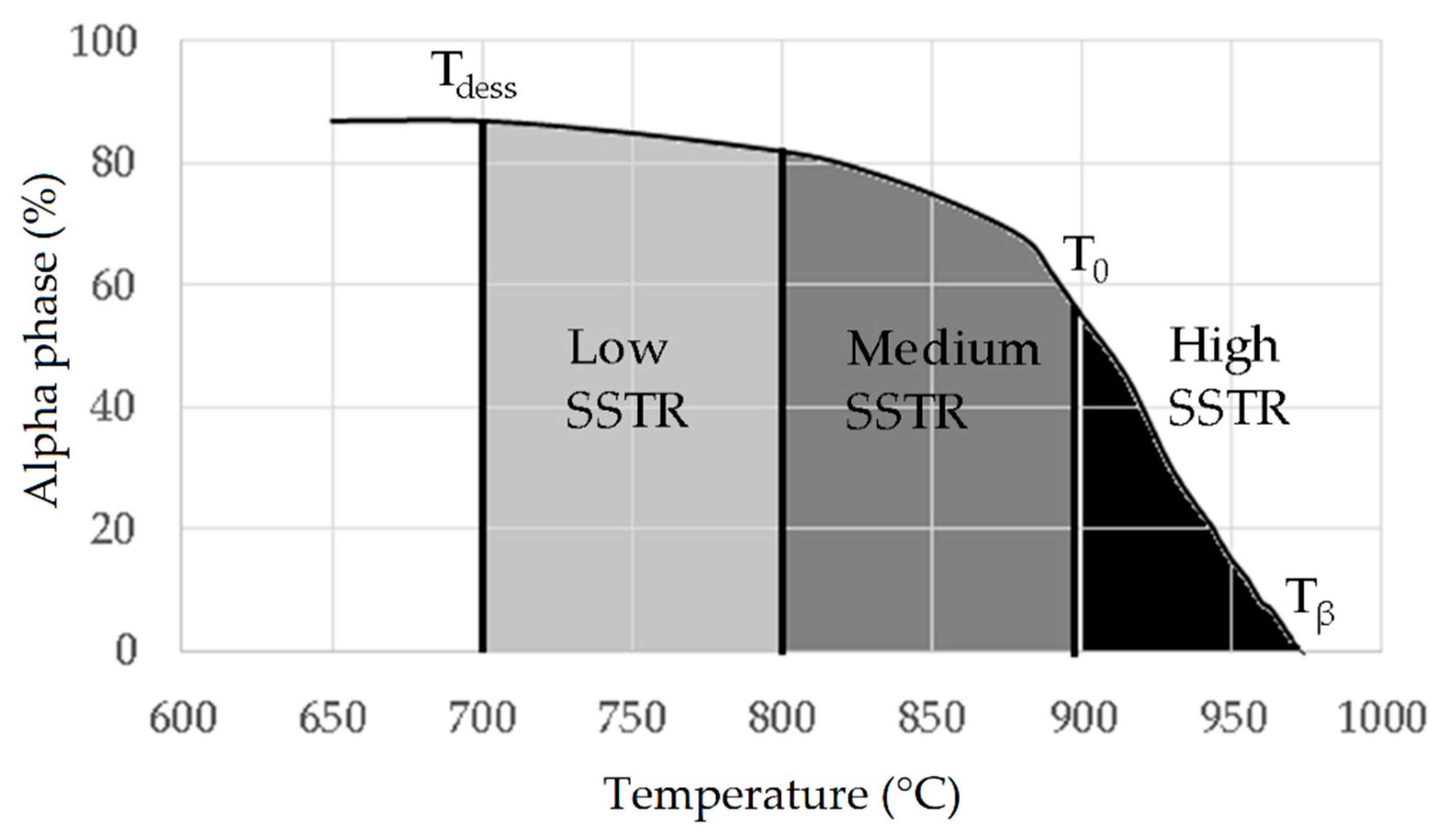
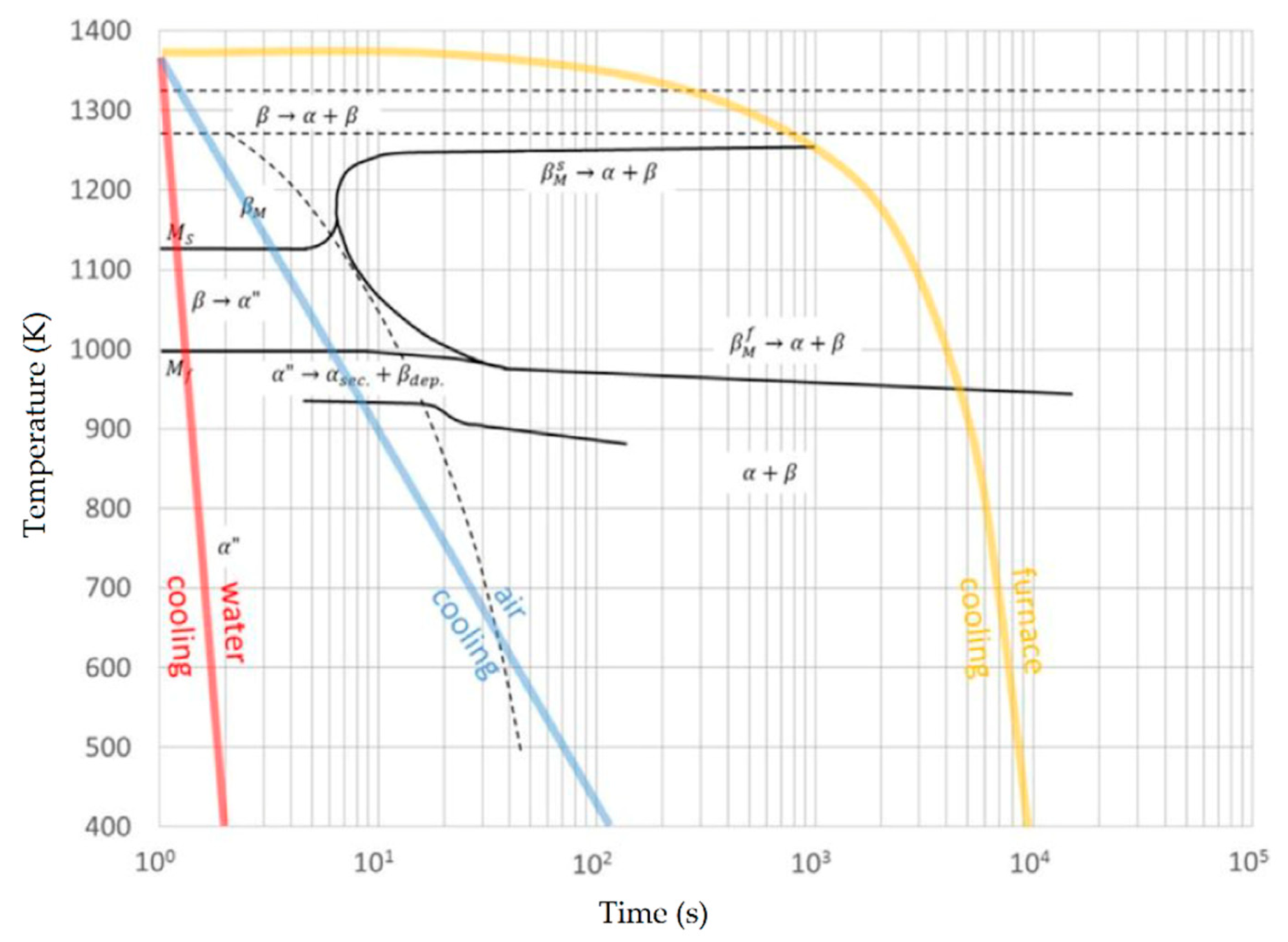
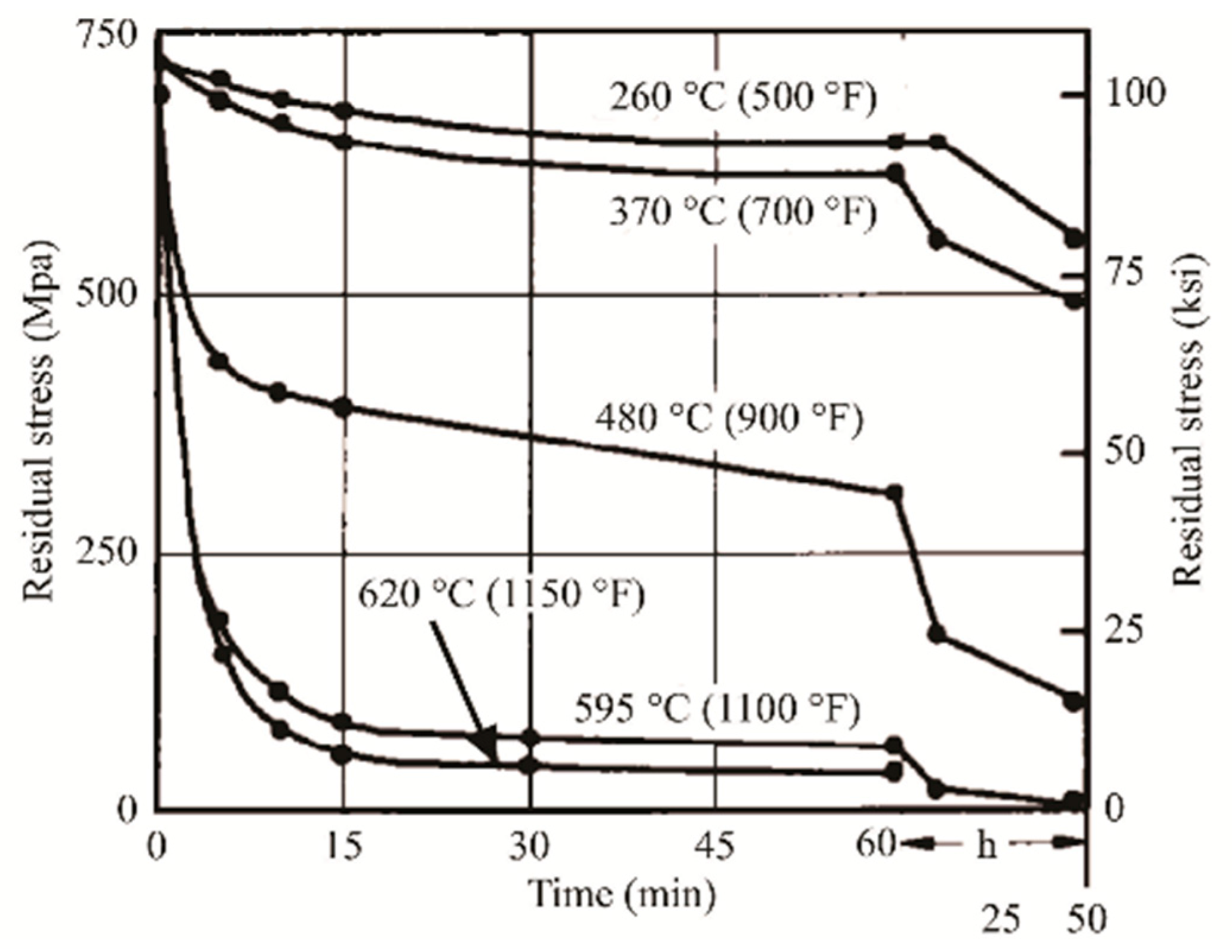
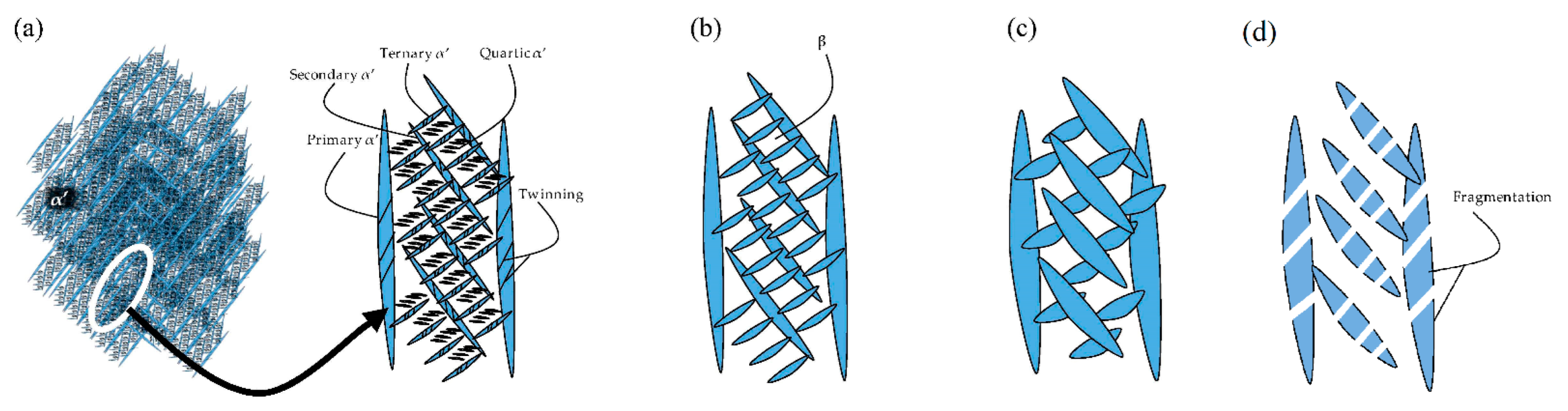

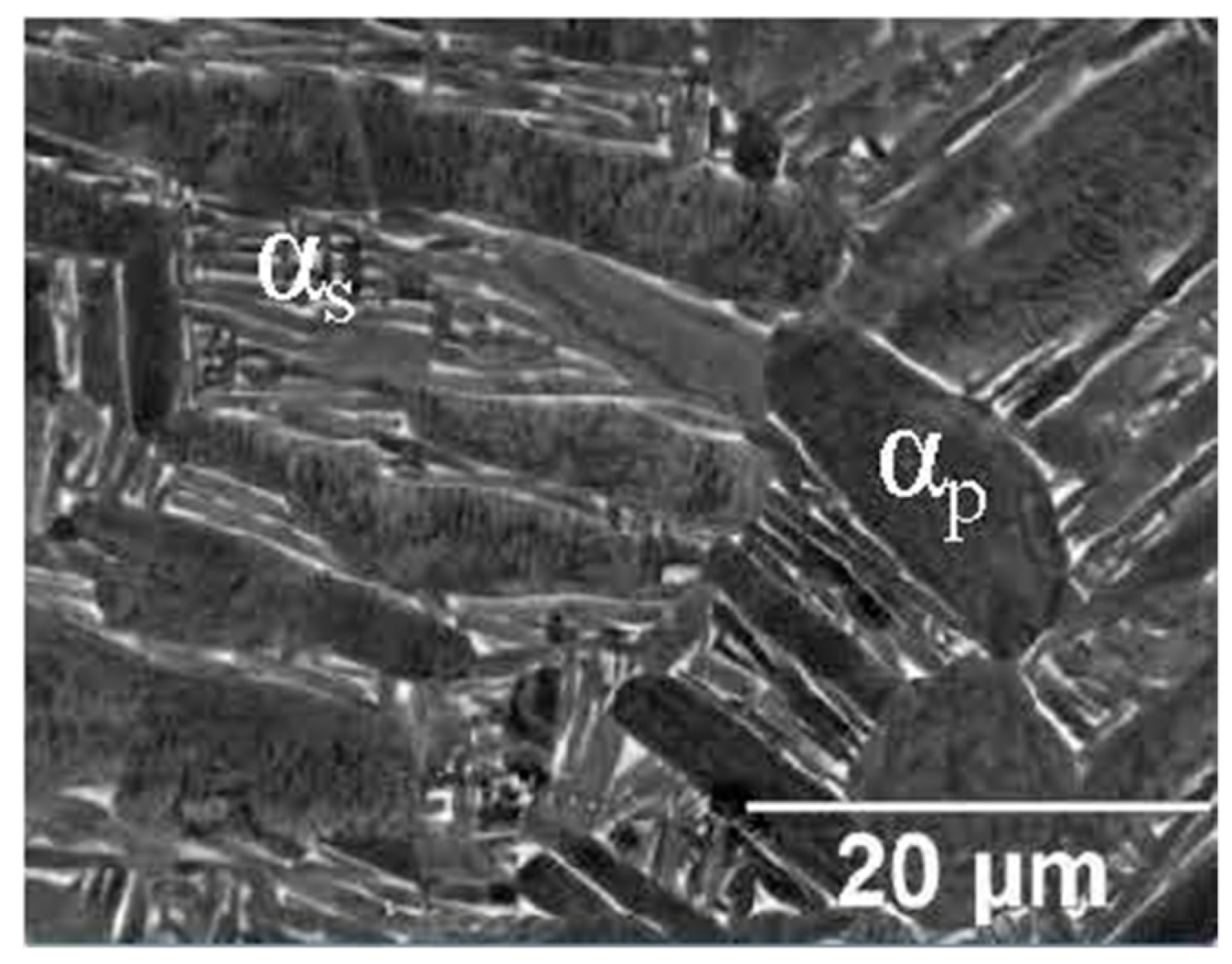

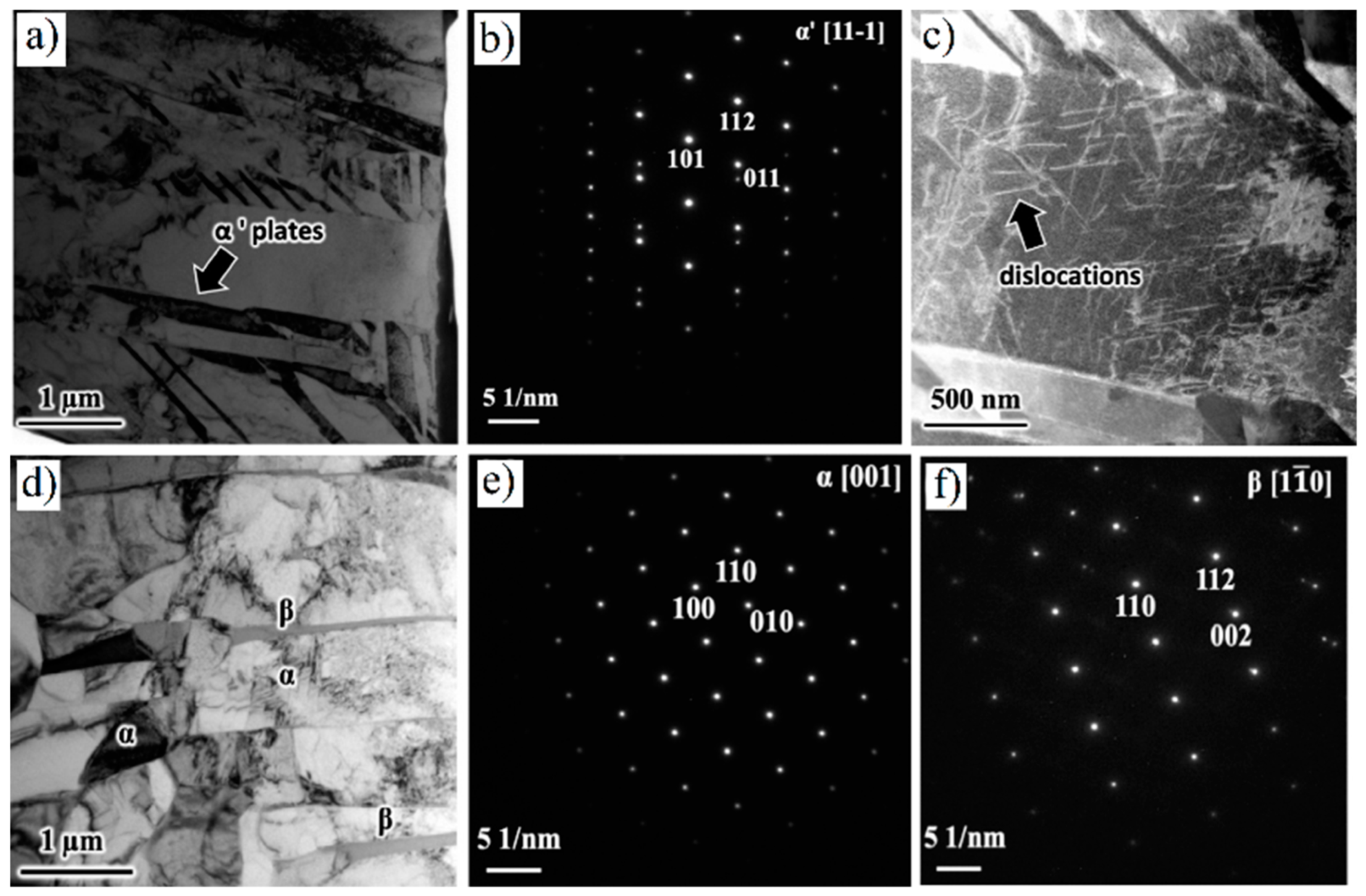
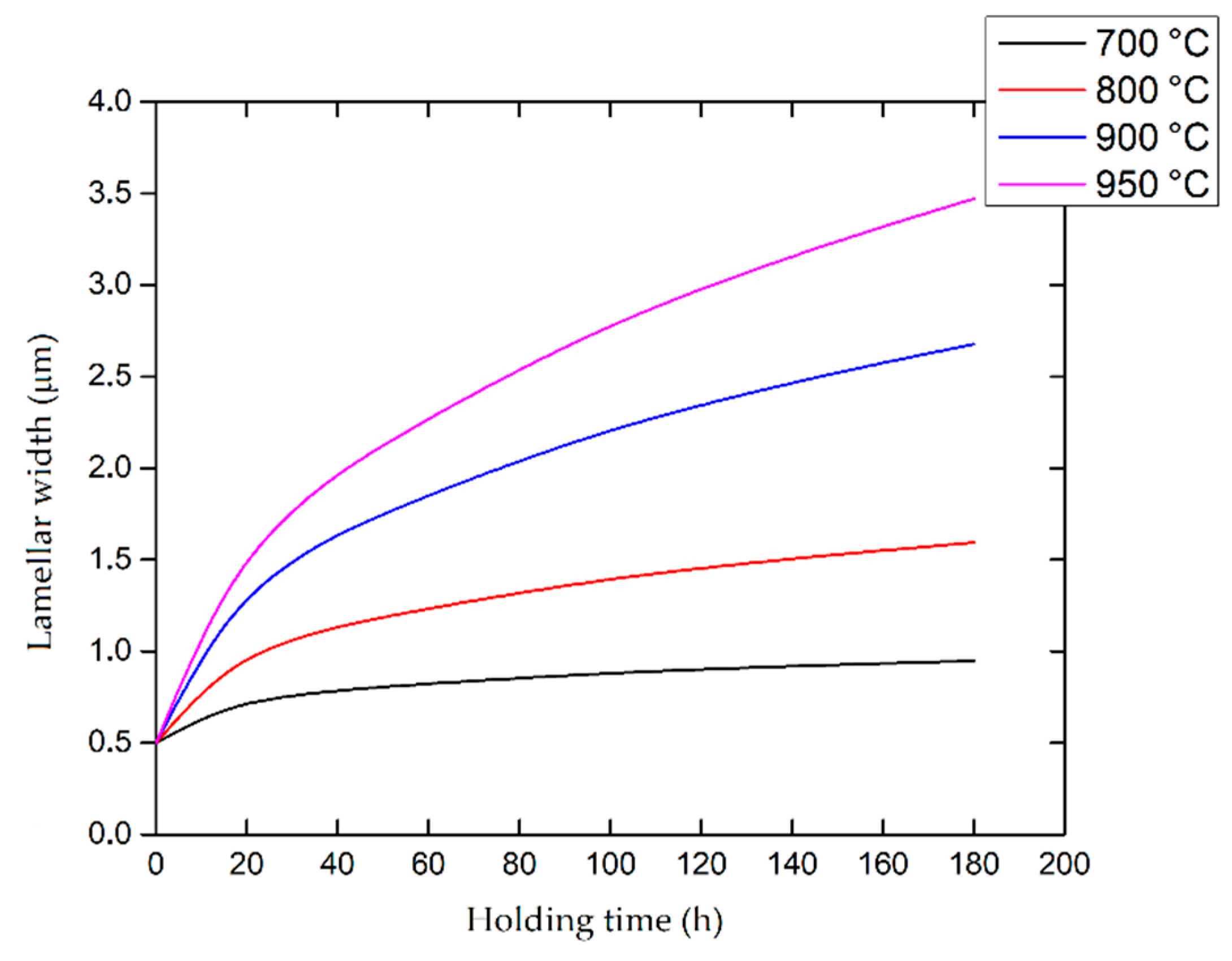
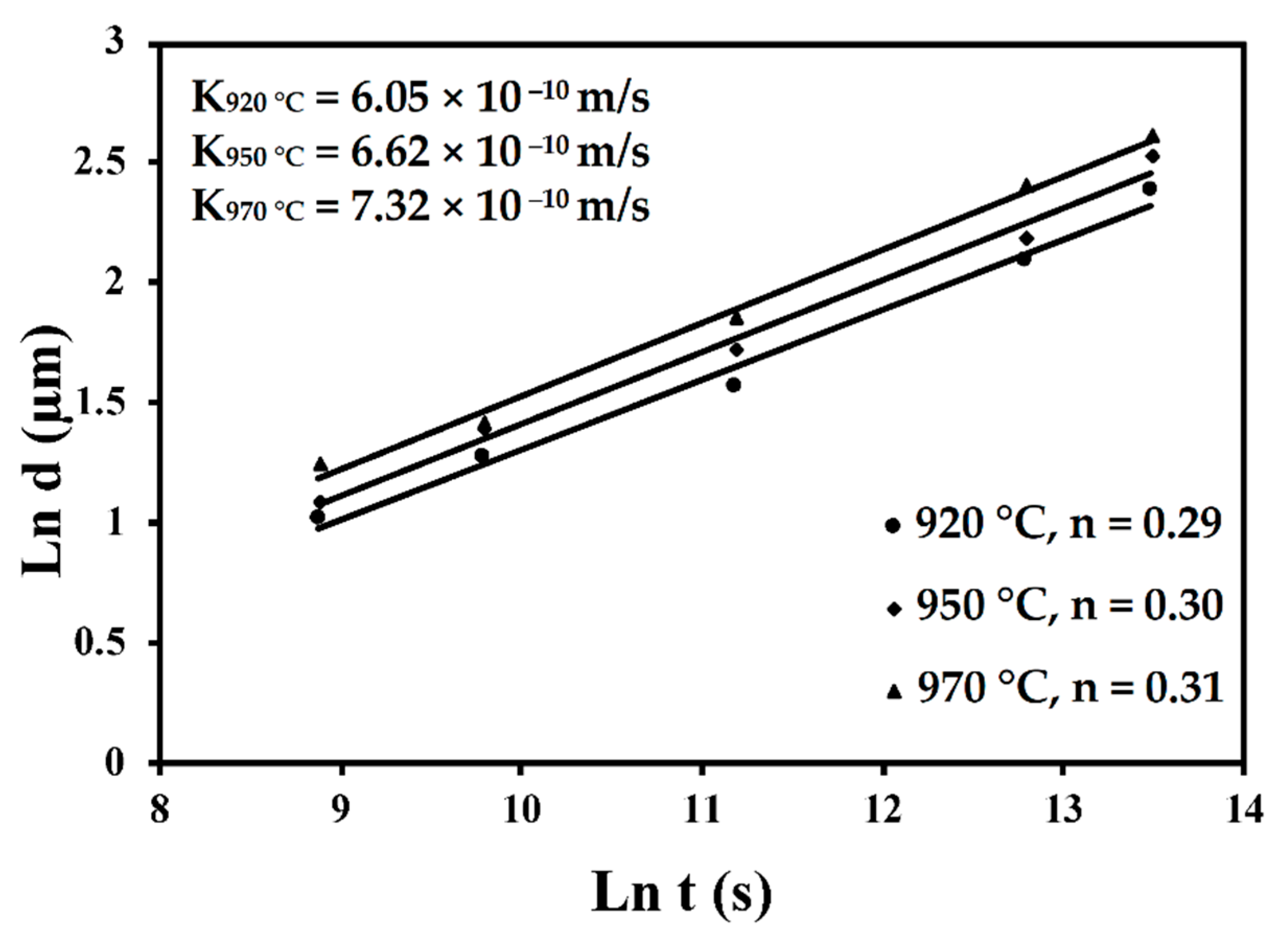

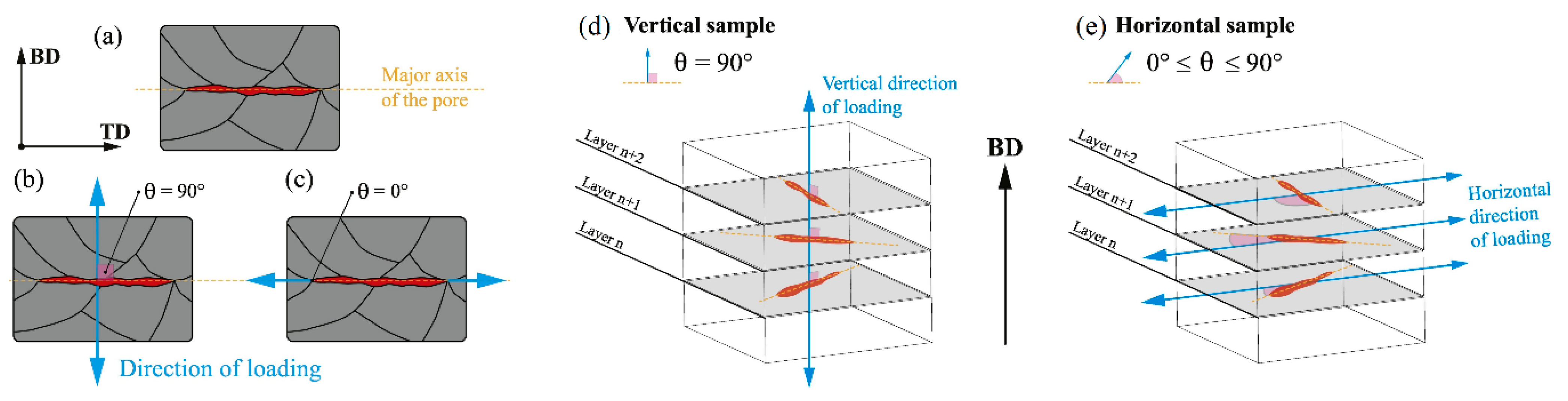
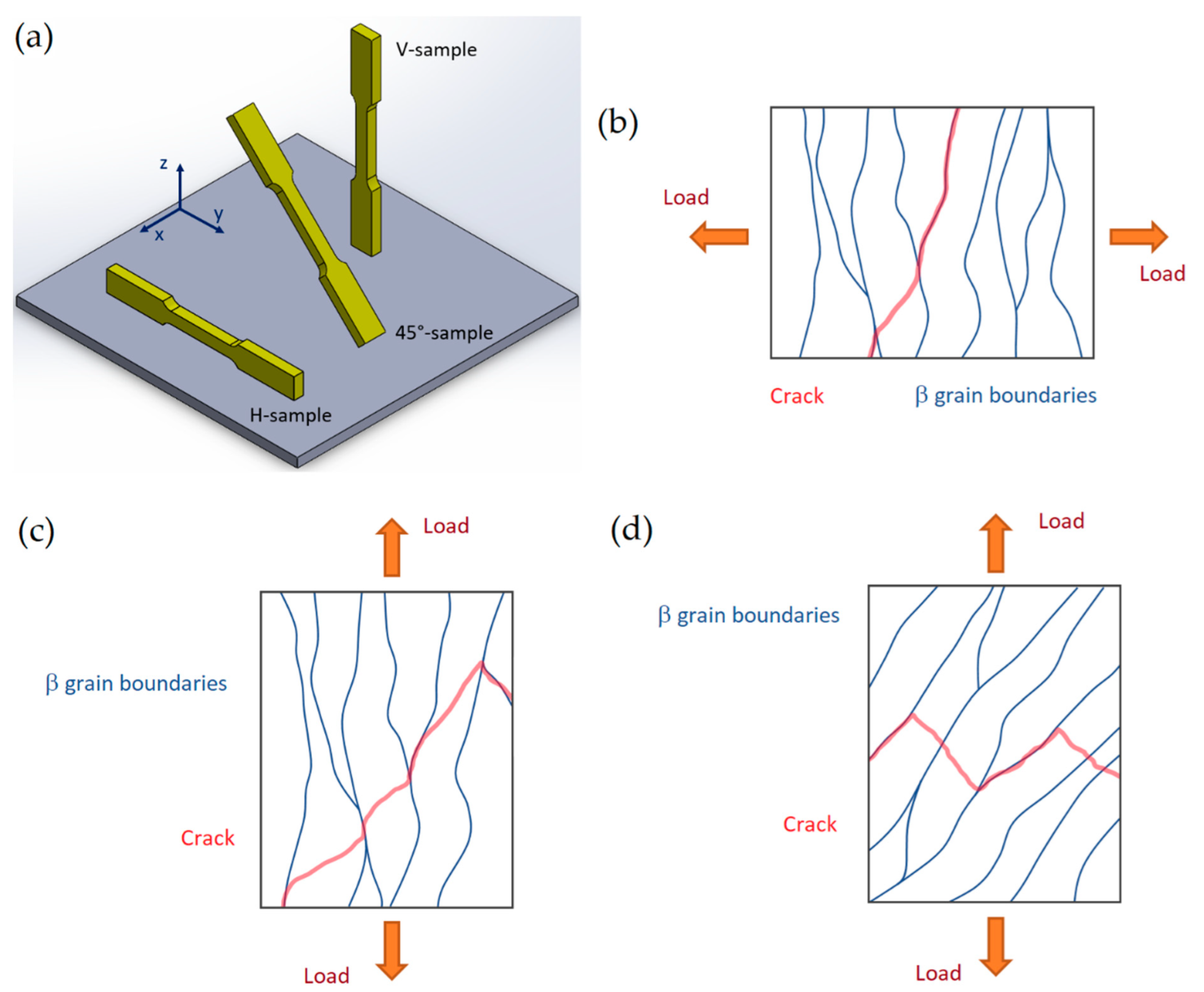
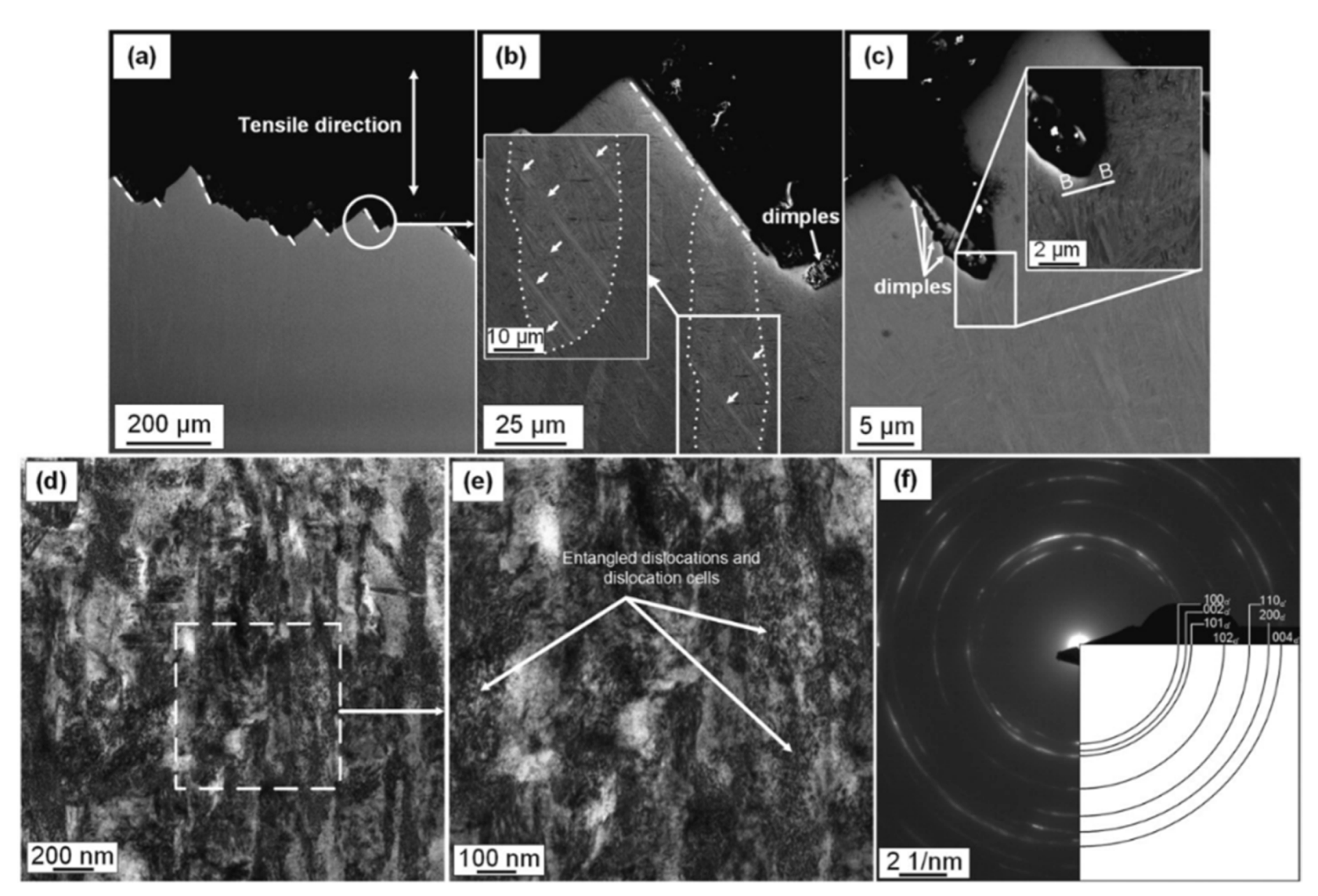
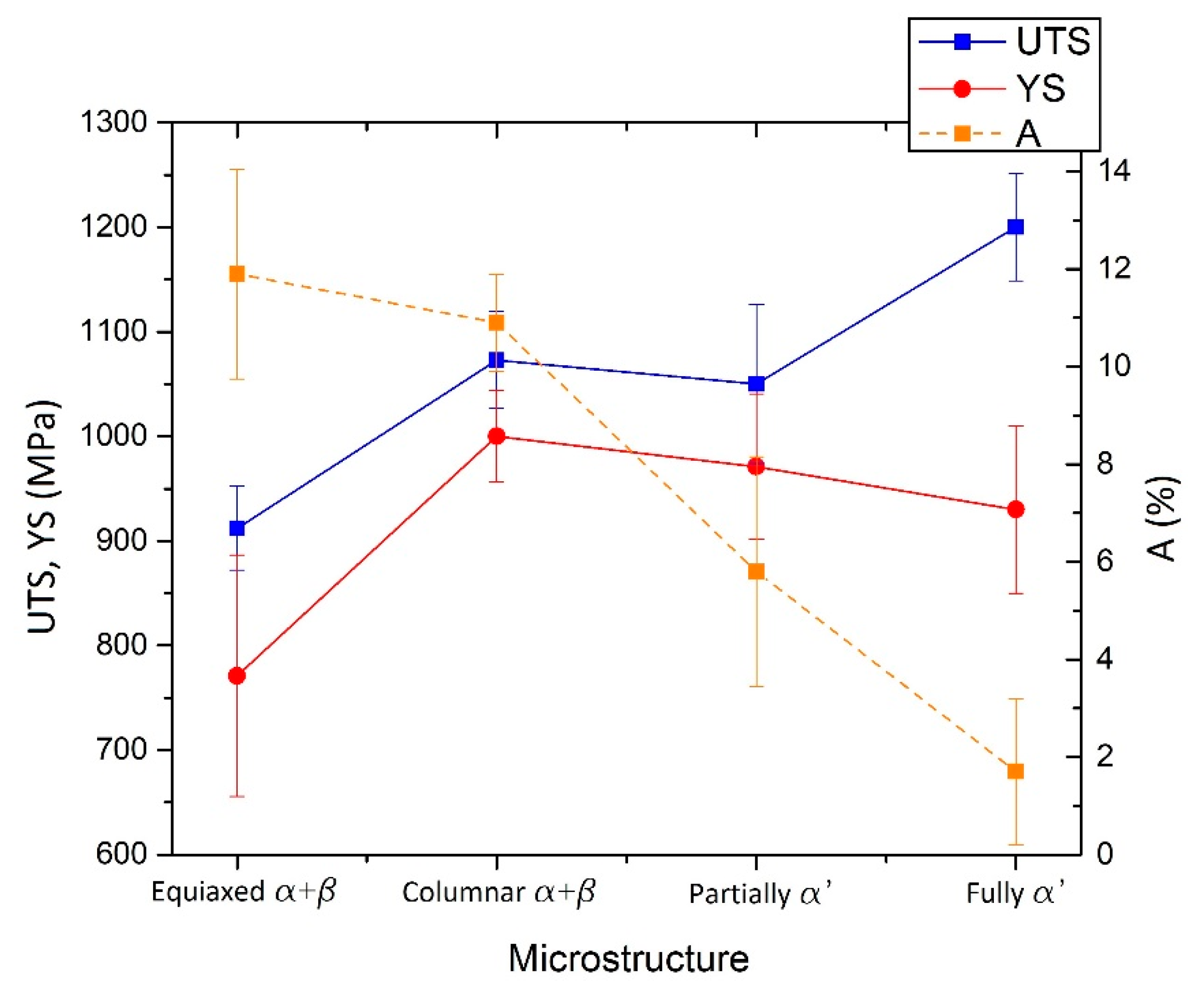
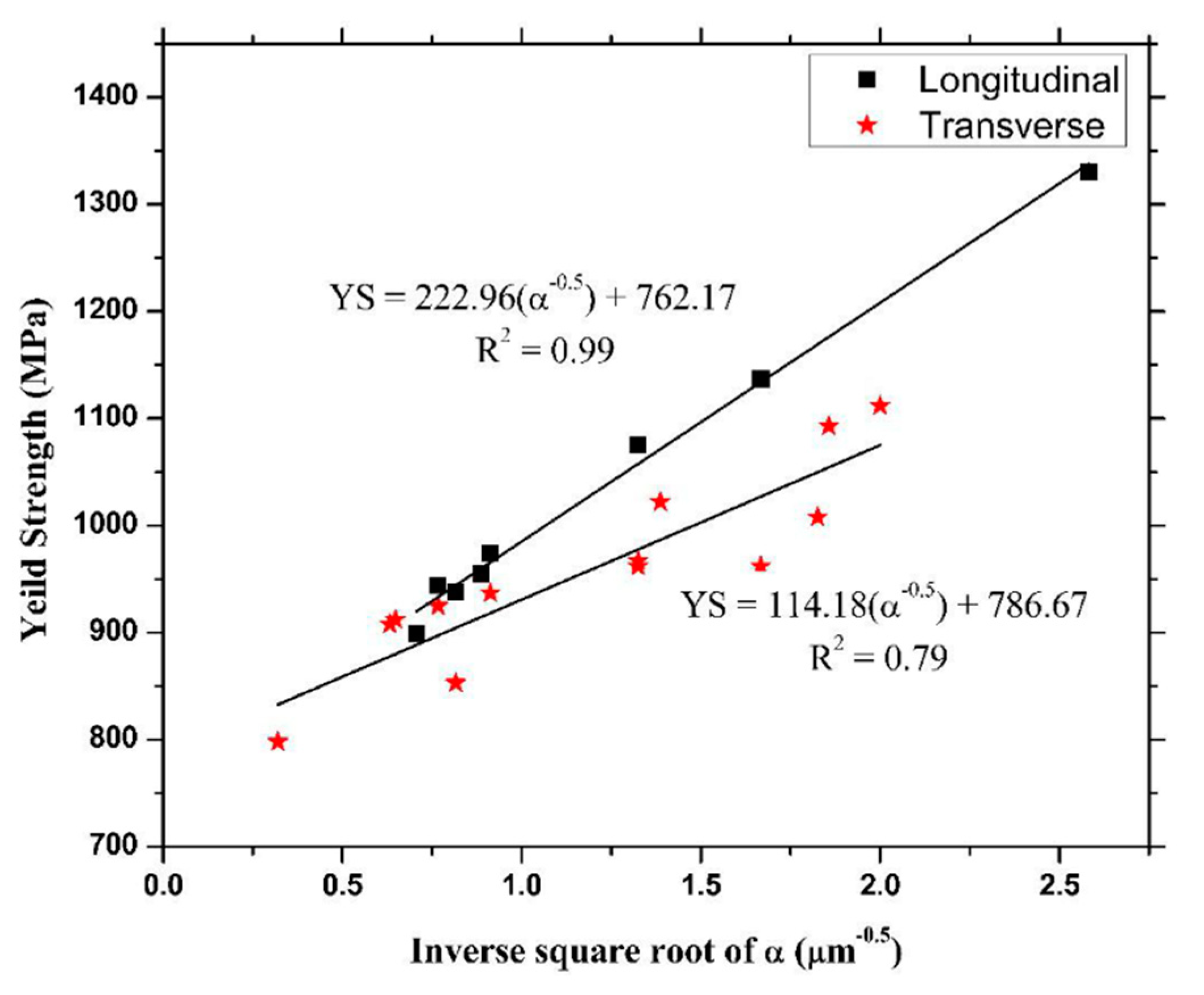


| Materials | P, (W) | v, (mm/s) | h, (μm) | t, (μm) | ED, (J/mm3) | Fully Dense, Dense, Porous | BR 1, (cm3/h) | Ref. |
|---|---|---|---|---|---|---|---|---|
| AlSi10Mg | 350 | 1050 | 170 | 50 | 39 | Fully dense | 32 | [14] |
| 788 | 1099 | 300 | 60 | 40 | Fully dense | 71 | [50] | |
| 463, 625, 788, 950 | 500, 800, 1099, 1400, 1700, 2000 | 300, 350, 400 | 17–75 | Dense | 38–174 | |||
| 300, 463, 788 | 800, 1400, 1700, 2300 | 300, 350, 400 | Porous | |||||
| 100 | 250, 500, 750, 1000 | 50, 100 | 40 | 25–200 | Dense | 2–14 | [51] | |
| 320, 360, 400 | 600, 750, 900 | 70.90–116.40 | 30 | 145–200 | Dense | 25–28 | [52] | |
| 370 | 1300 | 190 | 30 | 50 | Dense | 27 | [53] | |
| 240, 260, 320, 360, 400 | 1200, 1400, 1600, 1800, 2000 | 36, 40, 45, 51.4, 60 | 30 | 111 | Fully dense | 8–13 | [54] | |
| 400, 440 | 1350, 1500 | 105 | 50 | 38–56 | Fully dense | 25–28 | [55] | |
| 300 | 1230 | 105 | 50 | 68 | Dense | 23 | ||
| 150 | 500, 1500, 2500 | 45, 75, 105 | 30 | 74, 95, 222 | Fully dense | 2–28 | [56] | |
| 250 | 1500 | 75 | 30 | 74 | Dense | 12 | ||
| 150 | 2500 | 105, 150 | 30, 60 | 9, 13 | Porous | 28–81 | ||
| Ti6Al4V | 250 | 1600 | 60 | 30 | 87 | Fully dense | 10 | [32] |
| 170 | 1250 | 100 | 30 | 45 | Dense | 13 | [57] | |
| 200 | 200 | 180 | 50 | 111 | Dense | 6 | [58] | |
| 157 | 225 | 100 | 50 | 14 | Dense | 4 | [59] | |
| 100 | 700 | 75 | 30 | 6 | Dense | 6 | [60] | |
| Ti6Al4V | 260, 280, 300 | 1000, 1200, 1400 | 140 | 30 | 44–67 | Dense | 15–21 | [61] |
| 55–95 | 150–1000 | 49.5–99 | 25 | 148–269 | Fully dense | 1–11 | [62] | |
| 240, 300, 360 | 800, 1000, 1200 | 80, 100, 120 | 40 | 62.5–94 | Dense | 9–21 | [63] | |
| 200 | 800 | 80 | 30 | 10 | Porous | 7 | [64] | |
| 240 | 240 | 50 | 30 | 67 | Porous | 1 | [65] | |
| 100 | 400 | 70 | 50 | 7 | Porous | 5 | [66] | |
| 90 | 600 | 90 | 30 | 6 | Porous | 6 | [67] |
| Type of Defect | Cause | Remedy | Effects | Ref. |
|---|---|---|---|---|
| Lack-of-fusion (LOF) | Inhomogeneous distribution of the powder bed | Reduction in the layer thickness and increase of energy penetration | Decrease in mechanical properties and fatigue resistance | [51,59,70,71,72] |
| Non-optimization of the ED function | ||||
| Lack of material or low energy inducing no complete adherence of the melt to the surrounding material | ||||
| Keyhole pore | MP instability | Increase in energy depth penetration and laser power | Decrease in mechanical properties and fatigue resistance | [48,73] |
| Non-optimized process parameters | ||||
| Gas pores | Gas dissolution within the melt material | Reduction in the layer thickness, and the pressure into the chamber Reduction in the O2 Re-melting | Loss density, decrease in tensile strength and fatigue resistance. Gas pores are less critical in crack propagation than the LOF | [51,74,75,76,77,78,79] |
| Gas wrapped into the gas atomized particles | ||||
| Entrapment of the gas present into the build chamber | ||||
| Gas flow within the build chamber | ||||
| Anisotropy | Build orientation | HTs | Tensile properties correlated to the orientations | [59,80,81] |
| Preferential evaporation | Temperature-dependent vapor pressure | Reduction in linear energy density rather than the hatch spacing | Alloying elements loss and pore’s formation | [82,83,84] |
| Residual stress and distortion | High thermal gradient during the L-PBF process | Pre-heated BP Post-process HTs Opportune scan strategy and re-melting Sacrificial material and support structures | Sample distortion if residual stress is higher than the YS Alloying elements loss and pore’s formation Loss of tolerance requirement Reduction in fatigue resistance and tensile properties | [85,86,87,88] |
| Balling | Low viscosity of melt material | Reduction in ED value | Porosity | [62,89,90,91,92,93] |
| Excess of melt material | Stress Concentration point | |||
| MP instability: capillarity, Marangoni’s effect | Surface quality and roughness | |||
| Splashing of MP due to its high surface temperature | Intralayer connection |
| HTs | Temperature Used | Scopes | Ref. | ||
|---|---|---|---|---|---|
| Direct aging | DA | T ≤ 200 °C | Si-eutectic network is not destroyed (T ≤ 200 °C). Alloying strengthening | [7,9,14,138,139,155,156,157,158] | |
| Stress relief | SR | ~300 °C | Avoid deformations during sample removal from the BP Residual stress reduction | [139,143,155,159,160,161,162,163] | |
| Solution heat treatment | SHT | T6 | 400 < TSHT < Teutectic 1 TSHT > 480 °C 2 | Formation of SSS Melt of Si-eutectic (T > Teutectic) Increase of ductility | [7,143,157,162,163,164,165,166] |
| Artificial aging | AA | TAA ≤ 200 °C 160 < TAA < 180 °C 2 | Alloying strengthening | ||
| Hot Isostatic Pressing | HIP | 500 °C < T < Teutectic | Sample’s densification Increase of ductility | [107,109,165,167,168] | |
| Process Parameters | Directions | HT | UTS (MPa) | YS (MPa) | A (%) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P (W) | v (mm/s) | h (μm) | t (μm) | BP | |||||||
| 370 | 1400 | 70 | 90 | 150 °C | H | As-built | 441 ± 3 | 285 ± 6 | 6.6 ± 0.8 | [9] | |
| 411 ± 9 1 | 237 ± 6 | 7.0 ± 1.3 | |||||||||
| 750 | 1100 | -- | -- | RT | -- | As-built | 375 ± 18 | 225 ± 14 | 6.0 ± 2.5 | [13] | |
| 350 | 1150 | 170 | 50 | 150 °C | H | As-built | 430 ± 8 | 286 ± 8 | 7.0 ± 0.4 | [14] | |
| 365 ± 7 1 | 220 ± 2 | 7.2 ± 0.3 | |||||||||
| -- | -- | 190 | 60 | -- | H | As-built | 323 ± 2 | 190 ± 6 | 6.7 ± 0.2 | [137] | |
| 30 | 367 ± 4 | 244 ± 1 | 6.9 ± 1.0 | ||||||||
| 100 | 30 | 469 ± 4 | 314 ± 1 | 5.6 ± 0.6 | |||||||
| 190 | 60 | V | 340 ± 1 | 214 ± 6 | 3.2 ± 0.1 | ||||||
| 30 | 380 ± 2 | 233 ± 2 | 3.9 ± 0.2 | ||||||||
| 100 | 30 | 437 ± 4 | 278 ± 1 | 3.4 ± 0.1 | |||||||
| -- | -- | -- | 60 | RT | -- | As-built | 435 | 250 | 7.5 ± 2.5 | [139] | |
| 200 °C | 310 | 160 | 5.0 ± 1.0 | ||||||||
| 370 | 1300 | 190 | 30 | 165 °C | H | As-built | 429 ± 8 | 226 ± 7 | 4.0 ± 0.3 | [143] | |
| V | 418 ± 7 | 269 ± 6 | 7.8 ± 0.4 | ||||||||
| 340 | 1300 | 200 | 30 | 160 °C | H | As-built | 386 ± 3 | 248 ± 2 | 8.6 ± 1.4 | [138] | |
| V | 412 ± 5 | 228 ± 4 | 7.0 ± 0.1 | ||||||||
| 250 | 1400 | 130 | 30 | -- | -- | As-built | 448 | 264 | 9.8 | [158] | |
| 300 | 1000 | 130 | 40 | -- | -- | As-built | 463 ± 3 | 237 ± 4 | 7.6 ± 1.0 | [165] | |
| 350 | 1140 | 170 | 50 | 100 °C | -- | As-built | 434 ± 12 | 322 ± 8 | 5.3 ± 0.2 | [170] | |
| 200 | 1000 | 150 | 30 | -- | V | As-built | 465 ± 8 | 305 ± 4 | 8.6 ± 1.4 | [176] | |
| -- | -- | -- | -- | -- | H | As-built | 318 | 216 | 5.7 | [177] | |
| V | 320 | 221 | 5.4 | ||||||||
| 370 | 1300 | 190 | 30 | -- | H | As-built | 409 ± 2 | 242 ±2 | 10.9 ± 0.7 | [178] | |
| V | 410 ± 2 | 224 ± 1 | 6.7 ± 0.3 | ||||||||
| 390 | 1300 | 190 | 30 | RT | H | As-built | 525 ± 4 | 287± 2 | -- | [179] | |
| 350 | 1150 | 170 | 50 | 150 °C | V | As-built | 393 ± 20 | 273 ± 3 | 2.5 ± 0.4 | [180] | |
| 370 | 1400 | 70 | 90 | 150 °C | H | DA | 200 °C × 4 h | 374 ± 1 | 231 ± 2 | 8.2 ± 1.2 | [9] |
| 363 ± 8 1 | 219 ± 6 | 8.5 ± 0.6 | |||||||||
| 350 | 1150 | 170 | 50 | 150 °C | 371 ± 9 | 230 ± 8 | 8.2 ± 0.7 | ||||
| 348 ± 2 1 | 209 ± 2 | 7.6 ± 0.8 | |||||||||
| 750 | 1100 | -- | -- | RT | -- | DA | 160 °C × 8 h | 399 ± 13 | 284 ± 16 | 4.5 ± 1.9 | [13] |
| 350 | 1150 | 170 | 50 | 150 °C | H | DA | 175 °C × 6 h | 419 ± 16 | 258 ± 9 | 7.6 ± 0.4 | [14] |
| 396 ± 18 1 | 232 ± 11 | 7.6 ± 0.6 | |||||||||
| DA | 200 °C × 6 h | 395 ± 11 | 235 ± 12 | 9.0 ± 1.0 | |||||||
| 350 ± 9 1 | 199 ± 6 | 9.2 ± 0.5 | |||||||||
| DA | 225 °C × 6 h | 341 ± 15 | 199 ± 6 | 13.6 ± 1.1 | |||||||
| 331 ± 19 1 | 184 ± 5 | 15.2 ± 1.0 | |||||||||
| -- | -- | -- | 60 | RT | -- | DA | 170 °C × 6 h | 400 | 295 | 5.05 ± 0.5 | [139] |
| 340 | 1300 | 200 | 30 | RT | H | DA | 160 °C × 4 h | 471 ± 1 | 321 ± 2 | 8.6 ± 0.5 | [138] |
| V | 493 ± 1 | 292 ± 1 | 6.0 ± 0.6 | ||||||||
| 250 | 1400 | 130 | 30 | -- | -- | DA | 180 °C × 6 h | 452 | 310 | 6.2 | [158] |
| 300 | 1000 | 130 | 40 | -- | -- | DA1 | 177 °C × 10 h | 418 ± 9 | 233 ± 12 | 5.1 ± 0.8 | [165] |
| DA2 | 177 °C × 100 h | 403 ± 9 | 229 ± 12 | 4.2 ± 0.6 | |||||||
| DA3 | 177 °C × 1000 h | 391 ± 5 | 231 ± 9 | 4.6 ± 0.7 | |||||||
| 750 | 1000 | -- | -- | RT | -- | SR | 300 °C × 2 h | 225 ± 7 | 132 ± 9 | 11.5 ± 3.5 | [13] |
| 370 | 1300 | 190 | 30 | RT | -- | SR | 300 °C × 2 h | 302 ± 15 | 210 ± 16 | 10.7 ± 1.6 | [107] |
| -- | -- | -- | 60 | RT | -- | SR | 270 °C × 2 h | 335 | 210 | 12 ± 2 | [139] |
| 370 | 1300 | 190 | 30 | 165 | H | SR | 300 °C × 2 h | 257 ± 1 | 160 ± 1 | 18.1 ± 0.5 | [143] |
| V | 261 ± 3 | 170 ± 2 | 19.1 ± 1.0 | ||||||||
| 300 | 1000 | 130 | 40 | -- | -- | SR | 285 °C × 2 h | 249 ± 10 | 153 ± 8 | 21.3 ± 1.7 | [165] |
| SR+DA1 | 285 °C × 2 h + 177 °C × 10 h | 246 ± 9 | 154 ± 8 | 21.6 ± 1.8 | |||||||
| SR+DA2 | 285 °C × 2 h + 177 °C × 100 h | 271 ± 4 | 174 ± 3 | 16.5 ± 1.2 | |||||||
| SR+DA3 | 285 °C × 2 h + 177 °C × 1000 h | 245 ± 5 | 155 ± 2 | 14.8 ± 2.0 | |||||||
| SR | 190 °C × 2 h 2 | 443 ± 16 | 258 ± 4 | 4.7 ± 1.2 | |||||||
| SR + DA1 | 190 °C × 2 h + 177 °C × 10 h | 441 ± 9 | 231 ± 7 | 5.0 ± 0.8 | |||||||
| SR + DA2 | 190 °C × 2 h + 177 °C × 100 h | 407 ± 8 | 229 ± 7 | 5.0 ± 0.7 | |||||||
| SR + DA3 | 190 °C × 2 h + 177 °C × 1000 h | 387 ± 4 | 221 ± 6 | 5.3 ± 1.1 | |||||||
| 200 | 1000 | 150 | 30 | -- | V | SR | 300 °C × 4 min | 322 ± 5 | 220 ± 4 | 6.3 ± 0.2 | [176] |
| SR | 300 °C × 40 min | 282 ± 4 | 192 ± 6 | 12.8 ± 0.7 | |||||||
| 390 | 1300 | 190 | 30 | RT | H | SR | 250 °C × 2 h | 421 ± 2 | 249 ± 2 | -- | [179] |
| SR | 300 °C × 2 h | 341 ± 16 | 212 ± 12 | -- | |||||||
| -- | -- | -- | -- | 200 °C | H | SR | 300 °C × 2 h | 327 ± 3 | 209 ± 1 | -- | [181] |
| V | 350 ± 0 | 209 ± 0 | -- | ||||||||
| 370 | 1400 | 70 | 90 | 150 °C | H | T6 | 505 °C × 4 h + 175 °C × 4 h | 295 ± 2 | 239 ± 2 | 11.3 ± 2.5 | [9] |
| 292 ± 5 1 | 236 ± 5 | 9.5 ± 2.2 | |||||||||
| 750 | 1100 | -- | -- | RT | -- | SR + T6 | 300 °C × 2 h + 543 °C × 1 h + 180 °C × 1 2h | 329 ± 12 | 278 V 6 | 6.0 ± 1.4 | [13] |
| 300 °C × 2 h + 543 °C × 3 h + 180 °C × 8 h | 332 ± 11 | 292 ± 12 | 4.4 ± 0.8 | ||||||||
| 350 | 1150 | 170 | 50 | 150 °C | H | T6 | 505 °C × 4 h + 175 °C × 4 h | 274 ± 3 | 226 ± 2 | 8.4 ± 0.8 | [14] |
| 290 ± 4 1 | 238 ± 4 | 9.7 ± 1.0 | |||||||||
| 370 | 1300 | 190 | 30 | RT | -- | T6 | 540 °C × 2 h | 297 ± 10 | 234 ± 7 | 5.0 ± 1.5 | [107] |
| -- | -- | -- | -- | RT | -- | T6 | 540 °C × 8 h + 160 °C × 6 h | 225 | 180 | -- | [139] |
| 370 | 1300 | 190 | 30 | 165 °C | H | SHT | 500 °C × 2 h | 133 ± 1 | 78 ± 1 | 29 ± 1 | [143] |
| 340 | 1300 | 200 | 30 | RT | H | T6 | 540 °C × 1 h + 160 °C × 4 h | 323 ± 0 | 243 ± 0 | 15.3 ± 2.4 | [138] |
| V | 302 ± 2 | 223 ± 3 | 16.0 ± 1.4 | ||||||||
| 300 | 1000 | 130 | 40 | -- | -- | T6 | 530 °C × 6 h | 308 ± 8 | 240 ± 8 | 16.2 ± 1.5 | [165] |
| T6 + DA1 | 530 °C × 6 h + 160 °C × 6 h + 177 °C × 10 h | 283 ± 10 | 232 ± 7 | 14.5 ± 1.5 | |||||||
| T6 + DA2 | 530 °C × 6 h + 160 °C × 6 h + 177 °C × 100 h | 201 ± 2 | 159 ± 3 | 16.2 ± 0.6 | |||||||
| T6 + DA3 | 530 °C × 6 h + 160 °C × 6 h + 177 °C × 1000 h | 144 ± 6 | 94 ± 4 | 28.9 ± 2.7 | |||||||
| 350 | 1140 | 170 | 50 | 100 °C | -- | SHT | 450 °C × 2 h | 282 ± 6 | 197 ± 4 | 13.4 ± 0.5 | [170] |
| 500 °C × 2 h | 214 ± 5 | 126 ± 2 | 23.5 ± 0.8 | ||||||||
| 550 °C × 2 h | 168 ± 2 | 91 ± 2 | 23.7 ± 0.8 | ||||||||
| 200 | 1000 | 150 | 30 | -- | V | SHT | 540 °C × 2 h | 185 ± 7 | 98 ± 2 | 16.7 ± 0.5 | [176] |
| T6 | 540 °C × 2 h + 160 °C × 2 h | 254 ± 7 | 194 ± 5 | 7.0 ± 0.3 | |||||||
| 250 | 1400 | 130 | 30 | -- | -- | T6 | 520 °C × 2 h + 180 °C × 6 h | 242 | 180 | 9.6 | [180] |
| -- | -- | -- | -- | 200 °C | H | SR + T6 | 300 °C × 2 h + 540 °C × 8 h + 160 °C × 10 h | 337 ± 8 | 280 ± 5 | -- | [181] |
| V | 315 ± 15 | 267 ± 12 | -- | ||||||||
| 370 | 1300 | 190 | 30 | RT | -- | HIP | 500 °C × 75 min + 100 MPa | 176 ± 2 | 108 ± 3 | 25.0 ± 0.5 | [107] |
| SR +HIP + T6 | 300 °C × 2 h + 500 °C × 75 min + 100 MPa + 540 °C × 2 h + 180 °C × 4 h | 345 ± 1 | 308 ± 25 | 5.8 ± 1.7 | |||||||
| SR + HIP + T6 | 300 °C × 2 h + 500 °C × 75 min + 100 MPa + 540 °C × 2 h + 180 °C × 12 h | 306 ± 9 | 254 ± 9 | 8.7 ± 3.3 | |||||||
| 300 | 1000 | 130 | 40 | -- | -- | HIP | 515 °C × 3 h + 100 MPa | 144 ± 1 | 88 ± 4 | 32.2 ± 1.2 | [165] |
| 300 | 1000 | 130 | 40 | -- | -- | HIP + DA1 | 515 °C × 3 h + 100 MPa + 177 °C × 10 h | 144 ± 1 | 93 ± 2 | 31.8 V 1.0 | [165] |
| HIP + DA2 | 515 °C × 3 h + 100 MPa + 177 °C × 100 h | 138 ± 1 | 93 ± 1 | 30.5 ± 3.1 | |||||||
| HIP+DA3 | 515 °C × 3 h + 100 MPa + 177 °C × 1000 h | 127 ± 1 | 80 ± 1 | 33.3 ± 1.3 | |||||||
| Environment | Corrosion Related to | HT | Corrosion Characteristics | Ref. | |
|---|---|---|---|---|---|
| Harrison’s solution | Surface finish, build orientation | As built | Anisotropy of corrosion resistance between the xz and xy planes | Ecorr = −0.6 ÷ −0.7 V | [193] |
| Harrison’s solution | Surface finish and HTs | As built | The spontaneously passive layer formed in air is more protective than the same forming during the L-PBF process. | Ecorr/unpolished = −0.561 ÷ −0.649 V Ecorr/polished = −0.570 ÷ −0.758 V | [194] |
| 275 °C × 2 h (AC) | SR does not reduce the susceptibility to the corrosion attack penetration. | Ecorr/unpolished = −0.615 ÷ −0.869 V Ecorr/polished = −0.476 ÷ −0.624 V | |||
| 275 °C × 2 h (AC) + 525 °C × 1 h (WQ) | SR + SHT increase the local corrosion | Ecorr/unpolished = −0.600 ÷ −0.608 V Ecorr/polished = −0.610 ÷ −0.620 V | |||
| 01M NaCl | Scans strategy, layer thickness | As-built (BP T = 180 °C) | Pitting into MPBs, crack formation. Relation between the cellular grain size and the Volta potential between Si and α-Al. | Ecorr = −0.639 ÷ −0.650 V | [197] |
| 3.5 wt% NaCl | SLM/As cast | As built | As built lower mass loss than as cast | Ecorr = −0.73 V, icorr = 0.54 μA/cm2 | [199] |
| 3.5 wt% NaCl | Surface finish | 300 °C × 2 h | Higher resistance for the polished than unpolished samples | Unpolished 2.13 pit/cm2. Polished 0.93 pit/cm2 | [200] |
| Sample | ED (J/mm3) | Microstructure | Ref. |
|---|---|---|---|
| S1 | 68.47 | Acicular α′-martensite | [221] |
| S3 | 50.62 | Lamellar α + β | |
| S7 | 33.74 | Acicular α′-martensite + minority of α + β |
| Phases | a (Å) | c (Å) | c/a | Ref. |
|---|---|---|---|---|
| α-phase | 0.295 | 0.468 | 1.5896 | [243] |
| 0.294 | 0.467 | 1.588 | [254] | |
| 0.293 | 0.468 | 1.597 | [255] | |
| α′-martensite | 0.293 | 0.468 | 1.597 | [227] |
| 0.293 | 0.467 | 1.594 | [256] |
| HTs | Temperature Used | Scopes | Ref. | ||
|---|---|---|---|---|---|
| Stress Relief | SR | 400 < TSR < 800 °C 1 | Residual stress reduction Mechanical properties improvement Avoid distortions | [54,59,111,265,266,267,268,269,270] | |
| Annealing | ANN | 700 < TANN < 940 °C | Martensite decomposition Mechanical properties improvement (balance between strength and ductility: bi-modal structure) | [226,230,245,259,260,271] | |
| SHT | SHT | STA | 940 < TSHT < 970 °C 2 TβSHT > β-transus | Microstructural variation to improve ductility | [30,271,272,273,274,275,276] |
| Artificial aging | AA | TAA ≤ 700 °C 3 | Higher mechanical properties than the ANNed samples. α, β, α2-Ti3Al precipitation | ||
| Hot Isostatic Pressing | HIP | 900 < TSHT < 1050 °C (p ~ 100 MPa) 4 | Sample’s densification Increase in ductility and fatigue strength Improvement of biocompatibility | [110,111,269,276,277,278,279] | |
| HT | Cooling Method | Microstructure | Ref. |
|---|---|---|---|
| 500 °C × 10 h | FC | α′-martensite decomposition | [227] |
| T < 600 °C | WQ | No morphological changes of the as-built microstructure | [233] |
| α′-martensite decomposition in α platelet arranged in the same martensite orientation | |||
| 640–650 °C | - | Nucleation of β phase | [284] |
| 700 °C × 2 h | FC | Partially α′-martensite decomposition | [212] |
| Fine α needle-like phase | |||
| 700 °C × 2 h | FC | α′ → α + β transformation | [230] |
| Small fraction of β nano-particles | |||
| 704 °C × 2 h | FC | α′ → α + β transformation | [268] |
| 730 °C × 2 h | FC | α′ → α + β transformation and grain growth | [59] |
| α′ and α phases with the same length and width size | |||
| 750 °C × 2 h | WQ | α′ → α decomposition (same directions) | [233] |
| 750 °C × 8 h | AC | α′ → α + β transformation (quartic α′ → β; and primary, secondary and tertiary α′ → α) | [245] |
| Minimal grain growth | |||
| 750 °C × 10 h | FC | α′ → α + β fully transformed | [227] |
| 800 °C × 2 h | FC | α′ → α + β transformation does not reach the equilibrium Solute distribution into the matrix α′, α and β microstructure | [230] |
| 800 °C × 6 h | FC | Twins disappeared + recovered microstructure | |
| 800 °C × 10/20 min | AC | 10 min does not affected the microstructure, while 20 min induces α′ decomposition | [265] |
| 800 °C × 4 h | AC | Complete decomposition of α′ martensite | |
| Regular arrangement of α + β structure caused by self-accommodation α′ | |||
| 850 °C × 1 h | FC | α′ → α decomposition and β formation due to the V content α′, α and β microstructure | [270] |
| 910 °C × 0.5/2/8 h | WQ | Primary α′ → α lamella in β matrix, tertiary and quartic α′ into β 0.5 h is not sufficient to obtain equilibrium phases which are obtained after 2 h | [245] |
| 945 °C × 4 h | WQ | Large amount of grain growth | |
| 960 °C × 0.5/4 h | WQ | 0.5 h is similar to the 910 °C × 0.5 h (WQ). Globularisation of the α phase and significant grain growth. The α phase becomes elongated. | |
| 900 °C × 2 h | AC | α′ → α + β transformation with lamellar morphology | [285] |
| Heterogeneous distribution of alloying elements + secondary α | |||
| 950 °C × 2 h | FC | α′ → α + β lamella more stable and α′ is fully decomposed | [265] |
| β grains become equiaxed | |||
| 950 °C × 1 h | WQ | Primary α coarsened | [275] |
| Precipitate dissolve and a SSS is formed | |||
| 950 °C × 1 h | AC | β → α″ transformation | |
| Grain growth, but finer than FC Bi-lamella structure with secondary α + β nano-particles | |||
| 950 °C × 1 h | FC | Greater diffusion-controlled nucleation | |
| 1000 °C | WQ | Recrystallization (β equiaxed grain) + α′ martensite Colony-tipe α′ martensite → weave-type α′ martensite | [212] |
| 1000 °C × 2 h | FC | α′ → α + β lamellae stable transformation | |
| 1015 °C × 15 min | WQ | α′ martensite into columnar β grains | [268] |
| 1015 °C × 15 min | AC | α′ coarse needle + α coarse phase | |
| 1015 °C × 2 h | FC | α + β lamella | [32] |
| 1050 °C × 1 h | FC | Recrystallization (β equiaxed grain) + α crystalizes in the β grain boundaries α + β structure | [270] |
| 1050 °C × 1 h | AC | Recrystallization (equiaxed + half equiaxed β grains) | [265] |
| 1150 °C × 2 h | AC | Recrystallization (β equiaxed grain) + α crystalizes in the β grain boundaries α + β coarsened lamella structure | [285] |
| 920 °C × 2 h + 100 MPa + 920 °C × 12 h | AC + AC 1 | α′ → α + β equilibrium lamellar mixture without a significant dislocation density | [275] |
| 704 °C × 2 h + 920 °C × 2 h + 100 MPa | FC + AC | α′ → α + β equilibrium lamellar mixture | [268] |
| 955 °C × 1 h + 600 °C× 8 h | FC+WQ 2 + AC | α + β lamellar microstructure into semi-equiaxed grains | [286] |
| 850 °C × 2 h + (975 °C × 30 min → 875 °C → 975 °C × 30 min) 3 | FC + AC | α + β lamellar microstructure (width of α laths increases with temperature) Large primary + fine secondary α phases | [224] |
| 950 °C × 2 h + (975 °C × 30 min → 875 °C → 975 °C × 30 min) 3 | FC + AC | α + β lamellar microstructure (width of α laths increases with temperature) Large primary + fine secondary α phases | [224] |
| 1020 °C × 2h + (975 °C × 30 min → 875 °C → 975 °C × 30 min) 3 | FC + AC | ||
| 950 °C × 15 min + 500 °C × 8 h | WQ + AC | Acicular α′ + primary rod-like α phase | [287] |
| 950 °C × 4 h + 150 MPa | -- | Coarsened α + β lamellar | [288] |
| 900 °C × 2 h + 100 MPa | FC | α + β lamellar | [223] |
| 920 °C × 2 h + 100 MPa | -- | α + β lamellar and recrystallization process | [110] |
| 1050 °C × 2 h + 100 MPa | α + β lamellar more coarsened than 900 °C × 2 h |
| HT Region | HT | Microstructure 1 | Directions | E (GPa) | UTS (MPa) | YS (MPa) | A (%) | Ref. | |
|---|---|---|---|---|---|---|---|---|---|
| α + β | 540 °C × 5 h (WQ) | SR | -- | V | 113 ± 30 | 1223 ± 52 | 1118 ± 39 | 5.4 ± 2.0 | [32] |
| 611 °C × 2 h (AC) | SR | α′ martensite + β precipitate | V | -- | 1213 ± 20 | 1171 ± 40 | 13.3 ± 0.7 | [285] | |
| 640 °C × 2 h | ANN 2 | β(C) | 1225 ± 4 | 1104 ± 8 | 7.4 ± 1.6 | [319] | |||
| 1214 ± 24 | 1140 ± 43 | 3.2 ± 2.0 | |||||||
| 1256 ± 9 | 1152 ± 11 | 3.9 ± 1.2 | |||||||
| 650 °C × 3 h (FC) | SR | α′ martensite + α, β precipitation in β(C) | V | 111 ± 1 | 1101 ± 5 | 1040 ± 7 | 7.8 ± 0.7 | [271] | |
| 670 °C × 5 h (FC) | SR | Acicular α′ + α + β in β(C) | H(xz) | 116 ± 1 | 1170 ± 5 | 1112 ± 4 | 9.2 ± 0.2 | [111] | |
| H(xy) | 115 ± 1 | 1207 ± 5 | 1147 ± 7 | 7.9 ± 0.6 | |||||
| V | 121 ± 1 | 1193 ± 8 | 1164 ± 5 | 3.8 ± 0.1 | |||||
| 700 °C × 2 h (FC) | SR | α′ + α + β | H | -- | 1109 ± 18 | 1013 ± 17 | 13.5 ± 0.2 | [230] | |
| 700 °C × 1 h (10 K/min) | SR | α′ + α (fine needles) + β in β(C) | -- | 117.45 | 1115 | 1051 | 11.3 | [304] | |
| 705 °C × 3 h (AC) | ANN | β(C) | V | 115 ± 2 | 1082 ± 34 | 1026 ± 35 | 9.1 ± 2.0 | [32] | |
| 730 °C × 2 h (AC) | ANN | α′ + α + β in β(C) | H(xz) | 113 ± 9 | 1057 ± 8 | 958 ± 6 | 12.4 ± 0.7 | [59] | |
| α + β | 730 °C × 2 h (AC) | ANN | α′ + α + β in β(C) | H(xy) | 112 ± 6 | 1065 ± 21 | 974 ± 7 | 7.0 ± 0.5 | [59] |
| V | 117 ± 6 | 1052 ± 11 | 937 ± 9 | 9.6 ± 0.9 | |||||
| 730 °C × 2 h (AC) | ANN | H | 101 ± 4 | 1046 ± 6 | 965 ± 16 | 9.5 ± 1 | [325] | ||
| V | 110 ± 29 | 1000 ± 53 | 900± 101 | 1.9 ±8 0.8 | |||||
| 800 °C × 2 h (AC) | ANN | α′ + α (0.7μm) + β in β(C) | -- | -- | 1073 ± 9 | 1010 ± 11 | 17.0 ± 1.0 | [265] | |
| 800 °C × 2 h | SR | β(C) | V | -- | 1228 ± 32 | -- | 8.0 ± 1.5 | [110] | |
| 800 °C × 2 h (FC) | ANN | α + β lamellae | H | -- | 1024 ± 10 | 955 ± 9 | 14.7 ± 0.4 | [230] | |
| 800 °C × 6 h (FC) | ANN | H | -- | 1017 ± 5 | 928 ± 6 | 18.9 ± 1.3 | |||
| 800 °C × 12 h (FC) | ANN | H | -- | 1007 ± 4 | 923 ± 3 | 18.5 ± 0.6 | |||
| 850 °C × 1 h (FC) | ANN | α′ + α + β in β(C) | V | 114 ± 1 | 1003 ± 4 | 945 ± 6 | 8.1 ± 0.3 | [271] | |
| 850 °C × 2 h (FC) | ANN | α + β with increase of β fraction in β(C) | V | 115 ± 4 | 1004 ± 6 | 955 ± 6 | 12.8 ± 1.4 | [32] | |
| 850 °C × 5 h (FC) | ANN | α + β with increase of β fraction in β(C) | V | 112 ± 3 | 965 ± 20 | 909 ± 24 | -- | ||
| 900 °C × 30′ (AC) | ANN | α + β | V | -- | 1013 ± 23 | 981 ± 26 | 16.1 ± 2.9 | [285] | |
| 900 °C × 60′ (AC) | ANN | α + β | V | -- | 1026 ± 8 | 974 ± 1 | 15.5 ± 0.6 | ||
| 900 °C × 120′ (AC) | ANN | α + β | V | -- | 1021 ± 18 | 975 ± 16 | 15.0 ± 0.2 | ||
| 920 °C × 2 h + 100 MPa | HIP | β(E) | V | -- | 1086 ± 26 | -- | 13.8 ± 1.3 | [110] | |
| 930 °C × 2 h (FC) | ANN | α + β | V | -- | 968 ± 4 | 924 ± 8 | 19.5 ± 3.2 | [285] | |
| 930 °C × 2 h (AC) | ANN | -- | 1031 ± 44 | 991 ± 47 | 16.9 ± 1.9 | ||||
| 930 °C × 2 h (WQ) | ANN | -- | 1097 ± 12 | 1048 ± 15 | 9.2 ± 1.0 | ||||
| 940 °C × 1 h (AC) + 650 °C × 2 h (AC) | STA | α + β in β(C) | V | 116 ± 3 | 948 ± 27 | 899 ± 27 | 13.6 ± 0.3 | [32] | |
| 950 °C × 2 h (AC) | ANN | Bi-lamellar structure α (2.4 μm) + β in β(C) | -- | -- | 945 ± 5 | 893 ± 3 | 14.1 ± 1.5 | [265] | |
| 950 °C × 1 h (FC) | ANN | α′ + α + β coarsen in β(C→E) | V | 114 ± 2 | 926 ± 3 | 860 ± 5 | 10.5 ± 0.6 | [271] | |
| 950 °C × 1 h (WQ) + 700 °C × 2h(AC) | Mixed | α + β | H | 103 ± 11 | 1036 ± 30 | 944 ± 8 | 8.5 ± 1 | [325] | |
| V | 98 ± 3 | 1040 ± 4 | 924 ± 14 | 7.5 ± 2 | |||||
| 900 °C × 2 h + 700 °C × 1 h (10 K/min) | Mixed | α/β phase columnar in β(C) | -- | 118.8 | 988 | 980 | 9.5 | [303] | |
| 910 °C × 8 h (WQ) + 750 °C × 4 h (FC) | Mixed | Bimodal microstructure | -- | -- | ~950 | ~900 | ~18 | [245] | |
| 900 °C × 2 h + 100 MPa + 700 °C × 1 h (10 K/min) | HIP + ANN | α/β phase columnar in β(C) | -- | 115.4 | 973 ± 1 | 885 ± 3 | 19.0 ± 0.5 | [303] | |
| 920 °C × 2 h(AC) + 100 MPa + 920 °C × 12 h (AC) | HIP + ANN | Basketweave α + β in β(E) | H(xz) | 116 ± 1 | 1007 ± 1 | 937 ± 1 | 16.0 ± 0.4 | [111] | |
| H(xy) | 117 ± 2 | 1003 ± 1 | 936 ± 3 | 15.4 ± 0.3 | |||||
| V | 113 ± 1 | 999 ± 1 | 911 ± 4 | 16.9 ± 0.9 | |||||
| 920 °C × 2 h + 100 MPa | β(E) | -- | -- | 1089 ± 26 | -- | 13.8 ± 1.3 | [110] | ||
| See Table 9 | C ANN | Bimodal microstructure | H(xy) | -- | 1017 ± 16 | 865 ± 19 | 18 ± 1 | [305] | |
| V | -- | 1004 ± 23 | 849 ± 12 | 16 ± 1 | |||||
| α + β | 940 °C × 1 h + 650 °C × 2 h (AC) | STA | -- | V | 116 ± 2 | 948 ± 27 | 899 ± 27 | 13.6 ± 0.3 | [32] |
| β | 1020 °C × 2 h (FC) | SHT | α + β + α2-Ti3Al in β(E) | V | -- | 840 ± 27 | 760 ± 19 | 14.1 ± 2.5 | [32] |
| 1050 °C × 1 h (FC) | SHT | α + β + αp along β grain boundaries β(E) | V | 114 ± 1 | 869 ± 3 | 787 ± 4 | 11.5 ± 1.0 | [241] | |
| 1050 °C × 1 h (WQ) + 820 °C × 2 h (AC) | SHT + ANN | α + β | H | 96.7 ± 5 | 1019 ± 11 | 913 ± 7 | 8.9 ± 1.0 | [325] | |
| V | 95 ± 4 | 951 ± 55 | 869 ± 64 | 7.9 ± 2.0 | |||||
| 1050 °C × 2 h | SHT | β(C) | V | -- | 986 ± 45 | -- | 13.8 ± 0.8 | [110] | |
| 1050 °C × 2 h + 100 MPa | HIP | β(E) | V | -- | 1007 ± 15 | -- | 13.5 ± 0.7 | ||
| 1050 °C × 1 h (AC) | SHT | α (0.7μm) +β in β(E, hE) | -- | -- | 988 ± 8 | 869 ± 4 | 13.4 ± 0.7 | [265] | |
| 1050 °C × 1 h (WQ) + 990 °C × 30′ (AC) | SHT + ANN | Basketweave α + β in β(E, hE) | -- | -- | 962 ± 12 | 838 ± 6 | 12.0 ± 0.1 | ||
| 1150 °C × 2 h (AC) | SHT | α + β coarsen + α grain boundaries | V | -- | 1128 ± 8 | 1107 ± 10 | 4.9 ± | [285] | |
| β | 1200 °C × 1 h (AC) | SHT | α (0.9μm) + β in β(E, hE) | -- | -- | 988 ± 8 | 878 ± 7 | 11.2 ± 1.2 | [265] |
| β + α + β | 1015 °C × 30′ (AC) + 843 °C × 2 h (FC) | SHT + ANN | β(C) | V | -- | 874 ± 23 | 801 ± 20 | 13.5 ± 1.2 | [32] |
| 1015 °C × 30′ (AC) + 730 °C × 2 h (AC) | STA | β(E) | V | 113 ± 3 | 902 ± 19 | 822 ± 25 | 12.7 ± 0.6 | ||
| HT Region | Microstructural | Strength | Ductility | |
|---|---|---|---|---|
| α + β | T < low-SSTR | α′ → α decomposition Slight coarsening (high residence time) No effects of the cooling rate Ti3Al precipitation | ~as-built | |
| low- < T < medium-SSTR | α′ → α + β decomposition (~705 °C) Slight coarsening No effects of the cooling rate | ↓ | ↑ | |
| medium- < T < high-SSTR | α + β microstructure + α globularization Coarsening effects Effects of cooling tare (T → T0) | ↓ | ↑↑ | |
| β | T > | Recrystallization process (C → E) Coarsening effects Effects of cooling method | ↓↓ | ↑↑ |
| Acronyms | Meanings |
|---|---|
| A | Elongation at break (%) |
| AA | Artificial Aging |
| AC | Air Cooling |
| AM | Additive Manufacturing |
| ANN | Annealing |
| BFTEM | Bright-Field Transmission Electron Microscopy |
| BP | Build Platform |
| BR | Build Rate |
| C-ANN | Cycling Annealing |
| CP | Cold Platform |
| DA | Direct Aging |
| DED | Direct Energy Deposition |
| DSC | Differential Scanning Calorimetry |
| ED | Energy Density |
| EBSD | Electron Backscatter Diffraction |
| FC | Furnace Cooling |
| H | Horizontal |
| HAADF | High-Angle Annular Darf-Field |
| HAZ | Heat Affected Zone |
| HIP | Hot Isostatic Pressing |
| HP | Hot Platform |
| HRTEM | High-Resolution Transmission Electron Microscopy |
| HT | Heat Treatment(s) |
| L-PBF(ed) | Laser-Powder Bed Fusion/(Fused) |
| LSW | Lifshitz, Slyozov, Wagner |
| LOF | Lack-Of-Fusion |
| Ms, Mf | Martensite start, finish |
| MP | Melt/Molten pool |
| MPB | MP Boundary(s) |
| MPC | MP Center(s) |
| OM | Optical Microscope |
| PBF | Powder Bed Fusion |
| SAED | Selected Area Electron Diffraction |
| SCC | Stress Corrosion Cracking |
| SEM | Scanning Electron Microscopy |
| SDAS | Secondary Dendrite Arm Spacing |
| SHT | Solution Heat Treatment |
| SLM | Selective Laser Melting |
| SSTR | Solid Solution Temperature Region |
| STA | Solution Treated and Aged |
| STEM | Scanning Transmission Electron Microscope |
| TEM | Transmission Electron Microscope |
| UTS | Ultimate tensile strength |
| V | Vertical |
| XRD | X-Ray Diffraction |
| YS | Yield Strength |
| WQ | Water Quenching |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghio, E.; Cerri, E. Additive Manufacturing of AlSi10Mg and Ti6Al4V Lightweight Alloys via Laser Powder Bed Fusion: A Review of Heat Treatments Effects. Materials 2022, 15, 2047. https://doi.org/10.3390/ma15062047
Ghio E, Cerri E. Additive Manufacturing of AlSi10Mg and Ti6Al4V Lightweight Alloys via Laser Powder Bed Fusion: A Review of Heat Treatments Effects. Materials. 2022; 15(6):2047. https://doi.org/10.3390/ma15062047
Chicago/Turabian StyleGhio, Emanuele, and Emanuela Cerri. 2022. "Additive Manufacturing of AlSi10Mg and Ti6Al4V Lightweight Alloys via Laser Powder Bed Fusion: A Review of Heat Treatments Effects" Materials 15, no. 6: 2047. https://doi.org/10.3390/ma15062047
APA StyleGhio, E., & Cerri, E. (2022). Additive Manufacturing of AlSi10Mg and Ti6Al4V Lightweight Alloys via Laser Powder Bed Fusion: A Review of Heat Treatments Effects. Materials, 15(6), 2047. https://doi.org/10.3390/ma15062047







