Wide Concentration Range of Tb3+ Doping Influence on Scintillation Properties of (Ce, Tb, Gd)3Ga2Al3O12 Crystals Grown by the Optical Floating Zone Method
Abstract
:1. Introduction
2. Experimental Procedures
2.1. Crystal Growth
2.2. Characterization
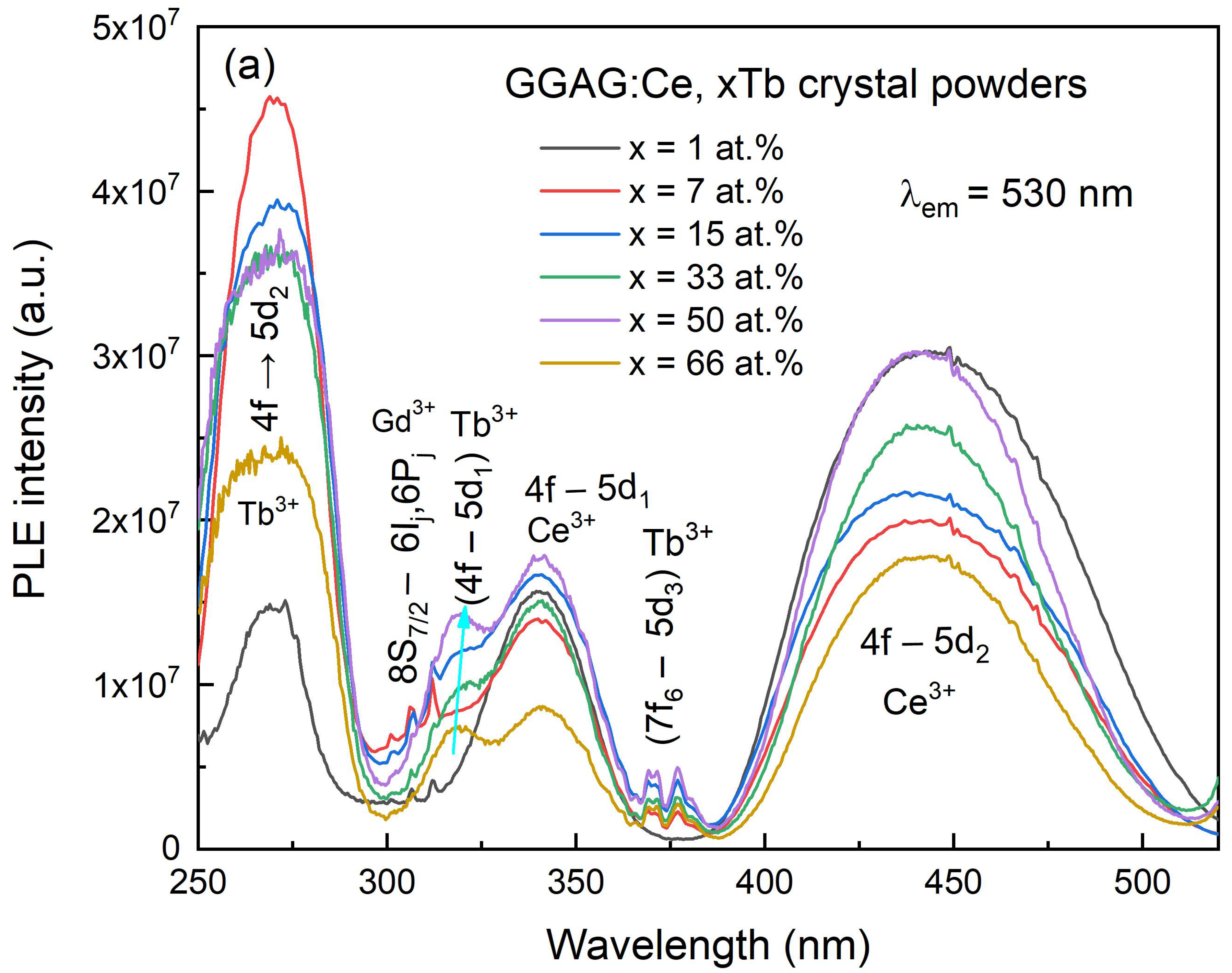
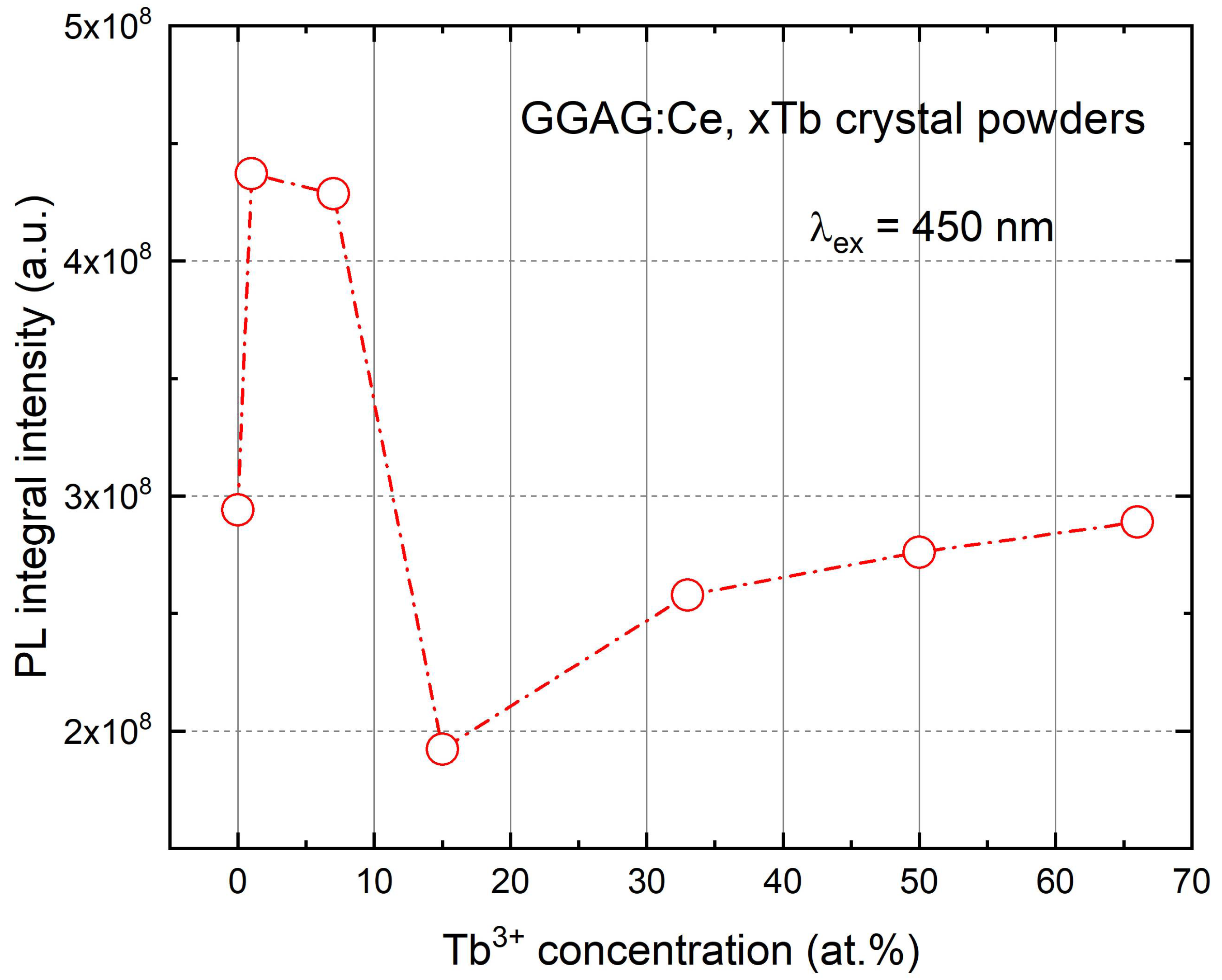
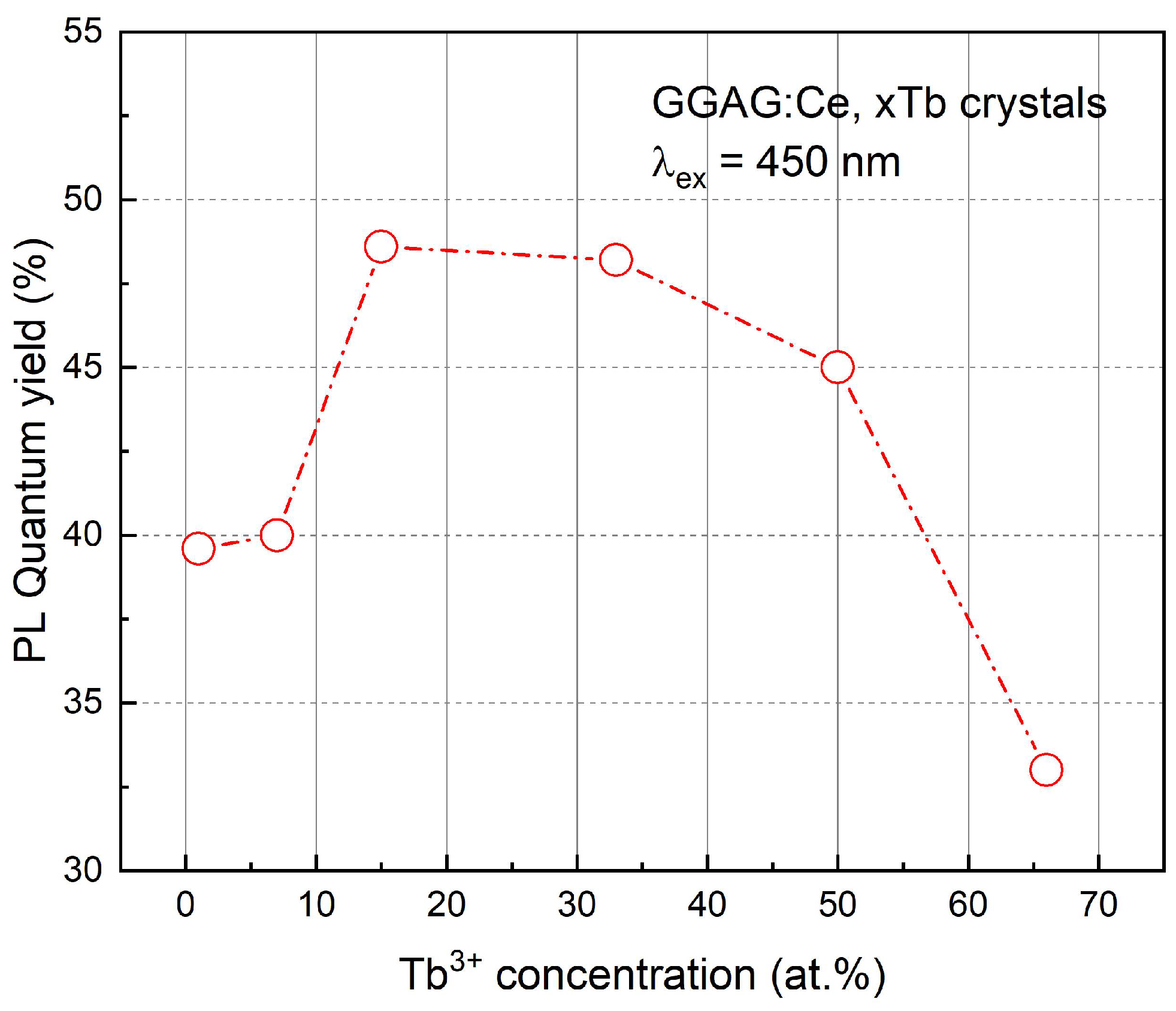
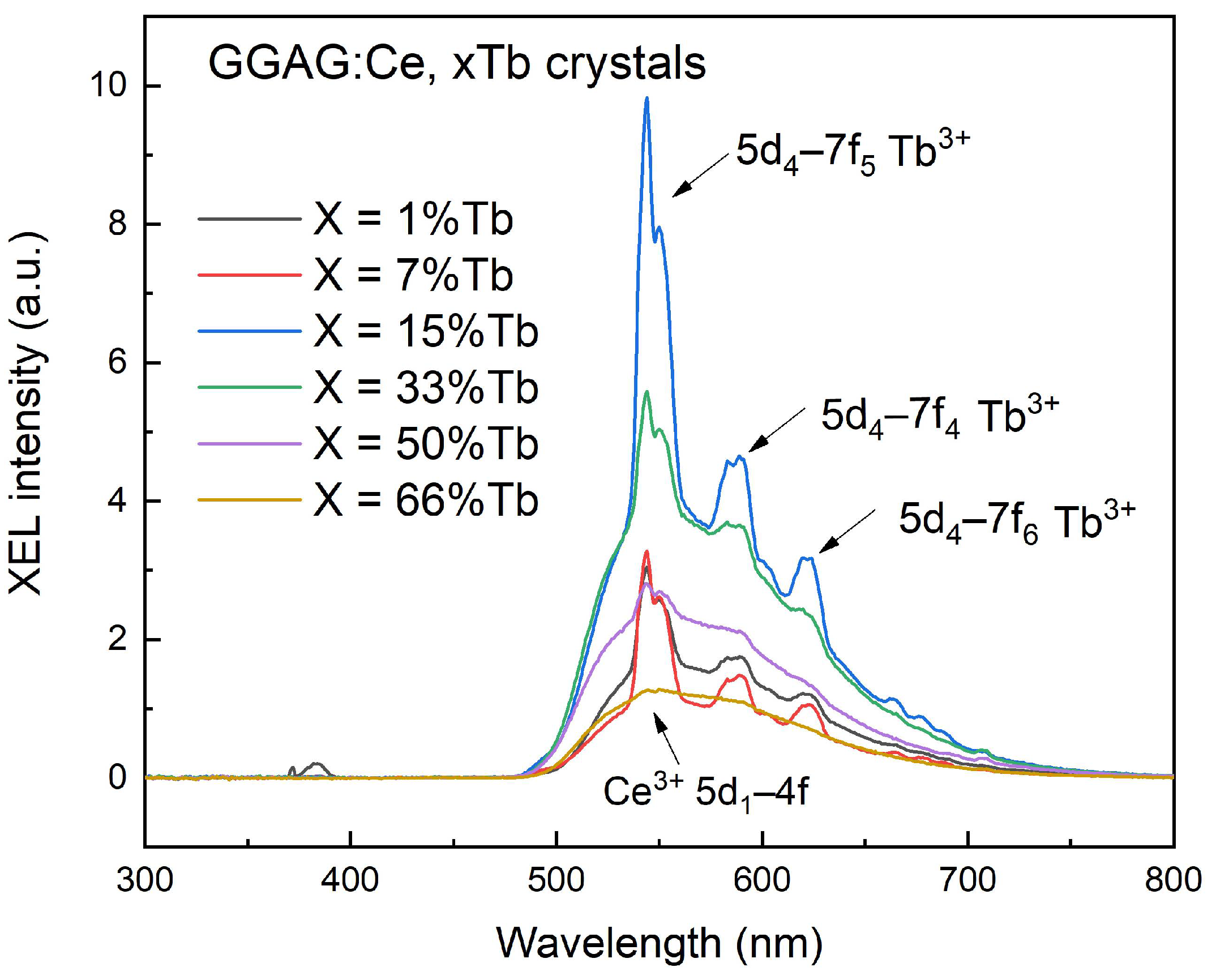
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hostaša, J.; Cova, F.; Piancastelli, A.; Fasoli, M.; Zanelli, C.; Vedda, A.; Biasini, V. Fabrication and luminescence of Ce-doped GGAG transparent ceramics, effect of sintering parameters and additives. Ceram. Int. 2019, 45, 23283–23288. [Google Scholar] [CrossRef]
- Boyarintseva, Y.; Neicheva, S.; Zhmurin, P.; Arhipov, P.; Gerasymov, I.; Tkachenko, S.; Sidletskiy, O.; Baumer, V.; Vovk, O.; Nizhankovskyi, S. Optical study of Y3-xGdxAl5O12:Ce crystals grown from the melt. Opt. Mater. 2019, 96, 109283. [Google Scholar] [CrossRef]
- Geras, Y.; Mov, I.; Nepokupnaya, T.; Boyarintsev, A.; Sidletskiy, O.; Tretyak, S.J.O.M. GAGG:Ce composite scintillator for X-ray imaging. Opt. Mater. 2020, 109, 110305. [Google Scholar]
- Dantelle, G.; Boulon, G.; Guyot, Y.; Testemale, D.; Guzik, M.; Kurosawa, S.; Kamada, K.; Yoshikawa, A. Research on Efficient Fast Scintillators: Evidence and X-ray Absorption Near Edge Spectroscopy Characterization of Ce4+ in Ce3+, Mg2+-co-Doped Gd3Al2Ga3O12 Garnet Crystal. Phys. Status Solidi (B) 2020, 257, 1900510. [Google Scholar] [CrossRef]
- Wu, Y.; Luo, Z.; Jiang, H.; Meng, F.; Koschan, M.; Melcher, C.L. Single crystal and optical ceramic multicomponent garnet scintillators: A comparative study. Nuclear Inst. Methods Phys. Res. A 2015, 780, 45–50. [Google Scholar] [CrossRef]
- Wu, T.; Wang, L.; Xu, T.; Wang, H.; Wang, C.; Huang, X.; Shen, H.; Shi, Y. Recent Progress of (Ce, Gd)3(Ga, Al)5O12 (Ce:GGAG)Scintillator for medical CT application. Chin. J. Technol. 2021, 21, 109–125. [Google Scholar]
- Suzuki, A.; Kurosawa, S.; Pejchal, J.; Babin, V.; Fujimoto, Y.; Yamaji, A.; Seki, M.; Futami, Y.; Yokota, Y.; Yubuta, K. The effect of different oxidative growth conditions on the scintillation properties of Ce:Gd3Al3Ga2O12crystal. Phys. Status Solidi 2012, 9, 2251–2254. [Google Scholar] [CrossRef]
- Kamada, K.; Yanagida, T.; Pejchal, J.; Nikl, M.; Endo, T.; Tsutumi, K.; Fujimoto, Y.; Fukabori, A.; Yoshikawa, A. Scintillator-oriented combinatorial search in Ce-doped (Y,Gd)3(Ga,Al)5O12 multicomponent garnet compounds. J. Phys. D Appl. Phys. 2011, 44, 505104. [Google Scholar] [CrossRef]
- Kochurikhin, V.; Kamada, K.; Jin Kim, K.; Ivanov, M.; Gushchina, L.; Shoji, Y.; Yoshino, M.; Yoshikawa, A. Czochralski growth of 4-inch diameter Ce:Gd3Al2Ga3O12 single crystals for scintillator applications. J. Cryst. Growth 2020, 531, 125384. [Google Scholar] [CrossRef]
- Wu, T.; Wang, L.; Shi, Y.; Xu, T.; Wang, H.; Fang, J.; Ni, J.; He, H.; Wang, C.; Wan, B.; et al. Fast (Ce,Gd)3Ga2Al3O12 Scintillators Grown by the Optical Floating Zone Method. Cryst. Growth Des. 2021, 22, 180–190. [Google Scholar] [CrossRef]
- Shishido, T.; Okamura, K.; Yajima, S. Gd3Al5O12 Phase Obtained by Crystallization of Amorphous Gd2O3·5/3Al2O3. J. Am. Ceram. Soc. 1978, 6, 373–375. [Google Scholar] [CrossRef]
- Kamada, K.; Kurosawa, S.; Prusa, P.; Nikl, M. Cz grown 2-in. size Ce:Gd3(Al,Ga)5O12 single crystal; relationship between Al, Ga site occupancy and scintillation properties. Opt. Mater. 2014, 36, 1942–1945. [Google Scholar] [CrossRef] [Green Version]
- Bartosiewicz, K.; Babin, V.; Kamada, K.; Yoshikawa, A.; Beitlerova, A.; Nikl, M. Effects of Gd/Lu ratio on the luminescence properties and garnet phase stability of Ce3+ activated GdxLu3-xAl5O12 single crystals. Opt. Mater. 2018, 80, 98–105. [Google Scholar] [CrossRef]
- Kamada, K.; Endo, T.; Tsutumi, K.; Yanagida, T.; Fujimoto, Y.; Fukabori, A.; Yoshikawa, A.; Pejchal, J.; Nikl, M. Composition Engineering in Cerium-Doped (Lu,Gd)3(Ga,Al)5O12 Single-Crystal Scintillators. Cryst. Growth Des. 2011, 11, 4484–4490. [Google Scholar] [CrossRef]
- Chewpraditkul, W.; Pattanaboonmee, N.; Sakthong, O.; Chewpraditkul, W.; Szczesniak, T.; Moszynski, M.; Kamada, K.; Yoshikawa, A.; Nikl, M. Luminescence and scintillation characteristics of (GdxY3-x)Al2Ga3O12:Ce (x = 1,2,3) single crystals. Opt. Mater. 2018, 76, 162–168. [Google Scholar] [CrossRef]
- Qi, Q.; Meng, M.; Ding, D.-z.; Zhao, S.-w.; Shi, J.-j.; Ren, G.-h. Effects of Trace MgO Addition on Optical and Scintillation Properties of GAGG:Ce Crystal. Chin.J. Lumin. 2021, 42, 28–36. [Google Scholar] [CrossRef]
- Mukherjee, P.; Hamilton, A.C.S.; Glass, H.F.J.; Dutton, S.E. Sensitivity of magnetic properties to chemical pressure in lanthanide garnets Ln3A2X3O12, Ln = Gd, Tb, Dy, Ho, A = Ga, Sc, In, Te, X = Ga, Al, Li. J. Phys.-Condens. Mat. 2017, 29, 405808. [Google Scholar] [CrossRef] [Green Version]
- Zorenko, T.; Gorbenko, V.; Witkiewicz-Lukaszek, S.; Zorenko, Y. Luminescent properties of (La,Lu,Gd)(3)(Al,Sc,Ga)(5)O-12:Ce mixed garnets under synchrotron radiation excitation. J. Lumin. 2018, 199, 483–487. [Google Scholar] [CrossRef]
- Jumpei Ueda, K.A. Setsuhisa Tanabe Temperature and compositional dependence of optical and optoelectronic properties in Ce3+-doped Y3Sc2Al3-xGaxO12 (x = 0,1,2,3). Opt. Mater. 2013, 35, 1952–1957. [Google Scholar] [CrossRef]
- Kanke, Y.; Navrotsky, A. A calorimetric study of the lanthanide aluminum oxides and the lanthanide gallium oxides: Stability of the perovskites and the garnets. J. Solid State Chem. 1998, 141, 424–436. [Google Scholar] [CrossRef]
- Ji-Guang, L.; Sakka, Y. Recent progress in advanced optical materials based on gadolinium aluminate garnet (Gd3Al5O12). Sci. Technol. Adv. Mat. 2015, 16, 14902–14919. [Google Scholar]
- Wu, T.; Wang, L.; He, H.; Wang, H.; Shen, H.; Liu, Q.; Shi, Y. Research progress of Lu3Al5O12-based scintillation ceramics. Chin. J. Lumin. 2021, 42, 917–937. [Google Scholar] [CrossRef]
- Setlur, A.A.; Shiang, J.J.; Vess, C.J. Transition from Long-Range to Short-Range Energy Transfer through Donor Migration in Garnet Hosts. J. Phys. Chem. C 2011, 115, 3475–3480. [Google Scholar] [CrossRef]
- Ogieglo, J.M.; Zych, A.; Ivanovskikh, K.V.; Justel, T.; Ronda, C.R.; Meijerink, A. Luminescence and Energy Transfer in Lu3Al5O12 Scintillators Co-Doped with Ce3+ and Tb3+. J. Phys. Chem. A 2012, 116, 8464–8474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teng, X.; Li, J.K.; Duan, G.B.; Liu, Z.M. Development of Tb3+ activated gadolinium aluminate garnet (Gd3Al5O12) as highly efficient green-emitting phosphors. J. Lumin. 2016, 179, 165–170. [Google Scholar] [CrossRef]
- Markovskyi, A.; Gieszczyk, W.; Bilski, P.; Fedorov, A.; Bartosiewicz, K.; Paprocki, K.; Zorenko, T.; Zorenko, Y. Composition engineering of Tb3-xGdxAl5-yGayO12:Ce single crystals and their luminescent, scintillation and photoconversion properties. J. Alloys Compd. 2020, 849, 155808. [Google Scholar] [CrossRef]
- Gorbenko, V.; Zorenko, T.; Witkiewicz, S.; Paprocki, K.; Sidletskiy, O.; Fedorov, A.; Bilski, P.; Twardak, A.; Zorenko, Y. LPE Growth of Single Crystalline Film Scintillators Based on Ce3+ Doped Tb3-xGdxAl5-yGayO12 Mixed Garnets. Crystals 2017, 7, 262. [Google Scholar] [CrossRef] [Green Version]
- Bartosiewicz, K.; Babin, V.; Mares, J.A.; Beitlerova, A.; Zorenko, Y.; Iskaliyeva, A.; Gorbenko, V.; Bryknar, Z.; Nikl, M.J.J.o.L. Luminescence and energy transfer processes in Ce3+ activated (Gd,Tb)3Al5O12 single crystalline films. J. Lumin. 2017, 188, 60–66. [Google Scholar] [CrossRef]
- Chen, J.; Tang, Y.R.; Yi, X.Z.; Tian, Y.N.; Ao, G.; Hao, D.M.; Lin, Y.D.; Zhou, S.M. Fabrication of (Tb, Gd)3Al5O12:Ce3+ phosphor ceramics for warm white light-emitting diodes application. Opt. Mater. Express 2019, 9, 3333–3341. [Google Scholar] [CrossRef]
- Babin, V.; Gorbenko, V.; Makhov, A.; Mares, J.A.; Nikl, M.; Zazubovich, S.; Zorenko, Y. Luminescence characteristics of Pb2+ centres in undoped and Ce3+-doped Lu3Al5O12 single-crystalline films and Pb2+-> Ce3+ energy transfer processes. J. Lumin. 2007, 127, 384–390. [Google Scholar] [CrossRef]
- Meng, M.; Qi, Q.; Ding, D.Z.; He, C.J.; Zhao, S.W.; Wan, B.; Chen, L.; Shi, J.J.; Ren, G.H. Twisted Growth, Component Segregation and Characteristics of Gd3(Al,Ga)5O12:Ce Scintillation Crystal. J. Inorg. Mater. 2021, 36, 188–196. [Google Scholar] [CrossRef]
- Velchuri, R.; Kumar, B.V.; Devi, V.R.; Prasad, G.; Prakash, D.J.; Vithal, M. Preparation and characterization of rare earth orthoborates, LnBO3 (Ln = Tb, La, Pr, Nd, Sm, Eu, Gd, Dy, Y) and LaBO3:Gd, Tb, Eu by metathesis reaction: ESR of LaBO3:Gd and luminescence of LaBO3:Tb, Eu. Mater. Res. Bull. 2011, 46, 1219–1226. [Google Scholar] [CrossRef]
- Xue, B.; Lyu, Q.; Wang, T.; Liu, L.; Wang, S.; Zhu, H.; Liu, S.; Sun, X. Research Progress of Gd3Al5O12 based Luminescent Materials. Chin. J. Lumin. 2020, 41, 1538–1553. [Google Scholar] [CrossRef]
- Liu, G.; Wang, B.; Li, J.; Cao, B.; Lu, Y.; Liu, Z. Research progress of gadolinium aluminum garnet based optical materials. Physica B 2021, 603, 412775. [Google Scholar] [CrossRef]
- Speight, J.G. LANGE’s Handbook of Chemistry, 16th ed.; CD&W Inc.: Laramie, WY, USA, 2008. [Google Scholar]
- Nagpure, I.M.; Pitale, S.S.; Coetsee, E.; Ntwaeaborwa, O.M.; Terblans, J.J.; Swart, H.C. Low voltage electron induced cathodoluminescence degradation and surface characterization of Sr3(PO4)2:Tb phosphor. Appl. Surf. Sci. 2011, 257, 10147–10155. [Google Scholar] [CrossRef]
- Kumar, V.; Luo, Z.P. A Review on X-ray Excited Emission Decay Dynamics in Inorganic Scintillator Materials. Photonics 2021, 8, 71. [Google Scholar] [CrossRef]
- Blasse, G.; Bril, A. Investigation of Some Ce3+-Activated Phosphors. J. Chem. Phys. 1967, 47, 5139–5145. [Google Scholar] [CrossRef]
- Kučera, M.; Nikl, M.; Hanuš, M.; Onderišinová, Z. Gd3+ to Ce3+ energy transfer in multi-component GdLuAG and GdYAG garnet scintillators. Phys. Status Solidi (RRL) 2013, 7, 571–574. [Google Scholar] [CrossRef]
- Dorenbos, P. The 4fn↔4fn-15d transitions of the trivalent lanthanides in halogenides and chalcogenides. J. Lumin. 2000, 91, 91–106. [Google Scholar] [CrossRef]
- Peng, G.H.; Zou, Z.G.; Li, J.Q.; Liao, J.S.; Wen, H.R. (Gd1-xTbx)3(Al1-yGay)5O12 green phosphors with high quantum yield and low thermal quenching via modulation the Ga3+ admixture. J. Lumin. 2021, 236, 118066. [Google Scholar] [CrossRef]
- Ueda, J.; Tanabe, S.; Nakanishi, T. Analysis of Ce3+ luminescence quenching in solid solutions between Y3Al5O12 and Y3Ga5O12 by temperature dependence of photoconductivity measurement. J. Appl. Phys. 2011, 110, 053102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorenbos, P. 5d-level energies of Ce3+ and the crystalline environment. IV. Aluminates and “simple” oxides. J. Lumin. 2002, 99, 283–299. [Google Scholar] [CrossRef]
- Wong, K.-L.; Bünzli, J.-C.G.; Tanner, P.A. Quantum yield and brightness. J. Lumin. 2020, 224, 117256. [Google Scholar] [CrossRef]
- Crosswhite, H.M.; Judd, B.R. The Spectra of Doubly and Triply Ionized Rare Earths. J. Opt. Soc. Am. 1961, 51, 820–827. [Google Scholar]
- Chen, X.P.; Hu, Z.W.; Dai, J.W.; Chen, H.H.; Shi, Y.; Kou, H.M.; Wang, T.T.; Vedda, A.; Beitlerova, A.; Kucerkova, R.; et al. The influence of air annealing on the microstructure and scintillation properties of Ce,Mg:LuAG ceramics. J. Am. Ceram. Soc. 2019, 102, 1805–1813. [Google Scholar] [CrossRef]


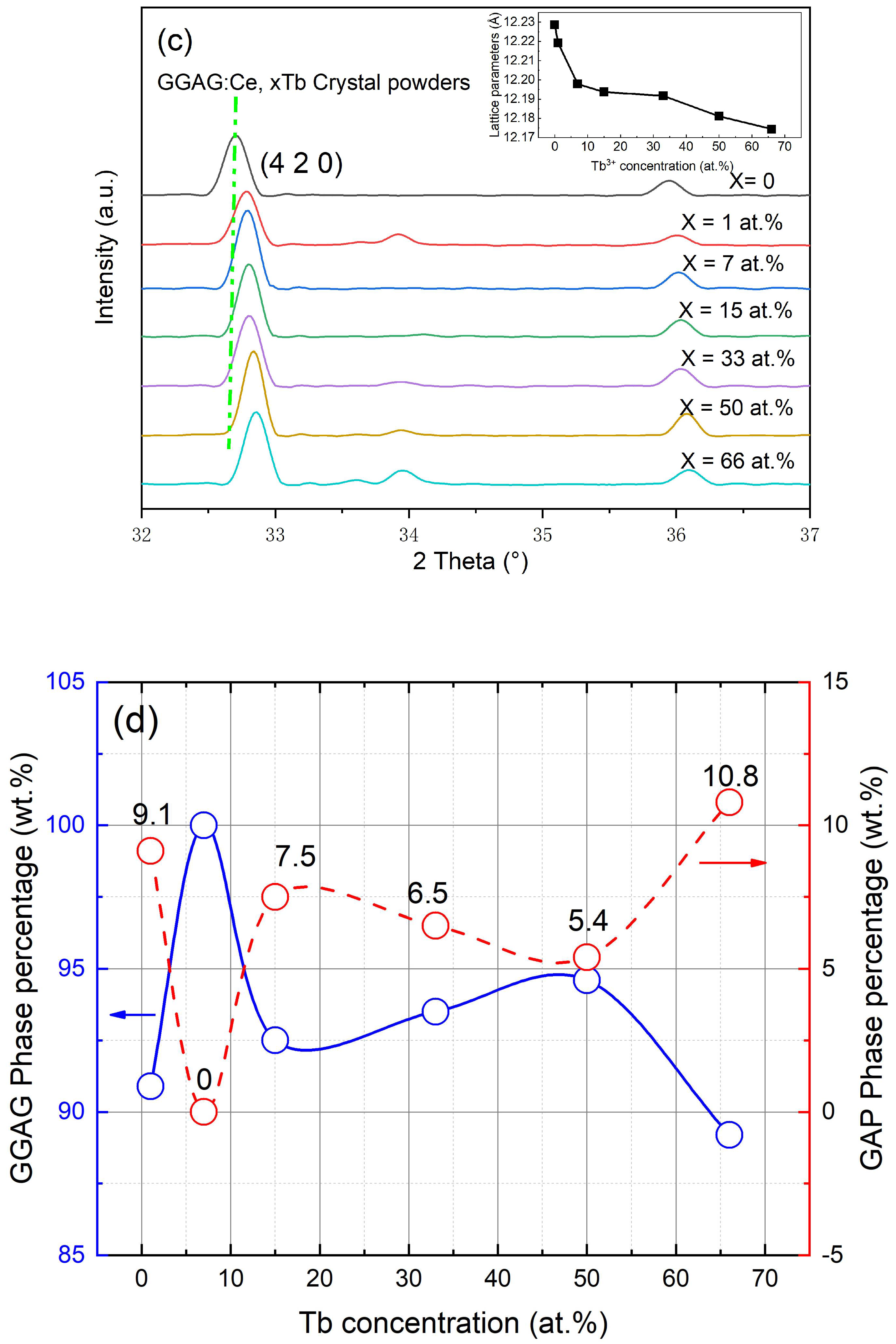
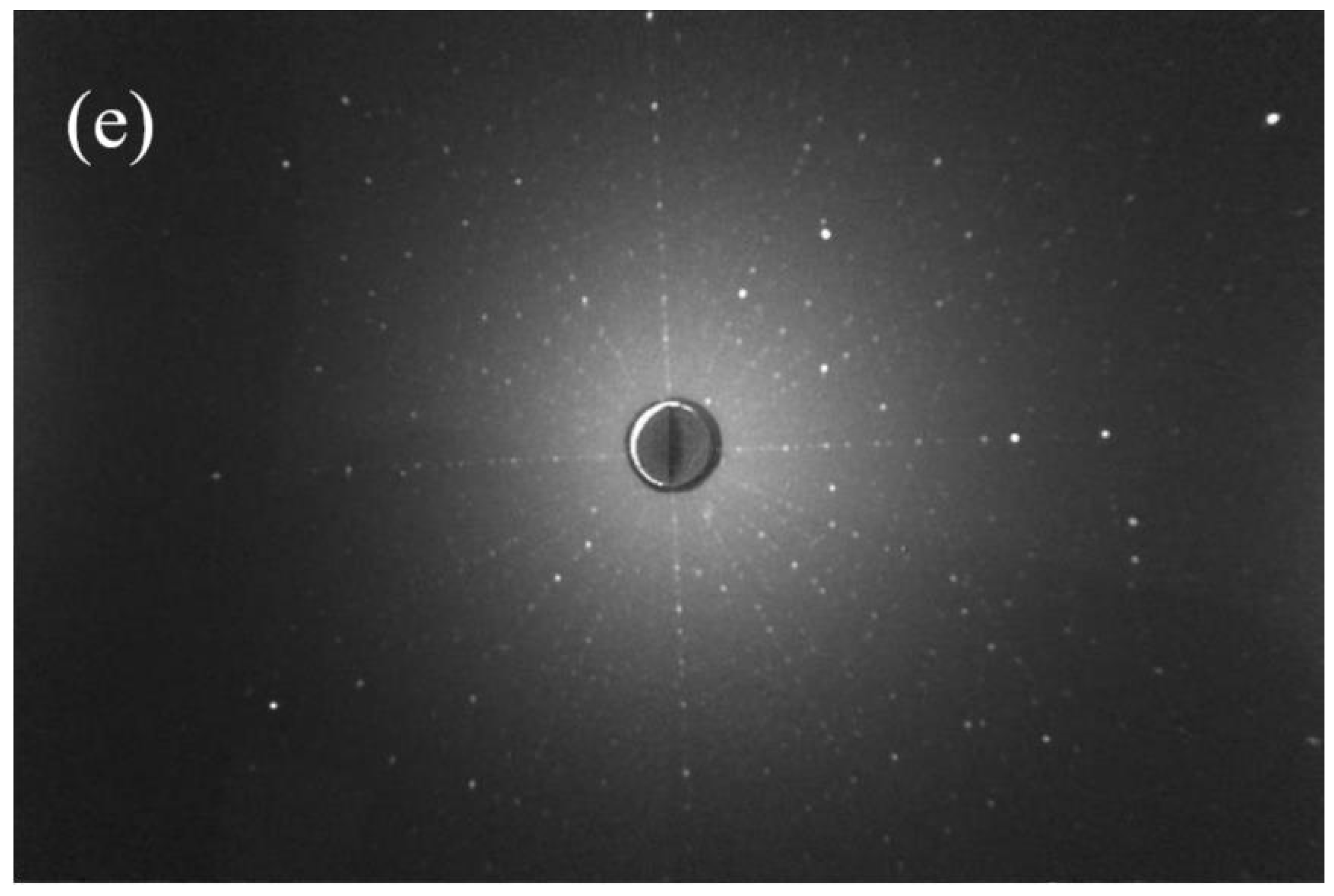
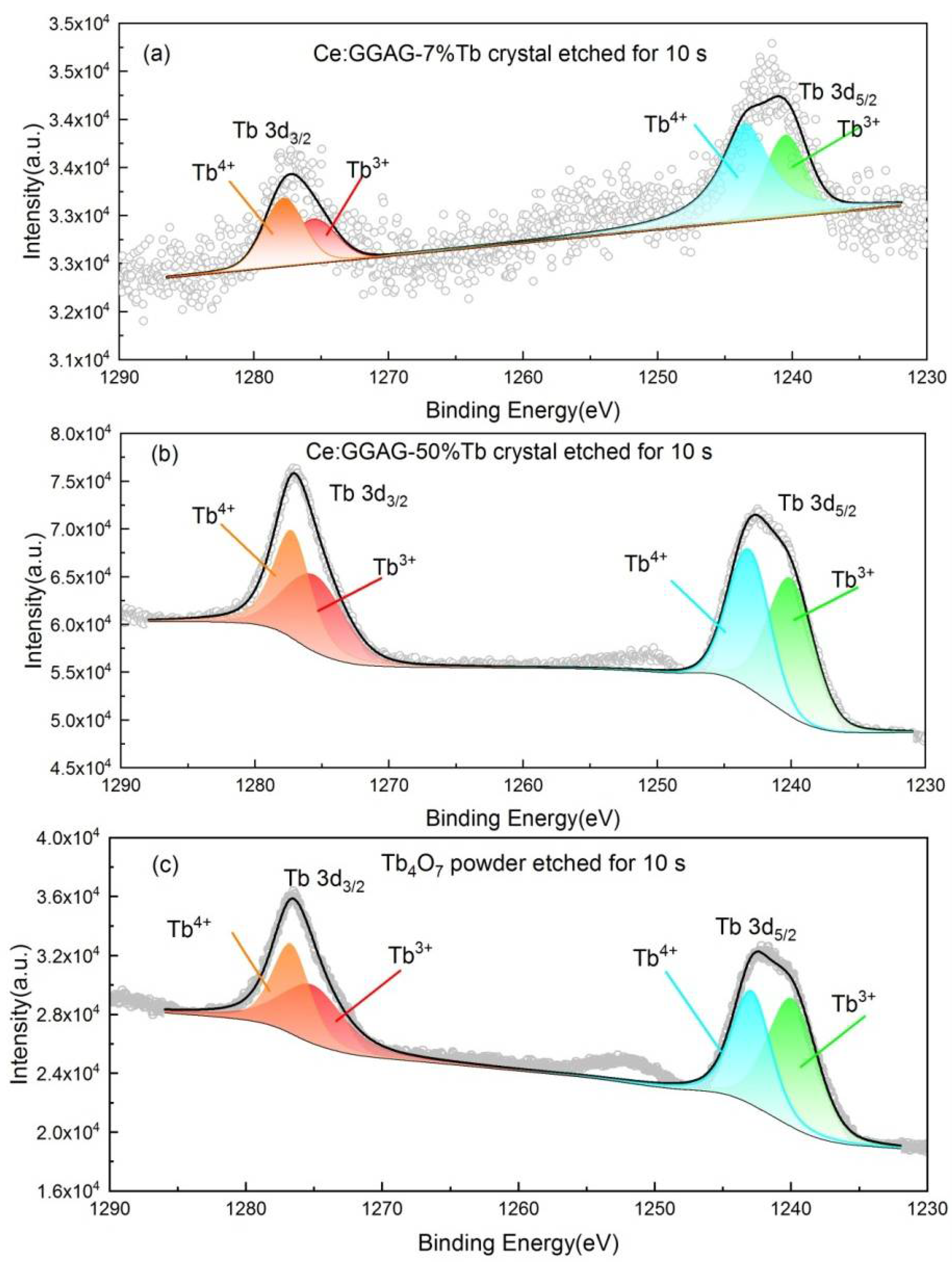
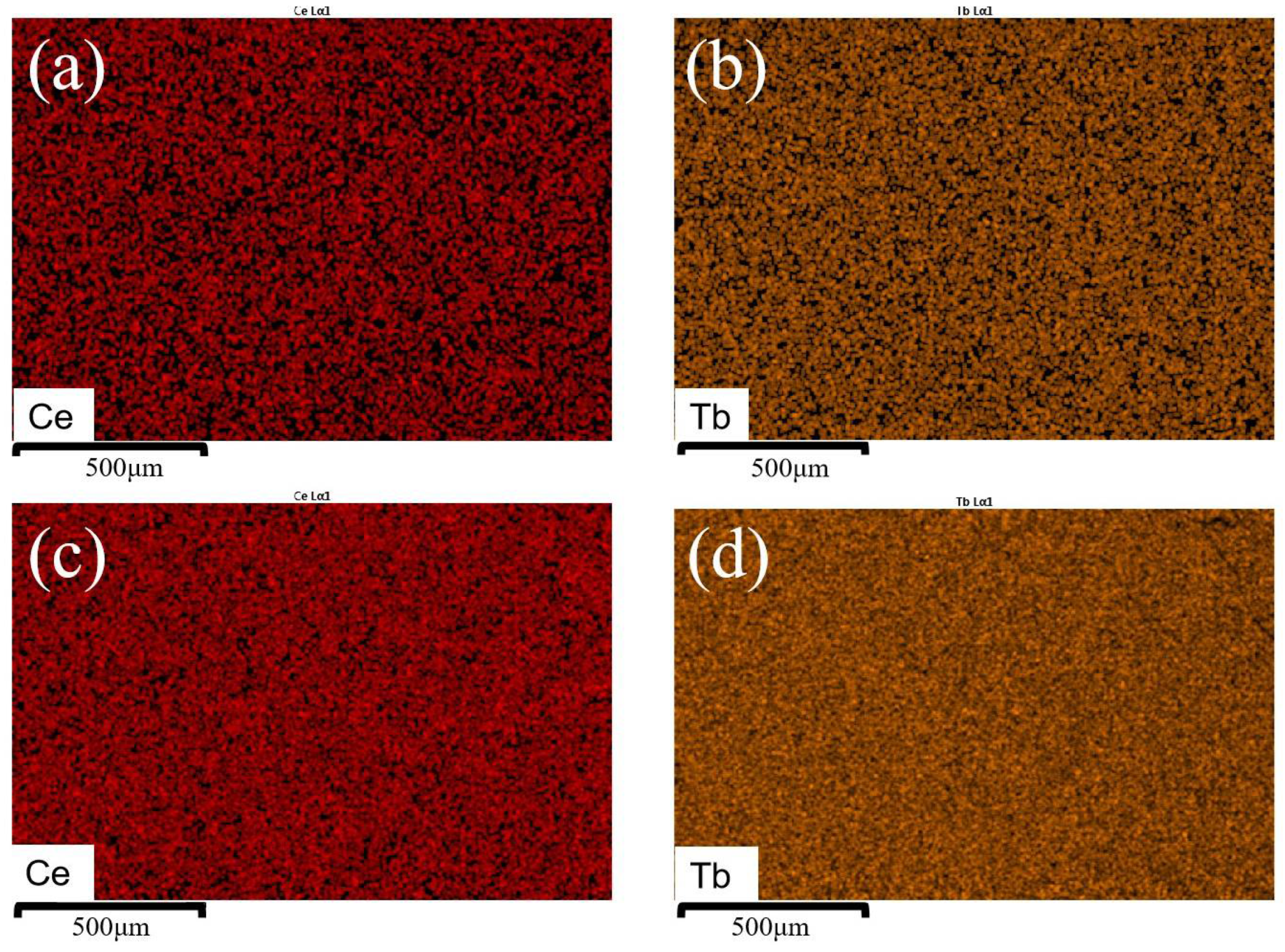




| Sample | Binding Energy (eV) | Radio | |||
|---|---|---|---|---|---|
| Tb 3d3/2 | Tb 3d5/2 | Tb3+/ (Tb3+ + Tb4+) | |||
| Tb4+ | Tb3+ | Tb4+ | Tb3+ | ||
| GGAG:Ce, 7% Tb | 1277.8 | 1275.6 | 1243.5 | 1240.5 | |
| Integral area | 2782 | 1968 | 6397 | 3205 | 36% |
| GGAG:Ce, 50% Tb | 1277.3 | 1275.6 | 1243.2 | 1240.1 | |
| Integral area | 47,177 | 46,831 | 57,342 | 66,188 | 52% |
| Tb4O7 powder | 1276.7 | 1275.1 | 1242.9 | 1239.9 | |
| Integral area | 24,442 | 26,652 | 31,212 | 41,573 | 55% |
| Nominal Chemical Composition of GGAG:Ce, xTb Crystals | Experimental Mol Value of Different Elements in Per Mol Compositions by SEM-EDS | ||||
|---|---|---|---|---|---|
| Ce | Tb | Gd | Ga | Al | |
| Ce0.03Tb0.03Gd2.94Ga2Al3O12 | 0.02 | 0.04 | 2.95 | 1.68 | 3.32 |
| Ce0.03Tb0.21Gd2.76Ga2Al3O12 | 0.02 | 0.20 | 2.71 | 1.88 | 3.18 |
| Ce0.03Tb0.45Gd2.52Ga2Al3O12 | 0.02 | 0.45 | 2.48 | 1.82 | 3.24 |
| Ce0.03Tb0.99Gd1.98Ga2Al3O12 | 0.02 | 0.96 | 1.94 | 1.84 | 3.24 |
| Ce0.03Tb1.485Gd1.485Ga2Al3O12 | 0.02 | 1.43 | 1.49 | 1.78 | 3.28 |
| Ce0.03Tb1.98Gd0.99Ga2A3O12 | 0.02 | 1.97 | 0.98 | 1.70 | 3.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Wang, L.; Shi, Y.; Huang, X.; Zhang, Q.; Xiong, Y.; Wang, H.; Fang, J.; Ni, J.; He, H.; et al. Wide Concentration Range of Tb3+ Doping Influence on Scintillation Properties of (Ce, Tb, Gd)3Ga2Al3O12 Crystals Grown by the Optical Floating Zone Method. Materials 2022, 15, 2044. https://doi.org/10.3390/ma15062044
Wu T, Wang L, Shi Y, Huang X, Zhang Q, Xiong Y, Wang H, Fang J, Ni J, He H, et al. Wide Concentration Range of Tb3+ Doping Influence on Scintillation Properties of (Ce, Tb, Gd)3Ga2Al3O12 Crystals Grown by the Optical Floating Zone Method. Materials. 2022; 15(6):2044. https://doi.org/10.3390/ma15062044
Chicago/Turabian StyleWu, Tong, Ling Wang, Yun Shi, Xintang Huang, Qian Zhang, Yifei Xiong, Hui Wang, Jinghong Fang, Jinqi Ni, Huan He, and et al. 2022. "Wide Concentration Range of Tb3+ Doping Influence on Scintillation Properties of (Ce, Tb, Gd)3Ga2Al3O12 Crystals Grown by the Optical Floating Zone Method" Materials 15, no. 6: 2044. https://doi.org/10.3390/ma15062044
APA StyleWu, T., Wang, L., Shi, Y., Huang, X., Zhang, Q., Xiong, Y., Wang, H., Fang, J., Ni, J., He, H., Wang, C., Zhou, Z., Liu, Q., Li, Q., Yu, J., Shichalin, O., & Papynov, E. (2022). Wide Concentration Range of Tb3+ Doping Influence on Scintillation Properties of (Ce, Tb, Gd)3Ga2Al3O12 Crystals Grown by the Optical Floating Zone Method. Materials, 15(6), 2044. https://doi.org/10.3390/ma15062044









