1. Introduction
Proper waste management should be directed on priority circular economy. The main principle of circular economy is to maintain the value of raw materials, ready-made goods, and finished products if possible while minimising waste. The generated waste, however, should be reused, i.e., recycled. In 2020, 123 million tons of waste was generated, including 109.47 thousand tons from various economic activity branches; as for the construction industry, 7.3 million tons was generated in 2020. It is an increase of 60% compared to 2019 [
1].
Mineral wool is a popular construction material, so it often functions in waste management obtained at the construction site. The producer is responsible for the entire waste management process (usually the contractor on the construction site) and becomes the owner of the waste at the time of its production. In addition, he is responsible for the costs associated with managing and storing the waste [
2].
According to the applicable regulations, construction waste management should be based on the waste management hierarchy. At the very beginning, the institution causing waste production should prevent its generation at every production stage (in the case of mineral wool, it is a factory producing it for the construction of buildings). In the next step, the waste owner should be prepared to reuse it, applying the principle of proximity (process them at the point of origin). In practice, many waste materials are reused at the construction site to save construction costs. However, it should be noted that the recycled material to be reused for construction purposes needs to undergo control tests determining its mechanical and physical properties. Ultimately, it all comes down to recycling, which is currently crucial to waste management. Unfortunately, wool as waste is generally put into storage. For the environment, it is the least favourable solution. It occupies space in landfills, thus leaving less and less room for people and all other creatures inhabiting the Earth.
As part of the search for a replacement, fewer emission binders for concrete production developed geopolymers in construction. However, the practical application of these materials is still minimal. The production of every ton of cement puts one tonne of CO
2 into the atmosphere [
1,
3,
4]. According to various estimates, the synthesis of geopolymers absorbs 2–3 times less energy than Portland cement and causes liberation of 4–8 times less CO
2 [
1,
5,
6,
7].
The first geopolymer applications can be dated to the beginning of the 1970s. At that time, fire-resistant chipboards were developed with a wooden core covered with two geopolymer coatings. In 1978–1980, a metakaolin-based liquid geopolymer binder containing a soluble alkali silicate was developed at the Cordis-Laboratory. It was the first mineral resin produced [
8,
9]. Rocla has developed a technology for producing sewage pipes from steel-reinforced geopolymers [
9]. They have successfully produced pipes with diameters ranging from 37.5 cm to 1.8 mm and adapted the conventional concrete prestressing technology to make railway sleepers from GPC prestressed geopolymer concrete. Geopolymer sleepers have been alternated with conventional sleepers on the main track line since 2002, and no problems were identified [
10]. Currently, low-profile sleepers made of GPC are being developed as an alternative to wooden sleepers. The Czech Republic has developed a new inorganic binder system for self-hardening masses based on a geopolymer binder, a viscous liquid with a low degree of polymerisation used to prepare self-hardening masses. As a result of the hardener, polymerisation increases and a polymer with a high binding capacity is formed. These materials enable foundries to produce cores from self-hardening materials ecologically and economically [
11]. Attempts have also been made to use geopolymer materials in drilling, mining, and hydro-technical construction for strengthening and sealing [
12,
13]. Alkaline activated geopolymer concrete has been commercialised in Australia under E-Crete (TM) name and is very popular among customers. E-Crete (TM) concrete reduced greenhouse gas emissions in concrete production by nearly 80% compared to concretes made of Portland cement [
1]. Until now, many different types of surfaces have been made of geopolymers, such as pavements, playgrounds slabs, building foundations, and many others. Rocla was the first company globally to launch geopolymer concrete products on a commercial scale [
3]. In Australia, at the University of Global Change Queensland (GCI), the world’s first public utility building was designed and constructed in 2013; the structural elements made of geopolymer concrete were used in building constructions.
Geopolymer mixtures have good fluidity, which allows for an accurate representation of the form and the possibility of obtaining surfaces with various degrees of smoothness [
3,
4,
12,
13,
14,
15,
16,
17,
18,
19,
20]. The concrete produced on the base of the geopolymer binder does not undergo the phenomenon of shrinkage cracks [
12,
18,
19]. Geopolymer concrete is characterised by high compressive strength, minimal shrinkage and negligible creep, better adhesion and high resistance to acid and sulphate corrosion [
1,
3,
4,
5,
6,
11,
12,
13,
16,
17,
18,
19,
21,
22,
23,
24,
25,
26,
27,
28,
29]. Some researchers also found that this concrete is also resistant to carbonate corrosion and possesses a very high fire resistance [
15] and a high resistance to UV radiation [
30]. Therefore, it is possible to the printing of geopolymers [
22]. Geopolymers have excellent pouring properties and perfectly match even complex forms. In addition to the applications mentioned above, they are also used to produce various types of small-sized products, such as decorative elements. They have also found their use as injections strengthening the soil. In addition, geopolymer concrete is used to immobilise hazardous waste, including radioactive waste. The availability of several geopolymer-based products will increase rapidly, mainly due to the properties of this material and the fact that an increasing number of enterprises and part of the society are beginning to become convinced of this type of product.
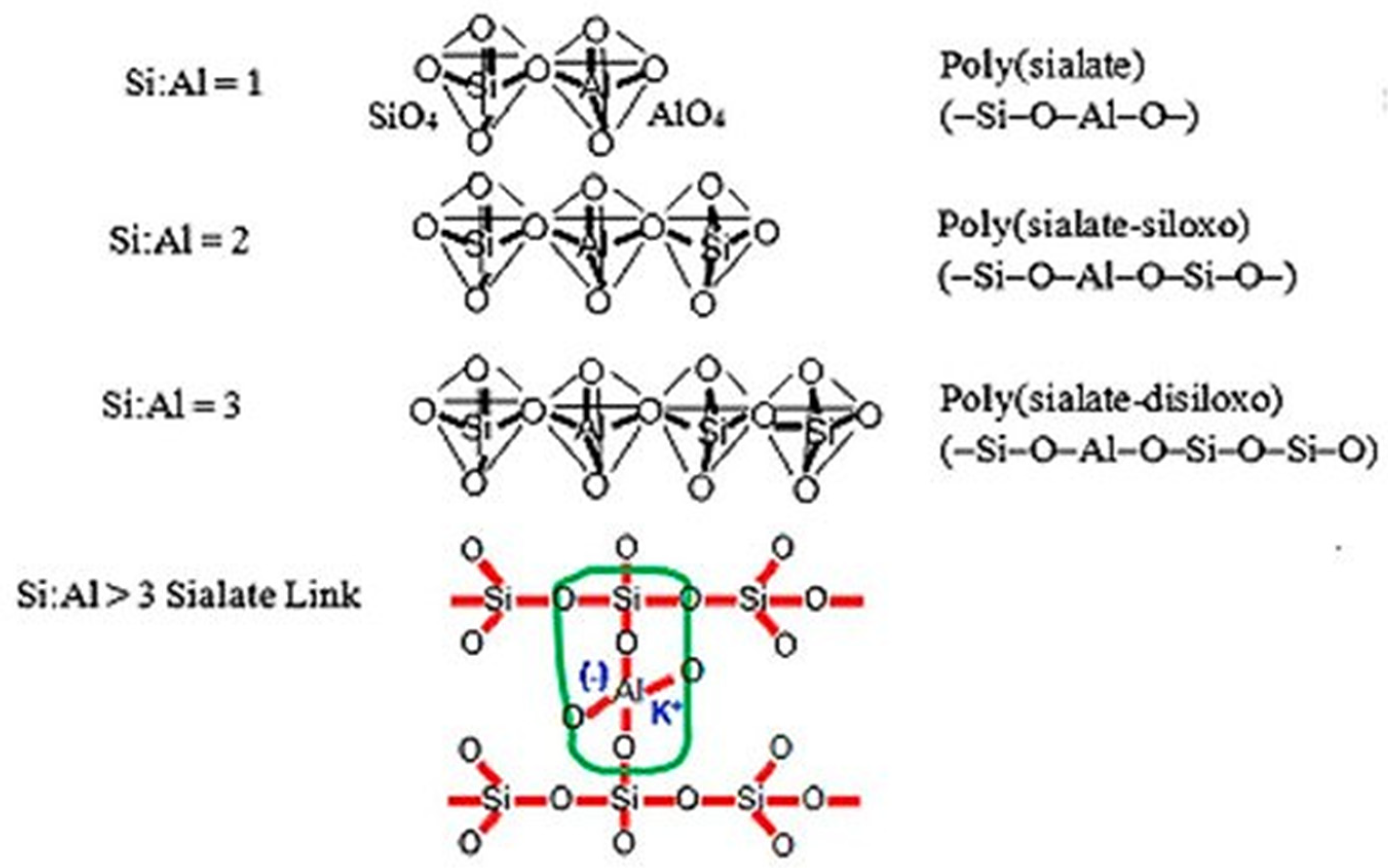
Geopolymers are inorganic polymers formed by combining aluminosilicate with an activator and water. A geopolymer recipe could be based on a variety of aluminosilicate materials. The geopolymer microstructure considers an aluminosilicate polymer, synthetically produced using silicon (Si) and aluminium (Al), geologically acquired from minerals. The chemical composition of the geopolymer is similar to the chemical composition of zeolite, but the structure of the geopolymer is different [
27]. The process of geopolymerisation is a chemical reaction between an alkali solution and a source material containing aluminosilicate (FA). It gives a three-dimensional polymeric chain and ring structure consisting of Si–O–Al–O bonds, as reported by
Scheme 1 [
30,
31]:
- -
PSDS Si-O-Al-O-Si-O-Si-O—poly(sialate-disiloxo),
- -
PSS Si-O-Al-O-Si-O—poly(sialate-siloxo),
- -
PS Si-O-Al-O—polysialate.
Scheme 1.
Structure of polysialates [
29].
Scheme 1.
Structure of polysialates [
29].
The reactions can occur at room temperature. Therefore, it can be considered energy and source efficient and much cleaner. Two main categories of the division of geopolymers are accepted. The first one considers the elementary units of polymeric chains [
5,
28,
29,
32].
The second classification of geopolymers concerns their origin and, more specifically, the type of pozzolanic aluminosilicate material. The oxides of silicon and aluminium constitute the bases of the geopolymer composition, and the additives of metal cations such as sodium or potassium constitute the stabilising material here.
Table 1 presents the chemical composition of the three most popular and most readily available materials from which geopolymeric cement can be produced. As it is evident, the contents of individual oxides are similar. All three are built on the base of silicon and aluminium oxides.
Due to this criterion, we distinguish geopolymers formed on the base of the following: fly ash, metakaolin, various types of rocks, volcanic agglomerates, silicas, fossil materials. Geopolymers are also from rock materials such as phosphorites [
34] or pozzolanic volcanic ashes [
35,
36]. An important step forward was the use of geopolymer technology in waste management. Establishing the geopolymer recipe on industrial waste brings many environmental benefits and reduces costs related to waste management [
3,
4,
12,
13,
14,
17,
18,
19,
20]. In producing geopolymers, such wastes as fly ash, diabase scrubbers [
37] or bio-carbon resulting from biomass [
38] are used.
From a chemical and mineralogical point of view, mineral rock wool is an ideal material for geopolymerisation (
Table 2).
However, in the case of glass wool, Al
2O
3 is too low, and it is necessary to add another waste or natural component rich in Al
2O
3. Moreover, the essential factors which affect the process of geopolymerisation are given below [
34,
35]:
Type of raw materials containing aluminosilicate
The surface area of solid raw materials
Glassy phase content in the raw material
Amount of aluminium and reactive silicon
Presence of iron, calcium, and inert particles in FA
Curing temperature and pressure
Duration of curing
Type of curing (conventional heating or microwave heating)
Type and concentration of alkalies
Alkaline liquid-to-raw material ratio
H2O to Na2O molar ratio
Water to Geopolymer solids ratio
Na2O to SiO2 ratio
SiO2 to Al2O3 ratio
The properties of geopolymers depend on the type of base material, the type and amount of activator used and their production technology, i.e., mixing time, material fragmentation, hardening temperature, humidity, hardening time and the amount of added water [
3,
4,
9,
12,
13,
14,
17,
18,
19,
20]. In addition, types of used alkaline activators, such as NaOH solution, KOH solution, a mixture of NaOH solution-water glass, and a mixture of KOH solution-water glass, are also essential to achieve a high compressive strength the geopolymers [
15].
Moreover, there is the possibility of adjusting the porosity (it is possible to attain low porosity below 2.5% or high porosity above 20%, depending on needs, up to foaming geopolymers (as insulating materials) [
33,
37]. If the geopolymer is foamed in the production process, it might become lightweight and partly insulating [
6,
36]. On the other hand, in an almost pore-free form, the geopolymer may be used to protect walls against moisture and fungi formation [
37,
38,
39].
Geopolymers are becoming essential in waste neutralisation technologies, especially in the neutralisation of hazardous waste [
3,
5,
29,
40]. Similarly, as material approved for use in construction, geopolymers should meet environmental regulations regarding the environment and human health safety.
The research involved geopolymer prepared from mineral rock and glass wool. The authors have already attempted to create a geopolymer from the mineral wool of the publication [
6,
7]. Depending on the production method, wool geopolymers may have different properties. For example, due to the high degree of grinding, the wools might have high strength as of 50 MPa [
6] or, due to the addition of other components, they may have a porous structure which causes the geopolymer to develop different properties [
6]. The rock wool has an adequate proportion of Si and Al to geopolymerisation, but glass wool has got to a low amount of Al. Therefore, the glass wool needs an additional component rich in Al
2O
3. As a source of Al, the bauxite was used. So far, glass wool with the mentioned additives has not been tested.
The leachability of wool and wool-based geopolymers was checked. Such research has not been conducted so far. Moreover, that is also new, and the article compares the vulnerability of mineral wool and geopolymer made of mineral wool to the leaching of soluble components. The geopolymer was washed out in a comminated form (crushed geopolymer pieces <10 mm). The study allowed us to determine the size and type of harmful substances that may negatively affect soil, groundwater, and surface waters.
The second part of the research investigated the mechanical properties of glass or rock wool-based binder and mortar.