Corrosion Behavior of an Mg2Sn Alloy
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
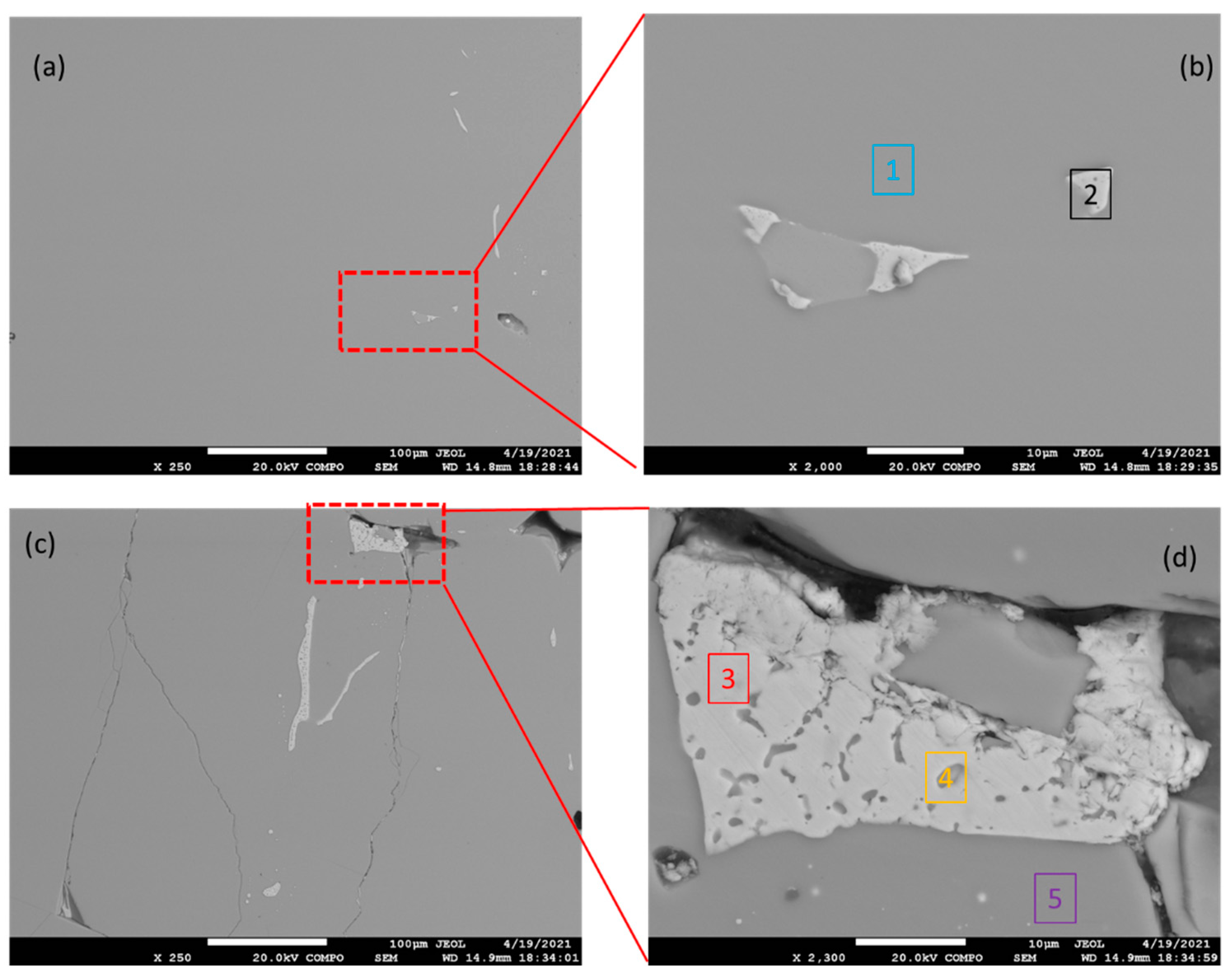
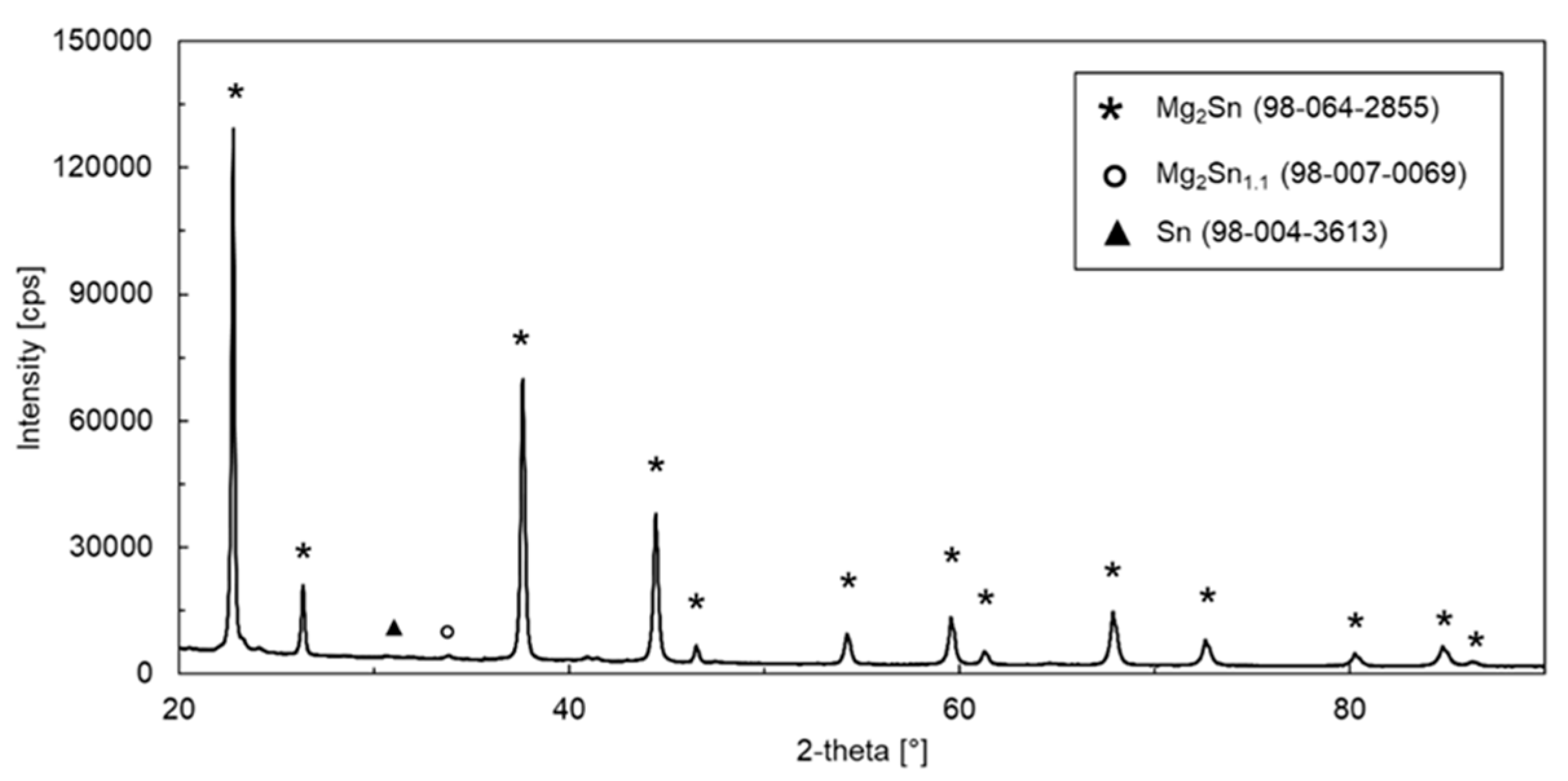
3.1. Microstructure and Phase Constitution of the Alloy
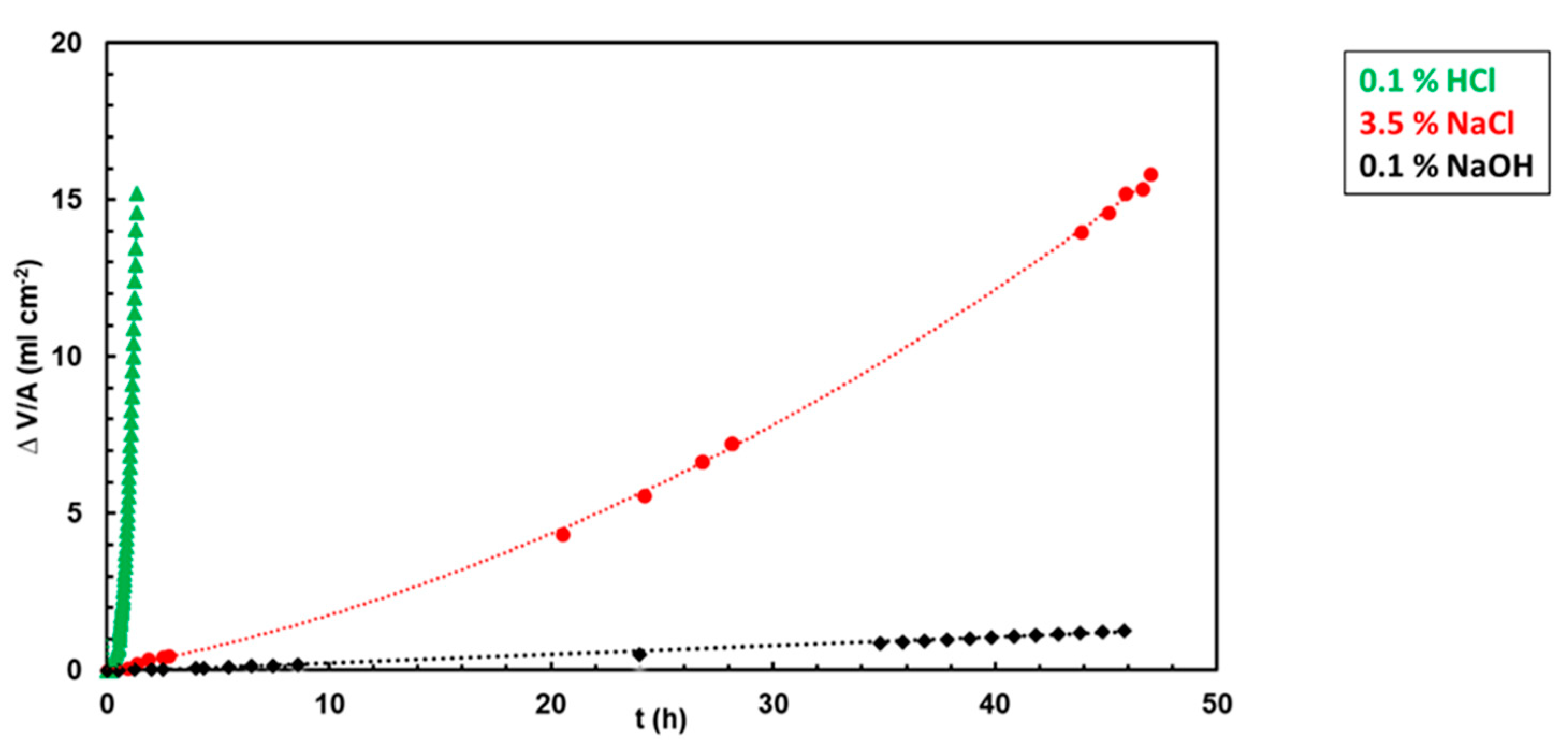
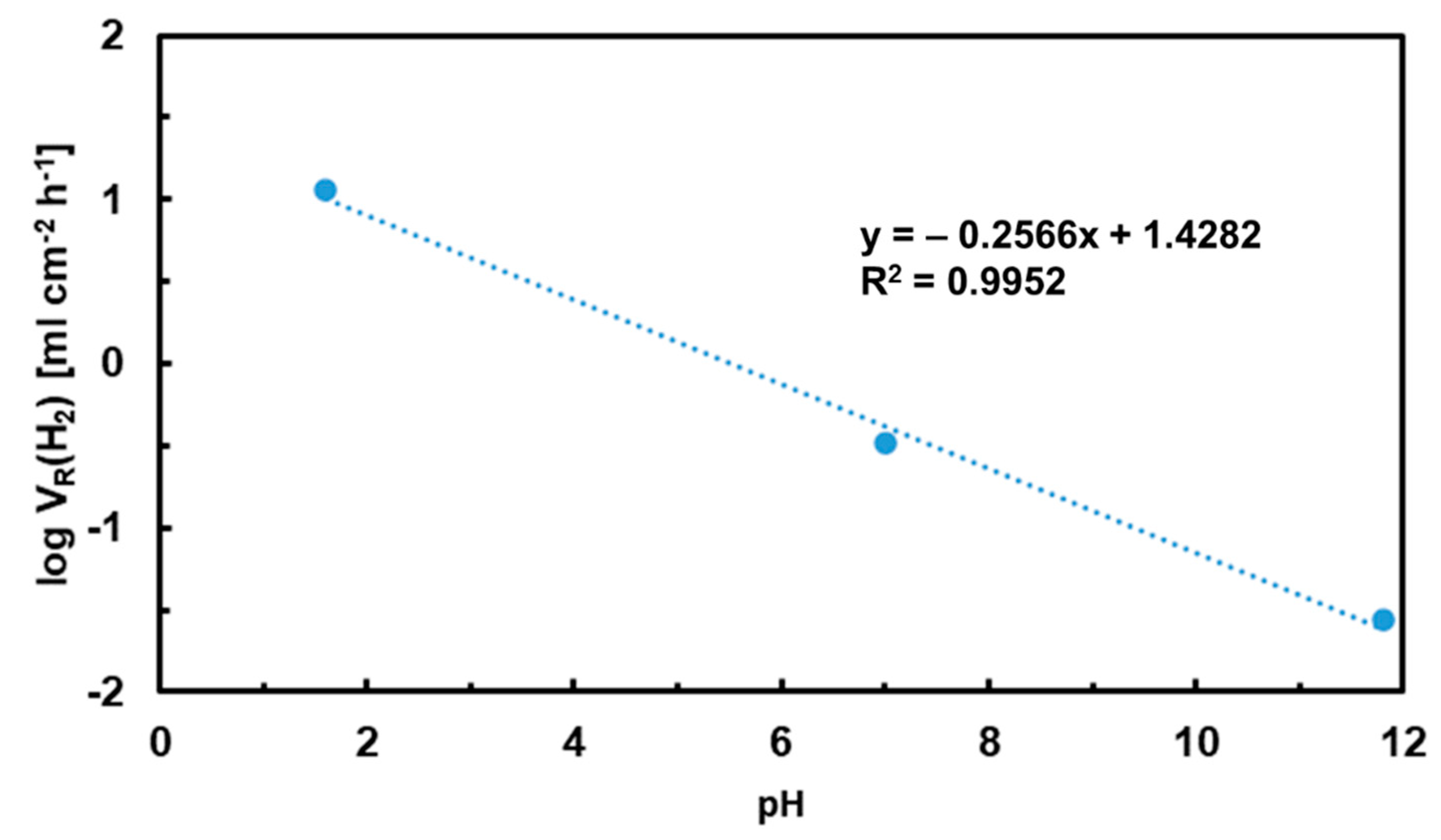
3.2. Hydrogen Evolution Experiments
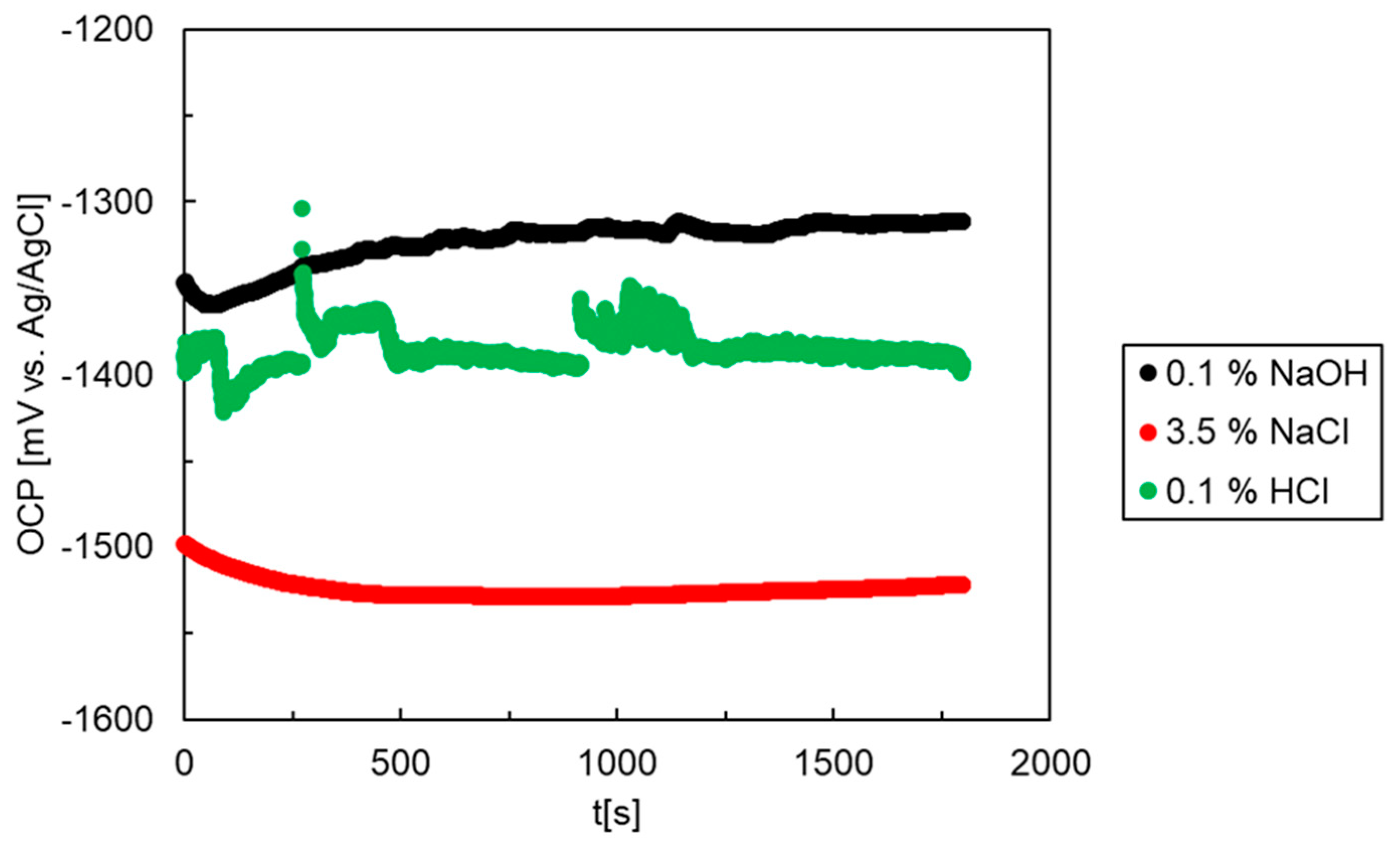
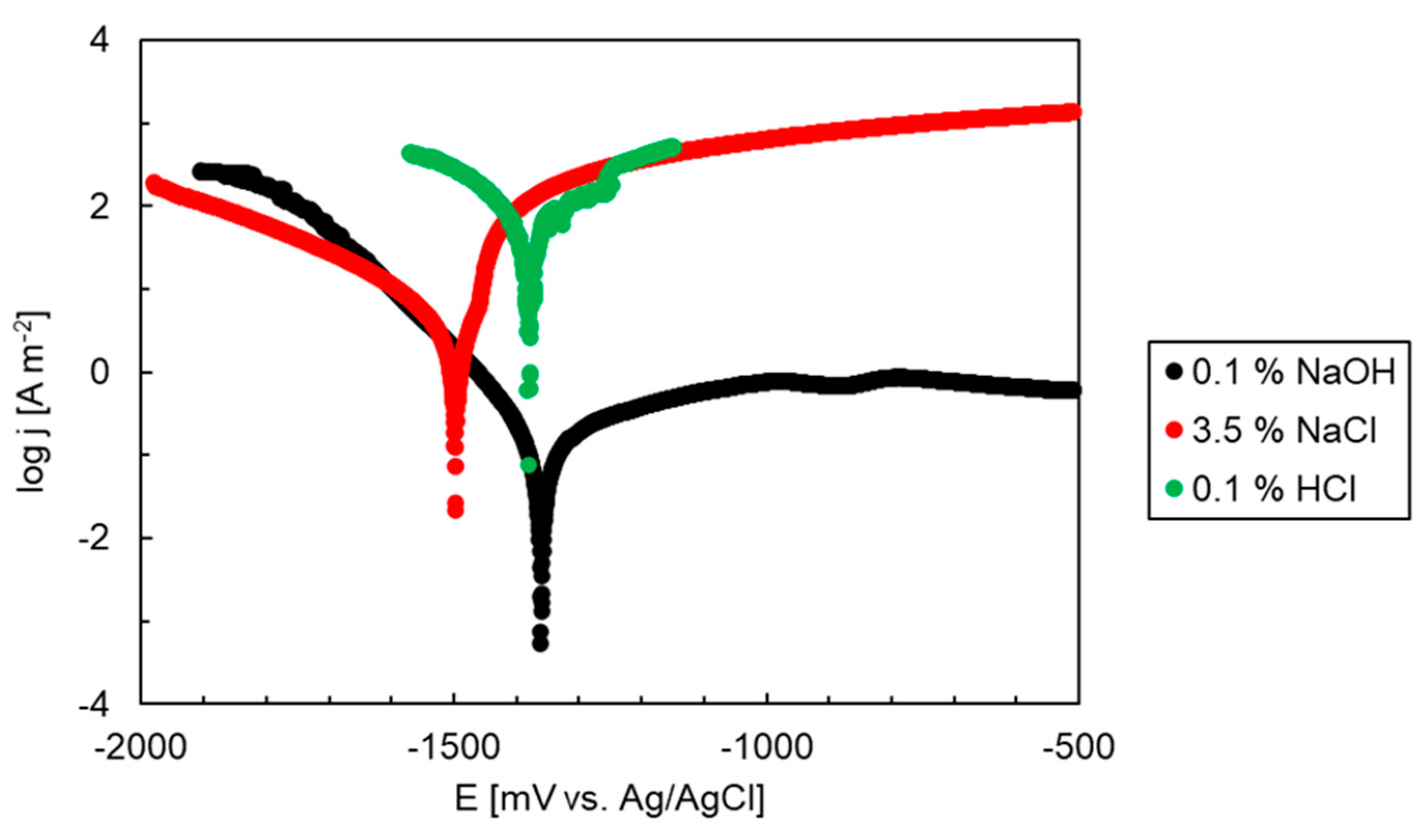
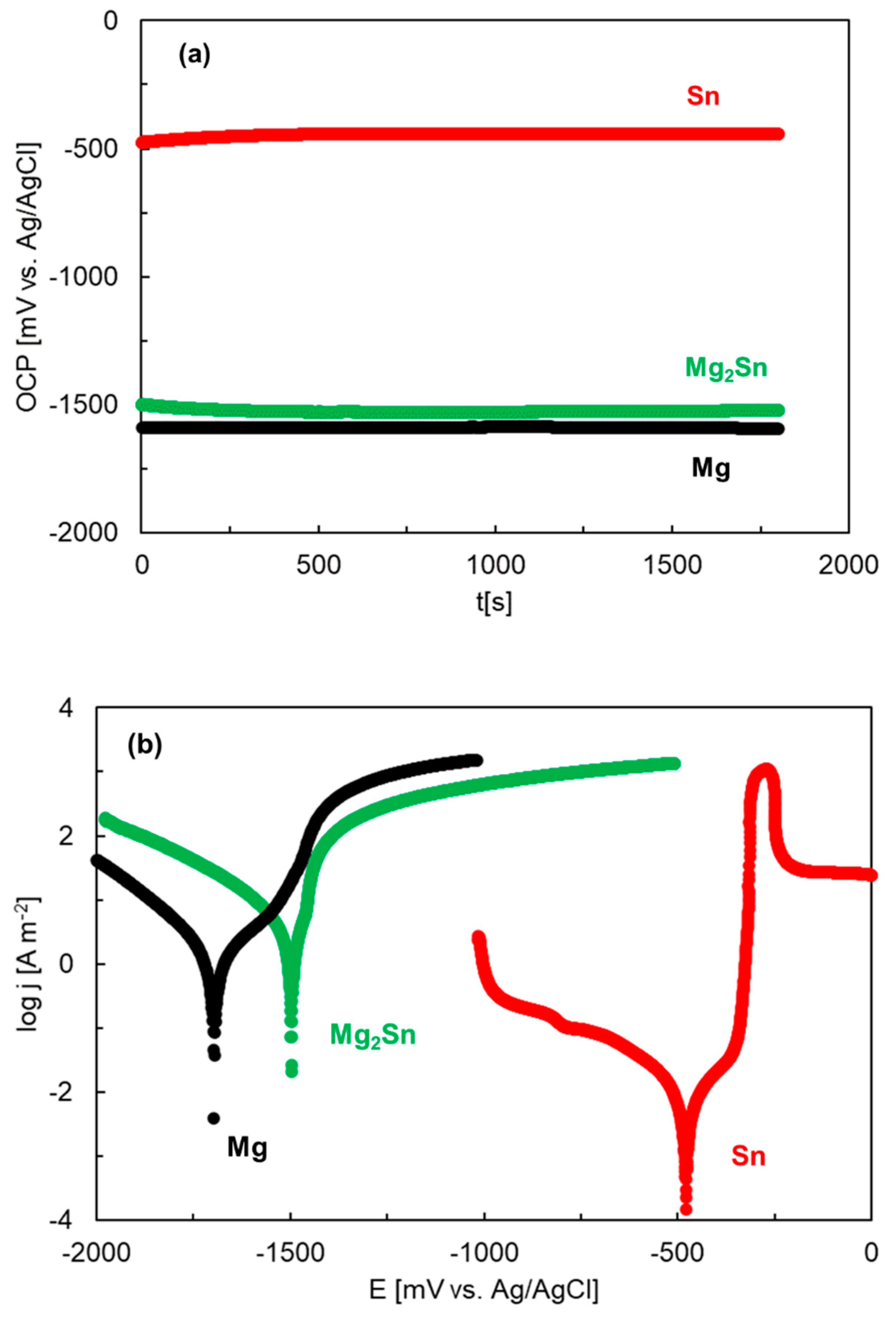
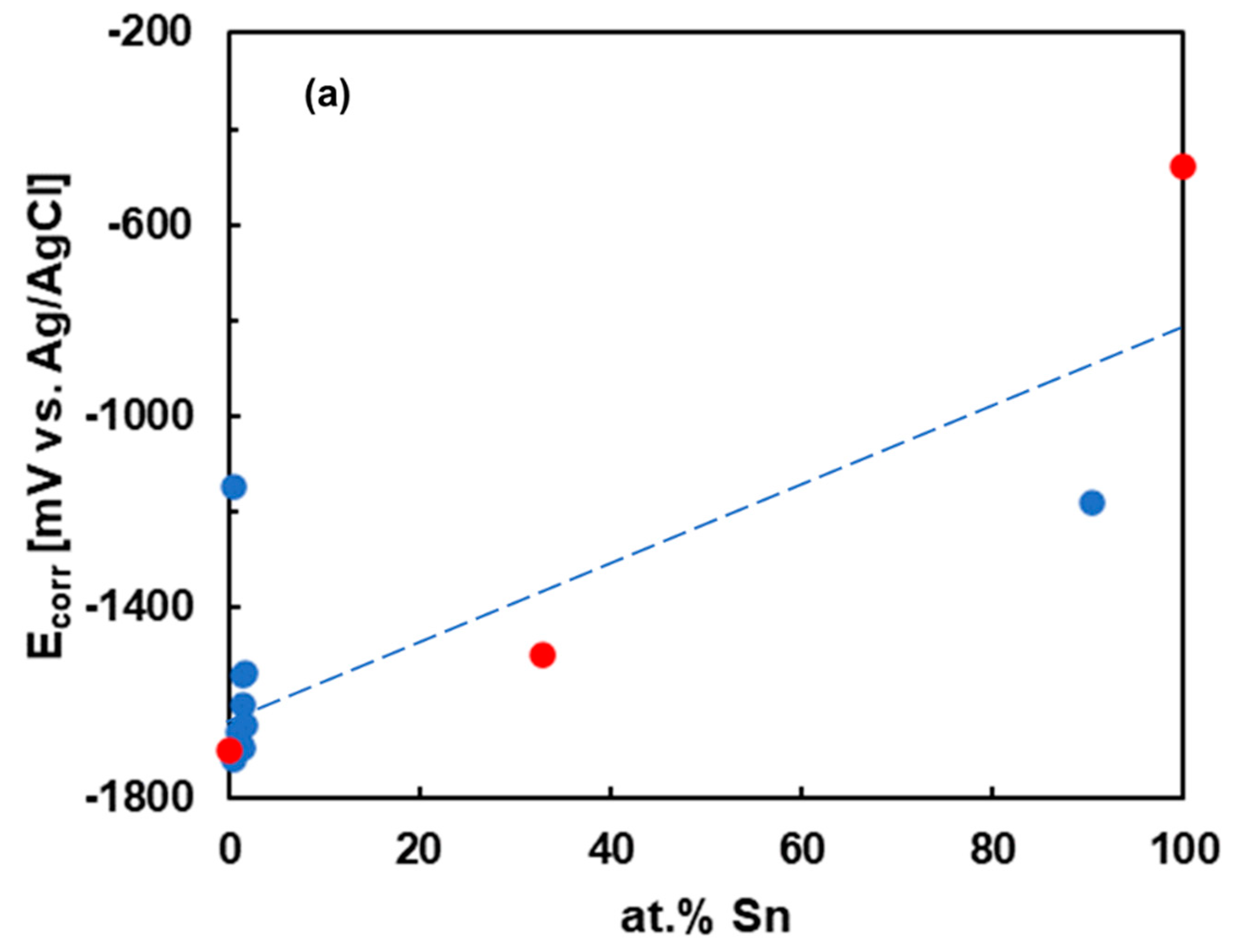
3.3. Electrochemical Corrosion Experiments (OCP, Polarization)
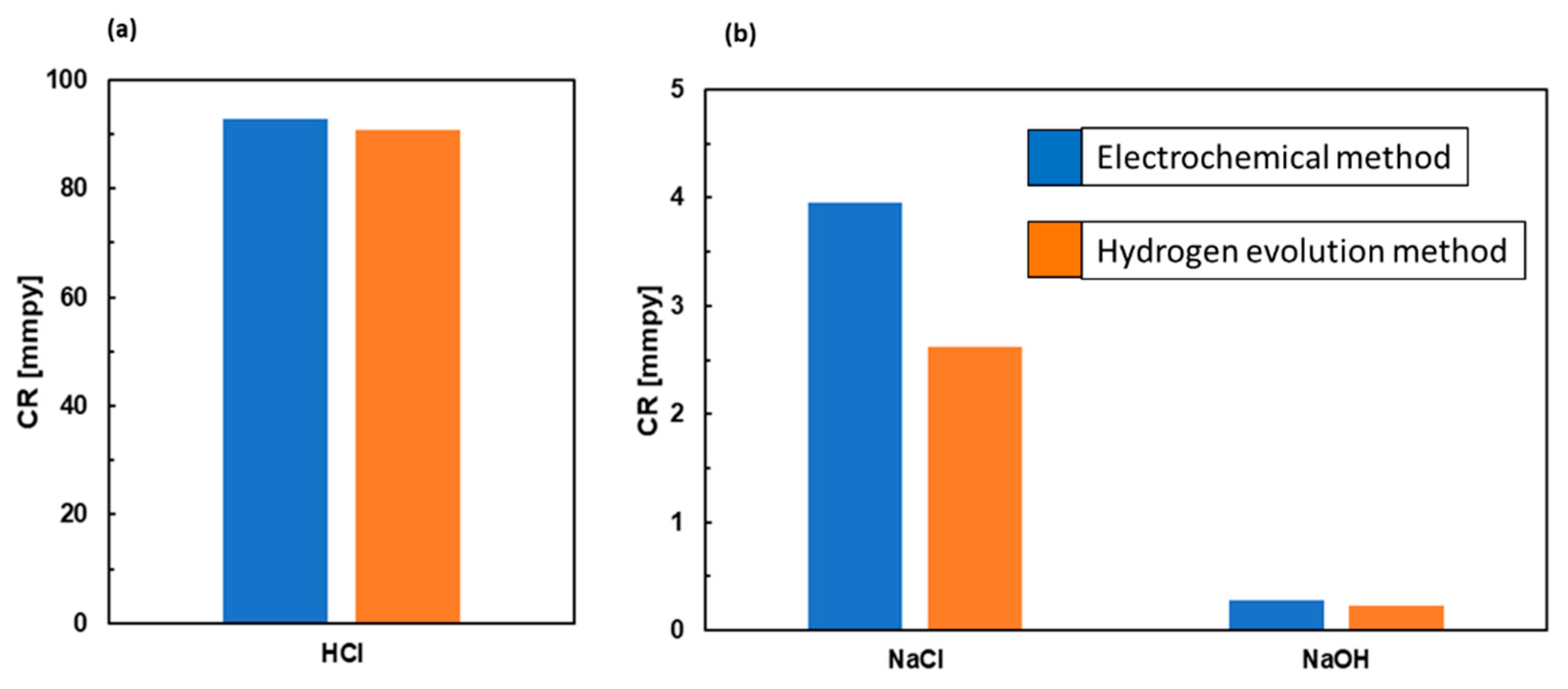
3.4. Calculation of Corrosion Rates
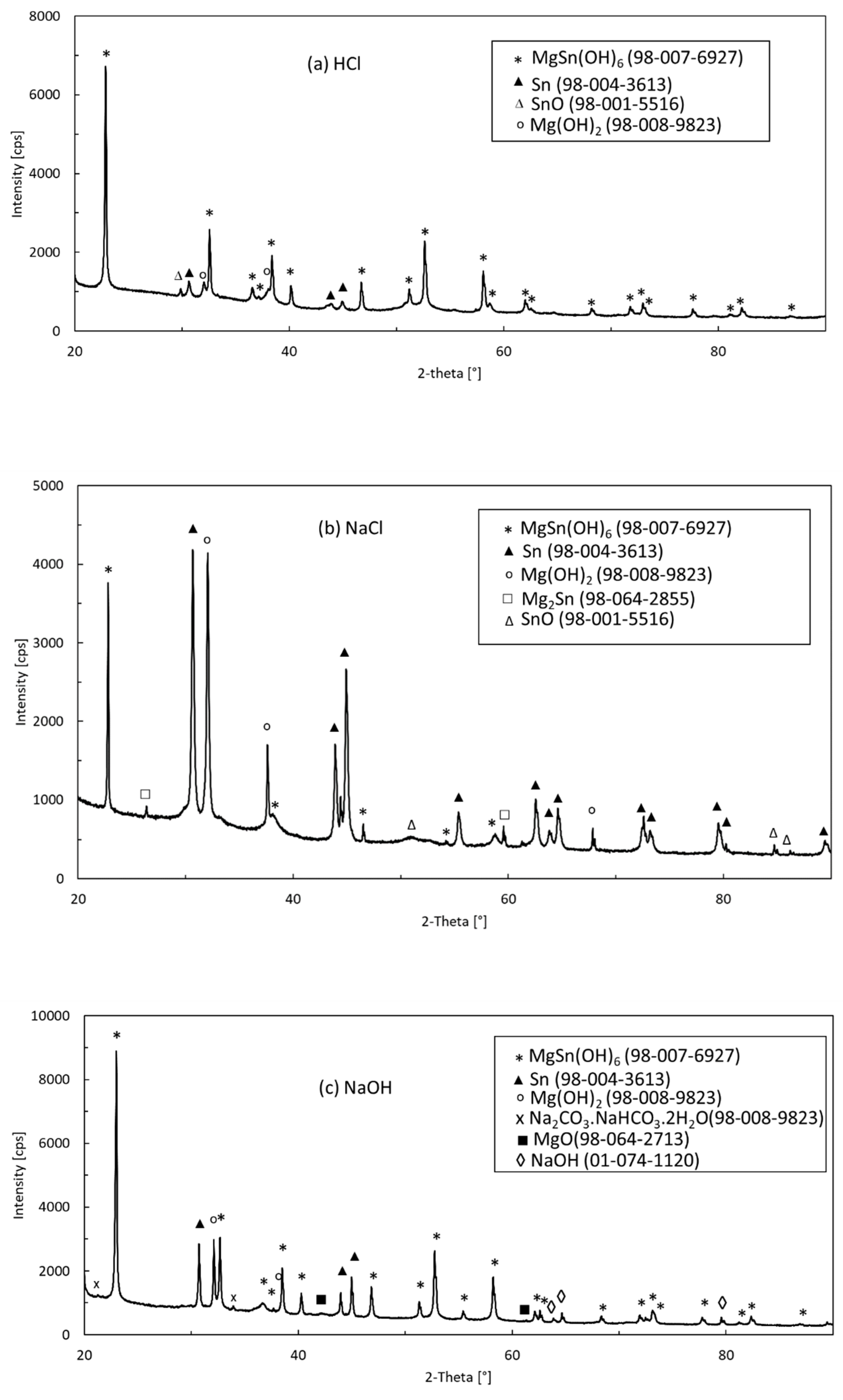
3.5. Microstructure and Phase Constitution of Corrosion Products
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bamberger, M.; Dehm, G. Trends in the development of new Mg alloys. Annu. Rev. Mater. Res. 2008, 38, 505–533. [Google Scholar] [CrossRef]
- Mezbahul-Islam, M.; Mostafa, A.O.; Medraj, M. Essential magnesium alloys binary phase diagrams and their thermochemical data. J. Mater. 2014, 2014, 704283. [Google Scholar] [CrossRef]
- Combronde, J.; Brebec, G. Heterodiffusion de Ag Cd, In, Sn et Sb dans le magnesium. Acta Metall. 1972, 20, 37–44. [Google Scholar] [CrossRef]
- Čermák, J.; Stloukal, I. Diffusion of 65Zn in Mg and in Mg–xAl solid solutions. Phys. Stat. Sol. 2006, 203, 2386–2392. [Google Scholar] [CrossRef]
- Gibson, M.A.; Fang, X.; Bettles, C.J.; Hutchinson, C.R. The effect of precipitate state on the creep resistance of Mg–Sn alloys. Scr. Mater. 2010, 63, 899–902. [Google Scholar] [CrossRef]
- Fu, P.; Peng, L.; Jiang, H.; Ding, W.; Zhai, C. Tensile properties of high strength cast Mg Alloys at room temperature: A review. China Foundry 2014, 11, 277–286. [Google Scholar]
- Weiler, J.P. Exploring the concept of castability in magnesium die-casting alloys. J. Magnes. Alloy. 2021, 9, 102–111. [Google Scholar] [CrossRef]
- Zhao, C.; Pan, F.; Zhao, S.; Pan, H.; Song, K.; Tang, A. Preparation and characterization of as-extruded Mg-Sn alloys for orthopedic application. Mater. Des. 2015, 70, 60–67. [Google Scholar] [CrossRef]
- Saatçi, B.; Ari, M.; Gündüz, M.; Türktekin, S.; Meydaneri, F.; Durmus, S.; Özdemir, M. Structural and transport properties of Sn–Mg alloys. Contin. Mech. Thermodyn. 2013, 25, 739–748. [Google Scholar] [CrossRef]
- Fang, D.; Cheng, X.; Li, Y.; Sun, Z. Microstructure and thermal characteristics of Mg-Sn alloys as phase change materials for thermal energy storage. RSC Adv. 2016, 6, 96327–96333. [Google Scholar] [CrossRef]
- Zhao, C.; Pan, F.; Zhao, S.; Pan, H.; Song, K.; Tang, A. Microstructure, corrosion behavior and cytotoxicity of biodegradable Mg–Sn implant alloys prepared by sub-rapid solidification. Mater. Sci. Eng. C 2015, 54, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Persaud-Sharma, D.; McGoron, A. Biodegradable magnesium alloys: A review of material development and applications. J. Biomim. Biomater. Tissue Eng. 2011, 12, 25–39. [Google Scholar] [CrossRef] [PubMed]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca stress simulation of metal-on-metal total hip arthroplasty during normal walking activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef] [PubMed]
- Jamari, J.; Ammarullah, M.I.; Saad, A.P.M.; Syahrom, A.; Uddin, M.; van der Heide, E.; Basri, H. The effect of bottom profile dimples on the femoral head on wear in metal-on-metal total hip arthroplasty. J. Funct. Biomater. 2021, 12, 38. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Shan, D.; Song, Y.; Chen, R.; Han, E. Influences of the quantity of Mg2Sn phase on the corrosion behavior of Mg–7Sn magnesium alloy. Electrochim. Acta 2011, 56, 2582–2590. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Kang, J.-Y.; Kim, S.G.; Kim, B.; Park, S.S.; Yim, C.D.; You, B.S. Influences of metallurgical factors on the corrosion behaviour of extruded binary Mg–Sn alloys. Corros. Sci. 2014, 82, 369–379. [Google Scholar] [CrossRef]
- Kubásek, J.; Vojtěch, D.; Lipov, J.; Ruml, T. Structure, mechanical properties, corrosion behavior and cytotoxicity of biodegradable Mg–X (X = Sn, Ga, In) alloys. Mater. Sci. Eng. C 2013, 33, 2421–2432. [Google Scholar] [CrossRef]
- Cain, T.W.; Glover, C.F.; Scully, J.R. The corrosion of solid solution Mg-Sn binary alloys in NaCl solutions. Electrochim. Acta 2019, 297, 564–575. [Google Scholar] [CrossRef]
- Gusieva, K.; Davies, C.H.J.; Scully, J.R.; Birbilis, N. Corrosion of magnesium alloys: The role of alloying. Int. Mater. Rev. 2015, 60, 169–194. [Google Scholar] [CrossRef]
- Gogola, P.; Gabalcová, Z.; Kusý, M.; Suchánek, H. The Effect of Sn Addition on Zn-Al-Mg Alloy; Part I: Microstructure and Phase Composition. Materials 2021, 14, 5404. [Google Scholar] [CrossRef]
- Gabalcová, Z.; Gogola, P.; Kusý, M.; Suchánek, H. The effect of Sn addition on Zn-Al-Mg alloy; Part II: Corrosion behaviour. Materials 2021, 14, 5290. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, J.; Zhao, W.; Liu, Q.; Jiang, D.; Guo, S. Effects of Sn addition on the mechanical properties and bio-corrosion behavior of cytocompatible Mg-4Zn alloys. J. Magnes. Alloy. 2019, 7, 15. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Q.; Chen, X. The effects of Sn content on the corrosion behavior and mechanical properties of Mg-5Gd-3Y-xSn-0.7Zr alloys. RSC Adv. 2021, 11, 1332. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Z.; Ren, J.; Kang, H.; Guo, E.; Li, J.; Wang, T. Corrosion behavior of as-cast Mg–5Sn based alloys with In additions in 3.5 wt% NaCl solution. Corros. Sci. 2020, 164, 108318. [Google Scholar] [CrossRef]
- Cruz, C.; Lima, T.; Soares, M.; Freitas, E.; Fujiwara, E.; Garcia, A.; Cheung, N. Effect of microstructure features on the corrosion behavior of the Sn-2.1 wt.% Mg solder alloy. Electron. Mater. Lett. 2020, 16, 276–292. [Google Scholar] [CrossRef]
- Claus, P.; Raif, F.; Cavet, S.; Demirel-Gülen, S.; Radnik, J.; Schreyer, M.; Fässler, T. From molecule to material: Mg2Sn as hydrogenation catalyst. Catal. Commun. 2006, 7, 618–622. [Google Scholar] [CrossRef]
- Sakaguchi, H.; Maeta, H.; Kubota, M.; Honda, H.; Esaka, T. Mg2Sn as a new lithium storage intermetallic compound. Electrochemistry 2000, 68, 632–636. [Google Scholar] [CrossRef][Green Version]
- Kim, H.; Kim, Y.-J.; Kim, D.G.; Sohn, H.-J.; Kang, T. Mechanochemical synthesis and electrochemical characteristics of Mg2Sn as an anode material for Li-ion batteries. Solid State Ion. 2001, 144, 41–49. [Google Scholar] [CrossRef]
- Luo, Z.; Song, K.; Li, G.; Yang, L. Hydrogen evolution ability of selected pure metals and galvanic corrosion behavior between the metals and magnesium. J. Electrochem. Sci. Technol. 2020, 11, 323–329. [Google Scholar] [CrossRef]
- Chen, X.; Wei, S.; Tong, F.; Taylor, M.P.; Cao, P. Electrochemical performance of Mg-Sn alloy anodes for magnesium rechargeable battery. Electrochim. Acta 2021, 398, 139336. [Google Scholar] [CrossRef]
- Ping, W.; Jianping, L.; Yongchun, G.; Zhong, Y.; Feng, X.; Jianli, W. Effect of Sn on microstructure and electrochemical properties of Mg alloy anode materials. Rare Met. Mater. Eng. 2012, 41, 2095–2099. [Google Scholar] [CrossRef]
- Ikhe, A.B.; Han, S.C.; Prabakar, S.J.R.; Park, W.B.; Sohn, K.-S.; Pyo, M. 3Mg/Mg2Sn anodes with unprecedented electrochemical performance towards viable magnesium-ion batteries. J. Mater. Chem. A 2020, 8, 14277–14286. [Google Scholar] [CrossRef]
- Tong, F.; Chen, X.; Teoh, T.E.; Wei, S.; Waterhouse, G.I.N.; Gao, W. Mg–Sn alloys as anodes for magnesium-air batteries. J. Electrochem. Soc. 2021, 168, 110531. [Google Scholar] [CrossRef]
- Yaghoobnejad Asl, H.; Fu, J.; Kumar, H.; Welborn, S.S.; Shenoy, V.B.; Detsi, E. In situ dealloying of bulk Mg2Sn in Mg-Ion half cell as an effective route to nanostructured Sn for high performance Mg-Ion battery anodes. Chem. Mater. 2018, 30, 1815–1824. [Google Scholar] [CrossRef]
- Jin, Y.R.; Feng, Z.Z.; Ye, L.Y.; Yan, Y.L.; Wang, Y.X. Mg2Sn: A potential mid-temperature thermoelectric material. RSC Adv. 2016, 6, 48728–48736. [Google Scholar] [CrossRef]
- Saito, W.; Hayashi, K.; Dong, J.; Li, J.-F.; Myazaki, Y. Control of the thermoelectric properties of Mg2Sn single crystals via point-defect engineering. Sci. Rep. 2020, 10, 2020. [Google Scholar] [CrossRef]
- ASTM G44-21; Standard Practice for Exposure of Metals and Alloys by Alternate Immersion in Neutral 3.5 % Sodium Chloride Solution. ASTM International: West Conshohocken, PA, USA, 2021. Available online: https://www.astm.org/g0044-21.html (accessed on 26 February 2022).
- Giannella, R.A.; Broitman, S.A.; Zamchek, N. Gastric acid barrier to ingested microorganisms in man: Studies in vivo and in vitro. Gut 1972, 13, 251–256. [Google Scholar] [CrossRef]
- McCafferty, E. Chapter 7 kinetics of corrosion. In Introduction to Corrosion Science; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Lutterotti, L.; Matthies, S.; Wenk, H.R. MAUD (Material Analysis Using Diffraction): A user friendly Java program for Rietveld Texture Analysis and more. In Proceedings of the 12th International Conference on Textures of Materials (ICOTOM-12), Montreal, QC, Canada, 9–13 August 1999; Volume 1, p. 1599. Available online: http://hdl.handle.net/11572/57067 (accessed on 25 March 2021).
- An, T.-H.; Choi, S.-M.; Kim, I.-H.; Kim, S.-U.; Seo, W.-S.; Kim, J.-Y.; Park, C. Thermoelectric properties of a doped Mg2Sn system. Renew. Energy 2012, 42, 23–27. [Google Scholar] [CrossRef]
- Esmaily, M.; Svensson, J.E.; Fajardo, S.; Birbilis, N.; Frankel, G.S.; Virtanen, S.; Arrabal, R.; Thomas, S.; Johansson, L.G. Fundamentals and advances in magnesium alloy corrosion. Prog. Mater. Sci. 2017, 89, 92–193. [Google Scholar] [CrossRef]
- Moon, S.; Nam, Y. Anodic oxidation of Mg–Sn alloys in alkaline solutions. Corros. Sci. 2012, 65, 494–501. [Google Scholar] [CrossRef]
- Palcut, M.; Priputen, P.; Šalgó, K.; Janovec, J. Phase constitution and corrosion resistance of Al-Co alloys. Mater. Chem. Phys. 2015, 166, 95–104. [Google Scholar] [CrossRef]
- Pourbaix. M. Atlas of Electrochemical Equilibria in Aqueous Solution, 2nd ed.; National Association of Corrosion Engineers: Houston, TX, USA, 1974; p. 644. [Google Scholar]
- Robertson, W.D.; Uhlig, H.H. An observation of corrosion cracking without stress. J. Appl. Phys. 1948, 19, 864–865. [Google Scholar] [CrossRef]
- Pechberty, C.; Klein, A.; Fraisse, B.; Stievano, L.; Berthelot, R. Mechanisms of electrochemical magnesium (de)alloying of Mg-Sn and Mg-Pb polymorphs. J. Magnes. Alloy. 2022, in press. [Google Scholar] [CrossRef]
- Lee, D.; Kim, B.; Beak, S.-M.; Kim, J.; Park, H.W.; Lee, J.G.; Park, S.S. Microstructure and corrosion resistance of a Mg2Sn-dispersed Mg alloy subjected to pulsed electron beam treatment. J. Magnes. Alloy. 2020, 8, 345–351. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, B.; Yang, H.; Yang, Q.; Xia, X.; Pan, F. High temperature oxidation behavior of Mg-Y-Sn, Mg-Y, Mg-Sn alloys and its effect on corrosion property. Appl. Surf. Sci. 2015, 353, 1013–1022. [Google Scholar] [CrossRef]
- Atrens, A.; Shi, Z.; Mehreen, S.U.; Johnston, S.; Song, G.-L.; Chen, X.; Pan, F. Review of Mg alloy corrosion rates. J. Magnes. Alloy. 2020, 8, 989–998. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Omiyale, B.O.; Ojo, O.T. Corrosion, corrosion fatigue, and protection of magnesium alloys: Mechanisms, measurements, and mitigation. J. Mater. Eng. Perform. 2021, 1–21. [Google Scholar] [CrossRef]
- ASTM G102–89; Standard Practice for Calculation of Corrosion Rates and Related Information from Electrochemical Measurements. ASTM International: West Conshohocken, PA, USA, 2015. Available online: https://www.astm.org/Standards/G102.htm (accessed on 6 August 2021).
- Song, G.; Atrens, A.; StJohn, D. A Hydrogen evolution method for the estimation of the corrosion rate of magnesium alloys. In Magnesium Technology; Hryn, J.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 255–262. [Google Scholar] [CrossRef]
- Bender, S.; Goellner, J.; Heyn, A.; Schmigalla, S. A new theory for the negative difference effect in magnesium corrosion. Mater. Corros. 2011, 62, 707–712. [Google Scholar] [CrossRef]
- Thomaz, T.R.; Weber, C.R.; Pelegrini, T., Jr.; Dick, L.F.P.; Knörnschild, G. The negative difference effect of magnesium and of the AZ91 alloy in chloride and stannate-containing solutions. Corros. Sci. 2010, 52, 2235–2243. [Google Scholar] [CrossRef]
- Liu, L.J.; Schlesinger, M. Corrosion of magnesium and its alloys. Corros. Sci. 2009, 51, 1733–1737. [Google Scholar] [CrossRef]
- Yang, J.; Yim, C.D.; You, B.S. Characteristics of surface films formed on Mg–Sn alloys in NaCl solution. J. Electrochem. Soc. 2016, 163, 395–401. [Google Scholar] [CrossRef]
- Lutterotti, L. Maud—Materials Analysis Using Diffraction. Available online: https://github.com/luttero/Maud (accessed on 25 March 2021).
- Basciano, L.C.; Peterson, R.C.; Roeder, P.L. Description of schoenfliesite, MgSn(OH)6, and roxbyite, Cu1.72S, from a 1375 BC shipwreck, and rietveld neutron-diffraction refinement of synthetic schoenfliesite, wickmanite, MnSn(OH)6, and burtite, CaSn(OH)6 Locality: A 1375 BC shipwreck. Can. Mineral. 1998, 36, 1203–1210. [Google Scholar]
- Faust, G.T.; Schaller, W.T. Schoenfliesite, MgSn(0H)6. Z. Krist. Cryst. Mater. 1971, 134, 116–141. [Google Scholar] [CrossRef]
- Haase, P.; Christensen, H.G.; Nielsen, U.G.; Koch, C.B.; Galazka, Z.; Mazlan, J. Stability and solubility of members of tin perovskites in the schenfliesite subgroup, □2(BSn4+)(OH,O)6 (B = Ca, Fe3+, Mg, Mn2+, Zn, Cu). Chem. Thermodyn. Therm. Anal. 2021, 1–2, 100005. [Google Scholar] [CrossRef]
- Mitchell, R.G.; Welch, M.D.; Chakhmouradian, A.R. Nomenclature of the perovskite supergroup: A hierarchical system of classification based on crystal structure and composition. Mineral. Mag. 2017, 81, 411–461. [Google Scholar] [CrossRef]
- Velu, S.; Suzuki, K.; Osaki, T.; Ohashi, F.; Tomura, S. Synthesis of new Sn incorporated layered double hydroxides and their evolution to mixed oxides. Mater. Res. Bull. 1999, 34, 1707–1717. [Google Scholar] [CrossRef]
- Velu, S.; Suzuki, K.; Okazaki, M.; Osaki, T.; Tomura, S.; Ohashi, F. Synthesis of new Sn-Incorporated layered double hydroxides and their thermal evolution to mixed oxides. Chem. Mater. 1999, 11, 2163–2172. [Google Scholar] [CrossRef]
- Barchiche, C.-E.; Rocca, E.; Hazan, J. Corrosion behaviour of Sn-containing oxide layer on AZ91D alloy formed by plasma electrolytic oxidation. Surf. Coat. Technol. 2008, 202, 4145–4152. [Google Scholar] [CrossRef]
- Demirci, E.E.; Arslan, E.; Ezirmik, K.V.; Baran, Ö.; Totik, Y.; Efeoglu, İ. Investigation of wear, corrosion and tribocorrosion properties of AZ91 Mg alloy coated by micro arc oxidation process in the different electrolyte solutions. Thin Solid Film. 2013, 528, 116–122. [Google Scholar] [CrossRef]




| Sample | Angle Range (2Theta) | Incident Beam | Diffracted Beam | Detector |
|---|---|---|---|---|
| Powdered Mg2Sn alloy | 20–90° | Divergence slit: 1/2° Soller slit: 0.04 rad Anti-scatter slit: 1° | Anti-scatter slit: 1° Soller slit: 0.04 rad | PIXcel3D detector in 1D scanning mode |
| Powdered corrosion products | 20–90° | Divergence slit: 1/4° Soller slit: 0.04 rad Anti-scatter slit: 1/2° | Anti-scatter slit: 1/2° Soller slit: 0.04 rad | PIXcel3D detector in 1D scanning mode |
| Sample | Point 1 | Point 2 | Point 3 | Point 4 | Point 5 |
|---|---|---|---|---|---|
| Mg [at.%] | 66.22 | 1.25 | 1.92 | 49.62 | 66.64 |
| Sn [at.%] | 33.78 | 98.75 | 98.08 | 50.38 | 33.36 |
| Chemical Formula | Reference Code–ICSD Database FIZ Karlsruhe | Crystal System | Space Group | Space Group Number | Fraction [Vol.%] |
|---|---|---|---|---|---|
| Mg2Sn | 98-064-2855 | Cubic | 225 | 95.75 | |
| Sn | 98-004-3613 | Tetragonal | I 41/a m d | 141 | 0.25 |
| Mg2Sn1.1 | 98-007-0069 | Hexagonal | 147 | 4.00 |
| OCP30 min [mV vs. Ag/AgCl] | Ecorr [mV vs. Ag/AgCl] | jcorr [A m−2] | Epitt [mV vs. Ag/AgCl] | |
|---|---|---|---|---|
| Mg2Sn | ||||
| NaCl | −1522 | −1498 | 2.10 | −1461 |
| HCl | −1393 | −1380 | 49.0 | −1255 |
| NaOH | −1311 | −1361 | 0.15 | - |
| Mg | ||||
| NaCl | −1592 | −1697 | 1.40 | −1551 |
| Sn | ||||
| NaCl | −441 | −479 | 0.012 | −358 |
| Compound Name | Formula | HCl | NaCl | NaOH |
|---|---|---|---|---|
| Schoenfliesite | MgSn(OH)6 | ■■■■ | ■■ | ■■■■ |
| Brucite | Mg(OH)2 | ■■ | ■■■■ | ■ |
| Romarchite | SnO | ■ | ■ | - |
| Periclase | MgO | - | - | ■ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabalcová, Z.; Gogola, P.; Gerhátová, Ž.; Palcut, M. Corrosion Behavior of an Mg2Sn Alloy. Materials 2022, 15, 2025. https://doi.org/10.3390/ma15062025
Gabalcová Z, Gogola P, Gerhátová Ž, Palcut M. Corrosion Behavior of an Mg2Sn Alloy. Materials. 2022; 15(6):2025. https://doi.org/10.3390/ma15062025
Chicago/Turabian StyleGabalcová, Zuzana, Peter Gogola, Žaneta Gerhátová, and Marián Palcut. 2022. "Corrosion Behavior of an Mg2Sn Alloy" Materials 15, no. 6: 2025. https://doi.org/10.3390/ma15062025
APA StyleGabalcová, Z., Gogola, P., Gerhátová, Ž., & Palcut, M. (2022). Corrosion Behavior of an Mg2Sn Alloy. Materials, 15(6), 2025. https://doi.org/10.3390/ma15062025







