In Vitro Toxicity of Bone Graft Materials to Human Mineralizing Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bone Graft Materials
2.2. Cells and Cell Culture
2.3. X-ray Fluorescence (XRF)
2.4. Raman Spectroscopy
2.5. Cell Plating and Bone Graft Materials Exposure
2.6. Cell Imaging and Cell Counting
2.7. Conditioned Media
2.8. Collagen Substrate
2.9. Statistical Analysis
2.10. Image Data Collection
3. Results
3.1. Analysis of the Bone Graft Materials
3.1.1. XRF
3.1.2. Raman
3.2. Tissue Culture Plastic (TCP) Substrate
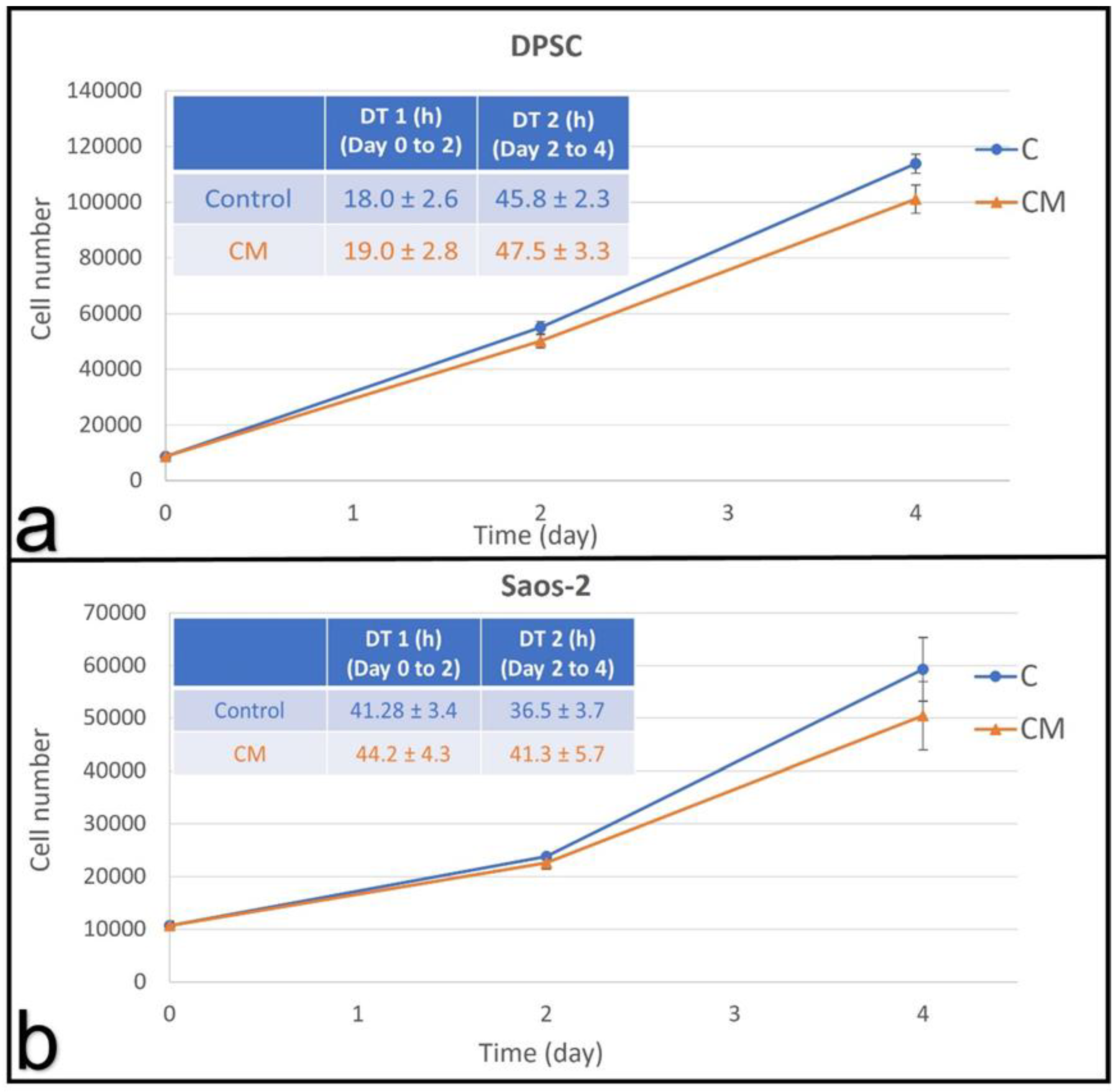
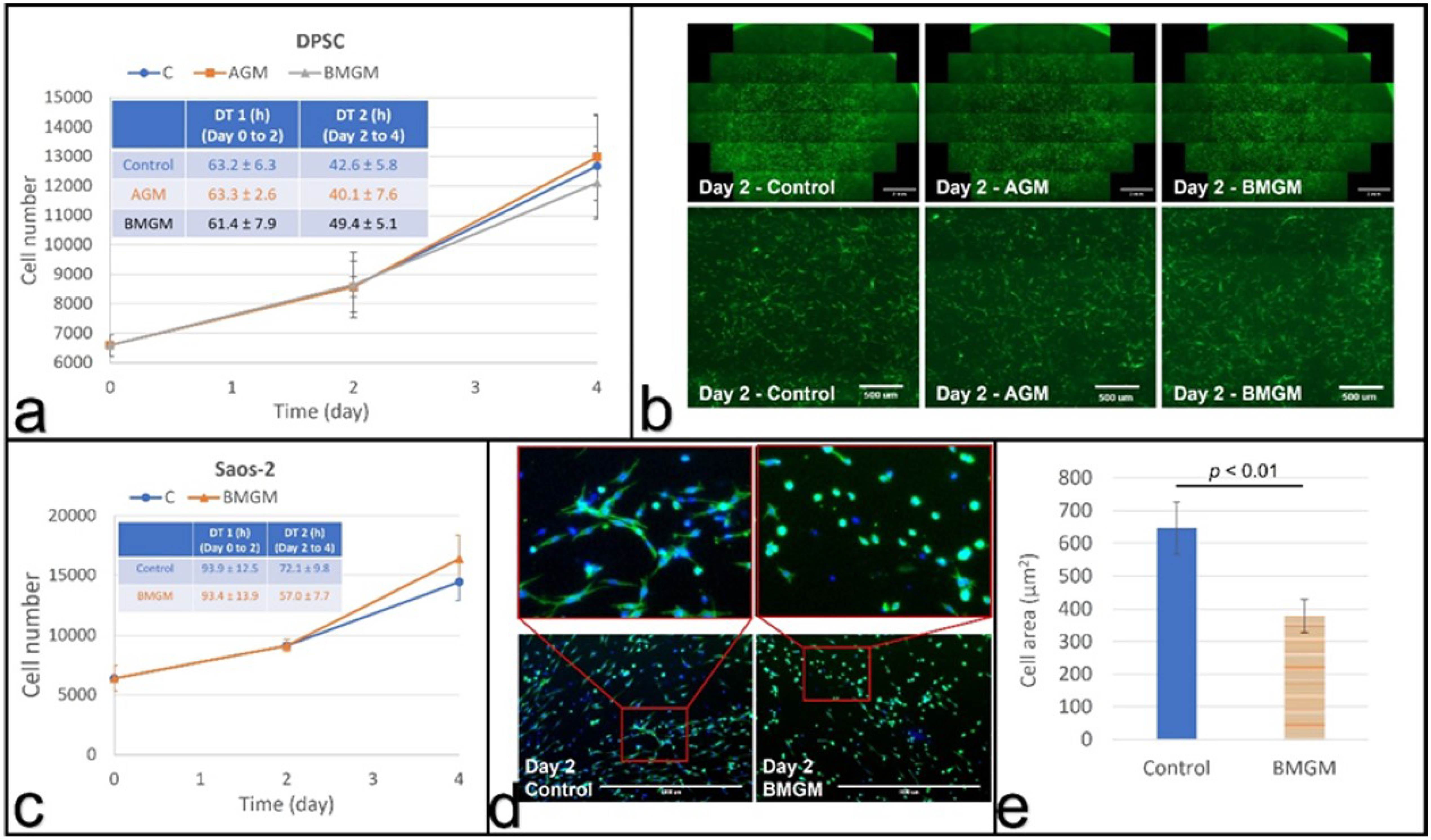
3.2.1. Dental Pulp Stem Cells
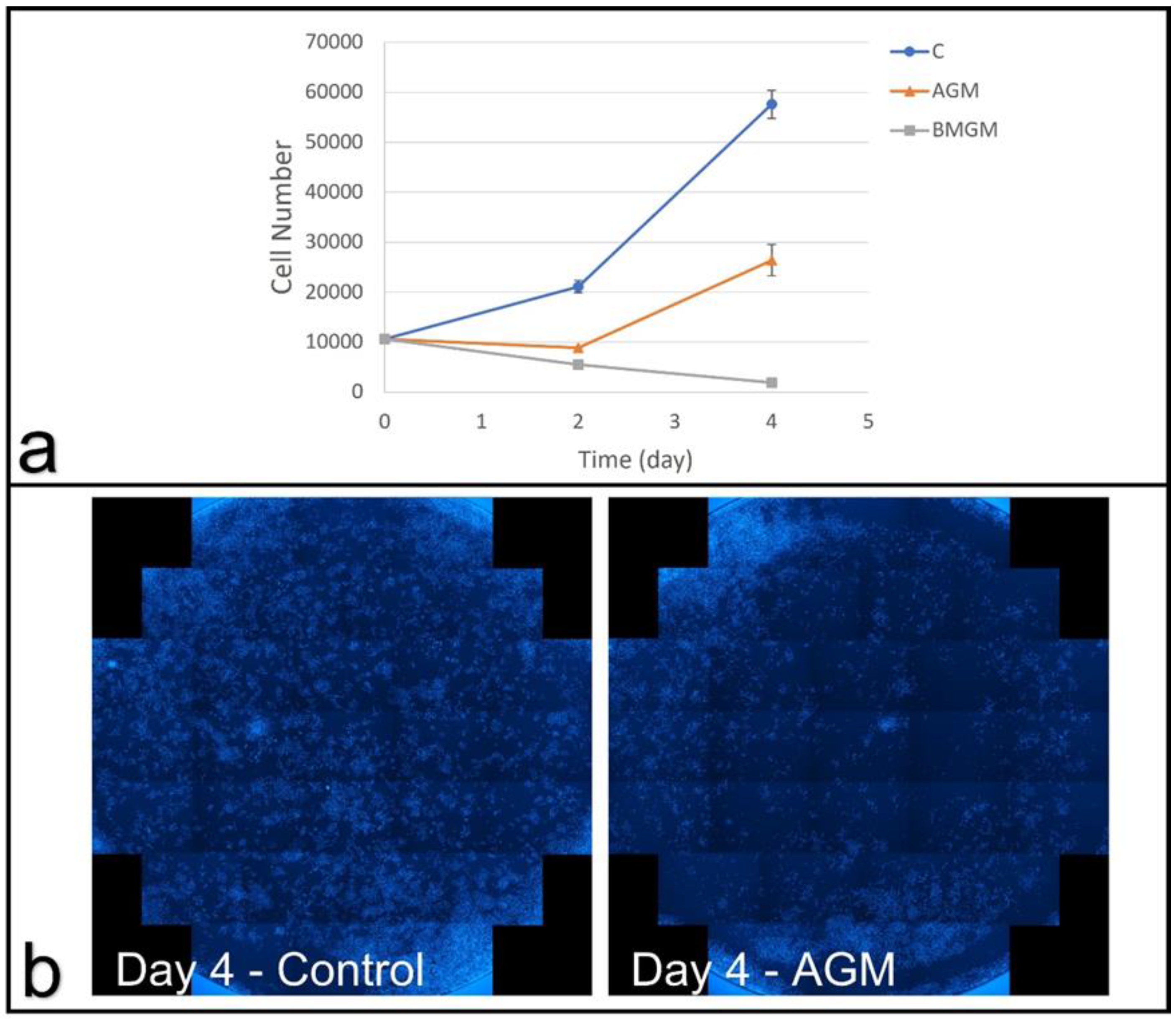
3.2.2. Osteosarcoma Cells
3.2.3. Conditioned Medium
3.3. Collagen Substrates
3.3.1. DPSC on Collagen
3.3.2. Saos-2 on Collagen
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aghaloo, T.L.; Moy, P.K. Which hard tissue augmentation techniques are the most successful in furnishing bony support for implant placement? Int. J. Oral. Maxillofac. Implants 2007, 22, 49–70. [Google Scholar]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 4, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Romanos, G.E. Anatomical and Biologic Considerations of Autogenous Bone Blocks Harvested from the Ramus Region. Int. J. Oral. Maxillofac. Implants 2019, 1, e1–e6. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Egusa, H. Current bone substitutes for implant dentistry. J. Prosthodont. Res. 2018, 2, 152–161. [Google Scholar] [CrossRef]
- Romanos, G.E.; Delgado-Ruiz, R.A.; Gómez-Moreno, G.; López-López, P.J.; Mate Sanchez de Val, J.E.; Calvo-Guirado, J.L. Role of mechanical compression on bone regeneration around a particulate bone graft material: An experimental study in rabbit calvaria. Clin. Oral. Implants Res. 2018, 6, 612–619. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Romanos, G.E.; Alexandre, G.S.; Gomez-Moreno, G.; Maté-Sánchez de Val, J.E.; Calvo-Guirado, J.L. Biological effects of compressive forces exerted on particulate bone grafts during socket preservation: Animal study. Clin. Oral. Implants Res. 2018, 7, 792–801. [Google Scholar] [CrossRef]
- Sallent, I.; Capella-Monsonís, H.; Procter, P.; Bozo, I.Y.; Deev, R.V.; Zubov, D.; Vasyliev, R.; Perale, G.; Pertici, G.; Baker, J.; et al. The Few Who Made It: Commercially and Clinically Successful Innovative Bone Grafts. Front. Bioeng. Biotechnol. 2020, 8, 952. [Google Scholar] [CrossRef]
- Delgado-Ruiz, R.A.; Calvo-Guirado, J.L.; Romanos, G.E. Bone grafting materials in critical defects in rabbit calvariae. A systematic review and quality evaluation using ARRIVE guidelines. Clin. Oral. Implants Res. 2018, 6, 620–634. [Google Scholar] [CrossRef]
- Ermakov, V.; Safonov, V.; Dogadkin, D. Characteristic features of molybdenum, copper, tungsten and rhenium accumulation in the environment. Innov. Infrastruct. Solut. 2021, 2, 1–7. [Google Scholar] [CrossRef]
- Honda, K.; Yamamoto, Y.; Hidaka, H.; Tatsukawa, R. Heavy metal accumulations in Adelie penguin, Pygoscelis adeliae, and their variations with the reproductive processes. Mem. Natl. Inst. Polar Res. Spec. Issue 1986, 40, 443–453. [Google Scholar]
- Lavery, T.J.; Kemper, C.M.; Sanderson, K.; Schultz, C.G.; Coyle, P.; Mitchell, J.G.; Seuront, L. Heavy metal toxicity of kidney and bone tissues in South Australian adult bottlenose dolphins (Tursiops aduncus). Mar. Environ. Res. 2009, 1, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perugini, M.; Visciano, P.; Manera, M.; Zaccaroni, A.; Olivieri, V.; Amorena, M. Heavy metal (As, Cd, Hg, Pb, Cu, Zn, Se) concentrations in muscle and bone of four commercial fish caught in the central Adriatic Sea, Italy. Environ. Monit. Assess. 2014, 4, 2205–2213. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.P.; VanderSchee, C.R.; Chou, H.; Bolt, A.; Epure, L.M.; Kuter, D.; Antoniou, J.; Bohle, S.; Mann, K.K.; Mwale, F. Tungsten accumulates in the intervertebral disc and vertebrae stimulating disc degeneration and upregulating markers of inflammation and pain. Eur. Cells Mater. 2021, 41, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Van der Voet, G.B.; Todorov, T.I.; Centeno, J.A.; Jonas, W.; Ives, J.; Mullick, F.G. Metals and health: A clinical toxicological perspective on tungsten and review of the literature. Mil. Med. 2007, 9, 1002–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chuang, Y.-C.; Wang, L.; Feng, K.-C.; Subramanian, A.; Chang, C.-C.; Simon, M.; Nam, C.-Y.; Rafailovich, M. The role of titania surface coating by atomic layer deposition in improving osteogenic differentiation and hard tissue formation of dental pulp stem cells. Adv. Eng. Mater. 2021, 9, 2100097. [Google Scholar] [CrossRef]
- Alliot-Licht, B.; Bluteau, G.; Magne, D.; Lopez-Cazaux, S.; Lieubeau, B.; Daculsi, G.; Guicheux, J. Dexamethasone stimulates differentiation of odontoblast-like cells in human dental pulp cultures. Cell Tissue Res. 2005, 3, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Anwar, H. Toxicity of Different Bone Grafting Materials In Vitro. Master’s Thesis, State University of New York at Stony Brook, New York, NY, USA, August 2018. [Google Scholar]
- Osterburg, A.R.; Robinson, C.T.; Schwemberger, S.; Mokashi, V.; Stockelman, M.; Babcock, G.F. Sodium tungstate (Na2WO4) exposure increases apoptosis in human peripheral blood lymphocytes. J. Immunotoxicol. 2010, 3, 174–182. [Google Scholar] [CrossRef]
- Gentleman, E.; Swain, R.; Evans, N.; Boonrunqsiman, S.; Jell, G.; Ball, M.D.; Shean, T.A.V.; Oyen, M.L.; Porter, A.; Stevens, M.M. Comparative materials differences revealed in engineered bone as a function of cell-specific differentiation. Nat. Mater. 2009, 8, 763–770. [Google Scholar] [CrossRef] [Green Version]
- Guandalini, G.S.; Zhang, L.; Fornero, E.; Centeno, J.A.; Mokashi, V.P.; Ortiz, P.A.; Stockelman, M.D.; Osterburg, A.R.; Chapman, G.G. Tissue distribution of tungsten in mice following oral exposure to sodium tungstate. Chem. Res. Toxicol. 2011, 4, 488–493. [Google Scholar] [CrossRef]
- Kelly, A.D.E.; Lemaire, M.; Young, Y.K.; Eustache, J.H.; Guilbert, C.; Molina, M.F.; Mann, K.K. In Vivo Tungsten Exposure Alters B-Cell Development and Increases DNA Damage in Murine Bone Marrow. Toxicol. Sci. 2013, 2, 434–446. [Google Scholar] [CrossRef]
- Leggett, R.W. A model of the distribution and retention of tungsten in the human body. Sci. Total. Environ. 1997, 2–3, 147–165. [Google Scholar] [CrossRef]
- Bíbr, B.; Deyl, Z.; Lener, J.; Kucera, J.; Simková, M. The mechanism of action of molybdenum and tungsten upon collagen structures in vivo. Physiol. Bohemoslov. 1987, 5, 417–424. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Li, K.; Fu, S.; Cuiffo, M.; Simon, M.; Rafailovich, M.; Romanos, G.E. In Vitro Toxicity of Bone Graft Materials to Human Mineralizing Cells. Materials 2022, 15, 1955. https://doi.org/10.3390/ma15051955
Yang F, Li K, Fu S, Cuiffo M, Simon M, Rafailovich M, Romanos GE. In Vitro Toxicity of Bone Graft Materials to Human Mineralizing Cells. Materials. 2022; 15(5):1955. https://doi.org/10.3390/ma15051955
Chicago/Turabian StyleYang, Fan, Kao Li, Shi Fu, Michael Cuiffo, Marcia Simon, Miriam Rafailovich, and Georgios E. Romanos. 2022. "In Vitro Toxicity of Bone Graft Materials to Human Mineralizing Cells" Materials 15, no. 5: 1955. https://doi.org/10.3390/ma15051955
APA StyleYang, F., Li, K., Fu, S., Cuiffo, M., Simon, M., Rafailovich, M., & Romanos, G. E. (2022). In Vitro Toxicity of Bone Graft Materials to Human Mineralizing Cells. Materials, 15(5), 1955. https://doi.org/10.3390/ma15051955







