Effect of Temperature Cycling Pretreatment on the Thermal Stability of Sm2(Co, Fe, Zr, Cu)17 Magnets in the Mild Temperature Range
Abstract
1. Introduction
2. Experiment and Characterization
2.1. Materials
2.2. High-Temperature Test
2.3. Temperature Cycling Test
2.4. Long-Term Isothermal Test
2.5. Characterization
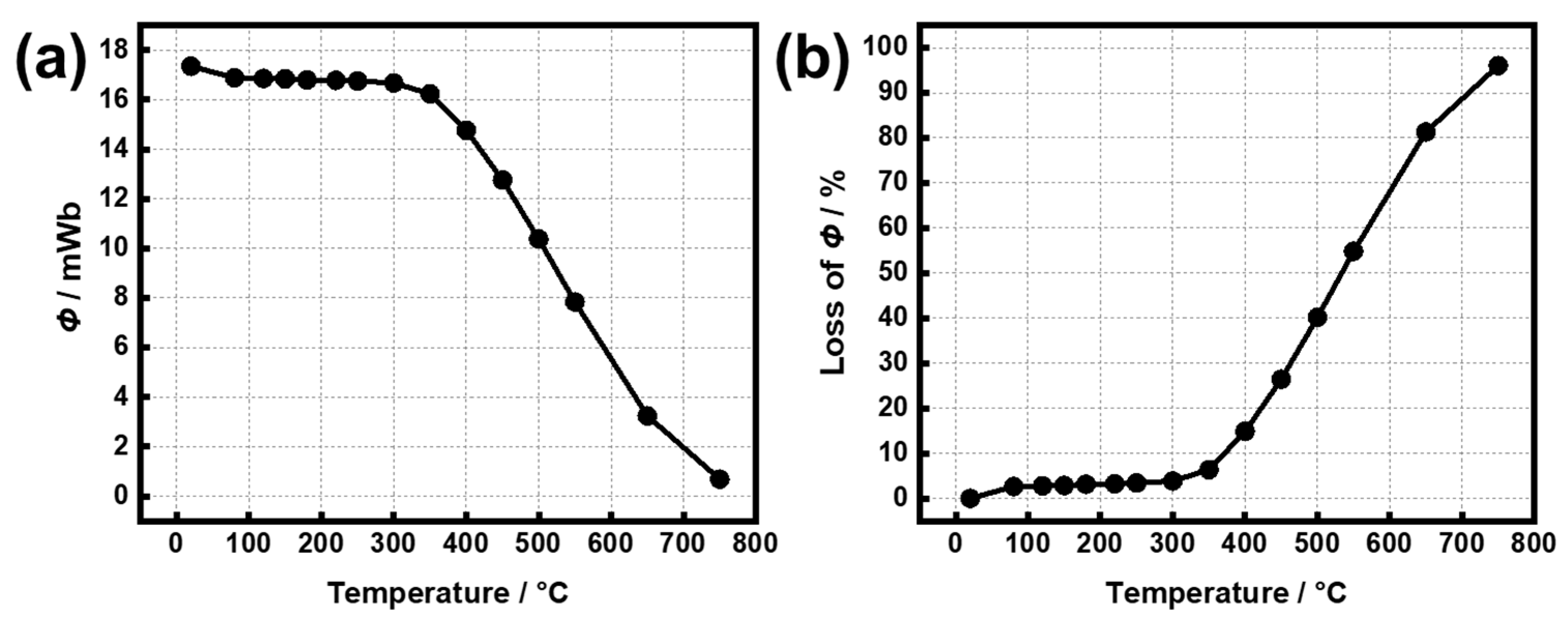
3. Results and Discussion
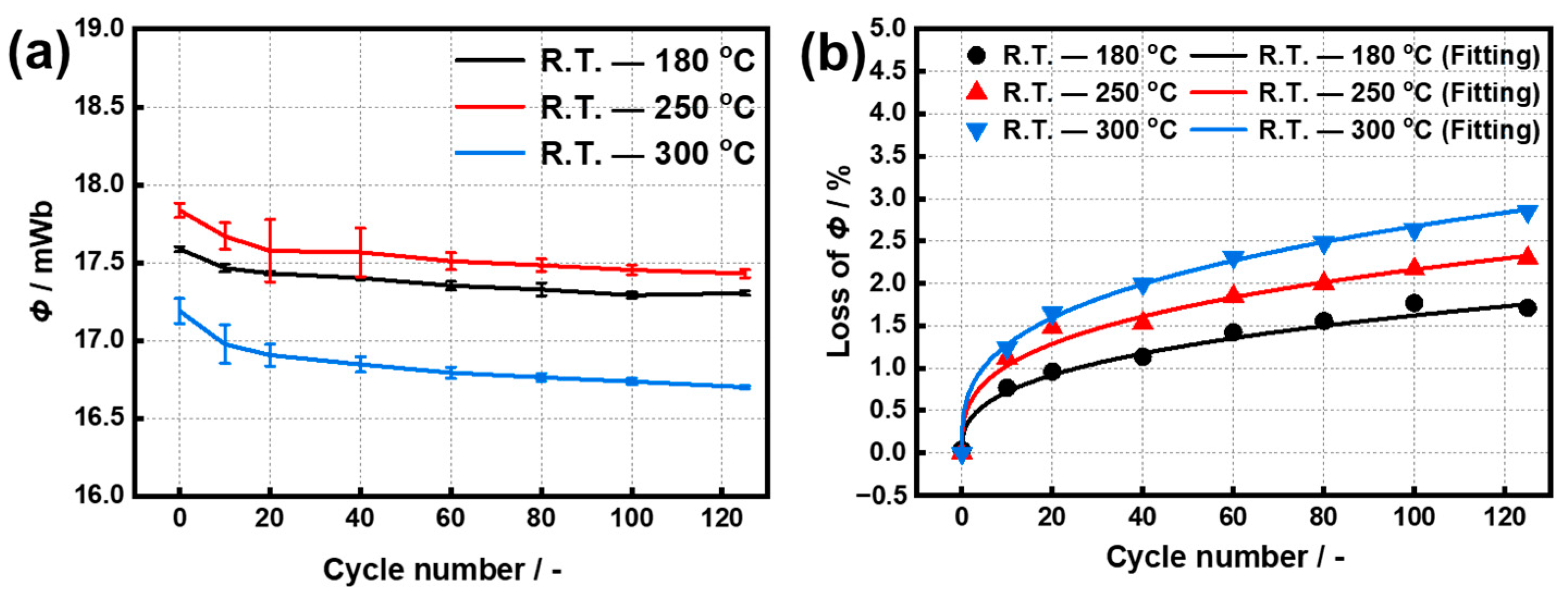
3.1. Temperature Cycling Test
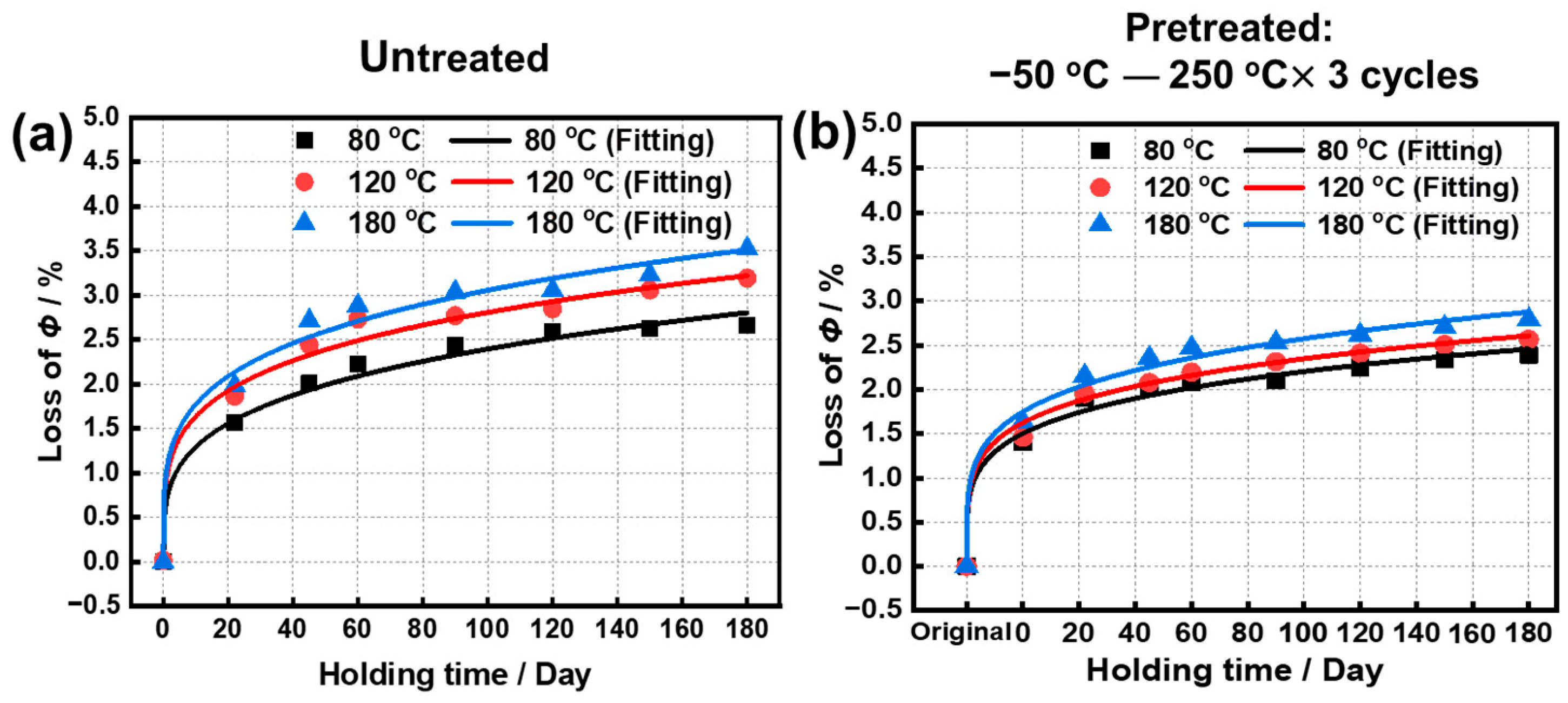
3.2. Long-Term Isothermal Test
4. Conclusions
- (1)
- For temperature cycling in ranges of R.T.–180 °C and R.T.–300 °C, the Φ losses of the original samples after 125th cycles were 0.73% and 1.24%, respectively, corresponding to Br attenuation rates of 19.7% and 29.9%, and Hcj attenuation rates of 1.77% and 6.62%.
- (2)
- The Φ losses of the original sample and the pretreated sample after oxidization at 180 °C reached 3.38% and 2.79% for 180 days, and further were simulated as 7.15% and 5.39% for 10 years. Meanwhile, Br and Hcj at 180 °C for 180 days were 0.99 T and 18.07 kOe for the original sample and were 1.05 T and 18.37 kOe for the pretreated samples.
- (3)
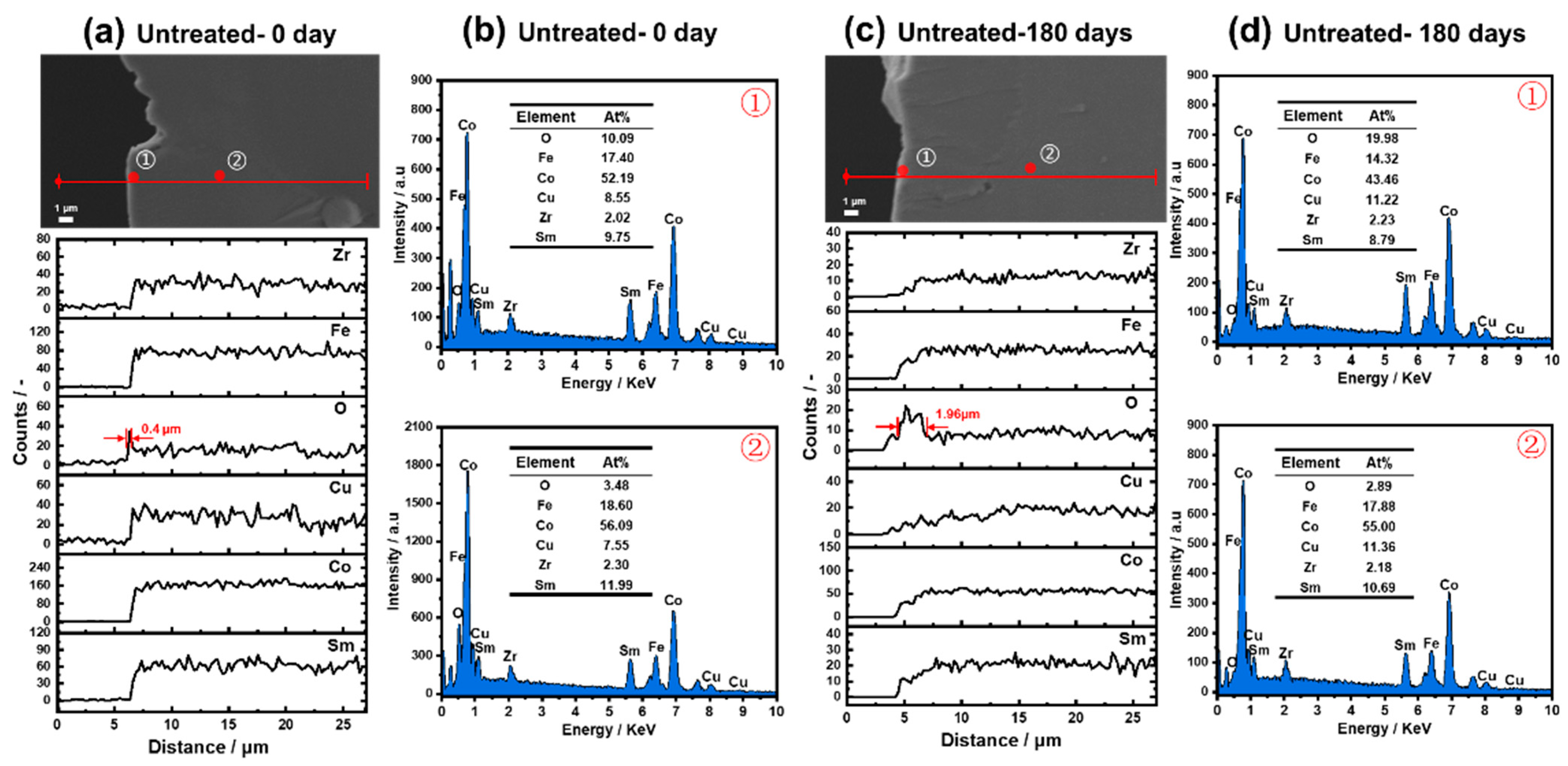
- After the original sample oxidized at 180 °C for 180 days, there was only an oxide outer layer with a thickness of 1.96 μm without the enrichment or precipitation of metal elements. The Cu, O-rich outer layer with a thickness of 0.72 μm formed through the pretreatment, and further extended to 1.28 μm after oxidization for 180 days, by which point the attenuation of magnetic properties was effectively inhibited.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Sarlioglu, B.; Jurkovic, S.; Patel, N.R.; Savagian, P. Analysis of Temperature Effects on Performance of Interior Permanent Magnet Machines for High Variable Temperature Applications. IEEE Trans. Ind. Appl. 2017, 53, 4923–4933. [Google Scholar] [CrossRef]
- Firdaus, M.; Rhamdhani, M.A.; Rankin, W.J.; Pownceby, M.; Webster, N.A.; D’Angelo, A.M.; Mcgregor, K. High-Temperature Oxidation of Rare Earth Permanent Magnets. Part 1—Microstructure Evolution and General Mechanism. Corros. Sci. 2018, 133, 374–385. [Google Scholar] [CrossRef]
- Pragnell, W.M.; Evans, H.E.; Williams, A.J. Oxidation Protection of Sm2Co17-Based Alloys. J. Alloys Compd. 2012, 517, 92–97. [Google Scholar] [CrossRef]
- Wang, C.; Shen, P.; Fang, Y.K.; Wang, S.; Li, Q.F.; Wang, L.; Li, W.; Zhu, M.G. Cellular Microstructure Modification and High Temperature Performance Enhancement for Sm2Co17-Based Magnets with Different Zr Contents. J. Mater. Sci. Technol. 2022, 120, 8–14. [Google Scholar] [CrossRef]
- Wang, C.; Yu, N.; Zhu, M.; Fang, Y.; Li, W. High-Temperature Properties Improvement and Microstructure Regulation of Sm2Co17 -Based Permanent Magnet. AIP Adv. 2019, 9, 125237. [Google Scholar] [CrossRef]
- Ramesh, P.; Lenin, N.C. High Power Density Electrical Machines for Electric Vehicles—Comprehensive Review Based on Material Technology. IEEE Trans. Magn. 2019, 55, 1–21. [Google Scholar] [CrossRef]
- Widmer, J.D.; Martin, R.; Kimiabeigi, M. Electric Vehicle Traction Motors without Rare Earth Magnets. Sustain. Mater. Technol. 2015, 3, 7–13. [Google Scholar] [CrossRef]
- Chen, M.Z. Developments in the Processing and Properties of NdFeB-Type Permanent Magnets. J. Magn. Magn. Mater. 2002, 248, 432–440. [Google Scholar]
- Sebastian, T. Temperature Effects on Torque Production and Efficiency of Pm Motors Using NdFeB Magnets. IEEE Trans. Ind. Appl. 1995, 31, 353–357. [Google Scholar] [CrossRef]
- Xia, W.; Zhang, T.L.; Liu, J.H.; Dong, Y.; Wang, H.; Jiang, C.B. Influence of the Final Heat Treatment Temperature on the Magnetic Property Losses of Sm(Co, Fe, Cu, Zr)z High Temperature Magnets. J. Magn. Magn. Mater. 2021, 528, 167763. [Google Scholar] [CrossRef]
- Pragnell, W.M.; Williams, A.J.; Evans, H.E. The Oxidation Morphology of SmCo Alloys. J. Alloys Compd. 2009, 487, 69–75. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, X.; Guo, Z.; Li, W.; Wang, F. Internal Oxidation of Sm(Cobalfe0.22Cu0.08Zr0.02)7.5 Alloy at 700 °C. Corros. Sci. 2013, 70, 260–267. [Google Scholar] [CrossRef]
- Peng, X.; Zhao, H.; Wang, X.; Guo, Z.; Li, W.; Wang, F. On High-Temperature Oxidation and Protection of 2:17-Type SmCo-Based Magnets. Corros. Rev. 2015, 33, 139–150. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, X.; Feng, Q.; Guo, Z.; Li, W.; Wang, F. The Mechanism of High-Temperature Oxidation of a SmCo-Based Magnetic Alloy. Corros. Sci. 2012, 61, 72–82. [Google Scholar] [CrossRef]
- Musa, M.; Song, X.; Liu, Y.; Wang, F.; Fan, J.P.; Ma, T.Y.; Ren, X.B. Effects of Pre-aging on Defects Evolution and Magnetic Properties of Sm-Co-Fe-Cu-Zr Magnet. J. Rare Earths 2021, 40, 1878–1884. [Google Scholar] [CrossRef]
- Campbell, A.R.; Zhang, Y.; Sheridan, R.S. The Effect of Grain Size on the Internal Oxidation of Sm2Co17-Type Permanent Magnets. J. Magn. Magn. Mater. 2022, 552, 169219. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, H.T.; Cao, X.; Ayan, B.; Gill, V.; Lambourne, A.; Huang, Y.Z. Atomic-Scale Oxidation of a Sm2Co17-Type Magnet. Acta Mater. 2021, 220, 117343. [Google Scholar] [CrossRef]
- Xu, C.; Wang, H.; Liu, B.J.; Xu, H.; Zhang, T.L.; Liu, J.H.; Jiang, C.B. The Formation Mechanism of 1: 5H Phase in Sm(Co, Fe, Cu, Zr)z Melt-Spun Ribbons with High Iron Content. J. Magn. Magn. Mater. 2020, 496, 165939. [Google Scholar] [CrossRef]
- Wang, X.; Peng, X.; Zhao, H.; Guo, Z.; Li, W.; Wang, F. High-Temperature Oxidation and Its Induced Coercivity Loss of a 2:17 Type SmCo-Based Magnet. J. Appl. Phys. 2015, 117, 093902. [Google Scholar] [CrossRef]
- Mao, S.; Yan, M.S.; Nie, X.; Sun, K.Q.; Jiang, J.J.; Song, Z.L. Evolution and Effects of Surface Degradation Layer of Sm2Co17 Magnets at High Temperatures. J. Appl. Phys. 2014, 115, 043912. [Google Scholar] [CrossRef]
- Zhang, Y.; Cao, X.; Tan, H.T.; Gill, V.; Lambourne, A.; Huang, Y.Z. Decomposition Behavior in the Early-Stage Oxidation of Sm2Co17-Type Magnets. Scr. Mater. 2021, 200, 113911. [Google Scholar] [CrossRef]
- Xu, H.; Wang, H.; Liu, J.; Zhang, T.; Zhou, C.; Jiang, C. Silicide Coating Stabilized High-Temperature Performance and Oxidation Resistance Mechanism of 2:17-Type SmCo Permanent Magnets—Sciencedirect. Corros. Sci. 2020, 173, 108752. [Google Scholar] [CrossRef]
- Safdar, S.; Jiang, C.; Rathore, F. High Thermal Stability and Corrosion Resistance of SmCo Based Magnets Surface Modified by Ni Coating. In Proceedings of the 2017 14th International Bhurban Conference on Applied Sciences and Technology (IBCAST), Islamabad, Pakistan, 10–14 January 2017; pp. 44–49. [Google Scholar]
- Liu, Z.; Zhang, C.; Wu, H.; Chen, R.; Yan, A. Enhancement of Domain Wall Pinning in High-Temperature Resistant Sm2Co17 Type Magnets by Addition of Y2O3. Materials 2022, 15, 5160. [Google Scholar] [CrossRef]
- Liu, L.; Jin, T.; Jiang, C. High-Temperature Oxidation Resistance and Magnetic Properties of Si-doped Sm2Co17-Type Magnets at 500 °C. J. Magn. Magn. Mater. 2012, 324, 2310–2314. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, B.; Wang, H.; He, Y.; Xu, C.; Wang, X.; Zhang, W.; Zhang, Z.; Jiang, C. Preparation of Low Remanence Temperature Coefficient (RT—300 °C) SmDy(Co, Fe, Cu, Zr)z Magnets and Molecular Field Analysis. J. Magn. Magn. Mater. 2018, 446, 38–43. [Google Scholar] [CrossRef]
- Zhang, T.L.; Zhang, B.; Wang, H.; Jiang, C.B.; Zhang, Z.H.; Wang, X.Q.; Zhang, W. Low Remanence Temperature Coefficient Sm1-xErx(Co, Fe, Cu, Zr)z Magnets Operating up to 400 °C. Rare Met. 2020, 39, 70–75. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Z.; Zhang, X.; Zhang, C.; Li, T.; Lee, D.; Yan, A. 2:17 type SmCo Magnets with Low-Temperature Coefficients of Remanence and Coercivity. J. Magn. Magn. Mater. 2019, 473, 376–380. [Google Scholar] [CrossRef]
- Wang, Q.Y.; Zheng, L.; An, S.Z.; Zhang, T.L.; Jiang, C.B. Thermal Stability of Surface Modified Sm2Co17-Type High Temperature Magnets. J. Magn. Magn. Mater. 2013, 331, 245–249. [Google Scholar] [CrossRef]
- Wu, H.L.; Long, Z.M.; Song, K.Q.; Li, C.Q.; Cong, D.L.; Shao, B.; Liu, X.W.; Ma, Y.L. The Effect of Temperature Cycling on the Magnetic Degradation and Microstructure of a Zn-Coated NdFeB Magnet. Coatings 2022, 12, 660. [Google Scholar] [CrossRef]
- Wang, J.; Chen, R.; Rong, C.; Zhuang, L.; Zhang, H.; Shen, B.; Yan, A. The Magnetization Behavior and Magnetic Viscosity of Sm(Co, Fe, Cu, Zr)Z Ribbons with Different Temperature Dependence of Coercivity. J. Appl. Phys. 2010, 107, 09A707. [Google Scholar] [CrossRef]



| Sample | Sm | Co | Fe | Cu | Zr |
|---|---|---|---|---|---|
| SmCo | 11.43 | 57.37 | 19.03 | 9.94 | 2.23 |
| Y | Temperature Range | Fitting Parameter | ||||||
|---|---|---|---|---|---|---|---|---|
| Xc | S.E.(Xc) | A | S.E.(A) | P | S.E.(P) | R2 | ||
| Loss of Φ | R.T.–180 °C | 0.00 | 7.79 | 0.32 | 0.13 | 0.35 | 0.08 | 0.99 |
| R.T.–250 °C | 0.00 | 8.38 | 0.49 | 0.20 | 0.32 | 0.09 | 0.98 | |
| R.T.–300 °C | 0.00 | 0.27 | 0.61 | 0.08 | 0.32 | 0.03 | 0.99 | |
| Y | Temperature Range | Simulated Value | |||
|---|---|---|---|---|---|
| 1st Cycle | 3rd Cycle | 5th Cycle | 5000th Cycle | ||
| Loss of Φ/% | R.T.–180 °C | 0.32 | 0.47 | 0.56 | 6.44 |
| R.T.–250 °C | 0.60 | 0.82 | 0.94 | 6.63 | |
| R.T.–300 °C | 0.61 | 0.87 | 1.02 | 9.38 | |
| Constant Temperature/°C | Pretreated | Fitting Parameter | ||||||
|---|---|---|---|---|---|---|---|---|
| Xc | S.E.(Xc) | A | S.E.(A) | P | S.E.(P) | R2 | ||
| 80 | / | 0.00 | 16.41 | 0.61 | 0.27 | 0.30 | 0.08 | 0.99 |
| −50–250 °C × 3 cycles | 0.00 | 17.26 | 0.79 | 0.27 | 0.21 | 0.07 | 0.99 | |
| 120 | / | 0.00 | 23.32 | 0.96 | 0.48 | 0.23 | 0.09 | 0.98 |
| −50–250 °C × 3 cycles | 0.00 | 7.84 | 0.88 | 0.13 | 0.21 | 0.03 | 0.99 | |
| 180 | / | 0.00 | 23.92 | 1.14 | 0.53 | 0.21 | 0.08 | 0.98 |
| −50–250 °C × 3 cycles | 0.00 | 14.86 | 0.96 | 0.28 | 0.20 | 0.05 | 0.99 | |
| Constant Temperature/°C | Pretreated | Loss of Φ/% (Simulated Value) | ||
|---|---|---|---|---|
| 2000th Day | 3650th Day | 6000th Day | ||
| 80 | / | 5.15 | 5.99 | 6.80 |
| −50–250 °C × 3 cycles | 4.03 | 4.58 | 5.09 | |
| 120 | / | 5.66 | 6.52 | 7.32 |
| −50–250 °C × 3 cycles | 4.43 | 5.08 | 5.76 | |
| 180 | / | 6.20 | 7.15 | 8.04 |
| −50–250 °C × 3 cycles | 4.74 | 5.39 | 6.00 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Long, Z.; Li, Z.; Song, K.; Li, C.; Cong, D.; Shao, B.; Liu, X.; Sun, J.; Ma, Y. Effect of Temperature Cycling Pretreatment on the Thermal Stability of Sm2(Co, Fe, Zr, Cu)17 Magnets in the Mild Temperature Range. Materials 2022, 15, 8830. https://doi.org/10.3390/ma15248830
Wu H, Long Z, Li Z, Song K, Li C, Cong D, Shao B, Liu X, Sun J, Ma Y. Effect of Temperature Cycling Pretreatment on the Thermal Stability of Sm2(Co, Fe, Zr, Cu)17 Magnets in the Mild Temperature Range. Materials. 2022; 15(24):8830. https://doi.org/10.3390/ma15248830
Chicago/Turabian StyleWu, Hulin, Zhimei Long, Zhongsheng Li, Kaiqiang Song, Chaoqun Li, Dalong Cong, Bin Shao, Xiaowei Liu, Jianchun Sun, and Yilong Ma. 2022. "Effect of Temperature Cycling Pretreatment on the Thermal Stability of Sm2(Co, Fe, Zr, Cu)17 Magnets in the Mild Temperature Range" Materials 15, no. 24: 8830. https://doi.org/10.3390/ma15248830
APA StyleWu, H., Long, Z., Li, Z., Song, K., Li, C., Cong, D., Shao, B., Liu, X., Sun, J., & Ma, Y. (2022). Effect of Temperature Cycling Pretreatment on the Thermal Stability of Sm2(Co, Fe, Zr, Cu)17 Magnets in the Mild Temperature Range. Materials, 15(24), 8830. https://doi.org/10.3390/ma15248830





