1. Introduction
Due to environmental pollution and the limitations of fossil fuel resources, solar energy has become an important part of human energy utilization [
1]. Modern solar energy technology can be affected by night, dust and clouds and cannot work continuously, which greatly reduces the efficiency of solar devices [
2]. In order to solve such problems, it is necessary to configure a set of energy storage devices to ensure continuous energy output. At present, nitrate is the main high-temperature energy storage medium.
The use target of the next generation of high-temperature energy storage media in solar-power-generation applications is 600–900 °C, which exceeds the stable temperature of nitrate [
3]. At high operating temperatures of solar power plants, 40 KCl + 60 MgCl
2 (wt%) molten salt has the advantages of low price, high density, high boiling point, low saturated steam pressure and good thermal stability. It is suitable for solar thermal power storage materials for use in tower and parabolic trough systems. However, chloride molten salt is highly corrosive; therefore, the corrosivity of Cl
− must be considered when designing energy storage materials [
4].
Chloride molten salt as an energy storage medium needs to work at high temperature, and thus its bearing equipment is mainly made of high-temperature-resistant materials. According to Sun et al. [
5], Inconel 625 alloy has good high-temperature corrosion resistance, and it is widely used in solar heat storage tubes. In order to explore the corrosion mechanism between Inconel 625 alloy and chloride energy storage medium, a large number of researchers have conducted relevant research.
Li, J.Y. [
6] studied the corrosion behavior of 316 stainless steel and Inconel 625 alloy in ZnCl
2, KCl and NaCl at 900 °C. He found that the two alloys were severely corroded in ternary chloride. A loose oxide layer was formed on the surface of the two alloys, and the oxide film did not protect the alloys. Ma, H.F. [
7] found that the corrosion rate of Inconel 625 alloy in alkaline earth metals was greater than that in alkali metals. Inconel 625 alloy needs to face the corrosion of molten salt during service, resulting in corresponding corrosion failure. If the corrosion pit exceeds the standard, it needs to be repaired to ensure the continued service of the product.
Inconel 625 flux-cored wire is the best choice for repairing castings. In recent years, the scarcity of nickel resources has led to the continuous rise in the price of Inconel 625 flux-cored wire. This has caused a large economic burden for solar power plants. In order to solve the problem of tight supply and high cost of nickel resources, it is urgent to find an element that can replace nickel. Through the literature, we found that N is a highly stable and austenitizing element [
8]. At the same time, it can effectively expand the range of the austenite zone in steel. Based on the design concept of low-nickel flux-cored wire and high-nitrogen steel, N is suggested to partially replace Ni in flux-cored wire.
In this paper, low-cost N is used to replace part of the Ni content. On the basis of the composition of Inconel 625 flux-cored wire, we reduce the content of Ni by 20%, increase the content of Cr, Fe, Mn, Al and W elements to balance and adjust the alloy powder and design a nitrogen-containing low-nickel flux-cored wire. Taking the corrosion performance of weld cladding prepared by Inconel 625 flux-cored wire as a reference, the corrosion environment in a solar energy heat storage pipe is simulated.
The high-temperature molten-salt corrosion depth and corrosion morphology of nitrogen-containing low-nickel weld cladding and Inconel 625 weld cladding in 40 KCl + 60 MgCl2 (wt%) at 900 °C are tested using the static immersion corrosion method. We explore the role of N in corrosion, provide theoretical guidance for the preparation of new welding materials with high-temperature corrosion performance not lower than that of nickel-based flux-cored wire and provide a material reference for the repair of solar energy heat storage pipes.
2. Materials and Methods
2.1. Experimental Materials and Methods
Schaeffler deeply studied the influence of alloy elements on the structure of stainless steel, converted it into the corresponding nickel equivalent Ni
eq and formed the famous Schaeffler diagram. However, this does not consider the effect of nitrogen. Speedel, M.O. [
9] studied the effects of N on Ni equivalent (Ni
eq) and corrected Ni
eq, providing guidance for the composition design of high-nitrogen stainless steel.
According to the modified nickel equivalent (Ni
eq) calculation Formula 1, every increase of 1 wt% N is equivalent to an increase of 18 wt% Ni [
8]. Co and C can also replace Ni to stabilize austenite. The increasing use of Co in batteries will also lead to higher prices in the next decade, and Co can cause allergic reactions, while C can enhance the formation trend of carbide and reduce corrosion resistance. It can be seen from Formula 1 that increasing the content of Mn cannot replace Ni but can reduce the Ni equivalent; therefore, N is the most reasonable element to replace Ni.
This paper involves four groups of flux-cored wires. The first three groups are nitrogen-containing low-nickel flux-cored wires, and the three weld claddings were marked as 1, 2 and 3, respectively. The fourth group was the comparison welding wire (weld cladding of Inconel 625) and was recorded as 4. Referring to the composition of Inconel 625 flux-cored wire, the appropriate content of Ni was controlled at 44.0%, the range of Cr was 20.0–24.0%, and the range of N was 0.15–0.21%. Carbon sulfur analysis and chemical methods were used to test the elemental content of the welding wire. The main components of the four sets of flux-cored wires are shown in
Table 1. The experimental material was the newly developed nitrogen-containing low-nickel flux-cored wire.
By adding manganese nitride and chromium nitride alloy powder to the core powder, N was used to partially replace Ni. Ni, Cr, Fe, Mn, Mo, Co, Cu, Ti, Al, Nb and W powders with purities above 99.9% were selected to adjust their element ratio. The powder mixer was used to uniformly mix the powder. Inconel 718 strip was selected for the preparation of flux-cored wire. The diameter of the newly developed nitrogen-containing low-nickel flux-cored wire was 2.55 mm.
The method of MIG welding was used for surfacing on a Q235 substrate. The size of the Q235 substrate was 180 × 100 × 20 mm. The normative reference of Q235 steel is shown in
Table 2. In order to avoid the influence of dilution of weld cladding, three layers of weld cladding with a height of 12 mm were prepared. The welding process parameters were as follows: the welding current was 180 A, welding voltage was 28 V, welding speed was 8 m/h, and gas flow was 10 L/min.
In order to reduce the splash overflow of N during welding, the shielding gas used in this test was 97%Ar + 3%N
2. According to Cheng J H [
10], when 3%N
2 was added, there were no pores in the weld cladding, and the N fixation effect was best. In the surfacing process, each layer of surfacing was equivalent to the heat treatment of the previous layer, which can play a role in refining the grain. Therefore, the particle size of the third layer was relatively large.
At the same time, in order to avoid the influence of dilution, it was not acceptable to sample from the surface of the first layer; thus, the sample was taken from the second layer of the surfacing layer. The sampling size was 10 × 10 × 2 mm. The weld cladding samples were polished with 200#–2000# metallographic abrasive paper, cleaned with acetone ultrasonic and dried for standby.
We referred to the national standards for corrosion test ASTM G31-2012a “Standard Guide for laboratory immersion corrosion testing of metals”. The static immersion corrosion method was used to simulate the working conditions of a solar heat storage pipe at 900 °C, and this was compared with Inconel 625 weld cladding. The molten salt used in this experiment was 40 KCl + 60 MgCl2 (wt%) (with purity greater than 99.5%).
The salt mixture was dried in oven at 300 °C for 48 h inside a glove box, which was filled with high purity Ar (the H
2O and O
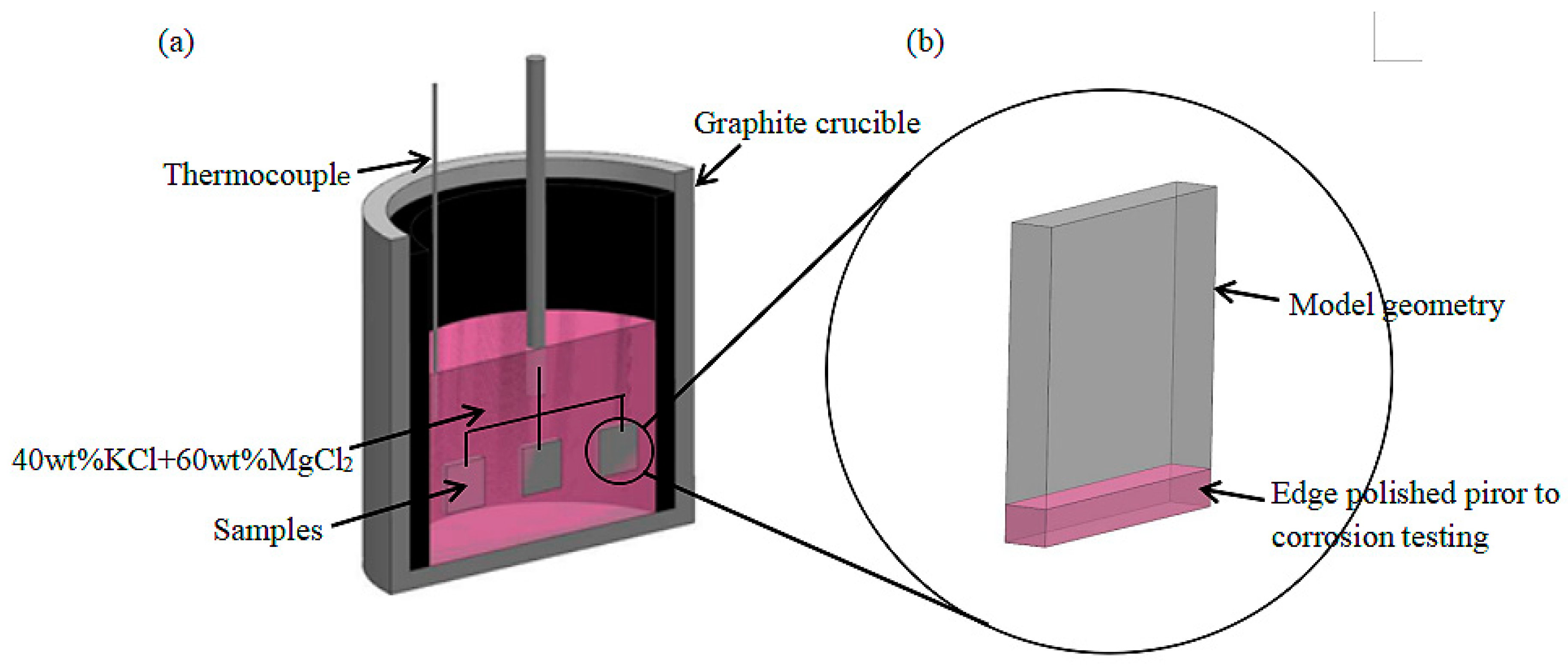
2 contents were maintained below 3 and 5 mg/kg, respectively), and was stored in the glove box before the corrosion test. For the high-temperature immersion corrosion test under an atmospheric environment, the thermocouple was placed in the drying tank to control the test temperature as shown in
Figure 1a. The weld cladding was placed in the molten salt for high-temperature corrosion while ensuring that the molten salt completely covered the sample.
It should be noted that the edges of the sample were polished before the high-temperature molten salt immersion corrosion test as shown in
Figure 1b. The corrosion temperature was set at 900 °C, and the holding times were 10, 20, 30, 40, 50 and 60 h. After the molten salt test, the corroded samples were ultrasonically cleaned with distilled water and acetone to remove the molten salt and loosely adhered corrosion products. Before and after the corrosion test, we used a 0.1 mg electronic balance to weigh the samples and calculate the weight loss. In order to ensure the representativeness of the test data, three samples were used in each group, and the test results are the average values.
2.2. Properties of Weld Cladding
Before high-temperature molten-salt corrosion, the weld cladding layer was cut into small pieces of 10 × 10 × 10 mm, and then we used 200#–2000# abrasive paper to polish the surface of the weld cladding. We then used water-soluble diamond grinding paste with a particle size of 1.5 to mechanically polish the sample. The treated samples were etched with 50 g CuCl2 + 100 mL alcohol + 100 mL concentrated hydrochloric acid for 25–35 s. After corrosion, the metallographic samples were immediately washed with tap water, then wiped with absolute ethanol and finally dried with a hair dryer for standby.
The microstructure of weld cladding was observed using a S-3400N scanning electron microscope, and the accelerating voltage was 30 KV. A JEM-2100 transmission electron microscope (TEM) was used to characterize the precipitated phase structure of the weld cladding to determine the influence of precipitation on the relative properties. The specific preparation process of TEM samples was as follows: a thickness of 600 μm was cut from the weld cladding and finally manually ground to 50 μm.
A puncher was used to prepare Φ 3 mm flakes, and then we ground off the burrs with 5000# sandpaper. A TJ100-SE electrolytic double jet thinning instrument was used to prepare the transmission samples. The thinning of the sheet outlet automatically stopped. The electrolyte was a mixture of alcohol and perchloric acid with a volume ratio of 9:1. The temperature was about −25 °C, the current was 120 mA, and the voltage was 39 V.
After the high-temperature molten salt immersion corrosion, the phase composition of the weld cladding was measured using an XRD-7000. The specific parameters were as follows: a pure Cu target, the tube voltage was 40 kV, the current was 30 mA, the scanning speed was 2°/min, and the scanning range was 20–90°. Cross-section specimens of welding cladding were prepared by using standard grinding and polishing procedures. A Geminisem 300 scanning electron microscope and EPMA-1720 electron probe were used to analyze the morphology and element distribution of the cross section of the weld cladding.
3. Experimental Results
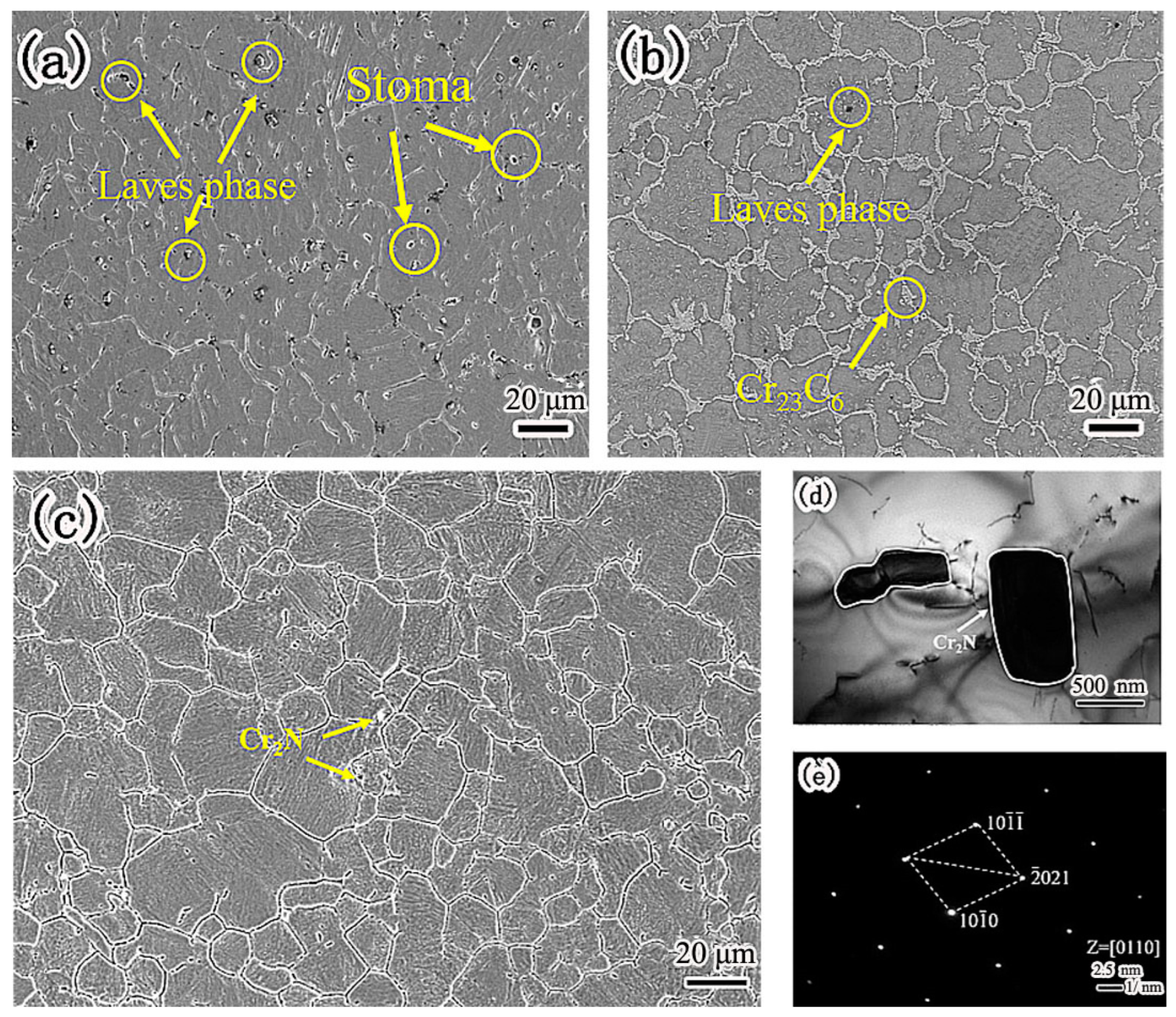
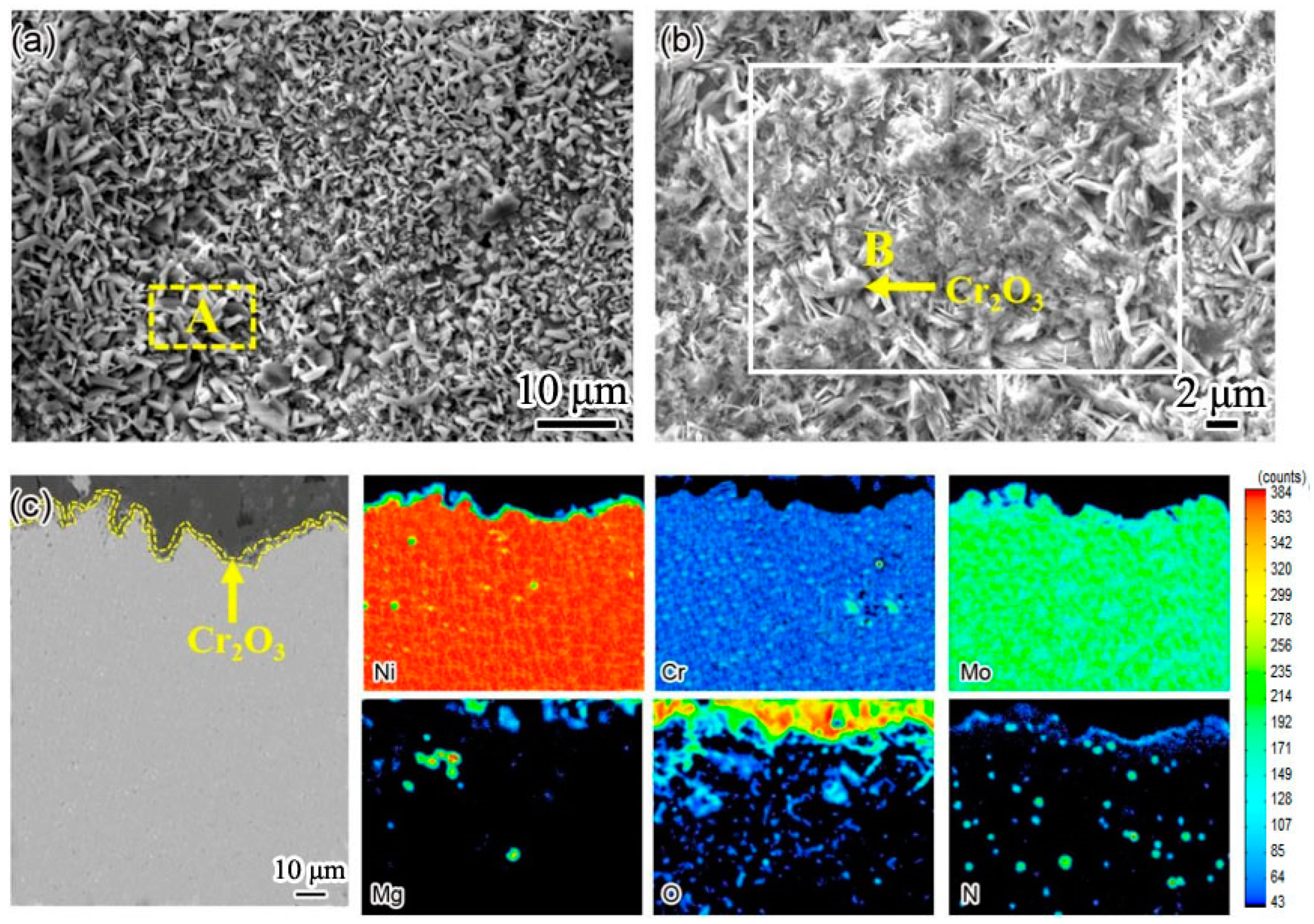
Figure 2 is the microstructure of nitrogen-containing low-nickel weld cladding. There were many massive precipitates of nitrogen-containing low-nickel weld cladding 1, which were arranged irregularly as shown in
Figure 2a. According to the energy spectrum analysis in
Table 3, the irregular precipitates contained a great deal of Ni, Cr, Mo and Nb elements. After calculation, the sum of the Ni, Fe and Cr atomic percentages was 58.48%. The sum of the Nb, Mo and Ti atomic percentages was 29.61%. The precipitates approximately conform to A
2B and have Laves (Ni, Fe, Cr)
2(Nb, Mo, Ti) structure. Therefore, it is judged that this phase is Laves phase.
In
Figure 2a, the N content is 0.21%. It can be seen from
Figure 2a that there are blowholes. In welding, the blowholes of the weld cladding have a great impact on the corrosion performance.
Figure 2b shows the microstructure of nitrogen-containing low-nickel weld cladding 2. According to the energy spectrum analysis of the precipitates, the precipitates are Laves phase + Cr
23C
6. In
Figure 2b, the content of N is 0.15%, the content of N is small, and no supersaturated N reacts with Cr to generate Cr
2N. N is dissolved in the matrix.
In nitrogen-containing low-nickel weld cladding 3, the content of N was 0.18%, and the supersaturated N combined with Cr to form Cr
2N. There is discontinuous precipitation along the grain boundary as shown in
Figure 2c. Through energy spectrum analysis, the atomic ratio of Cr to N was found to be 2:1, which shows that the flake precipitate is Cr
2N. In order to further explore the material of the precipitated phase, the crystal structure was analyzed by TEM.
Figure 2d shows the TEM morphology of grain boundary precipitates in nitrogen-containing low-nickel weld cladding 3. It can be seen from
Figure 2e that the precipitates had a hexagonal structure. After calibration, the spot of massive precipitate was Cr
2N, and the crystal band axis was [0110].
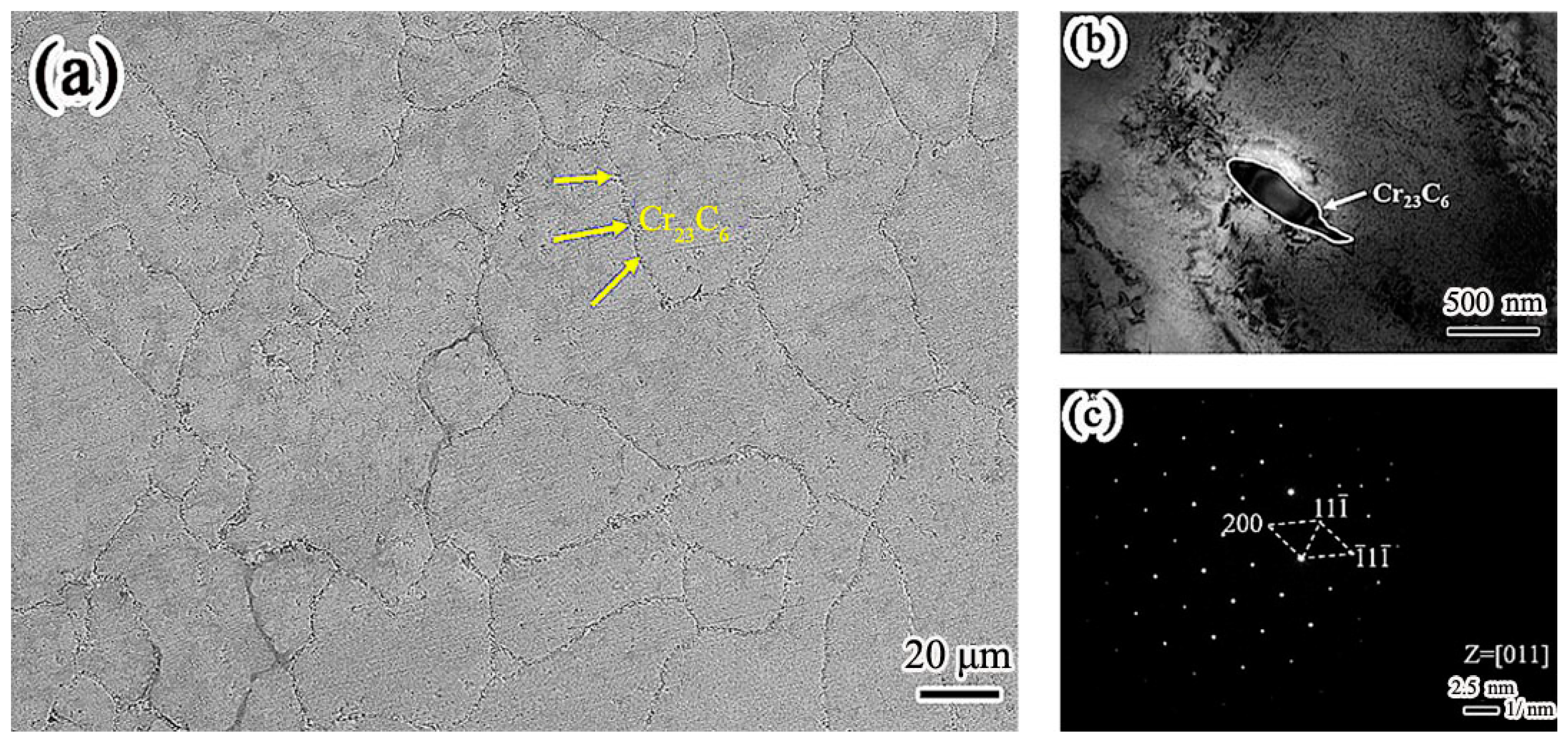
Figure 3 is the microstructure of the Inconel 625 weld cladding. In contrast, continuous precipitation was observed at the grain boundary of the Inconel 625 weld cladding as shown in
Figure 3a.
Figure 3b shows the TEM morphology of grain boundary precipitates in Inconel 625 weld cladding. The morphology of the precipitates is strip. It can be seen from
Figure 3c that the precipitates along the grain boundary have face-centered cubic (FCC) structure. After calibration, the spot was Cr
23C
6, and the crystal band axis was [011].
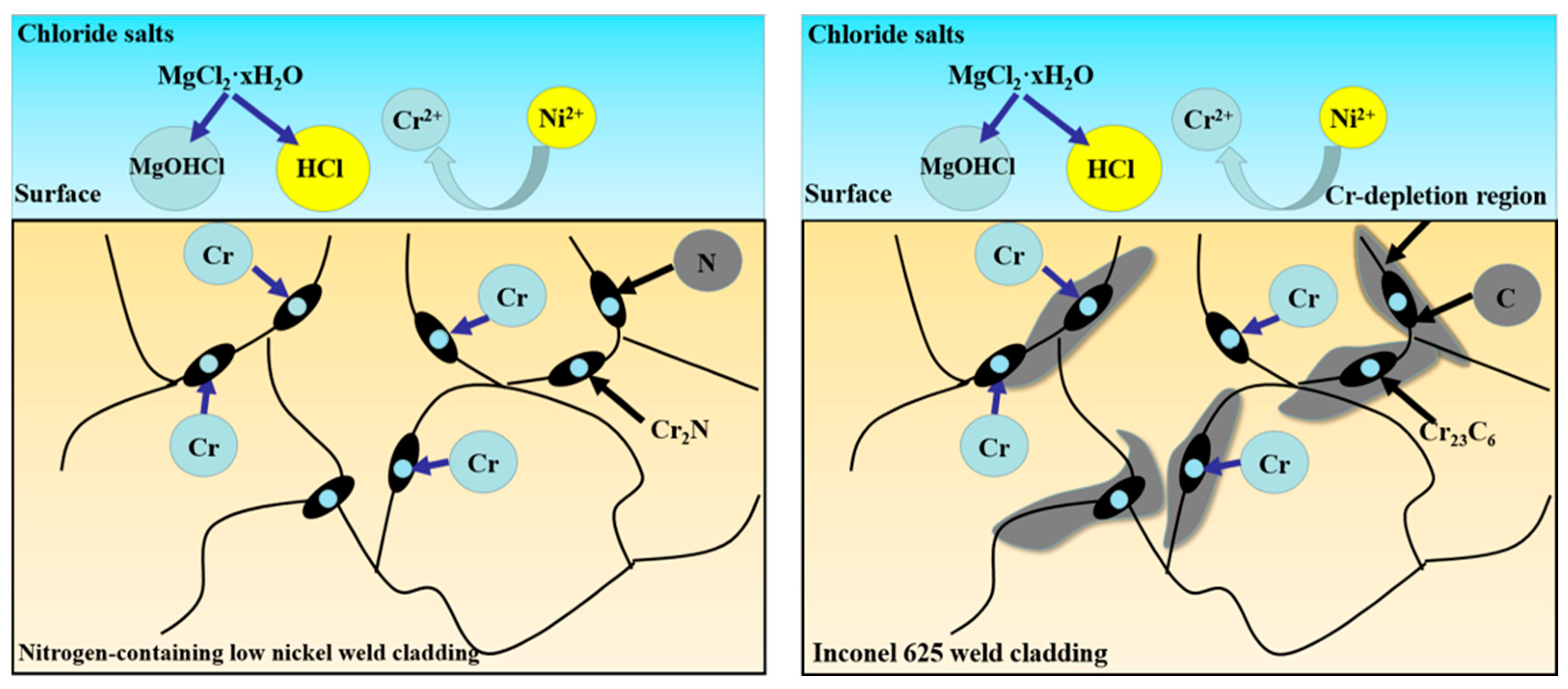
By comparing the microstructure of the weld claddings, it can be seen that both of them are austenite phase; however, the precipitates are different. This is the reason that the corrosion resistance of the weld claddings is different. Cr2N precipitated from nitrogen-containing low-nickel weld cladding will play a positive role in resisting high-temperature molten-salt corrosion.
Figure 4 is the corrosion weight loss curve and corrosion rate diagram of weld claddings 1, 2, 3 and 4. It can be seen from
Figure 4a that the corrosion weight loss of weld claddings 1, 2, 3 and 4 shows an increasing trend with the increase in corrosion time. However, the increasing range is different for different time intervals. The corrosion weight loss of weld cladding 1 increased sharply from 0 to 60 h, and the corrosion amount was large. It can be seen from the local enlarged drawing that the corrosion amount of weld claddings 2, 3 and 4 increased slowly before 10 h, and the corrosion weight loss increased sharply after 10 h.
We further clarify the influence of corrosion products on the corrosion rate. Before and after corrosion, the samples were weighed with an electronic balance (with an accuracy of 0.1 mg). Under the condition of uniform corrosion, the corrosion rate was calculated according to Formula (2).Weld claddings with corrosion times of 10, 30 and 60 h were selected to calculate the corrosion rate. The results are shown in
Figure 4b. It can be seen from the corrosion rate diagram that the corrosion rates of weld claddings 1, 2, 3 and 4 after 60 h of corrosion were 93.15, 10.63, 8.65 and 9.65 μm/year.
The corrosion rates of other weld claddings were low except for weld cladding 1. This is because the precipitated phase of weld cladding 1 was mainly Laves phase, and there were air holes; thus, the high-temperature corrosion resistance was poor. The maximum acceptable corrosion rate combined with solar-power-generation application is generally 30 µm/year, and the weld claddings 2, 3 and 4 meet the requirements of engineering applications. The corrosion rate of weld cladding 3 was lower than that of weld cladding 4, which indicates that it is feasible to increase N and reduce Ni in flux-cored wire.
CRcorr is the uniform corrosion rate (μm/year), m is the mass of the sample before immersion corrosion (g), mt is the mass of the sample after immersion corrosion (g), S1 is the total area of the immersed sample (cm2), t is the immersion corrosion test time (h), and ρ is the density of the metal (g/cm3).
In the corrosion process, with an increase in the corrosion time, the contact time between the weld-cladding surface and molten salt is prolonged, the corrosion reaction is intensified, and the products are increased. Most corrosion products are brittle oxides or compounds. In the subsequent cleaning process, the matrix cracks and peels off, resulting in an increase in the corrosion rate after 10 h. We found that 10 h was the abrupt change point of the corrosion behavior of weld claddings through the above curve of corrosion weight loss with time.
Therefore, the state of weld cladding after 10 h of corrosion was selected to analyze the corrosion products and corrosion mechanism.
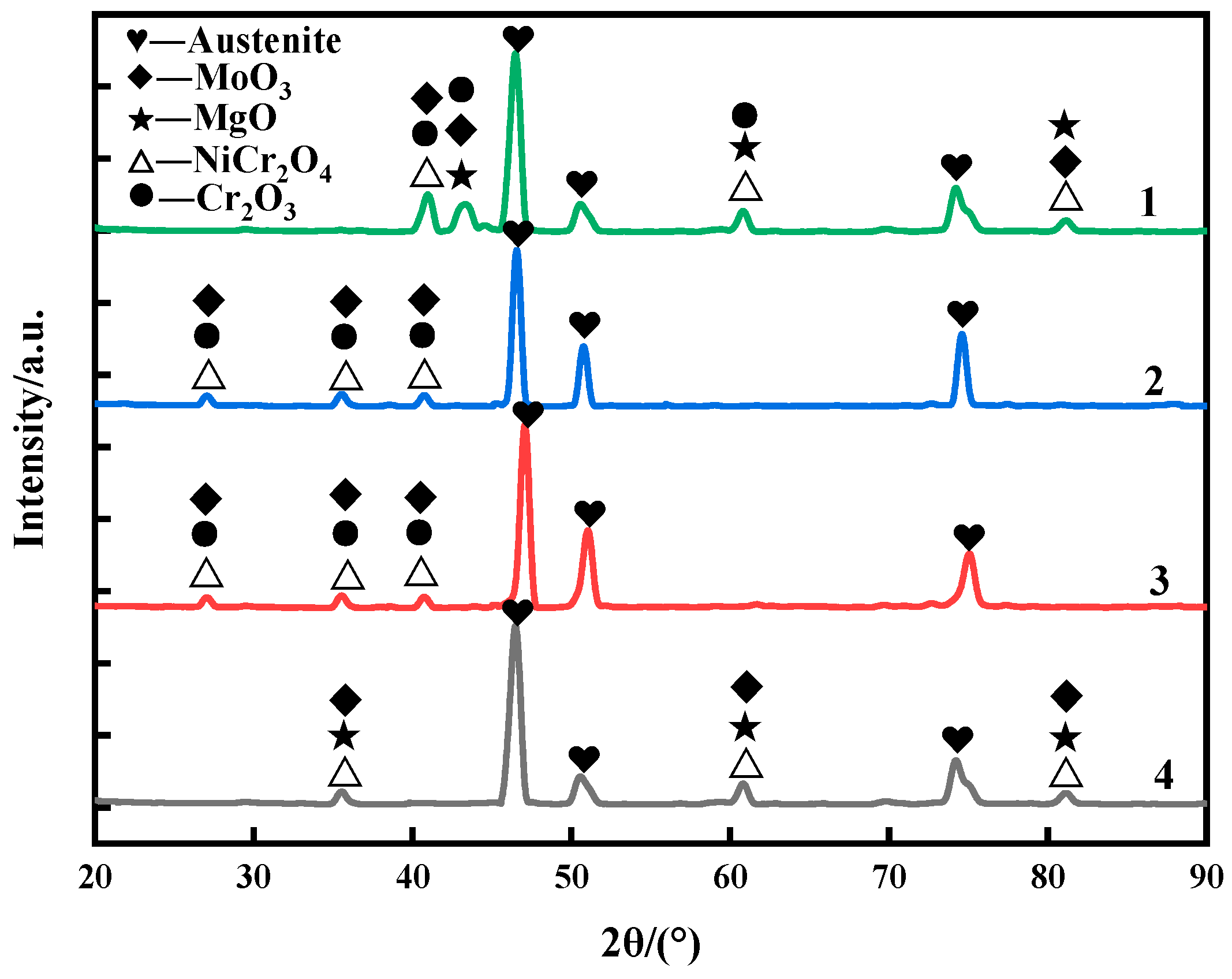
Figure 5 shows the surface phase composition of the four weld claddings after 10 h corrosion. It can be seen that the corrosion reaction was intense at 900 °C, and the weld claddings all correspond to the austenitic matrix. The corrosion products of nitrogen-containing low-nickel weld claddings 1, 2 and 3 contained MoO
3, NiCr
2O
4 and Cr
2O
3. The synergistic effect of N and Mo can stabilize the Cr
2O
3 protective layer [
10].
There is MgO precipitate on the surface of nitrogen-containing low-nickel weld cladding 1. MgO is unstable at high temperatures and will dissolve and fall off, which is the reason for the poor corrosion resistance of nitrogen-containing low-nickel weld cladding 1. Weld cladding 4 was only NiCr2O4, MoO3 and MgO. After corrosion, no Cr2O3 was detected on the surface of the Inconel 625 weld cladding, which means that the Cr2O3 protective film dissolved and fell off into the molten salt.
It can be seen from the local enlarged figure of corrosion loss and XRD that the corrosion resistance of nitrogen-containing low-nickel weld cladding 3 was better than that of the Inconel 625 weld cladding; therefore, 3 is the best formula for designing nitrogen-containing low-nickel flux-cored wire. Therefore, the corrosion mechanism of nitrogen-containing low-nickel weld cladding 3 and the Inconel 625 weld cladding will be discussed in the follow-up.
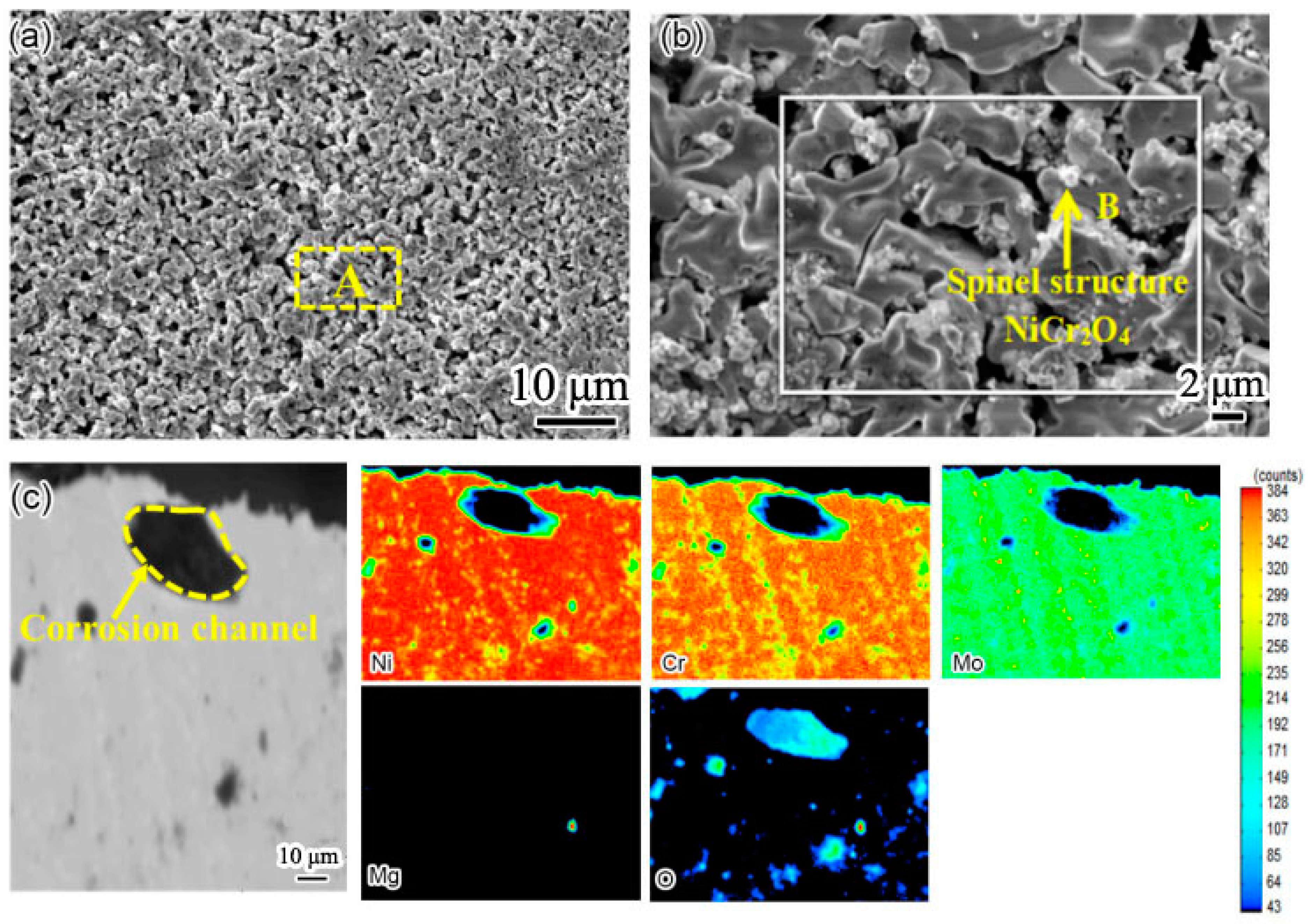
In order to clearly show the element distribution law of the two weld claddings sections, the cross sections of the two weld claddings after corrosion for 10 h were scanned and analyzed. The results are shown in
Figure 6 and
Figure 7.
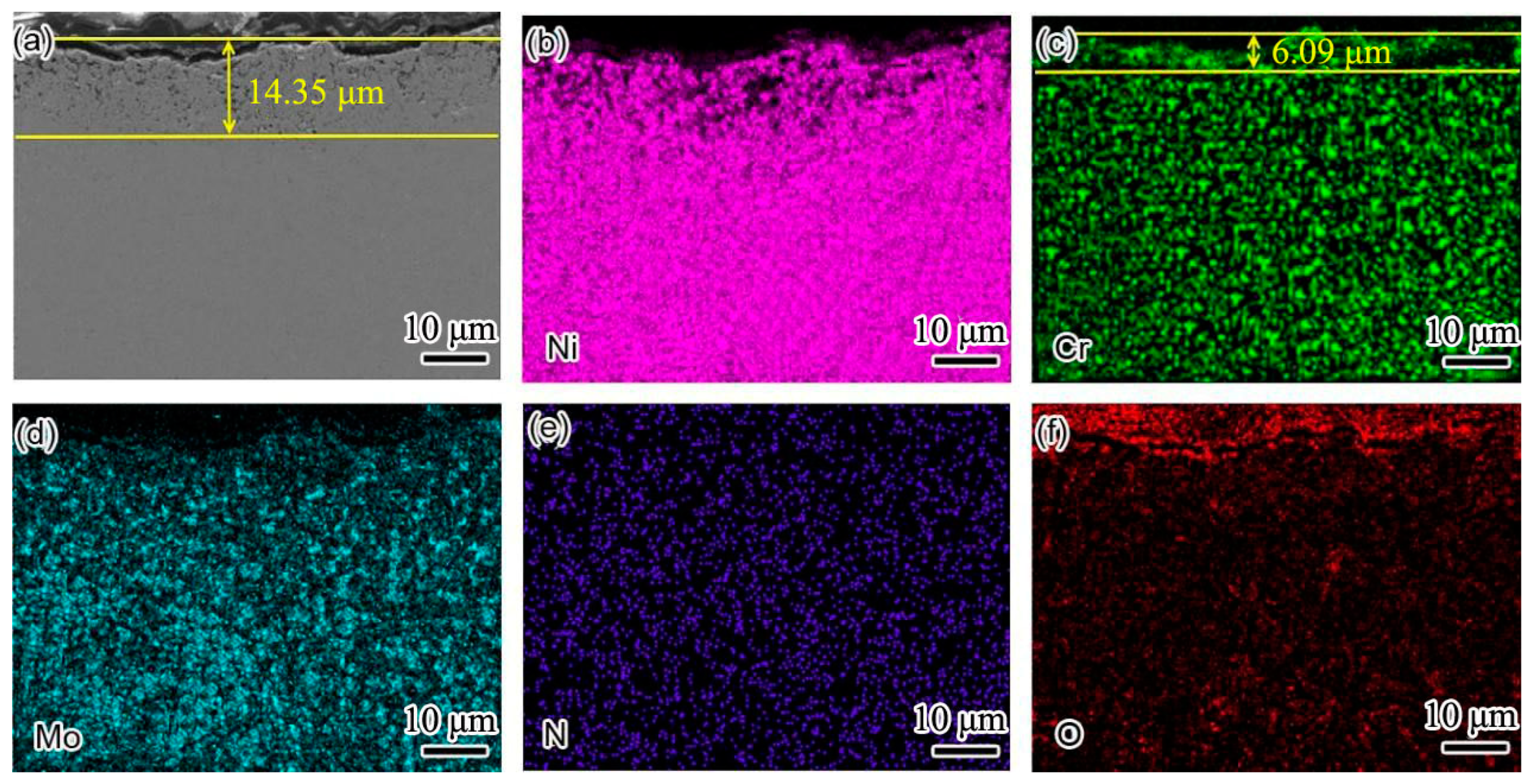
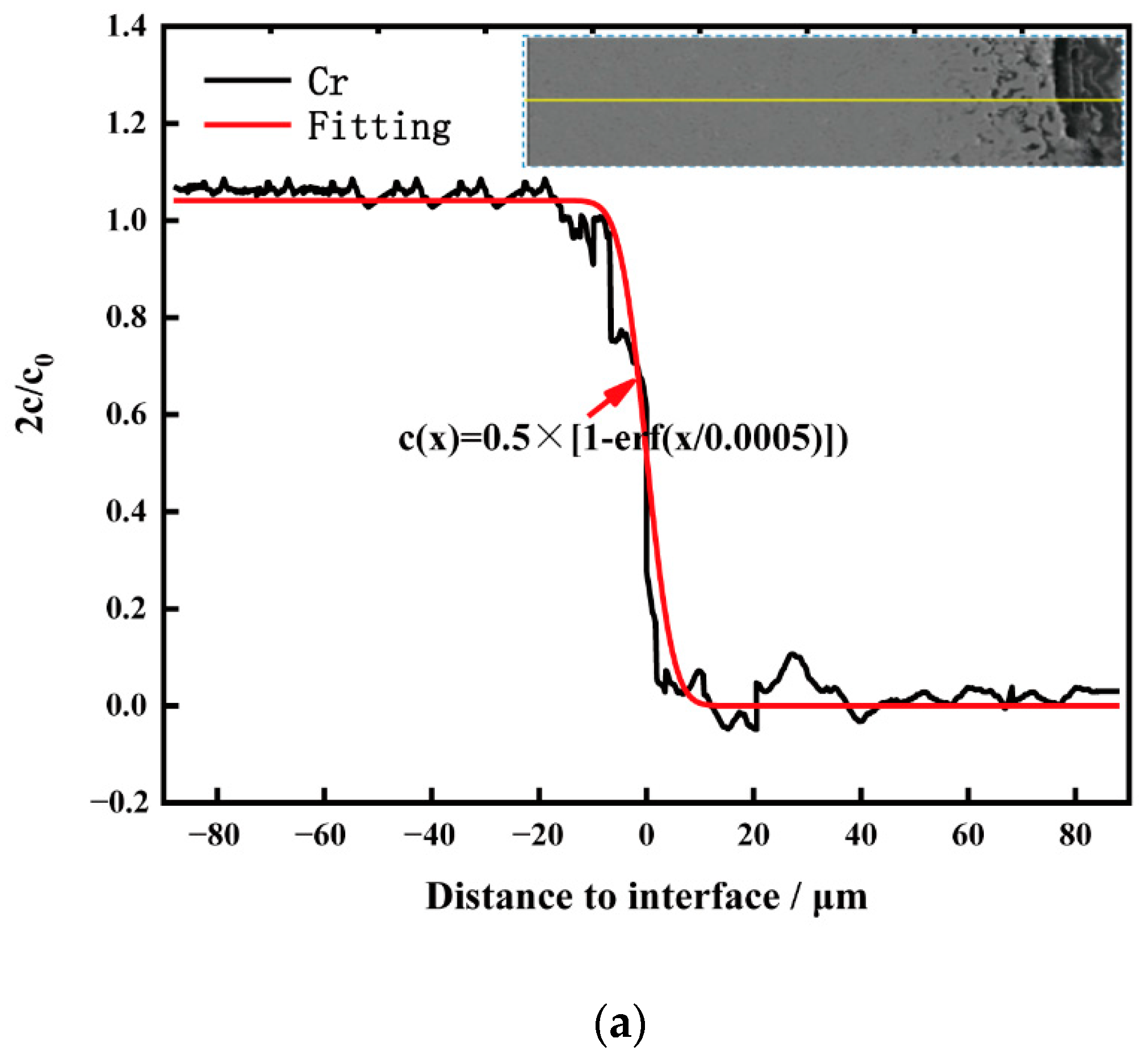
Figure 6 shows the cross-sectional element distribution of nitrogen-containing low-nickel weld cladding 3 after corrosion for 10 h. The distribution of Ni, Cr, N and Mo was uniform at 900 °C. The Cr diffused to the surface of the weld cladding and formed a 6.09 μm thick diffusion layer.
According to XRD analysis and the element distribution in
Figure 6, we determined that a Cr
2O
3 protective layer was formed on the weld-cladding surface. In addition, combined with the XRD results, we also found NiCr
2O
4 with a spinel structure on the weld-cladding surface. In the progress of corrosion reaction, the slight loss of Mo in the corrosion layer also shows that Mo elements migrated from the inside to the outside of the weld cladding. Mo elements first form a layer of MoO
2 at the interface of the weld cladding, and the MoO
2 oxide layer is oxidized to MoO
3 after cracking [
11].
D’Souza B [
12] proposed that, during the corrosion process, Mo can promote the corrosion inner layer to produce a large amount of oxygen, forming a stable Cr
2O
3 protective layer, which is consistent with the above results. According to
Figure 6e, N is dispersed in the weld cladding, and N is also enriched outside the corrosion layer. According to Wu, H.L. et al. [
9], N is an active element in the interface. It is easy to aggregate at the grain boundary, and some elements in the weld cladding can accelerate the dissolution of N, such as Cr, Mn and Mo.
Therefore, N is easy to combine with Cr and can precipitate at the grain boundary at an appropriate temperature to form Cr
2N. It is clear that N participates in the corrosion resistance reaction of the weld-cladding surface and greatly improves the corrosion resistance of nitrogen-containing low-nickel weld cladding. This result is consistent with the results obtained in [
11].
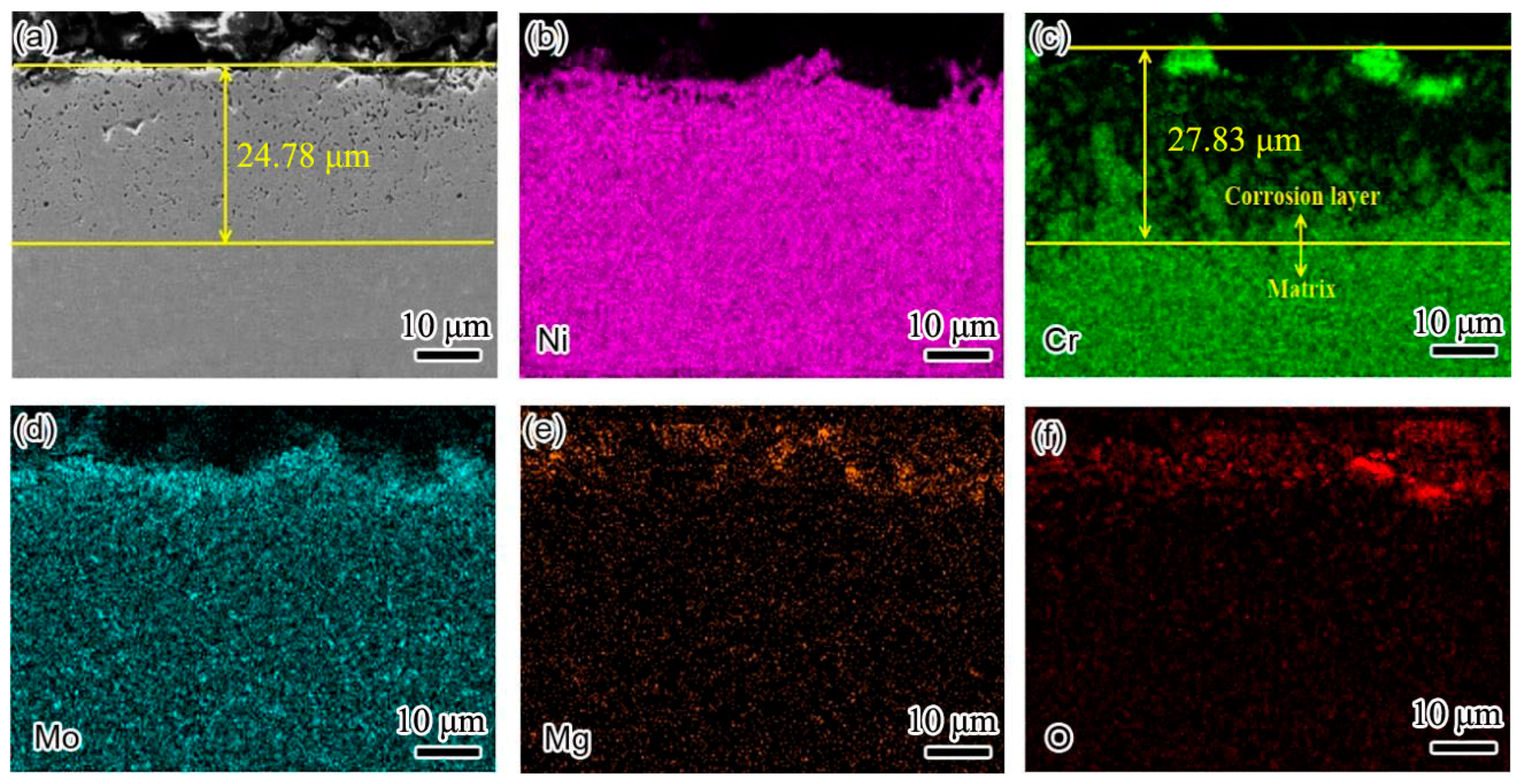
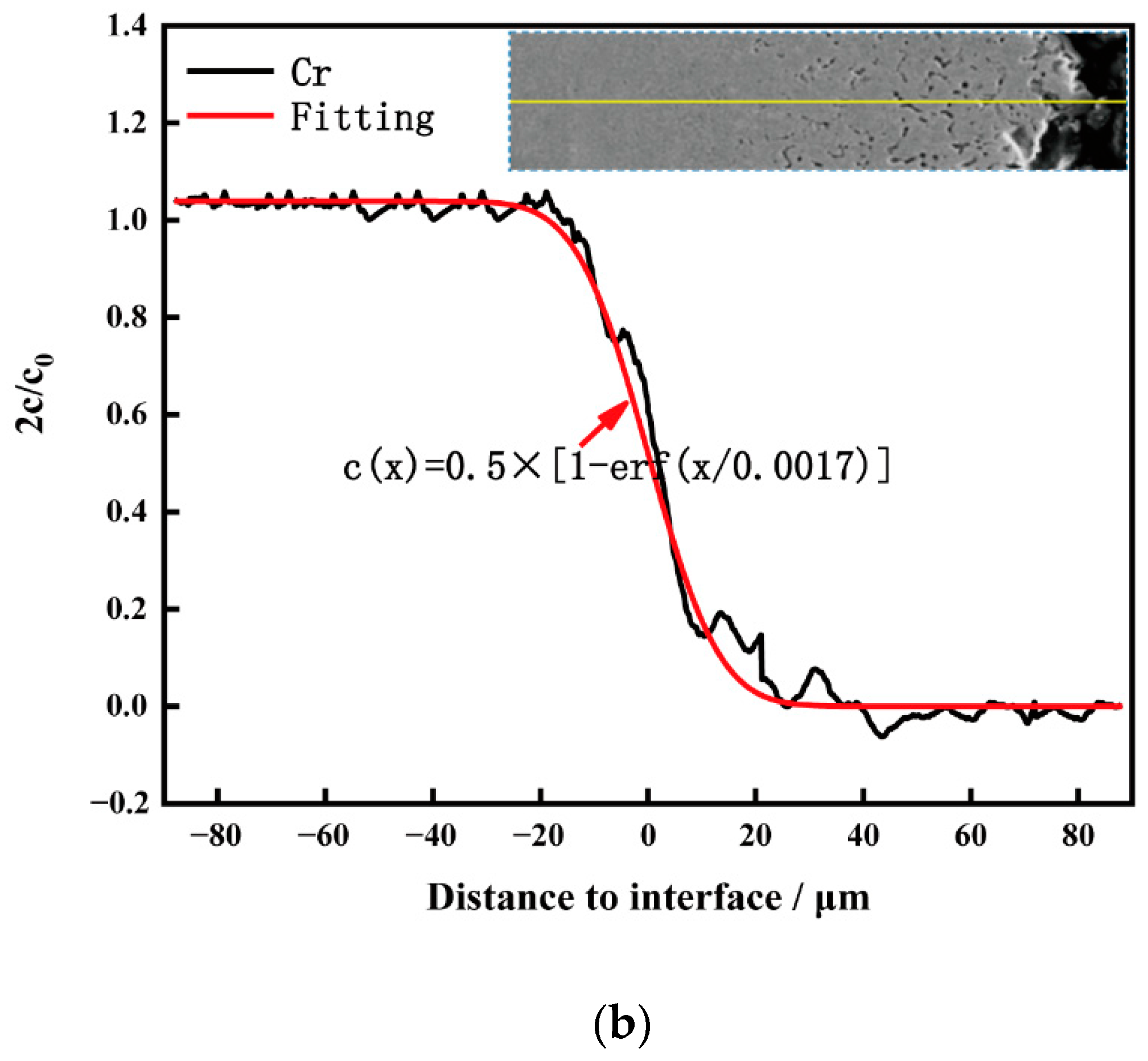
Figure 7 shows the cross-sectional element distribution of the Inconel 625 weld cladding after corrosion for 10 h. By comparing the corrosion depth in
Figure 6, the corrosion depth increased from 14.35 to 24.78 μm. This shows that the corrosion resistance of the weld cladding was improved by adding N. At 900 °C, the Inconel 625 weld cladding corroded for 10 h. The Inconel 625 weld cladding produced a 27.83 μm Cr-poor layer, which was also the main reason for the decline of its corrosion resistance. At 900 °C, there was still a small amount of Mg on the surface of the Inconel 625 weld cladding. According to XRD analysis, the surface corrosion products were mainly spinel NiCr
2O
4, MoO
3 and a small amount of MgO precipitation.