Thermal Properties of Porous Mullite Ceramics Modified with Microsized ZrO2 and WO3
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Proportions
2.3. Sample Preparation Methods
2.4. XRD Analysis
2.5. SEM Analysis
2.6. Apparent Porosity
2.7. Hg Porosimetry
2.8. Thermal Analysis
2.9. Thermal Shock Resistance Testing
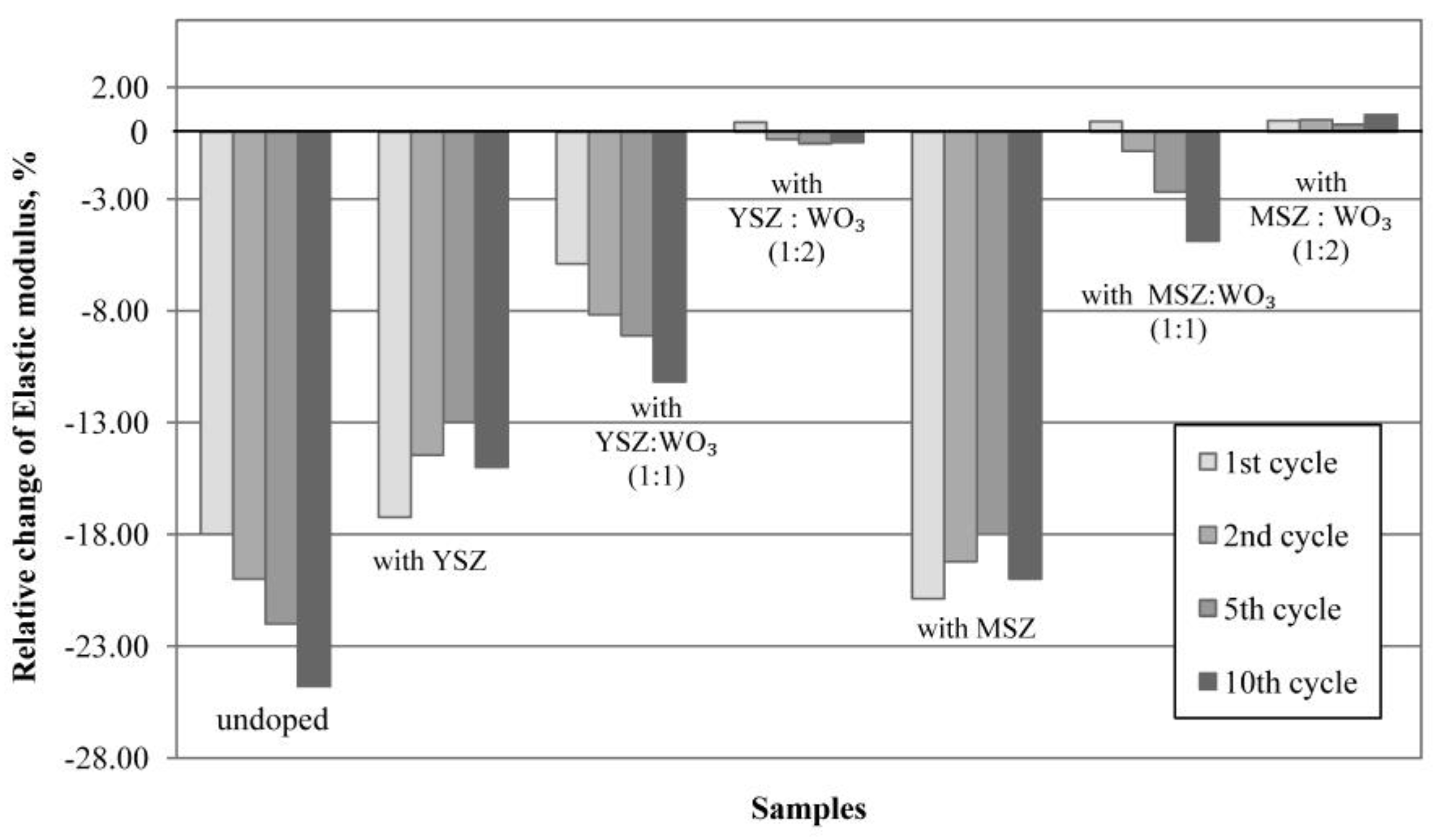
2.10. Elasticity Modulus Determination
3. Results and Discussion
3.1. Mineralogical Phase Composition
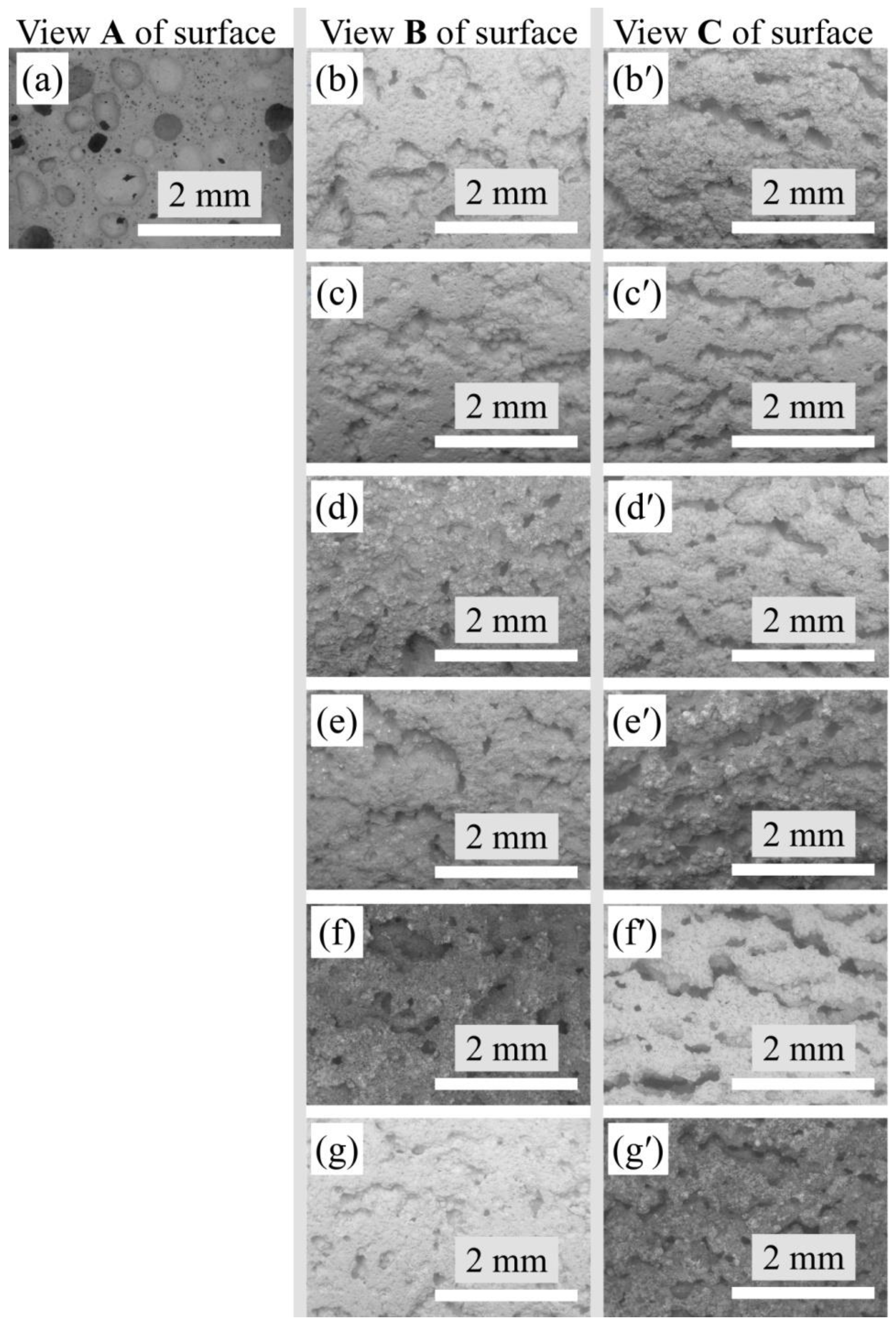
3.2. Macrostructure
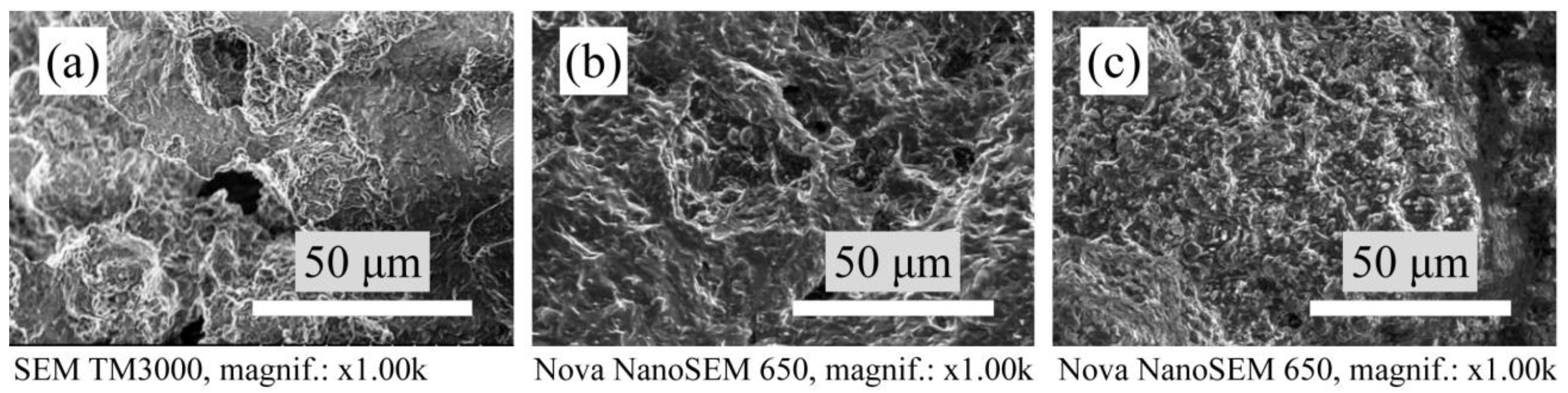
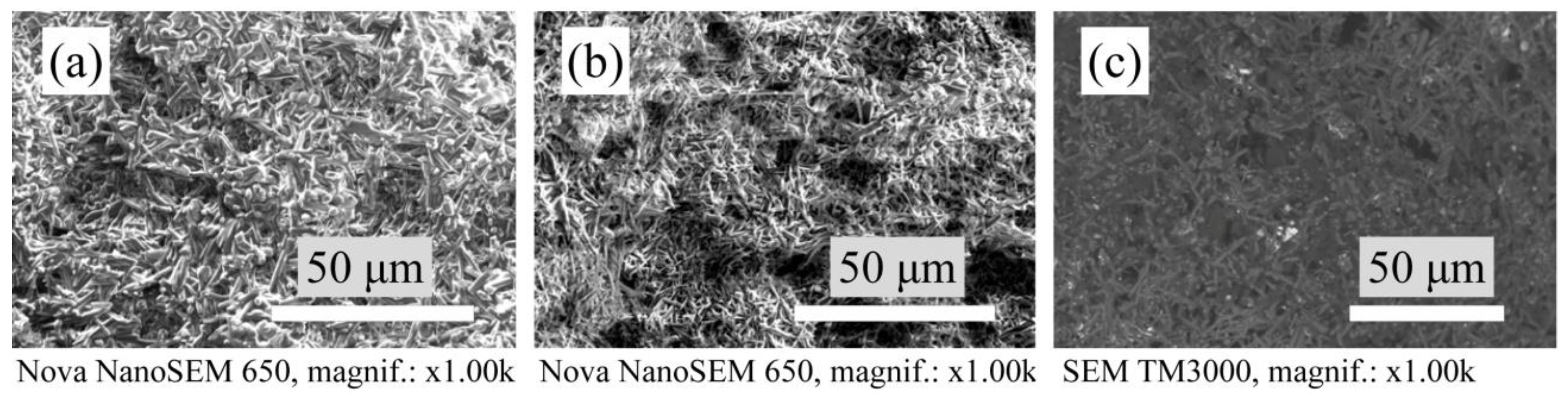
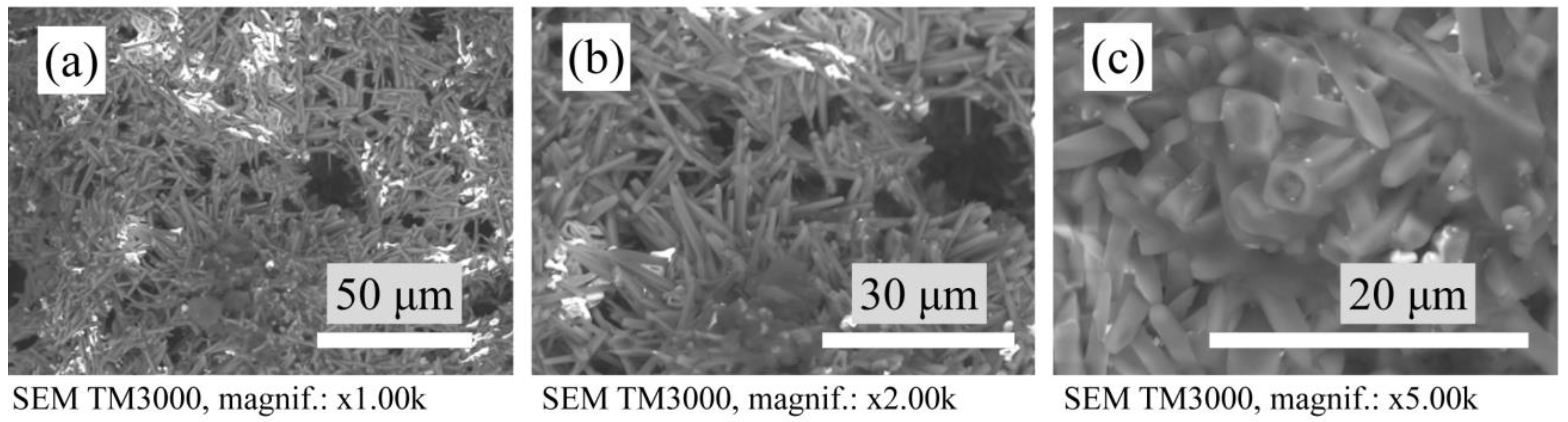
3.3. Microstructure
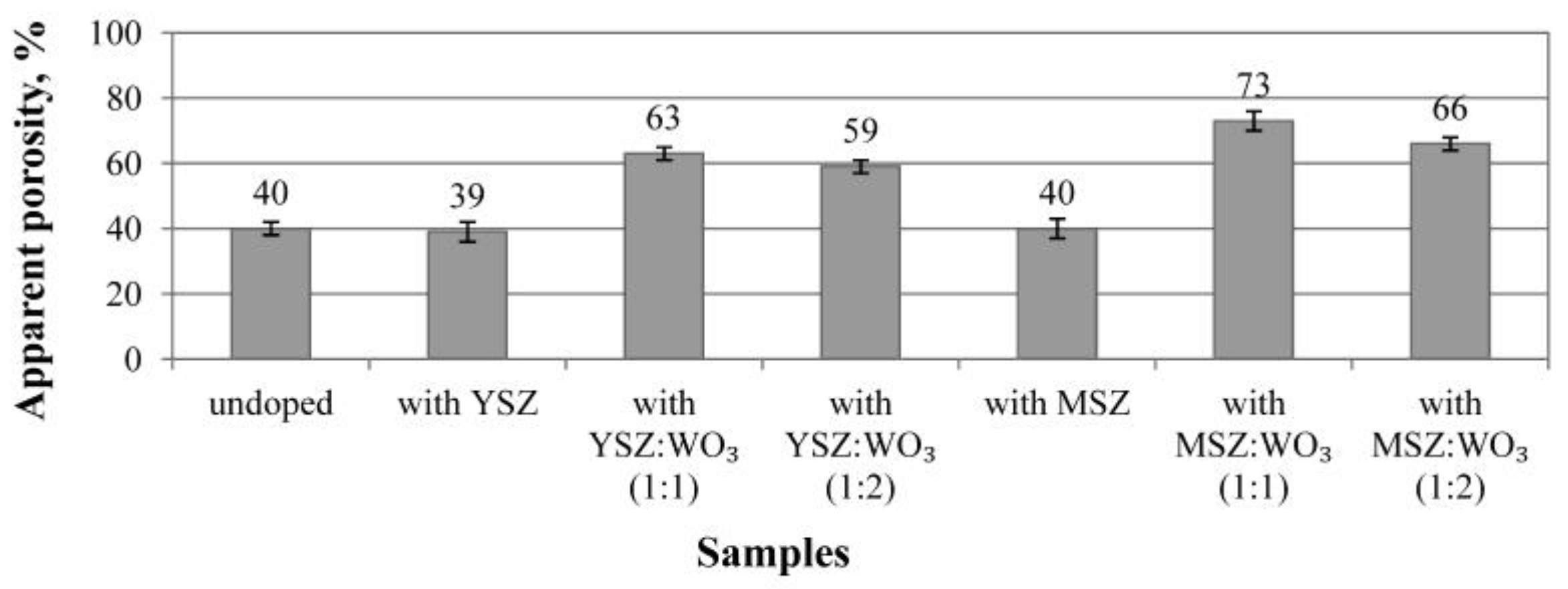
3.4. Porosity and Pore Size Distributions
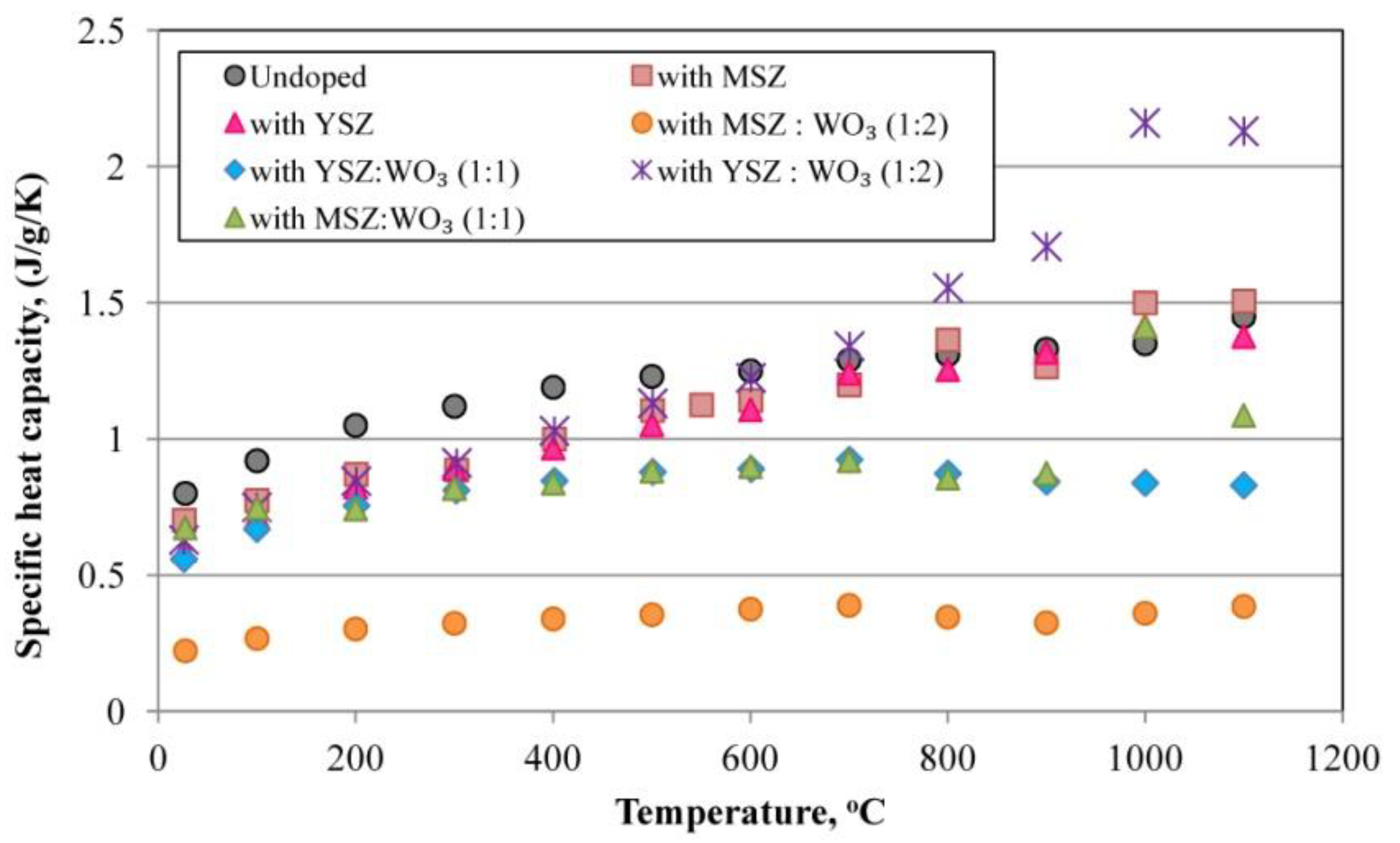
3.5. Specific Heat Capacity
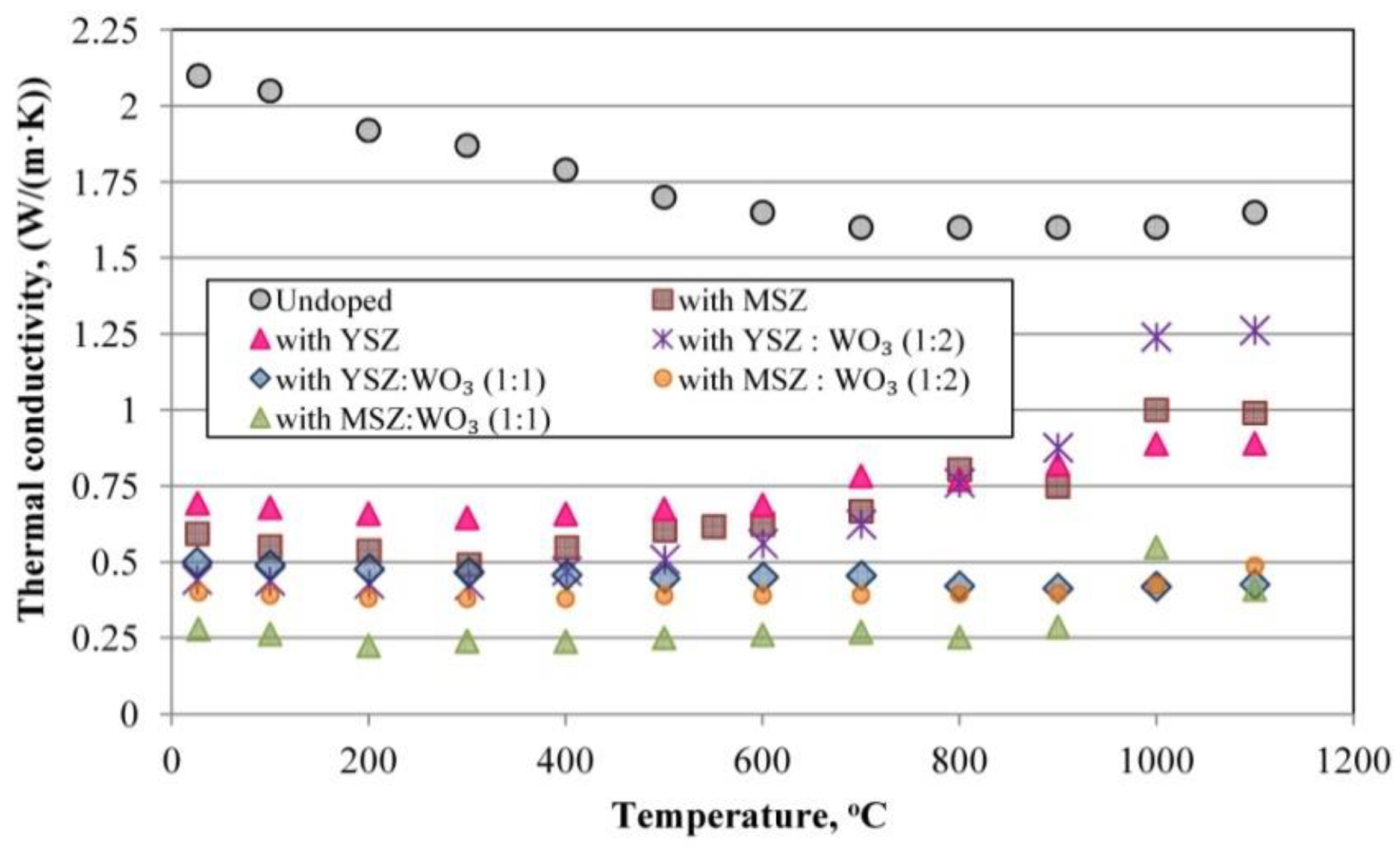
3.6. Thermal Conductivity
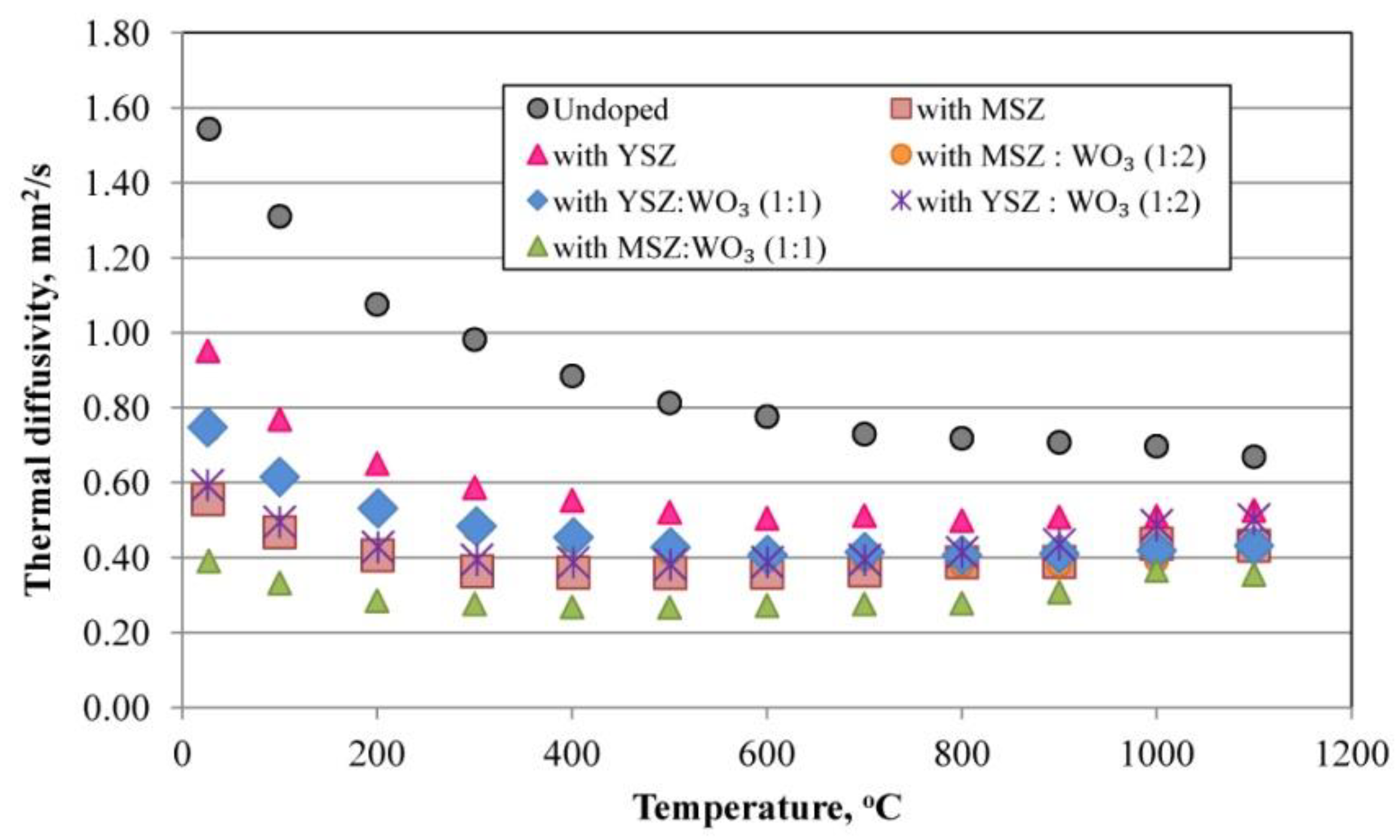
3.7. Thermal Diffusivity
3.8. Thermal Shock Resistance
4. Conclusions
- (a)
- The use of the microsized ZrO2 and WO3 additive promotes the formation of elongated partially networked pores with an orientation in a direction parallel to the base of the molds.
- (b)
- The thermal conductivity decreases with an increasing sample porosity and the randomness of the ceramics structure, as well as with the decreasing mullite crystal thickness.
- (c)
- The formation of the hollow mullite crystals decreases the thermal conductivity of the ceramics and stabilizes its temperature dependence.
- (d)
- The increase in the zircon content in the phase compositions of the porous mullite ceramic causes the decrease in the specific heat capacity of these ceramics.
- (e)
- The presence of zircon and aluminum tungstate in the phase compositions of the porous mullite ceramic improves the thermal shock resistance of the investigated ceramics.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dedinec, A.; Dedinec, A.; Taseska-Gjorgievska, V.; Markovska, N.; Kanevce, G. Energy transition of a developing country following the pillars of the EU Green Deal. Therm. Sci. 2022, 26, 1317–1329. [Google Scholar] [CrossRef]
- Salomão, R.; Oliveira, K.; Fernandes, L.; Tiba, P.; Prado, U. Porous refractory ceramics for high-temperature thermal insulation Part 1: The Science Behind Energy Saving. Interceram Int. Ceram. Rev. 2021, 70, 38–45. [Google Scholar] [CrossRef]
- Medvedovski, E. Alumina–mullite ceramics for structural applications. Ceram. Int. 2006, 32, 369–375. [Google Scholar] [CrossRef]
- Kurovics, E.; Kulkov, A.S.; Ibrahim, J.-E.F.M.; Kashin, A.D.; Pala, P.; Nagy, V.; Kulkov, S.N.; Gomze, L.A. Mechanical properties of mullite reinforced ceramics composite produced from kaolin and corn starch. Epitőanyag–JSBCM 2021, 73, 149–153. [Google Scholar] [CrossRef]
- Krenzel, T.F.; Schreuer, J.; Laubner, D.; Cichocki, M.; Schneider, H. Thermo-mechanical properties of mullite ceramics: New data. J. Am. Ceram. Soc. 2019, 10, 416–426. [Google Scholar] [CrossRef]
- Chen, S.; Wang, J.; Yuan, L.; Li, Q. Composite design of low thermal conductivity mullite brick for application to cement kiln. MATEC Web Conf. 2018, 142, 02008. [Google Scholar] [CrossRef][Green Version]
- Salomão, R.; Oliveira, K.; Fernandes, L.; Tiba, P.; Prado, U. Porous refractory ceramics for high-temperature thermal insulation—Part 2: The technology behind energy saving. Interceram Int. Ceram. Rev. 2022, 71, 38–50. [Google Scholar] [CrossRef]
- Wu, D.; Ren, H.; Wang, F.; Wang, H. High temperature thermal insulation performance of light nanomaterials for aerospace craft. Hangkong Xuebao/Acta Aeronaut. Astronaut. Sin. 2018, 39, 221636. [Google Scholar]
- Clarke, D.R. Materials selection guidelines for low thermal conductivity thermal barrier coatings. Surf. Coat. Technol. 2003, 163–164, 67–74. [Google Scholar] [CrossRef]
- Tavangarian, F.; Hui, D.; Li, G. Crack-healing in ceramics. Compos. Part B Eng. 2018, 144, 56–87. [Google Scholar] [CrossRef]
- Rendtorff, N.M.; Garrido, L.B.; Aglietti, E.F. Thermal shock resistance and fatigue of Zircon–Mullite composite materials. Ceram. Int. 2011, 137, 1427–1434. [Google Scholar] [CrossRef]
- Deng, Z.-Y.; Ferreira, J.M.F.; Tanaka, Y.; Isoda, Y. Microstructure and thermal conductivity of porous ZrO2 ceramics. Acta Mater. 2007, 55, 3663–3669. [Google Scholar] [CrossRef]
- Lee, W.J.; Cho, Y.J.; Lee, H.S.; Park, I.M.; Park, Y.H. Effect of pore morphology on elastic, heat conduction and thermal shock fracture behaviors of porous ceramics. Proc. Eng. 2011, 10, 2459–2463. [Google Scholar] [CrossRef]
- Gong, L.; Wang, Y.; Cheng, X.; Zhang, R.; Zhang, H. A novel effective medium theory for modelling the thermal conductivity of porous materials. Int. J. Heat Mass Transf. 2014, 68, 295–298. [Google Scholar] [CrossRef]
- Belogurova, O.A.; Grishin, N.N. Heat-insulating mullite-cordierite materials made from staurolite. Refract. Ind. Ceram. 2013, 54, 64–68. [Google Scholar] [CrossRef]
- Angle, J.P.; Wang, Z.; Dames, C.; Mecartney, M.L. Comparison of two-phase thermal conductivity models with experiments on dilute ceramic composites. J. Am. Ceram. Soc. 2013, 96, 2935–2942. [Google Scholar] [CrossRef]
- Feidt, M. Finite Physical Dimensions Optimal Thermodynamics 1, From Thermostatics to Non-Equilibrium Thermodynamics; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-1-78548-232-8. [Google Scholar]
- See, A.; Hassan, J.; Hashim, M.; Wahab, Z.A. Thermal diffusivity of kaolinite–mullite ceramic matrix composite with silicon nitride nanoparticle filler. Thermochim. Acta 2014, 593, 76–81. [Google Scholar] [CrossRef]
- Jannot, Y.; Degiovanni, A. Thermal Properties Measurement of Materials; John Wiley & Sons: Hoboken, NJ, USA, 2018; p. 342. ISBN 978-1-119-47505-7. [Google Scholar]
- Ilić, S.; Ivanovski, V.N.; Radovanović, Ž.; Egelja, A.; Kokunešoski, M.; Šaponjić, A.; Matović, B. Structural, microstructural and mechanical properties of sintered iron-doped mullite. Mater. Sci. Eng. B 2020, 256, 114543. [Google Scholar] [CrossRef]
- Aksel, C. The influence of zircon on the mechanical properties and thermal shock behaviour of slip-cast alumina–mullite refractories. Mater. Lett. 2002, 57, 992–997. [Google Scholar] [CrossRef]
- Kenawy, S.H.; Awaad, M.; Awad, H. In-situ mullite-zirconia composites from kaolin. Am. Ceram. Soc. Bull. 2006, 85, 9401–9406. [Google Scholar]
- Xu, X.; Xu, X.; Wu, J.; Lao, X.; Zhang, Y.; Li, K. Effect of Sm2O3 on microstructure, thermal shock resistance and thermal conductivity of cordierite-mullite-corundum composite ceramics for solar heat transmission pipeline. Ceram. Int. 2016, 42, 13525–13534. [Google Scholar] [CrossRef]
- Li, K.; Ge, S.; Yuan, G.; Zhang, H.; Zhang, J.; He, J.; Jia, Q.; Zhang, S. Effects of V2O5 addition on the synthesis of columnar self-reinforced mullite porous ceramics. Ceram. Int. 2021, 47, 11240–11248. [Google Scholar] [CrossRef]
- Wu, J.; Ding, C.; Xu, X.; Liu, Y.; Wang, Y. Microstructure and performances of corundum−mullite composite ceramics for heat transmission pipelines: Effects of Ho2O3 additive content. Ceram. Int. 2021, 47, 34794–34801. [Google Scholar] [CrossRef]
- Wu, J.; Ding, C.; Xu, X.; Zhou, S.; Zhou, Y.; Zhang, Q. Microstructure and performance of Cd2O3 added corundum-mullite ceramics composites for concentrated solar power application. Ceram. Int. 2021, 47, 17177–17185. [Google Scholar] [CrossRef]
- Gai, K.; Guan, B.; Liang, L.; Li, J.; Wang, Q.; Zhao, T. Continuous aluminum oxide-mullite-hafnium oxide composite ceramic fibers with high strength and thermal stability by melt-spinning from polymer precursor. J. Eur. Ceram. Soc. 2022, 42, 5911–5921. [Google Scholar] [CrossRef]
- Khorsand, A.; Majidian, H.; Farvizi, M. Wear behavior and microstructure of alumina-mullite- zirconia composites prepared by a novel method: Coating of zircon powder by aluminium alkoxide. Ceram. Int. 2022, 48, 33594–33603. [Google Scholar] [CrossRef]
- Reinders, L.; Pfeifer, S.; Kröner, S.; Stolpmann, H.; Renfftlen, A.; Greiler, L.C.; Clauß, B.; Buchmeiser, M.R. Development of mullite fibers and novel zirconia-toughened mullite fibres for high temperature applications. J. Eur. Ceram. Soc. 2021, 41, 3570–3580. [Google Scholar] [CrossRef]
- Weinberg, A.V.; Goeuriot, D.; Poirier, J.; Varona, C.; Chaucherie, X. Mullite–zirconia composite for the bonding phase of refractory bricks in hazardous waste incineration rotary kiln. J. Eur. Ceram. Soc. 2021, 41, 995–1002. [Google Scholar] [CrossRef]
- Kumar, P.; Nath, M.; Ghosh, A.; Tripathi, H.S. Thermo-mechanical properties of mullite—Zirconia composites derived from reaction sintering of zircon and sillimanite beach sand: Effect of CaO. Trans. Nonferrous Met. Soc. China 2016, 26, 2397–2403. [Google Scholar] [CrossRef]
- Bouchetou, M.L.; Poirier, J.; Morales, L.A.; Chotard, T.; Joubert, O.; Weissenbacher, M. Synthesis of an innovative zirconia-mullite raw material sintered from andalusite and zircon precursors and an evaluation of its corrosion and thermal shock performance. Ceram. Int. 2019, 45, 12832–12844. [Google Scholar] [CrossRef]
- Rendtorff, N.; Garrido, L.; Aglietti, E. Mullite-zirconia-zircon composites: Properties and thermal shock resistance. Ceram. Int. 2009, 35, 779–786. [Google Scholar] [CrossRef]
- ASTM Standards: E 1952–01; Standard Test Method for Thermal Conductivity and Thermal Diffusivity by Modulated Temperature Differential Scanning Calorimetry. ASTM: West Conshohocken, PA, USA, 2017.
- Camirand, C.P. Measurement of thermal conductivity by differential scanning calorimetry. Thermochim. Acta 2004, 417, 1–4. [Google Scholar] [CrossRef]
- ASTM Standards: E1269; Standard Test Method for Determining Specific Heat Capacity by Differential Scanning Calorimetry. ASTM: West Conshohocken, PA, USA, 2018.
- Robertis, E.D.; Cosme, E.H.H.; Neves, R.S.; Kuznetsov, A.Y.; Campos, A.P.C.; Landi, A.M.; Achete, C.A. Application of the modulated temperature differential scanning calorimetry technique for the determination of the specific heat of copper nanofluids. Appl. Therm. Eng. 2012, 41, 10–17. [Google Scholar] [CrossRef]
- ASTM Standards: C177-13; Standard Test Method for Steady-State Heat Flux Measurements and Thermal Transmission Properties by Means of the Guarded-Hot-Plate Apparatus. ASTM: West Conshohocken, PA, USA, 2019.
- ASTM Standards: C1044; Standard Practice for Using a Guarded-Hot-Plate Apparatus or Thin-Heater Apparatus in the Single-Sided Mode. ASTM: West Conshohocken, PA, USA, 2020.
- ASTM Standards: C1114; Standard Test Method for Steady-State Thermal Transmission Properties by Means of the Thin-Heater Apparatus. ASTM: West Conshohocken, PA, USA, 2019.
- ASTM Standards: C1113/C1113M-09; Standard Test Method for Thermal Conductivity of Refractories by Hot Wire (Platinum Resistance Thermometer Technique). ASTM: West Conshohocken, PA, USA, 2019.
- Codorniu, D.M.; Moyano, J.J.; Belmonte, M.; Osendi, M.I.; Miranzo, P. Thermal conduction in three-dimensional printed porous samples by high resolution infrared thermography. Open Ceram. 2020, 4, 100028. [Google Scholar] [CrossRef]
- ASTM Standards: E1225-13; Standard Test Method for Thermal Conductivity of Solids Using the Guarded-Comparative-Longitudinal Heat Flow Technique. ASTM: West Conshohocken, PA, USA, 2013.
- ASTM Standarts: E1461; Standard Test Method for Thermal Diffusivity by the Flash Method. ASTM: West Conshohocken, PA, USA, 2022.
- Rezaee, S.; Ranjbar, K. Thermal conductivity of porous Alumina-20 wt% zirconia ceramic composites. Ceram. Int. 2020, 46, 16564–16571. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, C.; Zhang, Z.; Tian, W.; Hui, B.; Zhang, J.; Zhang, K. Tungsten oxide polymorphs and their multifunctional applications. Adv. Coll. Int. Sci. 2022, 300, 102596. [Google Scholar] [CrossRef]
- Mahnicka-Goremikina, L.; Svinka, R.; Svinka, V.; Grase, L.; Goremikins, V. The formation of phases with low or negative linear thermal expansion coefficient in porous mullite ceramics. Epitőanyag–JSBCM 2020, 72, 91–98. [Google Scholar] [CrossRef]
- Achary, S.N.; Mukherjee, G.D.; Tyagi, A.K.; Vaidya, S.N. Preparation, thermal expansion, high pressure and high temperature behaviour of Al2(WO4)3. J. Mater. Sci. 2002, 37, 2501–2509. [Google Scholar] [CrossRef]
- Liu, H.; Xiong, X.; Li, M.; Wang, Z.; Wang, X.; Ma, Y.; Yuan, L. Fabrication and properties of mullite thermal insulation materials with in-situ synthesized mullite hollow whiskers. Ceram. Int. 2020, 46, 14474–14480. [Google Scholar] [CrossRef]
- Girolamo, G.D.; Blasi, C.; Pilloni, L.; Schioppa, M. Microstructural and thermal properties of plasma sprayed mullite coatings. Ceram. Int. 2010, 36, 1389–1395. [Google Scholar] [CrossRef]
- Chase, M.W., Jr. NIST-JANAF Thermochemical Tables, 4th ed.; American Institute of Physics: University Park, ML, USA, 1998; pp. 1–1951. [Google Scholar]
- Han, B.-y.; Khoroshilov, A.V.; Tyurin, A.V.; Baranchikov, A.E.; Razumov, M.I.; Ivanova, O.S.; Gavrichev, K.S.; Ivanov, V.K. WO3 thermodynamic properties at 80–1256 K revisited. J. Therm. Anal. Calorim. 2020, 142, 1533–1543. [Google Scholar] [CrossRef]
- Gong, L.; Wang, Y.; Cheng, X.; Zhang, R.; Zhang, H. Porous mullite ceramics with low thermal conductivity prepared by foaming and starch consolidation. J. Porous Mater. 2014, 21, 15–21. [Google Scholar] [CrossRef]
- Manga, M.; Jeanloz, R. Thermal conductivity of corundum and periclase and implications for the lower mantle. J. Geophys. Res. 1997, 102, 2999–3008. [Google Scholar] [CrossRef]
- Dietrich, B.; Kind, M.; Martin, H. Axial two-phase thermal conductivity of ceramic sponges—Experimental results and correlation. Int. J. Heat Mass Transf. 2011, 54, 2276–2282. [Google Scholar] [CrossRef]
- Mardare, C.C.; Hassel, A.W. Review on the versatility of tungsten oxide coatings. Phys. Status Solidi A 2019, 216, 1900047. [Google Scholar] [CrossRef]
- Rao, M.C. Structure and properties of WO3 thin films for electrochromic device application. J. Non-Oxide Glasses 2013, 5, 1–8. [Google Scholar]
- Zheng, H.; Zhen, O.J.; Strano, M.S.; Kaner, R.B.; Mitchell, A.; Kalantar-zadeh, K. Nanostructured tungsten oxide—Properties, synthesis, and applications. Adv. Funct. Mater. 2011, 21, 2175–2196. [Google Scholar] [CrossRef]
- Ning, S.; Huberman, S.C.; Ding, Z.; Nahm, Y.-H.; Kim, H.-S.; Chen, G.; Ross, C.A. Anomalous defect dependence of thermal conductivity in epitaxial WO3. Adv. Mater. 2019, 31, 1903738. [Google Scholar] [CrossRef]
- Wang, H.; Du, X.; Shi, Y.; Deng, M.; Wang, J.; Qi, J.; Huang, Z. Grain-size dependent thermal conductivity of Gd2Zr2O7 ceramics. Ceram. Int. 2022, 48, 16444–16448. [Google Scholar] [CrossRef]
- Giordano, R. Machinable zirconia. A study on the building blocks of restorative dentistry. Inside Dent. Technol. 2012, 3, 1–3. [Google Scholar]
- Žmak, I.; Ćorić, D.; Mandić, V.; Ćurković, L. Hardness and indentation fracture toughness of slip cast alumina and alumina-zirconia ceramics. Materials 2020, 13, 122. [Google Scholar] [CrossRef]
- Banerjee, S.; Mukhopadhyay, P. Chapter 4—Martensitic Transformations. In Phase Transformations. Examples from Titanium and Zirconium Alloys; Elsevier: Amsterdam, The Netherlands, 2007; Volume 12, pp. 1–813. ISBN 9780080548791. [Google Scholar]
- Zanelli, C.; Dondi, M.; Raimondo, M.; Guarini, G. Phase composition of alumina–mullite–zirconia refractory materials. J. Eur. Ceram. Soc. 2010, 30, 29–35. [Google Scholar] [CrossRef]
- Wei, J.; Bingqiang, H.; Wei, Y.; Li, N.; Miao, Z. Influence of phase evolution and thermal decomposition kinetics on the properties of zircon ceramic. Ceram. Int. 2021, 47, 27285–27293. [Google Scholar] [CrossRef]
- Cao, M.C.; Jin, X.X.; Dong, L.M.; Li, X.Y.; Zhang, X.Y. Effect of pore composition on thermal shock resistance of porous ceramics. Dig. J. Nanomater. Biostructures 2021, 16, 1–9. [Google Scholar] [CrossRef]



| Thermal Analysis Tests | References |
|---|---|
| Standard test method for thermal conductivity and thermal diffusivity by modulated temperature differential scanning calorimetry. | [34,35] |
| Standard test method for determining specific heat capacity by differential scanning calorimetry. | [36,37] |
| Hot-plate system. Guarded hot-plate systems are used to measure steady-state heat flow through materials with low thermal conductivity (insulators). | [38,39,40,41,42] |
| Heat flow system. Guarded Comparative–Longitudinal Heat Flow Technique. | [42,43] |
| The laser flash method and laser pulse: Laser Flash Thermal Conductivity. | [42,44,45] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahnicka-Goremikina, L.; Svinka, R.; Svinka, V.; Grase, L.; Juhnevica, I.; Rundans, M.; Goremikins, V.; Tolendiuly, S.; Fomenko, S. Thermal Properties of Porous Mullite Ceramics Modified with Microsized ZrO2 and WO3. Materials 2022, 15, 7935. https://doi.org/10.3390/ma15227935
Mahnicka-Goremikina L, Svinka R, Svinka V, Grase L, Juhnevica I, Rundans M, Goremikins V, Tolendiuly S, Fomenko S. Thermal Properties of Porous Mullite Ceramics Modified with Microsized ZrO2 and WO3. Materials. 2022; 15(22):7935. https://doi.org/10.3390/ma15227935
Chicago/Turabian StyleMahnicka-Goremikina, Ludmila, Ruta Svinka, Visvaldis Svinka, Liga Grase, Inna Juhnevica, Maris Rundans, Vadims Goremikins, Sanat Tolendiuly, and Sergey Fomenko. 2022. "Thermal Properties of Porous Mullite Ceramics Modified with Microsized ZrO2 and WO3" Materials 15, no. 22: 7935. https://doi.org/10.3390/ma15227935
APA StyleMahnicka-Goremikina, L., Svinka, R., Svinka, V., Grase, L., Juhnevica, I., Rundans, M., Goremikins, V., Tolendiuly, S., & Fomenko, S. (2022). Thermal Properties of Porous Mullite Ceramics Modified with Microsized ZrO2 and WO3. Materials, 15(22), 7935. https://doi.org/10.3390/ma15227935









