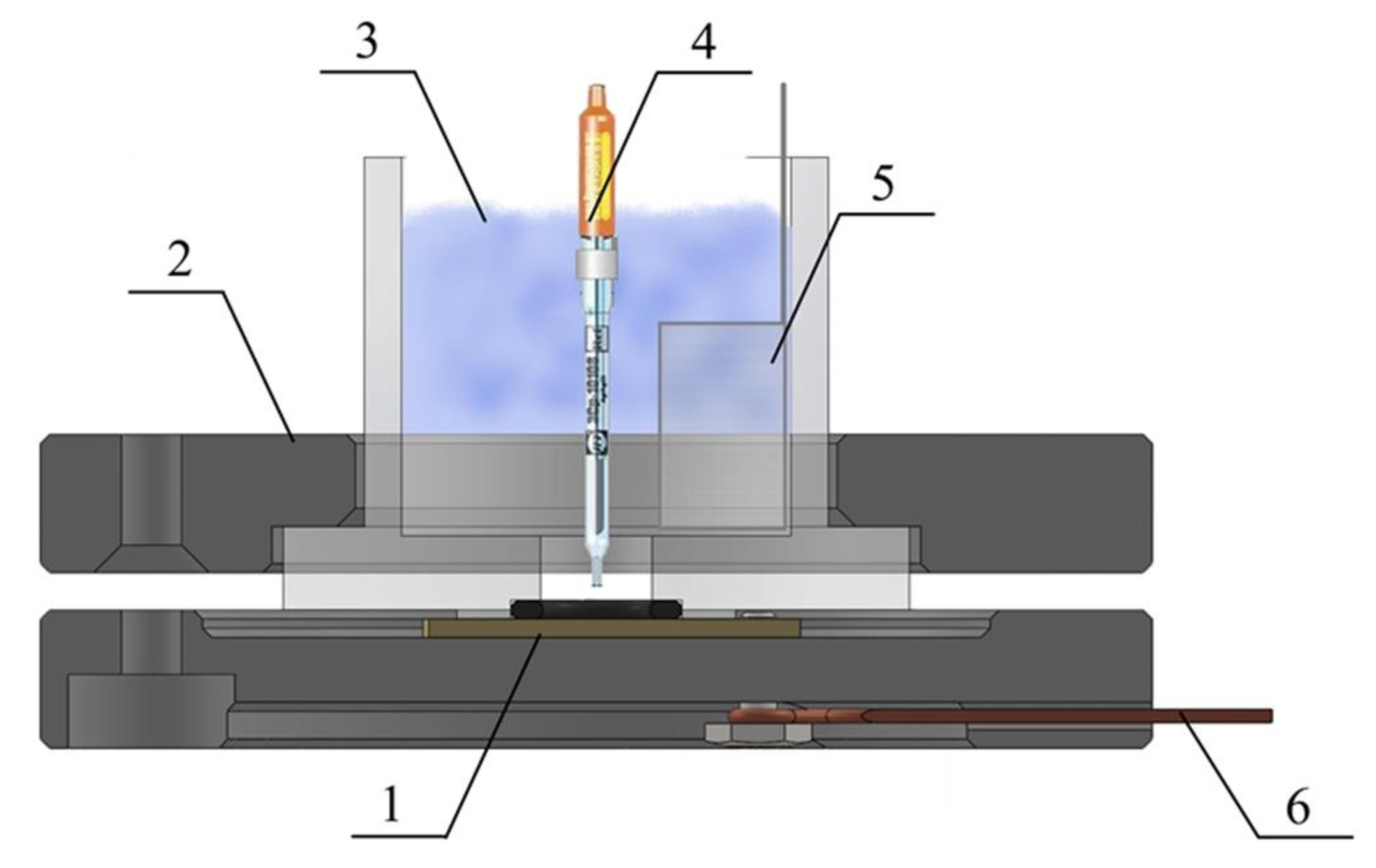
3.1. Plasma Electrolytic Oxidation (PEO)
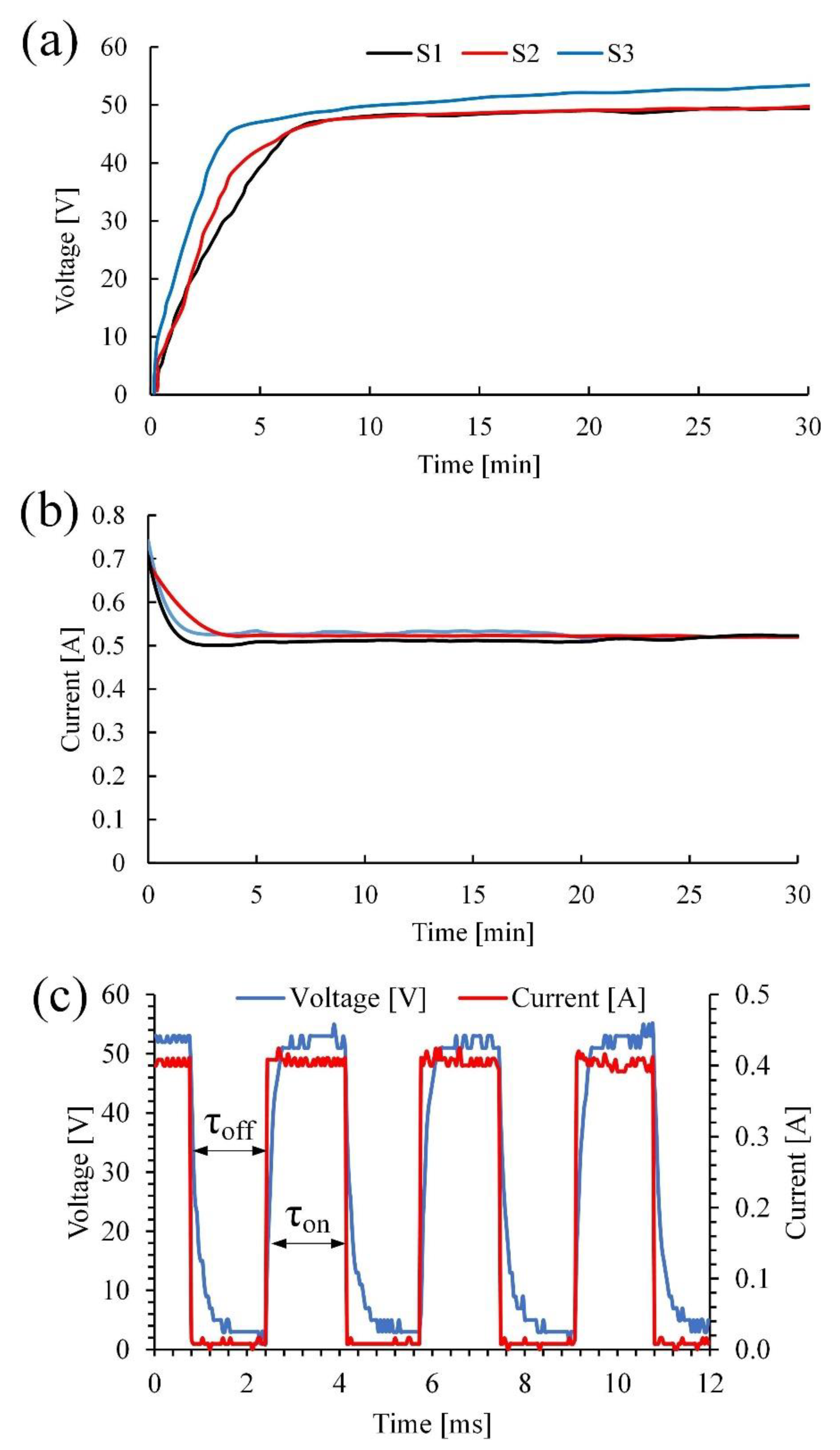
Figure 2 shows the electrical parameters for the PEO process.
The PEO process can be divided into three main stages [
45,
46], as shown in
Figure 2a,b. At the beginning of the surface treatment, the voltage is linearly increased up to a breakdown voltage of 45 V. The current efficiency is almost at the maximum in accordance with Faraday’s law and reaches a value of 0.7 A (0.045 A/cm
2). The process of forming an amorphous oxide film on the processed sample lasts 3 to 5 min. At this stage, active gas evolution occurs on the sample surface. Upon reaching 45–48 volts, the process passes to the second stage, which lasts approximately 10 min and is characterized by low-power microarc breakdowns. In addition, the resistance to charge transfer increases, and the current decreases from 0.7 to 0.5 A (
Figure 2b). This can be addressed by the transformation of the amorphous structure into a crystalline structure and the further growth of the coating.
In the third stage, the power of local breakdowns is increased, and their number is decreased, which corresponds to a compaction of the formed coating and an increase in its dielectric properties.
Depending on the conductivity of the oxide film and the properties of the semiconductor, the resulting two-junction metal-oxide-electrolyte system of the Schottky type can be shifted in the forward or reverse direction [
47,
48]. When the junction is forward-biased, the PEO process has a vapor gaseous envelope (VGE) surrounding the working electrode. With a reverse-biased junction, the PEO at the anode is characterized by forming an immobile oxide film, which accounts for most of the voltage drop. The most significant voltage drop occurs at the VGE, a non-stationary object with a strong electric field that contributes to the occurrence of plasma discharge in gaseous oxygen media. Micro discharges appear on its surface when the field reaches the breakdown point in this thin film. These phenomena distinguish PEO from other electrolytic processes and justify the term “plasma”.
The nature of the process itself can be described by the waveform plot shown in
Figure 2c. During the time period τ
off, when the voltage and current are not applied, Ti ions can produce a thin amorphous coating on the substrate surface. When an anode current and voltage (τ
on) are applied, dielectric breakdown occurs, leading to a micro discharge, which forms an oxide-ceramic coating on the metal surface.
It should be noted that the above stages of coating formation in molten salts have similar patterns in aqueous electrolytes. However, they proceed at lower voltages and amount to approximately 50 V at the final stage of the process compared to 175 V in aqueous electrolytes. This difference may occur due to the physical-chemical properties of the molten electrolyte, namely, a higher temperature of the electrolyte and conductivity that is an order of magnitude higher.
3.2. Morphology and Chemical Analysis
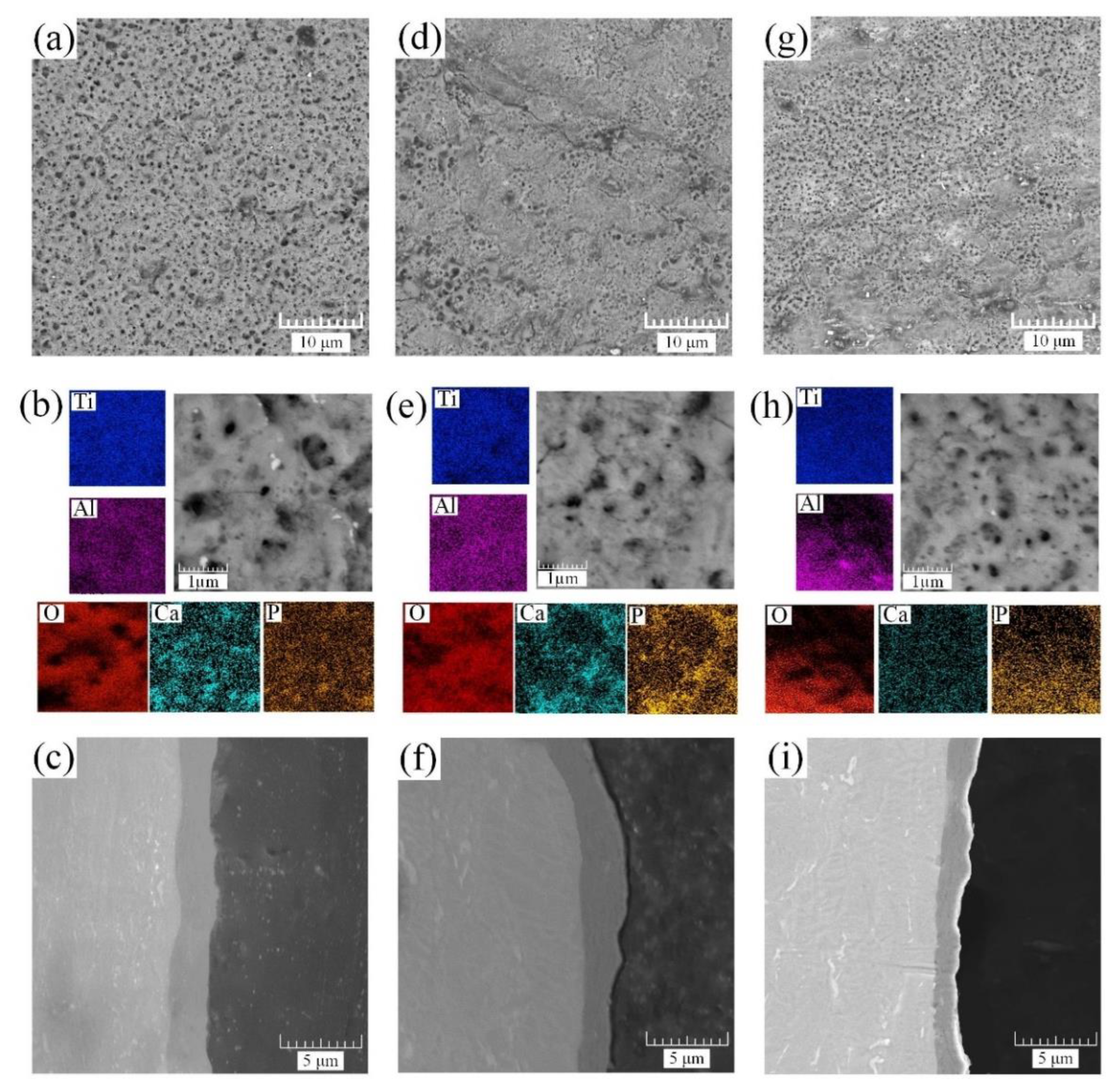
The coating morphology and cross-section obtained by SEM for the first step of the PEO process are illustrated in
Figure 3.
The study of the morphology of the coated surface reveals several changes associated with various electrical parameters used in the PEO process, particularly those associated with the magnitude and size of the coating porosity. The pores on the oxide surface are formed by various types of discharges classified by Hussein et al. [
49] in an aqueous electrolyte and by Sobolev et al. [
50] in molten salts (
Figure 3a,d,g). The formation of these discharges directly depends on their power density, and, consequently, on the morphology of the coating. The high-energy discharge creates micron-sized pores on the surface. With a decrease in the power density, and, as a result, a decrease in the electric field strength, the power of the discharges decreases. The size of the formed pores decreases to submicron sizes in the range of 0.1–0.2 μm. However, one should not forget that, usually, the formed coating has a heterogeneous structure and mixed coating morphology formed by both high- and low-energy breakdowns.
Evaluating the coating morphology of sample S1 (
Figure 3a,b) reveals a uniform distribution of micron and submicron surface pores formed by low- and high-energy breakdowns. The cross-section of the sample has a dense, nonporous structure with a coating thickness of 2.4–2.7 μm.
With an increase in the frequency of the current and voltage, both the duration of the micro arc discharge in the anode half-cycle and its single pulse energy decrease, resulting in a decrease in the power of the micro arc discharges. Further SEM study reveals that the surface structure formed for sample S2 (
Figure 3d,e) has a smaller number of micron pores and consists of ≈60% submicron pores. Additionally, due to a decrease in the energy of a single pulse, the coating growth rate slightly decreases and has a thickness of 2.0–2.5 μm. With a further increase in the frequency of the current and voltage (sample S3,
Figure 3g), micron-sized pores formed by high-energy pulses were not observed. The surface consists of ≈60% submicron pores and ≈40% nanopores (
Figure 3g,h). The thickness of the formed coating is decreased by almost 1.5 times compared to sample S1 with a value of 1.2–2.2 μm.
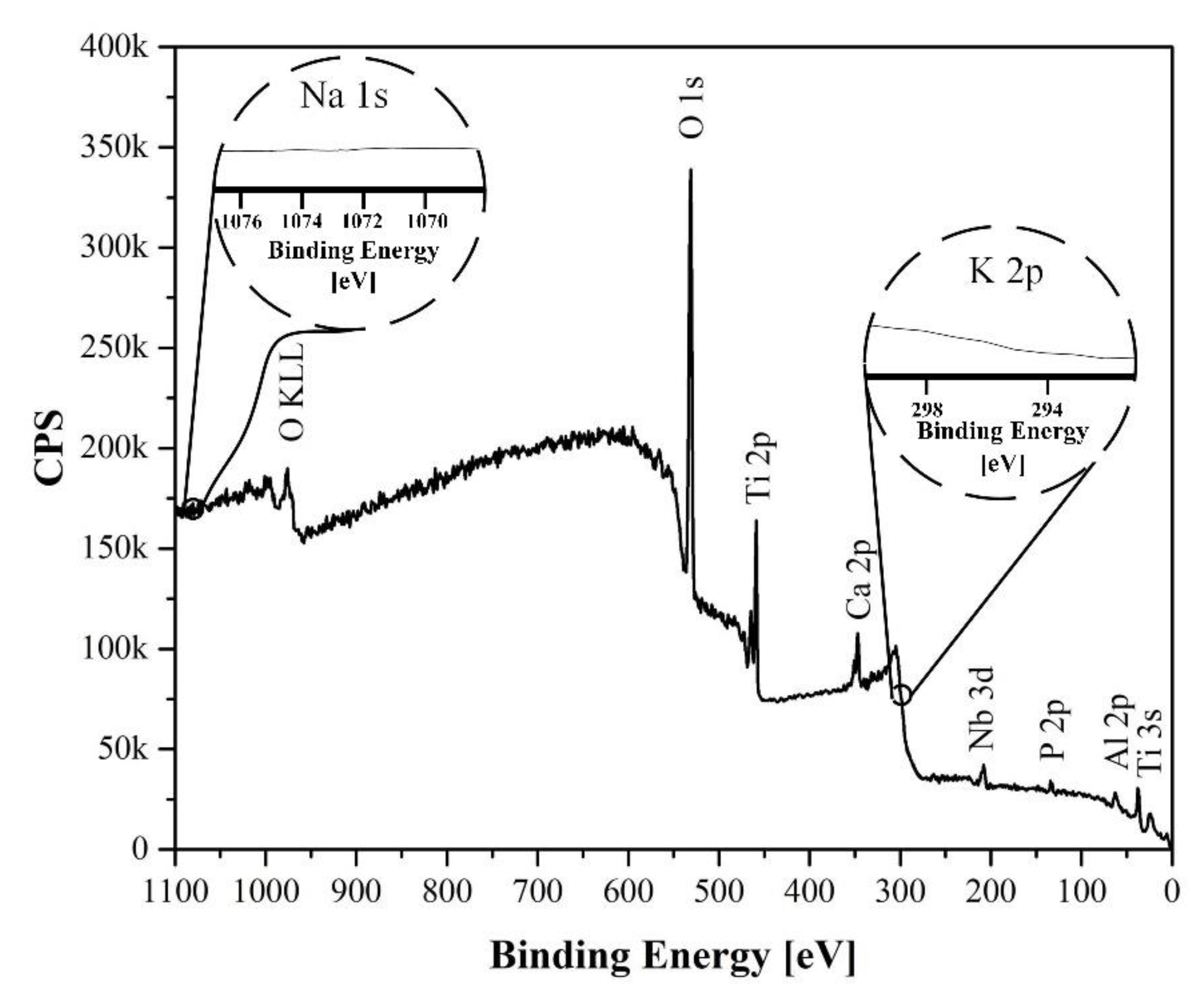
No significant changes in the chemical composition of the analyzed samples were detected, as can be observed in the elemental distribution mapping images (
Figure 3b,e,h) and the EDS elemental analysis results, which are shown in
Table 3.
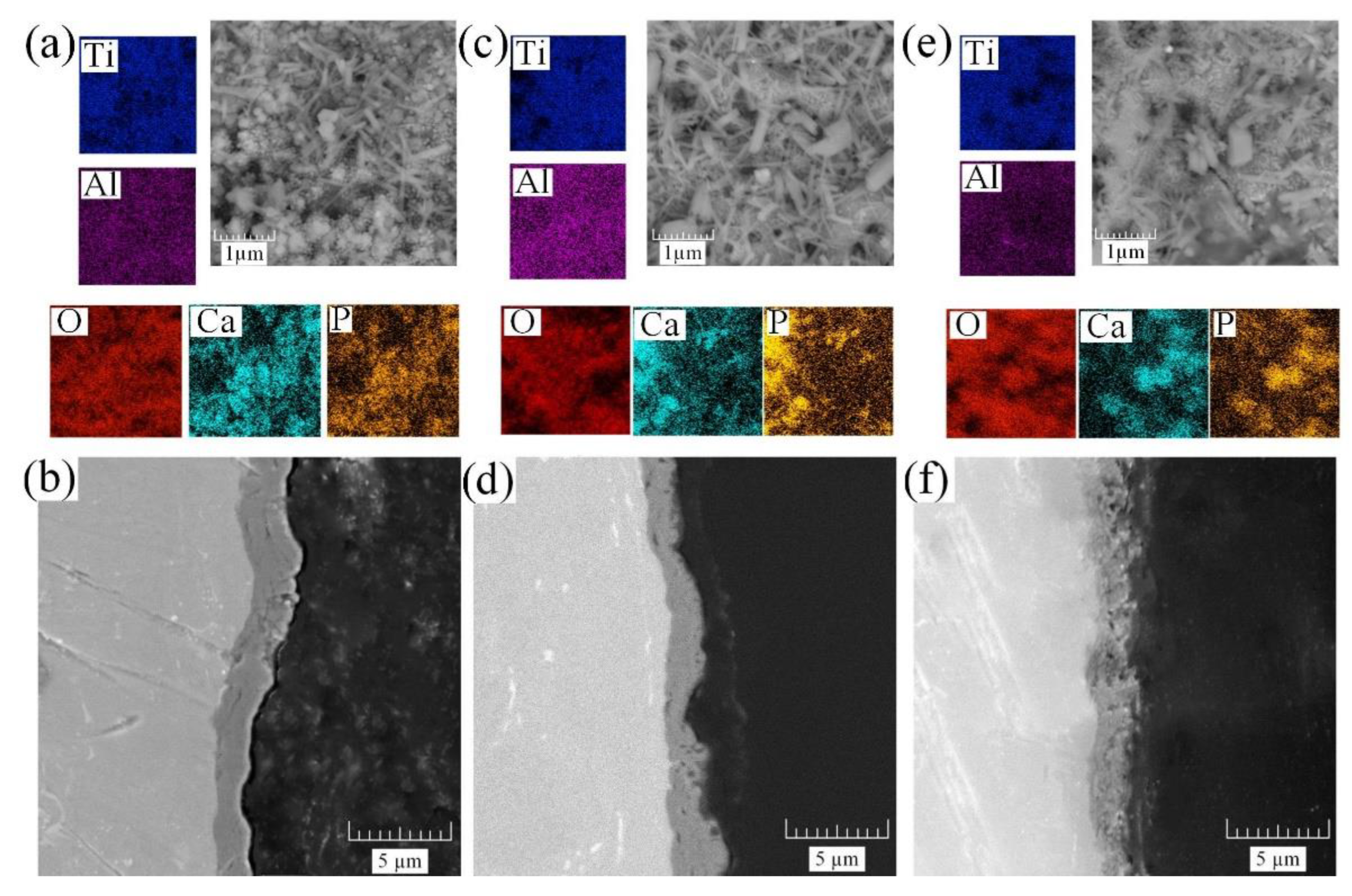
Furthermore, the obtained samples were treated by the HTT method for the formation and growth of HA crystals. Precursors were introduced into the coating in the form of a fine powder. The coating morphologies and cross-sections obtained by SEM are illustrated in
Figure 4.
After the HTT process, the previously introduced HA precursors were grown in the coating under the influence of temperature and pH [
40], resulting in the formation of hexagonal needle-like crystals with sizes of 0.5–1 μm. The morphology, presented earlier in
Figure 3, affects the surface filled with HA crystals, and, thereby, leads to the closure of previously formed pores. With this decrease in porosity (
Figure 3a,c,e), the number of crystals decreased, and their sizes increased, which is confirmed by the EDS mapping of calcium and phosphorus (
Figure 4a,c,e). The EDS chemical analysis data are presented in
Table 4.
The presented values in
Table 3 and
Table 4 indicate a difference in the surface compositions before and after the HTT process. The decrease in the fraction of Ti atoms is associated with the filling of the surface with HA crystals. This behavior is associated with the diffusion, nucleation, and crystallization processes on the sample surface. As described by Liu et al., Ca and P ions diffuse from the inner layer to the surface during HTT [
51], resulting in the formation of HA crystals. The optimal Ca/P ratio for HA is 1.67 [
44]. Most likely, the content of CaO crystals on the surface of the samples is increased simultaneously with an increase in the Ca/P ratio.
It should also be noted that HTT treatment did not significantly affect the structure of the coating, which has been confirmed by the cross-sections of samples S1–S3 (
Figure 3c,f,i) and S1 HTT–S3 HTT (
Figure 4b,d,f), respectively.
This study points out that the best surface is obtained using the current frequency of 100 Hz. The frequency increase negatively affects the structure of the oxide coating before and after HTT. With the increase in current frequency and voltage, the formed HA shows a nonstoichiometric composition and contains additional Ca ions, forming other compounds that differ from HA.
3.4. Phase Analyses
The phase compositions of the coatings were analyzed using the X-ray patterns presented in
Figure 6.
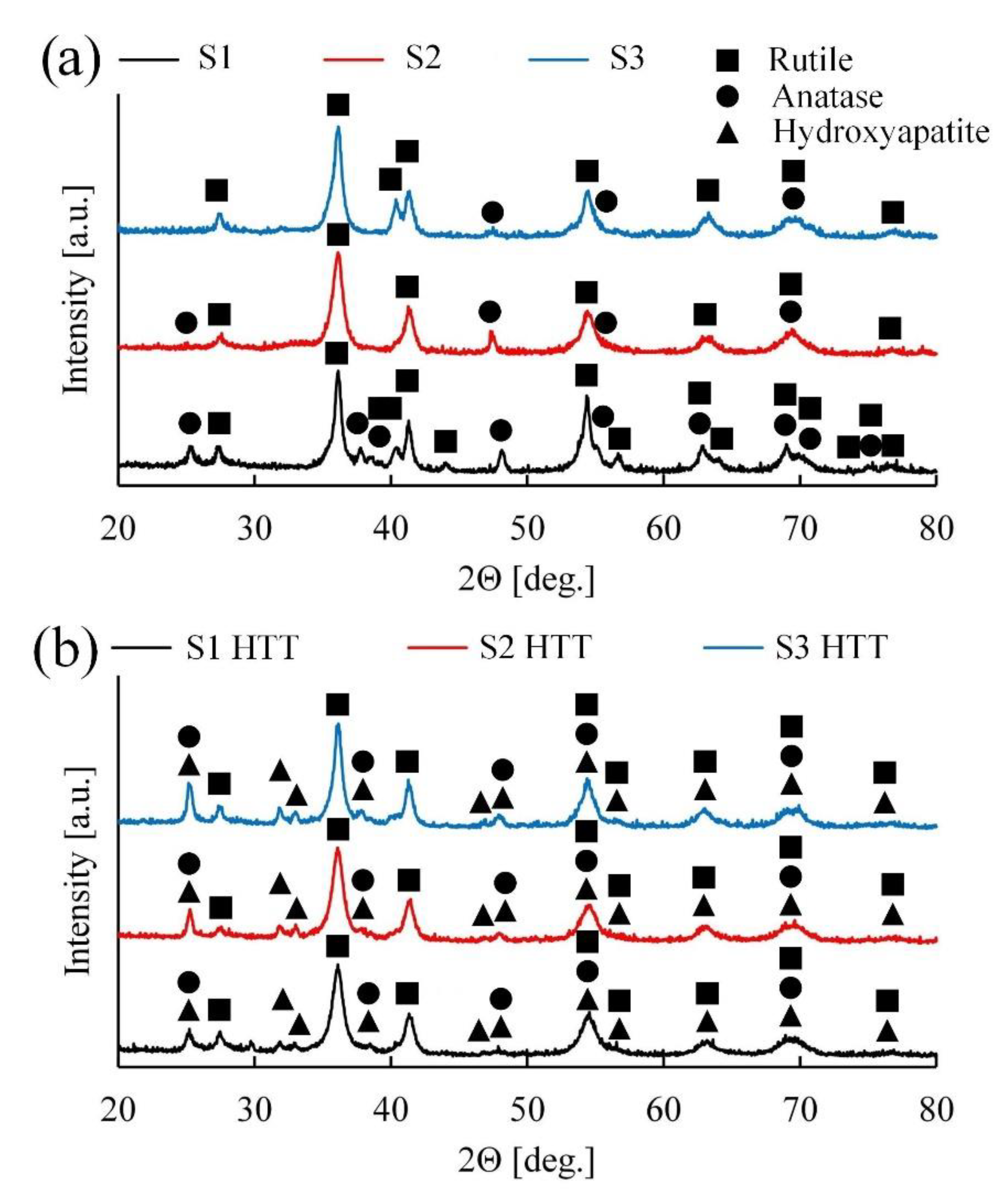
Based on
Figure 6a, it can be concluded that samples S1–S3 contain two phases of titanium oxide (anatase and rutile) and a low quantity of amorphous addition. Apparently, during the formation of the coating with an increase in the frequency of the current and voltage and a decrease in the power of a single pulse, the temperature in the discharge channel decreases, which contributes to the phase transition from the amorphous state of titanium oxide to the crystalline state [
52]. As is known [
53], anatase is a lower temperature phase of titanium oxide, and during the PEO process, under the influence of high temperatures, it is converted to rutile. Judging by the graphs shown in
Figure 6a, with a decrease in the power of a single breakdown (S1 > S2 > S3), the intensity of the anatase peaks decreases, and, as a consequence, the intensity of the rutile peaks increases.
As shown in
Figure 6b, after the HTT treatment, the intensity of the peaks of the rutile phase remains almost unchanged, but the amount of the anatase phase is increased. This dependence is conditioned because rutile, anatase, and an amorphous component has formed during the treatment of PEO, which, upon HTT, is reformed into the lowest temperature and stable crystalline phase of titanium oxide-anatase.
Apatite introduced earlier into the coating under the influence of temperature and pH forms individual HA phases on the surface of the coating in the form of hexagonal needles (
Figure 4), which is also confirmed by the EDS and XPS results. Upon further analysis of the X-ray patterns, it can be observed that the intensities of the HA peaks for the three samples (
Figure 6b) are of a similar order, and their concentrations in the coating are comparable.
Shin et al. [
54] showed that porous coatings of titanium oxide (rutile and anatase) formed on the surface of the implanted material could act as nuclei for the formation of HA in the simulated body fluid, thereby increasing the rate of osseointegration. However, titanium oxides themselves are not a bioactive material but rather only a substrate for the effective growth of HA; therefore, HA compounds were used as a dopant, which can increase the efficiency of osseointegration.
Additional phases consisting of electrolyte components were not found. It is difficult to determine the quantitative phase composition because of the inhomogeneous structure of the coatings, which affects the absorption of X-rays.
The XRD analysis revealed the presence of the following phases present in the coating: Rutile, anatase, amorphous titanium oxide, and crystalline HA. These data are necessary to consciously control the composition by varying the process parameters.
3.6. Electrochemical Measurements
The corrosion behavior of the untreated alloy (S0) and coating samples after PEO (S1–S3) and HTT treatment (S1 HTT–S3 HTT) were analyzed using PPT curves and EIS in Hanks’ solution.
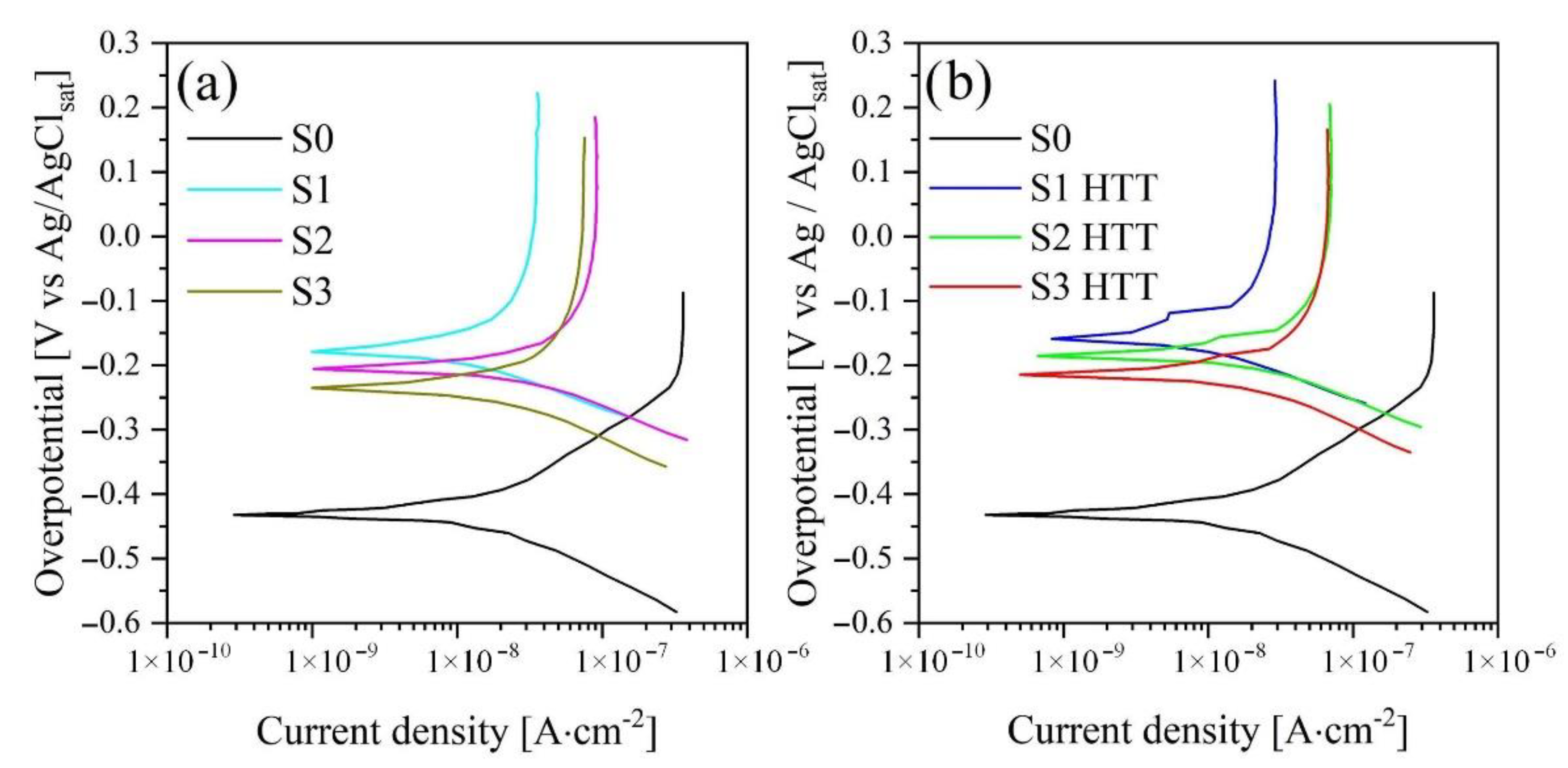
PPT curves are presented in
Figure 8, and their calculated values are given in
Table 6.
Based on the results of the potentiodynamic polarization curves, the corrosion potentials (
Ecorr), corrosion current densities (
icorr), and anodic and cathodic slopes of the linear sections (
βa and
βc) were determined by a graphical method. The polarization resistance (
Rp) values were calculated using the Stern-Geary equation [
59]:
Samples S1–S3 demonstrate an increase in the corrosion current and, as a consequence, a decrease in the polarization resistance in the order S1 > S2 > S3 and is 4.03 × 10
6, 2.29 × 10
6, and 2.14 × 10
6 Ohm∙cm
2, respectively. This pattern is directly related to the frequencies of current and voltage used in the formation of the coating, i.e., with an increase in the frequency of current and voltage, the power of a single breakdown decreases, which does not allow for a uniform oxide structure distributed over the entire area of the treated surface. The evolution of this phenomenon shows in
Figure 3.
After the HTT treatment, the regularity of the decrease in the polarization resistance in a series of samples (S1-HTT > S2-HTT > S3-HTT = 4.83 × 106 > 2.98 × 106 > 2.36 × 106 Ohm·cm2) remains. However, it has higher values of polarization resistance by 10–15% compared with samples processed in the PEO process. The persistence of this pattern can be explained by the effect of HTT treatment, which, as a result of high temperatures and pressure, transforms the amorphous phase of titanium oxide after the PEO process into a crystalline phase, and also accelerates the formation of HA crystals, which encapsulate coating defects, thereby increasing its corrosion resistance.
As noted earlier (
Section 3.3), HTT treatment does not have a significant effect on the cross-section of the samples and, therefore, on the internal structure, which is also confirmed by the analysis of the shape of PPT curves (before and after the HTT process) in semilogarithmic coordinates and its slopes of their linear sections presented in
Figure 8. The untreated alloy’s comparative performance has lower polarization resistance values and can be more easily attacked in Hanks’ solution.
The corrosion properties of the studied samples were also evaluated using EIS analysis. EIS analysis also makes it possible to study the influence of the structural features of the samples on their electrochemical behavior in Hanks’ solution. The most used and physically justified equivalent electrical circuits (EEC) for the corrosion process are shown in
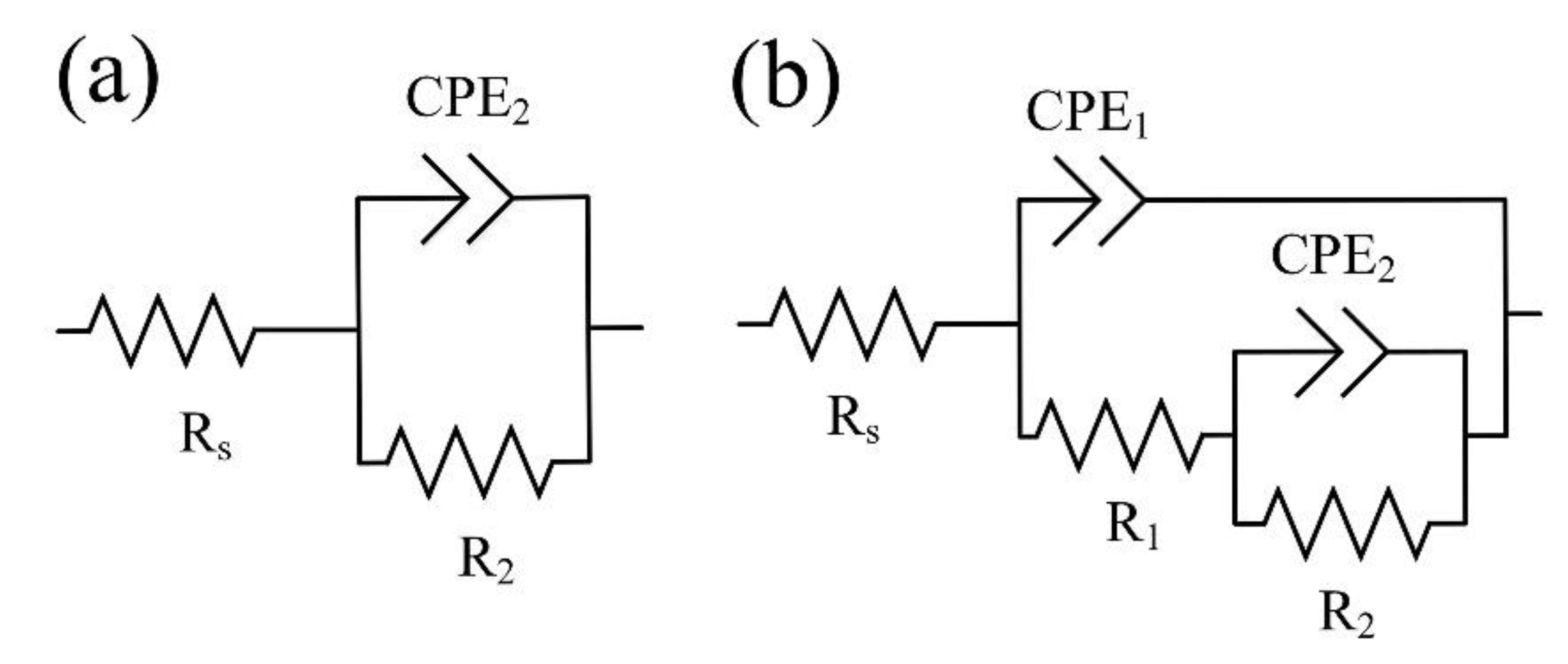
Figure 9.
Figure 9a presents the EEC that simulates the corrosion behavior of an S0 sample with a single “natural” layer, and
Figure 9b shows that of a bilayered oxide coating S1–S3 and S1 HTT–S3 HTT, the morphology and cross-section of which are presented in
Figure 3 and
Figure 4. Electrolyte/electrode interfaces consist of the electrolyte/porous layer and electrolyte/nonporous layer. The designations R
s, R
1, CPE
1, R
2, and CPE
2 describe the ECC main elements for this boundary. The R
s is the electrolyte resistance, which equals 37 ± 2 Ohm. The series-connected R
1 is the electrical resistance of the electrolyte in the pores, and CPE
1 is the geometric capacitance of the porous layer. The R
2-CPE
2 has described the process of charge transfer at the nonporous layer/electrolyte interface and represents the charge transfer resistance R
2 and electric double-layer capacitance CPE
2 [
60].
Based on the coating’s morphology, cross-section, and phase analyses, two equivalent circuits were chosen [
61,
62], as shown in
Figure 9.
Considering the nonideal behavior of the simulated interface, the capacitance can be represented by a constant phase element-CPE, which is described by the following equation [
63]:
where Z
CPE is the impedance of the CPE, Y
0 is the admittance of an ideal capacitance, j is the imaginary unit (√−1), ω is the angular frequency (2πf), and n is a dimensionless parameter that varies between 0 and 1. At n = 0, the CPE behaves as a resistor, and at n = 1, the CPE behaves as an ideal capacitor.
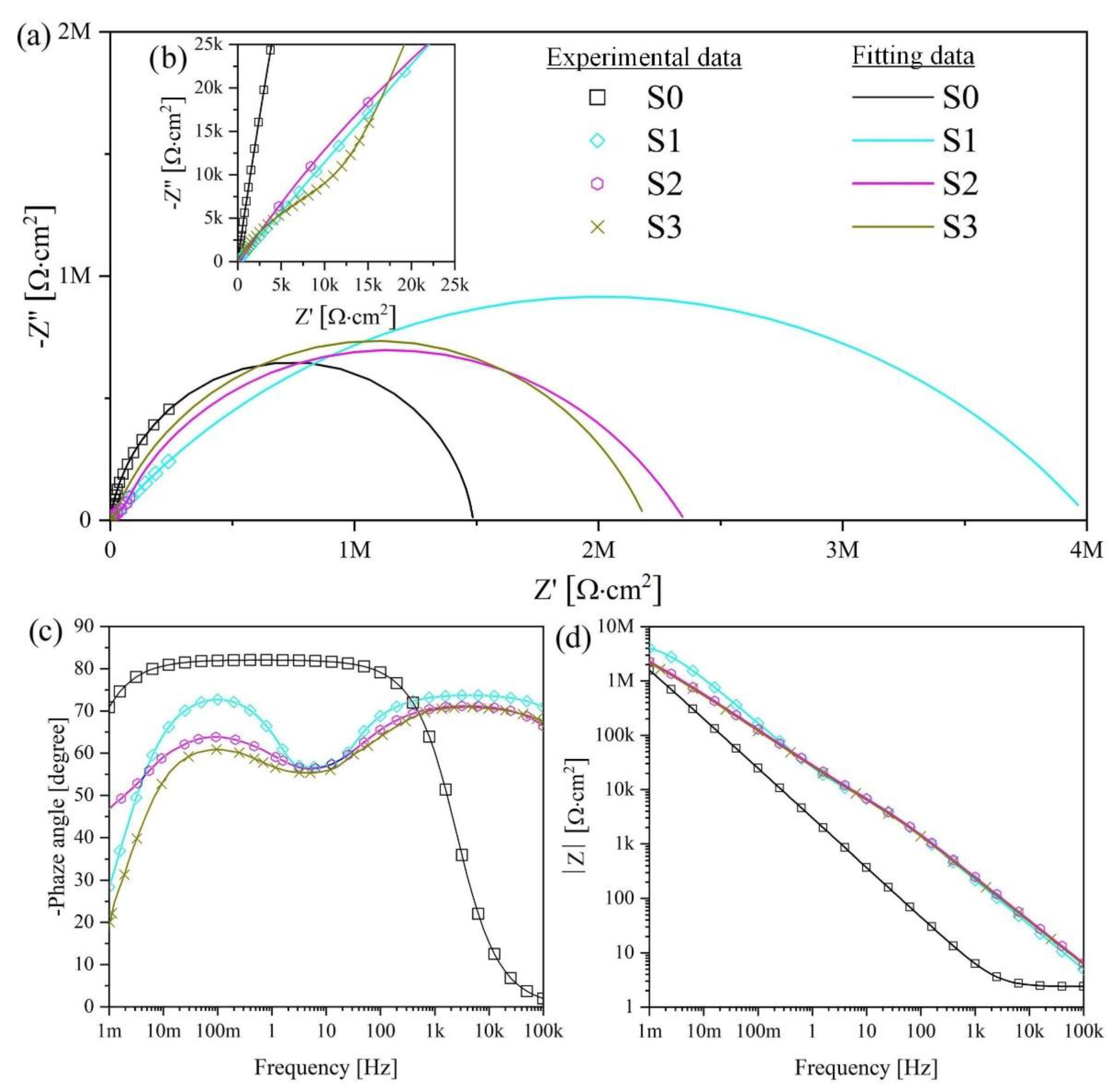
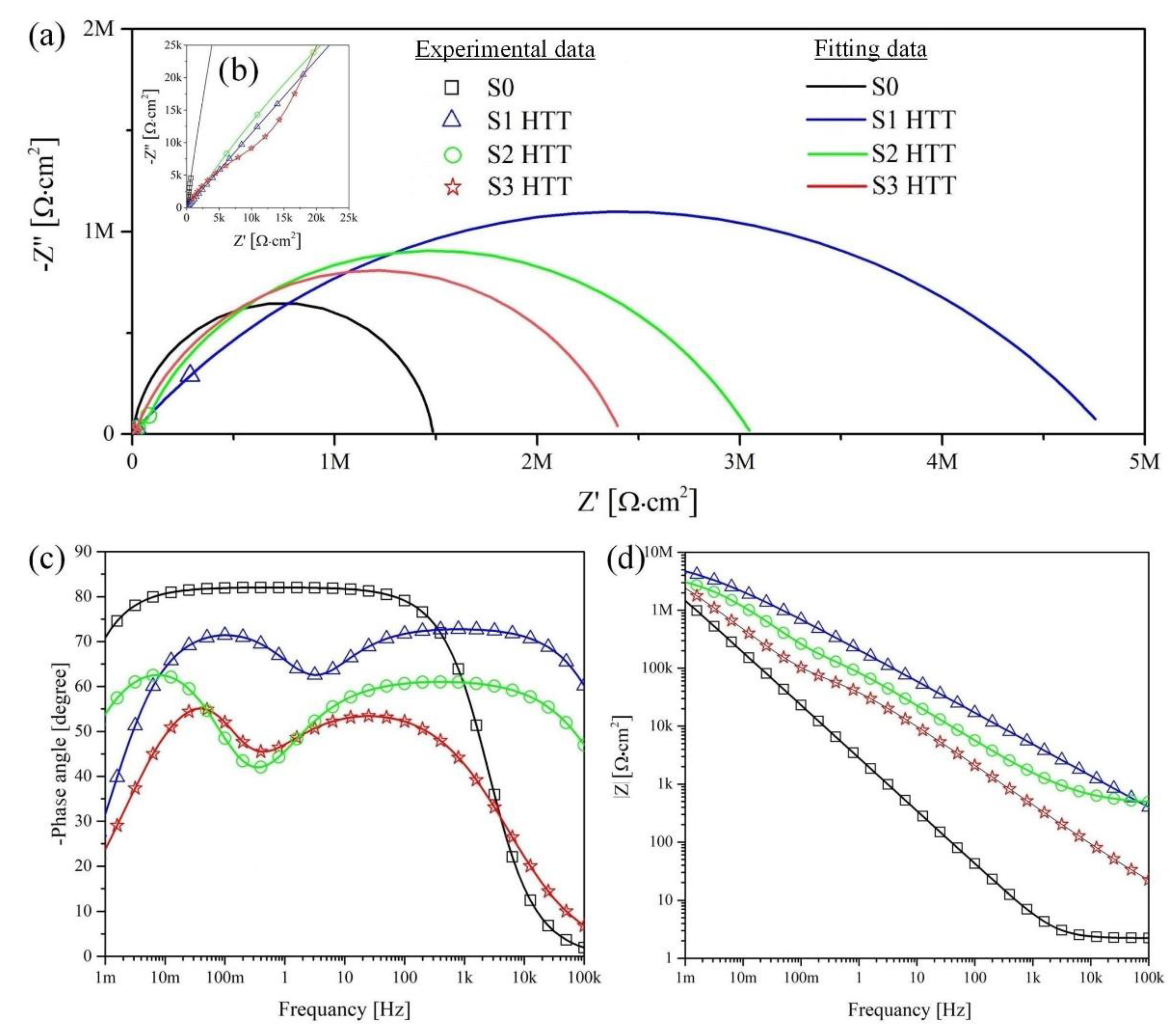
Figure 10 and
Figure 11 present the Nyquist and Bode plots for the S1–S3 and S1 HTT–S3HTT samples, respectively. The calculated values are presented in
Table 7.
The fitting of the Nyquist plots reveals a significant difference in corrosion properties between S0 and S1–S3, S1 HTT–S3 HTT samples. Sample S0 has characterized by one capacitive arch (
Figure 10 and
Figure 11a,b). This capacitive arch can be represented as the charge transfer resistance R
2 and the capacitance of the electric double layer CPE
2. The Bode plot has a mid-frequency band with a minimum at approximately −80° (
Figure 10 and
Figure 11c). The impedance module at low frequencies |Z|
f → 0 Hz = 1.49 × 10
6 Ohm·cm
2. Comparing the data obtained, the impedance spectrum of this sample can be represented by the equivalent electrical circuit shown in
Figure 9a.
From the Nyquist plot for the S1–S3 and S1 HTT–S3 HTT samples, two capacitive arches are found: The first (in the high-frequency range) characterizes the geometric capacitance of the porous layer (CPE
1) and the electrolyte resistance in the pores (R
1) [
64]. The second arch occurs in the mid- and low-frequency ranges (CPE
2), which can be attributed to the capacitance of the electrical double layer at the interface between the dense oxide layer and metal substrate and the charge transfer resistance R
2 [
65]. It can be observed from the frequency dependence on the phase angle that there are two characteristic bands (
Figure 10c): One is in the high-frequency region, and the second is in the mid-frequency region with 55–70° extrema (
Figure 10 and
Figure 11c) for both bands. The change in the phase angle (
Figure 10 and
Figure 11c) is associated with a decrease in the capacitive component of the S1–S3 and S1 HTT–S3 HTT samples, which directly depends on the change in morphology, surface porosity, and density of the resulting coating (
Figure 3 and
Figure 4).
The EIS data presented in
Figure 10a,b in the form of Nyquist and Bode diagrams indicate a positive effect of the formed PEO layer on the protective properties of the samples. Impedance modulus values measured at low frequencies |Z|
f = 0.001 Hz for the PEO coating are at least 1.3 times higher than for S0 (
Figure 10 and
Figure 11,
Table 7).
The comparative characteristic of the S1–S3 samples impedance modules has one order of magnitude in the range (2.09–3.51) × 106 Ohm·cm2. The main resistance to charge transfer is concentrated on the defects of the nonporous oxide layer, which is a barrier layer since the porous surface layer is characterized by resistance values in the range (0.22–5.21) × 105 Ohm·cm2, which are almost an order of magnitude lower than the resistance of the nonporous layer. Thus, the coating formation significantly reduces the possibility of charge transfer at the electrolyte/electrode (porous + nonporous layer) interface, decreasing the rate of corrosion processes.
Table 7 shows the calculated parameters of the electrical circuit elements for the corresponding EEC (
Figure 9). Based on the analysis of the presented data (
Table 7), it can be concluded that the R
2 parameter increases and the CPE
2 values decrease by at least 1.3 for samples with a PEO layer compared to S0. This is a consequence of the significantly greater thickness of the nonporous layer of the PEO coating compared to the thin “natural” oxide layer of the S0 sample. We also note that the values of n
2 decrease for the samples after being processed by the PEO method (
Table 7), which is associated with a reduction in the uniformity of the nonporous layer of the PEO coating compared to the oxide film on the uncoated surface S0.
Based on the analysis of the EIS results, it can be concluded that multistage processing (PEO + HTT) on S1 HTT–S3 HTT samples leads to an increase in the size of the capacitive arch (
Figure 11a,
Table 7) and an increase in the impedance modulus at low frequencies |Z|
f → 0 Hz by a factor of at least 1.2 (
Figure 11d,
Table 7) compared with samples S1–S3 (PEO). The comparative characteristic of the S1 HTT–S3 HTT and S1–S3 sample impedance modules is one order of magnitude in the range of (2.14–4.03) × 10
6 Ohm·cm
2 and (2.41–4.90) × 10
6 Ohm·cm
2, respectively. However, as mentioned earlier, the nature of this phenomenon is associated with the encapsulation of the surface pores of the porous layer and internal defects of the non-porous layer during the HTT process, which is also confirmed by an increase in the parameters R
1 and R
2 (
Table 7) characterizing the electrolyte resistance in the pores and the charge transfer resistance, respectively. Thus, it can be assumed that the decrease in the impedance modulus |Z|
f → 0 Hz in the presented samples is associated with a decrease in the available metal surface where the reactions can occur.
The experimental curves agree with the fitting results (
Table 7) since a chi-square value (χ
2) of approximately 10
−3 is obtained, which confirms the correctness of the ECC.
Note that the EIS data (
Figure 10 and
Figure 11,
Table 7) are in good agreement with the data obtained by the PPT (
Figure 8,
Table 6) and also confirm the conclusions made earlier when evaluating the results of SEM regarding the presence of a bilayer structure (
Figure 3 and
Figure 4) in the PEO coating.
Generally, the corrosion process on the metal/oxide surface proceeds according to the following reactions [
66,
67]:
With the following chemical dehydration reaction:
The cathode reaction (oxygen depolarization):
When the samples are immersed in Hanks’ solution with a “natural” oxide film (S0) and an oxide coating (S1–S3, S1 HTT–S3 HTT), an interaction between the substrate and electrolyte components occurs at the locations of defects. The anodic process can be divided into several stages with the formation of intermediate reaction products: At the first stage of the corrosion process, oxidation and hydrolysis occur with the formation of metastable titanium suboxides Ti
2O
3 (reaction 4). In the second stage, the metastable Ti
2O
3 is oxidized and hydrolyzed (5) until the most stable hydroxide complex TiO(OH)
2 is formed [
68], i.e., transformation into the most stable oxidation state Ti
3+ → Ti
4+ [
69]. The interaction of metal ions can also be represented as a one-stage reaction (6) forming a hydroxide complex. At the final stage of the process, because of dehydration, water molecules are split off with the formation of amorphous TiO
2 [
66]. The cathodic process (8) involves the depolarization of oxygen, usually occurring in electrolytes whose pH is close to neutral.
Previously obtained TiO(OH)
2 and TiO
2 interact with electrolyte components according to the adsorption-chemical mechanism [
70,
71] with the formation of HA occurring via the following reactions:
L. Frauchiger et al. [
72] detected that the phosphate anions can enter exchange reactions (9–11) with a hydrated surface to form complex titanium phosphates, which react with calcium ions to form HA and TiO
2. Furthermore, amorphous TiO
2 can adsorb Ca
2+ and PO
43− ions with HA formation. Therefore, it is logical to assume that the previously introduced apatite precursors have a positive effect on the bioactivity and the rate of osseointegration during the formation of the coating.
The complexity of the object under study, and the variety of chemical and electrochemical processes, both during the coating formation and when exposed to a corrosive environment, led to certain experimental limitations in the performance of the work. In particular, it was impossible to carry out measurements in the low-frequency range when using the EIS method. The results obtained do not yet allow us to conclude the mechanism of the corrosion failure of the coating. This requires special methods to identify the features of individual stages of the corrosion process and their flow regimes.