Snail Based Carbonated-Hydroxyapatite Material as Adsorbents for Water Iron (II)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. CHAp Adsorbent Preparation
2.3. Adsorption Experiments
2.4. Characterization Techniques
3. Results
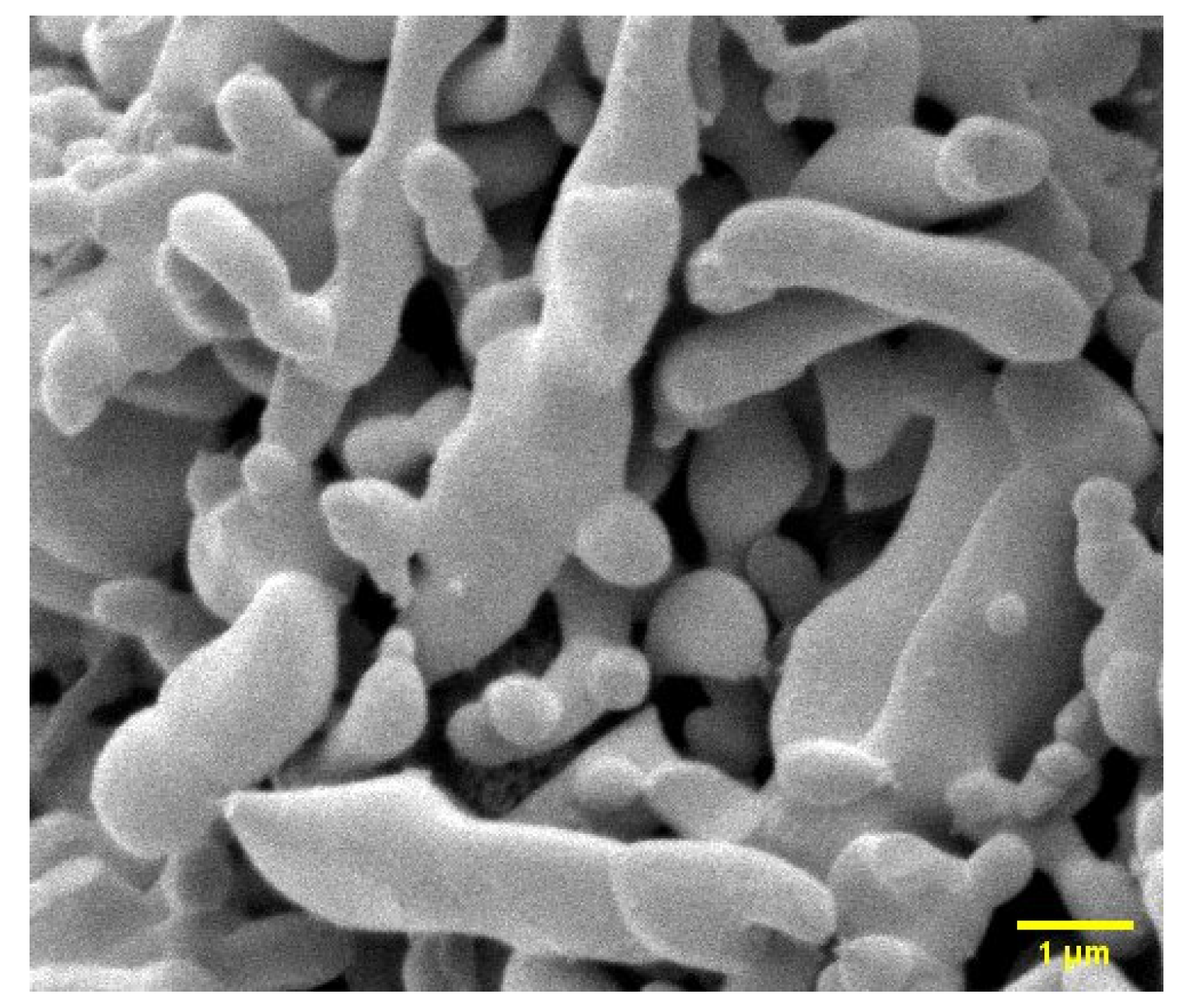
3.1. SEM Image of CHAp
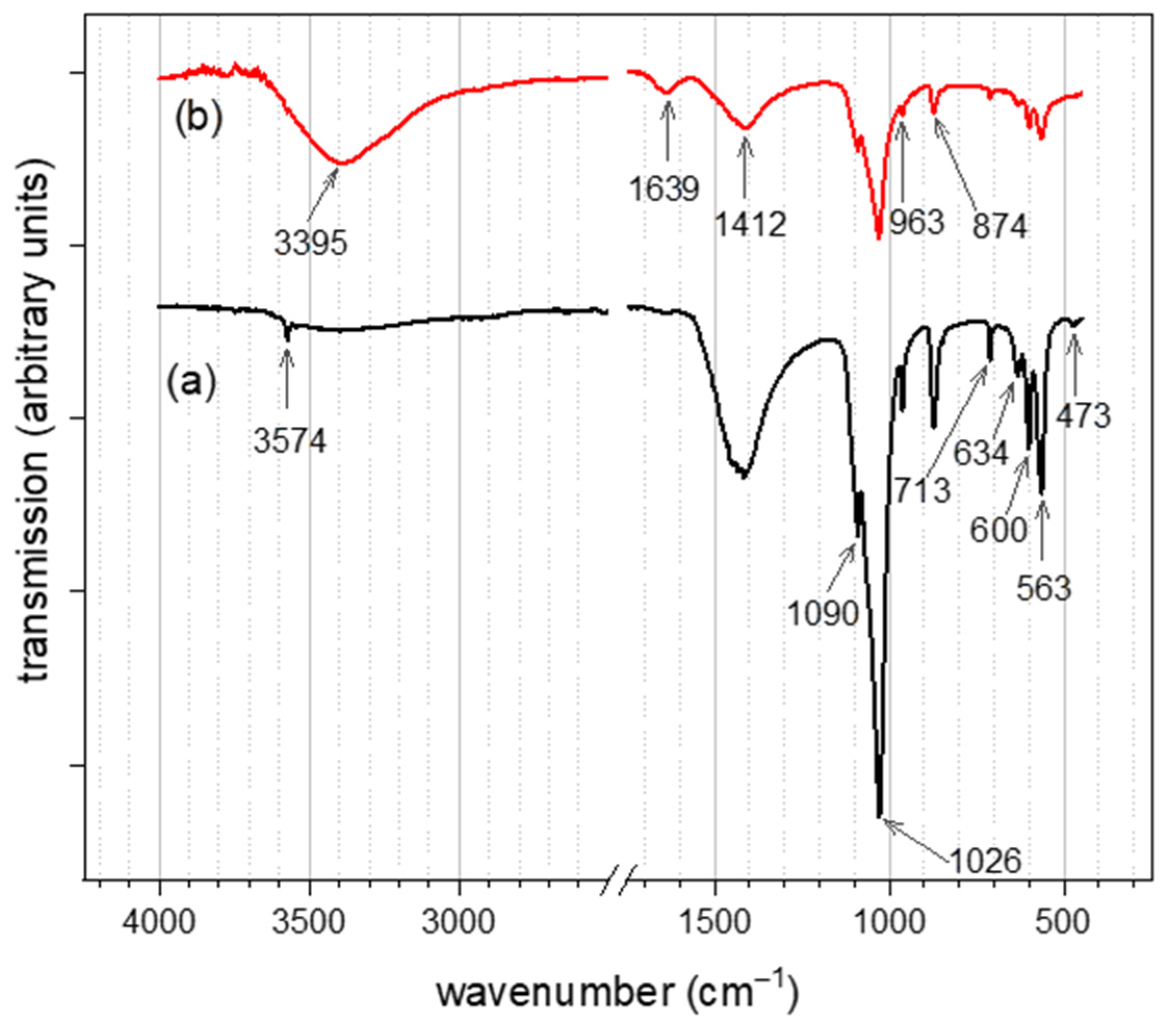
3.2. IR Spectra Analysis of CHAp
3.3. Raman Spectra Data Analysis and Spectroscopic Imaging of CHAp
3.4. Adsorption Test Results
3.4.1. Adsorption Kinetics
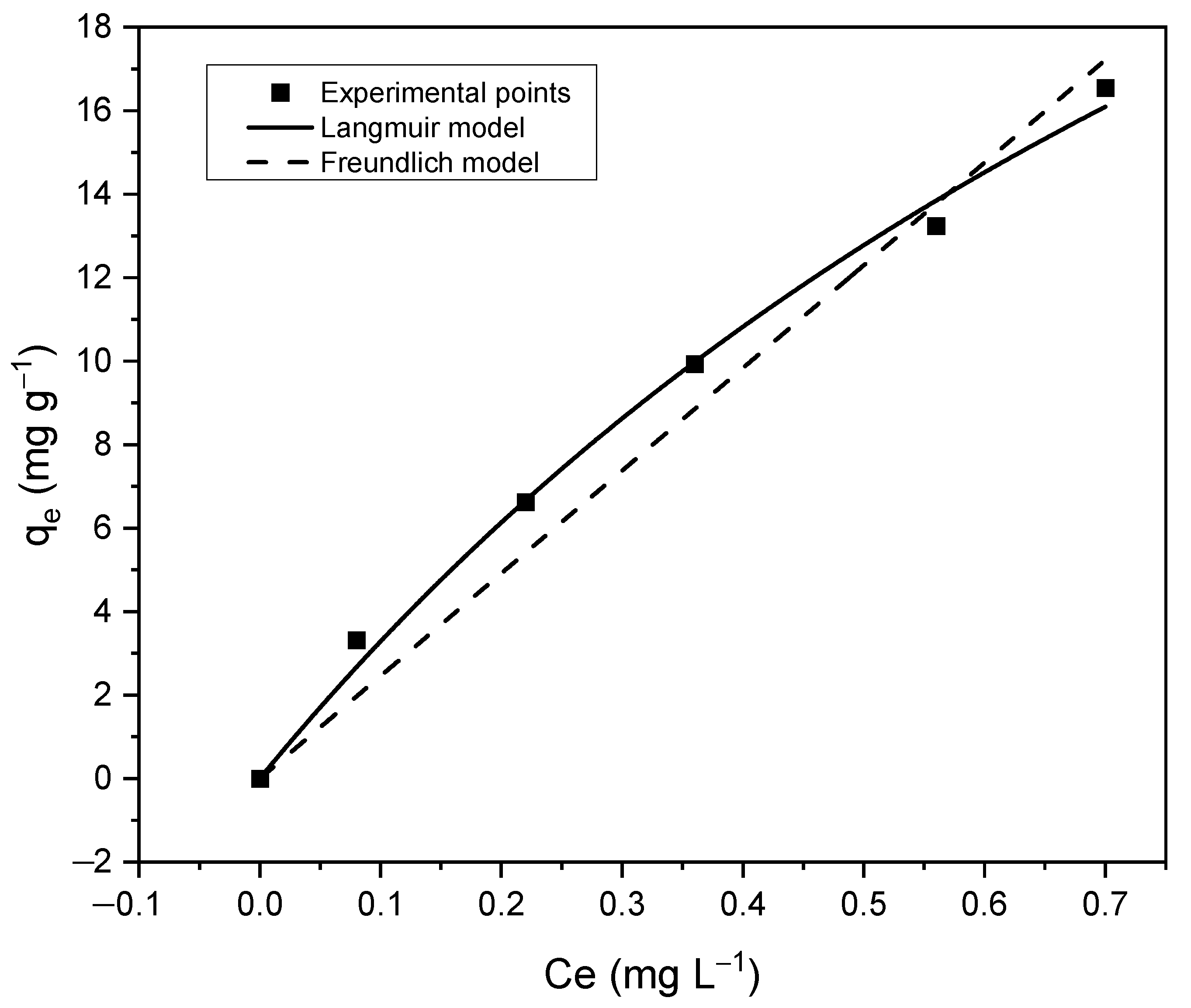
3.4.2. Adsorption Isotherms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olabiyi, O.G.; Adekola, F.A. Removal of Iron and Manganese from Aqueous Solution Using Hydroxyapatite Prepared from Cow Bone. Res. Rev. J. Mater. Sci. 2018, 6, 59–72. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N. Removal of Iron for Safe Drinking Water. Desalination 2012, 303, 1–11. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on Iron and Its Importance for Human Health. J. Res. Med. Sci. 2014, 19, 164–174. [Google Scholar] [PubMed]
- Galal-Gorchev, H. WHO Guidelines for Drinking-Water Quality. Water Supply 1993, 11, 1–16. [Google Scholar]
- Beenakumari, K.S. Removal of Iron from Water Using Modified Coconut Shell Charcoal as Adsorbent. Curr. World Environ. J. 2009, 4, 321–326. [Google Scholar]
- Plebani, M.; Laposata, M.; Lippi, G. A Manifesto for the Future of Laboratory Medicine Professionals. Clin. Chim. Acta 2019, 489, 49–52. [Google Scholar] [CrossRef]
- Saleh, H.N.; Panahande, M.; Yousefi, M.; Asghari, F.B.; Oliveri Conti, G.; Talaee, E.; Mohammadi, A.A. Carcinogenic and Non-Carcinogenic Risk Assessment of Heavy Metals in Groundwater Wells in Neyshabur Plain, Iran. Biol. Trace Elem. Res. 2019, 190, 251–261. [Google Scholar] [CrossRef]
- Kwakye-Awuah, B.; Sefa-Ntiri, B.; Von-Kiti, E.; Nkrumah, I.; Williams, C. Adsorptive Removal of Iron and Manganese from Groundwater Samples in Ghana by Zeolite Y Synthesized from Bauxite and Kaolin. Water 2019, 11, 1912. [Google Scholar] [CrossRef] [Green Version]
- Khatri, N.; Tyagi, S.; Rawtani, D. Recent Strategies for the Removal of Iron from Water: A Review. J. Water Process Eng. 2017, 19, 291–304. [Google Scholar] [CrossRef]
- Okoh, E.; Oruabena, B.; Amgbari, C.O.; Nelson, E.S. A Proposed Multi-Barrier Option for Removing Iron and Microbial Contamination from Yenagoa Borehole Waters; Springer: Singapore, 2019; ISBN 9789811370106. [Google Scholar]
- Dietrich, A.M.; Burlingame, G.A. A Review: The Challenge, Consensus, and Confusion of Describing Odors and Tastes in Drinking Water. Sci. Total Environ. 2020, 713, 135061. [Google Scholar] [CrossRef]
- Makvandi, P.; Iftekhar, S.; Pizzetti, F.; Zarepour, A.; Zare, E.N.; Ashrafizadeh, M.; Agarwal, T.; Padil, V.V.T.; Mohammadinejad, R.; Sillanpaa, M. Functionalization of Polymers and Nanomaterials for Water Treatment, Food Packaging, Textile and Biomedical Applications: A Review. Environ. Chem. Lett. 2021, 19, 583–611. [Google Scholar] [CrossRef]
- Asimeng, B.O.; Fianko, J.R.; Kaufmann, E.E.; Tiburu, E.K.; Hayford, C.F.; Anani, P.A.; Dzikunu, O.K. Preparation and Characterization of Hydroxyapatite from A Chatina Achatina Snail Shells: Effect of Carbonate Substitution and Trace Elements on Defluoridation of Water. J. Asian Ceram. Soc. 2018, 6, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-K.; Kim, H.-S.; Kwon, J.-H. The Removal of Heavy Metals Using Hydroxyapatite. Environ. Eng. Res. 2005, 10, 205–212. [Google Scholar] [CrossRef]
- Ishtiaque, M.S.; Das, H.; Hoque, S.M.; Choudhury, S. Synthesis of N-HAP Using Different Precursors and Dependence of Vickers Hardness on the Structure by Tuning Sintering Temperature. Int. J. Appl. Ceram. Technol. 2022, 19, 1281–1292. [Google Scholar] [CrossRef]
- Gomes, D.S.; Santos, A.M.C.; Neves, G.A.; Menezes, R.R. A Brief Review on Hydroxyapatite Production and Use in Biomedicine. Cerâmica 2019, 65, 282–302. [Google Scholar] [CrossRef] [Green Version]
- Peng, Q.; Yu, F.; Huang, B.; Huang, Y. Carbon-Containing Bone Hydroxyapatite Obtained from Tuna Fish Bone with High Adsorption Performance for Congo Red. RSC Adv. 2017, 7, 26968–26973. [Google Scholar] [CrossRef] [Green Version]
- Furko, M.; Horváth, Z.E.; Sulyok, A.; Kis, V.K.; Balázsi, K.; Mihály, J.; Balázsi, C. Preparation and Morphological Investigation on Bioactive Ion-Modified Carbonated Hydroxyapatite-Biopolymer Composite Ceramics as Coatings for Orthopaedic Implants. Ceram. Int. 2022, 48, 760–768. [Google Scholar] [CrossRef]
- Kadota, K.; Ibe, T.; Sugawara, Y.; Takano, H.; Yusof, Y.A.; Uchiyama, H.; Tozuka, Y.; Yamanaka, S. Water-Assisted Synthesis of Mesoporous Calcium Carbonate with a Controlled Specific Surface Area and Its Potential to Ferulic Acid Release. RSC Adv. 2020, 10, 28019–28025. [Google Scholar] [CrossRef]
- Hanesch, M. Raman Spectroscopy of Iron Oxides and (Oxy)Hydroxides at Low Laser Power and Possible Applications in Environmental Magnetic Studies. Geophys. J. Int. 2009, 177, 941–948. [Google Scholar] [CrossRef]
- Gunasekaran, S.; Anbalagan, G.; Pandi, S. Raman and Infrared Spectra of Carbonates of Calcite Structure. J. Raman Spectrosc. 2006, 37, 892–899. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, J.; Jiang, Z.; Shan, D.; Lu, Y. Biosorption of Fe(II) and Mn(II) Ions from Aqueous Solution by Rice Husk Ash. BioMed Res. Int. 2014, 2014, 973095. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Asimeng, B.O.; Tiburu, E.K.; Kan-Dapaah, K.; Efavi, J.K.; Asiamah, R.; Afeke, D.W. Influence of Preferred Orientation on the Bioactivity of Hydroxyapatite: A Potential Tooth Repair and Implant Surface Coating Material. Cerâmica 2020, 66, 340–346. [Google Scholar] [CrossRef]
- Hao, H.; Li, L.; Yuan, Z.; Patra, P.; Somasundaran, P. Adsorption Differences of Sodium Oleate on Siderite and Hematite. Miner. Eng. 2019, 137, 10–18. [Google Scholar] [CrossRef]
- Asimeng, B.O.; Karadag, I.; Iftekhar, S.; Xu, Y.; Czernuszka, J. XRD and IR Revelation of a Unique G-C3N4 Phase with Effects on Collagen/Hydroxyapatite Bone Scaffold Pore Geometry and Stiffness. SN Appl. Sci. 2020, 2, 1417. [Google Scholar] [CrossRef]
- Peccati, F.; Bernocco, C.; Ugliengo, P.; Corno, M. Properties and Reactivity toward Water of A Type Carbonated Apatite and Hydroxyapatite Surfaces. J. Phys. Chem. C 2018, 122, 3934–3944. [Google Scholar] [CrossRef] [Green Version]
- Giles, C.H.; Smith, D.; Huitson, A. A General Treatment and Classification of the Solute Adsorption Isotherm. I. Theoretical. J. Colloid Interface Sci. 1974, 47, 755–765. [Google Scholar] [CrossRef]
- Shikuku, V.O.; Zanella, R.; Kowenje, C.O.; Donato, F.F.; Bandeira, N.M.G.; Prestes, O.D. Single and Binary Adsorption of Sulfonamide Antibiotics onto Iron-Modified Clay: Linear and Nonlinear Isotherms, Kinetics, Thermodynamics, and Mechanistic Studies. Appl. Water Sci. 2018, 8, 175. [Google Scholar] [CrossRef] [Green Version]
- Ayash, M.A.A.; Ahmed, T.; Elnasr, S.; Soliman, M.H. Removing Iron Ions Contaminants from Groundwater Using Modified Nano-Hydroxyapatite by Nano Manganese Oxide. J. Water Resour. Prot. 2019, 11, 789–809. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, Nature and Utility of Universal Iron Chelator–Siderophore: A Review. Microbiol. Res. 2018, 212, 103–111. [Google Scholar] [CrossRef]
- Eberhardt, T.L.; Min, S.-H. Biosorbents Prepared from Wood Particles Treated with Anionic Polymer and Iron Salt: Effect of Particle Size on Phosphate Adsorption. Bioresour. Technol. 2008, 99, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Oliva, J.; De Pablo, J.; Cortina, J.-L.; Cama, J.; Ayora, C. The Use of Apatite IITM to Remove Divalent Metal Ions Zinc (II), Lead (II), Manganese (II) and Iron (II) from Water in Passive Treatment Systems: Column Experiments. J. Hazard. Mater. 2010, 184, 364–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Uliana, A.; Taylor, M.K.; Chakarawet, K.; Bandaru, S.R.S.; Gul, S.; Xu, J.; Ackerman, C.M.; Chatterjee, R.; Furukawa, H.; et al. Iron Detection and Remediation with a Functionalized Porous Polymer Applied to Environmental Water Samples. Chem. Sci. 2019, 10, 6651–6660. [Google Scholar] [CrossRef] [PubMed] [Green Version]

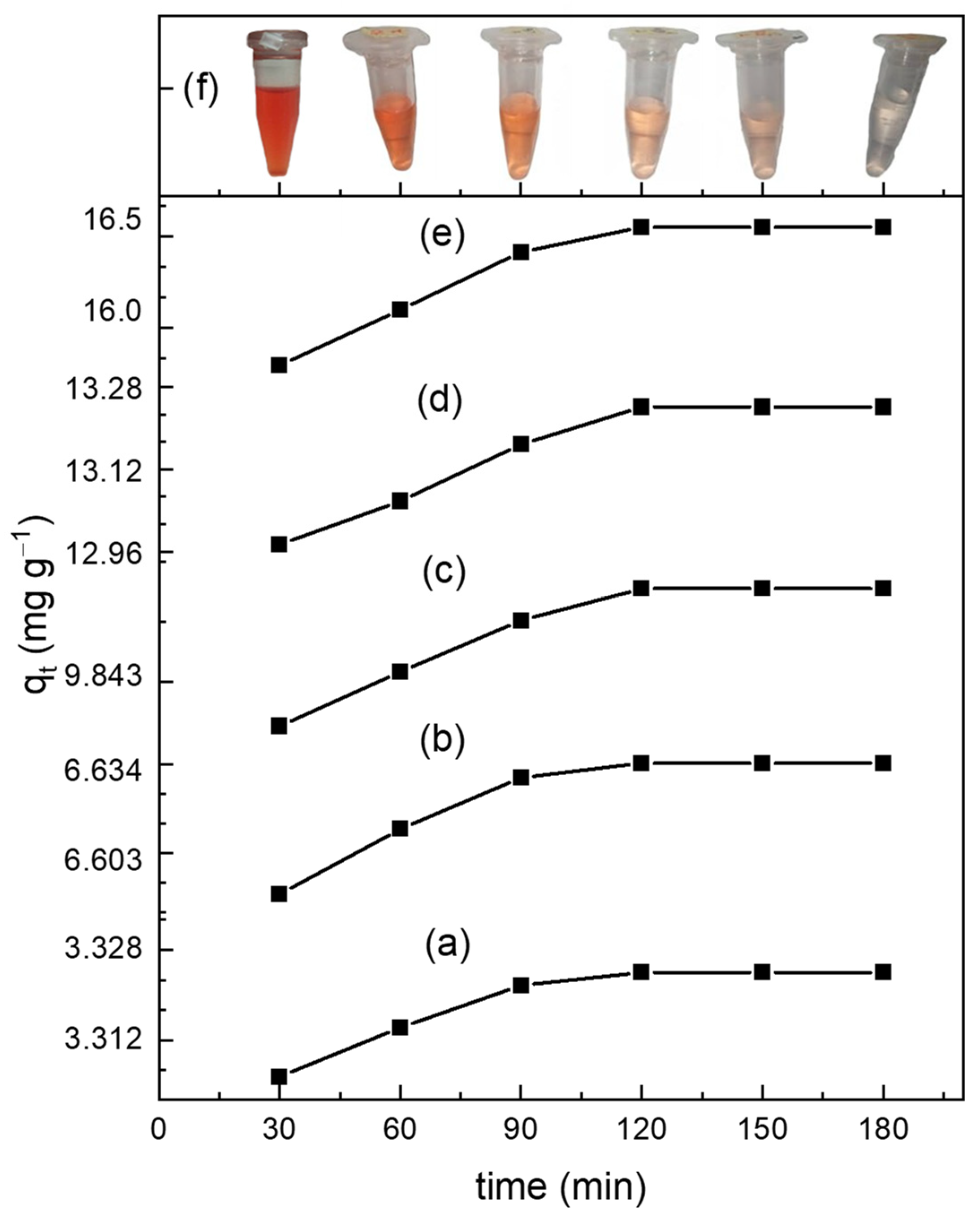

| Peudo-First-Order Model | Pseudo-Second-Order Model | ||||||
|---|---|---|---|---|---|---|---|
| qe-expa | qe-calb | R2 | qe-calb | R2 | |||
| 20 | 3.320 | 0.070 | −1.850 | 0.920 | 3.320 | 1.074 | 1.000 |
| 40 | 6.630 | 0.130 | −1.680 | 0.940 | 6.630 | 0.523 | 1.000 |
| 60 | 9.940 | 0.230 | −0.927 | 0.962 | 9.980 | 0.152 | 0.999 |
| 80 | 13.240 | 0.590 | −1.203 | 0.754 | 13.320 | 0.074 | 0.999 |
| 100 | 16.550 | 2.170 | −1.587 | 0.728 | 16.780 | 0.027 | 0.999 |
| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| KL | R2 | 1/n | KF | R2 | |
| 45.870 | 0.770 | 0.993 | 0.009 | 0.222 | 0.966 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asimeng, B.O.; Amenyaglo, E.K.; Dodoo-Arhin, D.; Efavi, J.K.; Kwakye-Awuah, B.; Tiburu, E.K.; Foster, E.J.; Czernuska, J. Snail Based Carbonated-Hydroxyapatite Material as Adsorbents for Water Iron (II). Materials 2022, 15, 3253. https://doi.org/10.3390/ma15093253
Asimeng BO, Amenyaglo EK, Dodoo-Arhin D, Efavi JK, Kwakye-Awuah B, Tiburu EK, Foster EJ, Czernuska J. Snail Based Carbonated-Hydroxyapatite Material as Adsorbents for Water Iron (II). Materials. 2022; 15(9):3253. https://doi.org/10.3390/ma15093253
Chicago/Turabian StyleAsimeng, Bernard Owusu, Edward Kwame Amenyaglo, David Dodoo-Arhin, Johnson Kwame Efavi, Bright Kwakye-Awuah, Elvis Kwason Tiburu, E. Johan Foster, and Jan Czernuska. 2022. "Snail Based Carbonated-Hydroxyapatite Material as Adsorbents for Water Iron (II)" Materials 15, no. 9: 3253. https://doi.org/10.3390/ma15093253
APA StyleAsimeng, B. O., Amenyaglo, E. K., Dodoo-Arhin, D., Efavi, J. K., Kwakye-Awuah, B., Tiburu, E. K., Foster, E. J., & Czernuska, J. (2022). Snail Based Carbonated-Hydroxyapatite Material as Adsorbents for Water Iron (II). Materials, 15(9), 3253. https://doi.org/10.3390/ma15093253







