Results at the 1-Year Follow-Up of a Prospective Cohort Study with Short, Zirconia Implants
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Intervention
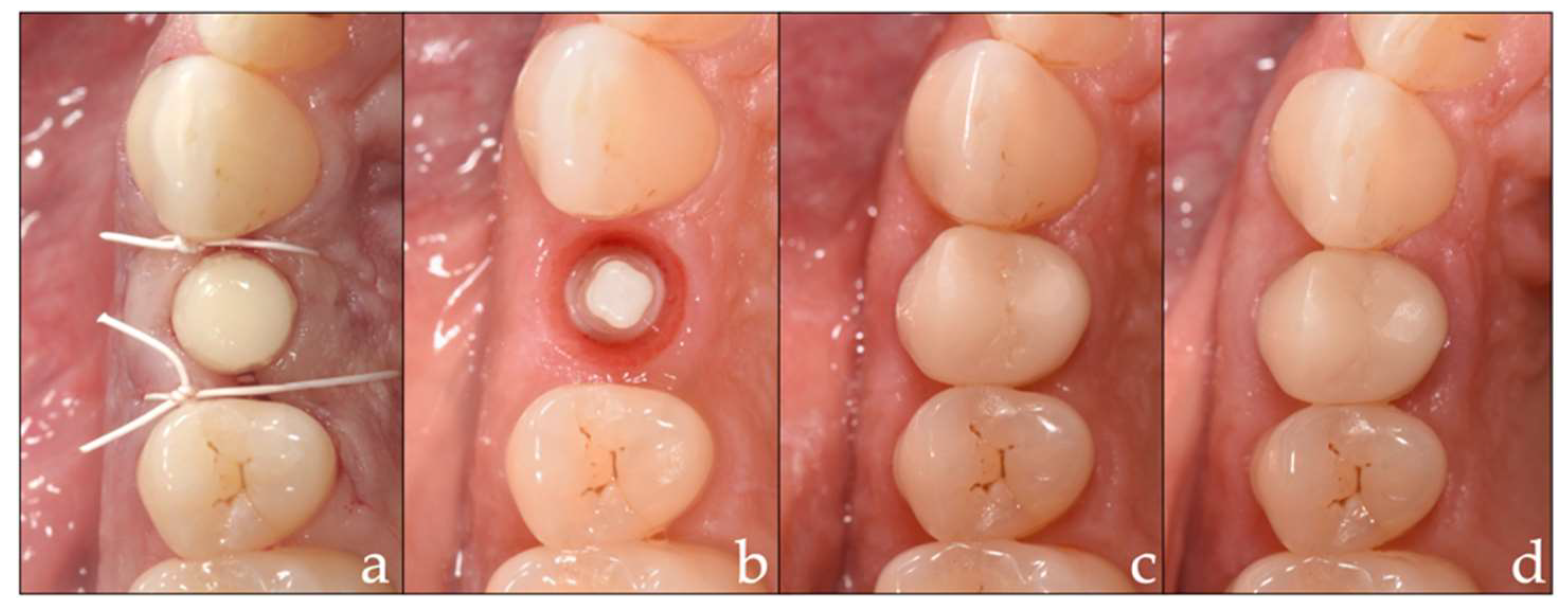
2.4. Prosthetic Insertion and Follow-Up
- 1.
- Restoration with monolithic zirconia (VITA YZ®, VITA Zahnfabrik)
- 2.
- Restoration with monolithic polymer-infiltrated ceramic (VITA ENAMIC®, VITA Zahnfabrik)
- 3.
- Restoration with monolithic polymer (VITA CAD-Temp®, VITA Zahnfabrik).
2.5. Examinations and Analyses
2.6. Statistical Analysis
3. Results
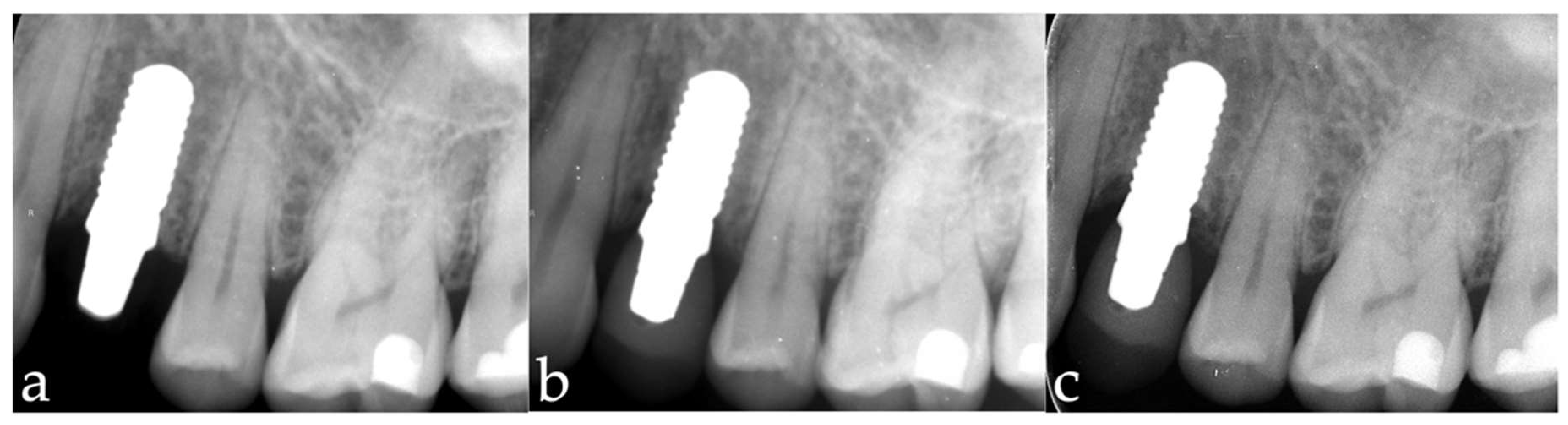
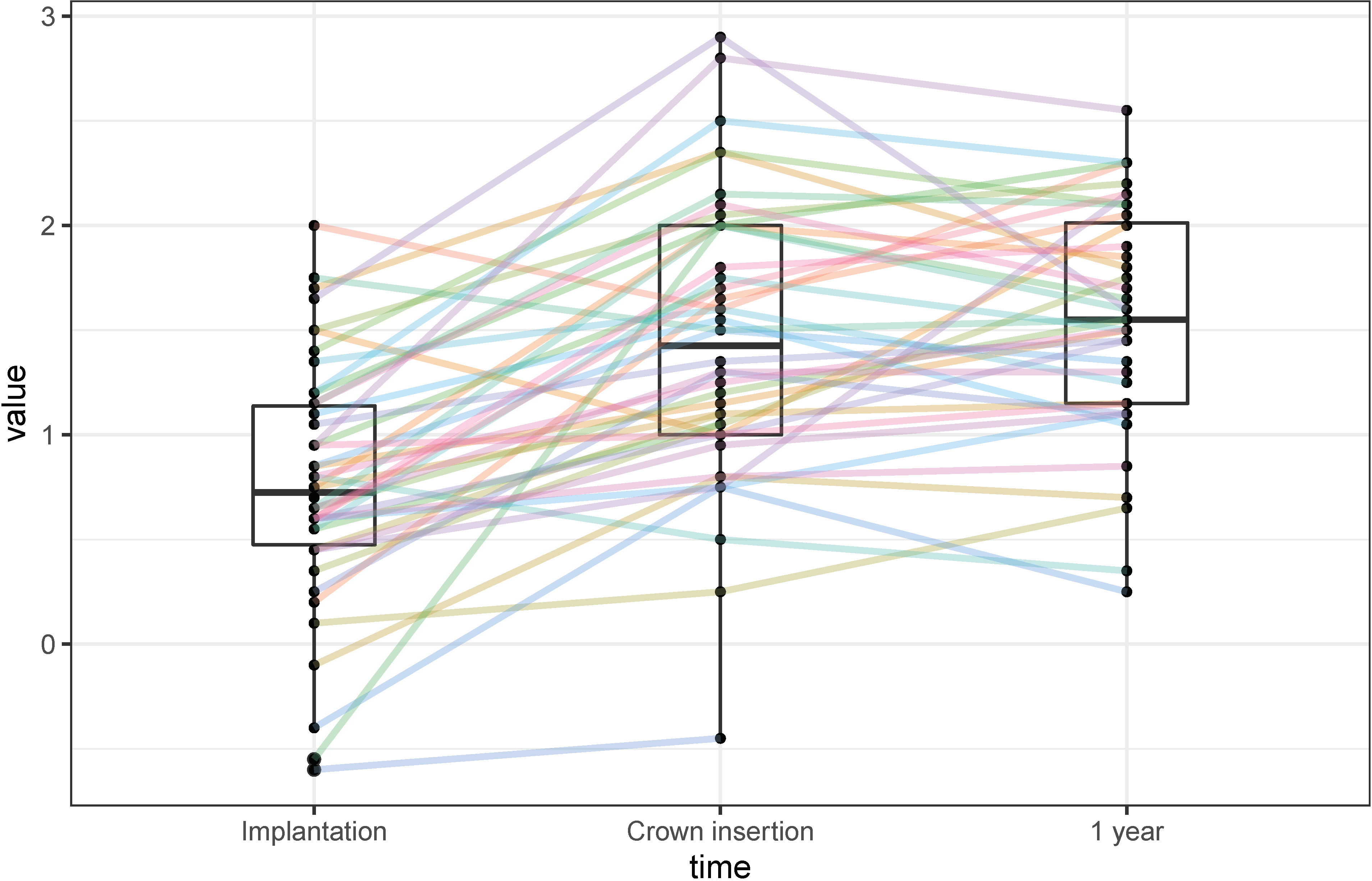
3.1. Marginal Bone-Level Changes
3.2. Implant Survival
3.3. Clinical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roehling, S.; Schlegel, K.A.; Woelfler, H.; Gahlert, M. Performance and outcome of zirconia dental implants in clinical studies: A meta-analysis. Clin. Oral Implants Res. 2018, 29 (Suppl. 16), 135–153. [Google Scholar] [CrossRef] [PubMed]
- Balmer, M.; Spies, B.C.; Kohal, R.J.; Hammerle, C.H.; Vach, K.; Jung, R.E. Zirconia implants restored with single crowns or fixed dental prostheses: 5-year results of a prospective cohort investigation. Clin. Oral Implants Res. 2020, 31, 452–462. [Google Scholar] [CrossRef]
- Lorenz, J.; Giulini, N.; Holscher, W.; Schwiertz, A.; Schwarz, F.; Sader, R. Prospective controlled clinical study investigating long-term clinical parameters, patient satisfaction, and microbial contamination of zirconia implants. Clin. Implant Dent. Relat. Res. 2019, 21, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Spitznagel, F.A.; Balmer, M.; Wiedemeier, D.B.; Jung, R.E.; Gierthmuehlen, P.C. Clinical outcomes of all-ceramic single crowns and fixed dental prostheses supported by ceramic implants: A systematic review and meta-analyses. Clin. Oral Implants Res. 2021, 33, 1–20. [Google Scholar] [CrossRef]
- Borgonovo, A.E.; Censi, R.; Vavassori, V.; Dolci, M.; Calvo-Guirado, J.L.; Delgado Ruiz, R.A.; Maiorana, C. Evaluation of the success criteria for zirconia dental implants: A four-year clinical and radiological study. Int. J. Dent. 2013, 2013, 463073. [Google Scholar] [CrossRef]
- Grassi, F.R.; Capogreco, M.; Consonni, D.; Bilardi, G.; Buti, J.; Kalemaj, Z. Immediate occlusal loading of one-piece zirconia implants: Five-year radiographic and clinical evaluation. Int. J. Oral Maxillofac. Implants 2015, 30, 671–680. [Google Scholar] [CrossRef]
- Kohal, R.J.; Spies, B.C.; Vach, K.; Balmer, M.; Pieralli, S. A Prospective Clinical Cohort Investigation on Zirconia Implants: 5-Year Results. J. Clin. Med. 2020, 9, 2585. [Google Scholar] [CrossRef]
- Renouard, F.; Nisand, D. Impact of implant length and diameter on survival rates. Clin. Oral Implants Res. 2006, 17 (Suppl. 2), 35–51. [Google Scholar] [CrossRef]
- Ravida, A.; Wang, I.C.; Barootchi, S.; Askar, H.; Tavelli, L.; Gargallo-Albiol, J.; Wang, H.L. Meta-analysis of randomized clinical trials comparing clinical and patient-reported outcomes between extra-short (</=6 mm) and longer (>/=10 mm) implants. J. Clin. Periodontol. 2019, 46, 118–142. [Google Scholar] [CrossRef]
- Srinivasan, M.; Vazquez, L.; Rieder, P.; Moraguez, O.; Bernard, J.P.; Belser, U.C. Survival rates of short (6 mm) micro-rough surface implants: A review of literature and meta-analysis. Clin. Oral Implants Res. 2014, 25, 539–545. [Google Scholar] [CrossRef]
- Sun, H.L.; Huang, C.; Wu, Y.R.; Shi, B. Failure rates of short (</=10 mm) dental implants and factors influencing their failure: A systematic review. Int. J. Oral Maxillofac. Implants 2011, 26, 816–825. [Google Scholar]
- Nisand, D.; Picard, N.; Rocchietta, I. Short implants compared to implants in vertically augmented bone: A systematic review. Clin. Oral Implants Res. 2015, 26 (Suppl. 11), 170–179. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Donos, N.; Alcoforado, G.; Balmer, M.; Gurzawska, K.; Mardas, N.; Milinkovic, I.; Nisand, D.; Rocchietta, I.; Stavropoulos, A.; et al. Therapeutic concepts and methods for improving dental implant outcomes. Summary and consensus statements. The 4th EAO Consensus Conference 2015. Clin. Oral Implants Res. 2015, 26 (Suppl. 11), 202–206. [Google Scholar] [CrossRef] [PubMed]
- Thoma, D.S.; Zeltner, M.; Husler, J.; Hammerle, C.H.; Jung, R.E. EAO Supplement Working Group 4—EAO CC 2015 Short implants versus sinus lifting with longer implants to restore the posterior maxilla: A systematic review. Clin. Oral Implants Res. 2015, 26 (Suppl. 11), 154–169. [Google Scholar] [CrossRef] [PubMed]
- Naenni, N.; Sahrmann, P.; Schmidlin, P.R.; Attin, T.; Wiedemeier, D.B.; Sapata, V.; Hammerle, C.H.F.; Jung, R.E. Five-Year Survival of Short Single-Tooth Implants (6 mm): A Randomized Controlled Clinical Trial. J. Dent. Res. 2018, 97, 887–892. [Google Scholar] [CrossRef]
- Rossi, F.; Botticelli, D.; Cesaretti, G.; De Santis, E.; Storelli, S.; Lang, N.P. Use of short implants (6 mm) in a single-tooth replacement: A 5-year follow-up prospective randomized controlled multicenter clinical study. Clin. Oral Implants Res. 2016, 27, 458–464. [Google Scholar] [CrossRef]
- Fischer, J.; Schott, A.; Martin, S. Surface micro-structuring of zirconia dental implants. Clin. Oral Implants Res. 2016, 27, 162–166. [Google Scholar] [CrossRef]
- ISO 14155:2020(en); Clinical Investigation of Medical Devices for Human Subjects—Good Clinical Practice. ISO: Geneva, Switzerland, 2020.
- Summers, R.B. A new concept in maxillary implant surgery: The osteotome technique. Compendium 1994, 15, 152–154. [Google Scholar]
- Rohr, N.; Balmer, M.; Jung, R.E.; Kohal, R.J.; Spies, B.C.; Hammerle, C.H.F.; Fischer, J. Influence of zirconia implant surface topography on first bone implant contact within a prospective cohort study. Clin. Implant Dent. Relat. Res. 2021, 23, 593–599. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; D’Agostino McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Mezzomo, L.A.; Miller, R.; Triches, D.; Alonso, F.; Shinkai, R.S. Meta-analysis of single crowns supported by short (<10 mm) implants in the posterior region. J. Clin. Periodontol. 2014, 41, 191–213. [Google Scholar] [CrossRef] [PubMed]
- Papaspyridakos, P.; De Souza, A.; Vazouras, K.; Gholami, H.; Pagni, S.; Weber, H.P. Survival rates of short dental implants (</=6 mm) compared with implants longer than 6 mm in posterior jaw areas: A meta-analysis. Clin. Oral Implants Res. 2018, 29 (Suppl. 16), 8–20. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S286–S291. [Google Scholar] [CrossRef] [PubMed]
- Borgonovo, A.E.; Censi, R.; Vavassori, V.; Arnaboldi, O.; Maiorana, C.; Re, D. Zirconia Implants in Esthetic Areas: 4-Year Follow-Up Evaluation Study. Int. J. Dent. 2015, 2015, 415029. [Google Scholar] [CrossRef]
- Bormann, K.H.; Gellrich, N.C.; Kniha, H.; Schild, S.; Weingart, D.; Gahlert, M. A prospective clinical study to evaluate the performance of zirconium dioxide dental implants in single-tooth edentulous area: 3-year follow-up. BMC Oral Health 2018, 18, 181. [Google Scholar] [CrossRef]
- Balmer, M.; Spies, B.C.; Vach, K.; Kohal, R.J.; Hammerle, C.H.F.; Jung, R.E. Three-year analysis of zirconia implants used for single-tooth replacement and three-unit fixed dental prostheses: A prospective multicenter study. Clin. Oral Implants Res. 2018, 29, 290–299. [Google Scholar] [CrossRef]
- Jung, R.E.; Grohmann, P.; Sailer, I.; Steinhart, Y.N.; Feher, A.; Hammerle, C.; Strub, J.R.; Kohal, R. Evaluation of a one-piece ceramic implant used for single-tooth replacement and three-unit fixed partial dentures: A prospective cohort clinical trial. Clin. Oral Implants Res. 2016, 27, 751–761. [Google Scholar] [CrossRef]
- Felice, P.; Checchi, L.; Barausse, C.; Pistilli, R.; Sammartino, G.; Masi, I.; Ippolito, D.R.; Esposito, M. Posterior jaws rehabilitated with partial prostheses supported by 4.0 × 4.0 mm or by longer implants: One-year post-loading results from a multicenter randomised controlled trial. Eur. J. Oral Implantol. 2016, 9, 35–45. [Google Scholar]
- Pohl, V.; Thoma, D.S.; Sporniak-Tutak, K.; Garcia-Garcia, A.; Taylor, T.D.; Haas, R.; Hammerle, C.H. Short dental implants (6 mm) versus long dental implants (11–15 mm) in combination with sinus floor elevation procedures: 3-year results from a multicentre, randomized, controlled clinical trial. J. Clin. Periodontol. 2017, 44, 438–445. [Google Scholar] [CrossRef]
- Hermann, J.S.; Buser, D.; Schenk, R.K.; Cochran, D.L. Crestal bone changes around titanium implants. A histometric evaluation of unloaded non-submerged and submerged implants in the canine mandible. J. Periodontol. 2000, 71, 1412–1424. [Google Scholar] [CrossRef]
- Kniha, K.; Schlegel, K.A.; Kniha, H.; Modabber, A.; Holzle, F.; Kniha, K. Evaluation of peri-implant bone levels and soft tissue dimensions around zirconia implants-a three-year follow-up study. Int. J. Oral Maxillofac. Surg. 2018, 47, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Pieralli, S.; Kohal, R.J.; Jung, R.E.; Vach, K.; Spies, B.C. Clinical Outcomes of Zirconia Dental Implants: A Systematic Review. J. Dent. Res. 2017, 96, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Cionca, N.; Hashim, D.; Mombelli, A. Two-piece zirconia implants supporting all-ceramic crowns: Six-year results of a prospective cohort study. Clin. Oral Implants Res. 2021, 32, 695–701. [Google Scholar] [CrossRef] [PubMed]


| Implant Position (FDI) | ||||||||
|---|---|---|---|---|---|---|---|---|
| 17 | 16 | 15 | 14 | 24 | 25 | 26 | 27 | |
| n implants (proportion of total implants) | 0 (0%) | 2 (4%) | 5 (11%) | 4 (9%) | 7 (15%) | 4 (9%) | 1 (2%) | 0 (0%) |
| n implants with dehiscence (proportion at each position) | 0 (0%) | 2 (40%) | 2 (50%) | 3 (43%) | 2 (50%) | 1 (100%) | ||
| Allocation of crown material n(Z/C/P) * | 0/0/2 | 2/0/3 | 1/3/0 | 1/1/5 | 2/2/0 | 1/0/0 | ||
| Implant Position (FDI) | ||||||||
| 47 | 46 | 45 | 44 | 34 | 35 | 36 | 37 | |
| n implants (proportion of total implants) | 0 (0%) | 10 (22%) | 4 (9%) | 2 (4%) | 0 (0%) | 0 (0%) | 7 (15%) | 0 (0%) |
| n implants with dehiscence (proportion at each position) | 3 (30%) | 4 (100%) | 1 (50%) | 2 (29%) | ||||
| Allocation of crown material n(Z/C/P) * | 3/4/3 | 2/1/1 | 0/2/0 | 3/2/2 | ||||
| Implants | Neighboring Teeth | |||||||
|---|---|---|---|---|---|---|---|---|
| PI | PD | BoP | Kerat. T. | PI | PD | BoP | Kerat. T. | |
| Baseline | 14 ± 25% | 3.3 ± 0.4 mm | 11 ± 22% | 3.3 ± 1.5 mm | 13 ± 17% | 2.6 ± 0.3 mm | 8 ± 10% | 3.5 ± 1.4 mm |
| 1 y Follow-up | 22 ± 20% | 3.4 ± 0.5 mm | 16 ± 16% | 3.4 ± 1.5 mm | 15 ± 15% | 2.6 ± 0.5 mm | 11 ± 11% | 3.5 ± 1.3 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balmer, M.; Fischer, C.; Pirc, M.; Hämmerle, C.H.F.; Jung, R.E. Results at the 1-Year Follow-Up of a Prospective Cohort Study with Short, Zirconia Implants. Materials 2022, 15, 5584. https://doi.org/10.3390/ma15165584
Balmer M, Fischer C, Pirc M, Hämmerle CHF, Jung RE. Results at the 1-Year Follow-Up of a Prospective Cohort Study with Short, Zirconia Implants. Materials. 2022; 15(16):5584. https://doi.org/10.3390/ma15165584
Chicago/Turabian StyleBalmer, Marc, Carolin Fischer, Miha Pirc, Christoph H. F. Hämmerle, and Ronald E. Jung. 2022. "Results at the 1-Year Follow-Up of a Prospective Cohort Study with Short, Zirconia Implants" Materials 15, no. 16: 5584. https://doi.org/10.3390/ma15165584
APA StyleBalmer, M., Fischer, C., Pirc, M., Hämmerle, C. H. F., & Jung, R. E. (2022). Results at the 1-Year Follow-Up of a Prospective Cohort Study with Short, Zirconia Implants. Materials, 15(16), 5584. https://doi.org/10.3390/ma15165584






