Preparation and Properties of Ce0.8Sm0.16Y0.03Gd0.01O1.9-BaIn0.3Ti0.7O2.85 Composite Electrolyte
Abstract
:1. Introduction
2. Experimental
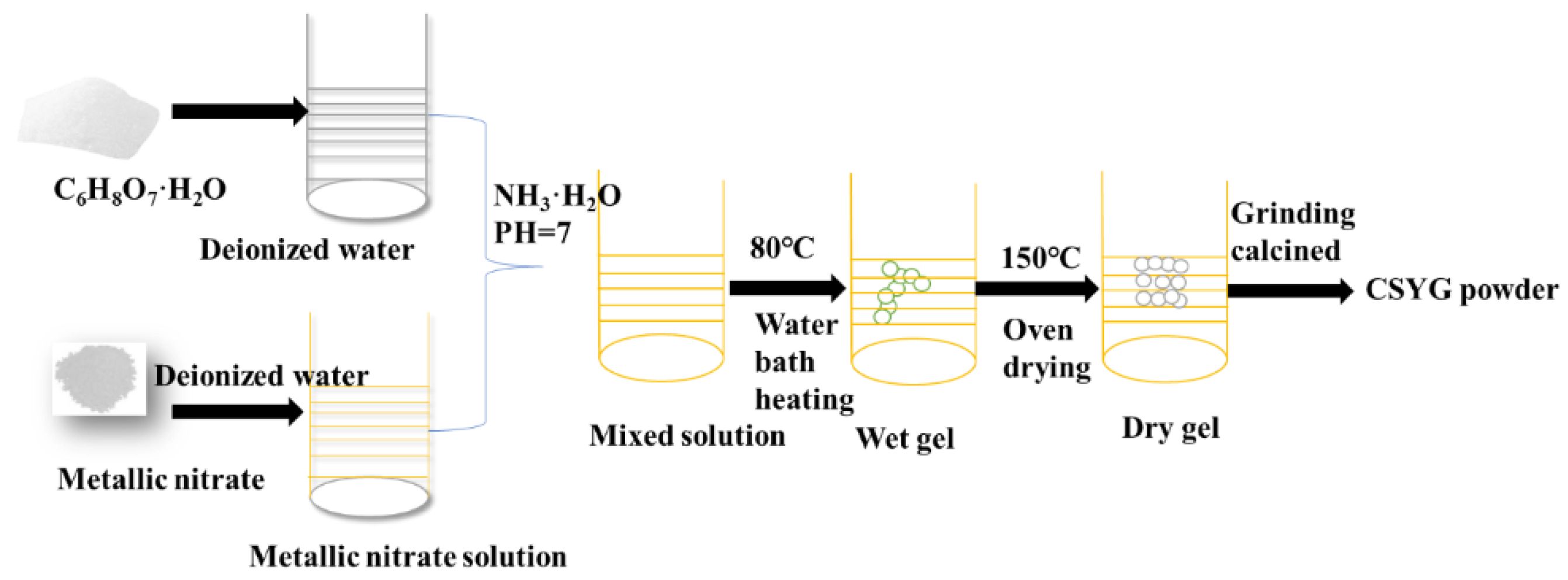
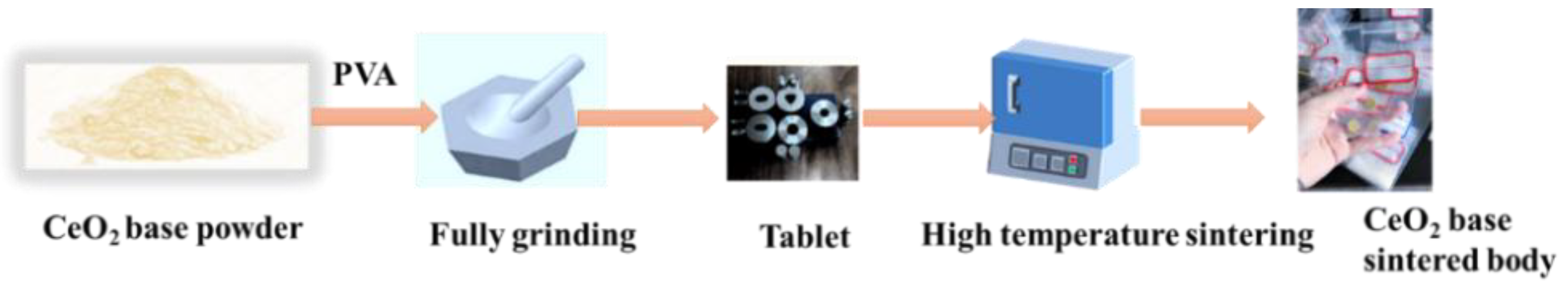
2.1. Sample Preparation
2.2. Characterizations
3. Results and Discussion
3.1. Structural Analysis
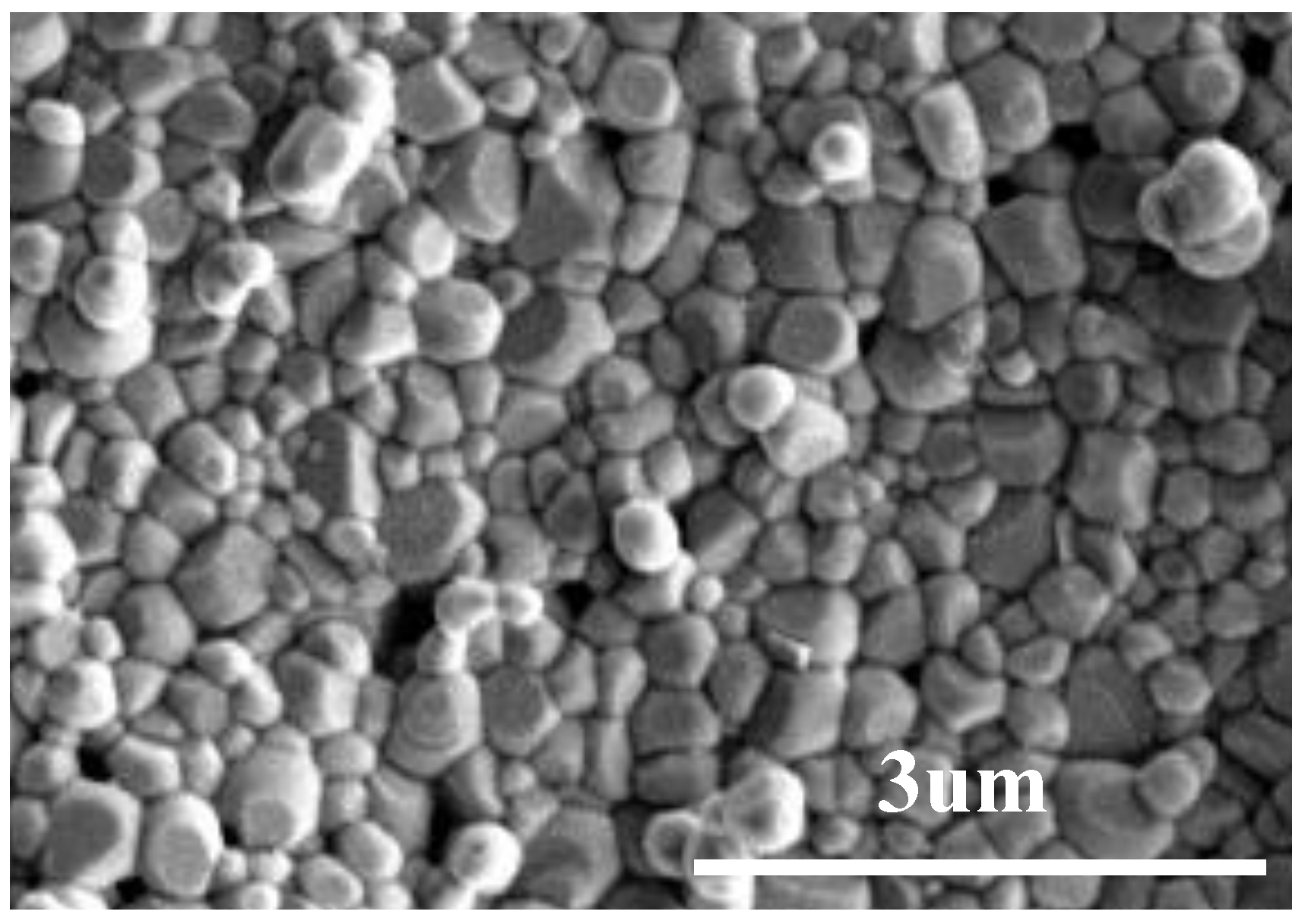
3.2. Density and Microstructure
3.3. Impedance Analysis
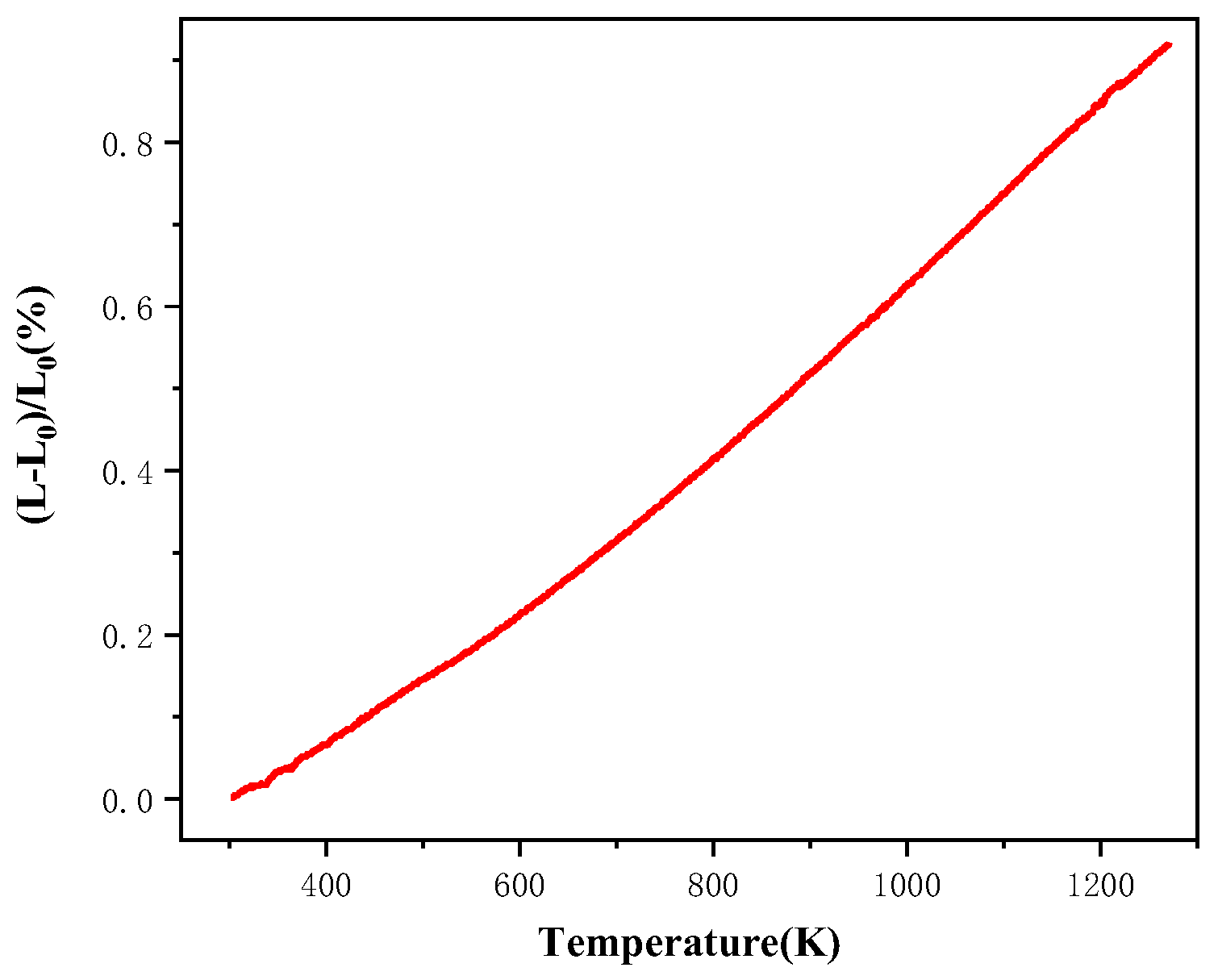
3.4. Thermal Expansion
3.5. Single Cell Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ivers-Tiffée, E.; Weber, A.; Herbstritt, D. Materials and technologies for SOFC-components. J. Eur. Ceram. Soc. 2001, 21, 1805–1811. [Google Scholar] [CrossRef]
- Peng, J.; Huang, J.; Wu, X.L.; Xu, Y.W.; Chen, H.; Li, X. Solid oxide fuel cell (SOFC) performance evaluation, fault diagnosis and health control: A review. J. Power Sources 2021, 505, 230058. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, K.; Kim, B.H.; Li, Q.; Huang, J. Electrochemical performance of solid oxide fuel cells with Sm, Nd co-doped Ce0.85(SmxNd1−x)0.15O2−δ electrolyte. Ceram. Int. 2015, 41, 6391–6397. [Google Scholar] [CrossRef]
- Saebea, D.; Authayanun, S.; Patcharavorachot, Y.; Chatrattanawet, N.; Arpornwichanop, A. Electrochemical performance assessment of low-temperature solid oxide fuel cell with YSZ-based and SDC-based electrolytes. Int. J. Hydrogen Energy 2018, 43, 921–931. [Google Scholar] [CrossRef]
- Singh, K.L.; Sharma, P.; Singh, A.P.; Kumar, A.; Sekhon, S.S. Structural and electrical analysis of microwave processed YSZ electrolytes for SOFC Prepared by co-precipitation method. JOM 2017, 69, 2448–2452. [Google Scholar] [CrossRef]
- Zhang, J.; Lenser, C.; Menzler, N.H.; Guillon, O. Comparison of solid oxide fuel cell (SOFC) electrolyte materials for operation at 500 °C. Solid State Ion. 2020, 344, 115138. [Google Scholar] [CrossRef]
- Hussain, S.; Yangping, L. Review of solid oxide fuel cell materials: Cathode, anode, and electrolyte. Energy Transit. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Xie, S.; Chen, L.; Wang, Y.; Meng, J.; Zhou, D. Bismuth tungstate/neodymium-doped ceria composite electrolyte for intermediate-temperature solid oxide fuel cell: Sintering aid and composite effect. J. Power Sources 2019, 428, 105–114. [Google Scholar] [CrossRef]
- Eressa, L.A.; Rao, P.V.B. Synthesis and characterization of Ytterbium and Samarium Co-doped ceria as an electrolyte for intermediate-temperature solid oxide fuel cell application. Mater. Chem. Phys. 2020, 242, 121914. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, H.; Li, W.; Ma, Z.; Ou, X.; Fan, Y.; Guo, Y.; Wang, X.; Ling, Y. Numerical study on the electron-blocking effect and optimized operation parameters of ceria-SOFCs with the pure Sm doping CeO2 electrolyte. Int. J. Hydrogen Energy 2021, 46, 13318–13329. [Google Scholar] [CrossRef]
- Santos, T.H.; Grilo, J.P.F.; Loureiro, F.J.A.; Fagg, D.P.; Fonseca, F.C.; Macedo, D.A. Structure, densification and electrical properties of Gd3+ and Cu2+ co-doped ceria solid electrolytes for SOFC applications: Effects of Gd2O3 content. Ceram. Int. 2018, 44, 2745–2751. [Google Scholar] [CrossRef]
- Anwar, M.; Ali, S.A.M.; Muchtar, A.; Somalu, M.R. Synthesis and characterization of M-doped ceria-ternary carbonate composite electrolytes (M = erbium, lanthanum and strontium) for low-temperature solid oxide fuel cells. J. Alloys Compd. 2019, 775, 571–580. [Google Scholar] [CrossRef]
- Buvat, G.; Quarez, E.; Joubert, O. Innovative solid oxide fuel cells based on BaIn0.3Ti0.7O2.85 electrolyte and La2Mo2O9 amorphous reduced phase as anode material. J. Power Sources 2016, 302, 107–113. [Google Scholar] [CrossRef]
- Benamira, M.; Thommy, L.; Moser, F.; Joubert, O.; Caldes, M.T. New anode materials for IT-SOFC derived from the electrolyte BaIn0.3Ti0.7O2.85 by lanthanum and manganese doping. Solid State Ion. 2014, 265, 38–45. [Google Scholar] [CrossRef]
- Prakash, D.; Delahaye, T.; Joubert, O.; Caldes, M.T.; Piffard, Y. Intermediate temperature solid oxide fuel cell based on BaIn0.3Ti0.7O2.85 electrolyte. J. Power Sources 2007, 167, 111–117. [Google Scholar] [CrossRef]
- Jayaraman, V.; Magrez, A.; Caldes, M.; Joubert, O.; Ganne, M.; Piffard, Y.; Brohan, L. Characterization of perovskite systems derived from Ba2In2O5: Part I: The oxygen-deficient Ba2In2(1−x)Ti2xO5+x rectangle(1−x) (0 ≤ x ≤ 1) compounds. Solid State Ion. 2004, 170, 17–24. [Google Scholar] [CrossRef]
- Letilly, M.; La Salle, A.L.G.; Lachgar, A.; Joubert, O. Synthesis, structural analysis and electrochemical performances of BLSITCFx as new cathode materials for solid oxide fuel cells (SOFC) based on BIT07 electrolyte. J. Power Sources 2010, 195, 4779–4784. [Google Scholar] [CrossRef]
- Letilly, M.; Le Gal La Salle, A.; Caldes, M.; Marrony, M.; Joubert, O. Validation of BaIn0.3Ti0.7O2.85 as SOFC Electrolyte with Nd2NiO4, LSM and LSCF as Cathodes. Fuel Cells 2009, 9, 622–629. [Google Scholar] [CrossRef]
- Jaiswal, N.; Kumar, D.; Upadhyay, S.; Parkash, O. High electrical conductivity of nanocomposites based on Ce0.82Sm0.16Sr0.02O1.90 and (Li/Na)2CO3 for low temperature solid oxide fuel cells. Ceram. Int. 2016, 42, 9004–9010. [Google Scholar] [CrossRef]
- Ricca, C.; Ringuedé, A.; Cassir, M.; Adamo, C.; Labat, F. Conduction mechanisms in oxide–carbonate electrolytes for SOFC: Highlighting the role of the interface from first-principles modeling. J. Phys. Chem. C 2018, 122, 10067–10077. [Google Scholar] [CrossRef]
- Ding, H.; Guo, X.; Li, J.; Hu, Q.; Sun, H.; Yang, Z.; Li, G.; Yu, F.; Li, C.; Wang, Y. Processing and characterization of novel Ce0.8Sm0.1Bi0.1O2−δ-BaCe0.8Sm0.1Bi0.1O3−δ (BiSDC-BCSBi) composite electrolytes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrog. Energy 2021, 46, 7515–7527. [Google Scholar] [CrossRef]
- Xu, S.; Lin, X.; Ge, B.; Ai, D.; Ma, J.; Peng, Z. Microstructure and electrical conductivity of La0.9Sr0.1Ga0.8Mg0.2O2.85-Ce0.8Gd0.2O1.9 composite electrolytes for SOFC s. Int. J. Appl. Ceram. Technol. 2019, 16, 108–118. [Google Scholar] [CrossRef]
- Dwivedi, S. Solid oxide fuel cell: Materials for anode, cathode and electrolyte. Int. J. Hydrogen Energy 2020, 45, 23988–24013. [Google Scholar] [CrossRef]
- Xu, D.; Liu, X.; Wang, D.; Yi, G.; Gao, Y.; Zhang, D.; Su, W. Fabrication and characterization of SDC–LSGM composite electrolytes material in IT-SOFCs. J. Alloys Compd. 2007, 429, 292–295. [Google Scholar] [CrossRef]
- Shri Prakash, B.; William Grips, V.K.; Aruna, S.T. A single step solution combustion approach for preparing gadolinium doped ceria solid oxide fuel cell electrolyte material suitable for wet powder and plasma spraying processes. J. Power Sources 2012, 214, 358–364. [Google Scholar] [CrossRef]
- Arabacı, A.; Altınçekiç, T.G.; Der, M.; Öksüzömer, M.A.F. Preparation and properties of ceramic electrolytes in the Nd and Gd Co-doped ceria systems prepared by polyol method. J. Alloys Compd. 2019, 792, 1141–1149. [Google Scholar] [CrossRef]
- Anjaneya, K.C.; Nayaka, G.P.; Manjanna, J.; Govindaraj, G.; Ganesha, K.N. Studies on structural, morphological and electrical properties of Ce0.8Ln0.2O2−δ (Ln = Y3+, Gd3+, Sm3+, Nd3+ and La3+) solid solutions prepared by citrate complexation method. J. Alloys Compd. 2014, 585, 594–601. [Google Scholar] [CrossRef]
- Moon, H.; Kim, S.D.; Park, E.W.; Hyun, S.H.; Kim, H.S. Characteristics of SOFC single cells with anode active layer via tape casting and co-firing. Int. J. Hydrog. Energy 2008, 33, 2826–2833. [Google Scholar] [CrossRef]
- Dai, H.; Chen, H.; He, S.; Cai, G.; Guo, L. Improving solid oxide fuel cell performance by a single-step co-firing process. J. Power Sources 2015, 286, 427–430. [Google Scholar] [CrossRef]
- Anjaneya, K.C.; Singh, M.P. Synthesis and properties of gadolinium doped ceria electrolyte for IT-SOFCs by EDTA-citrate complexing method. J. Alloys Compd. 2017, 695, 871–876. [Google Scholar] [CrossRef]
- Raghvendra; Singh, R.K.; Singh, P. Synthesis of La0.9Sr0.1Ga0.8Mg0.2O3−δ electrolyte via ethylene glycol route and its characterizations for IT-SOFC. Ceram. Int. 2014, 40, 7177–7184. [Google Scholar] [CrossRef]
- Benamira, M.; Letilly, M.; Quarez, E.; Joubert, O.; La Salle, A.L.G. Optimization of SOFC anode/electrolyte assembly based on BaIn0.3Ti0.7O2.85 (BIT07)/Ni-BIT07 using an interfacial anodic layer. J. Power Sources 2014, 251, 66–74. [Google Scholar] [CrossRef]
- Jiang, T.; Wang, Z.; Zhang, J.; Hao, X.; Rooney, D.; Liu, Y.; Sun, W.; Qiao, J.; Sun, K. Understanding the flash sintering of rare-earth-doped ceria for solid oxide fuel cell. J. Am. Ceram. Soc. 2015, 98, 1717–1723. [Google Scholar] [CrossRef]
- Wu, Y.C.; Liao, Y.Y. Effect of Ca2+ and Sr2+ doping on the microstructure and cell performance of samaria-doped ceria electrolytes used in solid oxide fuel cells. Int. J. Hydrog. Energy 2016, 41, 13591–13602. [Google Scholar] [CrossRef]
- Paydar, S.; Gholaminezad, I.; Shirani-Faradonbeh, H.; Imanlou, S.; Akbar, N.; Paydar, M.H. Evaluating the cathodic polarization of La0.7Sr0.3MnO3–Zr0.84−xCexY0.16O1.92 (x = 0, 0.42, 0.84) composites for SOFCs. J. Mater. Sci. Mater. Electron. 2021, 32, 11129–11144. [Google Scholar] [CrossRef]
- Fan, B.; Yan, J.; Yan, X. The ionic conductivity, thermal expansion behavior, and chemical compatibility of La0.54Sr0.44Co0.2Fe0.8O3−δ as SOFC cathode material. Solid State Sci. 2011, 13, 1835–1839. [Google Scholar] [CrossRef]
- Drożdż-Cieśla, E.; Wyrwa, J.; Pyda, W.; Rękas, M. A new method of preparing Ni/YSZ cermet materials. J. Mater. Sci. 2012, 47, 2807–2817. [Google Scholar] [CrossRef]
- Marina, O.A.; Canfield, N.L.; Stevenson, J.W. Thermal, electrical, and electrocatalytical properties of lanthanum-doped strontium titanate. Solid State Ion. 2002, 149, 21–28. [Google Scholar] [CrossRef]
- Venkataramana, K.; Madhuri, C.; Reddy, Y.S.; Bhikshamaiah, G.; Reddy, C.V. Structural, electrical and thermal expansion studies of tri-doped ceria electrolyte materials for IT-SOFCs. J. Alloys Compd. 2017, 719, 97–107. [Google Scholar] [CrossRef]
- Bi, H.; Liu, X.; Zhu, L.; Sun, J.; Yu, S.; Yu, H.; Pei, L. Effect of MgO addition and grain size on the electrical properties of Ce0.9Gd0.1O1.95 electrolyte for IT-SOFCs. Int. J. Hydrog. Energy 2017, 42, 11735–11744. [Google Scholar] [CrossRef]
- Wu, Y.C.; Huang, W.H. Processing improvement and performance analysis of La0.85Sr0.15Ga0.8Mg0.2O2.825 electrolyte-supported fuel cells. Ceram. Int. 2017, 43, S729–S738. [Google Scholar] [CrossRef]





| Composition (BIT07 Content) | Relative Density/% | Lattice Parameter/(Å) | σ700°C/(mS·cm−1) | σ800°C/(mS·cm−1) | Ea/(eV) |
|---|---|---|---|---|---|
| 5%BIT07 | 95.4 | 5.432 | 1.93 | 4.78 | 0.94 |
| 10%BIT07 | 95.6 | 5.436 | 2.32 | 5.67 | 0.99 |
| 15%BIT07 | 98.8 | 5.462 | 5.36 | 12.60 | 0.89 |
| 20%BIT07 | 96.7 | 5.443 | 3.92 | 9.31 | 0.92 |
| 25%BIT07 | 96.4 | 5.448 | 2.85 | 6.83 | 1.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Tian, C.; Zhu, M.; Yang, J.; Qu, X.; Chen, C.; Wang, C.; Liu, Y. Preparation and Properties of Ce0.8Sm0.16Y0.03Gd0.01O1.9-BaIn0.3Ti0.7O2.85 Composite Electrolyte. Materials 2022, 15, 5591. https://doi.org/10.3390/ma15165591
Wang Y, Tian C, Zhu M, Yang J, Qu X, Chen C, Wang C, Liu Y. Preparation and Properties of Ce0.8Sm0.16Y0.03Gd0.01O1.9-BaIn0.3Ti0.7O2.85 Composite Electrolyte. Materials. 2022; 15(16):5591. https://doi.org/10.3390/ma15165591
Chicago/Turabian StyleWang, Yajun, Changan Tian, Minzheng Zhu, Jie Yang, Xiaoling Qu, Cao Chen, Cao Wang, and Yang Liu. 2022. "Preparation and Properties of Ce0.8Sm0.16Y0.03Gd0.01O1.9-BaIn0.3Ti0.7O2.85 Composite Electrolyte" Materials 15, no. 16: 5591. https://doi.org/10.3390/ma15165591
APA StyleWang, Y., Tian, C., Zhu, M., Yang, J., Qu, X., Chen, C., Wang, C., & Liu, Y. (2022). Preparation and Properties of Ce0.8Sm0.16Y0.03Gd0.01O1.9-BaIn0.3Ti0.7O2.85 Composite Electrolyte. Materials, 15(16), 5591. https://doi.org/10.3390/ma15165591





