What Is the Cause of Toxicity of Silicone Oil?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. SilOil
2.1.2. Retinal Cell Lines
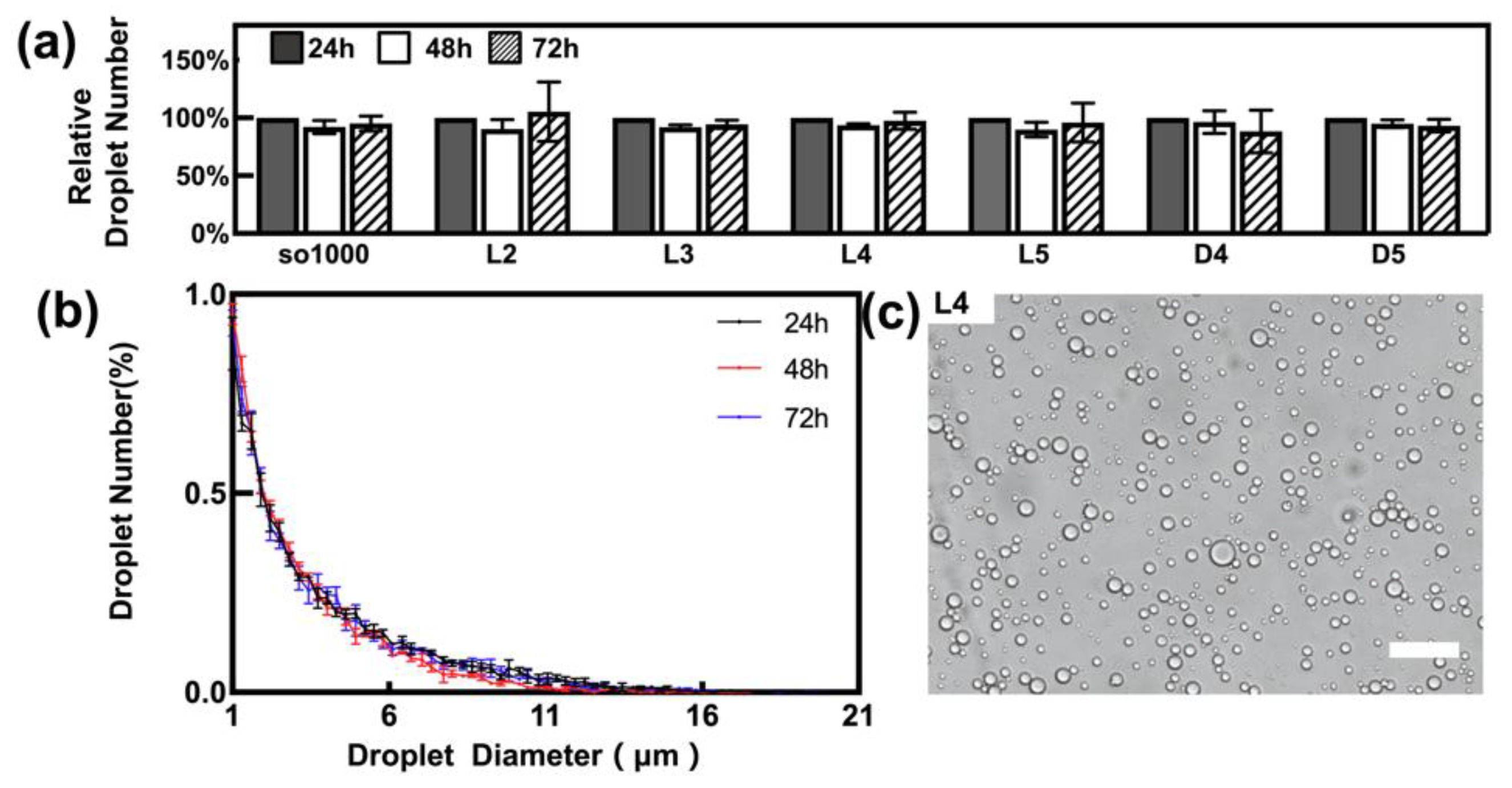
2.2. Preparation and Characterisation of Emulsified LMWC Droplets
2.3. The In Vitro Biocompatibility Tests
2.4. The Apoptotic Assay for Investigation of Cell Death Pathway
2.5. Statistical Tests
3. Results
3.1. Characterisation of Emulsified Droplets
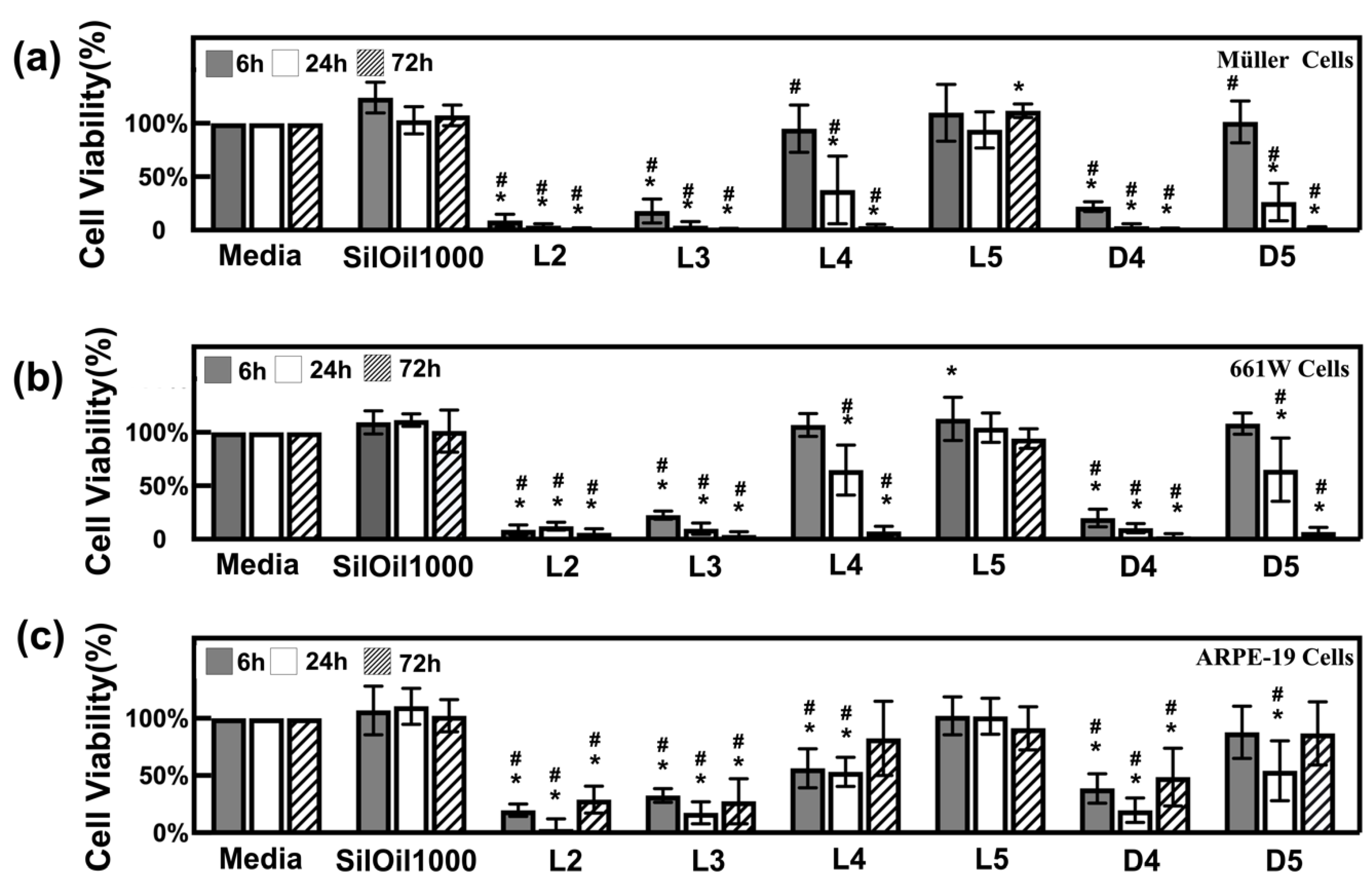
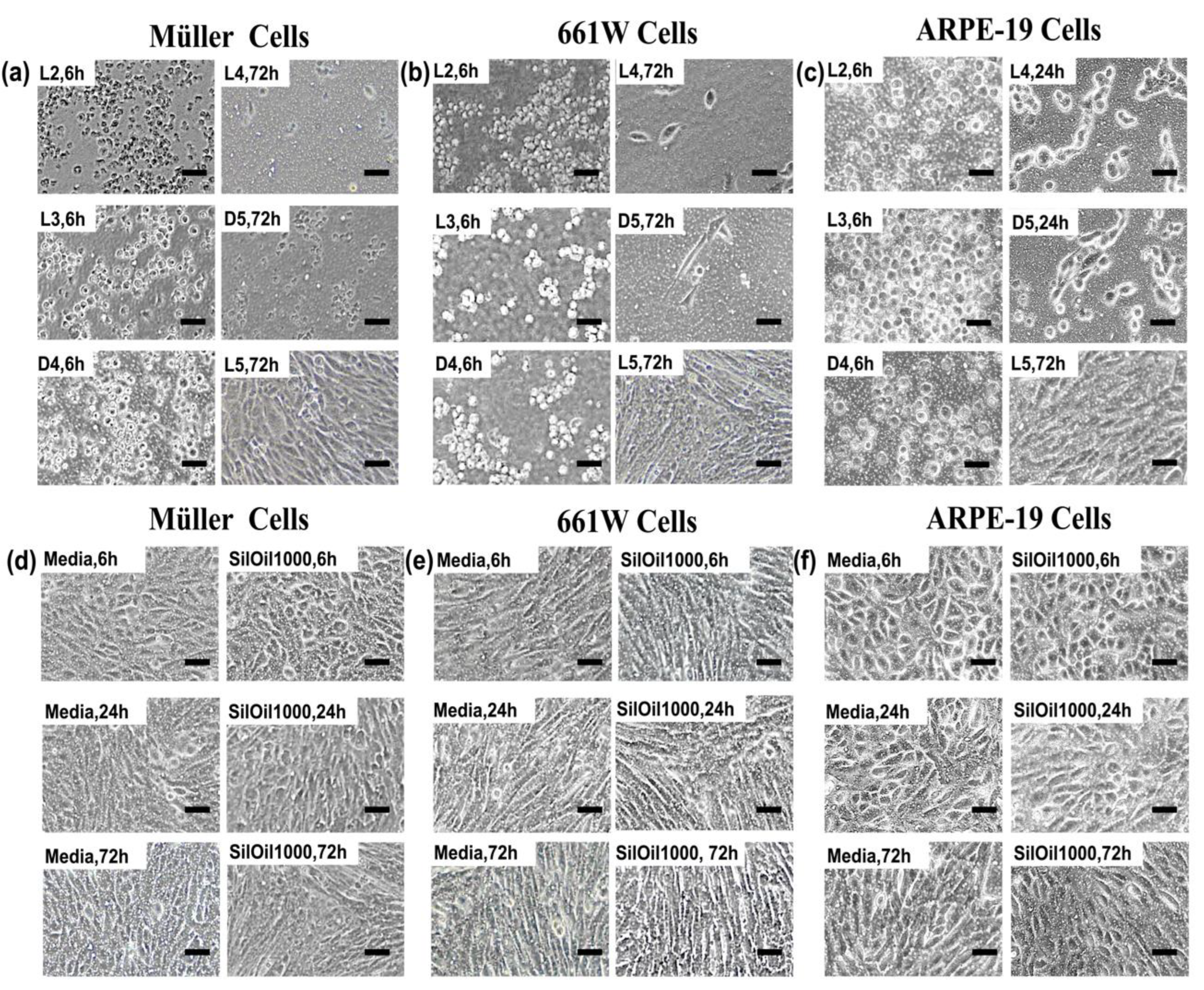
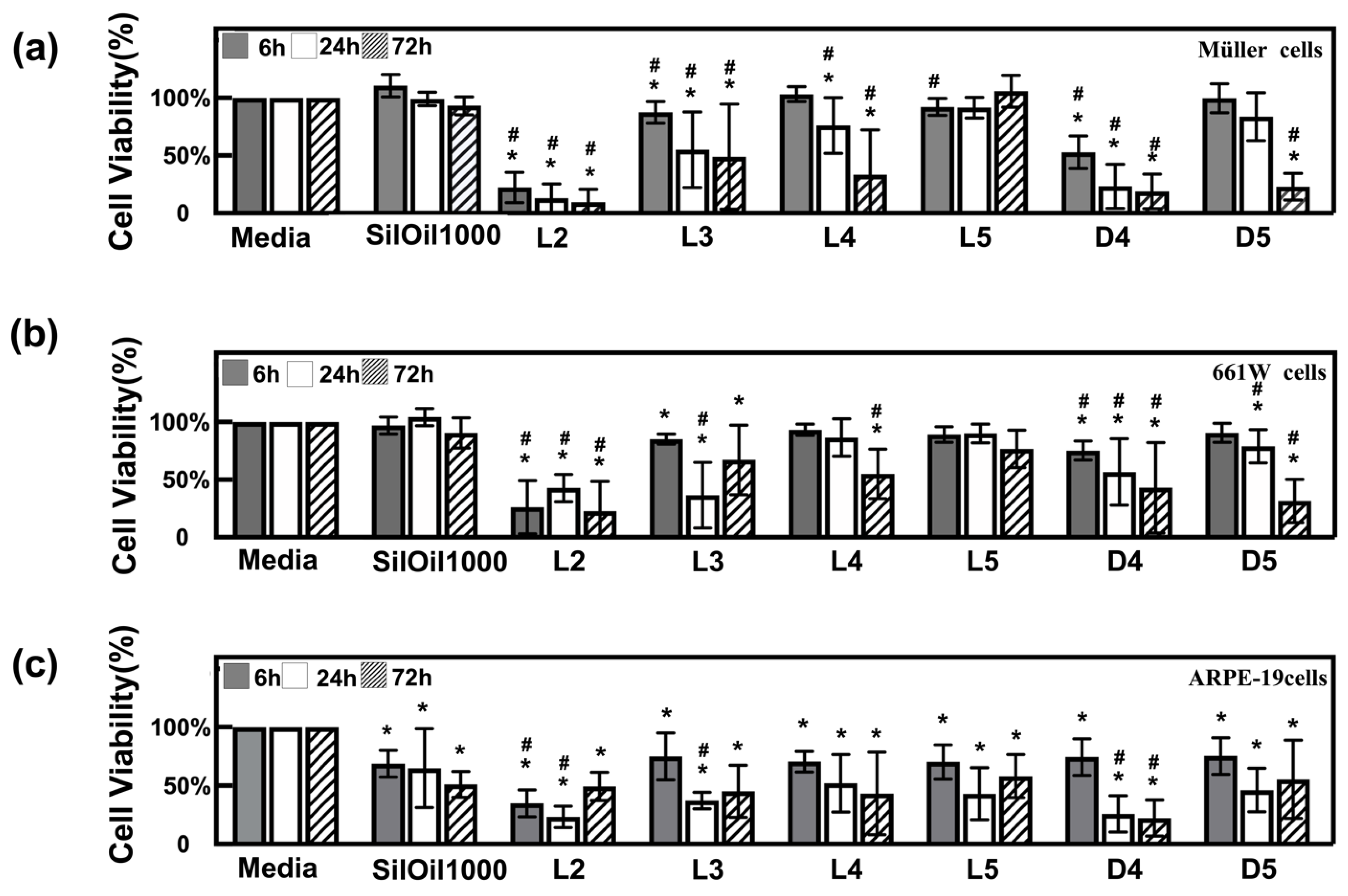
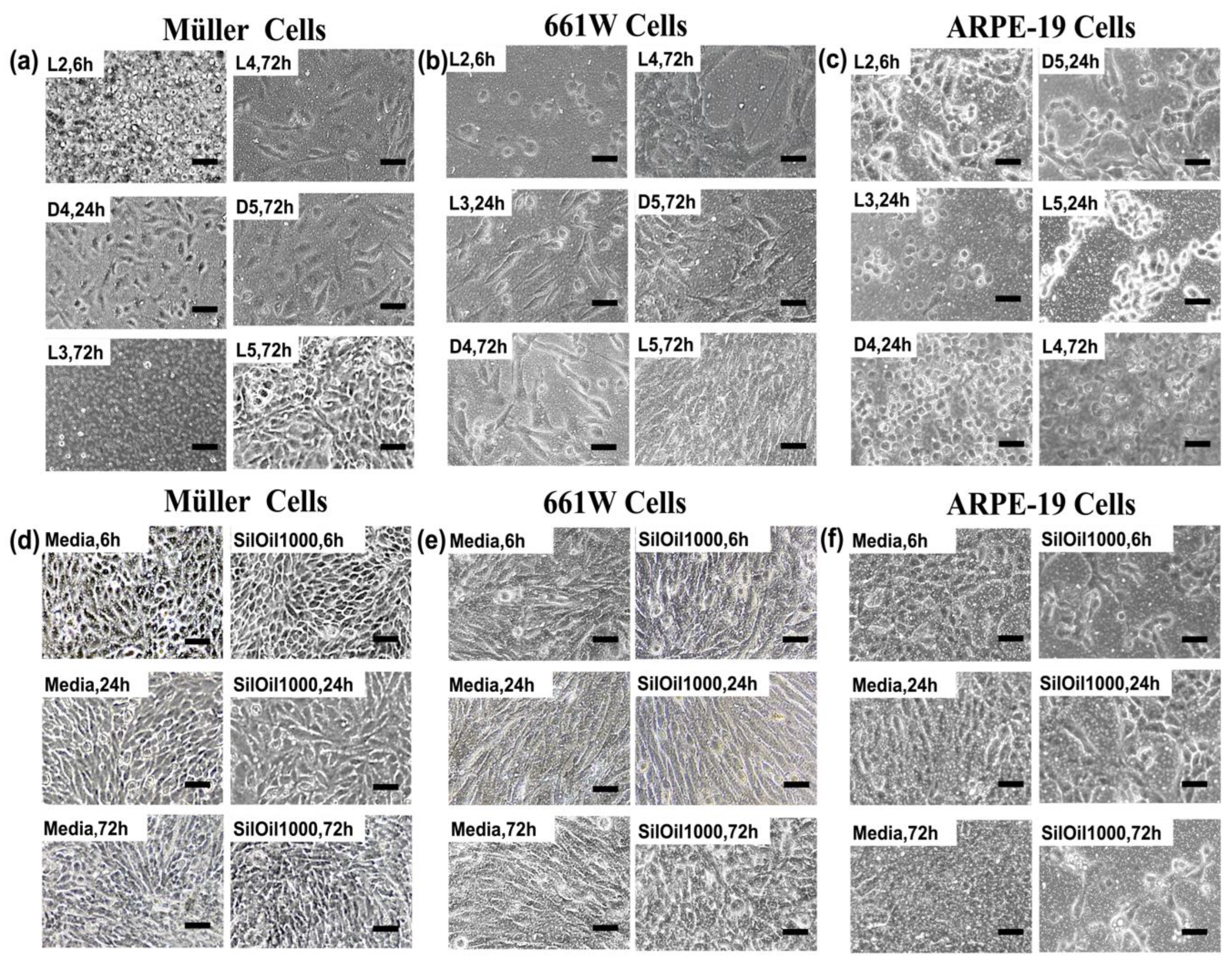
3.2. Toxicity of Liquid LMWCs on Retinal Cells
3.3. Toxicity of Emulsified LMWCs on Retinal Cells
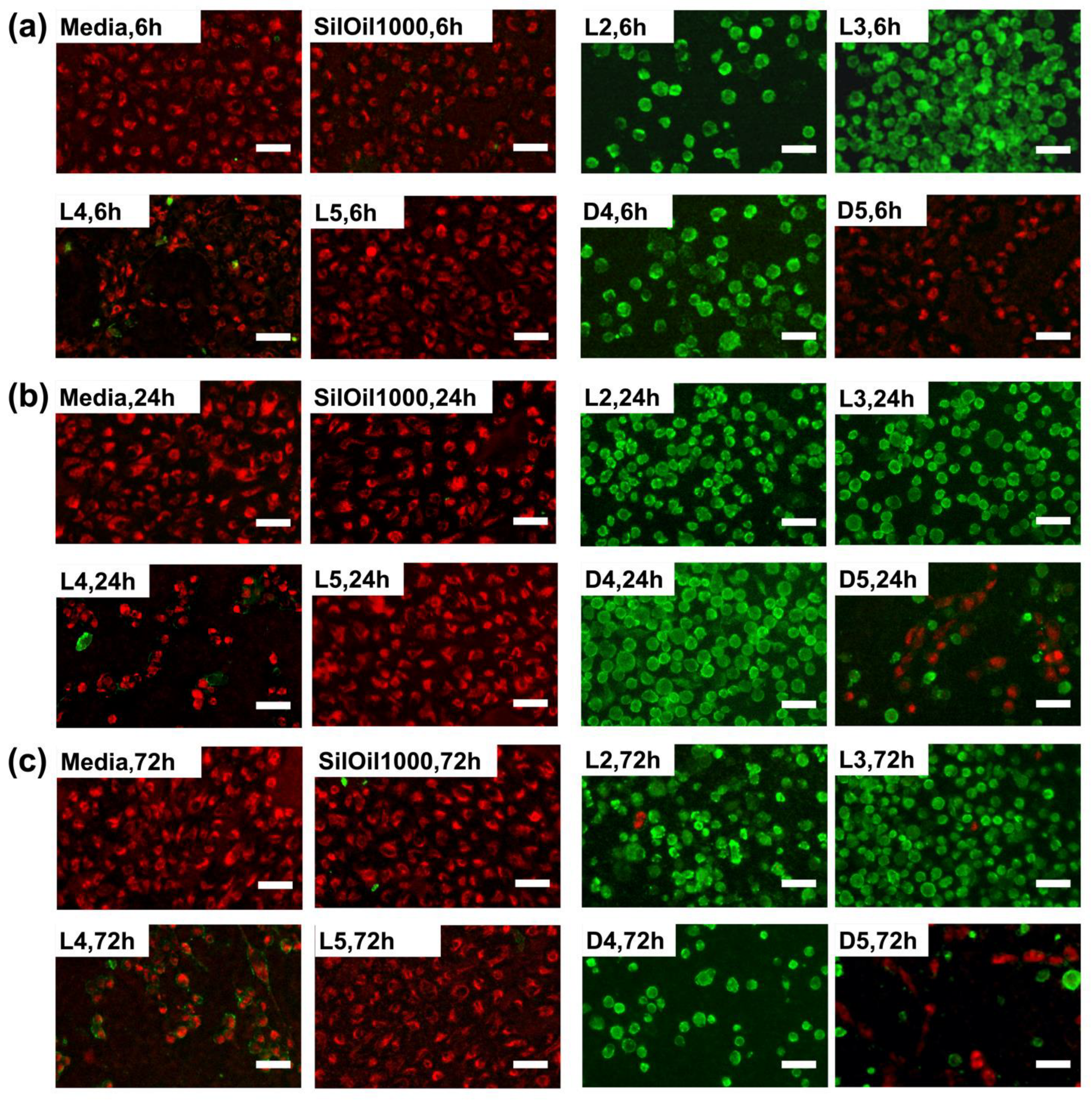
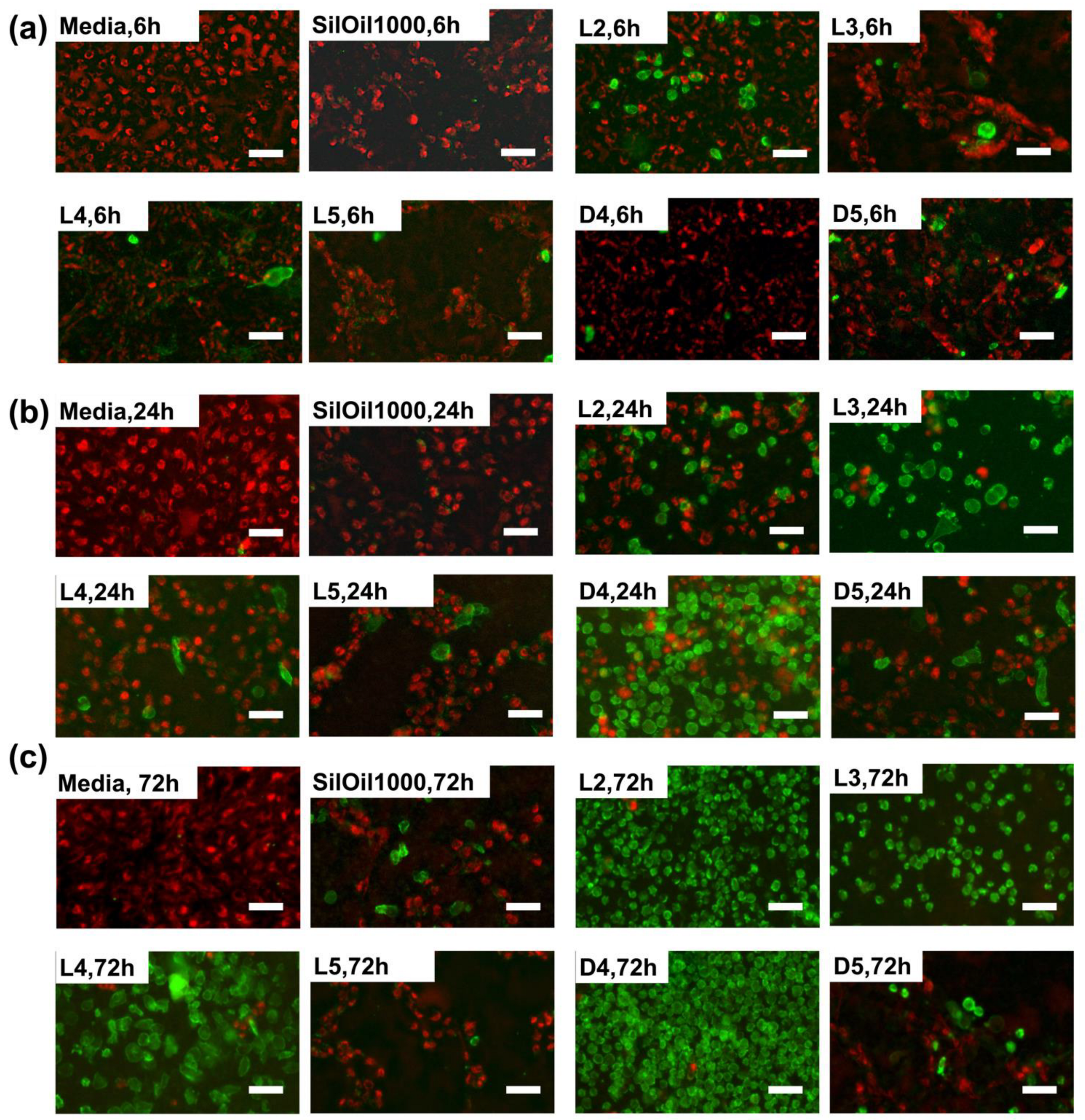
3.4. Apoptosis of ARPE-19 Cells after Treatment with Liquid LMWCs
3.5. Apoptosis of ARPE-19 Cells after Treatment with Emulsified LMWCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Federman, J.L.; Schubert, H.D. Complications associated with the use of silicone oil in 150 eyes after retina-vitreous surgery. Ophthalmology 1988, 95, 870–876. [Google Scholar] [CrossRef]
- Gonvers, M.; Hornung, J.P.; de Courten, C. The effect of liquid silicone on the rabbit retina. Histologic and ultrastructural study. Arch. Ophthalmol. 1986, 104, 1057–1062. [Google Scholar] [CrossRef]
- Papp, A.; Kiss, E.B.; Timar, O.; Szabo, E.; Berecki, A.; Toth, J.; Pali, J. Long-term exposure of the rabbit eye to silicone oil causes optic nerve atrophy. Brain Res. Bull. 2007, 74, 130–133. [Google Scholar] [CrossRef]
- Ni, C.; Wang, W.J.; Albert, D.M.; Schepens, C.L. Intravitreous silicone injection. Histopathologic findings in a human eye after 12 years. Arch. Ophthalmol. 1983, 101, 1399–1401. [Google Scholar] [CrossRef]
- Shields, C.L.; Eagle, R.C., Jr. Pseudo-Schnabel’s cavernous degeneration of the optic nerve secondary to intraocular silicone oil. Arch. Ophthalmol. 1989, 107, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, B.; Tavakolian, U.; Paulmann, H.; Heimann, K. Histopathological findings in eyes after silicone oil injection. Graefes Arch. Clin. Exp. Ophthalmol. 1986, 224, 34–37. [Google Scholar] [CrossRef]
- Wong, D.; Williams, R.; Stappler, T.; Groenewald, C. What pressure is exerted on the retina by heavy tamponade agents? Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 474–477. [Google Scholar] [CrossRef]
- Nakamura, K.; Refojo, M.F.; Crabtree, D.V.; Pastor, J.; Leong, F.L. Ocular toxicity of low-molecular-weight components of silicone and fluorosilicone oils. Investig. Ophthalmol. Vis. Sci. 1991, 32, 3007–3020. [Google Scholar]
- Gabel, V.P.; Kampik, A.; Burkhardt, J. Analysis of intraocularly applied silicone oils of various origins. Graefes Arch. Clin. Exp. Ophthalmol. 1987, 225, 160–162. [Google Scholar] [CrossRef]
- Dresp, J.H.; Menz, D.H. Interaction of different ocular endotamponades as a risk factor for silicone oil emulsification. Retina 2005, 25, 902–910. [Google Scholar] [CrossRef]
- Chan, Y.K.; Wong, D.; Yeung, H.K.; Man, P.K.; Shum, H.C. A low-molecular-weight oil cleaner for removal of leftover silicone oil intraocular tamponade. Investig. Ophthalmol. Vis. Sci. 2015, 56, 1014–1022. [Google Scholar] [CrossRef] [Green Version]
- Nakamura, K.; Refojo, M.F.; Crabtree, D.V. Factors contributing to the emulsification of intraocular silicone and fluorosilicone oils. Investig. Ophthalmol. Vis. Sci. 1990, 31, 647–656. [Google Scholar]
- Herbert, E.N.; Habib, M.; Steel, D.; Williamson, T.H. Central scotoma associated with intraocular silicone oil tamponade develops before oil removal. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Scheerlinck, L.M.; Schellekens, P.A.; Liem, A.T.; Steijns, D.; Leeuwen, R. Incidence, Risk Factors, and Clinical Characteristics of Unexplained Visual Loss after Intraocular Silicone Oil for Macula-on Retinal Detachment. Retina 2016, 36, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.B.; Papakostas, T.D.; Vavvas, D.G. Complications of emulsified silicone oil after retinal detachment repair. Semin. Ophthalmol. 2014, 29, 312–318. [Google Scholar] [CrossRef] [PubMed]
- Grzybowski, A.; Pieczynski, J.; Ascaso, F.J. Neuronal complications of intravitreal silicone oil: An updated review. Acta Ophthalmol. 2014, 92, 201–204. [Google Scholar] [CrossRef] [Green Version]
- Eckardt, C.; Nicolai, U.; Czank, M.; Schmidt, D. Identification of silicone oil in the retina after intravitreal injection. Retina 1992, 12, S17–S22. [Google Scholar] [CrossRef] [PubMed]
- Romano, M.R.; Ferrara, M.; Gatto, C.; Giurgola, L.; Zanoni, M.; Angi, M.; Rinaldi, M.; Borgia, A.; Sorrentino, T.; D’Amato Tothova, J. Safety of silicone oils as intraocular medical device: An in vitro cytotoxicity study. Exp. Eye Res. 2020, 194, 108018. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, L.; Chan, Y.K. Comment on “Safety of silicone oils as intraocular medical device: An in vitro cytotoxicity study” by M. R. Romano et al. (Exp. Eye Res. Vol 194, May 2020, 108018). Exp. Eye Res. 2020, 195, 108032. [Google Scholar] [CrossRef]
- Chen, Y.; Kearns, V.R.; Zhou, L.; Sandinha, M.T.; Lam, W.C.; Steel, D.H.; Chan, Y.K. Silicone oil in vitreoretinal surgery: Indications, complications, new developments and alternative long-term tamponade agents. Acta Ophthalmol. 2021, 99, 240–250. [Google Scholar] [CrossRef]
- al-Ubaidi, M.R.; Font, R.L.; Quiambao, A.B.; Keener, M.J.; Liou, G.I.; Overbeek, P.A.; Baehr, W. Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T antigen under control of the human interphotoreceptor retinoid-binding protein promoter. J. Cell Biol. 1992, 119, 1681–1687. [Google Scholar] [CrossRef]
- Sarthy, V.P.; Brodjian, S.J.; Dutt, K.; Kennedy, B.N.; French, R.P.; Crabb, J.W. Establishment and characterization of a retinal Muller cell line. Investig. Ophthalmol. Vis. Sci. 1998, 39, 212–216. [Google Scholar]
- Dunn, K.; Aotaki-Keen, A.; Putkey, F.; Hjelmeland, L. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996, 62, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.K.; Czanner, G.; Shum, H.C.; Williams, R.L.; Cheung, N.; Wong, D. Towards better characterization and quantification of emulsification of silicone oil in vitro. Acta Ophthalmol. 2017, 95, e385–e392. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.K.; Cheung, N.; Chan, W.S.; Wong, D. Quantifying silicone oil emulsification in patients: Are we only seeing the tip of the iceberg? Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1671–1675. [Google Scholar] [CrossRef]
- Steel, D.H.W.; Wong, D.; Sakamoto, T. Silicone oils compared and found wanting. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Mendichi, R.; Schieroni, A.; Piovani, D.; Allegrini, D.; Ferrara, M.; Romano, M. Comparative Study of Chemical Composition, Molecular and Rheological Properties of Silicone Oil Medical Devices. Transl. Vis. Sci. Technol. 2019, 8, 9. [Google Scholar] [CrossRef] [Green Version]
- Dresp, J.H. Benchmarking different brands of silicone oils. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 259, 13–20. [Google Scholar] [CrossRef]
- Van Bergen, N.J.; Wood, J.P.M.; Chidlow, G.; Trounce, I.A.; Casson, R.J.; Ju, W.-K.; Weinreb, R.N.; Crowston, J.G. Recharacterization of the RGC-5 Retinal Ganglion Cell Line. Investig. Ophthalmol. Vis. Sci. 2009, 50, 4267–4272. [Google Scholar] [CrossRef]
- Krishnamoorthy, R.R.; Clark, A.F.; Daudt, D.; Vishwanatha, J.K.; Yorio, T. A Forensic Path to RGC-5 Cell Line Identification: Lessons Learned. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5712–5719. [Google Scholar] [CrossRef] [Green Version]
- Brunner, S.; Izay, B.; Weidinger, B.; Maichel, B.; Binder, S. Chemical impurities and contaminants in different silicone oils in human eyes before and after prolonged use. Graefes Arch. Clin. Exp. Ophthalmol. 2011, 249, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Kociok, N.; Gavranic, C.; Kirchhof, B.; Joussen, A.M. Influence on membrane-mediated cell activation by vesicles of silicone oil or perfluorohexyloctane. Graefes Arch. Clin. Exp. Ophthalmol. 2005, 243, 345–358. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Refojo, M. Silicone oil as vitreous substitutes. Prog. Polym. Sci. 1998, 23, 509–532. [Google Scholar] [CrossRef]
- Fadok, V.; Bratton, D.; Frasch, S.; Warner, M.; Henson, P. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 1998, 5, 551–562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchetti, P.; Castedo, M.; Susin, S.; Zamzami, N.; Hirsch, T.; Macho, A.; Haeffner, A.; Hirsch, F.; Geuskens, M.; Kroemer, G. Mitochondrial permeability transition is a central coordinating event of apoptosis. J. Exp. Med. 1996, 184, 1155–1160. [Google Scholar] [CrossRef] [Green Version]
- Onnekink, C.; Kappel, R.; Boelens, W.; Pruijn, G. Low molecular weight silicones induce cell death in cultured cells. Sci. Rep. 2020, 10, 9558. [Google Scholar] [CrossRef] [PubMed]
| 6 h | 24 h | 72 h | |
|---|---|---|---|
| Photoreceptor cells (661W) | 1.0 × 105 | 5.0 × 104 | 2.5 × 104 |
| Müller Cell (rMC-1) | 9.0 × 104 | 4.5 × 104 | 2.0 × 104 |
| Retinal pigment epithelial cells (ARPE-19) | 1.2 × 105 | 7.0 × 104 | 4.0 × 104 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Lam Ip, Y.; Zhou, L.; Li, P.Y.; Chan, Y.M.; Lam, W.C.; Li, K.K.W.; Steel, D.H.; Chan, Y.K. What Is the Cause of Toxicity of Silicone Oil? Materials 2022, 15, 269. https://doi.org/10.3390/ma15010269
Chen Y, Lam Ip Y, Zhou L, Li PY, Chan YM, Lam WC, Li KKW, Steel DH, Chan YK. What Is the Cause of Toxicity of Silicone Oil? Materials. 2022; 15(1):269. https://doi.org/10.3390/ma15010269
Chicago/Turabian StyleChen, Ying, Yan Lam Ip, Liangyu Zhou, Pik Yi Li, Yee Mei Chan, Wai Ching Lam, Kenneth Kai Wang Li, David H. Steel, and Yau Kei Chan. 2022. "What Is the Cause of Toxicity of Silicone Oil?" Materials 15, no. 1: 269. https://doi.org/10.3390/ma15010269
APA StyleChen, Y., Lam Ip, Y., Zhou, L., Li, P. Y., Chan, Y. M., Lam, W. C., Li, K. K. W., Steel, D. H., & Chan, Y. K. (2022). What Is the Cause of Toxicity of Silicone Oil? Materials, 15(1), 269. https://doi.org/10.3390/ma15010269






