Hydrogen Sorption Behavior of Cast Ag-Mg Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
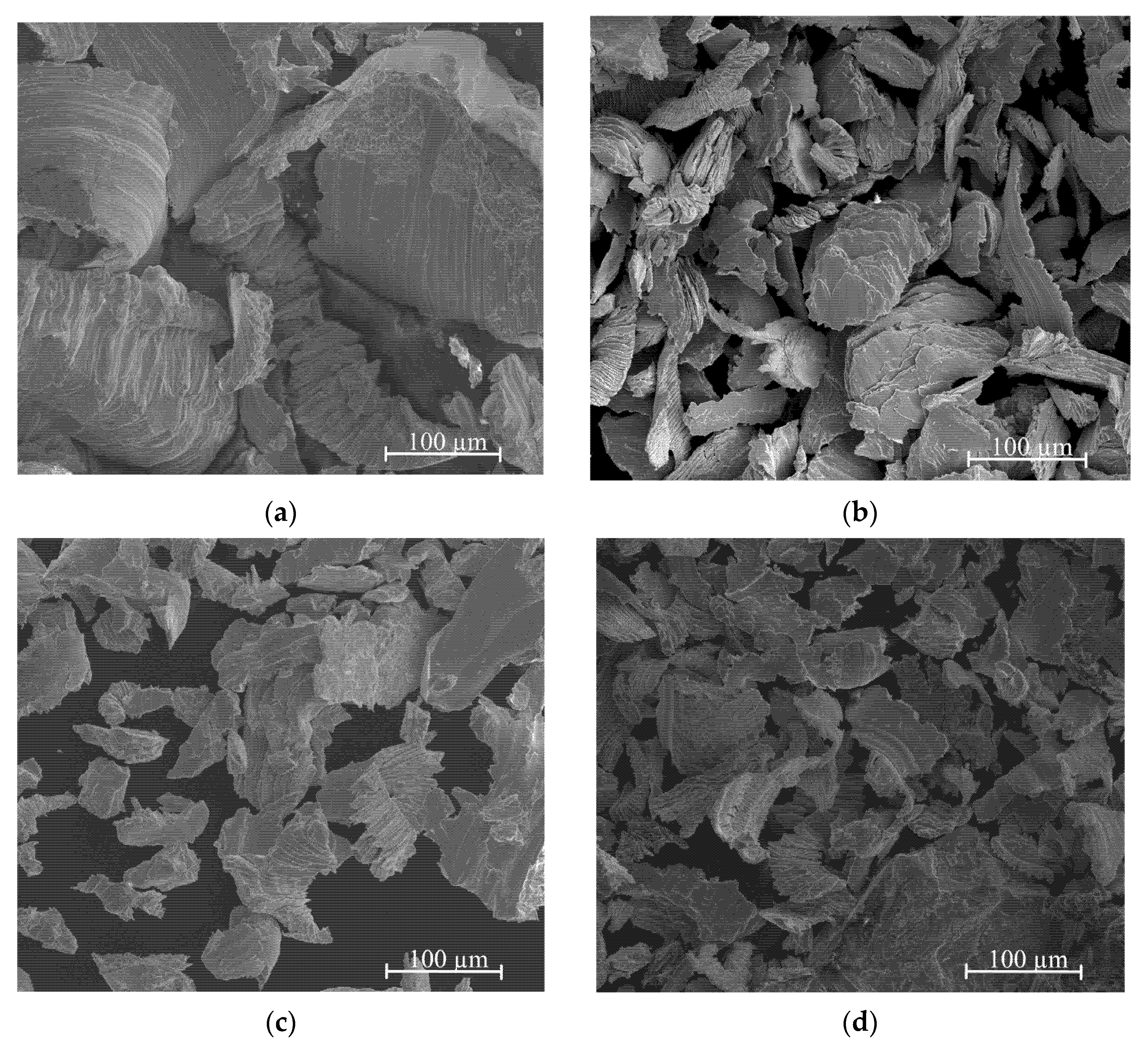
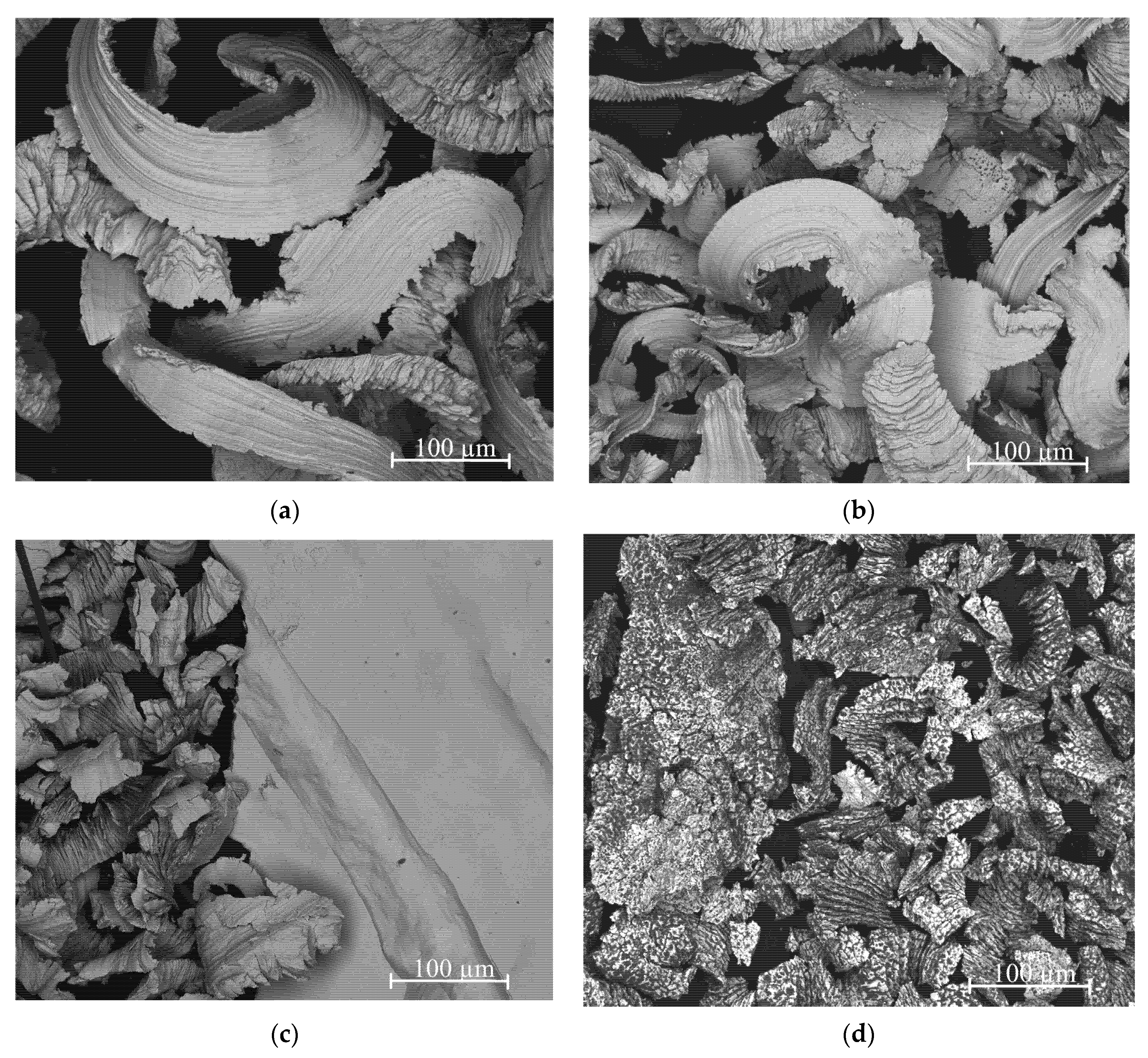
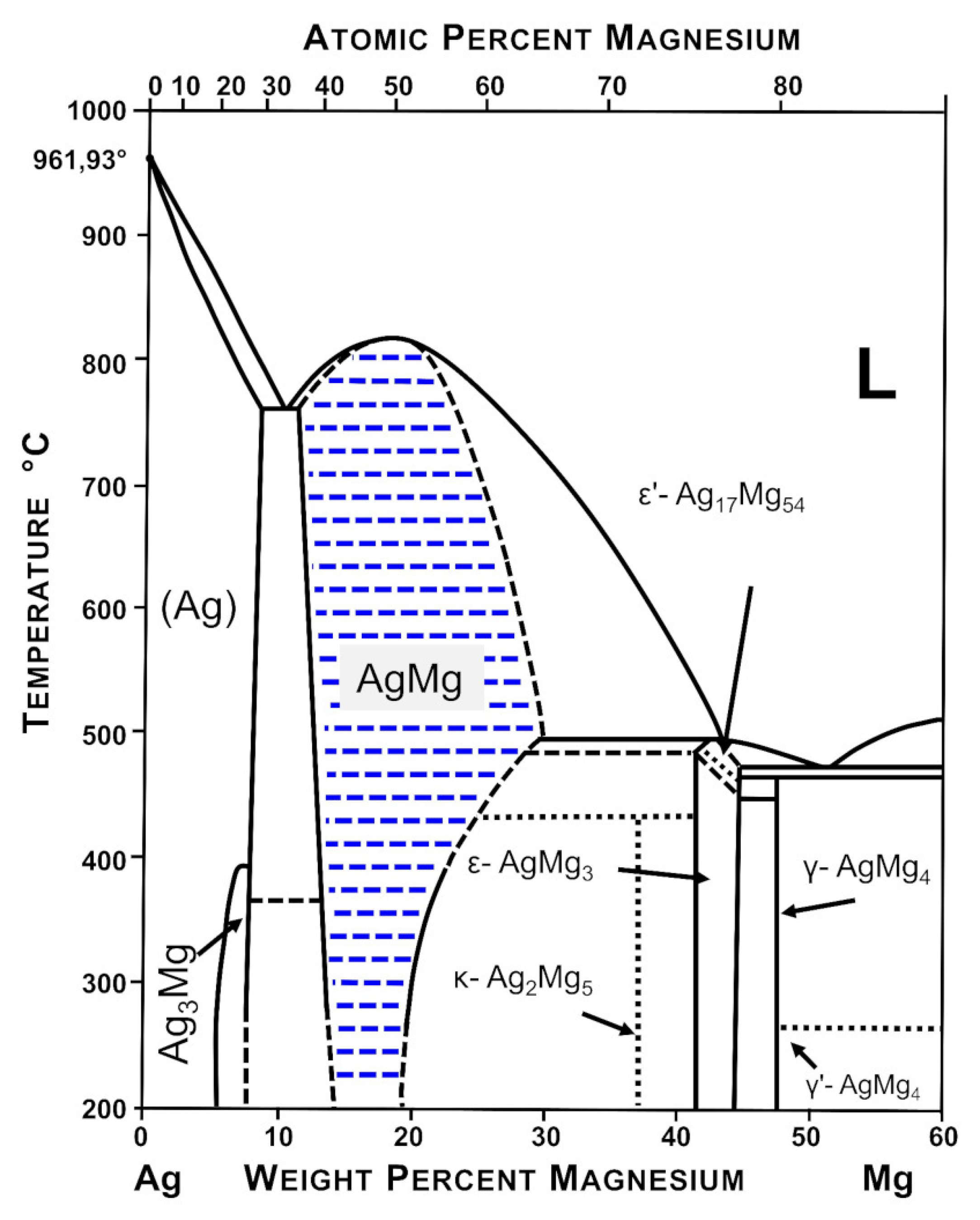
3.1. Morphology and Phase Composition of Ag-Mg Alloys before Hydrogenation
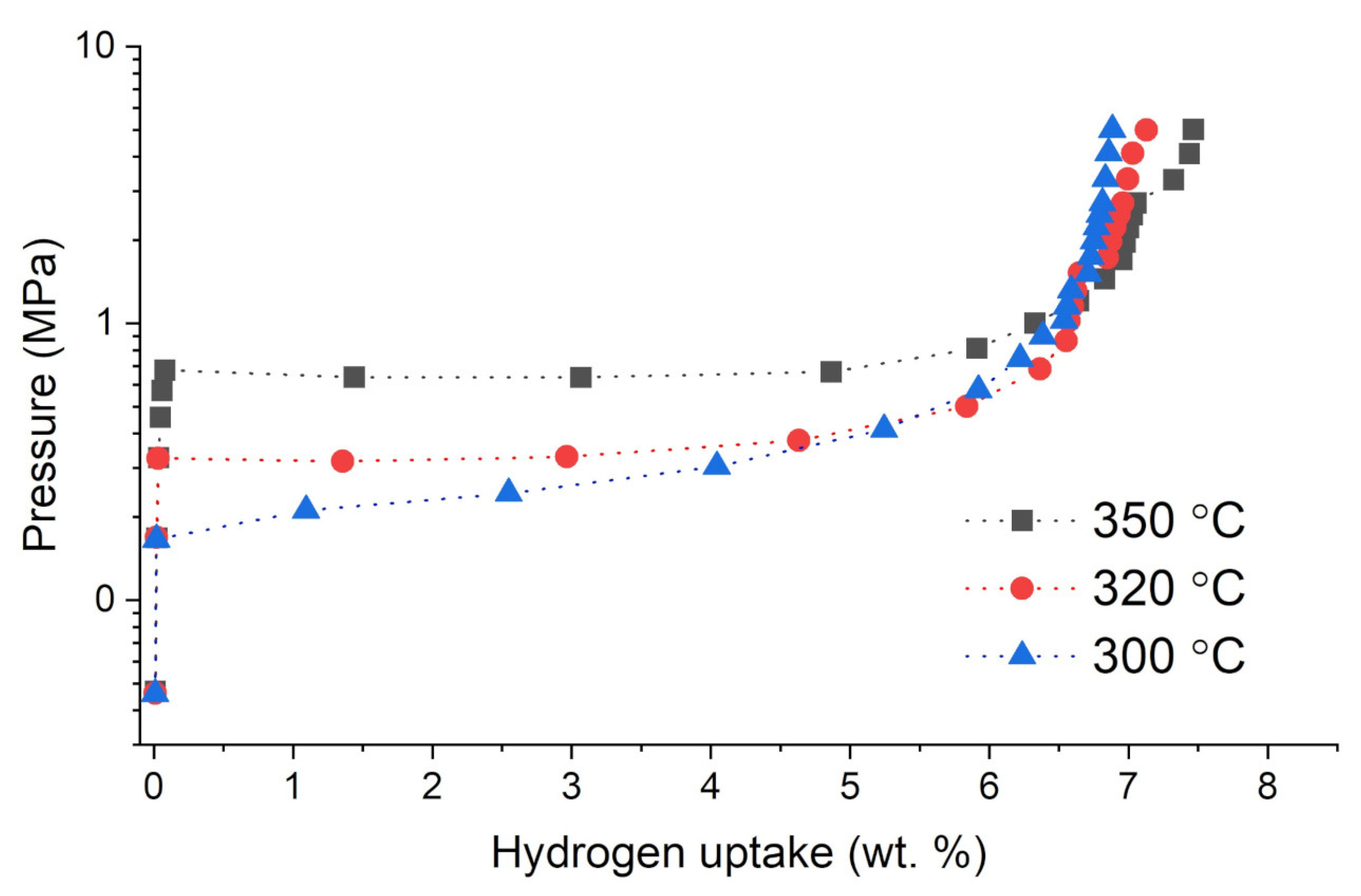
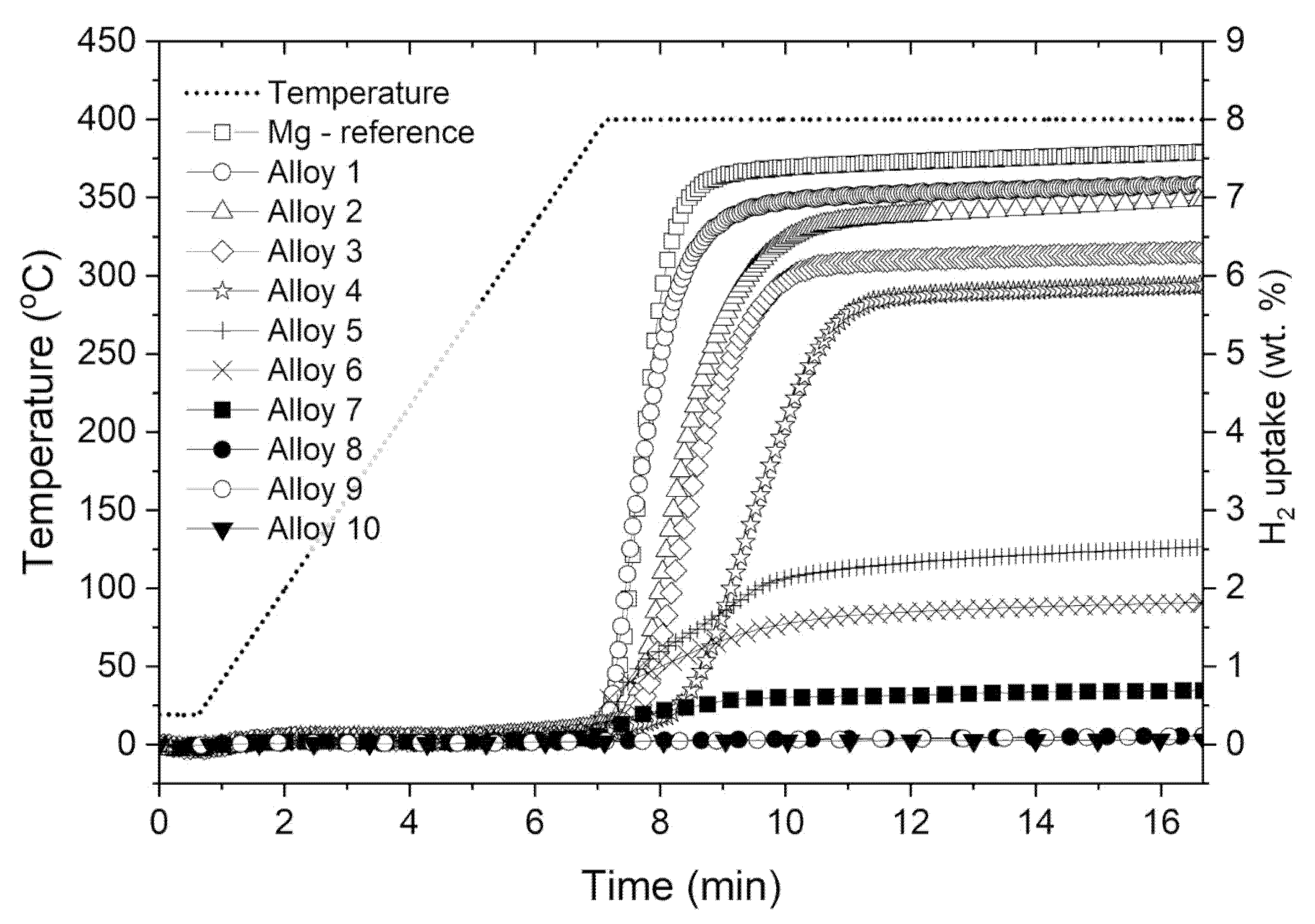
3.2. Hydrogen Absorption Studies of Analyzed Ag-Mg Alloys
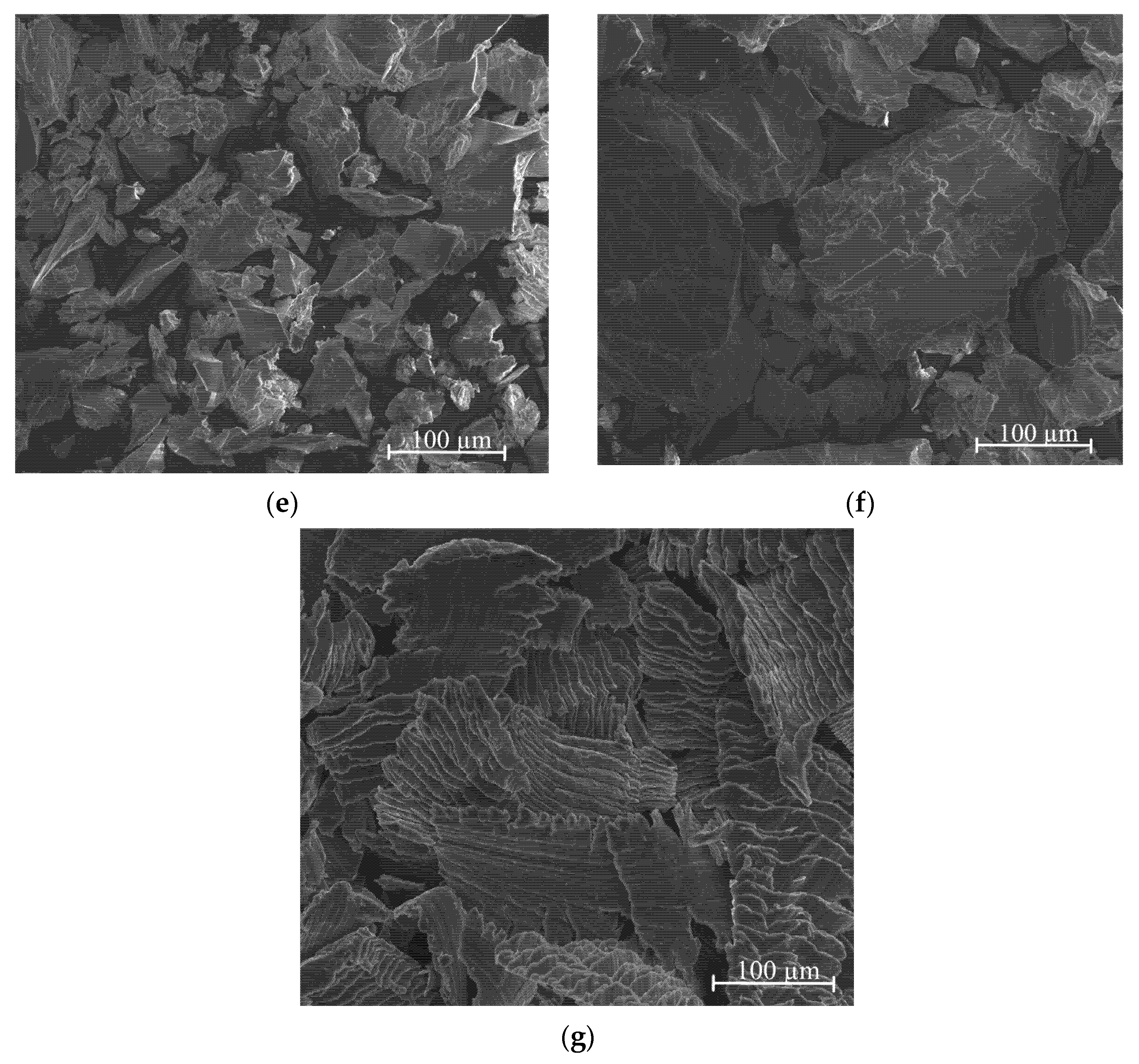
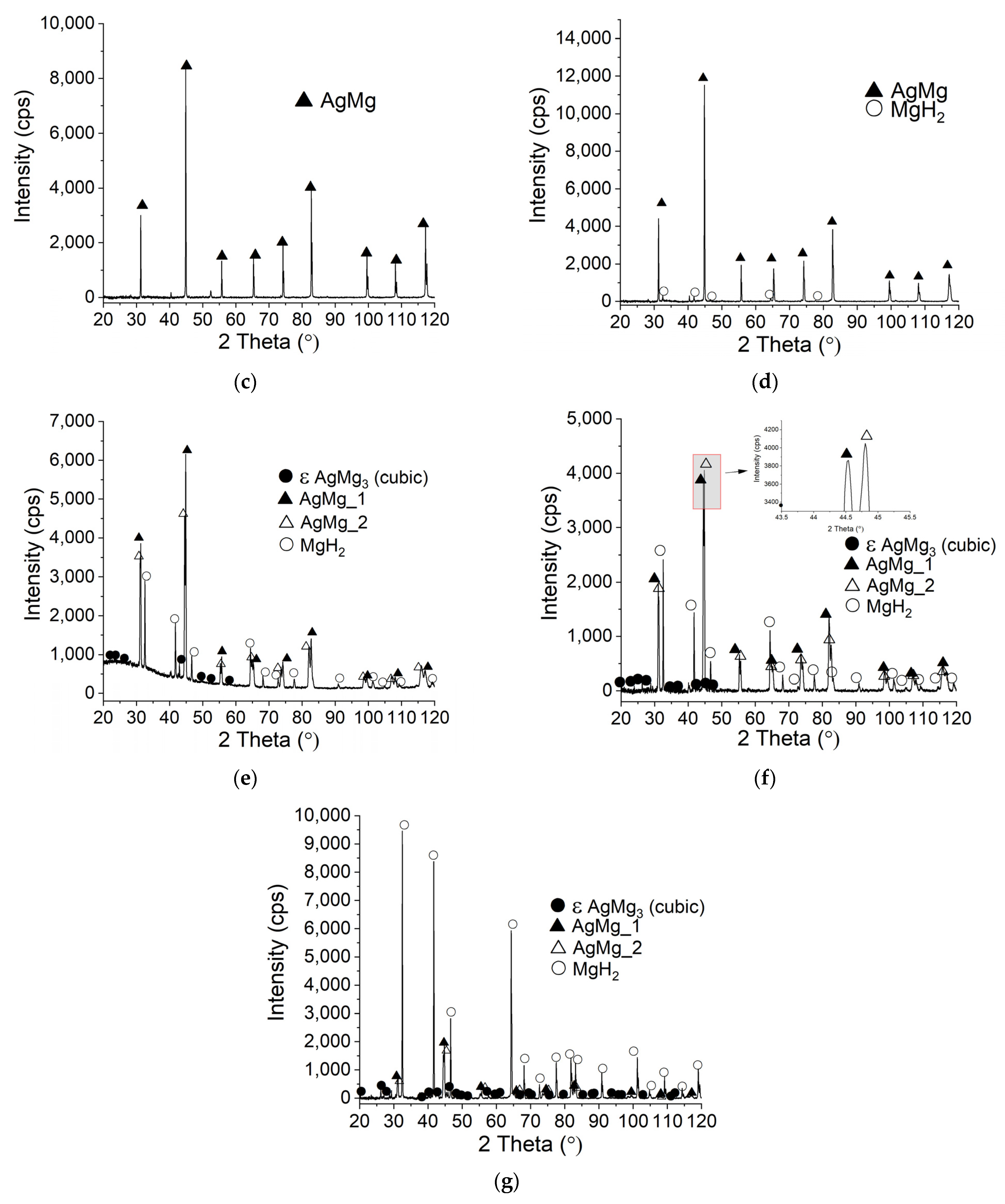
3.3. SEM and XRD Studies of Ag-Mg Alloys after Hydrogenation
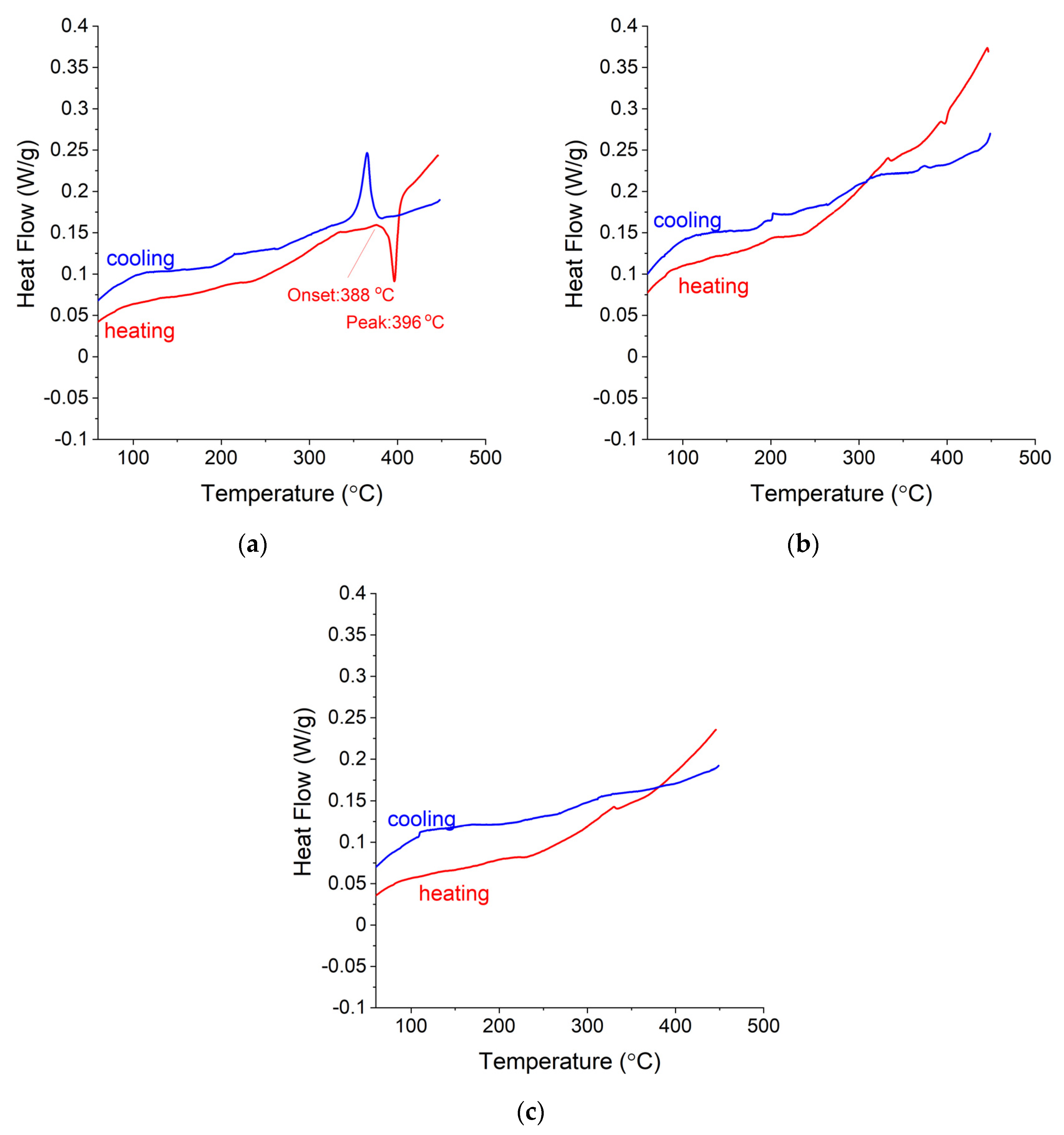
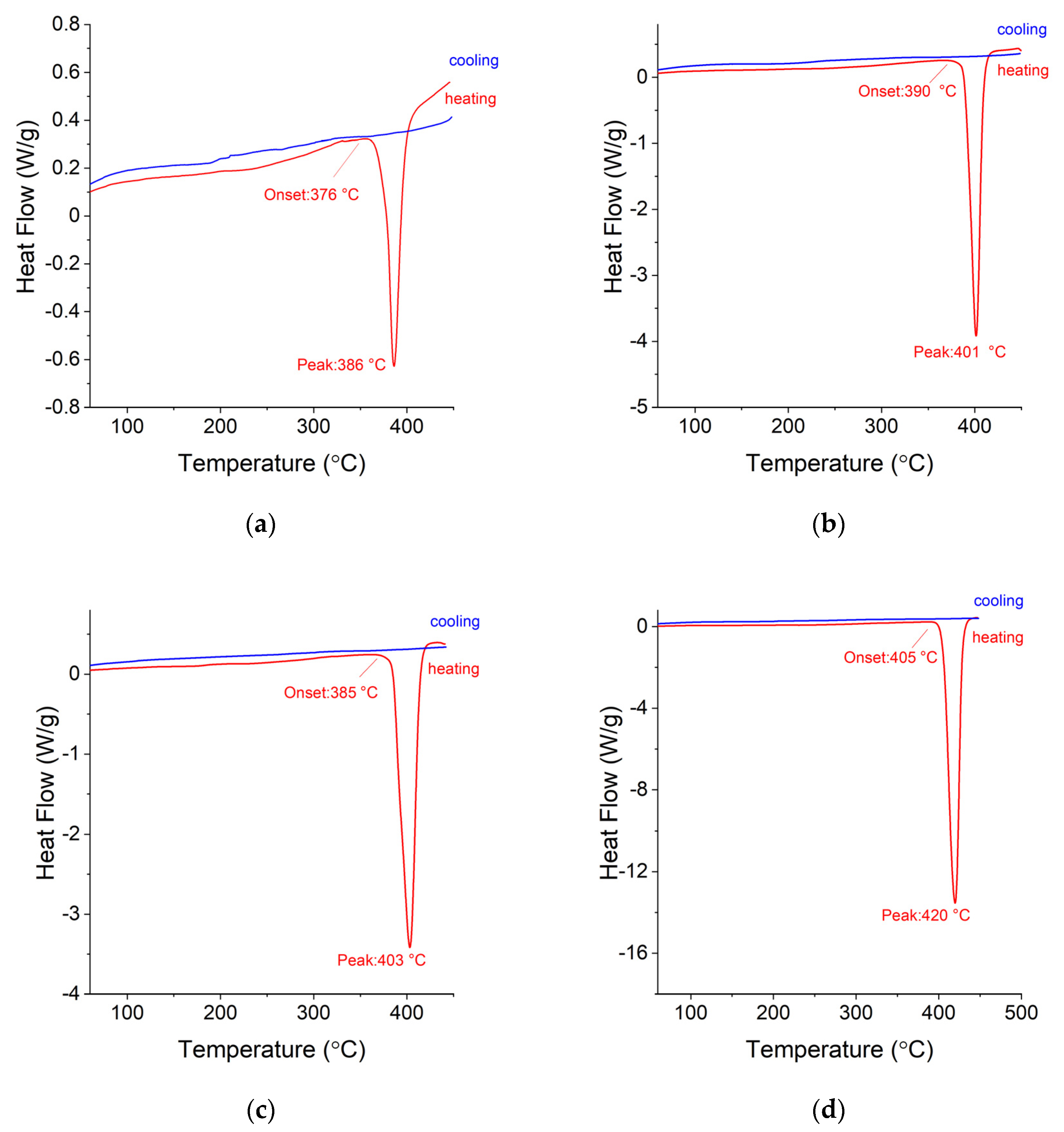
3.4. DSC and TG Measurements for Ag-Mg Alloys after Hydrogen Absorption
4. Conclusions
- (1)
- The filings produced for the sorption study were found to show different sizes depending on whether the alloy is from a single- or two-phase region, likely due to the different mechanical properties of the alloys. The filings produced from alloys from the two-phase region are wide and thin, while those from the one-phase region are much smaller.
- (2)
- A study of hydrogen absorption shows that alloys from the Ag-Mg system with concentrations of 50% at. Mg absorb hydrogen, and the amount of hydrogen absorbed depends on the Mg concentration in the alloy.
- (3)
- The higher the Mg concentration, the higher the amount of absorbed hydrogen.
- (4)
- During the reaction of alloys with hydrogen, the AgMg phase is formed, which remains in the product.
- (5)
- Two AgMg phases are observed with different lattice parameters (observed as a peak splitting).
- (6)
- The addition of Ag to Mg decreases the total absorption of hydrogen but slightly improves the decomposition temperature. However, the change in decomposition temperature is rather insignificant from the viewpoint of applications, especially considering the best currently known catalysts.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pistidda, C. Metals in Hydrogen Technology. Metals 2020, 10, 456. [Google Scholar] [CrossRef] [Green Version]
- Milanese, C.; Jensen, T.; Hauback, B.; Pistidda, C.; Dornheim, M.; Yang, H.; Lombardo, L.; Zuettel, A.; Filinchuk, Y.; Ngene, P. Complex hydrides for energy storage. Int. J. Hydrogen Energy 2019, 44, 7860–7874. [Google Scholar] [CrossRef] [Green Version]
- Dematteis, E.M.; Berti, N.; Cuevas, F.; Latroche, M.; Baricco, M. Substitutional effects in TiFe for hydrogen storage: A comprehensive review. Mater. Adv. 2021, 2, 2524–2560. [Google Scholar] [CrossRef]
- Abe, J.O.; Popoola, A.P.I.; Ajenifuja, E.; Popoola, O.M. Hydrogen energy, economy and storage: Review and recommendation. Int. J. Hydrogen Energy 2019, 44, 15072–15086. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, L.; Liu, F.; Wang, H.; Liu, J.; Yang, X.-S.; Sun, L.; Zhu, M. Magnesium-based hydrogen storage compounds: A review. J. Alloys Compd. 2020, 832, 154865. [Google Scholar] [CrossRef]
- Baran, A.; Polański, M. Magnesium-Based Materials for Hydrogen Storage—A Scope Review. Materials 2020, 13, 3993. [Google Scholar] [CrossRef]
- Crivello, J.C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; De Jongh, P.; Latroche, M.; Milanese, C. Review of magnesium hydride-based materials: Development and optimisation. Appl. Phys. A 2016, 122, 97. [Google Scholar] [CrossRef] [Green Version]
- Webb, C.J. A review of catalyst-enhanced magnesium hydride as a hydrogen storage material. J. Phys. Chem. Solids 2015, 84, 96–106. [Google Scholar] [CrossRef]
- Zhou, C.; Fang, Z.Z.; Ren, C.; Li, J.; Lu, J. Effect of Ti intermetallic catalysts on hydrogen storage properties of magnesium hydride. J. Phys. Chem. C 2013, 117, 12973–12980. [Google Scholar] [CrossRef]
- Wronski, Z.S.; Carpenter, G.J.C.; Czujko, T.; Varin, R.A. A new nanonickel catalyst for hydrogen storage in solid-state magnesium hydrides. Int. J. Hydrogen Energy 2011, 36, 1159–1166. [Google Scholar] [CrossRef]
- Bazzanella, N.; Checchetto, R.; Miotello, A. Atoms and Nanoparticles of Transition Metals as Catalysts for Hydrogen Desorption from Magnesium Hydride. J. Nanomater. 2011, 2011, 865969. [Google Scholar] [CrossRef] [Green Version]
- Huot, J.; Cuevas, F.; Deledda, S.; Edalati, K.; Filinchuk, Y.; Grosdidier, T.; Hauback, B.C.; Heere, M.; Jensen, T.R.; Latroche, M. Mechanochemistry of metal hydrides: Recent advances. Materials 2019, 12, 2778. [Google Scholar] [CrossRef] [Green Version]
- Si, T.Z.; Zhang, J.B.; Liu, D.M.; Zhang, Q.A. A new reversible Mg3Ag–H2 system for hydrogen storage. J. Alloys Compd. 2013, 581, 246–249. [Google Scholar] [CrossRef]
- Urretavizcaya, G.; Sarmiento Chávez, A.C.; Castro, F.J. Hydrogen absorption and desorption in the Mg–Ag system. J. Alloys Compd. 2014, 611, 202–209. [Google Scholar] [CrossRef]
- Pęska, M.; Smektalska, K.; Dworecka-Wójcik, J.; Terlicka, S.; Gąsior, W.; Gierlotka, W.; Dębski, A.; Polański, M. Hydrogen sorption behavior of mechanically synthesized Mg–Ag alloys. Int. J. Hydrogen Energy 2021, 46, 33152–33163. [Google Scholar] [CrossRef]
- Arakcheeva, A.W.; Karpinskyi, O.G.; Kolesnichenko, W.E. Crystal structure of Ag17Mg54. Crystallography 1988, 33, 1523–1525, (Translated from Russian). [Google Scholar]
- Ouyang, L.Z.; Cao, Z.J.; Yao, L.; Wang, H.; Liu, J.W.; Zhu, M. Comparative investigation on the hydrogenation/dehydrogenation characteristics and hydrogen storage properties of Mg3Ag and Mg3Y. Int. J. Hydrogen Energy 2014, 39, 13616–13621. [Google Scholar] [CrossRef]
- Si, T.; Cao, Y.; Zhang, Q.; Sun, D.; Ouyang, L.; Zhu, M. Enhanced hydrogen storage properties of a Mg-Ag alloy with solid dissolution of indium: A comparative study. Ournal Mater. Chem. A 2015, 3, 8581–8589. [Google Scholar] [CrossRef]
- Dębski, A.; Gierlotka, W.; Gąsior, W. Calorimetric studies and thermodynamic calculations of the Ag-Mg system. J. Alloys Compd. 2022, 891, 161937. [Google Scholar] [CrossRef]
- Kudla, C. Strukturell Komplexe Intermetallische. Phasen Untersuchungen an Binären und Ternären Phasen der Systeme Ag–Mg und Ag–Ga–Mg. Ph.D. Thesis, Technischen Universität Dresden, Dresden, Germany, 2007. [Google Scholar]
- Nayeb-Hashemi, A.A.; Clark, J.B. The Ag-Mg (Silver-Magnesium) System. Bull. Alloy. Phase Diagr. 1984, 5, 348–358. [Google Scholar] [CrossRef]






| Chemical Name | Source | Purity [wt. %] | Purification Method | Analysis Method |
|---|---|---|---|---|
| Silver | Innovator Sp. z o. o | 99.9 | None | Certified purity |
| Magnesium | Sigma Aldrich | 99.99 | None | Certified purity |
| Argon | Air Products | 99.9999 | None | Certified purity |
| Helium | Air Products | BIP (<10 ppb O2) | built-in filter in the bottle | Certified purity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębski, A.; Terlicka, S.; Sypien, A.; Gąsior, W.; Pęska, M.; Polański, M. Hydrogen Sorption Behavior of Cast Ag-Mg Alloys. Materials 2022, 15, 270. https://doi.org/10.3390/ma15010270
Dębski A, Terlicka S, Sypien A, Gąsior W, Pęska M, Polański M. Hydrogen Sorption Behavior of Cast Ag-Mg Alloys. Materials. 2022; 15(1):270. https://doi.org/10.3390/ma15010270
Chicago/Turabian StyleDębski, Adam, Sylwia Terlicka, Anna Sypien, Władysław Gąsior, Magda Pęska, and Marek Polański. 2022. "Hydrogen Sorption Behavior of Cast Ag-Mg Alloys" Materials 15, no. 1: 270. https://doi.org/10.3390/ma15010270
APA StyleDębski, A., Terlicka, S., Sypien, A., Gąsior, W., Pęska, M., & Polański, M. (2022). Hydrogen Sorption Behavior of Cast Ag-Mg Alloys. Materials, 15(1), 270. https://doi.org/10.3390/ma15010270







