Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections
Abstract
1. Introduction
2. Titanium and Its Alloys
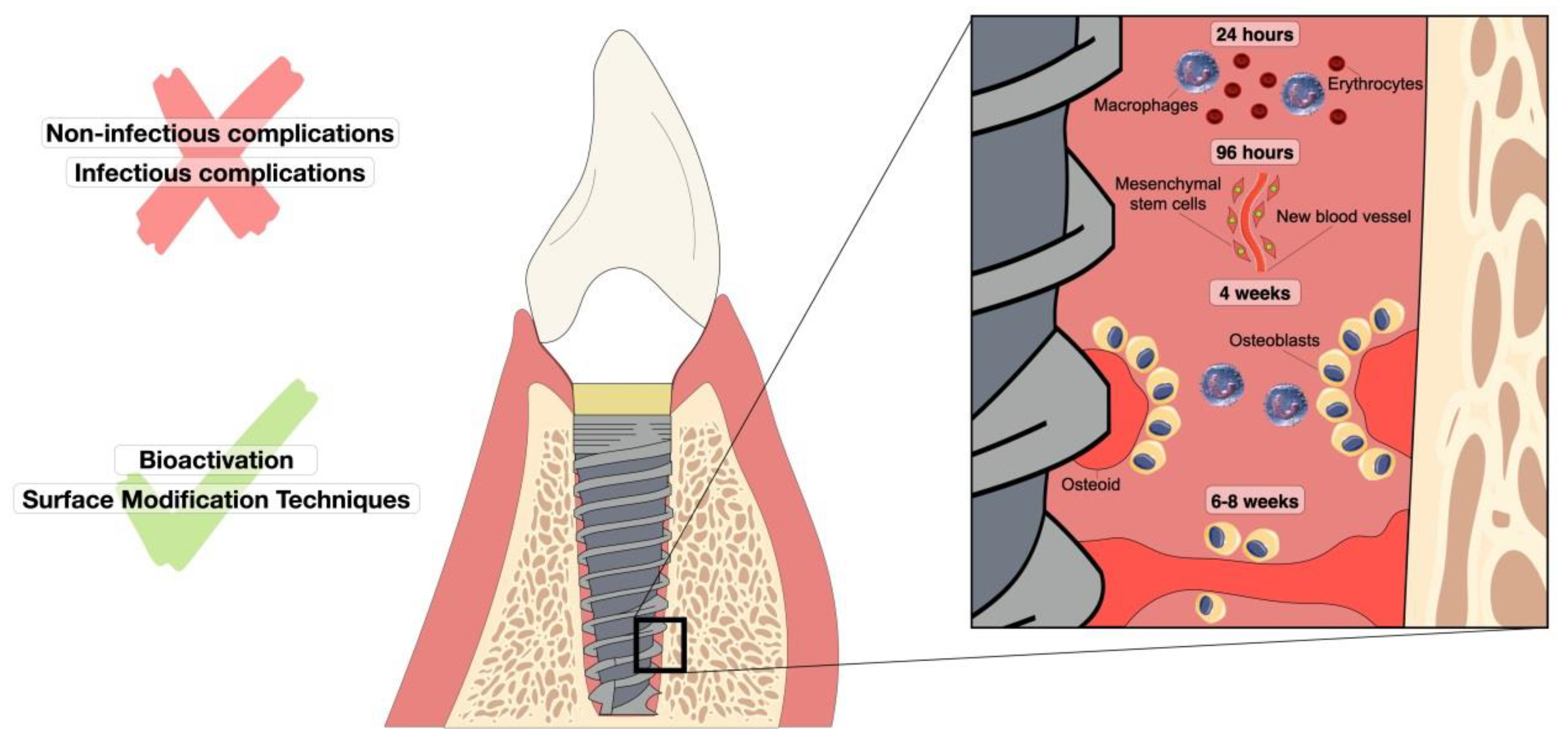
3. Osseointegration Process and Complications Associated with Dental Implants
3.1. Non-Infectious Complications
3.1.1. Patient’s Profile
3.1.2. Implant Profile
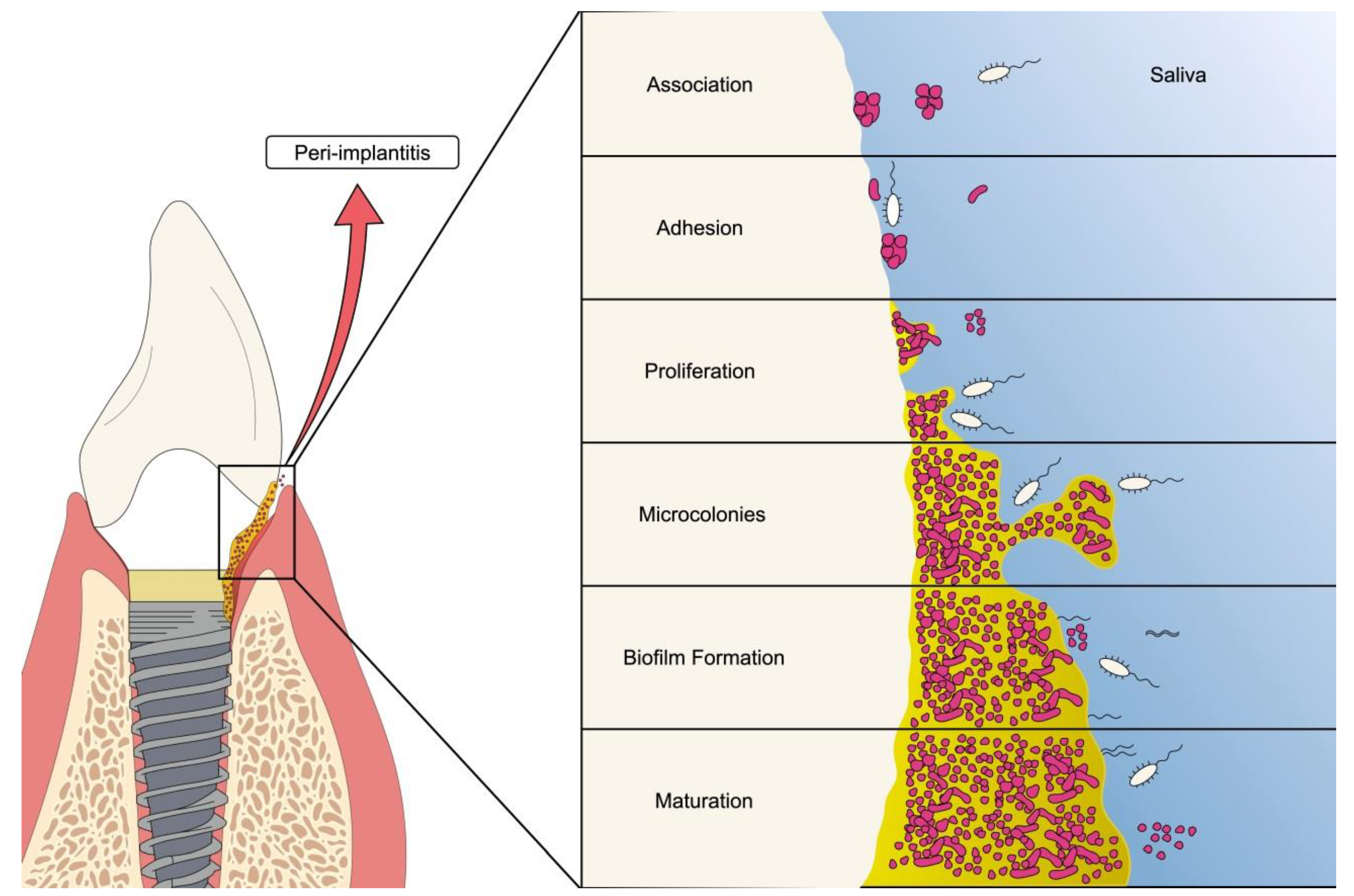
3.2. Infectious Complications
4. Nanotechnology for Promoting Osseointegration
4.1. Nanostructures on Titanium Surfaces
4.2. Surface Modification Techniques (Methods)
4.2.1. Mechanical Method
4.2.2. Chemical Methods
4.2.3. Electrochemical Methods
4.2.4. Layer-by-Layer Technique (LbL)
5. Biomimetic and Bioactive Surfaces
Surface Graded Functionalized
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Institute for Health Metrics and Evaluation (IHME). Findings from the Global Burden of Disease Study 2017; IHME: Seattle, WA, USA, 2018. [Google Scholar]
- Yano, Y.; Fan, J.; Dawsey, S.M.; Qiao, Y.; Abnet, C.C. A Long-Term Follow-up Analysis of Associations between Tooth Loss and Multiple Cancers in the Linxian General Population Cohort. J. Natl. Cancer Cent. 2021, 1, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, Y.-C.; Zhu, B.-L.; Wu, C.-C.; Lin, R.-F.; Zhang, X. Association between Periodontal Disease, Tooth Loss and Liver Diseases Risk. J. Clin. Periodontol. 2020, 47, 1053–1063. [Google Scholar] [CrossRef]
- Roberto, L.L.; Crespo, T.S.; Monteiro-Junior, R.S.; Martins, A.M.E.B.L.; De Paula, A.M.B.; Ferreira, E.F.; Haikal, D.S. Sociodemographic Determinants of Edentulism in the Elderly Population: A Systematic Review and Meta-Analysis. Gerodontology 2019, 36, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.; Saponaro, P.C. Management of Edentulous Patients. Dent. Clin. N. Am. 2019, 63, 249–261. [Google Scholar] [CrossRef]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface Modification Techniques of Titanium and Titanium Alloys for Biomedical Dental Applications: A Review. Mater. Today Proc. 2021, 39, 84–90. [Google Scholar] [CrossRef]
- Liaw, K.; Delfini, R.H.; Abrahams, J.J. Dental Implant Complications. Semin. Ultrasound CT MR 2015, 36, 427–433. [Google Scholar] [CrossRef]
- Lavenus, S.; Rozé, J.; Louarn, G.; Layrolle, P. Chapter 16—Impact of Nanotechnology on Dental Implants. In Nanobiomaterials in Clinical Dentistry, 2nd ed.; Subramani, K., Ahmed, W., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 385–399. ISBN 978-0-12-815886-9. [Google Scholar]
- da Silva, R.A.; da Silva Feltran, G.; Ferreira, M.R.; Wood, P.F.; Bezerra, F.; Zambuzzi, W.F. The Impact of Bioactive Surfaces in the Early Stages of Osseointegration: An In Vitro Comparative Study Evaluating the HAnano® and SLActive® Super Hydrophilic Surfaces. Biomed. Res. Int. 2020, 2020, 3026893. [Google Scholar] [CrossRef]
- Jones, J.C.R.; Colburn, Z.T. Biomolecules and Implant Materials. Ref. Modul. Mater. Sci. Mater. Eng. 2016, 1, 8–11. [Google Scholar] [CrossRef]
- Nicholson, J.W. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 11. [Google Scholar] [CrossRef]
- Haider, A.J.; Jameel, Z.N.; Al-Hussaini, I.H.M. Review on: Titanium Dioxide Applications. Energy Procedia 2019, 157, 17–29. [Google Scholar] [CrossRef]
- Shi, H.; Magaye, R.; Castranova, V.; Zhao, J. Titanium Dioxide Nanoparticles: A Review of Current Toxicological Data. Part. Fibre Toxicol. 2013, 10, 15. [Google Scholar] [CrossRef]
- Chen, X.; Mao, S.S. Titanium Dioxide Nanomaterials: Synthesis, Properties, Modifications, and Applications. Chem. Rev. 2007, 107, 2891–2959. [Google Scholar] [CrossRef]
- Dambournet, D.; Belharouak, I.; Amine, K. Tailored Preparation Methods of TiO2 Anatase, Rutile, Brookite: Mechanism of Formation and Electrochemical Properties. Chem. Mater. 2010, 22, 1173–1179. [Google Scholar] [CrossRef]
- Saini, M. Implant Biomaterials: A Comprehensive Review. World J. Clin. Cases 2015, 3, 52. [Google Scholar] [CrossRef]
- Osman, R.B.; Swain, M.V. A Critical Review of Dental Implant Materials with an Emphasis on Titanium versus Zirconia. Materials 2015, 8, 932–958. [Google Scholar] [CrossRef]
- Bodunrin, M.O.; Chown, L.H.; Omotoyinbo, J.A. Development of Low-Cost Titanium Alloys: A Chronicle of Challenges and Opportunities. Mater. Today Proc. 2021, 38, 564–569. [Google Scholar] [CrossRef]
- Kolli, R.P.; Devaraj, A. A Review of Metastable Beta Titanium Alloys. Metals 2018, 8, 506. [Google Scholar] [CrossRef]
- Fujii, H.; Fujisawa, K.; Ijii, M.; Yamashita, Y. Development of Low-Cost High-Strength Ti-Fe-O-N Alloy Series. Nippon. Steel Tech. Rep. 2002, 85, 107–112. [Google Scholar]
- Fujii, H.; Takahashi, K. Development of High Performance Ti-Fe-Al Alloy Series. Nippon Steel Tech. Rep. Overseas 2002, 85, 113–117. [Google Scholar]
- Fujii, H.; Maeda, T. Titanium Alloys Developed by Nippon Steel & Sumitomo Metal Corporation. Nippon Steel Sumitomo Met. Tech. Rep. 2014, 106, 16–21. [Google Scholar]
- Kania, A.; Szindler, M.M.; Szindler, M. Structure and Corrosion Behavior of TiO2 Thin Films Deposited by ALD on a Biomedical Magnesium Alloy. Coatings 2021, 11, 70. [Google Scholar] [CrossRef]
- Prando, D.; Brenna, A.; Diamanti, M.V.; Beretta, S.; Bolzoni, F.; Ormellese, M.; Pedeferri, M.P. Corrosion of Titanium: Part 2: Effects of Surface Treatments. J. Appl. Biomater. Funct. Mater. 2018, 16, 3–13. [Google Scholar] [CrossRef]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2003; ISBN 9783527602117. [Google Scholar]
- Dallago, M.; Fontanari, V.; Torresani, E.; Leoni, M.; Pederzolli, C.; Potrich, C.; Benedetti, M. Fatigue and Biological Properties of Ti-6Al-4V ELI Cellular Structures with Variously Arranged Cubic Cells Made by Selective Laser Melting; Elsevier: Amsterdam, The Netherlands, 2018; Volume 78, ISBN 3904612824. [Google Scholar]
- Ferraris, S.; Spriano, S.; Pan, G.; Venturello, A.; Bianchi, C.L.; Chiesa, R.; Faga, M.G.; Maina, G.; Vernè, E. Surface Modification of Ti-6Al-4V Alloy for Biomineralization and Specific Biological Response: Part I, Inorganic Modification. J. Mater. Sci. Mater. Med. 2011, 22, 533–545. [Google Scholar] [CrossRef]
- Ścibior, A.; Pietrzyk, Ł.; Plewa, Z.; Skiba, A. Vanadium: Risks and Possible Benefits in the Light of a Comprehensive Overview of Its Pharmacotoxicological Mechanisms and Multi-Applications with a Summary of Further Research Trends. J. Trace Elem. Med. Biol. 2020, 61, 126508. [Google Scholar] [CrossRef]
- Klein, G.L. Aluminum Toxicity to Bone: A Multisystem Effect? Osteoporos. Sarcopenia 2019, 5, 2–5. [Google Scholar] [CrossRef]
- Akagawa, Y.; Ichikawa, Y.; Nikai, H.; Tsuru, H. Interface Histology of Unloaded and Early Loaded Partially Stabilized Zirconia Endosseous Implant in Initial Bone Healing. J. Prosthet. Dent. 1993, 69, 599–604. [Google Scholar] [CrossRef]
- Akagawa, Y.; Hosokawa, R.; Sato, Y.; Kamayama, K. Comparison between Freestanding and Tooth-Connected Partially Stabilized Zirconia Implants after Two Years’ Function in Monkeys: A Clinical and Histologic Study. J. Prosthet. Dent. 1998, 80, 551–558. [Google Scholar] [CrossRef]
- Kohal, R.J.; Weng, D.; Bächle, M.; Strub, J.R. Loaded Custom-Made Zirconia and Titanium Implants Show Similar Osseointegration: An Animal Experiment. J. Periodontol. 2004, 75, 1262–1268. [Google Scholar] [CrossRef]
- Sivaraman, K.; Chopra, A.; Narayan, A.I.; Balakrishnan, D. Is Zirconia a Viable Alternative to Titanium for Oral Implant? A Critical Review. J. Prosthodont. Res. 2018, 62, 121–133. [Google Scholar] [CrossRef]
- Campbell, A.A. Bioceramics for Implant Coatings. Mater. Today 2003, 6, 26–30. [Google Scholar] [CrossRef]
- Velmurugan, D.; Masilamani Santha, A.; Gaurishankar Sarate, S. Dental Implant Materials, Implant Design, and Role of Fea- a Brief Review. J. Evol. Med. Dent. Sci. 2017, 6, 3487–3492. [Google Scholar] [CrossRef]
- Jorge, J.H.; Giampaolo, E.T.; Machado, A.L.; Vergani, C.E. Cytotoxicity of Denture Base Acrylic Resins: A Literature Review. J. Prosthet. Dent. 2003, 90, 190–193. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Darvell, B.W. Minimization of the Inevitable Residual Monomer in Denture Base Acrylic. Dent. Mater. 2005, 21, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Braun, K.O.; Mello, J.A.N.; Rached, R.N.; Del Bel Cury, A.A. Surface Texture and Some Properties of Acrylic Resins Submitted to Chemical Polishing. J. Oral Rehabil. 2003, 30, 91–98. [Google Scholar] [CrossRef]
- Jayesh, R.S.; Dhinakarsamy, V. Osseointegration. J. Pharm. Bioallied Sci. 2015, 7, S226–S229. [Google Scholar] [CrossRef]
- Branemark, P.-I. Osseointegration and Its Experimental Background. J. Prosthet. Dent. 1983, 50, 399–410. [Google Scholar] [CrossRef]
- Schnitman, P.A. Dental Implants: State of the Art, State of the Science. Int. J. Technol. Assess. Health Care 1990, 6, 528–544. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Miron, R.J. Health, Maintenance, and Recovery of Soft Tissues around Implants. Clin. Implant Dent. Relat. Res. 2016, 18, 618–634. [Google Scholar] [CrossRef]
- Insua, A.; Monje, A.; Wang, H.L.; Miron, R.J. Basis of Bone Metabolism around Dental Implants during Osseointegration and Peri-Implant Bone Loss. J. Biomed. Mater. Res. Part A 2017, 105, 2075–2089. [Google Scholar] [CrossRef]
- (Sam) Froes, F.H.; Qian, M. Preface. In Titanium in Medical and Dental Applications; Froes, F.H., Qian, M., Eds.; Woodhead Publishing: Sawston, UK, 2018; ISBN 978-0-12-812456-7. [Google Scholar]
- Papaspyridakos, P.; De Souza, A.; Vazouras, K.; Gholami, H.; Pagni, S.; Weber, H.P. Survival Rates of Short Dental Implants (≤6 Mm) Compared with Implants Longer than 6 Mm in Posterior Jaw Areas: A Meta-Analysis. Clin. Oral Implant. Res. 2018, 29, 8–20. [Google Scholar] [CrossRef]
- Koenig, V.; Vanheusden, A.J.; Le Goff, S.O.; Mainjot, A.K. Clinical Risk Factors Related to Failures with Zirconia-Based Restorations: An up to 9-Year Retrospective Study. J. Dent. 2013, 41, 1164–1174. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-L.; Lu, H.-K.; Ou, K.-L.; Su, P.-Y.; Chen, C.-M. Fractographic Analysis of Fractured Dental Implant Components. J. Dent. Sci. 2013, 8, 8–14. [Google Scholar] [CrossRef][Green Version]
- De Marco, G.; Di Francesco, F.; Lanza, A. Analysis and Management of Implant-Prosthetic Complications: Description of a Diagnostic and Therapeutic Algorithm with a Clinical Case. J. Prosthodont. Res. 2018, 62, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Lemos, C.A.A.; Verri, F.R.; de Souza Batista, V.E.; Júnior, J.F.S.; Mello, C.C.; Pellizzer, E.P. Complete Overdentures Retained by Mini Implants: A Systematic Review. J. Dent. 2017, 57, 4–13. [Google Scholar] [CrossRef]
- Pozzi, A.; Arcuri, L.; Fabbri, G.; Singer, G.; Londono, J. Long-Term Survival and Success of Zirconia Screw-Retained Implant-Supported Prostheses for up to 12 Years: A Retrospective Multicenter Study. J. Prosthet. Dent. 2021. [Google Scholar] [CrossRef] [PubMed]
- Compton, S.; Clark, D.; Chan, S.; Kuc, I.; Wubie, B.; Levin, L. Dental Implants in the Elderly Population: A Long-Term Follow-Up. Int. J. Oral Maxillofac. Implant. 2017, 32, 164–170. [Google Scholar] [CrossRef]
- Do, T.A.; Le, H.S.; Shen, Y.W.; Huang, H.L.; Fuh, L.J. Risk Factors Related to Late Failure of Dental Implant—A Systematic Review of Recent Studies. Int. J. Environ. Res. Public Health 2020, 17, 3931. [Google Scholar] [CrossRef]
- da Sales, P.H.H.; Barros, A.W.P.; de Lima, F.J.C.; de Carvalho, A.A.T.; Leão, J.C. Is Down Syndrome a Risk Factor or Contraindication for Dental Implants? A Systematic Review. J. Prosthet. Dent. 2021. [Google Scholar] [CrossRef]
- Hussein, M.O.; Alruthea, M.S. Marginal Bone Level Changes and Oral Health Impact Profile (14) Score of Smokers Treated by Mandibular Mini Implant Overdentures: A 5-Year Follow-up Study. Eur. J. Dent. 2020, 14, 590–597. [Google Scholar] [CrossRef]
- Pääsky, E.; Suomalainen, A.; Ventä, I. Are Women More Susceptible than Men to Iatrogenic Inferior Alveolar Nerve Injury in Dental Implant Surgery? Int. J. Oral Maxillofac. Surg. 2021, 51, 6–11. [Google Scholar] [CrossRef]
- Palareti, G.; Legnani, C.; Cosmi, B.; Antonucci, E.; Erba, N.; Poli, D.; Testa, S.; Tosetto, A. Comparison between Different D-Dimer Cutoff Values to Assess the Individual Risk of Recurrent Venous Thromboembolism: Analysis of Results Obtained in the DULCIS Study. Int. J. Lab. Hematol. 2016, 38, 42–49. [Google Scholar] [CrossRef]
- Dantas, M.V.M.; Verzola, M.H.A.; Sanitá, P.V.; Dovigo, L.N.; Cerri, P.S.; Gabrielli, M.A.C. The Influence of Cisplatin-Based Chemotherapy on the Osseointegration of Dental Implants: An in Vivo Mechanical and Histometrical Study. Clin. Oral Implant. Res. 2019, 30, 603–616. [Google Scholar] [CrossRef] [PubMed]
- Koivu, H.; MacKiewicz, Z.; Takakubo, Y.; Trokovic, N.; Pajarinen, J.; Konttinen, Y.T. RANKL in the Osteolysis of AES Total Ankle Replacement Implants. Bone 2012, 51, 546–552. [Google Scholar] [CrossRef]
- Chamanara, M.; Rashidian, A.; Mehr, S.E.; Dehpour, A.R.; Shirkohi, R.; Akbarian, R.; Abdollahi, A.; Rezayat, S.M. Melatonin Ameliorates TNBS-Induced Colitis in Rats through the Melatonin Receptors: Involvement of TLR4/MyD88/NF-ΚB Signalling Pathway. Inflammopharmacology 2019, 27, 361–371. [Google Scholar] [CrossRef]
- Wu, X.; Qiao, S.; Wang, W.; Zhang, Y.; Shi, J.; Zhang, X.; Gu, W.; Zhang, X.; Li, Y.; Ding, X.; et al. Melatonin Prevents Peri-implantitis via Suppression of TLR4/NF-ΚB. Acta Biomater. 2021, 134, 325–336. [Google Scholar] [CrossRef]
- de Medeiros, F.C.F.L.; Kudo, G.A.H.; Leme, B.G.; Saraiva, P.P.; Verri, F.R.; Honório, H.M.; Pellizzer, E.P.; Santiago Junior, J.F. Dental Implants in Patients with Osteoporosis: A Systematic Review with Meta-Analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 480–491. [Google Scholar] [CrossRef]
- Rosas, J.; Mayta-Tovalino, F.; Malpartida-Carrillo, V.; Degregori, A.M.; Mendoza, R.; Guerrero, M.E. Effect of Abutment Geometry and Luting Agents on the Vertical Marginal Discrepancy of Cast Copings on Implant Abutments: An In Vitro Study. Int. J. Dent. 2021, 2021, 9950972. [Google Scholar] [CrossRef]
- Schiegnitz, E.; Al-Nawas, B.; Tegner, A.; Sagheb, K.; Berres, M.; Kämmerer, P.W.; Wagner, W. Clinical and Radiological Long-Term Outcome of a Tapered Implant System with Special Emphasis on the Influence of Augmentation Procedures. Clin. Implant. Dent. Relat. Res. 2016, 18, 810–820. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H. 15-Year Follow-up of Short Dental Implants Placed in the Partially Edentulous Patient: Mandible Vs Maxilla. Ann. Anat. Anat. Anz. 2019, 222, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-Implant Health, Peri-Implant Mucositis, and Peri-Implantitis: Case Definitions and Diagnostic Considerations. J. Periodontol. 2018, 89, S304–S312. [Google Scholar] [CrossRef]
- Atieh, M.A.; Alsabeeha, N.H.M.; Faggion, C.M.J.; Duncan, W.J. The Frequency of Peri-Implant Diseases: A Systematic Review and Meta-Analysis. J. Periodontol. 2013, 84, 1586–1598. [Google Scholar] [CrossRef]
- Radaic, A.; Kapila, Y.L. The Oralome and Its Dysbiosis: New Insights into Oral Microbiome-Host Interactions. Comput. Struct. Biotechnol. J. 2021, 19, 1335–1360. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Nomura, N.; Suzuki, S. Biofilms: Hot Spots of Horizontal Gene Transfer (HGT) in Aquatic Environments, with a Focus on a New HGT Mechanism. FEMS Microbiol. Ecol. 2021, 96, fiaa031. [Google Scholar] [CrossRef]
- Worthington, R.J.; Richards, J.J.; Melander, C. Small Molecule Control of Bacterial Biofilms. Org. Biomol. Chem. 2012, 10, 7457–7474. [Google Scholar] [CrossRef] [PubMed]
- Pye, A.D.; Lockhart, D.E.A.; Dawson, M.P.; Murray, C.A.; Smith, A.J. A Review of Dental Implants and Infection. J. Hosp. Infect. 2009, 72, 104–110. [Google Scholar] [CrossRef]
- Dhir, S. Biofilm and Dental Implant: The Microbial Link. J. Indian Soc. Periodontol. 2013, 17, 5–11. [Google Scholar] [CrossRef]
- Flemming, H.-C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Fulaz, S.; Vitale, S.; Quinn, L.; Casey, E. Nanoparticle–Biofilm Interactions: The Role of the EPS Matrix. Trends Microbiol. 2019, 27, 915–926. [Google Scholar] [CrossRef] [PubMed]
- Whitchurch, C.B.; Tolker-Nielsen, T.; Ragas, P.C.; Mattick, J.S. Extracellular DNA Required for Bacterial Biofilm Formation. Science 2002, 295, 1487. [Google Scholar] [CrossRef]
- Toyofuku, M.; Inaba, T.; Kiyokawa, T.; Obana, N.; Yawata, Y.; Nomura, N. Environmental Factors That Shape Biofilm Formation. Biosci. Biotechnol. Biochem. 2016, 80, 7–12. [Google Scholar] [CrossRef]
- Yutaka, Y.; Nobuhiko, N.; Hiroo, U. Development of a Novel Biofilm Continuous Culture Method for Simultaneous Assessment of Architecture and Gaseous Metabolite Production. Appl. Environ. Microbiol. 2008, 74, 5429–5435. [Google Scholar] [CrossRef]
- Dongari-Bagtzoglou, A. Pathogenesis of Mucosal Biofilm Infections: Challenges and Progress. Expert Rev. Anti. Infect. Ther. 2008, 6, 201–208. [Google Scholar] [CrossRef]
- Nicolas, B.; Staffan, K.A.R.S.; Mahmoud, G.; Matthew, P.; Marvin, W.; Pranab, M. Dispersal from Microbial Biofilms. Microbiol. Spectr. 2015, 3, 343–362. [Google Scholar] [CrossRef]
- Nadell, C.D.; Xavier, J.B.; Levin, S.A.; Foster, K.R. The Evolution of Quorum Sensing in Bacterial Biofilms. PLoS Biol. 2008, 6, e14. [Google Scholar] [CrossRef]
- Koo, H.; Allan, R.N.; Howlin, R.P.; Stoodley, P.; Hall-Stoodley, L. Targeting Microbial Biofilms: Current and Prospective Therapeutic Strategies. Nat. Rev. Microbiol. 2017, 15, 740–755. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on Titanium and Titanium Based Alloys as Biomaterials for Orthopaedic Applications. Mater. Sci. Eng. C 2019, 102, 844–862. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 Nanotubes for Bone Regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef]
- Tan, A.W.; Pingguan-Murphy, B.; Ahmad, R.; Akbar, S.A. Review of Titania Nanotubes: Fabrication and Cellular Response. Ceram. Int. 2012, 38, 4421–4435. [Google Scholar] [CrossRef]
- Yoon, I.-K.; Hwang, J.-Y.; Jang, W.-C.; Kim, H.-W.; Shin, U.S. Natural Bone-like Biomimetic Surface Modification of Titanium. Appl. Surf. Sci. 2014, 301, 401–409. [Google Scholar] [CrossRef]
- Park, K.H.; Song, H.-J.; Park, Y.-J. Albumin Adsorption on Microwave-Treated Titanium Dioxide for Dental Implant Materials. Colloids Surf. B. Biointerfaces 2021, 208, 112124. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Z.; Zhu, X.; Jiang, L.; Shi, W.; Wang, Y.; Xu, N.; Gang, F.; Wang, X.; Zhao, L.; et al. Dual Directions to Address the Problem of Aseptic Loosening via Electrospun PLGA @ Aspirin Nanofiber Coatings on Titanium. Biomaterials 2020, 257, 120237. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Ivanovski, S. Dental Implants Modified with Drug Releasing Titania Nanotubes: Therapeutic Potential and Developmental Challenges. Expert Opin. Drug Deliv. 2017, 14, 1009–1024. [Google Scholar] [CrossRef] [PubMed]
- Dhatrak, P.; Shirsat, U.; Deshmukh, V. Fatigue Life Prediction of Commercial Dental Implants Based on Biomechanical Parameters: A Review. J. Mater. Sci. Surf. Eng. 2015, 3, 221–226. [Google Scholar]
- Granato, R.; Bonfante, E.A.; Castellano, A.; Khan, R.; Jimbo, R.; Marin, C.; Morsi, S.; Witek, L.; Coelho, P.G. Osteointegrative and Microgeometric Comparison between Micro-Blasted and Alumina Blasting/Acid Etching on Grade II and V Titanium Alloys (Ti-6Al-4V). J. Mech. Behav. Biomed. Mater. 2019, 97, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Nicoli, L.G.; Oliveira, G.J.P.L.; de Lopes, B.M.V.; Marcantonio, C.; Zandim-Barcelos, D.L.; Marcantonio, E.J. Survival/Success of Dental Implants with Acid-Etched Surfaces: A Retrospective Evaluation After 8 to 10 Years. Braz. Dent. J. 2017, 28, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Doe, Y.; Ida, H.; Seiryu, M.; Deguchi, T.; Takeshita, N.; Sasaki, S.; Sasaki, S.; Irie, D.; Tsuru, K.; Ishikawa, K.; et al. Titanium Surface Treatment by Calcium Modification with Acid-Etching Promotes Osteogenic Activity and Stability of Dental Implants. Materialia 2020, 12, 100801. [Google Scholar] [CrossRef]
- Minagar, S.; Berndt, C.C.; Wang, J.; Ivanova, E.; Wen, C. A Review of the Application of Anodization for the Fabrication of Nanotubes on Metal Implant Surfaces. Acta Biomater. 2012, 8, 2875–2888. [Google Scholar] [CrossRef]
- Alves, S.A.; Patel, S.B.; Sukotjo, C.; Mathew, M.T.; Filho, P.N.; Celis, J.-P.; Rocha, L.A.; Shokuhfar, T. Synthesis of Calcium-Phosphorous Doped TiO2 Nanotubes by Anodization and Reverse Polarization: A Promising Strategy for an Efficient Biofunctional Implant Surface. Appl. Surf. Sci. 2017, 399, 682–701. [Google Scholar] [CrossRef]
- Wang, B.; Wu, Z.; Lan, J.; Li, Y.; Xie, L.; Huang, X.; Zhang, A.; Qiao, H.; Chang, X.; Lin, H.; et al. Surface Modification of Titanium Implants by Silk Fibroin/Ag Co-Functionalized Strontium Titanate Nanotubes for Inhibition of Bacterial-Associated Infection and Enhancement of in Vivo Osseointegration. Surf. Coat. Technol. 2021, 405, 126700. [Google Scholar] [CrossRef]
- Li, B.; Hao, J.; Min, Y.; Xin, S.; Guo, L.; He, F.; Liang, C.; Wang, H.; Li, H. Biological Properties of Nanostructured Ti Incorporated with Ca, P and Ag by Electrochemical Method. Mater. Sci. Eng. C 2015, 51, 80–86. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Gonzalez Tenorio, R.; Mohedano, M.; Martinez-Campos, E.; Arrabal, R.; Matykina, E. Tailoring of Antibacterial and Osteogenic Properties of Ti6Al4V by Plasma Electrolytic Oxidation. Appl. Surf. Sci. 2018, 454, 157–172. [Google Scholar] [CrossRef]
- Kaseem, M.; Choe, H.C. The Effect of In-Situ Reactive Incorporation of MoOx on the Corrosion Behavior of Ti-6Al-4 V Alloy Coated via Micro-Arc Oxidation Coating. Corros. Sci. 2021, 192, 109764. [Google Scholar] [CrossRef]
- Kaseem, M.; Choe, H.C. Electrochemical and Bioactive Characteristics of the Porous Surface Formed on Ti-XNb Alloys via Plasma Electrolytic Oxidation. Surf. Coat. Technol. 2019, 378, 125027. [Google Scholar] [CrossRef]
- Santos-Coquillat, A.; Martínez-Campos, E.; Mohedano, M.; Martínez-Corriá, R.; Ramos, V.; Arrabal, R.; Matykina, E. In Vitro and in Vivo Evaluation of PEO-Modified Titanium for Bone Implant Applications. Surf. Coat. Technol. 2018, 347, 358–368. [Google Scholar] [CrossRef]
- Guzmán, E.; Rubio, R.G.; Ortega, F. A Closer Physico-Chemical Look to the Layer-by-Layer Electrostatic Self-Assembly of Polyelectrolyte Multilayers. Adv. Colloid Interface Sci. 2020, 282, 102197. [Google Scholar] [CrossRef]
- Chua, P.-H.; Neoh, K.-G.; Kang, E.-T.; Wang, W. Surface Functionalization of Titanium with Hyaluronic Acid/Chitosan Polyelectrolyte Multilayers and RGD for Promoting Osteoblast Functions and Inhibiting Bacterial Adhesion. Biomaterials 2008, 29, 1412–1421. [Google Scholar] [CrossRef]
- Lemons, J.E. Dental Implant Biomaterials. J. Am. Dent. Assoc. 1990, 121, 716–719. [Google Scholar] [CrossRef]
- Mueller, C.K.; Solcher, P.; Peisker, A.; Mtsariashvilli, M.; Schlegel, K.A.; Hildebrand, G.; Rost, J.; Liefeith, K.; Chen, J.; Schultze-Mosgau, S. Analysis of the Influence of the Macro- and Microstructure of Dental Zirconium Implants on Osseointegration: A Minipig Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e1–e8. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Uludag, H. A Review of Nanostructured Surfaces and Materials for Dental Implants: Surface Coating, Patterning and Functionalization for Improved Performance. Biomater. Sci. 2018, 6, 1312–1338. [Google Scholar] [CrossRef]
- Kunrath, M.F.; Muradás, T.C.; Penha, N.; Campos, M.M. Innovative Surfaces and Alloys for Dental Implants: What about Biointerface-Safety Concerns? Dent. Mater. 2021, 37, 1447–1462. [Google Scholar] [CrossRef]
- Bauer, S.; Schmuki, P.; von der Mark, K.; Park, J. Engineering Biocompatible Implant Surfaces: Part I: Materials and Surfaces. Prog. Mater. Sci. 2013, 58, 261–326. [Google Scholar] [CrossRef]
- Le Guéhennec, L.; Soueidan, A.; Layrolle, P.; Amouriq, Y. Surface Treatments of Titanium Dental Implants for Rapid Osseointegration. Dent. Mater. 2007, 23, 844–854. [Google Scholar] [CrossRef]
- Hanawa, T. A Comprehensive Review of Techniques for Biofunctionalization of Titanium. J Periodontal Implant. Sci. 2011, 41, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Sartori, M.; Giavaresi, G.; Parrilli, A.; Ferrari, A.; Aldini, N.N.; Morra, M.; Cassinelli, C.; Bollati, D.; Fini, M. Collagen Type I Coating Stimulates Bone Regeneration and Osteointegration of Titanium Implants in the Osteopenic Rat. Int. Orthop. 2015, 39, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Guo, Y.; Liu, R.; Wu, S.; Fang, J.; Huang, B.; Li, Z.; Chen, Z.; Chen, Z. Tuning Surface Properties of Bone Biomaterials to Manipulate Osteoblastic Cell Adhesion and the Signaling Pathways for the Enhancement of Early Osseointegration. Colloids Surf. B Biointerfaces 2018, 164, 58–69. [Google Scholar] [CrossRef]
- Chang, Y.-C.; Ho, K.-N.; Feng, S.-W.; Huang, H.-M.; Chang, C.-H.; Lin, C.-T.; Teng, N.-C.; Pan, Y.H.; Chang, W.-J. Fibronectin-Grafted Titanium Dental Implants: An In Vivo Study. Biomed. Res. Int. 2016, 2016, 2414809. [Google Scholar] [CrossRef][Green Version]
- Sverzut, A.T.; Crippa, G.E.; Morra, M.; de Oliveira, P.T.; Beloti, M.M.; Rosa, A.L. Effects of Type I Collagen Coating on Titanium Osseointegration: Histomorphometric, Cellular and Molecular Analyses. Biomed. Mater. 2012, 7, 35007. [Google Scholar] [CrossRef]
- Marín-Pareja, N.; Salvagni, E.; Guillem-Marti, J.; Aparicio, C.; Ginebra, M.-P. Collagen-Functionalised Titanium Surfaces for Biological Sealing of Dental Implants: Effect of Immobilisation Process on Fibroblasts Response. Colloids Surf. B Biointerfaces 2014, 122, 601–610. [Google Scholar] [CrossRef]
- Heller, M.; Kumar, V.V.; Pabst, A.; Brieger, J.; Al-Nawas, B.; Kämmerer, P.W. Osseous Response on Linear and Cyclic RGD-Peptides Immobilized on Titanium Surfaces in Vitro and in Vivo. J. Biomed. Mater. Res. Part A 2018, 106, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Vines, J.B.; Lim, D.-J.; Anderson, J.M.; Jun, H.-W. Hydroxyapatite Nanoparticle Reinforced Peptide Amphiphile Nanomatrix Enhances the Osteogenic Differentiation of Mesenchymal Stem Cells by Compositional Ratios. Acta Biomater. 2012, 8, 4053–4063. [Google Scholar] [CrossRef]
- Yazdani, J.; Ahmadian, E.; Sharifi, S.; Shahi, S.; Maleki Dizaj, S. A Short View on Nanohydroxyapatite as Coating of Dental Implants. Biomed. Pharmacother. 2018, 105, 553–557. [Google Scholar] [CrossRef]
- Tapsir, Z.; Jamaludin, F.H.; Pingguan-Murphy, B.; Saidin, S. Immobilisation of Hydroxyapatite-Collagen on Polydopamine Grafted Stainless Steel 316L: Coating Adhesion and in Vitro Cells Evaluation. J. Biomater. Appl. 2017, 32, 987–995. [Google Scholar] [CrossRef]
- Lee, S.-W.; Hahn, B.-D.; Kang, T.Y.; Lee, M.-J.; Choi, J.-Y.; Kim, M.-K.; Kim, S.-G. Hydroxyapatite and Collagen Combination-Coated Dental Implants Display Better Bone Formation in the Peri-Implant Area than the Same Combination plus Bone Morphogenetic Protein-2-Coated Implants, Hydroxyapatite Only Coated Implants, and Uncoated Implants. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2014, 72, 53–60. [Google Scholar] [CrossRef]
- Rodriguez, G.M.; Bowen, J.; Grossin, D.; Ben-Nissan, B.; Stamboulis, A. Functionalisation of Ti6Al4V and Hydroxyapatite Surfaces with Combined Peptides Based on KKLPDA and E Peptides. Colloids Surf. B Biointerfaces 2017, 160, 154–160. [Google Scholar] [CrossRef]
- Townsend, L.; Williams, R.L.; Anuforom, O.; Berwick, M.R.; Halstead, F.; Hughes, E.; Stamboulis, A.; Oppenheim, B.; Gough, J.; Grover, L.; et al. Antimicrobial Peptide Coatings for Hydroxyapatite: Electrostatic and Covalent Attachment of Antimicrobial Peptides to Surfaces. J. R. Soc. Interface 2017, 14, 20160657. [Google Scholar] [CrossRef]
- Ke, D.; Vu, A.A.; Bandyopadhyay, A.; Bose, S. Compositionally Graded Doped Hydroxyapatite Coating on Titanium Using Laser and Plasma Spray Deposition for Bone Implants. Acta Biomater. 2019, 84, 414–423. [Google Scholar] [CrossRef]
- Javed, F.; Akram, Z.; Khan, J.; Zafar, M.S. Growth Factors and Guided Bone Regeneration. In Dental Implants; Woodhead Publishing Series in Biomaterials; Woodhead Publishing: Sawston, UK, 2020; pp. 133–143. ISBN 978-0-12-819586-4. [Google Scholar]
- Cao, Y.; Tan, Q.; Li, J.; Wang, J. Bone Morphogenetic Proteins 2, 6, and 9 Differentially Regulate the Osteogenic Differentiation of Immortalized Preodontoblasts. Braz. J. Med. Biol. Res. 2020, 53, e9750. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, S.; Li, M.; Zhang, J.; Bi, Y.; He, Y.; Liu, X.; Nan, G.; Su, Y.; Zhu, G.; et al. Noggin Resistance Contributes to the Potent Osteogenic Capability of BMP9 in Mesenchymal Stem Cells. J. Orthop. Res. 2013, 31, 1796–1803. [Google Scholar] [CrossRef]
- Teng, F.-Y.; Tai, I.-C.; Ho, M.-L.; Wang, J.-W.; Weng, L.W.; Wang, Y.J.; Wang, M.-W.; Tseng, C.-C. Controlled Release of BMP-2 from Titanium with Electrodeposition Modification Enhancing Critical Size Bone Formation. Mater. Sci. Eng. C 2019, 105, 109879. [Google Scholar] [CrossRef] [PubMed]
- Niino, M.; Maeda, S. Recent Development Status of Functionally Gradient Materials. ISIJ Int. 1990, 30, 699–703. [Google Scholar] [CrossRef]
- Sadollah, A.; Bahreininejad, A. Optimum Gradient Material for a Functionally Graded Dental Implant Using Metaheuristic Algorithms. J. Mech. Behav. Biomed. Mater. 2011, 4, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Khan, T.; Guo, H.; Shi, S.; Zhong, W.; Zhang, W. Functionally Graded Materials: An Overview of Stability, Buckling, and Free Vibration Analysis. Adv. Mater. Sci. Eng. 2019, 2019, 1354150. [Google Scholar] [CrossRef]
- Rupp, F.; Liang, L.; Geis-Gerstorfer, J.; Scheideler, L.; Hüttig, F. Surface Characteristics of Dental Implants: A Review. Dent. Mater. 2018, 34, 40–57. [Google Scholar] [CrossRef]
- Glied, A.; Mundiya, J. Implant Material Sciences. Dent. Clin. N. Am. 2021, 65, 81–88. [Google Scholar] [CrossRef]
| Nanostructure | Material | Method | Application | Ref. |
|---|---|---|---|---|
| Nanotubes | TiO2 | Anodization | Experimental optimization | [79] |
| TiO2/nano Brushite | Hydrothermal treatment/Anodization | Implant material/Bone regeneration | [80] | |
| Silicate nanoparticle | TiO2 | Acid etching/Electrospray deposition | Orthopedic and dental implants | [81] |
| Nanotubes/ Porous | Calcium phosphate-Sr-Si/TiO2 | 3D printing/ Anodization | Orthopedic and dental implants | [82] |
| Nanoparticles | Silver nanoparticles | Electrodeposition | Antibacterial property/Implant material | [83] |
| Nanowires | Zn-Ti | Acid etching/ Chemical treatment | Biocompatibility and antibacterial activity/ Implant material | [84] |
| Nanowire/coating | Na2Ti3O7/SrTiO3 | Chemical treatment | Implant material | [85] |
| Nanofibers | Keratin/Ti | Mechanical treatment | Peri-implantitis/ Dental implants | [86] |
| Nanopores | TiO2 | Chemical and electrochemical treatment | Biological integration/Dental implants | [87] |
| Nanotubes | TiO2/Hydroxyapatite/Chitosan | Electrochemical treatment | Dental implants | [88] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, R.C.S.; Agrelli, A.; Andrade, A.N.; Mendes-Marques, C.L.; Arruda, I.R.S.; Santos, L.R.L.; Vasconcelos, N.F.; Machado, G. Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections. Materials 2022, 15, 3150. https://doi.org/10.3390/ma15093150
Silva RCS, Agrelli A, Andrade AN, Mendes-Marques CL, Arruda IRS, Santos LRL, Vasconcelos NF, Machado G. Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections. Materials. 2022; 15(9):3150. https://doi.org/10.3390/ma15093150
Chicago/Turabian StyleSilva, Rayane C. S., Almerinda Agrelli, Audrey N. Andrade, Carina L. Mendes-Marques, Isabel R. S. Arruda, Luzia R. L. Santos, Niedja F. Vasconcelos, and Giovanna Machado. 2022. "Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections" Materials 15, no. 9: 3150. https://doi.org/10.3390/ma15093150
APA StyleSilva, R. C. S., Agrelli, A., Andrade, A. N., Mendes-Marques, C. L., Arruda, I. R. S., Santos, L. R. L., Vasconcelos, N. F., & Machado, G. (2022). Titanium Dental Implants: An Overview of Applied Nanobiotechnology to Improve Biocompatibility and Prevent Infections. Materials, 15(9), 3150. https://doi.org/10.3390/ma15093150








