A Critical Review of the Design, Manufacture, and Evaluation of Bone Joint Replacements for Bone Repair
Abstract
:1. Introduction
2. Review of the Design of the Bone Joint Replacement
| Development Stage | Representative Structure | Representative Example | References | |
|---|---|---|---|---|
| Early stage (approximately before 2002) | Typical structure | 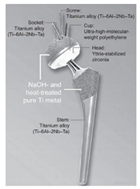 | [12] | |
| Nowadays (approximately 2002 to present) | Porous materials | Internal cellular structure |  | [16] |
| Internal TPMS structure |  | [17] | ||
| Functionally graded implant | Functionally graded material |  | [9] | |
| Functionally graded microstructure |  | [8] | ||
3. Review of the Manufacturing of the Bone Joint Replacement
4. Review of the Evaluation of the Performance of the Bone Joint Replacement
5. Conclusions and Future Perspectives
- (1)
- The use of the machine learning in the optimization of the joint replacement.
- (2)
- The use of advanced measurement techniques to validate the computational models and to evaluate the performance of the joint replacement.
- (3)
- The consideration of the long-term dynamic behavior of the joint replacement and the surrounding environment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferguson, R.J.; Palmer, A.J.; Taylor, A.; Porter, M.L.; Malchau, H.; Glyn-Jones, S. Hip replacement. Lancet 2018, 392, 1662–1671. [Google Scholar] [CrossRef]
- Pivec, R.; Johnson, A.J.; Mears, S.C.; Mont, M.A. Hip arthroplasty. Lancet 2012, 380, 1768–1777. [Google Scholar] [CrossRef]
- Matsoukas, G.; Willing, R.; Kim, I.Y. Total hip wear assessment: A comparison between computational and in vitro wear assessment techniques using ISO 14242 loading and kinematics. J. Biomech. Eng. 2009, 131, 41011–41022. [Google Scholar] [CrossRef] [PubMed]
- Barrack, R.L. Dislocation after total hip arthroplasty: Implant design and orientation. Jaaos-J. Am. Acad. Orthop. Surg. 2003, 11, 89–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Xu, S.; Zhou, S.; Xu, W.; Leary, M.; Choong, P.; Xie, Y. Topological design and additive manufacturing of porous metals for bone scaffolds and orthopaedic implants: A review. Biomaterials 2016, 83, 127–141. [Google Scholar] [CrossRef]
- Ścigała, K.; Będziński, R.; Filipiak, J.; Chlebus, E.; Dybała, B. Application of generative technologies in the design of reduced stiffness stems of hip joint endoprosthesis. Arch. Civ. Mech. Eng. 2011, 11, 753–767. [Google Scholar] [CrossRef]
- Shaik, S.A.; Bose, K.; Cherukuri, H.P. A study of durability of hip implants. Mater. Des. 2012, 42, 230–237. [Google Scholar] [CrossRef]
- Kladovasilakis, N.; Tsongas, K.; Tzetzis, D. Finite element analysis of orthopedic hip implant with functionally graded bioinspired lattice structures. Biomimetics 2020, 5, 44. [Google Scholar] [CrossRef] [PubMed]
- Abdellah, A.M.; Fischer, J.; Yadav, R.; Khandaker, M. Minimizing stress shielding and cement damage in cemented femoral component of a hip prosthesis through computational design optimization. Adv. Orthop. 2017, 2017, 8437956. [Google Scholar] [CrossRef] [Green Version]
- Nowak, M.; Sokołowski, J.; Żochowski, A. New aspects of the trabecular bone remodeling regulatory model resulting from the shape optimization studies. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2020, 234, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Nowak, M.; Boguszewski, A. Topology optimization without volume constraint–the new paradigm for lightweight design. Bull. Pol. Acad. Sci. Tech. Sci. 2021, 69. [Google Scholar]
- Kokubo, T.; Yamaguchi, S. Biomineralization of metals using chemical and heat treatments. Biominer. Biomater. Woodhead Publ. 2016, 339–364. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, K.; Yu, Z. Porous Structure Design in Tissue Engineering Using Anisotropic Radial Basis Functions. In International Symposium on Visual Computing; Springer: Cham, Switzerland, 2018; Volume 11241, pp. 79–90. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.M. Bioinspired Design of Materials. Surf. Biol. Des. Mater. 2019, 27–97. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Abu Al-Rub, R.K. Multifunctional mechanical metamaterials based on triply periodic minimal surface lattices. Adv. Eng. Mater. 2019, 21, 1900524. [Google Scholar] [CrossRef]
- Kolken, H.M.A.; de Jonge, C.P.; van der Sloten, T.; Garcia, A.F.; Pouran, B.; Willemsen, K.; Zadpoor, A.A. Additively manufactured space-filling meta-implants. Acta Biomater. 2021, 125, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Bruno, J.; Brailovski, V.; Simoneau, C.; Dumas, M.; Terriault, P. Development and in vitro validation of a simplified numerical model for the design of a biomimetic femoral stem. J. Mech. Behav. Biomed. Mater. 2018, 77, 539–550. [Google Scholar] [CrossRef]
- Zhong, Z. Progress in the study of mechanics problems of functionally graded materials and structures. Prog. Mech. 2010, 40, 528–541. [Google Scholar] [CrossRef]
- Lin, D.; Li, Q.; Li, W.; Zhou, S.; Swain, M.V. Design optimization of functionally graded dental implant for bone remodeling. Compos. Part B Eng. 2009, 40, 668–675. [Google Scholar] [CrossRef]
- Singh, G.; Marwaha, A. A review of metamaterials and its applications. IJETT 2015, 19, 305–310. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, H. A review on auxetic structures and polymeric materials. Sci. Res. Essays 2010, 5, 1052–1063. [Google Scholar] [CrossRef]
- Hou, X.; Silberschmidt, V.V. Metamaterials with negative poisson’s ratio: A review of mechanical properties and deformation mechanisms. Mech. Adv. Mater. 2015, 155–179. [Google Scholar] [CrossRef]
- Kolken, H.M.A.; Garcia, A.F.; Du Plessis, A.; Rans, C.; Mirzaali, M.J.; Zadpoor, A.A. Fatigue performance of auxetic meta-biomaterials. Acta Biomater. 2021, 126, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, L.; Li, J.; Tian, S.; Zhang, M.; Fan, Y. A novel auxetic structure based bone screw design: Tensile mechanical characterization and pullout fixation strength evaluation. Mater. Des. 2020, 188, 108424. [Google Scholar] [CrossRef]
- Bucher, I.; Ewins, D.J. Modal analysis and testing of rotating structures. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Eng. Sci. 2001, 359, 61–96. [Google Scholar] [CrossRef]
- Gu, Y. Meshfree methods and their comparisons. Int. J. Comput. Methods 2005, 2, 477–515. [Google Scholar] [CrossRef]
- Liu, G.R.; Zhang, G.Y.; Gu, Y.; Wang, Y.Y. A meshfree radial point interpolation method (RPIM) for three-dimensional solids. Comput. Mech. 2005, 36, 421–430. [Google Scholar] [CrossRef]
- Liu, G.R. Meshfree Methods: Moving Beyond the Finite Element Method; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar] [CrossRef]
- Kononenko, I. Machine learning for medical diagnosis: History, state of the art and perspective. Artif. Intell. Med. 2001, 23, 89–109. [Google Scholar] [CrossRef] [Green Version]
- Cilla, M.; Checa, S.; Preininger, B.; Winkler, T.; Perka, C.; Duda, G.N.; Pumberger, M. Femoral head necrosis: A finite element analysis of common and novel surgical techniques. Clin. Biomech. 2017, 48, 49–56. [Google Scholar] [CrossRef]
- Hussin, M.S.; Fernandez, J.; Ramezani, M.; Kumar, P.; Kelly, P.A. Analytical and computational sliding wear prediction in a novel knee implant: A case study. Comput. Methods Biomech. Biomed. Eng. 2020, 23, 143–154. [Google Scholar] [CrossRef]
- Belinha, J. Dinis, L.M.D.J.S.; Jorge, R.N. The analysis of the bone remodelling around femoral stems: A meshless approach. Math. Comput. Simul. 2016, 121, 64–94. [Google Scholar] [CrossRef]
- Cilla, M.; Borgiani, E.; Martínez, J.; Duda, G.N.; Checa, S. Machine learning techniques for the optimization of joint replacements: Application to a short-stem hip implant. PLoS ONE 2017, 12, e0183755. [Google Scholar] [CrossRef] [Green Version]
- Bhaskar, B.; Arun, S.; Sreekanth, P.R.; Kanagaraj, S. Biomaterials in Total Hip Joint Replacements: The Evolution of Basic Concepts, Trends, and Current Limitations—A Review. Trends Biomater. 2016, 2, 175–210. [Google Scholar]
- Woesz, A.; Rumpler, M.; Stampfl, J.; Varga, F.; Fratzl-Zelman, N.; Roschger, P.; Fratzl, P. Towards bone replacement materials from calcium phosphates via rapid prototyping and ceramic gelcasting. Mater. Sci. Eng. C 2005, 25, 181–186. [Google Scholar] [CrossRef]
- Ruyu, M.; Wendong, X.; Dongmei, W.; Kerong, D.; Chengtao, W. Design and manufacture of custom hip prostheses based on standard X-ray films. Int. J. Adv. Manuf. Technol. 2005, 27, 70–74. [Google Scholar] [CrossRef]
- Marcin, K.; Wach, T.; Szymor, P.; Zieliński, R. Two different techniques of manufacturing TMJ replacements–a technical report. J. Cranio-Maxillofac. Surg. 2017, 45, 1432–1437. [Google Scholar] [CrossRef]
- Bidyut, P.; Gupta, S. The Relevance of Biomechanical Analysis in Joint Replacements: A Review. J. Inst. Eng. Ser. C 2020, 101, 913–927. [Google Scholar] [CrossRef]
- Wauthle, R.; Van Der Stok, J.; Yavari S, A.; Van Humbeeck, J.; Kruth, J.P.; Zadpoor, A.A.; Schrooten, J. Additively manufactured porous tantalum implants. Acta Biomater. 2015, 14, 217–225. [Google Scholar] [CrossRef]
- Ataee, A.; Li, Y.; Fraser, D.; Song, G.; Wen, C. Anisotropic Ti-6Al-4V gyroid scaffolds manufactured by electron beam melting (EBM) for bone implant applications. Mater. Des. 2018, 137, 345–354. [Google Scholar] [CrossRef]
- Duan, B.; Wang, M.; Zhou, W.; Cheung, W.L.; Li, Z.; Lu, W. Three-dimensional nanocomposite scaffolds fabricated via selective laser sintering for bone tissue engineering. Acta Biomater. 2010, 6, 4495–4505. [Google Scholar] [CrossRef]
- Arabnejad, S.; Johnston, R.B.; Pura, J.A.; Singh, B.; Tanzer, M.; Pasini, D. High-strength porous biomaterials for bone replacement: A strategy to assess the interplay between cell morphology, mechanical properties, bone ingrowth and manufacturing constraints. Acta Biomater. 2016, 30, 345–356. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Cheng, G.; Xu, L. Topology optimization considering overhang constraint in additive manufacturing. Comput. Struct. 2019, 212, 86–100. [Google Scholar] [CrossRef]
- Navacchia, A.; Hume, D.R.; Rullkoetter, P.J.; Shelburne, K.B. A computationally efficient strategy to estimate muscle forces in a finite element musculoskeletal model of the lower limb. J. Biomech. 2019, 84, 94–102. [Google Scholar] [CrossRef]
- Kebbach, M.; Grawe, R.; Geier, A.; Winter, E.; Bergschmidt, P.; Kluess, D.; Bader, R. Effect of surgical parameters on the biomechanical behaviour of bicondylar total knee endoprostheses–A robot-assisted test method based on a musculoskeletal model. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Shu, L.; Yamamoto, K.; Yao, J.; Saraswat, P.; Liu, Y.; Mitsuishi, M.; Sugita, N. A subject-specific finite element musculoskeletal framework for mechanics analysis of a total knee replacement. J. Biomech. 2018, 77, 146–154. [Google Scholar] [CrossRef]
- Li, J.; Lu, Y.; Miller, S.C.; Jin, Z.; Hua, X. Development of a finite element musculoskeletal model with the ability to predict contractions of three-dimensional muscles. J. Biomech. 2019, 94, 230–234. [Google Scholar] [CrossRef]
- Shriram, D.; Kumar, G.P.; Cui, F.; Lee, Y.H.D.; Subburaj, K. Evaluating the effects of material properties of artificial meniscal implant in the human knee joint using finite element analysis. Sci. Rep. 2017, 7, 6011. [Google Scholar] [CrossRef]
- Beidokhti, H.N.; Janssen, D.; van de Groes, S.; Hazrati, J.; Van den Boogaard, T.; Verdonschot, N. The influence of ligament modelling strategies on the predictive capability of finite element models of the human knee joint. J. Biomech. 2017, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, G.; Kim, I.Y. Design optimization of a total hip prosthesis for wear reduction. J. Biomech. Eng. 2009, 131, 051003–051015. [Google Scholar] [CrossRef] [PubMed]
- Katz, Y.; Lubovsky, O.; Yosibash, Z. Patient-specific finite element analysis of femurs with cemented hip implants. Clin. Biomech. 2018, 58, 74–89. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Zhang, D.; Arola, D.D. Fatigue of the bone/cement interface and loosening of total joint replacements. Int. J. Fatigue 2010, 32, 1639–1649. [Google Scholar] [CrossRef]
- Zohar, Y.; Katz, A.; Milgrom, C. Toward verified and validated FE simulations of a femur with a cemented hip prosthesis. Med Eng. Phys. 2013, 35, 978–987. [Google Scholar] [CrossRef]
- Li, G.; Wang, L.; Pan, W.; Yang, F.; Jiang, W.; Wu, X.; Kong, X.; Dai, K.; Hao, Y. In vitro and in vivo study of additive manufactured porous Ti6Al4V scaffolds for repairing bone defects. Sci. Rep. 2016, 6, 34072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taniguchi, N.; Fujibayashi, S.; Takemoto, M.; Sasaki, K.; Otsuki, B.; Nakamura, T.; Matsuda, S. Effect of pore size on bone ingrowth into porous titanium implants fabricated by additive manufacturing: An in vivo experiment. Mater. Sci. Eng. C 2016, 59, 690–701. [Google Scholar] [CrossRef] [Green Version]
- Ackland, D.C.; Robinson, D.; Redhead, M.; Lee, P.V.S.; Moskaljuk, A.; Dimitroulis, G. A personalized 3D-printed prosthetic joint replacement for the human temporomandibular joint: From implant design to implantation. J. Mech. Behav. Biomed. Mater. 2017, 69, 404–411. [Google Scholar] [CrossRef]
- Wegener, B.; Sichler, A.; Milz, S.; Sprecher, C.; Pieper, K.; Hermanns, W.; Quadbeck, P. Development of a novel biodegradable porous iron-based implant for bone replacement. Sci. Rep. 2020, 10, 9141. [Google Scholar] [CrossRef] [PubMed]
- Raina, D.B.; Larsson, D.; Sezgin, E.A.; Isaksson, H.; Tägil, M.; Lidgren, L. Biomodulation of an implant for enhanced bone-implant anchorage. Acta Biomater. 2019, 96, 619–630. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H.; Yang, Q.; Wang, S.; Wang, J.; Ma, J.; Zhang, S. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials 2017, 137, 37–48. [Google Scholar] [CrossRef]
- Warnke, P.H.; Springer, I.N.G.; Wiltfang, J.; Acil, Y.; Eufinger, H.; Wehmöller, M.; Terheyden, H. Growth and transplantation of a custom vascularised bone graft in a man. Lancet 2004, 364, 766–770. [Google Scholar] [CrossRef]
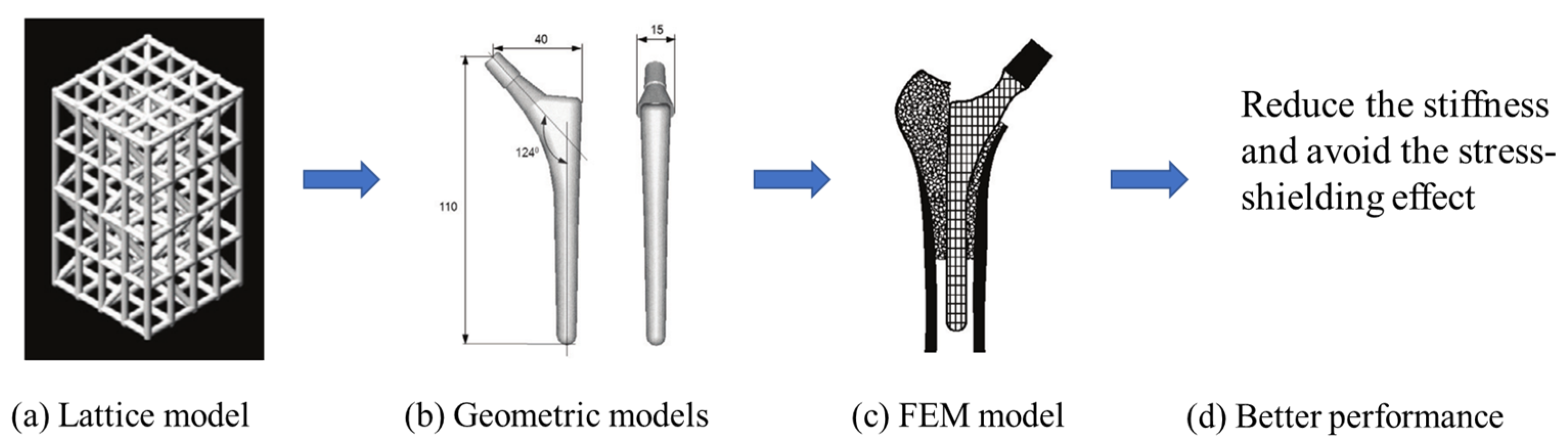
| Representative Study | Design Objective | Design Variable | Design Constraints | References |
|---|---|---|---|---|
 | Minimize the stiffness of hip endoprosthesis | The diameter of the internal structure of the stem of the hip joint prosthesis | The thickness of the structure manufactured by AM no less than 0.2 mm due to the manufacturing technique | [6] |
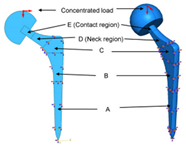 | Maximize the durability of the implant | Femoral ball sizes | The natural size of the femoral ball usually ranges from 40 to 54 mm | [7] |
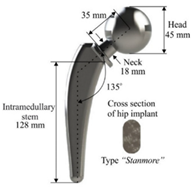 | Minimize the weight | The head diameter, the diameter, and length of the neck | The relative density should be 50% due to the femur bone | [8] |
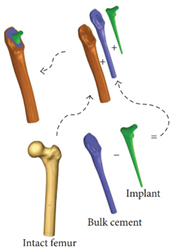 | Minimize stress shielding | Shapes and sizes of the cross sections | The accuracy of the computational model needs to be verified | [9] |
| Type of Auxetic Structures | Application in Bone Implants | Advantages and Disadvantages | References |
|---|---|---|---|
| Re-entrant | Bone-implant contact; medical screw | Good NPR effect; longer fatigue life | [22,24] |
| Chiral | Bone scaffold; medical screw | High fracture toughness; limited by chirality | [24] |
| Rotating | Auxetic materials fabrication; medical screw | Better auxetic performance; low stability | [24] |
| Optimization Method | Advantage and Disadvantage | Representative Study | References | |
|---|---|---|---|---|
| Finite element method | Geometrical model | Further in vivo tests are needed |  | [30] |
| Computational model | The model is verified by in vivo testing | 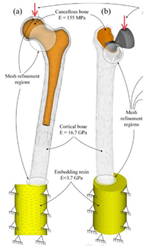 | [17] | |
| Meshless method | More accurate; difficult to establish a model | 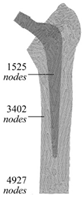 | [32] | |
| Machine learning techniques | Increase of diagnostic accuracy; not mature enough | 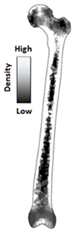 | [33] | |
| Manufacturing Method | Representative Example | Advantages and Disadvantages | References | |
|---|---|---|---|---|
| Traditional manufacturing techniques | Rapid prototyping (RP) |  | Advantages: Relative high precision; Disadvantages: The processing route is not easy to control; the cost is high | [35,37] |
| Computer Numerical Control (CNC) |  | |||
| Additive manufacturing techniques | Selective laser melting |  | Advantages: Easy to Relative high precisionincorporate multiple materials, no support structure; Relative high precisionDisadvantages: Relatively poor mechanical properties | [39,40,41] |
| Electron beam melting |  | |||
| Selective laser sintering |  | |||
| Type of Method | Advantages and Disadvantages | Representative Example | References |
|---|---|---|---|
| Musculoskeletal model | Able to simulate the activities of the human body; wear mechanism was not considered | 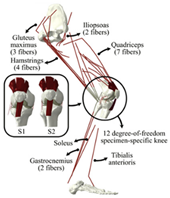 | [44] |
| Combines the advantages of numerical and experimental methods | 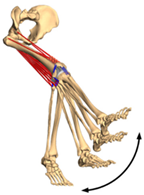 | [45] | |
| Coupled musculoskeletal-FE model | Reflects the stress state more comprehensively; lack of in vivo measurements |  | [46] |
| Deformable contact models of the hip are considered; the accuracy can be improved |  | [47] | |
| FE model | The mechanical information is comprehensive; difficult to develop the model | 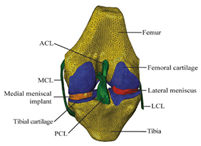 | [48] |
| The effect of simulation prediction is good | 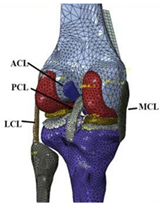 | [49] |
| Type of Method | Performance to Be Evaluated | Representative Study | References |
|---|---|---|---|
| Uniaxial tensile test | Strains on bones’ and prosthesis’ surfaces |  | [51] |
| Static mechanical test | Stress shielding |  | [53] |
| Laxity Test | The stress on the bone |  | [49] |
| Fatigue test | The fatigue strength of the bone interface | 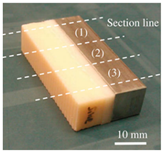 | [52] |
| Type of Method | Subjects to Be Used | Performance to Be Evaluated | Conclusion of Study | References |
|---|---|---|---|---|
| Animal testing | Goat metatarsus | Implant stability | The implant was identified as achieving favorable implant stability | [54] |
| Tibia of rats | Prosthesis stress; implant stability | Implants with a pore size of 600 µm showed higher fixation ability than those with a pore size of 300 µm | [55] | |
| Tibia of rats | Release of osteopromotive molecules | Local controlled delivery of ZA alone can enhance bone implant anchorage | [58] | |
| Tibia of merino sheep | The rate of degradation; prosthesis stress | Iron-based porous materials can be candidates for the development of self-degrading bone replacement materials | [57] | |
| Knee of rats | The cytocompatibility | The multilayer scaffold could induce osteochondral repair | [59] | |
| Clinical trial | Human temporomandibular joint | Prosthesis stress | The new implant has improved clinical and biomechanical joint function compared to the stock device | [56] |
| Human mandibular | Bone formation | It is possible to form a mandibular replacement inside the latissimus dorsi muscle in a human being | [60] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huo, Y.; Lyu, Y.; Bosiakov, S.; Han, F. A Critical Review of the Design, Manufacture, and Evaluation of Bone Joint Replacements for Bone Repair. Materials 2022, 15, 153. https://doi.org/10.3390/ma15010153
Huo Y, Lyu Y, Bosiakov S, Han F. A Critical Review of the Design, Manufacture, and Evaluation of Bone Joint Replacements for Bone Repair. Materials. 2022; 15(1):153. https://doi.org/10.3390/ma15010153
Chicago/Turabian StyleHuo, Yi, Yongtao Lyu, Sergei Bosiakov, and Feng Han. 2022. "A Critical Review of the Design, Manufacture, and Evaluation of Bone Joint Replacements for Bone Repair" Materials 15, no. 1: 153. https://doi.org/10.3390/ma15010153
APA StyleHuo, Y., Lyu, Y., Bosiakov, S., & Han, F. (2022). A Critical Review of the Design, Manufacture, and Evaluation of Bone Joint Replacements for Bone Repair. Materials, 15(1), 153. https://doi.org/10.3390/ma15010153







