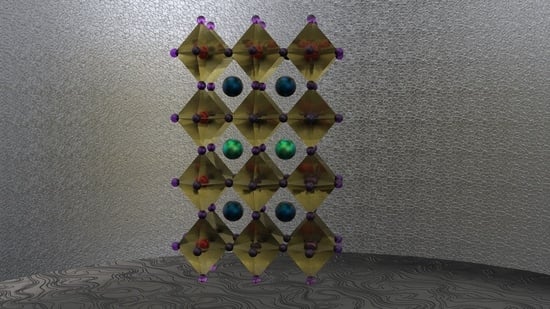
Layered Oxygen-Deficient Double Perovskites as Promising Cathode Materials for Solid Oxide Fuel Cells
Abstract
1. Introduction
2. Cathode Materials for IT–SOFCs: Past, Present, and Future
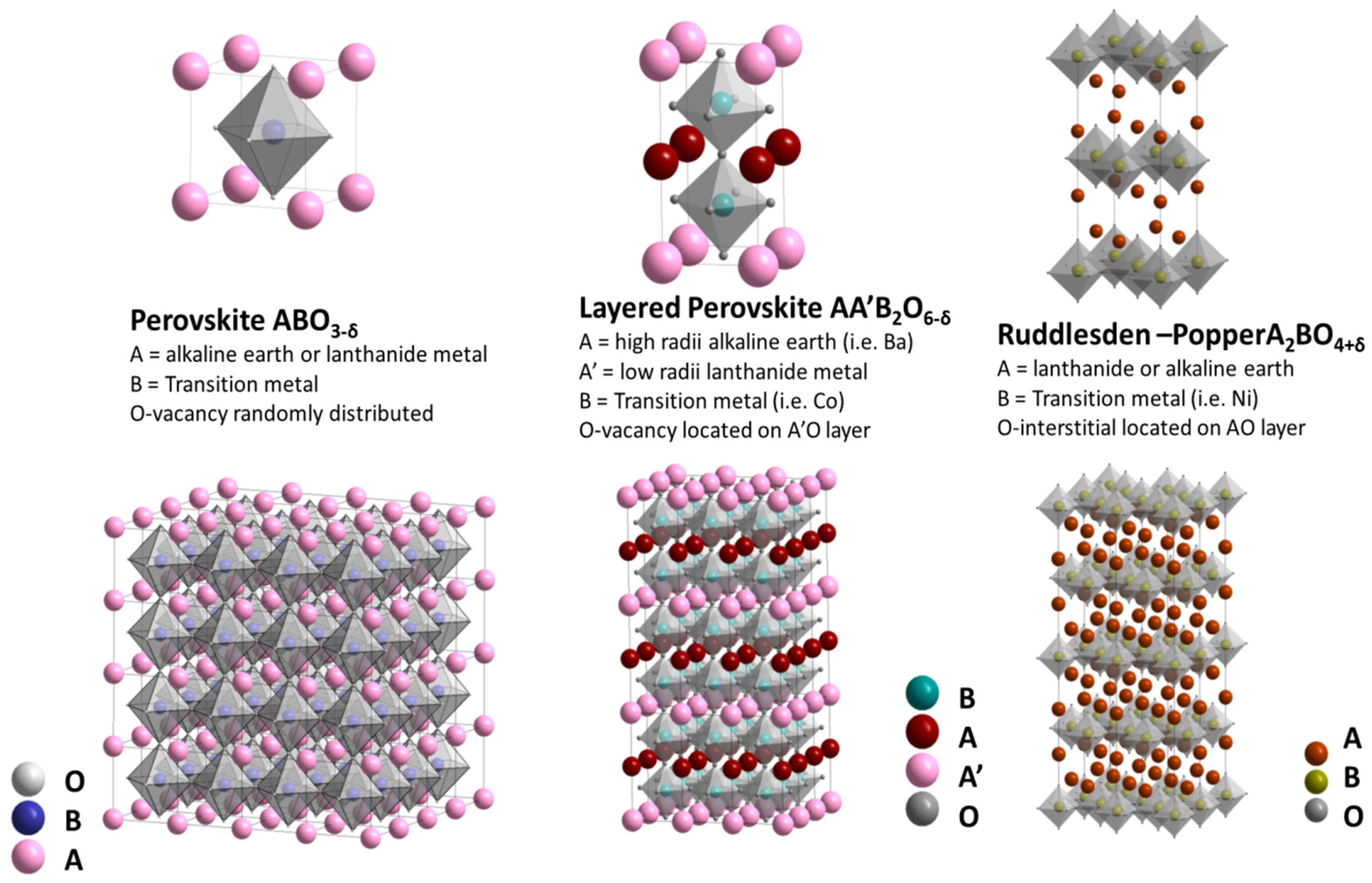
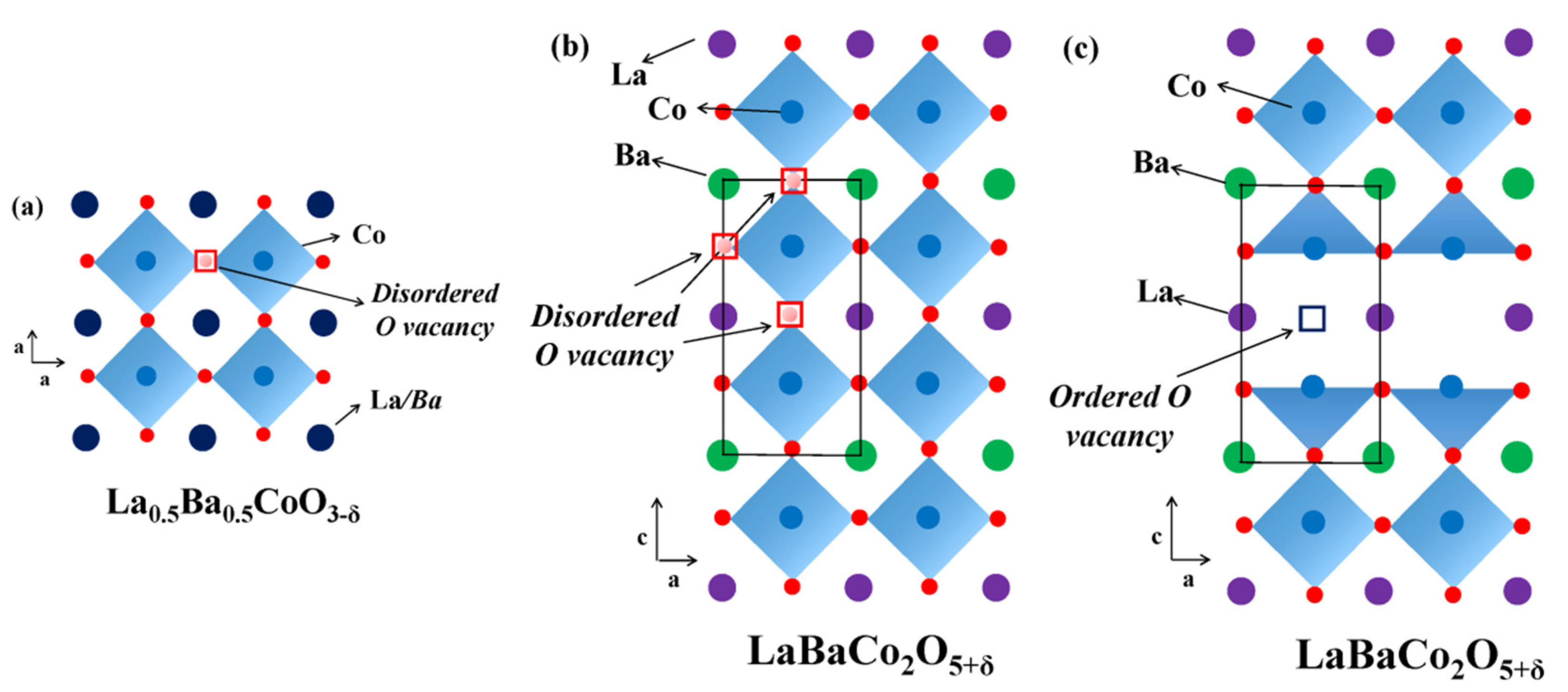
3. Crystal Structure, Phase Transitions, and Physicochemical and Functional Properties of Layered Oxygen-Deficient Double Perovskites
4. Electrochemical Performance of Layered Oxygen-Deficient Perovskites
4.1. Layered Cobaltites of REEs and Barium
4.2. A-Site-Deficient and A-Site-Substituted LnBaCo2O5+δ Layered Perovskites
4.3. B-Site Deficient and B-Site Substituted LnBaCo2O5+δ Layered Perovskites
4.4. Composites Based on LnBaCo2O5+δ Layered Perovskites
4.5. LnBaMe’Me”O5+δ Layered Perovskites and Their Solid Solutions
4.6. The Other Layered Oxygen-Deficient Double Perovskites
4.7. Short Resume
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lenser, C.; Udomsilp, D.; Menzler, N.H.; Holtappels, P.; Fujisaki, L.; Matsumoto, H.; Sabato, A.G.; Smeacetto, F.; Chrysanthou, A.; Molin, S. Solid oxide fuel and electrolysis cells. In Advanced Ceramics for Energy Conversion and Storage; Guillon, O., Ed.; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2019; pp. 387–547. [Google Scholar]
- Lyu, Y.; Xie, J.; Wang, D.; Wang, J. Review of cell performance in solid oxide fuel cells. J. Mater. Sci. 2020, 55, 7184–7207. [Google Scholar] [CrossRef]
- Filippov, S.P.; Yaroslavtsev, A.B. Hydrogen energy: Development prospects and materials. Russ. Chem. Rev. 2021, 90, 627–643. [Google Scholar] [CrossRef]
- Istomin, S.Y.; Lyskov, N.V.; Mazo, G.N.; Antipov, E.V. Electrode materials based on complex d-metal oxides for symmetrical solid oxide fuel cells. Russ. Chem. Rev. 2021, 90, 644–676. [Google Scholar] [CrossRef]
- Kharton, V.V.; Marques, F.M.B.; Atkinson, A. Transport properties of solid oxide electrolyte ceramics: A brief review. Solid State Ion. 2004, 174, 135–149. [Google Scholar] [CrossRef]
- Jacobson, A.J. Materials for Solid Oxide Fuel Cells. Chem. Mater. 2010, 22, 660–674. [Google Scholar] [CrossRef]
- Irshad, M.; Siraj, K.; Raza, R.; Ali, A.; Tiwari, P.; Zhu, B.; Rafique, A.; Ali, A.; Ullah, M.K.; Usman, A. A Brief Desription of High Temperature Solid Oxide Fuel Cell’s Operation, Materials, Design, Fabrication Technologies and Performance. Appl. Sci. 2016, 6, 75. [Google Scholar] [CrossRef]
- da Silva, F.S.; de Souza, T.M. Novel materials for solid oxide fuel cell technologies: A literature review. Int. J. Hydrogen Energy 2017, 42, 26020–26036. [Google Scholar] [CrossRef]
- Hussain, S.; Yangping, L. Review of solid oxide fuel cell materials: Cathode, anode, and electrolyte. Energy Transit. 2020, 4, 113–126. [Google Scholar] [CrossRef]
- Vostakola, M.F.; Horri, B.A. Progress in Materials Development for Low-Temperature Solid Oxide Fuel Cells: A Review. Energies 2021, 14, 1280. [Google Scholar] [CrossRef]
- Dudek, M.; Lis, B.; Lach, R.; Daugela, S.; Šalkus, T.; Kežionis, A.; Mosiałek, M.; Socha, R.; Morgiel, J.; Gajek, M.; et al. Ba0.95Ca0.05Ce0.9Y0.1O3 as electrolyte for proton-conducting ceramic fuel cells. Electrochim. Acta 2019, 304, 70–79. [Google Scholar] [CrossRef]
- Molenda, J.; Kupecki, J.; Baron, R.; Blesznowski, M.; Brus, G.; Brylewski, T.; Busko, M.; Chmielowec, J.; Cwieka, K.; Gazda, M.; et al. Status report on high temperature fuel cells in Poland—Recent advances and achievements. Int. J. Hydrogen Energy 2017, 42, 4366–4403. [Google Scholar] [CrossRef]
- Plazaola, A.A.; Labella, A.C.; Liu, Y.; Porras, N.B.; Tanaka, D.A.P.; Annaland, M.V.S.; Gallucci, F. Mixed Ionic–Electronic Conducting Membranes (MIEC) for Their Application in Membrane Reactors: A Review. Processes 2019, 7, 128. [Google Scholar] [CrossRef]
- Khan, M.S.; Xu, X.; Knibbe, R.; Zhu, Z. Air electrodes and related degradation mechanisms in solid oxide electrolysis and reversible solid oxide fuel cells. Renew. Sust. Energy Rev. 2021, 143, 110918. [Google Scholar] [CrossRef]
- Wang, R.-T.; Chang, H.-Y.; Wang, J.-C. An Overview on the Novel Core-Shell Electrodes for solid Oxide Fuel Cell (SOFC) Using Polymeric Methodology. Polymers 2021, 13, 2774. [Google Scholar] [CrossRef]
- Lu, Y.; Mi, Y.; Li, J.; Qi, F.; Yan, S.; Dong, W. Recent Progress in Semiconductor-Ionic Conductor Nanomaterial as a Membrane for Low-Temperature Solid Oxide Fuel Cells. Nanomaterials 2021, 11, 2290. [Google Scholar] [CrossRef]
- Kalinina, E.; Pikalova, E. Opportunities, Challenges and Prospects for Electrodeposition of Thin-Film Functional Layers in Solid Oxide Fuel Cell Technology. Materials 2021, 14, 5584. [Google Scholar] [CrossRef]
- Liu, J.; Ma, J.; Zhang, Z.; Qin, Y.; Wang, Y.-J.; Wang, Y.; Tan, R.; Duan, X.; Tian, T.Z.; Zhang, C.H.; et al. 2021 Roadmap: Electrocatalysis for green catalytic processes. J. Phys. Mater. 2021, 4, 022004. [Google Scholar] [CrossRef]
- Chen, G.; Feldhoff, A.; Weidenkaff, A.; Li, C.; Liu, S.; Zhu, X.; Sunarso, J.; Huang, K.; Wu, X.-Y.; Ghoniem, A.F.; et al. Roadmap ob Sustaninable Mixed Ionic-Electronic Conducting Membranes. Adv. Funct. Mater. 2021, 31, 2105702. [Google Scholar] [CrossRef]
- Kim, S.; Kim, G.; Manthiram, A. A review on infiltration technoques for energy conversion and storage devices: From fundamentals fo applications. Sust. Energy Fuels 2021, 5, 5024–5037. [Google Scholar] [CrossRef]
- Mendonça, C.; Ferreira, A.; Santos, D.M.F. Towards the Commercialization of Solid Oxide Fuel Cells: Recent Advances in Materials and Integration Strategies. Fuels 2021, 2, 393–419. [Google Scholar] [CrossRef]
- Hanif, M.B.; Rauf, S.; Motola, M.; Babar, Z.U.D.; Li, C.-J. Recent progress of perovskite-based electrolyte materials for solid oxide fuel cells and performance optimizing strategies for energy storage applications. Mat. Res. Bull. 2022, 146, 111612. [Google Scholar] [CrossRef]
- Cigolotti, V.; Genovese, M.; Fragiacomo, P. Comprehensive Review on Fuel Cell Technology for Stationary Applications as Sustainable and Efficient Poly-Generation Energy Systems. Energies 2021, 14, 4963. [Google Scholar] [CrossRef]
- Skutina, L.S.; Vylkov, A.A.; Kuznetsov, D.K.; Medvedev, D.A.; Shur, Y. Tailoring Ni and Sr2Mg0.25Ni0.75MoO6–δ Cermet Compositions for Designing the Fuel Electrodes of Solid Oxide Electrochemical Cells. Energies 2019, 12, 2394. [Google Scholar] [CrossRef]
- Skutina, L.; Filonova, E.; Medvedev, D.; Maignan, A. Undoped Sr2MMoO6 Double Perovskite Molybdates (M = Ni, Mg, Fe) as Promising Anode Materials for Solid Oxide Fuel Cells. Materials 2021, 14, 1715. [Google Scholar] [CrossRef] [PubMed]
- Klyndyuk, A.I. Layered Perovskite–Like Oxides 0112 Type: Structure, Properties, and Possible Applications. In Advanced in Chemistry Research; Taylor, J.C., Ed.; Nova Science Publishers: New York, NY, USA, 2010; pp. 59–105. [Google Scholar]
- Sun, C.; Hui, R.; Roller, J. Cathode materials for solid oxide fuel cells: A review. J. Solid State Electrochem. 2010, 14, 1125–1144. [Google Scholar] [CrossRef]
- Tahir, N.N.M.; Baharuddin, N.A.; Samat, A.A.; Osman, N.; Somalu, M.R. A review on cahtode materials for conventional and proton-conducting solid oxide fuel cells. J. Alloys Compd. 2021, 894, 162458. [Google Scholar] [CrossRef]
- Khan, M.Z.; Song, R.-H.; Mehran, M.T.; Lee, S.-B.; Lim, T.-H. Controlling cation migration and inter-diffusion across cathode/interlayer/electrolyte interfaces of solid oxide fuel cells: A review. Ceram. Int. 2021, 47, 5839–5869. [Google Scholar] [CrossRef]
- Wang, F.; Kishimoto, H.; Ishihara, T.; Develos-Bagarinao, K.; Yamaji, K.; Horita, T.; Yokokawa, H. A review of sulfur poisoning of solid oxide fuel cell cathode materials for solid oxide fuel cells. J. Power Source 2020, 478, 228763. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, P. Progress and challenges of cathode contact layer for solid oxide fuel cell. Int. J. Hydrogen Energy 2020, 45, 33876–33894. [Google Scholar] [CrossRef]
- Aziz, A.J.A.; Baharuddin, N.A.; Somalu, M.R.; Muchtar, A. Review of composite cathodes for intermediate-temperature solid oxide fuel cell applications. Ceram. Int. 2020, 46, 23314–23325. [Google Scholar] [CrossRef]
- Qiu, P.; Yang, X.; Zhu, T.; Sun, S.; Jia, L.; Li, J. Review on core-shell structured cathode for intermediate temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 23160–23173. [Google Scholar] [CrossRef]
- Kaur, P.; Singh, K. Review of perovskite-structure related cathode materials for solid oxide fuel cells. Ceram. Int. 2020, 46, 5521–5535. [Google Scholar] [CrossRef]
- Yatoo, M.A.; Kawale, S.S.; Skinner, S.J. Perovskite and layered oxide materials for intermediate temperature solid oxide fuel cells. In Intermediate Temperature Solid Oxide Fuel Cells; Kaur, G., Ed.; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2020; pp. 315–346. [Google Scholar]
- Irvine, J.; Rupp, J.L.M.; Liu, G.; Xu, X.; Haile, S.; Qian, X.; Snyder, A.; Freer, R.; Efren, D.; Skinner, S.; et al. Roadmap on inorganic perovskites for energy applications. J. Phys. Energy 2021, 3, 031502. [Google Scholar] [CrossRef]
- Su, C.; Wang, W.; Shao, Z. Cation-Deficient Perovskites for Clean Energy Conversion. Acc. Mater. Res. 2021, 2, 477–488. [Google Scholar] [CrossRef]
- Nechache, A.; Hody, S. Alternative and innovative solid oxide electrolysis cell materials: A review. Renew. Sust. Energy Rev. 2021, 149, 111322. [Google Scholar] [CrossRef]
- Hanif, M.B.; Motola, M.; Rauf, S.; Li, C.-J.; Li, C.-X. Recent advancements, doping strategies and the future prespective of perovskite-based solid oxide fuel cells for energy conversion. Chem. Eng. J. 2022, 428, 132603. [Google Scholar] [CrossRef]
- Gao, Z.; Mogni, L.V.; Miller, E.C.; Railsback, J.C.; Barnett, S.A. A perspective on low-temperature solid oxide fuel cells. Energy Environ. Sci. 2016, 9, 1602–1644. [Google Scholar] [CrossRef]
- Afroze, S.; Karim, A.H.; Cheok, Q.; Eriksson, S.; Azad, A.K. Latest development of double perovskite electrode materials for solid oxide fuel cells: A review. Front. Energy 2019, 13, 770–797. [Google Scholar] [CrossRef]
- Bello, I.T.; Zhai, S.; He, Q.; Xu, Q.; Ni, M. Scientometric review of advancements in the development of high-performance cathode for low and intermediate temperature solid oxide fuel cells: Three decades in retrospect. Int. J. Hydrogen Energy 2021, 46, 26518–26536. [Google Scholar] [CrossRef]
- Kasyanova, A.V.; Tarutina, L.R.; Rudenko, A.O.; Lyagaeva, J.G.; Medvedev, D.A. Ba(Ce,Zr)O3–based electrodes for protonic ceramic electrochemical cells: Towards highly compatible functionality and triple-conducting behaviour. Russ. Chem. Rev. 2020, 89, 667–692. [Google Scholar] [CrossRef]
- Mather, G.C.; Muñoz-Gil, D.; Zamudio-García, J.; Porras-Vázquez, J.M.; Marrero-López, D.; Pérez-Coll, D. Perspectives on Cathodes for Protonic Ceramic Fuel Cells. Appl. Sci. 2021, 11, 5363. [Google Scholar] [CrossRef]
- Seong, A.; Kim, J.; Jeong, D.; Sengodan, S.; Liu, M.; Choi, S.; Kim, G. Electrokinetic Proton Transport in Triple (H+/O2–/e–) Conducting Oxides as a Key Descriptor for Highly Efficient Protonic Ceramic Fuel Cells. Adv. Sci. 2021, 8, 2004099. [Google Scholar] [CrossRef] [PubMed]
- Samat, A.A.; Darus, M.; Osman, N.; Baharuddin, N.A.; Anwar, M. A Short Review on Triple Conducting Oxide Cathode Materials for Proton onducting Oxide Fuel Cell. AIP Conf. Proc. 2021, 2339, 020233. [Google Scholar]
- Wang, N.; Hinokuma, S.; Ina, T.; Zhu, C.; Habazaki, H.; Aoki, Y. Mixed Proton–electron–oxide ion Triple Conducting Manganite as Efficient Cobalt-free Cathode for Protonic ceramic Fuel Cells. J. Mater. Chem. A 2020, 8, 11043–11055. [Google Scholar] [CrossRef]
- Malyshkin, D.; Novikov, A.; Ivanov, I.; Sereda, V.; Tsvetkov, D.; Zuev, A. The origin of triple conductivity and water uptake in layered double perovskites: A case study on lanthanum-substituted GdBaCo2O6–δ. J. Alloys Compd. 2020, 845, 156309. [Google Scholar] [CrossRef]
- Xiang, H.; Xing, Y.; Dai, F.-z.; Wang, H.; Su, L.; Miao, L.; Zhang, G.; Wang, Y.; Qi, X.; Yao, L.; et al. High-entropy ceramics: Present status, challenges, and a look forward. J. Adv. Ceram. 2021, 10, 385–441. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Wang, Q.; Schweider, S.; Botros, M.; Fu, T.; Hahn, H.; Drezesinski, T.; Vreitung, B. High-entropy energy materials: Challenges and new opportunities. Energy Environ. Sci. 2021, 14, 2883–2905. [Google Scholar] [CrossRef]
- Wright, A.J.; Luo, J. A step forward from high-entropy ceramics to compositionally complex ceramics: A new perspective. J. Mater. Sci. 2020, 55, 9812–9827. [Google Scholar] [CrossRef]
- Shen, L.; Du, Z.; Zhang, Y.; Dong, X.; Zhao, H. Medium-Entropy perovskites Sr(FeαTiβCoγMnζ)O3–δ as promising cathodes for intermediate temperature solid oxide fuel cell. Appl. Catal. B. Environ. 2021, 295, 120264. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Olszewska, A.; Falkenstein, A.; Shwab, C.; Szymczak, M.; Zajusz, M.; Moździerz, M.; Mikuła, A.; Zielińska, K.; Berent, K.; et al. An innovative approach to design SOFC air electrode materials: High entropy La1–xSrx(Co,Cr,Fe,Mn,Ni)O3–δ (x = 0., 0.1, 0.2, 0.3) perovskites synthesized by the sol–gel method. J. Mater. Chem. A 2020, 8, 24455. [Google Scholar] [CrossRef]
- Dąbrowa, J.; Zielińska, K.; Stepien, A.; Zajusz, M.; Nowakowska, M.; Moździerz, M.; Berent, K.; Szymczak, M.; Świerczek, K. Formation of Solid Solutions and Physicochemical Properties of the High-Entropy Ln1–xSrx(Co,Cr,Fe,Mn,Ni)O3–δ (Ln = La, Pr, Nd, Sm or Gd) Perovskites. Materials 2021, 14, 5264. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wang, G.; Wu, H.; Bashiwork, B.A.; Tian, D.; Zhu, S.; Yang, Y.; Lu, Y.; Ding, Y.; Ling, Y.; et al. A high-entropy perovskite cathode for solid oxide fuel cells. J. Alloys Compd. 2021, 872, 159633. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Høydalsvik, K.; Einarsrud, M.-A.; Grande, T. Effect of A-Site Ordering on Chemical Stability, Oxygen Stoichiometry and Electrical Conductivity in Layered LaBaCo2O5+δ Double Perovskite. Materials 2016, 9, 154. [Google Scholar] [CrossRef]
- Malyshkin, D.A.; Novikov, A.Y.; Sereda, V.V.; Ivanov, I.L.; Tsvetkov, D.S.; Zuev, A.Y. In Situ and ex Situ Study of Cubic La0.5Ba0.5CoO3–δ to Double Perovskite LaBaCo2O6–δ Transition and Formation of Domain Textured Phases with Fast Oxygen Exchange Capability. Inorg. Chem. 2018, 57, 12409–12416. [Google Scholar] [CrossRef] [PubMed]
- Tsvetkov, D.S.; Ivanov, I.L.; Malyshkin, D.A.; Sednev, A.L.; Sereda, V.V.; Zuev, A.Y. Double perovskites REBaCo2–xMxO6–δ (RE = La, Pr, Nd, Eu, Gd; M = Fe, Mn) as energy-related materials: An overview. Pure Appl. Chem. 2019, 91, 923–940. [Google Scholar] [CrossRef]
- Yasodha, P.; Premila, M.; Bharathi, A.; Valsakimar, M.C.; Rajaraman, R.; Sundar, C.S. Infrared spectroscopic study of the local structural changes across the metal insulator transition in nickel-doped GdBaCo2O5.5. J. Solid State Chem. 2010, 183, 2602–2608. [Google Scholar] [CrossRef]
- Kundu, A.; Pralong, V.; Raveau, B.; Caignaert, V. Magnetic and electrical properties of ordered 112-type perovskite LnBaCoMnO5+δ (Ln = Nd, Eu). J. Mater. Sci. 2011, 46, 681–687. [Google Scholar] [CrossRef][Green Version]
- Konne, J.L.; Davis, S.A.; Glatzel, S.; Hall, S.R. Synthesis of phase pure praseodimium barium copper iron oxide. Chem. Commun. 2013, 49, 5477. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Chizhova, E.A. Synthesis and Properties of LnBaFeCoO5+δ (Ln = Nd, Sm, Gd). Inorg. Mater. 2013, 49, 319–324. [Google Scholar] [CrossRef]
- Zhuravleva, T.A. Electrophysical Properties of Layered Perovskites LnBaCo2–xCuxO5+δ (Ln = Sm, Nd) for Solid Oxide Fuel Cells. Rus. J. Electrochem. 2011, 47, 676–680. [Google Scholar] [CrossRef]
- Cherepanov, V.A.; Aksenova, T.V.; Gavrilova, L.Y.; Mikhaleva, K.N. Structure, nonstoichiometry of the NdBa(Co,Fe)2O5+δ layered perovskite. Solid State Ion. 2011, 188, 53–57. [Google Scholar] [CrossRef]
- Świerczek, K. Physico-chemical properties of Ln0.5A0.5Co0.5Fe0.5O3–δ (Ln: La, Sm; A: Sr, Ba) cathode materials and their performance in electrolyte-supported Intermediate Temperature Solid Oxide Fuel Cell. J. Power Source 2011, 196, 7110–7116. [Google Scholar] [CrossRef]
- Szpunar, I.; Strandbakke, R.; Sørby, M.H.; Wachowsky, S.L.; Balaguer, M.; Tarach, M.; Serra, J.M.; Witkowska, A.; Dzik, E.; Norby, T.; et al. High-Temperature Structural and Electrical Properties of BaLnCo2O6Positrodes. Materials 2020, 13, 4044. [Google Scholar] [CrossRef]
- Galeano, V.; Zapata, V.H.; Ostos, C.; Morán, O. On the electrical properties of textured YBaCo2O5+δ thin layers tested by means of complex impedance spectroscopy. Vacuum 2020, 181, 109595. [Google Scholar] [CrossRef]
- Taskin, A.; Lavrov, A. Origin of the large thermoelectric power in oxygen-variable RBaCo2O5 + x(R = Gd,Nd). Phys. Rev. 2006, 73, 1211101. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Chizhova, Y.e.A.; Sazanovich, N.V.; Krasutskaya, N.S. Thermoelectric Properties of Some Perovskite Oxides. J. Thermoelectr. 2009, 3, 73–80. [Google Scholar]
- Klyndyuk, A.I. Thermoelectric Properties of Lauered Ferrocuprates LnBaCuFeO5+δ (Ln = La, Pr, Nd, Sm, Gd–Lu). Phys. Solid State 2009, 51, 250–254. [Google Scholar] [CrossRef]
- Zeng, C.; Liu, Y.; Lan, J.; Ren, G.; Lin, Y.; Li, M.; Nan, C. Thermoelectric properties of Sm1–xLaxBaCuFeO5 ceramics. Mat. Res. Bull. 2015, 69, 46–50. [Google Scholar] [CrossRef]
- Zeng, C.; Zhan, B.; Butt, S.; Liu, Y.; Ren, G.; Lin, Y.-H.; Li, M.; Nan, C.-W. Electrical and Thermal Conduction Behaviors in La-Substituted GdBaCuFeO5+δ Ceramics. J. Am. Ceram. Soc. 2015, 98, 437–442. [Google Scholar] [CrossRef]
- Wu, T.; Gao, P. Development of Perovskite-Type Materials for Thermoelectric Application. Materials 2018, 11, 999. [Google Scholar] [CrossRef]
- Han, B.; Li, Y.; Chen, N.; Deng, D.; Xinxin, X.; Wang, Y. Preparation and Photocatalytic Properties of LnBaCo2O5+δ (Ln = Eu, Gd, and Sm). J. Mat. Sci. Chem. Eng. 2015, 3, 17–25. [Google Scholar]
- Løken, A.; Ricote, S.; Wachowski, S. Thermal and Chemical Expansion in Proton Ceramic Electrolytes and Compatible Electrodes. Crystals 2018, 8, 365. [Google Scholar] [CrossRef]
- Galin, M.Z.; Ivanov-Schitz, A.K.; Mazo, G.N. Molecular Dynamics Simulation of Structural and Transport Properties of Solid Solutions of Double Perovskites Based on PrBaCo2O5.5. Crystallography 2020, 65, 289–296. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Ivanov, I.L.; Malyshkin, D.A.; Sereda, V.V.; Zuev, A.Y. Mechano-Chemical Coupling in Double Perovskites as Energy Related Materials. ECS Transact. 2016, 72, 21–35. [Google Scholar] [CrossRef]
- Klyndyuk, A.I. Thermal and Chemical Expansion of LnBaCuFeO5+δ (Ln = La, Pr, Gd) Ferrocuprates and LaBa0.75Sr0.25CuFeO5+δ Solid Solution. Rus. J. Inorg. Chem. 2007, 52, 1343–1349. [Google Scholar] [CrossRef]
- Klyndyuk, A.I. New Perovskite Oxides LaBaMCoO5+δ (M = Fe, Cu): Synthesis, Structure, and Properties. Phys. Solid State 2009, 51, 270–274. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Chizhova, E.A. Properties of Perovskite-Like Phases LnBaCuFeO5+δ (Ln = La, Pr). Glass Phys. Chem. 2008, 34, 313–318. [Google Scholar] [CrossRef]
- Zhu, L.; Wei, B.; Lü, Z.; Feng, J.; Xu, L.; Gao, H.; Zhang, Y.; Huang, X. Performance degradation of double-perovskite PrBaCo2O5+δ oxygen electrode in CO2containing atmospheres. Appl. Surf. Sci. 2017, 416, 649–655. [Google Scholar] [CrossRef]
- Tellez, H.; Druce, J.; Ju, Y.-W.; Kilner, J.; Ushihara, T. Surface chemistry evolution in LnBaCo2O5+δ double perovskites for oxygen electrodes. Int.J. Hydrogen Energy 2014, 39, 20856–20863. [Google Scholar] [CrossRef]
- Perry, N.H.; Ishihara, T. Roles of Bulk and Surface Chemistry in the Oxygen Exchange Kinetics and Related Properties of Mixed Conducting Perovskite Oxide Electrodes. Materials 2016, 9, 858. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Sadovskaya, E.M.; Eremeev, N.F.; Skriabin, P.I.; Krasnov, A.V.; Bespalko, Y.N.; Pavlova, S.N.; Fedorova, Y.E.; Pikalova, E.Y.; Shlyakhtina, A.V. Oxygen Mobility in the Materials for Solid Oxide Fuel Cells and Catalytic Membranes (Review). Rus. J. Electrochim. 2019, 55, 701–718. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, G.; Dai, R.; Lv, X.; Yang, D.; Geng, S. A review of the chemical compatibility between oxide electrodes and electrolytes in solid oxide fuel cells. J. Power Source 2021, 492, 229630. [Google Scholar] [CrossRef]
- Liu, J.; Jin, F.; Yang, X.; Niu, B.; Li, Y.; He, T. YBaCo2O5+δ-based double-perovskite cathodes for intermediate-temperature solid oxide fuel cells with simultaneously improved structural stability and thermal expansion properties. Electrochim. Acta 2019, 297, 344–354. [Google Scholar] [CrossRef]
- Tsvetkov, D.; Tsvetkova, N.; Ivanov, I.; Malyshkin, D.; Sereda, V.; Zuev, A. PrBaCo2O6–δ–Ce0.8Sm0.2O1.9 Composite Cathodes for Intermediate-Temperature Solid Oxide Fuel Cells: Stability and Cation Interdifffusion. Energies 2019, 12, 417. [Google Scholar] [CrossRef]
- Zvonareva, I.; Fu, X.-Z.; Medvedev, D.; Shao, Z. Electrochemistry and energy conversion features of protonic ceramic cells with mixed ionic-electronic electrolytes. Energy Environ. Sci. 2021; in press. [Google Scholar] [CrossRef]
- Mao, X.; Ma, G. Performance of cobalt-free double-perovskite NdBaFe2–xMnxO5+δ cathode materials for proton-conducting IT-SOFC. J. Alloys Compd. 2015, 637, 286–296. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, F.; Hao, X.; Niu, B.; Lyu, P.; He, T. B–site-ordered Co-based double perovskites Sr2Co1–xNbxFeO5+δ as active and stable cathodes for intermediate-temperature solid oxide fuel cells. J. Alloys Compd. 2020, 829, 154470. [Google Scholar] [CrossRef]
- Chang, A.; Skinner, S.J.; Kilner, J.A. Electrical properties of GdBaCo2O5+x for ITSOFC applications. Solid State Ion. 2006, 177, 2009–2011. [Google Scholar] [CrossRef]
- Tarancon, A.; Morata, A.; Dezanneau, G.; Skinner, S.J.; Kilner, J.A.; Estradé, S.; Hernández-Ramírez, F.; Peiró, F.; Morante, J.R. GdBaCo2O5+x layered perovskites as an intermediate temperature solid oxide fuel cell cathode. J. Power Source 2007, 174, 255–263. [Google Scholar] [CrossRef]
- Zhang, K.; Ge, L.; Ran, R.; Shao, Z.; Liu, S. Synthesis, characterization and evaluation of cation-ordered LnBaCo2O5+δ as materials of oxygen permeation membranes and cathodes of SOFCs. Acta Mater. 2008, 56, 4876–4889. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. LnBaCo2O5+δ Oxides as cathodes for Intermediate-Temperature Solid Oxide Fuel Cells. J. Electrochem. Soc. 2008, 155, B385–B390. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, X.; Yi, C.; Yan, D.; Su, W. Electrochemical performance of PrBaCo2O5+δ layered perovskite as an intermediate-temperature solid oxide fuel cell cathode. J. Power Source 2008, 185, 193–196. [Google Scholar] [CrossRef]
- Gu, H.; Chen, H.; Gao, L.; Zheng, Y.; Zhu, X.; Guo, L. Oxygen reduction mechanism of NdBaCo2O5+δ cathode for intermediate-temperature solid oxide fuel cells under cathodicpolarization. Int. J. Hydrogen Energy 2009, 34, 2416–2420. [Google Scholar] [CrossRef]
- Liu, Y. YBaCo2O5+δ as a new cathode material for zirconia-based solid oxide fuel cells. J. Alloys Compd. 2009, 477, 860–862. [Google Scholar] [CrossRef]
- Jin, M.; Zhang, X.; Qiu, Y.; Sheng, J. Layered PrBaCo2O5+δperovskite as a cathode for proton-conducting solid oxide fuel cells. J. Alloys Compd. 2010, 494, 359–361. [Google Scholar] [CrossRef]
- Pang, S.; Jiang, X.; Li, X.; Su, Z.; Xu, H.; Xu, Q.; Chen, C. Characterization of cation-orderedperovskite oxide LaBaCo2O5+δ as cathode of intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2012, 37, 6836–6843. [Google Scholar] [CrossRef]
- Kim, J.-H.; Manthiram, A. Layered LnBaCo2O5+δPerovskite Cathodes for Solid Oxide Fuel Cells: An Overview and Perspective. J. Mater. Chem. A 2015, 3, 24195–24210. [Google Scholar] [CrossRef]
- Wang, S.; Zan, J.; Qiu, W.; Zheng, D.; Li, F.; Chen, W.; Pei, Q.; Jiang, L. Evaluation of perovskite oxides LnBaCo2O5+δ (Ln = La, Pr, Nd and Sm) as cathode material for IT–SOFC. J. Electroanalyt. Chem. 2021, 886, 115144. [Google Scholar] [CrossRef]
- Bernuy-Lopez, C.; Rioja-Monnlor, L.; Nakamura, T.; Ricote, S.; O’Hayre, R.; Amezawa, K.; Einarsrud, M.-A.; Grande, T. Effect of Cation Ordering on the Performance and Chemical Stability of Layered Double Perovskite Cathodes. Materials 2018, 11, 196. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Xie, Y.; Hao, X.; Xia, T.; Li, Q.; Wang, J.; Huo, L.; Zhao, H. Addressing electrocatalytic activity and stability of LnBaCo2O5+δperovskites for hydrogen evolution reaction by structural and electronic features. Appl. Catal. B Environment. 2021, 297, 120403. [Google Scholar] [CrossRef]
- Jiang, X.; Shi, Y.; Zhou, W.; Li, X.; Su, Z.; Pang, S.; Jiang, L. Effects of Pr3+-deficiency on structure and properties of PrBaCo2O5+δ cathode material—A comparison with Ba2+-deficiency case. J. Power Source 2014, 272, 371–377. [Google Scholar] [CrossRef]
- Yi, K.; Sun, L.; Li, Q.; Xia, T.; Huo, L.; Zhao, H.; Li, J.; Lü, Z.; Bassat, J.-M.; Rougier, A.; et al. Effect of Nd-deficiency on electrochemical properties of NdBaCo2O6–δ cathode for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2016, 41, 10228–10238. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, Q.; Shi, Y.; Li, X.; Zhou, W.; Xu, H.; Zhang, Q. Synthesis and properties of Sm3+-deficient Sm1–xBaCo2O5+δperovskite oxides as cathode materials. Int. J. Hydrogen Energy 2014, 39, 10817–10823. [Google Scholar] [CrossRef]
- Kim, C.G.; Woo, S.H.; Song, K.E.; Baek, S.-W.; Kang, H.; Choi, W.S.; Kim, J.H. Enhanced Electrochemical Properties of Non-stoichiometric Layered Perovskites, Sm1–xBaCo2O5+d, for IT–SOFC Cathodes. Front. Chem. 2021, 9, 633868. [Google Scholar] [CrossRef] [PubMed]
- Pang, S.L.; Jiang, X.N.; Li, X.N.; Xu, H.X.; Jiang, L.; Xu, Q.L.; Shi, Y.C.; Zhang, Q.Y. Structure and properties of layered-perovskite LaBa1–xCo2O5+δ (x = 0–0.15) as intermediate-temperature cathode material. J. Power Source 2013, 240, 54–59. [Google Scholar] [CrossRef]
- Pang, S.; Jiang, X.; Li, X.; Wang, Q.; Su, Z. Characterization of Ba-deficient PrBa1–xCo2O5+δ as cathode intermediate temperature solid oxide fuel cells. J. Power Source 2012, 204, 53–59. [Google Scholar] [CrossRef]
- Wang, J.; Meng, F.; Xia, T.; Shi, Z.; Lian, J.; Xu, C.; Zhao, H.; Bassat, J.-M.; Grenier, J.-C. Superior electrochemical performance and oxygen reduction kinetics of layered perovskite PrBaxCo2O5+δ (x = 0.90–1.0) oxides as cathode materials for intermediate for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 18392–18404. [Google Scholar] [CrossRef]
- Donazzi, A.; Pelosato, R.; Cordaro, G.; Stucchi, D.; Cristiani, C.; Dotelli, G.; Sopra, I.N. Evaluation of Ba deficient NdBaCo2O5+δ oxide as cathode material for IT-SOFC. Electrochim. Acta 2015, 182, 573–587. [Google Scholar] [CrossRef]
- Pang, S.; Yang, G.; Su, Y.; Xu, J.; Shen, X.; Zhu, M.; Wu, X.; Li, S.; Chen, C. A-site cation deficiency tuned oxygen transport dynamics of perovskite Pr0.5Ba0.25–xCa0.25CoO3–δ for intermediate temperature solid oxide fuel cells. Ceram. Int. 2019, 45, 14602–14607. [Google Scholar] [CrossRef]
- Yao, C.; Yang, J.; Zhang, H.; Chen, S.; Lang, X.; Meng, J.; Cai, K. Evaluation of A-site Ba-deficient PrBa0.5–xSr0.5Co2O5+δ (x = 0, 0.4, and 0.08) as cathode material for solid oxide fuel cells. J. Alloys Compd. 2021, 883, 160759. [Google Scholar] [CrossRef]
- Pang, S.; Wang, W.; Chen, T.; Wang, Y.; Xu, K.; Shen, X.; Xi, X.; Fan, J. The effect of potassium on the properties of PrBa1–xCo2O5+δ (x = 0.00–0.10) cathodes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2016, 41, 13705–13714. [Google Scholar] [CrossRef]
- Bangwal, A.S.; Jha, P.K.; Chauchan, M.; Singh, S.; Sinha, A.S.K.; Jha, P.A.; Singh, P. Compositional effect on oxygen reduction reaction in Pr excess double perovskite Pr1+xBa1–xCo2O6–δcathode materials. Int. J. Hydrogen Energy 2020, 45, 23378–23390. [Google Scholar] [CrossRef]
- Lu, F.; Xia, T.; Li, Q.; Wang, J.; Huo, L.; Zhao, H. Heterostructured simple perovskitenanorod-decorated double perovskite cathode for solid oxide fuel cells: Highly catalytic activity, stability and CO2-durability for oxygen reduction reaction. Appl. Catal. B Environ. 2019, 249, 19–31. [Google Scholar] [CrossRef]
- Azad, A.K.; Kim, J.H.; Irvine, J.T.S. Structure–property relationship in layered perovskite cathode LnBa0.5Sr0.5Co2O5+δ (Ln = Pr, Nd) for solid oxide fuel cells. J. Power Source 2011, 196, 7333–7337. [Google Scholar] [CrossRef]
- Xia, W.; Liu, X.; Jin, F.; Jia, X.; Shen, Y. Evaluation of calcium codoping in double perovskite PrBaCo2O5+δ as cathode for IT–SOFCs. Electrochim. Acta 2020, 464, 137274. [Google Scholar] [CrossRef]
- Subardi, A.; Liao, K.-Y.; Fu, Y.-P. Oxygen transport, thermal and electrochemical properties of NdBa0.5Sr0.5Co2O5+δ cathode for SOFCs. J. Eur. Ceram. Soc. 2019, 39, 30–40. [Google Scholar] [CrossRef]
- Yao, C.; Zhang, H.; Liu, X.; Meng, J.; Zhang, X.; Meng, F.; Meng, J. Ivestigation of layered perovskite NdBa0.5Sr0.25Ca0.25Co2O5+δ as cathode for solid oxide fuel cells. Ceram. Int. 2018, 44, 12048–12054. [Google Scholar] [CrossRef]
- Yoo, S.; Jun, A.; Ju, Y.-W.; Odkhuu, D.; Hyodo, J.; Jeong, H.Y.; Park, N.; Shin, J.; Ishihara, T.; Kim, G. Development of Double-Perovskite Compoundes as Cathode Materials for Low-Temperature Solid Oxide Fuel Cells. Angew. Chem. Int. Ed. 2014, 53, 13064–13067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jin, M.; Sheng, J. Layered GdBa0.5Sr0.5Co2O5+δ as a cathode for proton-conducting solid oxide fuel cells with stable BaCe0.5Zr0.3Y0.16Zn0.04O3–δ electrolyte. J. Alloys Compd. 2010, 496, 241–243. [Google Scholar] [CrossRef]
- Meng, F.; Xia, T.; Wang, J.; Shi, Z.; Lian, J.; Zhao, H.; Bassat, J.-M.; Grenier, J.-C. Evaluation of layered perovskites YBa1–xSrxCo2O5+δ as cathodes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 4531–4543. [Google Scholar] [CrossRef]
- Xue, J.; Shen, Y.; He, T. Performance of double-perovskite YBa0.5Sr0.5Co2O5+δ as cathode material for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2011, 36, 6894–6898. [Google Scholar] [CrossRef]
- Woo, S.H.; Song, K.E.; Baek, S.-W.; Kang, H.; Choi, W.; Shin, T.H.; Park, J.-Y.; Kim, J.H. Pr- and Sm-Substituted Layered Perovskite Oxide Systems for IT–SOFC Cathodes. Energies 2021, 14, 6739. [Google Scholar] [CrossRef]
- Subardi, A.; Cheng, M.-H.; Fu, Y.-P. Chemical bulk diffusion and electrochemical properties of SmBa0.6Sr0.4Co2O5+δ cathode for intermediate solid oxide fuel cells. Int. J. Hydrogen Energy 2014, 39, 20783–20790. [Google Scholar] [CrossRef]
- Subardi, A.; Chen, C.-C.; Fu, Y.-P. Oxygen transportation, electrical conductivity and electrochemical properties of layered perovskite SmBa0.5Sr0.5Co2O5+δ. Int. J. Hydrogen Energy 2017, 42, 5284–5294. [Google Scholar] [CrossRef]
- Zhang, L.; Li, S.; Xia, T.; Sun, L.; Huo, L.; Zhao, H. Co-deficient PrBaCo2–xO6–δperovskites as cathode materials for intermediate-temperature solid oxide fuel cells: Enhanced electrochemical performance and oxygen reduction kinetics. Int. J. Hydrogen Energy 2018, 43, 3761–3775. [Google Scholar] [CrossRef]
- Che, X.; Shen, Y.; Li, H.; He, T. Assessment of LnBaCo1.6Ni0.4O5+δ (Ln = Pr, Nd, and Sm)double-perovskites as cathodes for intermediate-temperature solid-oxide fuel cells. J. Power Source 2013, 222, 288–293. [Google Scholar] [CrossRef]
- Liu, L.; Guo, R.; Wang, S.; Yang, Y.; Yin, D. Synthesis and characterization of PrBa0.5Sr0.5Co2–xNixO5+δ (x = 0.1, 0.2 and 0.3) cathodes for intermediate temperature SOFCs. Ceram. Int. 2014, 40, 16393–16398. [Google Scholar] [CrossRef]
- Urusova, A.S.; Cherepanov, V.A.; Lebedev, O.I.; Aksenova, T.V.; Gavrilova, L.Y.; Caignaert, V.; Raveau, B. Tuning oxygen content and distribution by substitution at Co site in 112 YBaCo2O5+δ: Impact on transport and thermal properties. Chem. Mater. A 2014, 2, 8823. [Google Scholar] [CrossRef]
- Liu, B.; Yang, J.; Yan, D.; Jia, L.; Chi, B.; Pu, J.; Li, J. Novel PrBa0.9Ca0.1Co2–xZnxO5+δ double-perovskite as an active cathode materialfor high-performance proton-conducting solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 31009–31016. [Google Scholar] [CrossRef]
- Jun, A.; Shin, J.; Kim, G. High redox and performance stability of layered SmBa0.5Sr0.5Co1.5Cu0.5O5+δperovskite cathodes for intermediate-temperature solid oxide fuel cells. Phys. Chem. Chem. Phys. 2013, 15, 19906–19912. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Lü, S.; Meng, X.; Zhao, X.; Ji, Y.; Wang, Y.; Fu, C.; Liu, X.; Li, X.; et al. Effect of Cu doping on YBaCo2O5+δ as cathode for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2014, 134, 107–115. [Google Scholar] [CrossRef]
- Klyndyuk, A.I.; Mosiałek, M.; Kharitonov, D.S.; Chizhova, E.A.; Zimowska, M.; Socha, R.; Komenda, A. Structural and electrochemical characterization of YBa(Fe,Co,Cu)2O5+δ layered perovskites as cathode materials for solid oxide fuel cells. Int. J. Hydrogen Energy 2021, 46, 16977–16988. [Google Scholar] [CrossRef]
- Lu, F.; Xia, T.; Li, Q.; Sun, L.; Huo, L.; Zhao, H. Ta-doped PrBa0.94Co2–xTaxO5+δ as promising oxygen electrodes: A focused study on catalytic oxygen reduction reaction activity, stability and CO2-durability. J. Power Source 2019, 417, 42–52. [Google Scholar] [CrossRef]
- Xu, J.; Cai, H.; Hao, G.; Zhang, L.; Song, Z.; Long, W.; Zhang, L.; Mang, L. Characterization of high-valence Mo-doped PrBaCo2O5+δ cathodes for IT–SOFCs. J. Alloys Compd. 2020, 842, 155600. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Lü, S.; Meng, X.; Zhang, Y.; Yu, B.; Li, X.; Sui, Y.; Yang, J.; Fu, C.; et al. Synthesis and characterization of SmSrCo2–xMnxO5+δ (x = 0.0, 0.2, 0.4, 0.6, 0.8, 1.0) cathode materials for intermediate-temperature solid-oxide fuel cells. Ceram. Int. 2014, 40, 11343–11350. [Google Scholar] [CrossRef]
- Muñoz-Gil, D.; Urones-Garrote, E.; Pérez-Coll, D.; Amador, U.; García-Martin, S. Crystal structure and compositional effectson the electrical and electrochemical properties of GdBaCo2–xMnxO5+δ (0 ≤ x ≤ 2) oxides for use as air electrodes in solid oxide fuel cells. J. Mater. Chem. A 2018, 6, 5452–5460. [Google Scholar] [CrossRef]
- Olszewska, A.; Świerczek, K. ReBaCo2–xMnxO5+δ (Re: Rare earth element) layered perovskites for application as cathodes in Solid Oxide Fuel Cells. E3S Web Conf. 2019, 108, 01020. [Google Scholar] [CrossRef]
- Olszewska, A.; Świerczek, K.; Niemczyk, A. Peculiar Properties of Electrochemically Oxidized SmBaCo2–xMnxO5+δ (x = 0; 0.5 and 1) A-Site Ordered Perovskites. Crystals 2020, 10, 205. [Google Scholar] [CrossRef]
- Kim, Y.N.; Kim, J.-H.; Manthiram, A. Effect of fe substitution on the structure and properties of LnBaCo2–xFexO5+δ (Ln = Nd and Gd) cathodes. J. Power Source 2010, 195, 6411–6419. [Google Scholar] [CrossRef]
- Zhao, L.; Shen, J.; He, B.; Chen, F.; Xia, C. Synthesis, characterization and evaluation of PrBaCo2–xFexO5+δ as cathodes for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2011, 36, 3658–3665. [Google Scholar] [CrossRef]
- Xue, J.; He, T. Double-perovskites YBaCo2–xFexO5+δ cathodes for intermadiate-temperature solid oxide fuel cells. J. Power Source 2011, 196, 3729–3735. [Google Scholar] [CrossRef]
- Zou, J.; Park, J.; Kwak, B.; Yoon, H.; Chung, J. Effect of Fe doping on PrBaCo2O5+δ as cathode for intermediate-temperature solid oxide fuel cells. Solid State Ion. 2012, 206, 112–119. [Google Scholar] [CrossRef]
- Choi, S.; Shin, J.; Kim, G. The electrochemical and thermodynamic characterization of PrBaCo2–xFexO5+δ (x = 0, 0.5, 1) infiltrated into yttria-stabilized zirconia scaffold as cathodes for solid oxide fuel cells. J. Power Source 2012, 201, 10–17. [Google Scholar] [CrossRef]
- Jiang, L.; Wei, T.; Zeng, R.; Zhang, W.-X.; Huang, Y.-H. Thermal and electrochemical properties of PrBa0.5Sr0.5Co2–xFexO5+δ (x = 0.5, 1.0, 1.5) cathode materials for solid-oxide fuel cells. J. Power Source 2013, 232, 279–285. [Google Scholar] [CrossRef]
- Yoo, C.-Y.; Joo, J.H.; Lee, H.J.; Yu, J.H. The effects of Fe-substitution on the crystal structure and oxygen permeability of PrBaCo2O5+δ. Mat. Lett. 2013, 108, 65–68. [Google Scholar] [CrossRef]
- Anjum, U.; Vashishta, S.; Sinha, N.; Haider, M.A. Role of oxygen anion diffusion in improved electrochemical performance of layered perovskite LnBa1–ySryCo2–xFexO5+δ (Ln = Pr, Nd, Gd) electrodes. Solid State Ion. 2015, 280, 24–29. [Google Scholar] [CrossRef]
- Strandbakke, R.; Cherepanov, V.A.; Zuev, A.Y.; Tsvetkov, D.S.; Argirusis, C.; Sourkoni, G.; Prüne, S.; Norby, T. Gd- and Pr-based double perovskitecobaltites as oxygen electrodes for proton ceramic fuel cells and electrolyser cells. Solid State Ion. 2015, 278, 120–132. [Google Scholar] [CrossRef]
- Lee, T.-H.; Parl, K.-Y.; Kim, N.-I.; Dong, S.-J.; Hong, K.-H.; Ahn, D.; Azad, A.K.; Hwang, J.; Bhattacharjee, S.; Lee, S.-C.; et al. Robust NdBa0.5Sr0.5Co1.5Fe0.5O5+δ cathode material and its degradation prevention operating logic for intermediate-temperature solid oxide fuel cells. J. Power Source 2016, 331, 495–506. [Google Scholar] [CrossRef]
- Anjum, U.; Vashishtha, S.; Agarwal, M.; Tiwari, P.; Sinha, N.; Agrawal, A.; Basu, S.; Haider, M.A. Oxygen anion diffusion in double perovskite GdBaCo2O5+δ and LnBa0.5Sr0.5Co2–xFexO5+δ (Ln = Gd, Pr, Nd) electrodes. Int. J. Hydrogen Energy 2016, 41, 7631–7640. [Google Scholar] [CrossRef]
- Muñoz-Gil, D.; Pérez-Coll, D.; Urones-Garrote, E.; Amador, U.; Garcia-Martin, S. Influence of the synthesis conditions on the crystal structure and properties of GdBaCo2–xFexO5+δ oxides as air-electrodes for Intermediate Temperature Solid Oxide Fuel Cells. J. Mater. Chem. A 2017, 5, 12550–12556. [Google Scholar] [CrossRef]
- Zhang, S.-L.; Chen, K.; Zhang, A.-P.; Li, C.-X.; Li, C.-J. Effect of Fe doping on the performance of suspension plasma-sprayed PrBa0.5Sr0.5Co2–xFexO5+δ cathodes for intermediate-temperature solid oxide fuel cells. Ceram. Int. 2017, 43, 11648–11655. [Google Scholar] [CrossRef]
- Cordaro, G.; Donazzi, A.; Pelosato, R.; Cristiani, C.; Dotelli, G.; Sora, I.N. Electrochemical and Chemical Characterization of NdBa1–xCo2–yFeyO5+δ Cathodes for IT–SOFCs. ECS Transact. 2017, 78, 507–520. [Google Scholar] [CrossRef]
- Kim, B.-J.; Fabbri, E.; Castelli, I.E.; Borlaf, M.; Graule, T.; Nachtegaal, M.; Schmidt, T.J. Fe-Doping in Double Perovskite PrBaCo2(1–x)Fe2xO6–δ: Insights into Structural and Electronic Effects to Enhance Oxygen Evolution Catalyst Stability. Catalysts 2019, 9, 263. [Google Scholar] [CrossRef]
- Cordaro, G.; Donazzi, A.; Pelosato, R.; Mastropasqua, L.; Cristiani, C.; Sora, I.N.; Dotelli, G. Structural and Electrochemical Characterization of NdBa1–xCo2–yFeyO5+δ as cathode for Intermediate Temperature Solid Oxide Fuel Cells. J. Electrochem. Soc. 2020, 167, 024502. [Google Scholar] [CrossRef]
- Lu, C.; Niu, B.; Yi, W.; Ji, Y.; Xu, B. Efficient symmetrical electrodes of PrBaFe2–xCoxO5+δ (x = 0, 0.2, 0.4) for solid oxide fuel cells and solid oxide electrolysis cells. Electrochim. Acta 2020, 358, 136916. [Google Scholar] [CrossRef]
- Jin, F.; Shen, Y.; Wang, R.; He, T. Double-perovskite PrBaCo2/3Fe2/3Cu2/3O5+δ as cathode material for intermediate-temperature solid oxide fuel cells. J. Power Source 2013, 234, 244–251. [Google Scholar] [CrossRef]
- Jin, F.; Li, L.; He, T. NdBaCo2/3Fe2/3Cu2/3O5+δ double perovskites as a novel cathode material for CeO2- and LaGaO3-based solid oxide fuel cells. J. Power Source 2015, 273, 591–599. [Google Scholar] [CrossRef]
- Jin, F.; Li, J.; Wang, Y.; Chu, X.; Xu, M.; Zhai, Y.; Zhang, Y.; Fang, W.; Zou, P.; He, T. Evaluation of Fe and Mn co-doped layered perovskite PrBaCo2/3Fe2/3Mn1/2O5+δ as a novel cathode for intermediate-temperature solid-oxide fuel cell. Ceram. Int. 2018, 44, 22489–22496. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, D.S.; Muralidharan, P.; Jo, S.H.; Kim, D.K. Improved electrochemical performance and thermal compatibility of Fe- and Cu-doped SmBaCo2O5+δ–Ce0.9Gd0.1O1.95 composite cathode for intermediate-temperature solid oxide fuel cells. J. Power Source 2011, 196, 3095–3098. [Google Scholar] [CrossRef]
- Li, R.; Wang, D.; Ge, L.; He, S.; Chen, H.; Guo, L. Effect of Bi2O3 on the electrochemical performance of LaBaCo2O5+δ cathode for intermediate-temperature solid oxide fuel cells. Ceram. Int. 2014, 40, 2599–2603. [Google Scholar] [CrossRef]
- Tsvetkov, D.S.; Tsvetkova, N.S.; Ivanov, I.L.; Malyshkin, D.A.; Sereda, V.V.; Zuev, A.Y. PrBaCo2O6–δ–Ce0.8Sm0.2O1.9 Composite Cathodes for Intermediate Temperature Solid Oxide Fuel cells. ECS Transact. 2015, 68, 965–976. [Google Scholar] [CrossRef]
- Li, S.; Zhang, L.; Xia, T.; Li, Q.; Sun, L.; Huo, L.; Zhao, H. Synergistic effect study of EuBa0.98Co2O5+δ–Ce0.8Sm0.2O1.9 composite cathodes for intermediate-temperature solid oxide fuel cells. J. Alloys Compd. 2019, 771, 513–521. [Google Scholar] [CrossRef]
- Kim, J.-H.; Irvine, J. Characterization of layered perovskite oxides NdBa1−xSrxCo2O5+δ (x = 0 and 0.5) as cathode materials for IT-SOFC. Int. J. Hydrogen Energy 2012, 37, 5920–5929. [Google Scholar] [CrossRef]
- Kim, J.-H.; Kim, Y.N.; Bi, Z.; Manthiram, A.; Paranthaman, M.P.; Huq, A. Overcoming phase instability of RBaCo2O5+δ (R = Y and Ho) by Sr substitution for application as cathodes in solid oxide fuel cells. Solid State Ion. 2013, 253, 81–87. [Google Scholar] [CrossRef]
- Idrees, A.; Jiang, X.; Liu, G.; Luo, H.; Zhang, Q.; Jiang, L.; Li, X.; Xu, B. An optimized synthesis route for high performance composite cathode based on a layered perovskite oxide of PrBa0.92Co2O6–δ with cationic deficiency. Int. J. Hydrogen Energy 2019, 44, 4271–4280. [Google Scholar] [CrossRef]
- Idrees, A.; Jiang, X.; Jiang, L.; Zhang, Q. Properties of composite cathodes composed of Pr3+-deficient oxide and ionic conductor Ce0.8Sm0.2O1.9. Ceram. Int. 2020, 46, 17532–17539. [Google Scholar] [CrossRef]
- Rioja-Monnlor, L.; Ricote, S.; Bernuy-Lopez, C.; Grande, T.; O’Hayre, R.; Einarsrud, M.-A. High-Performance La0.5Ba0.5Co1/3Mn1/3Fe1/3O3–δ–BaZr1–zYzO3–δ Cathode Composites via an Exsolution Mechanism for protonic Ceramic Fuel Cells. Inorg. 2018, 6, 83. [Google Scholar] [CrossRef]
- Rioja-Monnlor, L.; Bernuy-Lopez, C.; Fontaine, M.-L.; Grande, T.; Einarsrud, M.-A. Microstructural and compositional optimization of La0.5Ba0.5CoO3–δ–BaZr1–zYzO3–δ (z = 0, 0,05 and 0.1) nanocomposite cathodes for protonic ceramic fuel cells. J. Phys. Energy 2020, 2, 015001. [Google Scholar] [CrossRef]
- Choi, M.-B.; Jeon, S.-Y.; Hwang, H.-J.; Park, J.-Y.; Song, S.-J. Composite of Ce0.8Gd0.2O2–δ and GdBaCo2O5+δ as oxygen separation membranes. Solid State Ion. 2010, 181, 1680–1684. [Google Scholar] [CrossRef]
- Chen, T.; Zhao, H.; Xie, Z.; Feng, L.; Lu, X.; Ding, W.; Li, F. Electrical conductivity and oxygen permeability of Ce0.8Sm0.2O2–δ–PrBaCo2O5+δ dual-phase composites. Int. J. Hydrogen Energy 2012, 37, 5277–5285. [Google Scholar] [CrossRef]
- Liu, X.; Jin, F.; Sun, N.; Li, J.; Shen, Y.; Wng, F.; Li, J. Nd3+-deficiency double perovskite Nd1–xBaCo2O5+δ and performance optimization as cathode materials for intermediate-temperature solid oxide fuel cells. Ceram. Int. 2021, 47, 33886–33896. [Google Scholar] [CrossRef]
- Wang, S.-F.; Hsu, Y.-F.; Liao, Y.-L.; Huang, S.-T.; Jasinski, P. High-performance NdSrCo2O5+δ–Ce0.8Gd0.2O2–δ composite cathodes for electrolyte-supported microtubular solid oxide fuel cells. Int. J. Hydrogen Energy 2021, 46, 31778–31787. [Google Scholar] [CrossRef]
- Zhou, Q.; He, T.; He, Q.; Ji, Y. Electrochemical performances of LaBaCuFeO5+x and LaBaCuCoO5+x as potential cathode materials for intermediate-temperature solid oxide fuel cells. Electrochem. Commun. 2009, 11, 80–83. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, Y.; Shen, Y.; He, T. Layered Perovskite GdBaCuCoO5+δ Cathode Material for Intermediate-Temperature Solid Oxide Fuel Cells. J. Electrochem. Soc. 2010, 157, B628–B632. [Google Scholar] [CrossRef]
- Zhu, Z.; Tao, Z.; Bi, L.; Liu, W. Investigation of SmBaCuCoO5+δ as cathode for proton-conducting solid oxide fuel cells. Mat. Res. Bull. 2010, 45, 1771–1774. [Google Scholar] [CrossRef]
- Zhao, L.; Nian, Q.; He, B.; Lin, B.; Ding, H.; Wang, S.; Peng, R.; Meng, G.; Liu, X. Novel layered perovskite oxide PrBaCuCoO5+δ ad a potential cathode for intermadiate-temperature solid oxide fuel cells. J. Power Source 2010, 195, 453–456. [Google Scholar] [CrossRef]
- Nian, Q.; Zhao, L.; He, B.; Lin, B.; Peng, R.; Meng, G.; Liu, X. Layered SmBaCuCoO5+δ and SmBaCuFeO5+δperovskite oxides as cathode materials for proton-conducting SOFCs. J. Alloys Compd. 2010, 492, 291–294. [Google Scholar] [CrossRef]
- Ling, Y.; Lin, B.; Zhao, L.; Zhang, X.; Yu, J.; Peng, R.; Meng, G.; Liu, X. Layered perovskite LaBaCuMO5+x (M = Fe, Co) cathodes for intermediate-temperature protonic ceramic membrane fuel cells. J. Alloys Compd. 2010, 493, 252–255. [Google Scholar] [CrossRef]
- Zhou, Q.; Wei, T.; Guo, S.; Qi, X.; Ruan, R.; Li, Y.; Wu, Y.; Liu, Q. Evaluation of GdBaCuCo0.5Fe0.5O5+δ as cathode material for intermediate temperature solid oxide fuel cells. Ceram. Int. 2012, 38, 2899–2903. [Google Scholar] [CrossRef]
- Yang, Z.; Ding, Z.; Xiao, J.; Zhang, H.; Ma, G.; Zhou, Z. A novel cobalt-free layered perovskite-type GdBaFeNiO5+δ cathode material for proton-conducting intermediate-temperature solid oxide fuel cells. J. Power Source 2012, 220, 15–19. [Google Scholar] [CrossRef]
- Jin, F.; Xu, H.; Long, W.; Shen, Y.; He, T. Characterization and evaluation of double perovskites LnBaCoFeO5+δ (Ln = Pr and Nd) as intermediate-temperature solid oxide fuel cell cathodes. J. Power Source 2013, 243, 10–18. [Google Scholar] [CrossRef]
- Li, L.; Jin, F.; Shen, Y.; He, T. Cobalt-free double perovskite cathode GdBaFeNiO5+δ and electrochemical performance improvement by Ce0.8Sm0.2O1.9 impregnation for intermeduate-temperature solid oxide fuel cells. Electrochim. Acta 2015, 182, 682–692. [Google Scholar] [CrossRef]
- Li, R.; Jin, F.; Zhang, Y.; Niu, B.; Liu, J.; He, T. Performance and optimization of perovskite-type La1.4Ca0.6CoMnO5+δ cathode for intermediate-temperature solid oxide fuel cells. Int. J. Hydrogen Energy 2019, 44, 8467–8478. [Google Scholar] [CrossRef]
- Pang, S.; Su, Y.; Yang, G.; Shen, X.; Zhu, M.; Wu, X.; Li, S.; Yang, X.; Xi, X. Enhanced electrochemical performance of Ca-doped NdBa1–xCaxCoCuO5+δ as cathode materials for intermediate-temperature solid oxide fuel cells. Ceram. Int. 2018, 44, 21902–21907. [Google Scholar] [CrossRef]
- Lü, S.; Meng, X.; Fu, X.; Liu, M.; Sui, Y.; Chen, Y.; Cao, J.; Sun, Y.; Ji, Y.; Yang, L. The evolution of structure and electrochemical properties of Y-site deficiency Y1–xBaCoCuO5+δ cathode for solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 23227–23236. [Google Scholar] [CrossRef]
- Costilla-Aquillar, S.U.; Escudero, M.J.; Cienfuegos-Pelaes, R.F.; Aguillar-Martinez, J.A. Double perovskite La1.8Sr0.2CoFeO5+δ as a cathode material for intermediate temperature solid oxide fuel cells. J. Alloys Compd. 2021, 862, 158025. [Google Scholar] [CrossRef]
- Jin, F.; Liu, X.; Chu, X.; Shen, Y.; Li, J. Effect of nonequivalent substitution of Pr3+/4+ with Ca2+ in PrBaCoFeO5+δ as cathodes for IT–SOFC. J. Mater. Sci. 2021, 56, 1147–1161. [Google Scholar] [CrossRef]
- Hashim, S.S.; Liang, F.; Zhou, W.; Sunarso, J. Cobalt-Free Perovskite Cathodes for Solid Oxide Fuel Cells. Chem. Electro. Chem. 2019, 6, 3549–3569. [Google Scholar] [CrossRef]
- Chen, D.; Wang, F.; Shi, H.; Ran, R.; Shao, Z. Systematic evaluation of Co-free LnBaFe2O5+δ (Ln = Lanthanides or Y) oxides towards the application as cathodes for intermediate-temperature solid oxide fuel cells. Electrochim. Acta 2012, 78, 466–474. [Google Scholar] [CrossRef]
- Li, F.; Tao, Z.; Dai, H.; Xi, X.; Ding, H. A high-performing proton-conducting solid oxide fuel cell with layered perovskite cathode in intermediate temperatures. Int. J. Hydrogen Energy 2018, 43, 19757–19762. [Google Scholar] [CrossRef]
- Li, H.; Lü, Z. A highly stable cobalt-free LaBa0.5Sr0.5Fe2O6–δ oxide as a high performance cathode material for solid oxide fuel cells. Int. J. Hydrogen Energy 2020, 45, 19831–19839. [Google Scholar] [CrossRef]
- Zhang, Y.; Niu, B.; Hao, X.; Wang, Y.; Liu, J.; Jiang, P.; He, T. Layered oxygen-deficient double perovskite GdBaFe2O5+δ as electrode material for symmetrical solid-oxide fuel cells. Electrochim. Acta 2021, 370, 137807. [Google Scholar] [CrossRef]
- Li, H.; Lű, Z. High-perfromance fluorine-doped cobalt-free oxide as a potential cathode material for solid oxide fuel cells. Int. J. Hydrogen Energy 2021, 46, 2503–2510. [Google Scholar] [CrossRef]
- Kim, D.; Son, S.J.; Kim, M.; Park, H.J.; Joo, J.H. PrBaFe2O5+δ promising electrode for redox-stable symmetrical proton-conducting solid oxide fuel cells. J. Eur. Ceram. Soc. 2021, 41, 5939–5946. [Google Scholar] [CrossRef]
- Son, S.J.; Kim, D.; Park, H.J.; Joo, J.H. Investigation of oxygen ion transport and surface exchange properties of PrBaFe2O5+δ. J. Eur. Ceram. Soc. 2021, 41, 2691–2698. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Sayagués, M.J.; Gotor, F.J. Novel, Simple and Highly Efficient Route to Obtain PrBaMn2O5+δDouble Perovskite: Mechanochemical Synthesis. Nanomaterials 2021, 11, 380. [Google Scholar] [CrossRef] [PubMed]
- Kudyakova, V.S.; Shalamova, A.M.; Chukin, A.V.; Suntsov, A.Y. Enhanced thermal stability and red-ox activity of PrBaMn2–xFexO6–δ oxides. Mat. Res. Bull. 2021, 140, 111309. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, W.; Li, Y.; Yue, W.; Geng, G.; Yu, B. Enhancing CO2 catalytic activation and direct electroreduction on in-situ exsolved Fe/MnOx nanoparticles from (Pr,Ba)2Mn2–yFeyO5+δ layered perovskites for SOEC cathodes. Appl. Catal. B Environ. 2020, 268, 118389. [Google Scholar] [CrossRef]
- Fu, R.; Jiang, P.; Xu, H.; Niu, B.; Jiang, F.; Yang, L.; Feng, T.; He, T. Performance of Pd-impregnated Sr1.9FeNb0.9Mo0.1O6–δ double perovskite as symmetrical electrodes for direct hydrocarbon solid oxide fuel cells. Int. J. Hydrogen Energy 2019, 44, 31394–31405. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhou, C.; Zhou, W.; Yang, N. Textured Sr2Sc0.1Nb0.1Co1.5Fe0.3O6–2δ Thin Film Cathodes for IT–SOFCs. Materials 2019, 12, 777. [Google Scholar] [CrossRef]
- Gómez-Pérez, A.; Azcondo, M.T.; Yuste, M.; Pérez-Flores, J.; Bonanos, N.; Porcher, F.; Muñoz-Noval, A.; Hoelzel, M.; García-Alvarado, F.; Amador, U. The A-cation deficient perovskite series La2–xCoTiO6–δ (0 ≤ x ≤ 0.20): New components for potential SOFC composite cathodes. J. Mater. Chem. A 2016, 4, 3386–3397. [Google Scholar] [CrossRef]
- Yue, Z.; Jiang, L.; Ai, N.; Guan, C.; Jiang, S.P.; Sun, X.; Rickard, W.D.A.; Wang, X.; Shao, Y.; Chen, K. Facile co-synthesis and utilization of ultrafine and highly active PrBa0.8Ca0.2Co2O5+δ-Gd0.2Ce0.8O1.9 composite cathodes for solid oxide fuel cells. Electrochim. Acta 2022, 403, 139673. [Google Scholar] [CrossRef]
- Tolstov, K.Y.; Politov, B.V.; Zhukov, V.P.; Chulkov, E.V.; Kozhevnikov, V.L. The impact of atomic defects on high-temperature stability and electron transport properties in Sr2Mg1−xNixMoO6–δ solid solutions. J. Alloys Compd. 2021, 883, 160821. [Google Scholar] [CrossRef]
- Wu, M.; Li, H.; Ma, S.; Chen, S.; Xiang, W. Boosting the surface oxygen activity for high performance Iron-based perovskite oxide. Sci. Total Environ. 2021, 795, 148904. [Google Scholar] [CrossRef]
- Sednev-Lugovets, A.L.; Sereda, V.V.; Malyshkin, D.A.; Tsvetkov, D.S.; Ivanov, I.L.; Zuev, A.Y.; Maignan, A. Defect chemistry and high-temperature thermodynamics of PrBaCo2O6-δ. J. Chem. Thermodynam. 2021, 161, 106523. [Google Scholar] [CrossRef]
- Gumeci, C.; Parrondo, J.; Hussain, A.M.; Thompson, D.; Dale, N. Praseodymium based double-perovskite cathode nanofibers for intermediate temperature solid oxide fuel cells (IT-SOFC). Int. J. Hydrogen Energy 2021, 46, 71798–71806. [Google Scholar] [CrossRef]
- Sereda, V.V.; Malyshkin, D.A.; Ivanov, I.L.; Tsvetkov, D.S.; Zuev, A.Y.; Maignan, A. Redox Thermochemistry, Thermodynamics, and Solar Energy Conversion and Storage Capability of Some Double Perovskite Cobaltites. Inorg. Chem. 2021, 60, 18141–18153. [Google Scholar] [CrossRef]
- Vinoth Kumar, R.; Khandale, A.P. A review on recent progress and selection of cobalt-based cathode materials for low temperature-solid oxide fuel cells. Renew. Sust. Energy 2022, 156, 111985. [Google Scholar] [CrossRef]
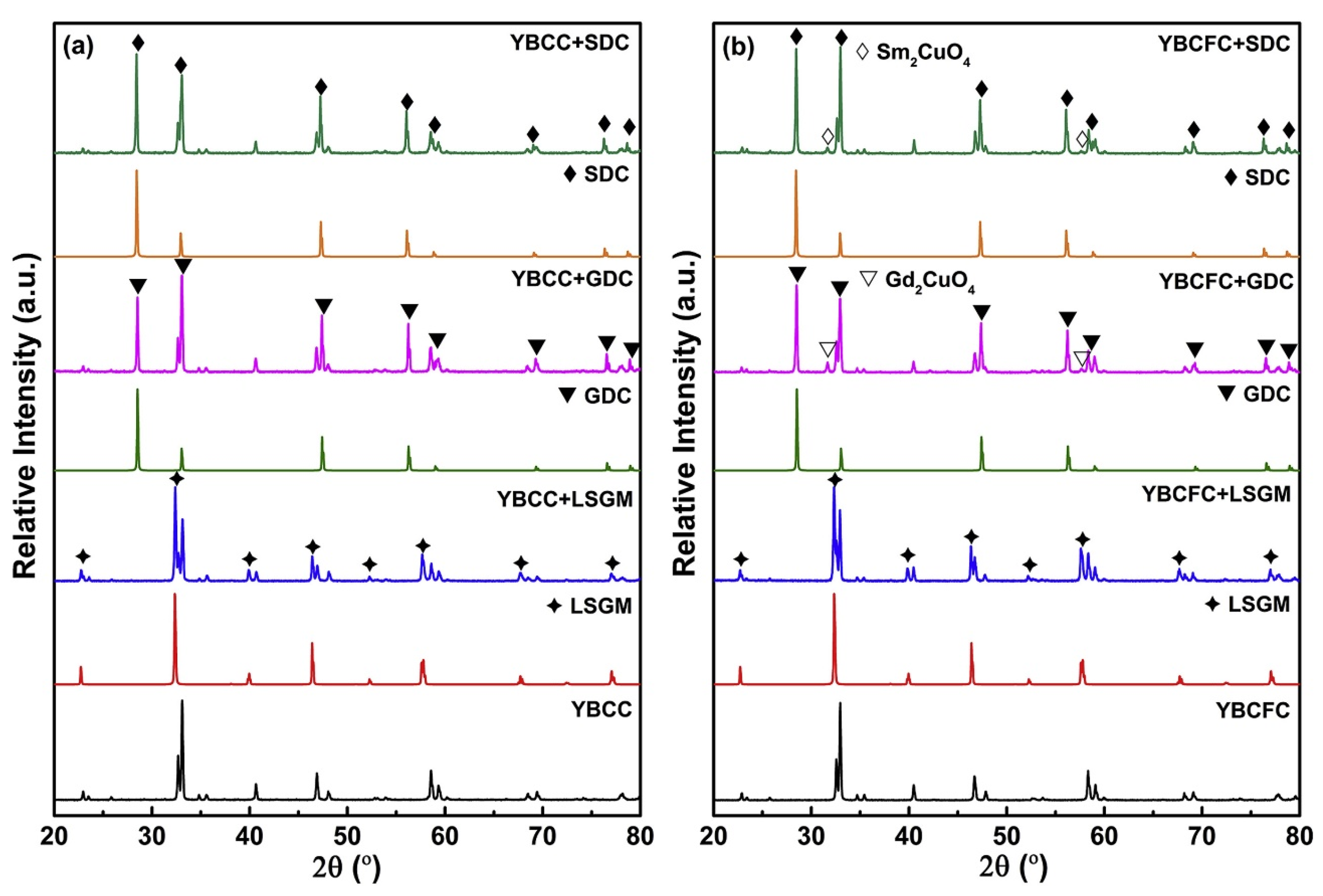

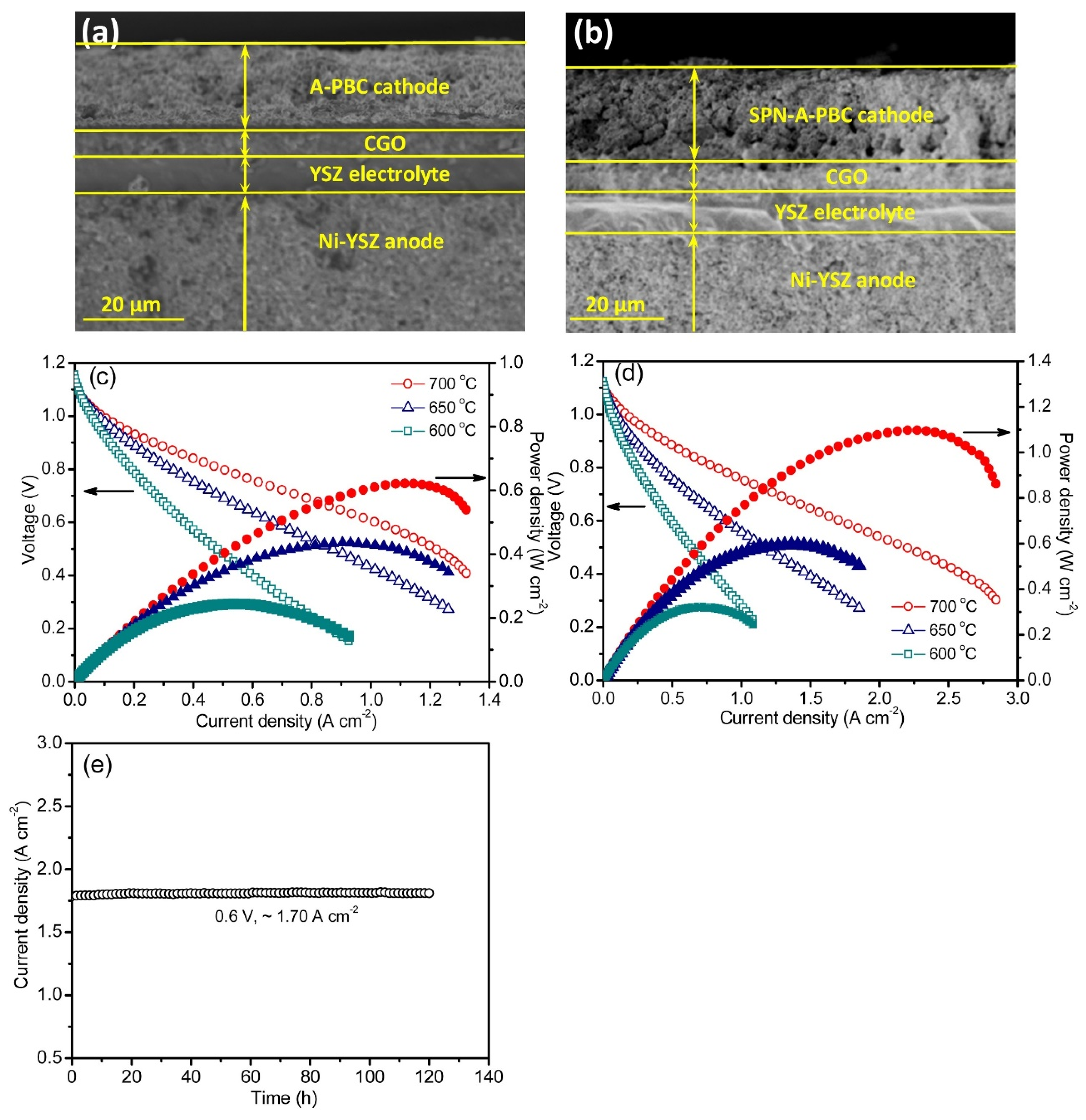
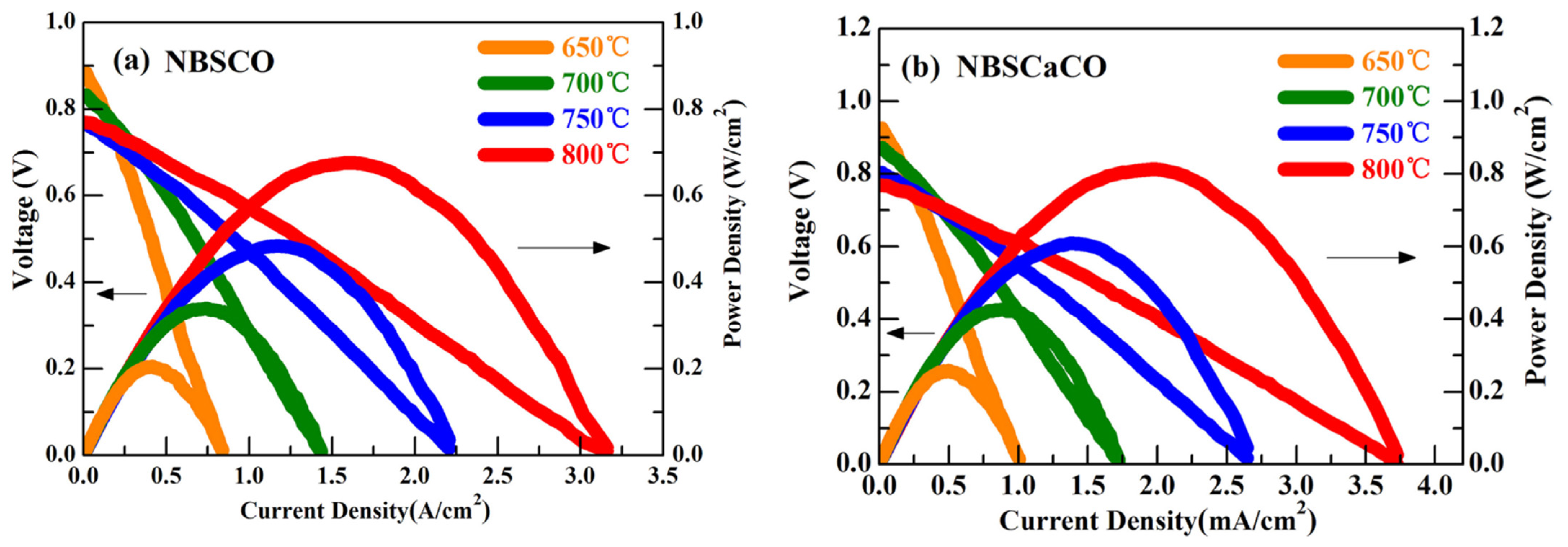
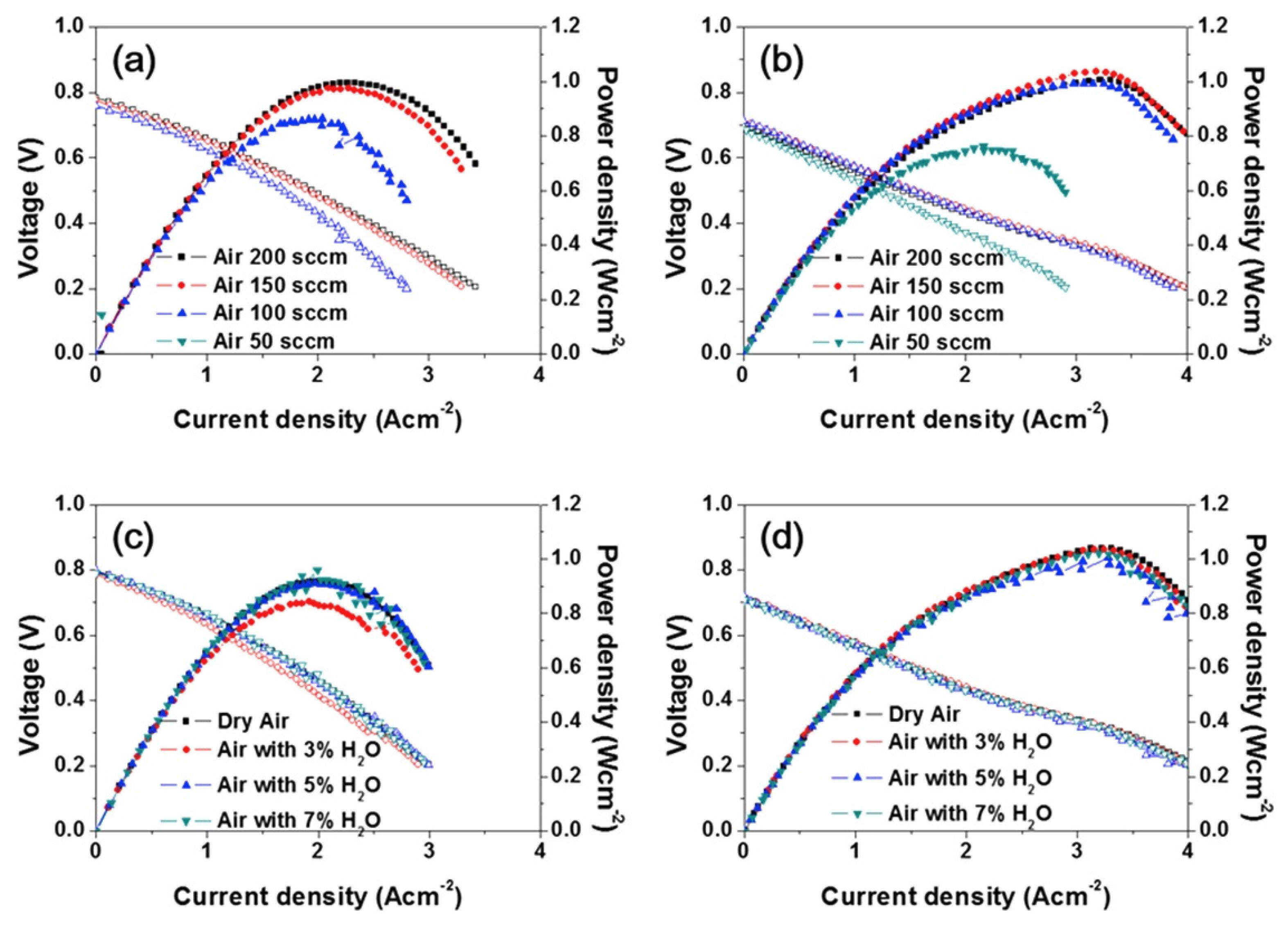

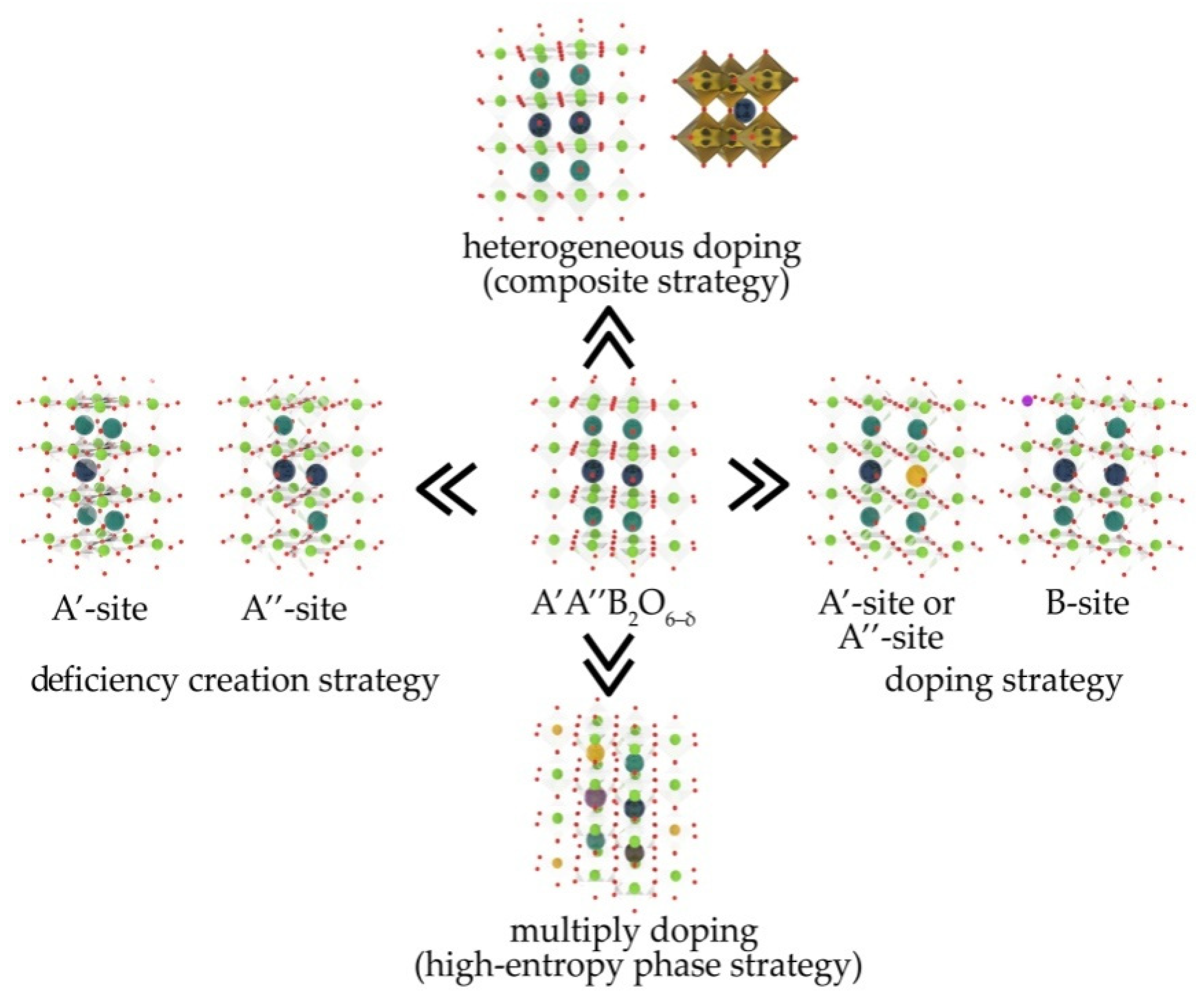
| Compound (Space Group) | TEC 106, K−1 | Temperature Interval, K | Refs. |
|---|---|---|---|
| NdBaCoFeO5+δ (P4/mmm) | 16.6 | 300–653 | [62] |
| 26.5 | 653–1100 | ||
| SmBaCoFeO5+δ (P4/mmm) | 13.6 | 300–518 | [62] |
| 19.3 | 518–1100 | ||
| GdBaCoFeO5+δ (P4/mmm) | 12.9 | 300–553 | [62] |
| 19.9 | 553–1100 | ||
| SmBaCo2O5+δ (Pmmm) | 21.2 | 293–1173 | [63] |
| SmBaCoCuO5+δ (Pmmm) | 15.0 | 293–1173 | [63] |
| NdBaCo2O5+δ (P4/mmm) | 19.7 | 293–1173 | [63] |
| NdBaCoCuO5+δ (P4/mmm) | 16.5 | 293–1173 | [63] |
| NdBaCo2O5+δ (P4/mmm) | 18.3 | 300–530 | [64] |
| 23.8 | 530–1300 | ||
| NdBaCo1.8Fe0.2O5+δ (P4/mmm) | 18.8 | 300–530 | [64] |
| 21.9 | 530–1300 | ||
| NdBaCo1.6Fe0.4O5+δ (P4/mmm) | 18.9 | 300–530 | [64] |
| 21.9 | 530–1300 | ||
| NdBaCo1.4Fe0.6O5+δ (P4/mmm) | 18.3 | 300–530 | [64] |
| 22.1 | 530–1300 | ||
| NdBaCo1.2Fe0.8O5+δ (P4/mmm) | 18.4 | 300–530 | [64] |
| 21.9 | 530–1300 | ||
| PrBaCo2O6–δ (Pmmm) | 16.8 | 298–473 | [66] |
| 21.6 | 473–1273 | ||
| NdBaCo2O6–δ (Pmmm) | 16.3 | 298–473 | [66] |
| 21.6 | 473–1273 | ||
| NdBaCo2O6–δ (Pm3m) | 11.9 | 298–473 | [66] |
| 22.6 | 473–1273 |
| Compound (Space Group) | CEC 103 | Direction | Refs. |
|---|---|---|---|
| NdBaCoFeO5+δ (P4/mmm) | 11.04 | in-plane | [62] |
| −17.27 | out-of-plane | ||
| 4.59 | volume | ||
| SmBaCoFeO5+δ (P4/mmm) | 20.72 | in-plane | [62] |
| −18.00 | out-of-plane | ||
| 23.94 | volume | ||
| GdBaCoFeO5+δ (P4/mmm) | 17.78 | in-plane | [62] |
| −12.36 | out-of-plane | ||
| 25.97 | volume | ||
| LaBaCuFeO5+δ (Pm3m) | 9.20 | volume | [78] |
| LaBa0.75Sr0.25CuFeO5+δ (Pm3m) | 7.55 | volume | [78] |
| PrBaCuFeO5+δ (P4/mmm) | 8.72 | volume | [78] |
| LaBaCoFeO5+δ (Pm3m) | 17.3 | volume | [79] |
| LaBaCoCuO5+δ (Pm3m) | 15.7 | volume | [79] |
| PrBaCuFeO5+δ (P4/mmm) | 12.0 | volume | [80] |
| Cathode | Electrolyte | ASR, Ω cm2 (T, K) | MPD, mW cm−2 (T, K) | Refs. |
|---|---|---|---|---|
| GdBaCo2O5+δ | Ce0.2Gd0.2O2–δ | 0.534 (918) | – | [91] |
| GdBaCo2O5+δ | YSZ | 0.25 (998) | 250 (1073) 500 (1073) * | [92] |
| PrBaCo2O5+δ | GDC | 0.23 (873) | – | [93] |
| LaBaCo2O5+δ | LSCM | – | 516 (1073) | [94] |
| GdBaCo2O5+δ | LSCM | – | 443 (1073) | [94] |
| PrBaCo2O5+δ | SDC | – | 866 (923) | [95] |
| NdBaCo2O5+δ | SDC | 0.08 (973) | – | [96] |
| YBaCo2O5+δ | YSZ | 2.03 (1053) | – | [97] |
| PrBaCo2O5+δ | BaCe0.5Zr0.3Y0.16Zn0.04O3–δ | 0.12 (973) | 361 (973) | [98] |
| LaBaCo2O5+δ | Gd0.1Ce0.9O1.95 | 0.0086 (1073) | – | [99] |
| Cathode | Electrolyte | ASR, Ω cm2 (T, K) | MPD, mW cm−2 (T, K) | Refs. |
|---|---|---|---|---|
| Pr0.95BaCo2O5+δ | Ce0.9Gd0.1O1.95 | 0.054 (923) | – | [104] |
| Nd0.96BaCo2O5+δ | Ce0.9Gd0.1O1.95 | 0.043 (973) | 600 (973) | [105] |
| Sm0.95BaCo2O5+δ | GDC | 0.038 (1023) | – | [106] |
| Sm0.90BaCo2O5+δ | Ce0.9Gd0.1O2–δ | 0.035 (973) | – | [107] |
| LaBa0.90Co2O5+δ | Ce0.9Gd0.1O1.95 | 0.023 (973) | – | [108] |
| PrBa0.92Co2O5+δ | Ce0.9Gd0.1O1.95 | 0.093 (873) | – | [109] |
| PrBa0.94Co2O5+δ | Ce0.9Gd0.1O1.95 | 0.042 (873) | 1030 (973) | [110] |
| NdBa0.90Co2O5+δ | Ce0.9Gd0.1O2 | 0.1 (973) | – | [111] |
| Pr0.5Ba0.245Ca0.25CoO3–δ | Ce0.9Gd0.1O1.95 | – | 2080 (1073) | [112] |
| PrBa0.42Sr0.5Co2O5+δ | La0.8Sr0.2Ga0.8Mg0.2O3–δ | 0.082 (1023) | – | [113] |
| PrBa0.5Sr0.5Co2O5+δ | Ce0.9Gd0.1O2–δ | 0.286 (873) | – | [117] |
| Pr0.9Ca0.1Ba0.8Ca0.2Co2O5+δ | SDC | 0.069 (973) | 712 (1073) | [118] |
| NdBa0.5Sr0.5Co2O5+δ | SDC | 0.09 (1073) | 341 (1073) | [119] |
| NdBa0.5Sr0.25Ca0.25Co2O5+δ | Sm0.2Ce0.8O1.9 | 0.062 (1073) | 812 (1073) * | [120] |
| NdBa0.75Ca0.25Co2O5+δ | GDC | 0.066 (873) | 2114 (873) | [121] |
| GdBa0.5Sr0.5Co2O5+δ | BaCe0.5Zr0.3Y0.16Zn0.04O3–δ | 0.15 (973) | 350 (973) | [122] |
| YBa0.5Sr0.5Co2O5+δ | La0.9Sr0.1Ga0.8Mg0.115Co0.085O2.85 | – | 650 (1073) | [123] |
| YBa0.8Sr0.2Co2O5+δ | Ce0.9Gd0.1O2–δ | 0.21 (973) | – | [124] |
| Pr0.5Sm0.5Ba0.5Sr0.5Co2O5+δ | Ce0.9Gd0.1O2 | 0.10 (973) | [125] |
| Cathode | Electrolyte | ASR, Ω cm2 (T, K) | MPD, m Wcm−2 (T, K) | Refs. |
|---|---|---|---|---|
| PrBaCo1.6Ni0.4O5+δ | Ce0.8Sm0.2O1.9 | 0.018 (1073) | 732 (1073) | [129] |
| PrBa0.5Sr0.5Co1.9Ni0.1O5+δ | YSZ | 0.297 (1073) | 120 (1073) | [130] |
| PrBa0.9Ca0.1Co1.85Zn0.15O5+δ | BZCYYb | 0.04 (1023) * | 876 (1023) * | [132] |
| SmBa0.5Sr0.5Co1.5Cu0.5O5+δ | GDC | 0.201 (873) | 1760 (923) | [133] |
| YBaCo1.4Cu0.6O5+δ | La0.9Sr0.1Ga0.8Mg0.2O3–δ | 0.076 (1023) | 815 (1123) | [134] |
| YBaCoCuO5+δ | La0.9Sr0.1Ga0.8Mg0.2O3–δ | 0.138 (973) | 543 (1073) | [86] |
| PrBa0.94Co1.96Ta0.4O5+δ | GCO | 0.020 (973) | 1050 (973) | [136] |
| PrBaCo1.97Mo0.03O5+δ | Sm0.2Ce0.8O1.9 | 0.067 (973) | 339 (973) | [137] |
| SmSrCo0.8Mn0.2O5+δ | Ce0.9Gd0.1O1.95 | 0.078 (973) | – | [138] |
| GdBaCo1.8Mn0.2O5+δ | Ce0.9Gd0.1O2–δ | 0.078 (92) | – | [139] |
| GdBaCo1.5Mn0.5O5+δ | LSGM | 0.040 (1123) | – | [140] |
| PrBaFe2O5+δ | Sm0.2Ce0.8O1.9 | 0.18 (973) | – | [143] |
| YBaCo1.8Fe0.2O5+δ | La0.8Sr0.2Ga0.8Mg0.115Co0.085O2.85 | 0.13 (973) | 768 (1073) | [145] |
| PrBaCo1.6Fe0.4O5+δ | Ce0.8Sm0.2O2–γ | 0.13 (973) | 446.4 (973) | [145] |
| PrBaCoFeO5+δ | YSZ | – | 720 (973) ** | [146] |
| PrBaCo1.5Fe0.5O5+δ | La0.8Sr0.2Ga0.83Mg0.17O2.815 | 0.07 (1123) | 697 (1123) | [147] |
| NdBa0.5Sr0.5Co1.5Fe0.5O5+δ | Nd0.1Ce0.9O2–δ | – | 1010 (923) # | [151] |
| GdBaCo1.4Fe0.6O5+δ | Ce0.9Gd0.1O2–δ | 0.096 (923) | – | [153] |
| PrBa0.5Sr0.5Co1.8Fe0.2O5+δ | ScSZ | 0.012 (973) | 1350 (973) | [154] |
| NdBaCo1.6Fe0.4O5+δ | Gd0.1Ce0.9O2 | 0.17 (973) | – | [155] |
| NdBa0.9Co1.9Fe0.1O5+δ | Ce0.9Gd0.1O2–δ | 0.14 (973) | – | [157] |
| PrBaCo0.2Fe1.8O5+δ | La0.9Sr0.1Ga0.8Mg0.2O3 | – | 735 (1123) | [158] |
| PrBaCo2/3Fe2/3Cu2/3O5+δ | Ce0.9Gd0.1O1.95 | 0.038 (1073) | 659 (1073) | [159] |
| NdBaCo2/3Fe2/3Cu2/3O5+δ | La0.9Sr0.1Ga0.8Mg0.2O3–δ | 0.023 (1073) | 719 (1073) | [160] |
| PrBaCo2/3Fe2/3Mn1/2O5+δ | Sm0.2Ce0.8O1.9 | 0.028 (1073) | 588 (1073) | [161] |
| Material Composition | Diffusion Coefficient (cm2 s−1) | T, K | Activation Energy (kJ mol−1) | Refs. |
|---|---|---|---|---|
| PrBaCo2O5+δ | 3.0·10−8 | 873 | 40.5 | [149] |
| PrBa0.5Sr0.5Co2O5+δ | 8.33·10−8 | 873 | 28.9 | [149] |
| PrBaCo1.5Fe0.5O5+δ | 8.0·10−8 | 873 | 24.12 | [149] |
| PrBa0.5Sr0.5CoFeO5+δ | 5.50·10−8 | 873 | 41.6 | [152] |
| PrBa0.5Sr0.5Co1.5Fe0.5O5+δ | 1.18·10−7 | 873 | 30.9 | [149] |
| GdBa0.5Sr0.5Co2O5+δ | 4·10−8 | 923 | 47.2 | [152] |
| GdBa0.5Sr0.5Co1.5Fe0.5O5+δ | 3.0·10−8 | 873 | 34.7 | [149] |
| GdBa0.5Sr0.5Co1.5Fe0.5O5+δ | 5.13·10−8 | 923 | 40.6 | [152] |
| GdBa0.5Sr0.5CoFeO5+δ | 7.5·10−8 | 923 | 44.9 | [149] |
| NdBa0.5Sr0.5Co2O5+δ | 4·10−8 | 873 | 28.7 | [152] |
| NdBa0.5Sr0.5Co1.5Fe0.5O5+δ | 5.16·10−8 | 873 | 48.0 | [149] |
| NdBa0.5Sr0.5CoFeO5+δ | 3.8·10−8 | 873 | 28.6 | [152] |
| Cathode | Electrolyte | ASR, Ω cm2 (T, K) | MPD, mW cm–2 (T, K) | Refs. |
|---|---|---|---|---|
| LaBaCuFeO5+δ | Ce0.8Sm0.2O1.9 | 0.21 (973) | 557 (1073) | [176] |
| LaBaCuCoO5+δ | Ce0.8Sm0.2O1.9 | 0.11 (973) | 603 (1073) | [176] |
| GdBaCuCoO5+δ | La0.9Sr0.1Ga0.8Mg0.2O3–δ | 0.091 (1023) | 545 (1073) | [177] |
| GdBaCuCoO5+δ | Sm0.2Ce0.8O1.9 | 0.129 (1023) | 528 (1073) | [177] |
| SmBaCuCoO5+δ | BaCe0.7Zr0.1Y0.2O3–δ | 0.22 (973) | 355 (973) | [178] |
| PrBaCuCoO5+δ | Sm0.2Ce0.8O1.9 | 0.047 (973) | 791 (973) | [179] |
| LaBaCuFeO5+δ | BaZr0.1Ce0.7Y0.2O3–δ | 0.27 (973) | 327 (973) | [181] |
| LaBaCuCoO5+δ | BaZr0.1Ce0.7Y0.2O3–δ | 0.15 (973) | 432 (973) | [181] |
| GdBaFeNiO5+δ | BaZr0.1Ce0.7Y0.2O3–δ | 0.15 (973) | 456 (973) | [183] |
| PrBaCoFeO5+δ | La0.9Sr0.1Ga0.8Mg0.2O3–δ | 0.049 (1073) | 749 (1073) | [184] |
| NdBaCoFeO5+δ | La0.9Sr0.1Ga0.8Mg0.2O3–δ | 0.062 (1073) | 669 (1073) | [184] |
| La1.4Ca0.6CoMnO5+δ | La0.9Sr0.1Ga0.8Mg0.2O3–δ | – | 445 (1073) | [186] |
| NdBa0.7Ca0.3CoCuO5+δ | Gd0.1Ce0.9O1.95 | 0.038 (973) | 1840 (1073) | [187] |
| Y0.93BaCoCuO5+δ | La0.9Sr0.1Ga0.8Mg0.2O3–δ | 0.029 (1073) | 862 (1123) | [188] |
| Pr0.9Ca0.1BaCoFeO5+δ | La0.9Sr0.1Ga0.8Mg0.2O3–δ | 0.027 (1073) | 728 (1073) | [190] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klyndyuk, A.I.; Chizhova, E.A.; Kharytonau, D.S.; Medvedev, D.A. Layered Oxygen-Deficient Double Perovskites as Promising Cathode Materials for Solid Oxide Fuel Cells. Materials 2022, 15, 141. https://doi.org/10.3390/ma15010141
Klyndyuk AI, Chizhova EA, Kharytonau DS, Medvedev DA. Layered Oxygen-Deficient Double Perovskites as Promising Cathode Materials for Solid Oxide Fuel Cells. Materials. 2022; 15(1):141. https://doi.org/10.3390/ma15010141
Chicago/Turabian StyleKlyndyuk, Andrei I., Ekaterina A. Chizhova, Dzmitry S. Kharytonau, and Dmitry A. Medvedev. 2022. "Layered Oxygen-Deficient Double Perovskites as Promising Cathode Materials for Solid Oxide Fuel Cells" Materials 15, no. 1: 141. https://doi.org/10.3390/ma15010141
APA StyleKlyndyuk, A. I., Chizhova, E. A., Kharytonau, D. S., & Medvedev, D. A. (2022). Layered Oxygen-Deficient Double Perovskites as Promising Cathode Materials for Solid Oxide Fuel Cells. Materials, 15(1), 141. https://doi.org/10.3390/ma15010141