Platelet-Derived Growth Factor-Modulated Guided Tissue Regeneration with a Bioresorbable Membrane in Class III Furcation Defects: A Histometric Study in the Monkey
Abstract
1. Introduction
2. Materials and Methods
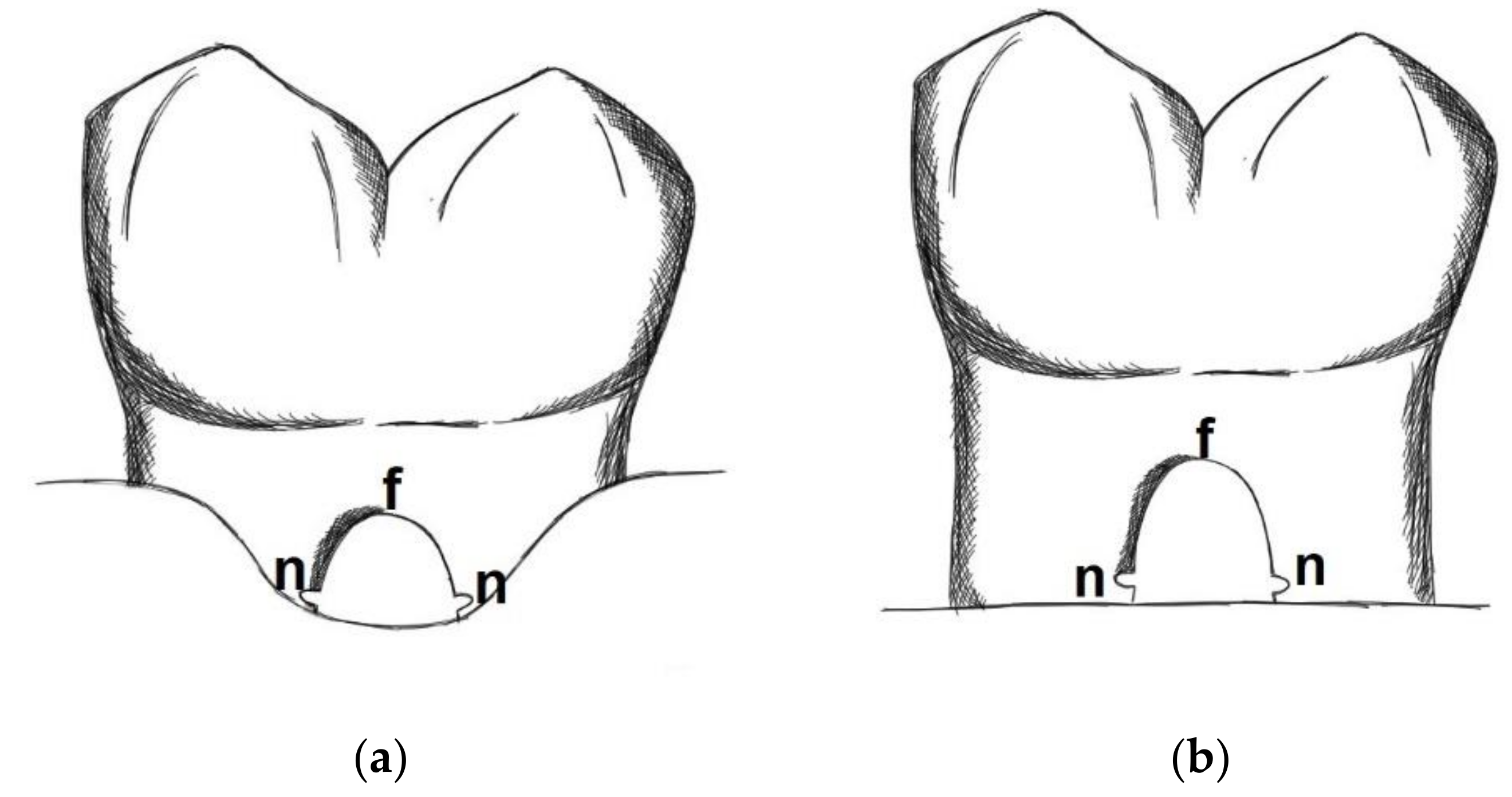
- Defect height: Vertical distance between apical end of root notch (n) and coronal end of furcation (f)
- New bone height: Vertical distance between apical end of root notch (n) and coronal end of newly formed bone (b)
- New cementum height: Vertical distance between apical end of root notch (n) and coronal end of newly formed cementum (c)
- New connective tissue height: Vertical distance between apical end of root notch (n) and apical end of epithelium (e)
- Epithelial height: Vertical distance between apical end of epithelium (e) and coronal end of furcation (f)
- 6.
- Defect area: Area outlined by a line between the apical ends of the notches and the root surfaces along the furcation.
- 7.
- New bone area: Area occupied by newly formed bone within the defect area
- 8.
- New cementum area: Area occupied by newly formed cementum within the defect area
- 9.
- New soft connective tissue area: Area occupied by newly formed soft connective tissue within the defect area
- 10.
- Epithelium area: Area occupied by epithelium within the defect area
- 11.
- Free bone graft area: Area occupied within the defect area by bone graft particles not embedded into new bone
3. Results
3.1. Clinical Findings
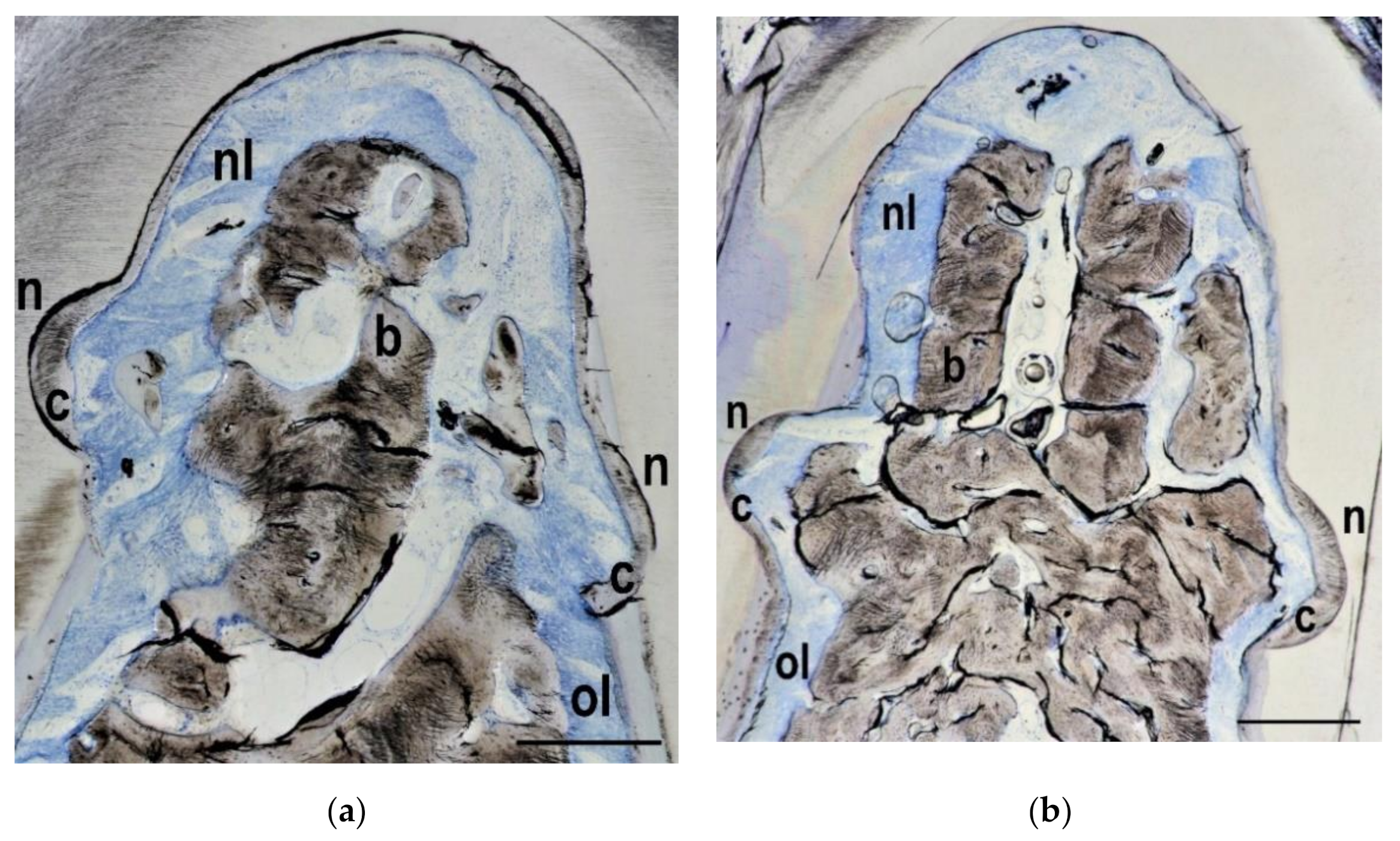
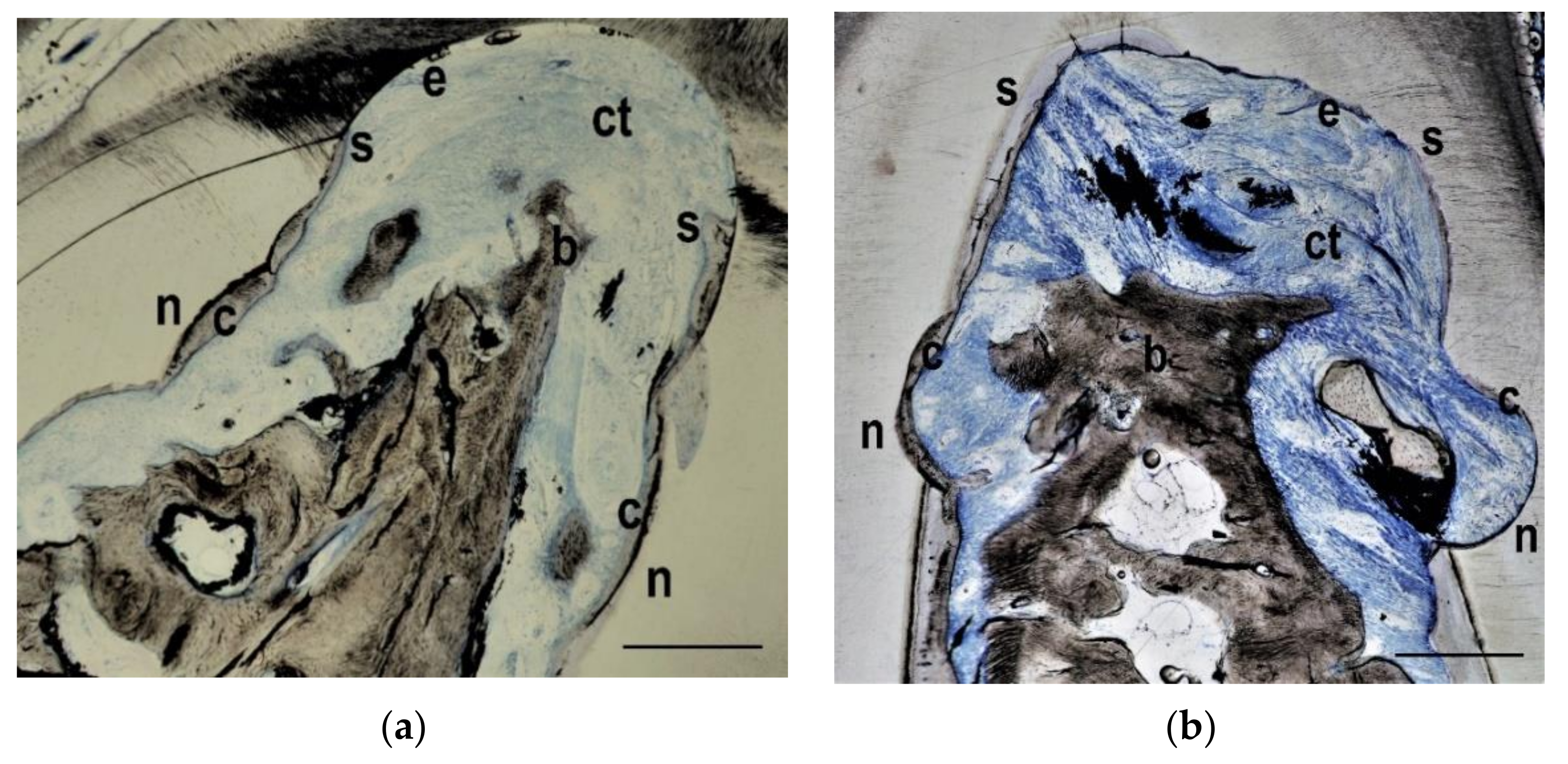
3.2. Histological Findings
3.3. Histometrical Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carranza, F.A., Jr.; Jolkovsky, D.L. Current status of periodontal therapy for furcation involvements. Dent. Clin. N. Am. 1991, 35, 555–570. [Google Scholar] [PubMed]
- Pontoriero, R.; Nyman, S.; Lindhe, J.; Rosenberg, E.; Sanavi, F. Guided tissue regeneration in the treatment of furcation defects in man. J. Clin. Periodontol. 1987, 14, 618–620. [Google Scholar] [CrossRef] [PubMed]
- Pontoriero, R.; Lindhe, J.; Nyman, S.; Karring, T.; Rosenberg, E.; Sanavi, F. Guided tissue regeneration in degree II furcation-involved mandibular molars. A clinical study. J. Clin. Periodontol. 1988, 15, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Caffesse, R.G.; Smith, B.A.; Duff, B.; Morrison, E.C.; Merrill, D.; Becker, W. Class II furcations treated by guided tissue regeneration in humans: Case reports. J. Periodontol. 1990, 61, 510–514. [Google Scholar] [CrossRef]
- Mardam-Bey, W.; Majzoub, Z.; Kon, S. Anatomic considerations in the etiology and management of maxillary and mandibular molars with furcation involvement. Int. J. Periodontics Restor. Dent. 1991, 11, 398–409. [Google Scholar]
- Reddy, M.S.; Aichelmann-Reidy, M.E.; Avila-Ortiz, G.; Klokkevold, P.R.; Murphy, K.G.; Rosen, P.S.; Schallhorn, R.G.; Sculean, A.; Wang, H.L. Periodontal regeneration—Furcation defects: A consensus report from the AAP Regeneration Workshop. J. Periodontol. 2015, 86 (Suppl. 2), S131–S133. [Google Scholar] [CrossRef]
- Pontoriero, R.; Nyman, S.; Ericsson, I.; Lindhe, J. Guided tissue regeneration in surgically-produced furcation defects. An experimental study in the beagle dog. J. Clin. Periodontol. 1992, 19, 159–163. [Google Scholar] [CrossRef]
- Becker, W.; Becker, B.E.; Prichard, J.F.; Caffesse, R.; Rosenberg, E.; Gian-Grasso, J. Root isolation for new attachment procedures. A surgical and suturing method: Three case reports. J. Periodontol. 1987, 58, 819–826. [Google Scholar] [CrossRef]
- Pontoriero, R.; Lindhe, J.; Nyman, S.; Karring, T.; Rosenberg, E.; Sanavi, F. Guided tissue regeneration in the treatment of furcation defects in mandibular molars. A clinical study of degree III involvements. J. Clin. Periodontol. 1989, 16, 170–174. [Google Scholar] [CrossRef]
- Kocher, T.; Kuhrau, N.; Plagmann, H.C. Guided tissue regeneration for the treatment of different periodontal defects. A clinical study. Dtsch. Zahnarztl. Z. 1991, 46, 423–425. [Google Scholar]
- Garrett, S.; Gantes, B.; Zimmerman, G.; Egelberg, J. Treatment of mandibular class III periodontal furcation defects. Coronally positioned flaps with and without expanded polytetrafluoroethylene membranes. J. Periodontol. 1994, 65, 592–597. [Google Scholar] [CrossRef]
- Pontoriero, R.; Lindhe, J. Guided tissue regeneration in the treatment of degree III furcation defects in maxillary molars. J. Clin. Periodontol. 1995, 22, 810–812. [Google Scholar] [CrossRef]
- Yamanouchi, K.; Chang, C.Y.; Yamada, S. A clinical evaluation of guided tissue regeneration in the treatment of class II and class III furcation bony defects. Bull. Tokyo Dent. Coll. 1995, 36, 9–17. [Google Scholar]
- Araujo, M.; Berglundh, T.; Lindhe, J. The periodontal tissues in healed degree III furcation defects. An experimental study in dogs. J. Clin. Periodontol. 1996, 23, 532–541. [Google Scholar] [CrossRef]
- Lindhe, J.; Pontoriero, R.; Berglundh, T.; Araujo, M. The effect of flap management and bioresorbable occlusive devices in GTR treatment of degree III furcation defects. An experimental study in dogs. J. Clin. Periodontol. 1995, 22, 276–283. [Google Scholar] [CrossRef]
- Eickholz, P.; Kim, T.S.; Holle, R. Guided tissue regeneration with non-resorbable and biodegradable barriers: 6 months results. J. Clin. Periodontol. 1997, 24, 92–101. [Google Scholar] [CrossRef]
- Da Costa-Noble, R.; Soustre, E.C.; Cadot, S.; Lauverjat, Y.; Lefebvre, F.; Rabaud, M. Evaluation of bioabsorbable elastin-fibrin matrix as a barrier in surgical periodontal treatment. J. Periodontol. 1996, 67, 927–934. [Google Scholar] [CrossRef]
- Dogan, A.; Taner, L.; Oygür, T.; Balos, K. Effects of fibrin adhesive material (Tissucol) application on furcation defects in dogs. J. Nihon Univ. Sch. Dent. 1992, 34, 34–41. [Google Scholar] [CrossRef][Green Version]
- Anderegg, C.R.; Martin, S.J.; Gray, J.L.; Mellonig, J.T.; Gher, M.E. Clinical evaluation of the use of decalcified freeze-dried bone allograft with guided tissue regeneration in the treatment of molar furcation invasions. J. Periodontol. 1991, 62, 264–268. [Google Scholar] [CrossRef]
- Gantes, B.G.; Synowski, B.N.; Garrett, S.; Egelberg, J.H. Treatment of periodontal furcation defects. Mandibular class III defects. J. Periodontol. 1991, 62, 361–365. [Google Scholar] [CrossRef]
- Liao, C.S.; Liu, C.M.; Wong, M.Y.; Hou, L.T.; Chang, W.K. Guided tissue regeneration demineralized freeze-dried bone allograft: Treatment of furcation defects in mandibular molars. J. Formos. Med. Assoc. 1995, 94, 406–413. [Google Scholar] [PubMed]
- Yaegashi, T. Effect of hydroxylapatite particles during healing of experimental furcation involvement in beagle dogs. Nihon Shishubyo Gakkai Kaishi 1989, 31, 83–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pepelassi, E.M.; Bissada, N.F.; Greenwell, H.; Farah, C.F. Doxycycline-tricalcium phosphate composite graft facilitates osseous healing in advanced periodontal furcation defects. J. Periodontol. 1991, 62, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Caffesse, R.G.; Quinones, C.R. Polypeptide growth factors and attachment proteins in periodontal wound healing and regeneration. Periodontol. 2000 1993, 1, 69–79. [Google Scholar] [CrossRef]
- Lynch, S.E.; Williams, R.C.; Polson, A.M.; Howell, T.H.; Reddy, M.S.; Zappa, U.E.; Antoniades, H.N. A combination of platelet-derived and insulin-like growth factors enhances periodontal regeneration. J. Clin. Periodontol. 1989, 16, 545–548. [Google Scholar] [CrossRef]
- Lynch, S.E.; de Castilla, G.R.; Williams, R.C.; Kiritsy, C.P.; Howell, T.H.; Reddy, M.S.; Antoniades, H.N. The effects of short-term application of a combination of platelet-derived and insulin-like growth factors on periodontal wound healing. J. Periodontol. 1991, 62, 458–467. [Google Scholar] [CrossRef]
- Rutherford, R.B.; Niekrash, C.E.; Kennedy, J.E.; Charette, M.F. Platelet-derived and insulin-like growth factors stimulate regeneration of periodontal attachment in monkeys. J. Periodontal Res. 1992, 27, 285–290. [Google Scholar] [CrossRef]
- Rutherford, R.B.; Ryan, M.E.; Kennedy, J.E.; Tucker, M.M.; Charette, M.F. Platelet-derived growth factor and dexamethasone combined with a collagen matrix induce regeneration of the periodontium in monkeys. J. Clin. Periodontol. 1993, 20, 537–544. [Google Scholar] [CrossRef]
- Park, J.B.; Matsuura, M.; Han, K.Y.; Norderyd, O.; Lin, W.L.; Genco, R.J.; Cho, M.I. Periodontal regeneration in class III furcation defects of beagle dogs using guided tissue regenerative therapy with platelet-derived growth factor. J. Periodontol. 1995, 66, 462–477. [Google Scholar] [CrossRef]
- Cho, M.I.; Lin, W.L.; Genco, R.J. Platelet-derived growth factor-modulated guided tissue regenerative therapy. J. Periodontol. 1995, 66, 522–530. [Google Scholar] [CrossRef]
- Bennardo, F.; Liborio, F.; Barone, S.; Antonelli, A.; Buffone, C.; Fortunato, L.; Guidice, A. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: A pilot study. Clin. Oral Investig. 2021. [Google Scholar] [CrossRef]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part A: Intra-bony defects, furcation defects and periodontal plastic surgery. A systematic review and meta-analysis. J. Clin. Periodontol. 2017, 44, 67–82. [Google Scholar] [CrossRef]
- Laugisch, O.; Cosgarea, R.; Nikou, G.; Nikolidakis, D.; Donos, N.; Salvi, G.E.; Stavropoulos, A.; Jepsen, S.; Sculean, A. Histologic evidence of periodontal regeneration in furcation defects: A systematic review. Clin. Oral Investig. 2019, 23, 2861–2906. [Google Scholar] [CrossRef]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The Säge-Schliff (sawing and grinding) technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef]
- Wang, H.L.; Greenwell, H.; Fiorellini, J.; Giannobile, W.; Offenbacher, S.; Salkin, L.; Townsend, C.; Sheridan, P.; Genco, R.J. Periodontal Regeneration. J. Periodontol. 2005, 76, 1601–1622. [Google Scholar] [CrossRef]
- Górski, B.; Kaczyński, T.; Miskiewicz, A.; Górska, R. Early postoperative healing following guided tissue regeneration in aggressive periodontitis patients. Dent. Med. Probl. 2018, 55, 289–297. [Google Scholar] [CrossRef]
- Wachtel, H.; Schenk, G.; Böhm, S.; Weng, D.; Zuhr, O.; Hürzeler, M.B. Microsurgical access flap and enamel matrix derivative for the treatment of periodontal intrabony defects: A controlled clinical study. J. Clin. Periodontol. 2003, 30, 496–504. [Google Scholar] [CrossRef]
- Majzoub, J.; Barootchi, S.; Tavelli, L.; Wang, C.W.; Travan, S.; Wang, H.L. Treatment effect of guided tissue regeneration on the horizontal and vertical components of furcation defects: A retrospective study. J. Periodontol. 2020, 91, 1148–1158. [Google Scholar] [CrossRef]
- Ling, L.J.; Hung, S.L.; Lee, C.F.; Chen, Y.T.; Wu, K.M. The influence of membrane exposure on the outcomes of guided tissue regeneration: Clinical and microbiological aspects. J. Periodontal Res. 2003, 38, 57–63. [Google Scholar] [CrossRef]
- Majzoub, J.; Barootchi, S.; Tavelli, L.; Wang, C.W.; Chan, H.L.; Wang, H.L. Guided tissue regeneration combined with bone allograft in infrabony defects: Clinical outcomes and assessment of prognostic factors. J. Periodontol. 2020, 91, 746–755. [Google Scholar] [CrossRef]
- Soldatos, N.K.; Stylianou, P.; Koidou, V.P.; Angelov, N.; Yukna, R.; Romanos, G.E. Limitations and options using resorbable versus nonresorbable membranes for successful guided bone regeneration. Quintessence Int. 2017, 48, 131–147. [Google Scholar] [CrossRef] [PubMed]


| Histometric Parameters | GTR (n = 5) | PDGF (n = 5) | Control (n = 2) |
|---|---|---|---|
| Defect height (in mm) | 2.2 ± 0.5 mm | 2.2 ± 0.6 mm | 2.3 ± 0.0 mm |
| New bone height (in % of defect height) | 51 ± 17% | 42 ± 30% | 77 ± 4% |
| New cementum height (in % of defect height) | 44 ± 27% | 42 ± 25% | 66 ± 6% |
| New connective tissue height (in % of defect height) | 92 ± 11% | 77 ± 37% | 96 ± 6% |
| Epithelium height (in % of defect height) | 6 ± 11% | 20 ± 39% | 3 ± 4% |
| Defect area (in mm2) | 3.5 ± 1.2 mm2 | 3.1 ± 0.9 mm2 | 3.5 ± 0.0 mm2 |
| New bone area (in % of defect area) | 27 ± 18% | 23 ± 15% | 57 ± 13% |
| New cementum area (in % of defect area) | 5 ± 3% | 4 ± 3% | 7 ± 2% |
| New connective tissue area (in % of defect area) | 63 ± 16% | 55 ± 27% | 35 ± 9% |
| Epithelium area (in % of defect area) | 4 ± 7% | 19 ± 40% | 1 ± 2% |
| Free bone graft area (in % of defect area) | 2 ± 2% | 1 ± 1% | 0 ± 0% |
| Histometric Parameters | GTR (n = 5) | PDGF (n = 5) | Control (n = 2) |
|---|---|---|---|
| Defect height (in mm) | 2.2 ± 0.5 mm | 2.3 ± 0.5 mm | 2.6 ± 0.01 mm |
| New bone height (in % of defect height) | 57 ± 20% | 52 ± 23% | 80 ± 2% |
| New cementum height (in % of defect height) | 53 ± 15% | 40 ± 30% | 87 ± 19% |
| New connective tissue height (in % of defect height) | 93 ± 8% | 89 ± 7% | 97 ± 2% |
| Epithelium height (in % of defect height) | 5 ± 8% | 10 ± 9% | 0 ± 0% |
| Defect area (in mm2) | 3.6 ± 1.3 mm2 | 4.1 ± 1.4 mm2 | 4.3 ± 0.4 mm2 |
| New bone area (in % of defect area) | 35 ± 15% | 27 ± 15% | 55 ± 10% |
| New cementum area (in % of defect area) | 5 ± 2% | 7 ± 5% | 6 ± 1% |
| New connective tissue area (in % of defect area) | 56 ± 19% | 60 ± 17% | 40 ± 11% |
| Epithelium area (in % of defect area) | 2 ± 4% | 6 ± 4% | 0 ± 0% |
| Free bone graft area (in % of defect area) | 1 ± 1% | 1 ± 1% | 0 ± 0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weng, D.; Stapf, L.; Kern, M.; Kohal, R.-J. Platelet-Derived Growth Factor-Modulated Guided Tissue Regeneration with a Bioresorbable Membrane in Class III Furcation Defects: A Histometric Study in the Monkey. Materials 2021, 14, 2420. https://doi.org/10.3390/ma14092420
Weng D, Stapf L, Kern M, Kohal R-J. Platelet-Derived Growth Factor-Modulated Guided Tissue Regeneration with a Bioresorbable Membrane in Class III Furcation Defects: A Histometric Study in the Monkey. Materials. 2021; 14(9):2420. https://doi.org/10.3390/ma14092420
Chicago/Turabian StyleWeng, Dietmar, Lina Stapf, Matthias Kern, and Ralf-Joachim Kohal. 2021. "Platelet-Derived Growth Factor-Modulated Guided Tissue Regeneration with a Bioresorbable Membrane in Class III Furcation Defects: A Histometric Study in the Monkey" Materials 14, no. 9: 2420. https://doi.org/10.3390/ma14092420
APA StyleWeng, D., Stapf, L., Kern, M., & Kohal, R.-J. (2021). Platelet-Derived Growth Factor-Modulated Guided Tissue Regeneration with a Bioresorbable Membrane in Class III Furcation Defects: A Histometric Study in the Monkey. Materials, 14(9), 2420. https://doi.org/10.3390/ma14092420








