Preparation of UiO-66-NH2@PDA under Water System for Chemical Warfare Agents Degradation
Abstract
1. Introduction
2. Experimental Section
2.1. Chemicals and Materials
2.2. Synthesis of UiO-66-NH2
2.3. Preparation of Buffer Solution
2.4. Synthesis of UiO-66-NH2@PDA Composites
2.5. Characterizations
2.6. Degradation of DMNP
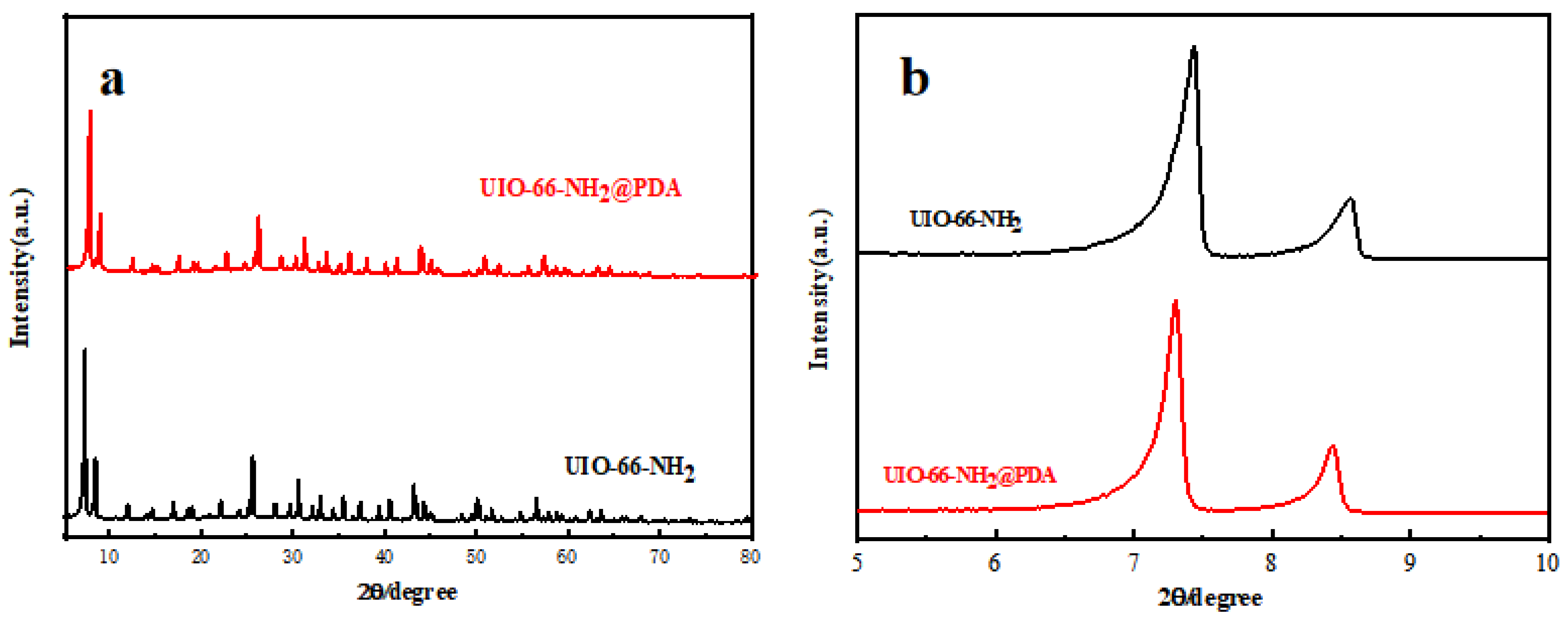
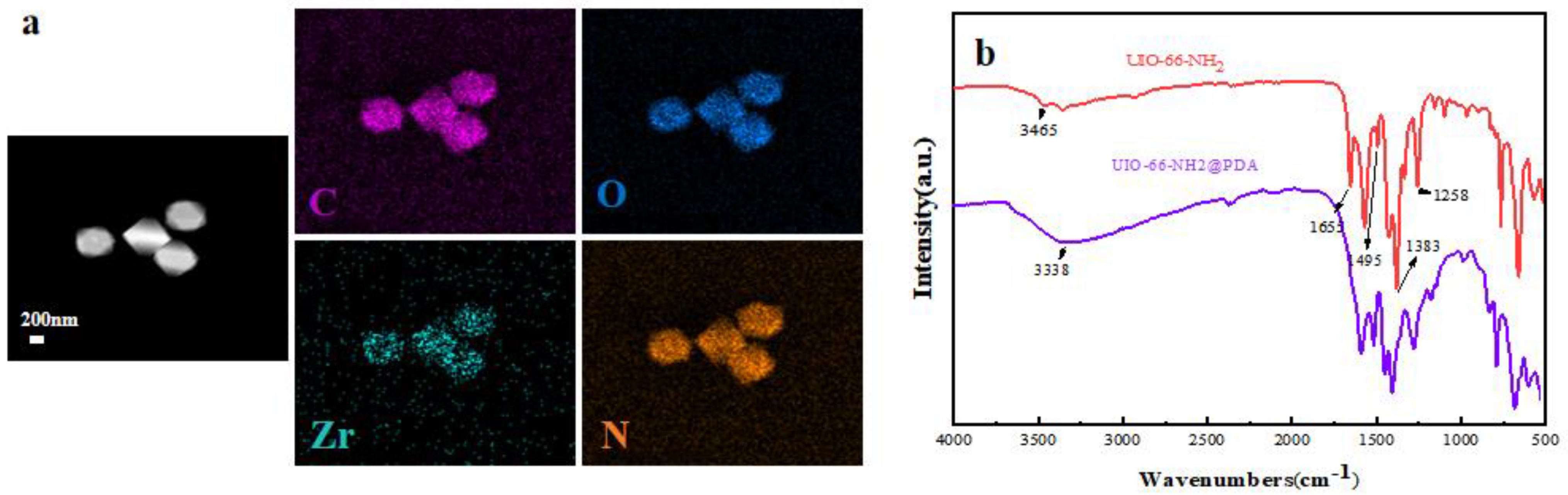
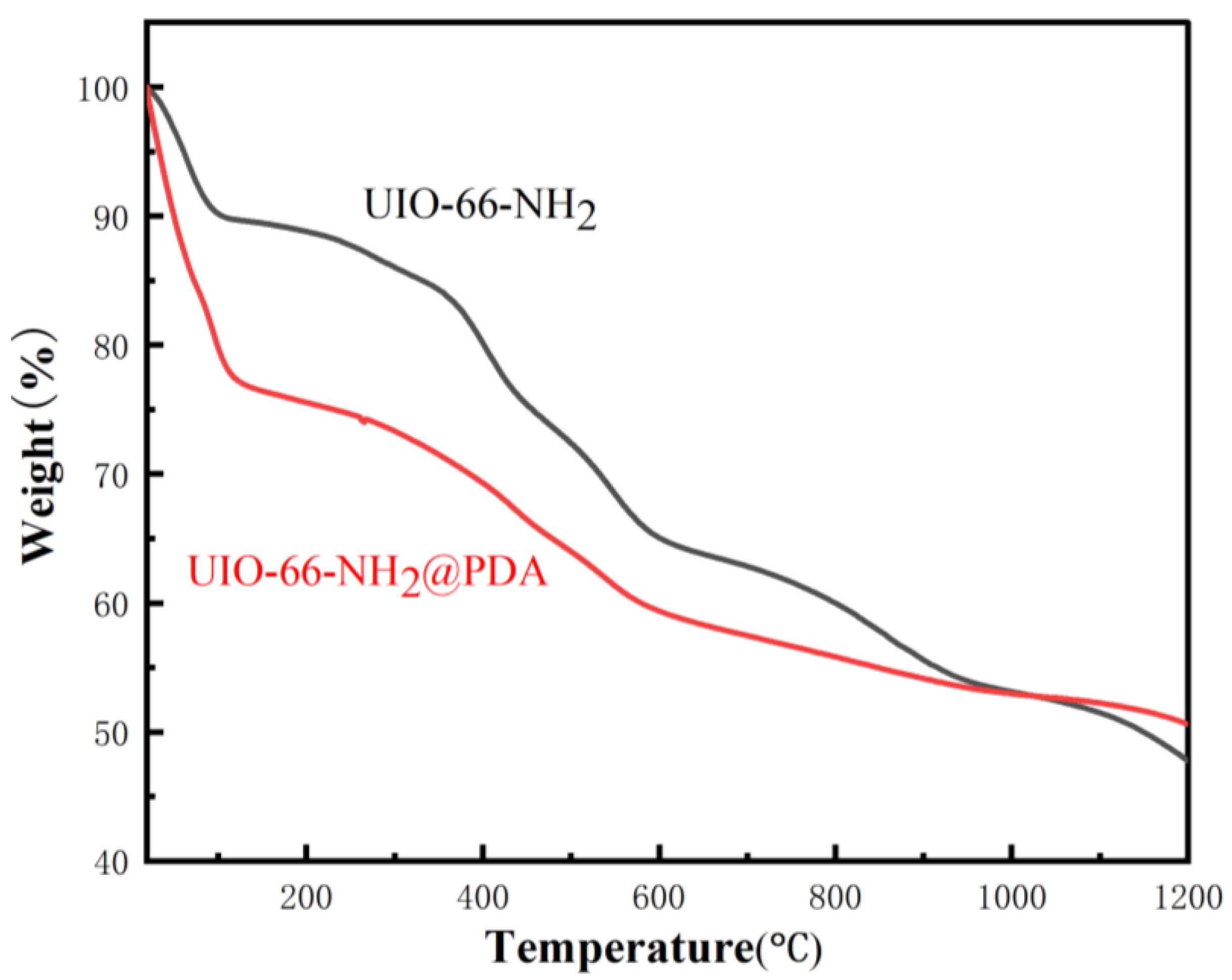
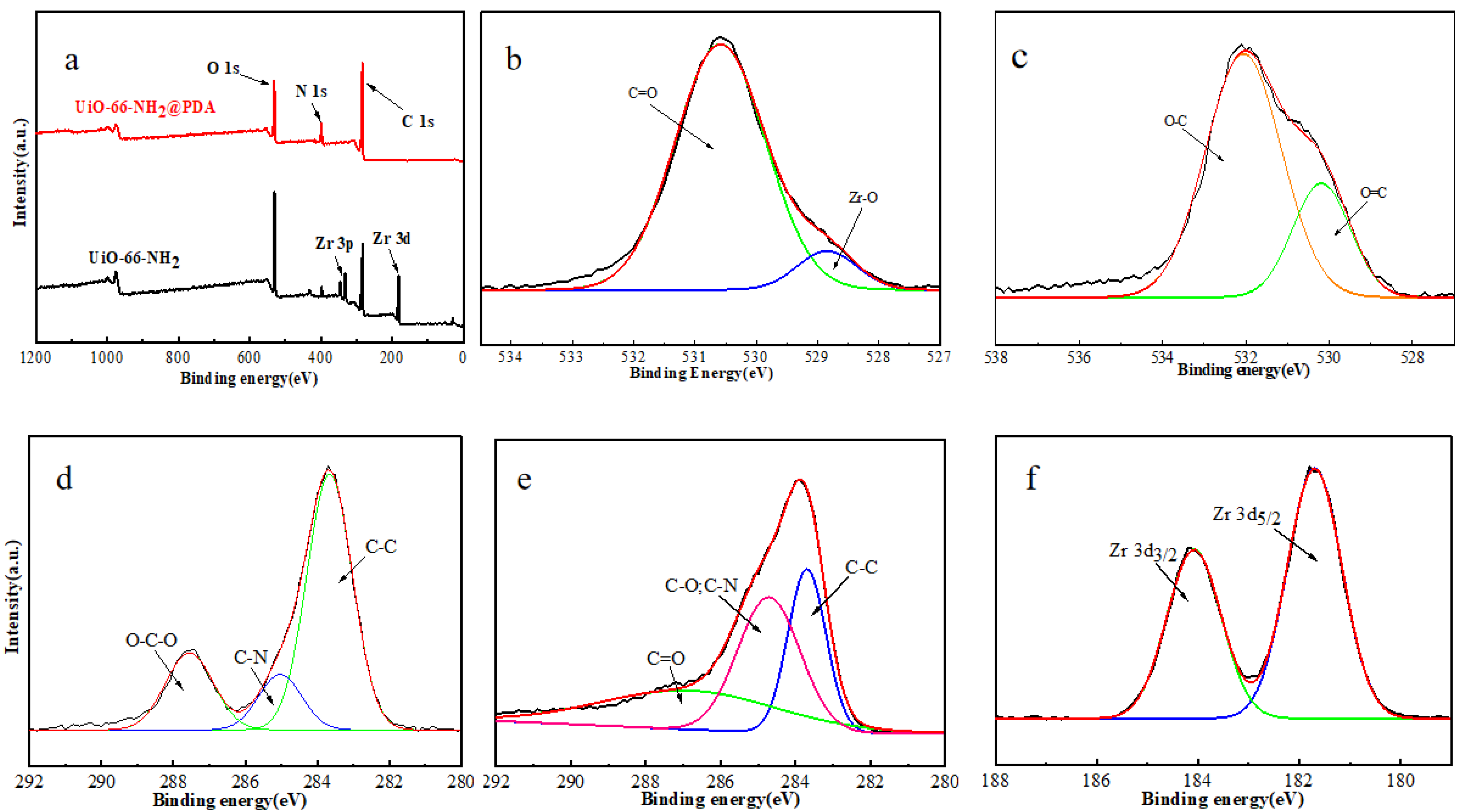
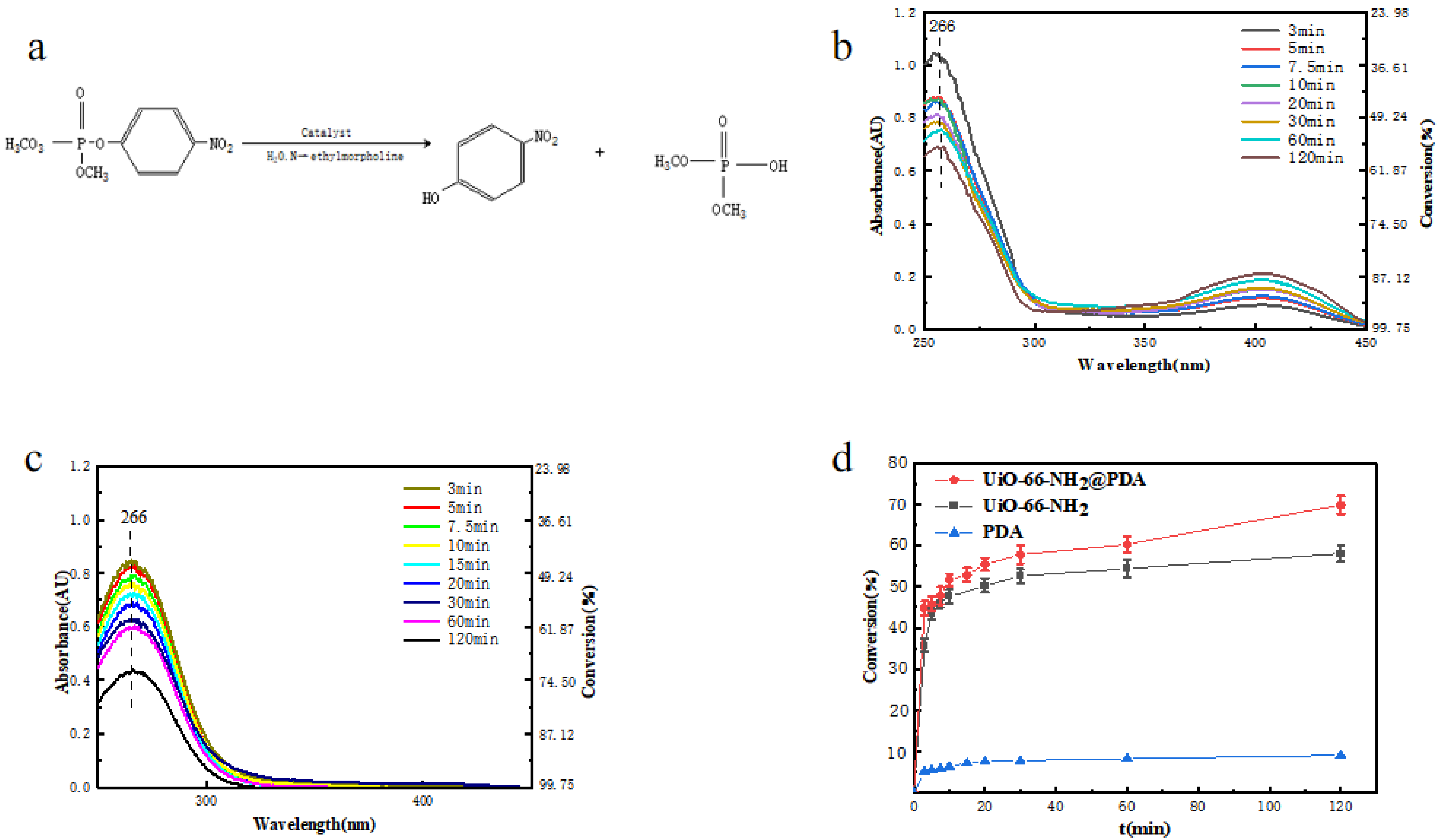
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Enserink, M. UN Taps Special Labs to Investigate Syrian Attack. Science 2013, 341, 1050–1051. [Google Scholar] [CrossRef] [PubMed]
- Bajgar, J. Organophosphates/Nerve Agent Poisoning: Mechanism of Action, Diagnosis, Prophylaxis, and Treatment. Adv. Clin. Chem. 2004, 38, 151–216. [Google Scholar] [PubMed]
- Raushel, F.M. Catalytic Detoxification. Nature 2011, 469, 310–311. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G. Current Issues in Organophosphate Toxicology. Clin. Chim. Acta 2006, 366, 11–13. [Google Scholar] [CrossRef]
- Casida, J.E. Organophosphate Toxicology: Safety Aspects of Nonacetylcholinesterase Secondary Targets. Chem. Res. Toxicol. 2004, 17, 983–998. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, D.L.; Liu, J.; Dwyer, D.B.; Troiano, J.L.; Boyer, S.M.; Decoste, J.B.; Bernier, W.E.; Jones, W.E., Jr. Electrospun Metal-Organic Framework Polymer Composites for the Catalytic Degradation of Methyl Paraoxon. New J. Chem. 2017, 41, 8748–8753. [Google Scholar] [CrossRef]
- Kitagawa, S.; Kitaura, R.; Noro, S.-I. Functional Porous Coordination Polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef]
- Zhou, H.-C.; Long, J.R.; Yaghi, O.M. Introduction to Metal–Organic Frameworks. Chem. Rev. 2012, 112, 673–674. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 6149. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Yao, A.; Jiao, X.; Li, C.; Chen, D. Fast and Sustained Degradation of Chemical Warfare Agent Simulants Using Flexible Self-Supported Metal–Organic Framework Filters. ACS Appl. Mater. Interfaces 2018, 10, 20396–20403. [Google Scholar] [CrossRef] [PubMed]
- Lu, A.X.; McEntee, M.; Browe, M.A.; Hall, M.G.; Decoste, J.B.; Peterson, G.W. Mofabric: Electrospun Nanofiber Mats from PVDF/UiO-66-NH2 for Chemical Protection and Decontamination. ACS Appl. Mater. Interfaces 2017, 9, 13632–13636. [Google Scholar] [CrossRef]
- Kitao, T.; Zhang, Y.; Kitagawa, S.; Wang, B.; Uemura, T. Hybridization of MOFs and Polymers. Chem. Soc. Rev. 2017, 46, 3108–3133. [Google Scholar] [CrossRef]
- Decoste, J.B.; Denny, J.M.S.; Peterson, G.W.; Mahle, J.J.; Cohen, S.M. Enhanced Aging Properties of HKUST-1 in Hydrophobic Mixed-Matrix Membranes for Ammonia Adsorption. Chem. Sci. 2016, 7, 2711–2716. [Google Scholar] [CrossRef]
- Qin, L.; Huang, D.; Xu, P.; Zeng, G.; Lai, C.; Fu, Y.; Yi, H.; Li, B.; Zhang, C.; Cheng, M. In-Situ Deposition of Gold Nanoparticles onto Polydopamine-Decorated G-C3N4 for Highly Efficient Reduction of Nitroaromatics in Environmental Water Purification. J. Colloid Interface Sci. 2019, 534, 357–369. [Google Scholar] [CrossRef]
- He, F.; Chen, G.; Yu, Y.; Zhou, Y.; Zheng, Y.; Hao, S. The Synthesis of Condensed C-PDA–g-C3N4 Composites with Superior Photocatalytic Performance. Chem. Commun. 2015, 51, 6824–6827. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-Inspired Surface Chemistry for Multifunctional Coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Sun, Y.; Zhou, J.; Xu, R.; Duan, H. Mussel-Inspired Synthesis of Polydopamine-Functionalized Graphene Hydrogel as Reusable Adsorbents for Water Purification. ACS Appl. Mater. Interfaces 2013, 5, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Fei, B.; Qian, B.; Yang, Z.; Wang, R.; Liu, W.C.; Mak, C.L.; Xin, J.H. Coating Carbon Nanotubes by Spontaneous Oxidative Polymerization of Dopamine. Carbon 2008, 46, 1795–1797. [Google Scholar] [CrossRef]
- Black, K.C.L.; Liu, Z.; Messersmith, P.B. Catechol Redox Induced Formation of Metal Core—Polymer Shell Nanoparticles. Chem. Mater. 2011, 23, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired Catecholic Chemistry for Surface Modification. Chem. Soc. Rev. 2011, 40, 4244–4258. [Google Scholar] [CrossRef]
- Farnad, N.; Farhadi, K.; Voelcker, N.H. Polydopamine Nanoparticles as a New and Highly Selective Biosorbent for the Removal of Copper (Ii) Ions from Aqueous Solutions. Water Air Soil Pollut. 2012, 223, 3535–3544. [Google Scholar] [CrossRef]
- Lu, G.; Cui, C.; Zhang, W.; Liu, Y.; Huo, F. Synthesis and Self-Assembly of Monodispersed Metal-Organic Framework Microcrystals. Chem. Asian J. 2013, 8, 69–72. [Google Scholar] [CrossRef]
- Mondloch, J.E.; Katz, M.J.; Planas, N.; Semrouni, D.; Gagliardi, L.; Hupp, J.T.; Farha, O.K. Are Zr6-Based MOFs Water Stable? Linker Hydrolysis vs. Capillary-Force-Driven Channel Collapse. Chem. Commun. 2014, 50, 8944–8946. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zhang, Z.; Liu, H.; Wang, Y. Cd0.2Zn0.8S@ UiO-66-NH2 Nanocomposites as Efficient and Stable Visible-Light-Driven Photocatalyst for H2 Evolution and CO2 Reduction. Appl. Catal. B Environ. 2017, 200, 448–457. [Google Scholar] [CrossRef]
- Huang, A.; Wan, L.; Caro, J. Microwave-Assisted Synthesis of Well-Shaped UiO-66-NH2 with High CO2 Adsorption Capacity. Mater. Res. Bull. 2018, 98, 308–313. [Google Scholar] [CrossRef]
- Lin, K.-Y.A.; Liu, Y.-T.; Chen, S.-Y. Adsorption of Fluoride to UiO-66-NH2 in Water: Stability, Kinetic, Isotherm and Thermodynamic Studies. J. Colloid Interface Sci. 2016, 461, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Shanahan, J.; Kissel, D.S.; Sullivan, E. Pani@ UiO-66 and Pani@ UiO-66-NH2 Polymer-MOF Hybrid Composites as Tunable Semiconducting Materials. ACS Omega 2020, 5, 6395–6404. [Google Scholar] [CrossRef]
- Yao, A.; Jiao, X.; Chen, D. Bio-Inspired Polydopamine-Mediated Zr-MOF Fabrics for Solar Photothermal-Driven Instantaneous Detoxification of Chemical Warfare Agent Simulants. ACS Appl. Mater. Interfaces 2020, 12, 18437–18445. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Ren, S.; Zhao, H.; Xue, Q.; Wang, L. Polydopamine Coated Graphene Oxide for Anticorrosive Reinforcement of Water-Borne Epoxy Coating. Chem. Eng. J. 2018, 335, 255–266. [Google Scholar] [CrossRef]
- Wang, H.; Wei, L.; Wang, Z.; Chen, S. Preparation, Characterization and Long-Term Antibacterial Activity of Ag–Poly (Dopamine)–TiO2 Nanotube Composites. RSC Adv. 2016, 6, 14097–14104. [Google Scholar] [CrossRef]
- Celebi, N.; Aydin, M.Y.; Soysal, F.; Yıldız, N.; Salimi, K. Core/Shell PDA@ UiO-66 Metal–Organic Framework Nanoparticles for Efficient Visible-Light Photodegradation of Organic Dyes. ACS Appl. Nano Mater. 2020, 3, 11543–11554. [Google Scholar] [CrossRef]
- Wang, R.; Gu, L.; Zhou, J.; Liu, X.; Teng, F.; Li, C.; Shen, Y.; Yuan, Y. Quasi-Polymeric Metal–Organic Framework UiO-66/G-C3N4 Heterojunctions for Enhanced Photocatalytic Hydrogen Evolution under Visible Light Irradiation. Adv. Mater. Interfaces 2015, 2, 1500037. [Google Scholar] [CrossRef]
- Sun, D.T.; Peng, L.; Reeder, W.S.; Moosavi, S.M.; Tiana, D.; Britt, D.K.; Oveisi, E.; Queen, W.L. Rapid, Selective Heavy Metal Removal from Water by a Metal–Organic Framework/Polydopamine Composite. ACS Cent. Sci. 2018, 4, 349–356. [Google Scholar] [CrossRef]
- Liang, Q.; Zhang, M.; Zhang, Z.; Liu, C.; Xu, S.; Li, Z. Zinc Phthalocyanine Coupled with UiO-66 (NH2) Via a Facile Condensation Process for Enhanced Visible-Light-Driven Photocatalysis. J. Alloy Compd. 2017, 690, 123–130. [Google Scholar] [CrossRef]
- Wan, S.; Ou, M.; Zhong, Q.; Wang, X. Perovskite-Type CsPbBr3 Quantum Dots/UiO-66 (NH2) Nanojunction as Efficient Visible-Light-Driven Photocatalyst for CO2 Reduction. Chem. Eng. J. 2019, 358, 1287–1295. [Google Scholar] [CrossRef]
- Katz, M.J.; Moon, S.-Y.; Mondloch, J.E.; Beyzavi, M.H.; Stephenson, C.J.; Hupp, J.T.; Farha, O.K. Exploiting Parameter Space in MOFs: A 20-Fold Enhancement of Phosphate-Ester Hydrolysis with UiO-66-NH2. Chem. Sci. 2015, 6, 2286–2291. [Google Scholar] [CrossRef]
- Katz, M.J.; Mondloch, J.E.; Totten, R.K.; Park, J.K.; Nguyen, S.T.; Farha, O.K.; Hupp, J.T. Simple and Compelling Biomimetic Metal-Organic Framework Catalyst for the Degradation of Nerve Agent Simulants. Angew. Chem. 2014, 126, 507–511. [Google Scholar] [CrossRef]
- Yao, A.; Jiao, X.; Chen, D.; Li, C. Photothermally Enhanced Detoxification of Chemical Warfare Agent Simulants Using Bioinspired Core–Shell Dopamine–Melanin@ Metal–Organic Frameworks and Their Fabrics. ACS Appl. Mater. Interfaces 2019, 11, 7927–7935. [Google Scholar] [CrossRef]
- Moon, S.-Y.; Proussaloglou, E.; Peterson, G.W.; DeCoste, J.B.; Hall, M.G.; Howarth, A.J.; Hupp, J.T.; Farha, O.K. Detoxification of Chemical Warfare Agents Using a Zr6-Based Metal-Organic Framework/Polymer Mixture. Chem. Eur. J. 2016, 22, 14864–14868. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Tu, Y.; Wu, S. Preparation of UiO-66-NH2@PDA under Water System for Chemical Warfare Agents Degradation. Materials 2021, 14, 2419. https://doi.org/10.3390/ma14092419
Chen M, Tu Y, Wu S. Preparation of UiO-66-NH2@PDA under Water System for Chemical Warfare Agents Degradation. Materials. 2021; 14(9):2419. https://doi.org/10.3390/ma14092419
Chicago/Turabian StyleChen, Mingfei, Yingxue Tu, and Songhai Wu. 2021. "Preparation of UiO-66-NH2@PDA under Water System for Chemical Warfare Agents Degradation" Materials 14, no. 9: 2419. https://doi.org/10.3390/ma14092419
APA StyleChen, M., Tu, Y., & Wu, S. (2021). Preparation of UiO-66-NH2@PDA under Water System for Chemical Warfare Agents Degradation. Materials, 14(9), 2419. https://doi.org/10.3390/ma14092419




