Use of Therapeutic Pathogen Recognition Receptor Ligands for Osteo-Immunomodulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Reagents
2.3. Cell Sources and Culture Conditions
2.4. hMSC Osteogenic Differentiation
2.5. Osteoclast Differentiation Assay
2.6. Cytokine Expression
2.7. Statistical Analysis
3. Results
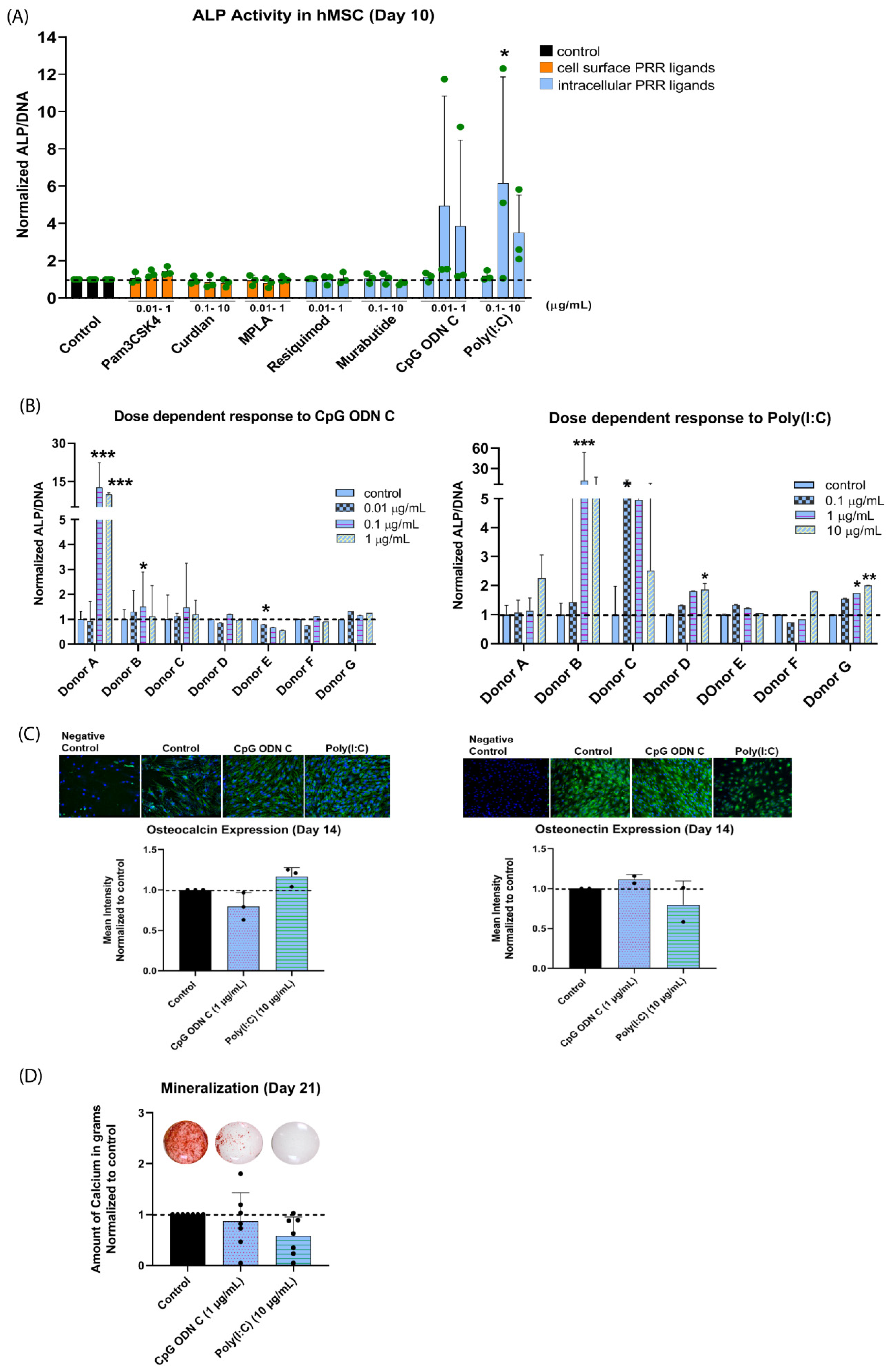
3.1. Effect of PRR Ligands on hMSC Osteogenic Differentiation
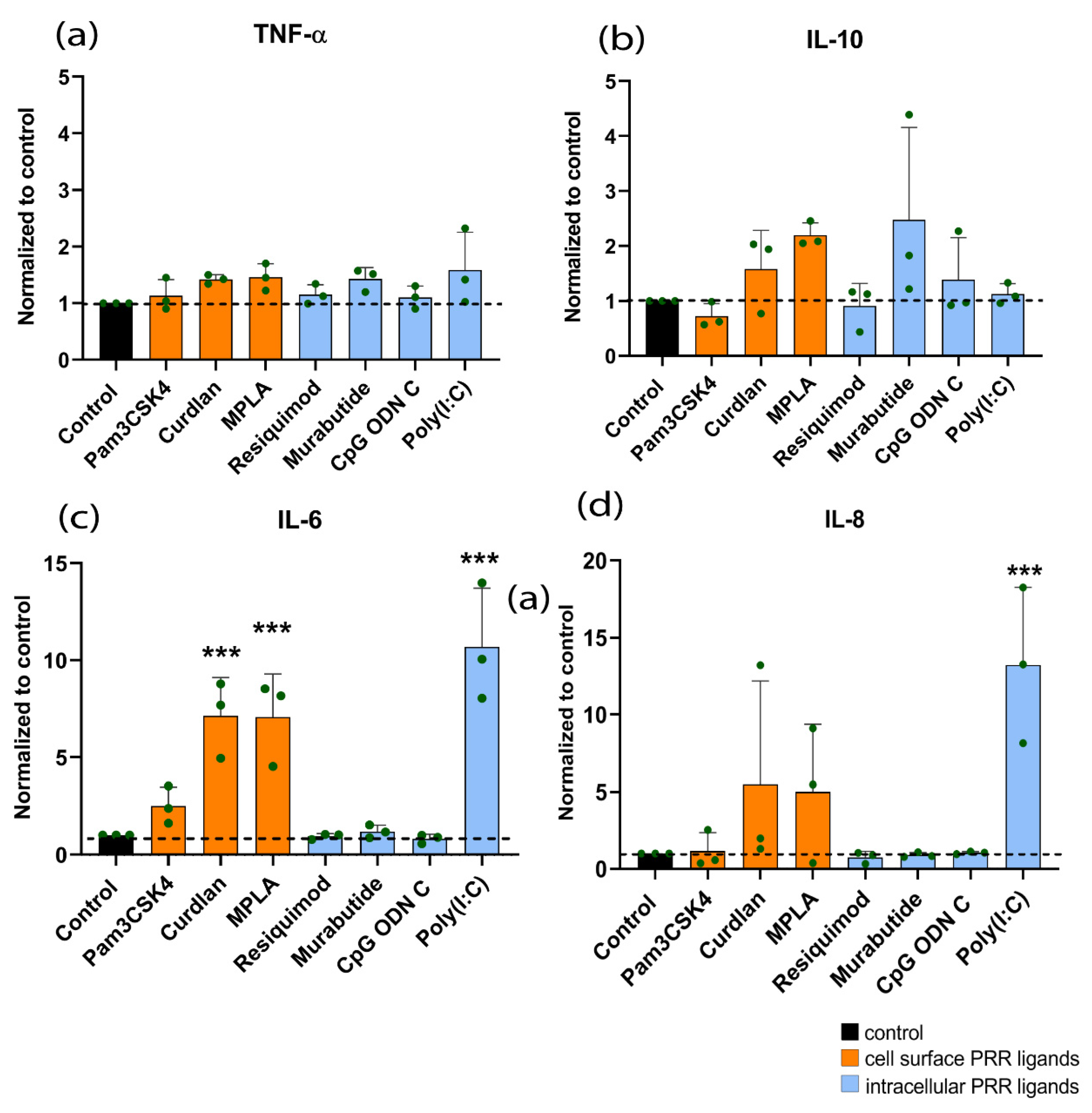
3.2. Effect of PRR Ligands on Cytokine Expression of hMSCs
3.3. Effect of PRR Ligands on Human Osteoclast Differentiation
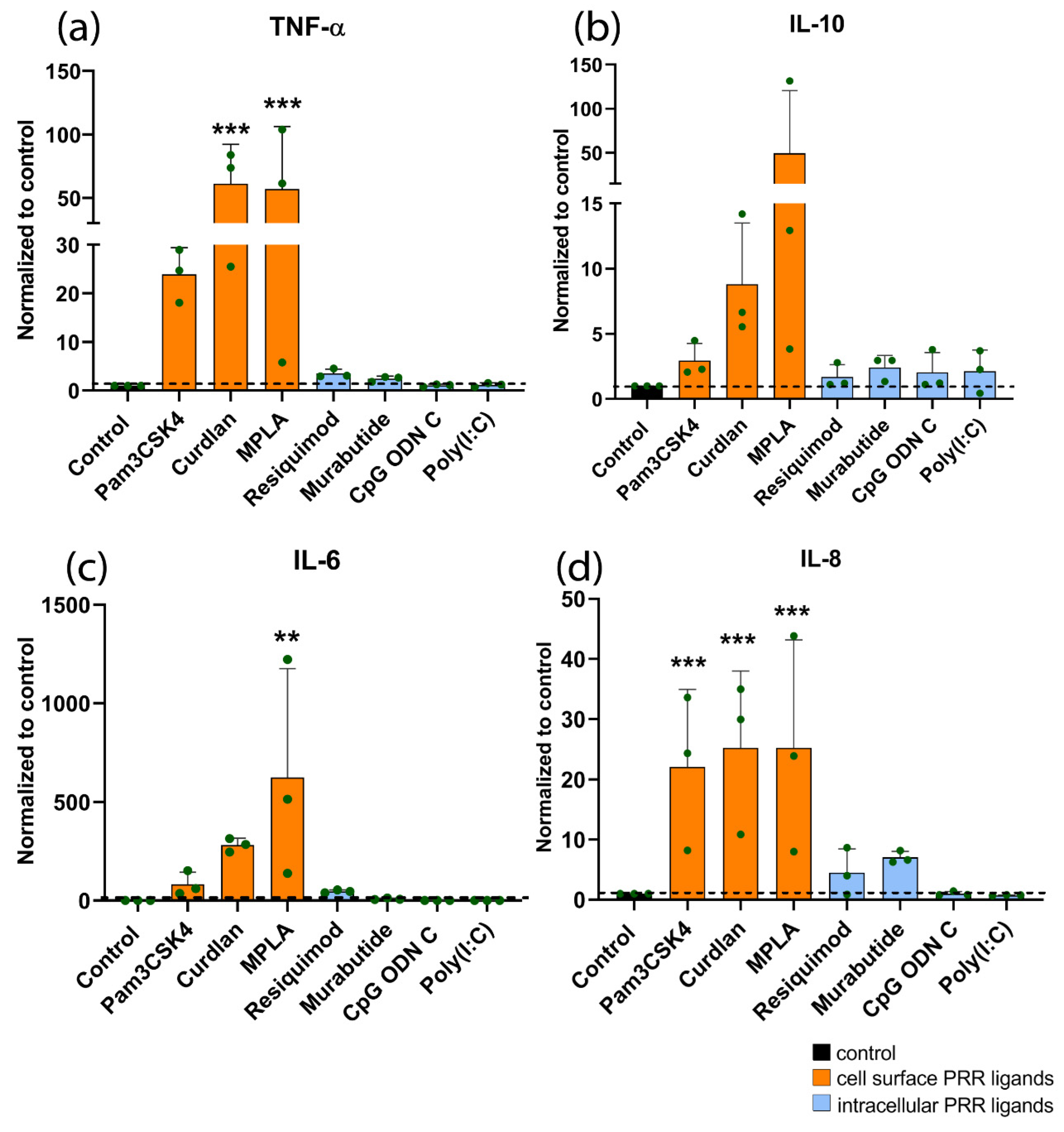
3.4. Effect of PRR Ligands on Cytokine Expression of Human Monocytes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone Substitutes in Orthopaedic Surgery: From Basic Science to Clinical Practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Kolk, A.; Handschel, J.; Drescher, W.; Rothamel, D.; Kloss, F.; Blessmann, M.; Heiland, M.; Wolff, K.-D.; Smeets, R. Current Trends and Future Perspectives of Bone Substitute Materials – From Space Holders to Innovative Biomaterials. J. Cranio-Maxillofac. Surg. 2012, 40, 706–718. [Google Scholar] [CrossRef]
- Barradas, A.M.C.; Yuan, H.; Blitterswijk, C.A.V.; Habibovic, P.; Medicine, T. Osteoinductive Biomaterials: Current Knowledge of Properties, Experimental Models and Biological Mechanisms. 2011, 21, 407–429. [Google Scholar] [CrossRef]
- Lehr, A.M.; Oner, F.C.; Delawi, D.; Stellato, R.K.; Hoebink, E.A.; Kempen, D.H.R.; van Susante, J.L.C.; Castelein, R.M.; Kruyt, M.C. Efficacy of a Standalone Microporous Ceramic Versus Autograft in Instrumented Posterolateral Spinal Fusion. Spine 2020, 45, 944–951. [Google Scholar] [CrossRef]
- Morris, M.T.; Tarpada, S.P.; Cho, W. Bone Graft Materials for Posterolateral Fusion Made Simple: A Systematic Review. Eur. Spine J. 2018, 27, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Spiller, K.L.; Nassiri, S.; Witherel, C.E.; Anfang, R.R.; Ng, J.; Nakazawa, K.R.; Yu, T.; Vunjak-Novakovic, G. Sequential Delivery of Immunomodulatory Cytokines to Facilitate the M1-to-M2 Transition of Macrophages and Enhance Vascularization of Bone Scaffolds. Biomaterials 2015, 37, 194–207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mountziaris, P.M.; Spicer, P.P.; Kasper, F.K.; Mikos, A.G. Harnessing and Modulating Inflammation in Strategies for Bone Regeneration. Tissue Eng. Part B Rev. 2011, 17, 393–402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastidas-Coral, A.P.; Bakker, A.D.; Zandieh-Doulabi, B.; Kleverlaan, C.J.; Bravenboer, N.; Forouzanfar, T.; Klein-Nulend, J. Cytokines TNF-α, IL-6, IL-17F, and IL-4 Differentially Affect Osteogenic Differentiation of Human Adipose Stem Cells. Stem Cells Int. 2016, 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croes, M.; Kruyt, M.C.; Groen, W.M.; van Dorenmalen, K.M.A.; Dhert, W.J.A.; Öner, F.C.; Alblas, J. Interleukin 17 Enhances Bone Morphogenetic Protein-2-Induced Ectopic Bone Formation. Sci. Rep. 2018, 8, 7269. [Google Scholar] [CrossRef] [PubMed]
- Claes, L.; Recknagel, S.; Ignatius, A. Fracture Healing under Healthy and Inflammatory Conditions. Nat. Rev. Rheumatol. 2012, 8, 133–143. [Google Scholar] [CrossRef]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal Stem Cell-Macrophage Crosstalk and Bone Healing. Biomaterials 2019, 196, 80–89. [Google Scholar] [CrossRef]
- Croes, M.; Kruyt, M.C.; Loozen, L.; Kragten, A.H.; Yuan, H.; Dhert, W.J.; Öner, F.C.; Alblas, J. Local Induction of Inflammation Affects Bone Formation. Eur. Cell. Mater. 2017, 33, 211–226. [Google Scholar] [CrossRef]
- O’Neill, L.A.J.; Golenbock, D.; Bowie, A.G. The History of Toll-like Receptors — Redefining Innate Immunity. Nat. Rev. Immunol. 2013, 13, 453–460. [Google Scholar] [CrossRef]
- Hwa Cho, H.; Bae, Y.C.; Jung, J.S. Role of Toll-Like Receptors on Human Adipose-Derived Stromal Cells. Stem Cells 2006, 24, 2744–2752. [Google Scholar] [CrossRef]
- Croes, M.; Kruyt, M.C.; Boot, W.; Pouran, B.; Braham, M.V.; Pakpahan, S.A.; Weinans, H.; Vogely, H.C.; Fluit, A.C.; Dhert, W.J.; et al. The Role of Bacterial Stimuli in Inflammation-Driven Bone Formation. Eur. Cell. Mater. 2019, 37, 402–419. [Google Scholar] [CrossRef]
- Croes, M.; Wal, B.C.H.; Vogely, H.C. Impact of Bacterial Infections on Osteogenesis: Evidence From In Vivo Studies. J. Orthop. Res. 2019, 37, 2067–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croes, M.; Boot, W.; Kruyt, M.C.; Weinans, H.; Pouran, B.; van der Helm, Y.J.M.; Gawlitta, D.; Vogely, H.C.; Alblas, J.; Dhert, W.J.A.; et al. Inflammation-Induced Osteogenesis in a Rabbit Tibia Model. Tissue Eng. Part C Methods 2017, 23, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Dowling, J.K.; Mansell, A. Toll-like Receptors: The Swiss Army Knife of Immunity and Vaccine Development. Clin Transl Immunol. 2016, 5, e85. [Google Scholar] [CrossRef] [PubMed]
- Hussein, W.M.; Liu, T.Y.; Skwarczynski, M.; Toth, I. Toll-like Receptor Agonists: A Patent Review (2011–2013). Expert Opin Ther Pat 2014, 24, 453–470. [Google Scholar] [CrossRef]
- Basith, S.; Manavalan, B.; Lee, G.; Kim, S.G.; Choi, S. Toll-like Receptor Modulators: A Patent Review (2006–2010). Expert Opin. Ther. Pat. 2011, 21, 927–944. [Google Scholar] [CrossRef]
- Kline, J.N. Eat Dirt: CpG DNA and Immunomodulation of Asthma. Proc. Am. Thorac. Soc. 2007, 4, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Colnot, C. Skeletal Cell Fate Decisions within Periosteum and Bone Marrow during Bone Regeneration. J. Bone Miner. Res. 2009, 24, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, J.; Liu, S.; Jin, Y. Stem Cell-Based Bone and Dental Regeneration: A View of Microenvironmental Modulation. Int. J. Oral Sci. 2019, 11, 23. [Google Scholar] [CrossRef] [Green Version]
- Qi, C.; Xiaofeng, X.; Xiaoguang, W. Effects of Toll-like Receptors 3 and 4 in the Osteogenesis of Stem Cells. Stem Cells Int. 2014, 2014, 917168. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Lee, J.-H.; Kim, H.-N.; Kwak, H.B.; Kim, H.-M.; Lee, S.E.; Rhee, J.H.; Kim, H.-H.; Lee, Z.H. Stimulation by TLR5 Modulates Osteoclast Differentiation through STAT1/IFN-β. J. Immunol. 2008, 180, 1382–1389. [Google Scholar] [CrossRef] [Green Version]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast Differentiation and Activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef]
- Ducy, P.; Karsenty, G. Two Distinct Osteoblast-Specific Cis-Acting Elements Control Expression of a Mouse Osteocalcin Gene. Mol. Cell. Biol. 1995, 15, 1858–1869. [Google Scholar] [CrossRef] [Green Version]
- Davison, N.L.; Gamblin, A.L.; Layrolle, P.; Yuan, H.; de Bruijn, J.D.; Barrère-de Groot, F. Liposomal Clodronate Inhibition of Osteoclastogenesis and Osteoinduction by Submicrostructured Beta-Tricalcium Phosphate. Biomaterials 2014, 35, 5088–5097. [Google Scholar] [CrossRef]
- Soto-Peñaloza, D.; Martín-de-Llano, J.J.; Carda-Batalla, C.; Peñarrocha-Diago, M.; Peñarrocha-Oltra, D. Basic Bone Biology Healing During Osseointegration of Titanium Dental Implants. Atlas Immed. Dent. Implant Load. 2019, 17–28. [Google Scholar]
- Akhavan, B.; Croes, M.; Wise, S.G.; Zhai, C.; Hung, J.; Stewart, C.; Ionescu, M.; Weinans, H.; Gan, Y.; Amin Yavari, S.; et al. Radical-Functionalized Plasma Polymers: Stable Biomimetic Interfaces for Bone Implant Applications. Appl. Mater. Today 2019, 16, 456–473. [Google Scholar] [CrossRef]
- Croes, M.; Oner, F.C.; Kruyt, M.C.; Blokhuis, T.J.; Bastian, O.; Dhert, W.J.A.; Alblas, J. Proinflammatory Mediators Enhance the Osteogenesis of Human Mesenchymal Stem Cells after Lineage Commitment. PLoS ONE 2015, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Terheyden, H.; Lang, N.P.; Bierbaum, S.; Stadlinger, B. Osseointegration-Communication of Cells. Clin. Oral Implants Res. 2012, 23, 1127–1135. [Google Scholar] [CrossRef]
- Souza, P.P.C.; Lerner, U.H. The Role of Cytokines in Inflammatory Bone Loss. Immunol. Investig. 2013, 42, 555–622. [Google Scholar] [CrossRef]
- Guihard, P.; Danger, Y.; Brounais, B.; David, E.; Brion, R.; Delecrin, J.; Richards, C.D.; Chevalier, S.; Rédini, F.; Heymann, D.; et al. Induction of Osteogenesis in Mesenchymal Stem Cells by Activated Monocytes/Macrophages Depends on Oncostatin M Signaling. Stem Cells 2012, 30, 762–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pennings, I.; van Dijk, L.A.; van Huuksloot, J.; Fledderus, J.O.; Schepers, K.; Braat, A.K.; Hsiao, E.C.; Barruet, E.; Morales, B.M.; Verhaar, M.C.; et al. Effect of Donor Variation on Osteogenesis and Vasculogenesis in Hydrogel Cocultures. J. Tissue Eng. Regen. Med. 2019, 13, 433–445. [Google Scholar] [CrossRef] [Green Version]
- Krug, A.; Rothenfusser, S.; Hornung, V.; Jahrsdörfer, B.; Blackwell, S.; Ballas, Z.K.; Endres, S.; Krieg, A.M.; Hartmann, G. Identification of CpG Oligonucleotide Sequences with High Induction of IFN-Alpha/Beta in Plasmacytoid Dendritic Cells. Eur. J. Immunol. 2001, 31, 2154–2163. [Google Scholar] [CrossRef]
- Romieu-Mourez, R.; François, M.; Boivin, M.-N.; Bouchentouf, M.; Spaner, D.E.; Galipeau, J. Cytokine Modulation of TLR Expression and Activation in Mesenchymal Stromal Cells Leads to a Proinflammatory Phenotype. J. Immunol. 2009, 182, 7963–7973. [Google Scholar] [CrossRef]
- Yang, A.; Lu, Y.; Xing, J.; Li, Z.; Yin, X.; Dou, C.; Dong, S.; Luo, F.; Xie, Z.; Hou, T.; et al. IL-8 Enhances Therapeutic Effects of BMSCs on Bone Regeneration via CXCR2-Mediated PI3k/Akt Signaling Pathway. Cell. Physiol. Biochem. 2018, 48, 361–370. [Google Scholar] [CrossRef]
- Huh, J.-E.; Lee, S.Y. IL-6 Is Produced by Adipose-Derived Stromal Cells and Promotes Osteogenesis. Biochim. Biophys. Acta BBA Mol. Cell Res. 2013, 1833, 2608–2616. [Google Scholar] [CrossRef] [Green Version]
- Dumitru, C.A.; Hemeda, H.; Jakob, M.; Lang, S.; Brandau, S. Stimulation of Mesenchymal Stromal Cells (MSCs) via TLR3 Reveals a Novel Mechanism of Autocrine Priming. FASEB J. 2014, 28, 3856–3866. [Google Scholar] [CrossRef] [Green Version]
- Glass, G.E.; Chan, J.K.; Freidin, A.; Feldmann, M.; Horwood, N.J. TNF-α Promotes Fracture Repair by Augmenting the Recruitment and Differentiation of Muscle-Derived Stromal Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 1585–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayringer, I.; Reindl, M.; Berger, T. A Critical Comparison of Frequently Used Methods for the Analysis of Tumor Necrosis Factor-α Expression by Human Immune Cells. J. Immunol. Methods 2000, 235, 33–40. [Google Scholar] [CrossRef]
- Takami, M.; Kim, N.; Rho, J.; Choi, Y. Stimulation by Toll-Like Receptors Inhibits Osteoclast Differentiation. J. Immunol. 2002, 169, 1516–1523. [Google Scholar] [CrossRef] [Green Version]
- Souza, P.P.C.; Lerner, U.H. Finding a Toll on the Route: The Fate of Osteoclast Progenitors after Toll-like Receptor Activation. Front. Immunol. 2019, 10, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Zou, W.; Bar-Shavit, Z. Dual Modulation of Osteoclast Differentiation by Lipopolysaccharide. J. Bone Miner. Res. 2002, 17, 1211–1218. [Google Scholar] [CrossRef] [PubMed]
- Prins, H.-J.; Braat, A.K.; Gawlitta, D.; Dhert, W.J.A.; Egan, D.A.; Tijssen-Slump, E.; Yuan, H.; Coffer, P.J.; Rozemuller, H.; Martens, A.C. In Vitro Induction of Alkaline Phosphatase Levels Predicts in Vivo Bone Forming Capacity of Human Bone Marrow Stromal Cells. Stem Cell Res. 2014, 12, 428–440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoemann, C.D.; El-Gabalawy, H.; McKee, M.D. In Vitro Osteogenesis Assays: Influence of the Primary Cell Source on Alkaline Phosphatase Activity and Mineralization. Pathol. Biol. 2009, 57, 318–323. [Google Scholar] [CrossRef]
- Dickie, L.J.; Church, L.D.; Coulthard, L.R.; Mathews, R.J.; Emery, P.; McDermott, M.F. Vitamin D3 Down-Regulates Intracellular Toll-like Receptor 9 Expression and Toll-like Receptor 9-Induced IL-6 Production in Human Monocytes. Rheumatol. Oxf. Engl. 2010, 49, 1466–1471. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Zhao, N.; Xu, X.; Xu, Y.; Li, S.; Zhang, J.; Yang, P. Dose-Specific Effects of Tumor Necrosis Factor Alpha on Osteogenic Differentiation of Mesenchymal Stem Cells. Cell Prolif. 2011, 44, 420–427. [Google Scholar] [CrossRef]
- Kovach, T.K.; Dighe, A.S.; Lobo, P.I.; Cui, Q. Interactions between MSCs and Immune Cells: Implications for Bone Healing. J. Immunol. Res. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Takahashi, N.; Jimi, E.; Udagawa, N.; Takami, M.; Kotake, S.; Nakagawa, N.; Kinosaki, M.; Yamaguchi, K.; Shima, N.; et al. Tumor Necrosis Factor α Stimulates Osteoclast Differentiation by a Mechanism Independent of the ODF/RANKL-RANK Interaction. J. Exp. Med. 2000, 191, 275–285. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Nijnik, A.; Philpott, D.J. Modulating Immunity as a Therapy for Bacterial Infections. Nat. Rev. Microbiol. 2012, 10, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Wakita, D.; Chamoto, K.; Narita, Y.; Tsuji, T.; Takeshima, T.; Gyobu, H.; Kawarada, Y.; Kondo, S.; Akira, S.; et al. Liposome-Encapsulated CpG Oligodeoxynucleotides as a Potent Adjuvant for Inducing Type 1 Innate Immunity. Cancer Res. 2004, 64, 8754–8760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hornung, V.; Rothenfusser, S.; Britsch, S.; Krug, A.; Jahrsdörfer, B.; Giese, T.; Endres, S.; Hartmann, G. Quantitative Expression of Toll-Like Receptor 1–10 MRNA in Cellular Subsets of Human Peripheral Blood Mononuclear Cells and Sensitivity to CpG Oligodeoxynucleotides. J. Immunol. 2002, 168, 4531–4537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzio, M.; Bosisio, D.; Polentarutti, N.; D’amico, G.; Stoppacciaro, A.; Mancinelli, R.; van’t Veer, C.; Penton-Rol, G.; Ruco, L.P.; Allavena, P.; et al. Differential Expression and Regulation of Toll-Like Receptors (TLR) in Human Leukocytes: Selective Expression of TLR3 in Dendritic Cells. J. Immunol. 2000, 164, 5998–6004. [Google Scholar] [CrossRef] [Green Version]
- Gale, E.C.; Roth, G.A.; Smith, A.A.A.; Alcántara-Hernández, M.; Idoyaga, J.; Appel, E.A. A Nanoparticle Platform for Improved Potency, Stability, and Adjuvanticity of Poly(I:C). Adv. Ther. 2020, 3, 1900174. [Google Scholar] [CrossRef]
- Scharnweber, D.; Bierbaum, S.; Wolf-Brandstetter, C. Utilizing DNA for Functionalization of Biomaterial Surfaces. FEBS Lett. 2018, 592, 2181–2196. [Google Scholar] [CrossRef] [Green Version]
- Amin Yavari, S.; Croes, M.; Akhavan, B.; Jahanmard, F.; Eigenhuis, C.C.; Dadbakhsh, S.; Vogely, H.C.; Bilek, M.M.; Fluit, A.C.; Boel, C.H.E.; et al. Layer by Layer Coating for Bio-Functionalization of Additively Manufactured Meta-Biomaterials. Addit. Manuf. 2020, 32, 100991. [Google Scholar] [CrossRef]
- Marsell, R.; Einhorn, T.A. The Biology of Fracture Healing. Injury 2011, 42, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Irie, K.; Alpaslan, C.; Takahashi, K.; Kondo, Y.; Izumi, N.; Sakakura, Y.; Tsuruga, E.; Nakajima, T.; Ejiri, S.; Ozawa, H.; et al. Osteoclast Differentiation in Ectopic Bone Formation Induced by Recombinant Human Bone Morphogenetic Protein 2 (RhBMP-2). J. Bone Miner. Metab. 2003, 21, 363–369. [Google Scholar] [CrossRef]
- Inzana, J.A.; Schwarz, E.M.; Kates, S.L.; Awad, H.A. Biomaterials Approaches to Treating Implant-Associated Osteomyelitis. Biomaterials 2016, 81, 58–71. [Google Scholar] [CrossRef] [Green Version]
- Yavari, S.A.; Castenmiller, S.M.; van Strijp, J.A.; Croes, M. Combating Implant Infections: Shifting Focus from Bacteria to Host. Adv. Mater. 2020, 32, 2002962. [Google Scholar] [CrossRef] [PubMed]
- Weighardt, H.; Feterowski, C.; Veit, M.; Rump, M.; Wagner, H.; Holzmann, B. Increased Resistance Against Acute Polymicrobial Sepsis in Mice Challenged with Immunostimulatory CpG Oligodeoxynucleotides Is Related to an Enhanced Innate Effector Cell Response. J. Immunol. 2000, 165, 4537–4543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, S.; Thormann, U.; Sommer, U.; Stotzel, S.; Mohamed, W.; Schnettler, R.; Domann, E.; Chakraborty, T.; Alt, V. Impact of Prophylactic CpG Oligodeoxynucleotide Application on Implant-Associated Staphylococcus Aureus Bone Infection. Bone 2015, 78, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Ribes, S.; Arcilla, C.; Ott, M.; Schütze, S.; Hanisch, U.-K.; Nessler, S.; Nau, R. Pre-Treatment with the Viral Toll-like Receptor 3 Agonist Poly(I:C) Modulates Innate Immunity and Protects Neutropenic Mice Infected Intracerebrally with Escherichia Coli. J. Neuroinflammation 2020, 17, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, W.; Domann, E.; Chakraborty, T.; Mannala, G.; Lips, K.S.; Heiss, C.; Schnettler, R.; Alt, V. TLR9 Mediates S. Aureus Killing inside Osteoblasts via Induction of Oxidative Stress. BMC Microbiol. 2016, 16, 230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.-M.; Wang, J.; Zhang, B.; Fang, L.; Xu, K.; Liu, R.-Y. CpG-ODN Promotes Phagocytosis and Autophagy through JNK/P38 Signal Pathway in Staphylococcus Aureus-Stimulated Macrophage. Life Sci. 2016, 161, 51–59. [Google Scholar] [CrossRef]
- Sun, K.; Metzger, D.W. Inhibition of Pulmonary Antibacterial Defense by Interferon-γ during Recovery from Influenza Infection. Nat. Med. 2008, 14, 558–564. [Google Scholar] [CrossRef]
- Ruiz, J.; Kanagavelu, S.; Flores, C.; Romero, L.; Riveron, R.; Shih, D.Q.; Fukata, M. Systemic Activation of TLR3-Dependent TRIF Signaling Confers Host Defense against Gram-Negative Bacteria in the Intestine. Front. Cell. Infect. Microbiol. 2016, 5. [Google Scholar] [CrossRef] [Green Version]

| Therapeutic PRR Ligand | Receptor | Natural Ligand | Concentration |
|---|---|---|---|
| Pam3CSK4 | TLR1/2 a | Bacterial lipoproteins | 0.01–1 μg/mL |
| Curdlan | Dectin-1 a | n/a | 0.1–10 μg/mL |
| MPLA | TLR4 a | Gram-negative bacterial Lipid A | 0.01–1 μg/mL |
| Resiquimod | TLR7/8 b | Microbial single-stranded RNA | 0.01–1 μg/mL |
| Murabutide | NOD2 b | Bacterial peptidoglycan | 0.1–10 μg/mL |
| CpG ODN C | TLR9 b | Microbial DNA | 0.01–1 μg/mL |
| Poly(I:C) | TLR3 b | Microbial double-stranded RNA | 0.1–10 μg/mL |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khokhani, P.; Rahmani, N.R.; Kok, A.; Öner, F.C.; Alblas, J.; Weinans, H.; Kruyt, M.C.; Croes, M. Use of Therapeutic Pathogen Recognition Receptor Ligands for Osteo-Immunomodulation. Materials 2021, 14, 1119. https://doi.org/10.3390/ma14051119
Khokhani P, Rahmani NR, Kok A, Öner FC, Alblas J, Weinans H, Kruyt MC, Croes M. Use of Therapeutic Pathogen Recognition Receptor Ligands for Osteo-Immunomodulation. Materials. 2021; 14(5):1119. https://doi.org/10.3390/ma14051119
Chicago/Turabian StyleKhokhani, Paree, Nada R. Rahmani, Anne Kok, F. Cumhur Öner, Jacqueline Alblas, Harrie Weinans, Moyo C. Kruyt, and Michiel Croes. 2021. "Use of Therapeutic Pathogen Recognition Receptor Ligands for Osteo-Immunomodulation" Materials 14, no. 5: 1119. https://doi.org/10.3390/ma14051119
APA StyleKhokhani, P., Rahmani, N. R., Kok, A., Öner, F. C., Alblas, J., Weinans, H., Kruyt, M. C., & Croes, M. (2021). Use of Therapeutic Pathogen Recognition Receptor Ligands for Osteo-Immunomodulation. Materials, 14(5), 1119. https://doi.org/10.3390/ma14051119







