Synthesis, Characterization and Testing of Antimicrobial Activity of Composites of Unsaturated Polyester Resins with Wood Flour and Silver Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Composites
2.3. Research Methods
2.3.1. Preparation of Composite Samples
2.3.2. Accelerated Aging Test
2.3.3. Mechanical and Thermomechanical Properties
2.3.4. Determination of Gloss
2.3.5. Bacterial Strains
2.3.6. Anti-Biofilm Activity of Tested Materials
2.3.7. Confocal Laser Scanning Microscopy—Biofilm Visualization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Penczek, P.; Czub, P.; Pielichowski, J. Unsaturated polyester resins: Chemistry and technology. Adv. Polym. Sci. 2005, 184. [Google Scholar] [CrossRef]
- Johnson, K.G.; Yang, L.S. Preparation, in Properties and Applications of Unsaturated Polyesters. In Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters; Scheirs, J., Long, T.E., Eds.; John Wiley & Sons Ltd.: Chichester, UK, 2003; pp. 699–714. [Google Scholar]
- Unsaturated Polyester Resin Market Forecast, Trend Analysis & Competition Tracking—Global Market Insights 2020 to 2030. Available online: https://www.factmr.com/report/4731/unsaturated-polyester-resin-market (accessed on 12 January 2021).
- Cherian, B.; Thachil, E.T. Synthesis of Unsaturated Polyester Resin—Effect of Sequence of Addition of Reactants. Polym. Plast. Technol. Eng. 2007, 44, 931–938. [Google Scholar] [CrossRef]
- Fink, J.K. Unsaturated Polyester Resins. In Reactive Polymers Fundamentals and Applications, 2nd ed.; Fink, J.K., Ed.; William Andrew Inc.: San Francisco, CA, USA, 2005; pp. 1–67. [Google Scholar]
- Chabros, A.; Gawdzik, B.; Podkościelna, B.; Goliszek, M.; Pączkowski, P. Composites of unsaturated poliester resins with cellulose and its derivatives. Materials 2020, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.A.; Baig, F.K.; Mehboob, R. Nosocomial infections: Epidemiology, prevention, control and surveillance. Asian Pac. J. Trop. Biomed. 2017, 7, 478–482. [Google Scholar] [CrossRef]
- Khan, H.; Ahmad, A.; Mehboob, R. Nosocomial infections and their control strategies. Asian Pac. J. Trop. Biomed. 2015, 5, 509–514. [Google Scholar] [CrossRef]
- Joshi, M.; Kaur, S.; Kaur, H.P.; Mishra, T. Nosocomial infection: Source and prevention. Int. J. Pharm. Sci. Res. 2019, 10, 1613–1624. [Google Scholar]
- Kalwar, K.; Shan, D. Antimicrobial effect of silver nanoparticles (AgNPs) and their mechanism—A mini review. Micro Nano Lett. 2018, 13, 277–280. [Google Scholar] [CrossRef]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver nanoparticles as an effective disinfectant: A review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.-H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef]
- Dai, T.; Vrahas, M.S.; Murray, C.K.; Hamblin, M.R. Ultraviolet C irradiation: An alternative antimicrobial approach to localized infections? Expert Rev. Anti Infect. Ther. 2012, 10, 185–195. [Google Scholar] [CrossRef]
- Memarzadeh, F.; Olmsted, R.N.; Bartley, J.M. Applications of ultraviolet germicidal irradiation disinfection in health care facilities: Effective adjunct, but not stand-alone technology. Am. J. Infect. Control. 2010, 38, S13–S24. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Wu, U.I.; Tai, H.M.; Sheng, W.H. Effectiveness of an ultraviolet-C disinfection system for reduction of healthcare-associated pathogens. J. Microbiol. Immunol. Infect. 2019, 52, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Weber, D.J.; Rutala, W.A.; Anderson, D.J.; Chen, L.F.; Sickbert-Bennett, E.E.; Boyce, J.M. Effectiveness of ultraviolet devices and hydrogen peroxide systems for terminal room decontamination: Focus on clinical trials. Am. J. Infect. Control 2016, 44, e77–e84. [Google Scholar] [CrossRef]
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks). Opinion on Nanosilver: Safety, Health and Environmental Effects and Role in Antimicrobial Resistance; European Union: Brussels, Belgium, 2013; ISBN 978-92-79-30132-2. [Google Scholar] [CrossRef]
- SCCS (Scientific Committee on Consumer Safety). Opinion on Colloidal Silver; European Union: Brussels, Belgium, 2018; ISBN 978-92-76-00236-9. [Google Scholar] [CrossRef]
- Ahamed, M.; AlSalhi, M.S.; Siddiqui, M.K.J. Silver nanoparticle applications and human health. Clin. Chim. Acta 2010, 411, 1841–1848. [Google Scholar] [CrossRef] [PubMed]
- Pączkowski, P.; Puszka, A.; Gawdzik, B. Green Composites Based on Unsaturated Polyester Resin from Recycled Poly (Ethylene Terephthalate) with Wood Flour as Filler—Synthesis, Characterization and Aging Effect. Polymers 2020, 12, 2966. [Google Scholar] [CrossRef] [PubMed]
- EN ISO. 4892-2:2013 Plastics—Methods of Exposure to Laboratory Light Sources—Part 2: Xenon-arc Lamps; International Organization of Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- EN ISO. 178:2019 Plastics—Determination of Flexural Properties; International Organization of Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- ASTM. D2583, Standard Test Method for Indentation Hardness of Rigid Plastics by Means of a Barcol Impressor; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- EN ISO. 6721-1:2019 Plastics—Determination of Dynamic Mechanical Properties—Part 1: General Principles; International Organization of Standardization: Geneva, Switzerland, 2019. [Google Scholar]
- ASTM. D2457, Standard Test Method for Specular Gloss of Plastic Films and Solid Plastics; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Miazga-Karska, M.; Michalak, K.; Ginalska, G. Anti-Acne Action of Peptides Isolated from Burdock Root—Preliminary Studies and Pilot Testing. Molecules 2020, 25, 2027. [Google Scholar] [CrossRef]
- Kommerein, N.; Doll, K.; Stumpp, N.S.; Stiesch, M. Development and characterization of an oral multispecies biofilm implant flow chamber model. PLoS ONE 2018, 13, e0196967. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Siracusa, V. Microbial Degradation of Synthetic Biopolymers Waste. Polymers 2019, 11, 1066. [Google Scholar] [CrossRef]
- Shenov, M.A.; D’Melo, D.J. Evaluation of mechanical properties of unsaturated polyester-guar gum/hydroxypropyl guar gum composites. EXPRESS Polym. Lett. 2007, 1, 622–628. [Google Scholar] [CrossRef]
- Mohammed, M.H.; Dauda, B. Unsaturated Polyester Resin Reinforced with Chemically Modified Natural Fibre. IOSR J. Polymer. Text. Eng. 2014, 1, 31–38. [Google Scholar]
- Rahman, M.R.; Hamdan, S.; Hasan, M.; Baini, R.; Salleh, A.A. Physical, mechanical, and thermal of wood flour reinforced maleic anhydride grafted unsaturated polyester (UP) biocomposites. BioResources 2015, 10, 4557–4568. [Google Scholar] [CrossRef]
- Sen, M. Nanocomposite materials. In Nanotechnology and the Environment; Sen, M., Ed.; IntechOpen: London, UK, 2020. [Google Scholar]
- Zhao, Q.; Jia, Z.; Li, X.; Ye, Z. Surface degradation of unsaturated poliester resin in Xe artificial 309 weathering environment. Mater. Design. 2010, 31, 4457–4460. [Google Scholar] [CrossRef]
- Sanyasi, S.; Majhi, R.K.; Kumar, S.; Mishra, M.; Ghosh, A.; Suar, M.; Satyam, P.V.; Mohapatra, H.; Goswami, C.; Goswami, L. Polysaccharide-capped silver Nanoparticles inhibit biofilm formation and eliminate multi-drug-resistant bacteria by disrupting bacterial cytoskeleton with reduced cytotoxicity towards mammalian cells. Sci. Rep. 2016, 6, 24929. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, H.S.; Ryu, D.S.; Choi, S.J.; Lee, D.S. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Korean J. Microbiol. Biotechnol. 2011, 39, 77–85. [Google Scholar]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef]
- Furno, F.; Morley, K.S.; Wong, B.; Sharp, B.L.; Arnold, P.L.; Howdle, S.M.; Bayston, R.; Brown, P.D.; Winship, P.D.; Reid, H.J. Silver nanoparticles and polymeric medical devices: A new approach to prevention of infection? J. Antimicrob. Chemother. 2004, 54, 1019–1024. [Google Scholar] [CrossRef]
- Singh, P.; Pandit, S.; Garnæs, J.; Tunjic, S.; Mokkapati, V.R.; Sultan, A.; Thygesen, A.; Mackevica, A.; Mateiu, R.V.; Daugaard, A.E.; et al. Green synthesis of gold and silver nanoparticles from Cannabis sativa (industrial hemp) and their capacity for biofilm inhibition. Int. J. Nanomed. 2018, 13, 3571–3591. [Google Scholar] [CrossRef]
- Hussain, A.; Alajmi, M.F.; Khan, M.A.; Pervez, S.A.; Ahmed, F.; Amir, S.; Husain, F.M.; Khan, M.S.; Shaik, G.M.; Hassan, I.; et al. Biosynthesized Silver Nanoparticle (AgNP) From Pandanus odorifer Leaf Extract Exhibits Anti-metastasis and Anti-biofilm Potentials. Front. Microbiol. 2019, 10, 8. [Google Scholar] [CrossRef]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the anti-bacterial activity of silver nanoparticles depend on the shape of the nanoparticle? A study of the Gram-negative bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Pazos-Ortiz, E.; Roque-Ruiz, J.H.; Hinojos-Márquez, E.A.; López-Esparza, J.; Donohué-Cornejo, A.; Cuevas-González, J.C.; Espinosa-Cristóbal, L.F.; Reyes-López, S.Y. Dose-dependent Antimicrobial Activity of Silver Nanoparticles on Polycaprolactone Fibers against Gram-positive and Gram-negative Bacteria. J. Nanomater. 2017, 6. [Google Scholar] [CrossRef]



| Sample | UPR (wt%) | WF (wt%) | AgNPs aq. sol. (wt%) |
|---|---|---|---|
| 1 | 100.0 | - | - |
| 2 | 99.0 | 1.0 | - |
| 3 | 98.0 | 2.0 | - |
| 4 | 95.0 | 5.0 | - |
| 5 | 99.8 | - | 0.2 |
| 6 | 99.5 | - | 0.5 |
| 7 | 99.0 | - | 1.0 |
| 8 | 94.0 | 5.0 | 1.0 |
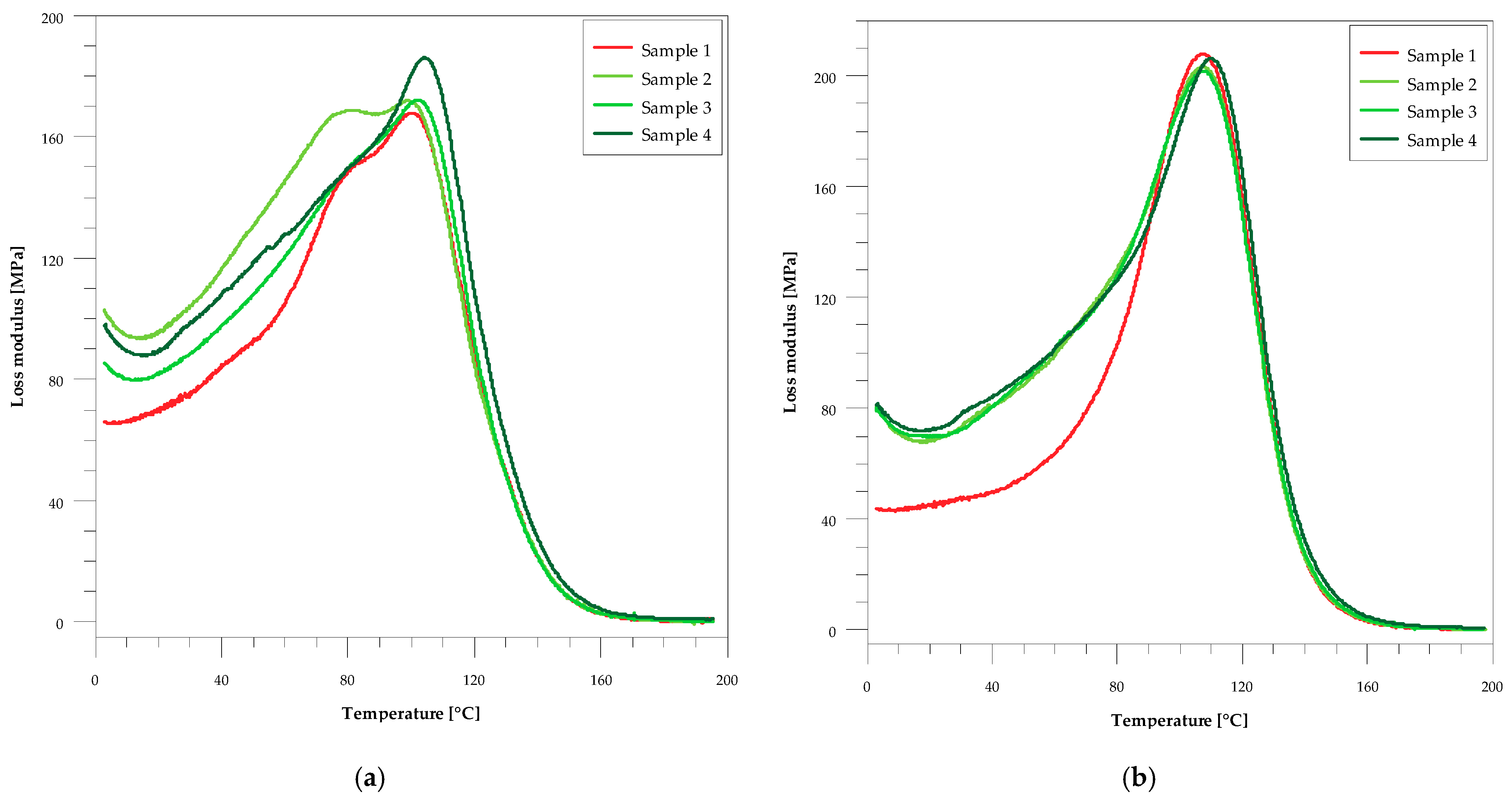
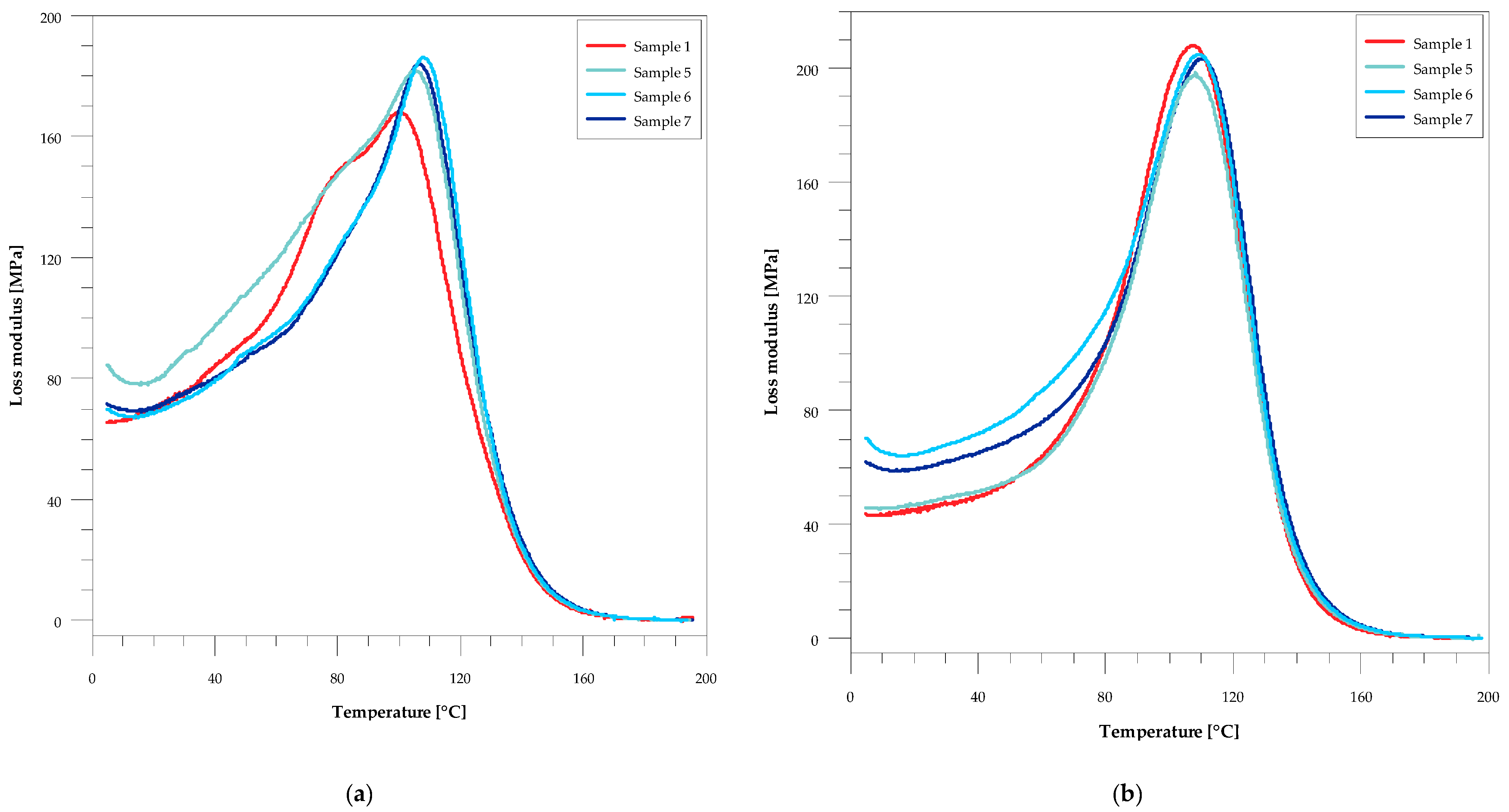
| Sample | Flexural Strength, σf (MPa) | Barcol Hardness, HBa (°B) | Mechanical Loss Factor, Tan δmax | Full Width at Half Maximum, FWHM (°C) | ||||
|---|---|---|---|---|---|---|---|---|
| before | after | before | after | before | after | before | after | |
| 1 | 108.19 ± 3.77 | 69.26 ± 4.35 | 36.0 ± 1.0 | 56.4 ± 0.4 | 0.4579 | 0.4839 | 46.1 | 39.7 |
| 2 | 81.96 ± 2.71 | 50.03 ± 3.33 | 39.5 ± 0.5 | 55.7 ± 0.7 | 0.4419 | 0.4510 | 45.9 | 39.6 |
| 3 | 76.83 ± 2.65 | 40.95 ± 4.27 | 40.3 ± 0.5 | 54.1 ± 0.6 | 0.4368 | 0.4397 | 43.4 | 40.0 |
| 4 | 74.61 ± 1.48 | 39.43 ± 4.39 | 45.2 ± 0.7 | 51.3 ± 0.5 | 0.4227 | 0.4131 | 42.4 | 38.5 |
| 5 | 119.05 ± 2.65 | 72.31 ± 4.82 | 48.8 ± 0.8 | 57.0 ± 1.0 | 0.4543 | 0.4575 | 40.6 | 41.9 |
| 6 | 121.81 ± 1.97 | 70.71 ± 4.45 | 49.2 ± 0.2 | 57.5 ± 0.5 | 0.4513 | 0.4427 | 42.0 | 41.2 |
| 7 | 128.74 ± 1.27 | 70.03 ± 3.69 | 53.3 ± 0.3 | 58.8 ± 0.8 | 0.4479 | 0.4408 | 42.7 | 41.1 |
| 8 | 76.69 ± 1.90 | 51.95 ± 3.51 | 55.0 ± 1.0 | 60.6 ± 0.6 | 0.3383 | 0.3328 | 47.6 | 46.2 |
| Sample | Glass-Transition Temperature, Tg (°C) | Storage Modulus, E′ | ||||||
|---|---|---|---|---|---|---|---|---|
| From tan δ | From Loss Modulus Curve, E″ | Glassy, E′(20 °C) (GPa) | Rubbery, E′(180 °C) (MPa) | |||||
| before | after | before | after | before | after | before | after | |
| 1 | 127 | 132 | 100 | 107 | 2.91 | 2.81 | 24.06 | 25.24 |
| 2 | 126 | 131 | 101 | 108 | 3.17 | 2.78 | 31.45 | 23.78 |
| 3 | 126 | 131 | 103 | 108 | 2.94 | 2.81 | 34.68 | 28.50 |
| 4 | 126 | 130 | 105 | 110 | 2.86 | 2.90 | 49.08 | 40.04 |
| 5 | 128 | 131 | 106 | 108 | 2.79 | 2.73 | 27.13 | 22.05 |
| 6 | 129 | 131 | 108 | 110 | 2.78 | 2.83 | 27.53 | 23.33 |
| 7 | 130 | 132 | 109 | 111 | 2.75 | 2.86 | 27.75 | 25.75 |
| 8 | 126 | 129 | 104 | 107 | 2.77 | 2.83 | 46.11 | 41.20 |
| Sample | Gloss (GU) | |||||
|---|---|---|---|---|---|---|
| 20° | 60° | 85° | 20° | 60° | 85° | |
| before Aging | after Aging | |||||
| 1 | 101.3 | 112.4 | 100.4 | 30.6 | 50.2 | 81.1 |
| 2 | 96.8 | 110.4 | 98.5 | 63.3 | 92.2 | 96.0 |
| 3 | 96.4 | 101.8 | 99.2 | 57.5 | 84.0 | 90.3 |
| 4 | 76.8 | 95.3 | 96.1 | 55.3 | 81.0 | 87.6 |
| 5 | 113.3 | 121.8 | 99.9 | 85.9 | 97.3 | 98.1 |
| 6 | 128.3 | 133.9 | 102.9 | 83.9 | 96.8 | 98.1 |
| 7 | 107.1 | 121.1 | 102.0 | 83.4 | 91.6 | 94.6 |
| 8 | 94.7 | 100.4 | 100.3 | 73.5 | 90.2 | 95.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pączkowski, P.; Puszka, A.; Miazga-Karska, M.; Ginalska, G.; Gawdzik, B. Synthesis, Characterization and Testing of Antimicrobial Activity of Composites of Unsaturated Polyester Resins with Wood Flour and Silver Nanoparticles. Materials 2021, 14, 1122. https://doi.org/10.3390/ma14051122
Pączkowski P, Puszka A, Miazga-Karska M, Ginalska G, Gawdzik B. Synthesis, Characterization and Testing of Antimicrobial Activity of Composites of Unsaturated Polyester Resins with Wood Flour and Silver Nanoparticles. Materials. 2021; 14(5):1122. https://doi.org/10.3390/ma14051122
Chicago/Turabian StylePączkowski, Przemysław, Andrzej Puszka, Malgorzata Miazga-Karska, Grażyna Ginalska, and Barbara Gawdzik. 2021. "Synthesis, Characterization and Testing of Antimicrobial Activity of Composites of Unsaturated Polyester Resins with Wood Flour and Silver Nanoparticles" Materials 14, no. 5: 1122. https://doi.org/10.3390/ma14051122
APA StylePączkowski, P., Puszka, A., Miazga-Karska, M., Ginalska, G., & Gawdzik, B. (2021). Synthesis, Characterization and Testing of Antimicrobial Activity of Composites of Unsaturated Polyester Resins with Wood Flour and Silver Nanoparticles. Materials, 14(5), 1122. https://doi.org/10.3390/ma14051122








