Chemical Reaction and Ion Bombardment Effects of Plasma Radicals on Optoelectrical Properties of SnO2 Thin Films via Atomic Layer Deposition
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and PEALD Process
2.2. Characteristic Measurements
3. Results and Discussion
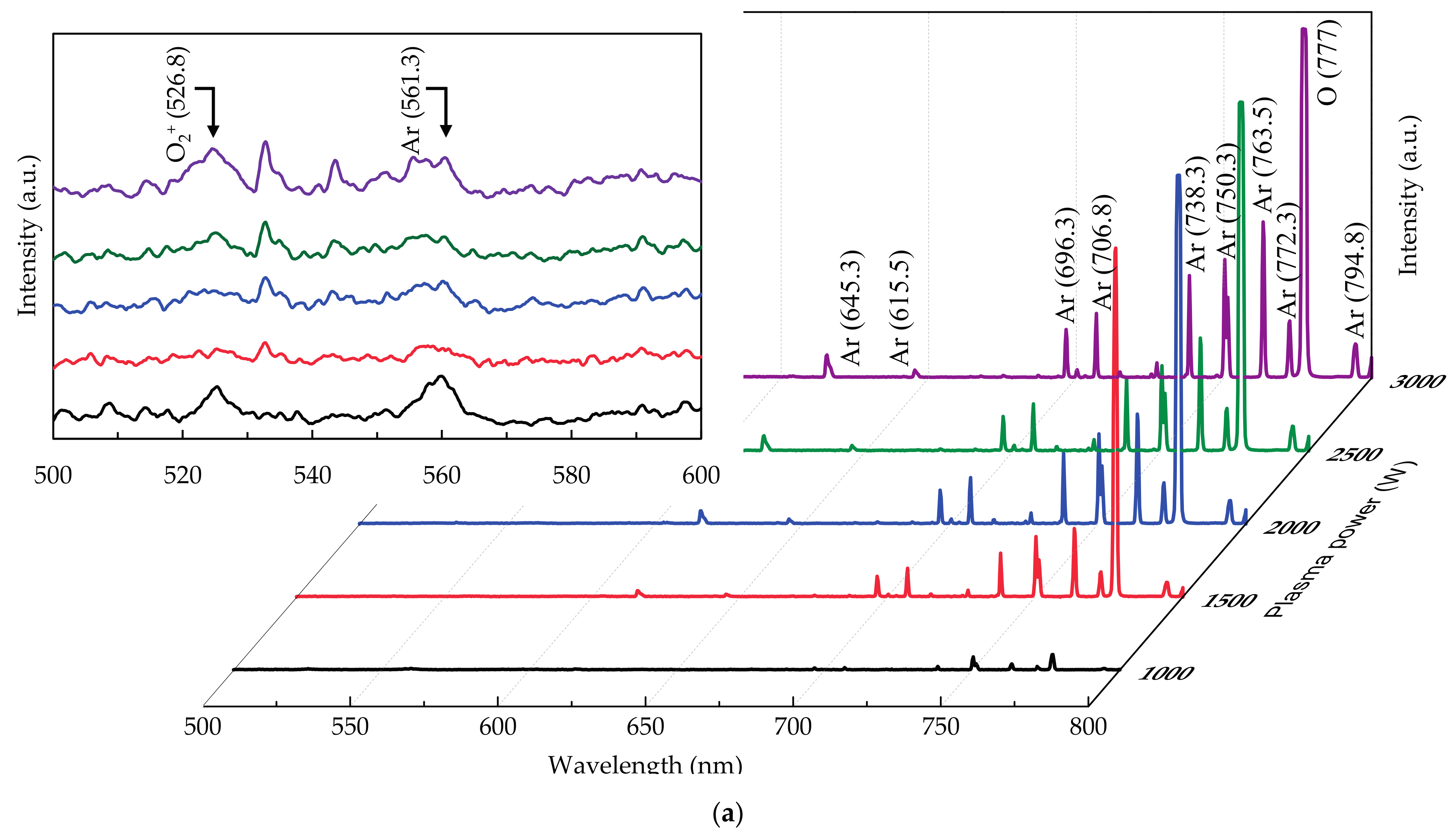
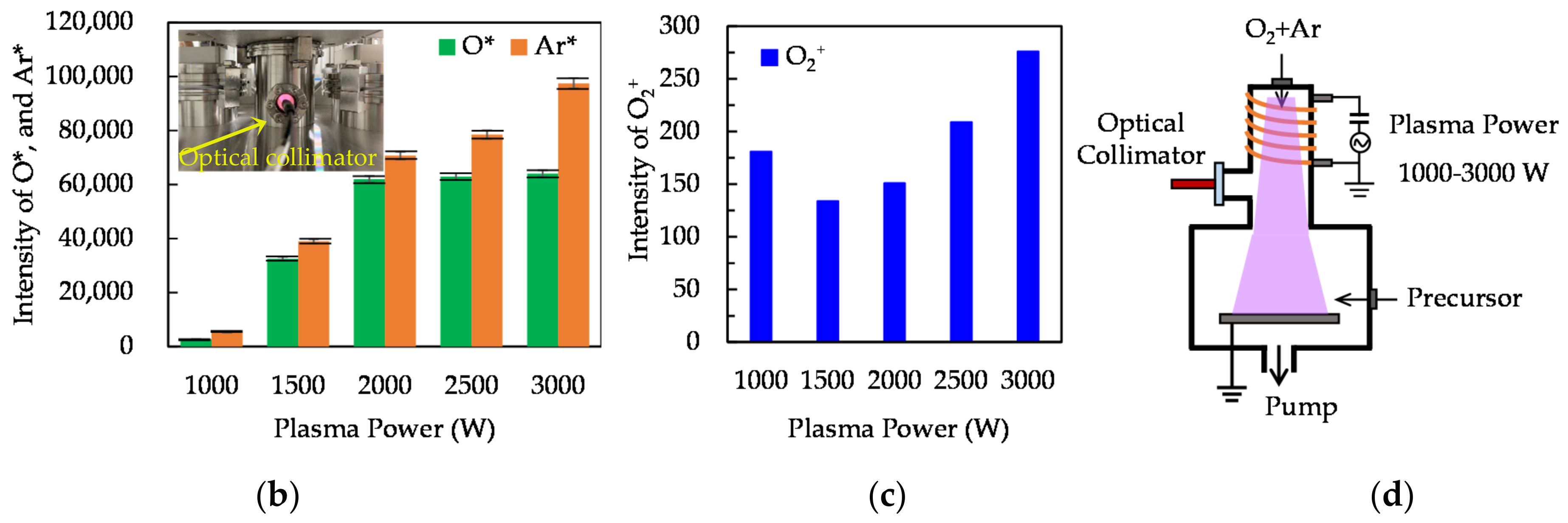
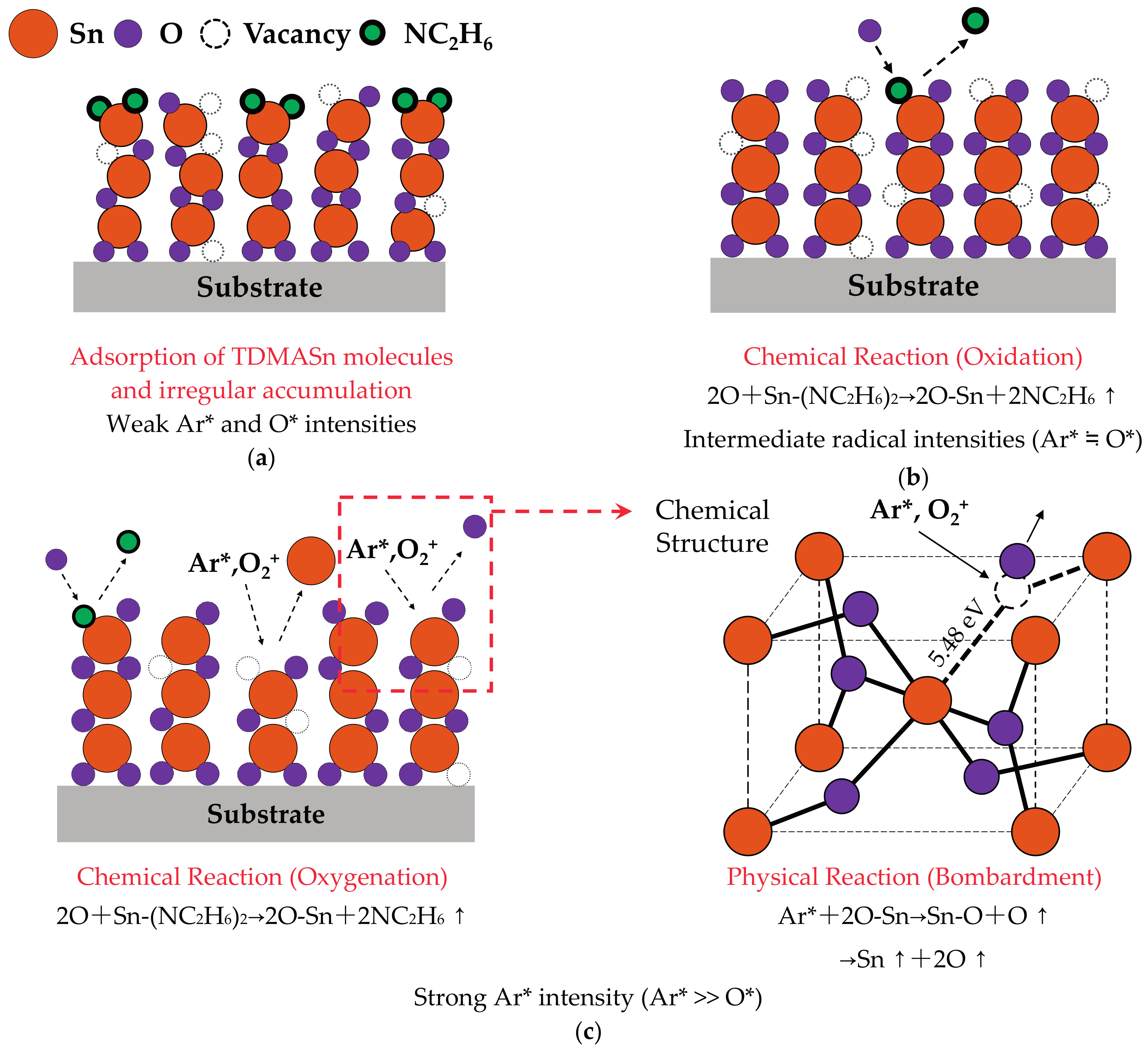
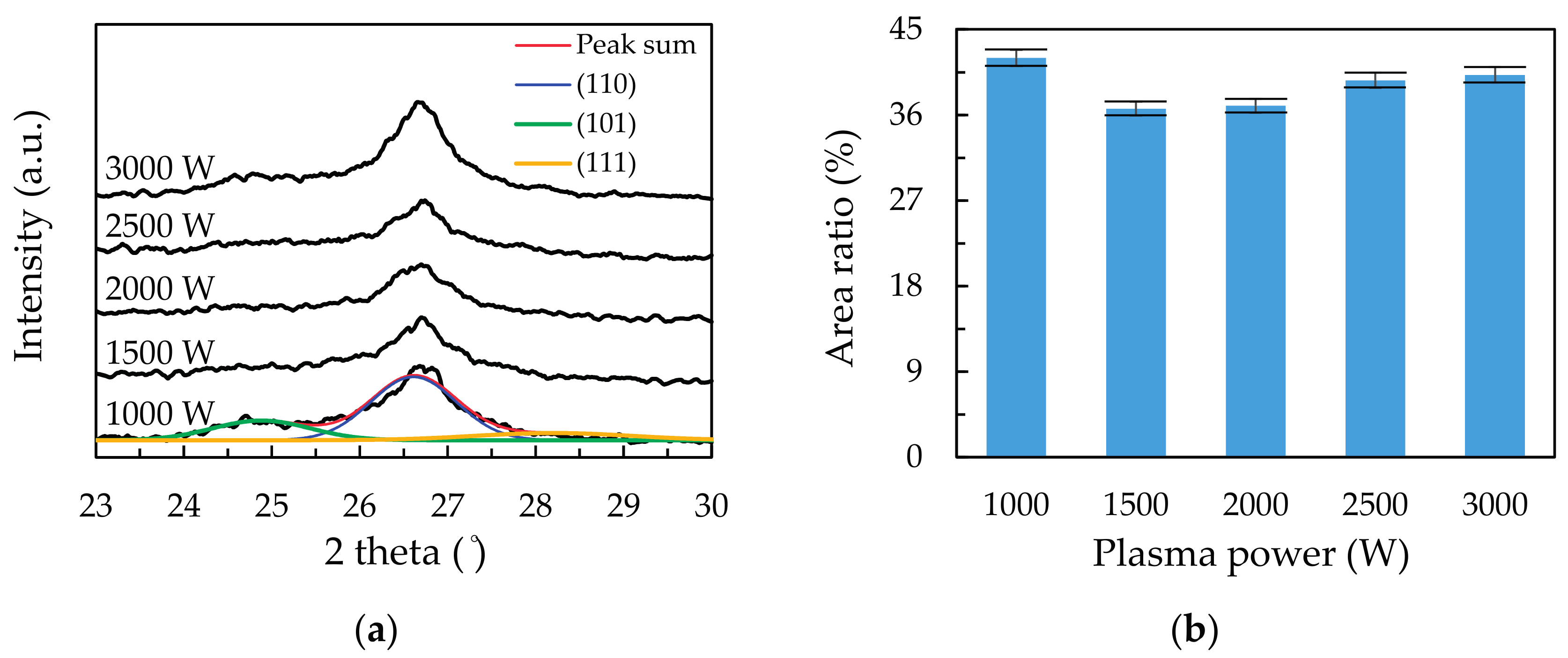
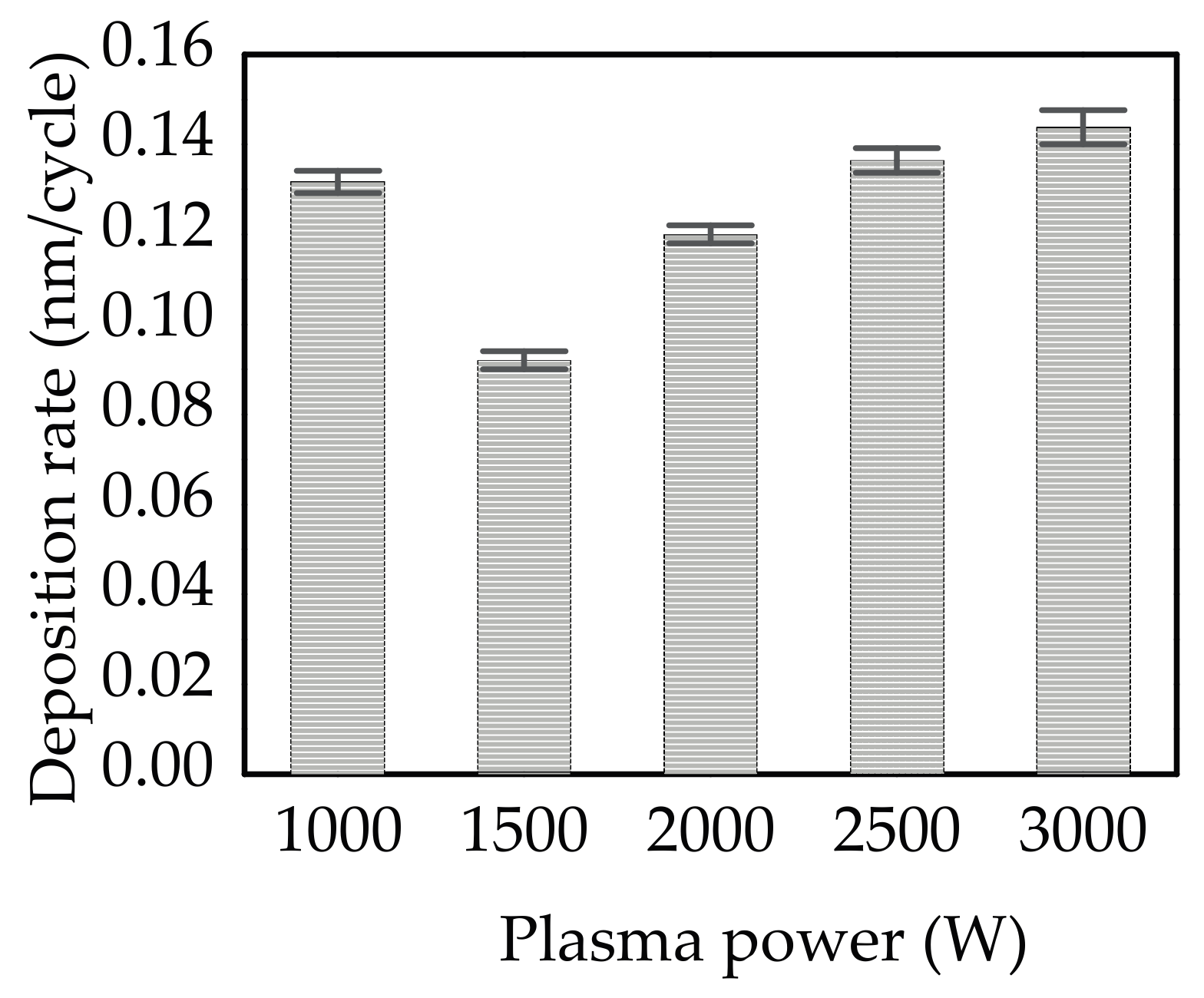
3.1. Deposition Mechanism
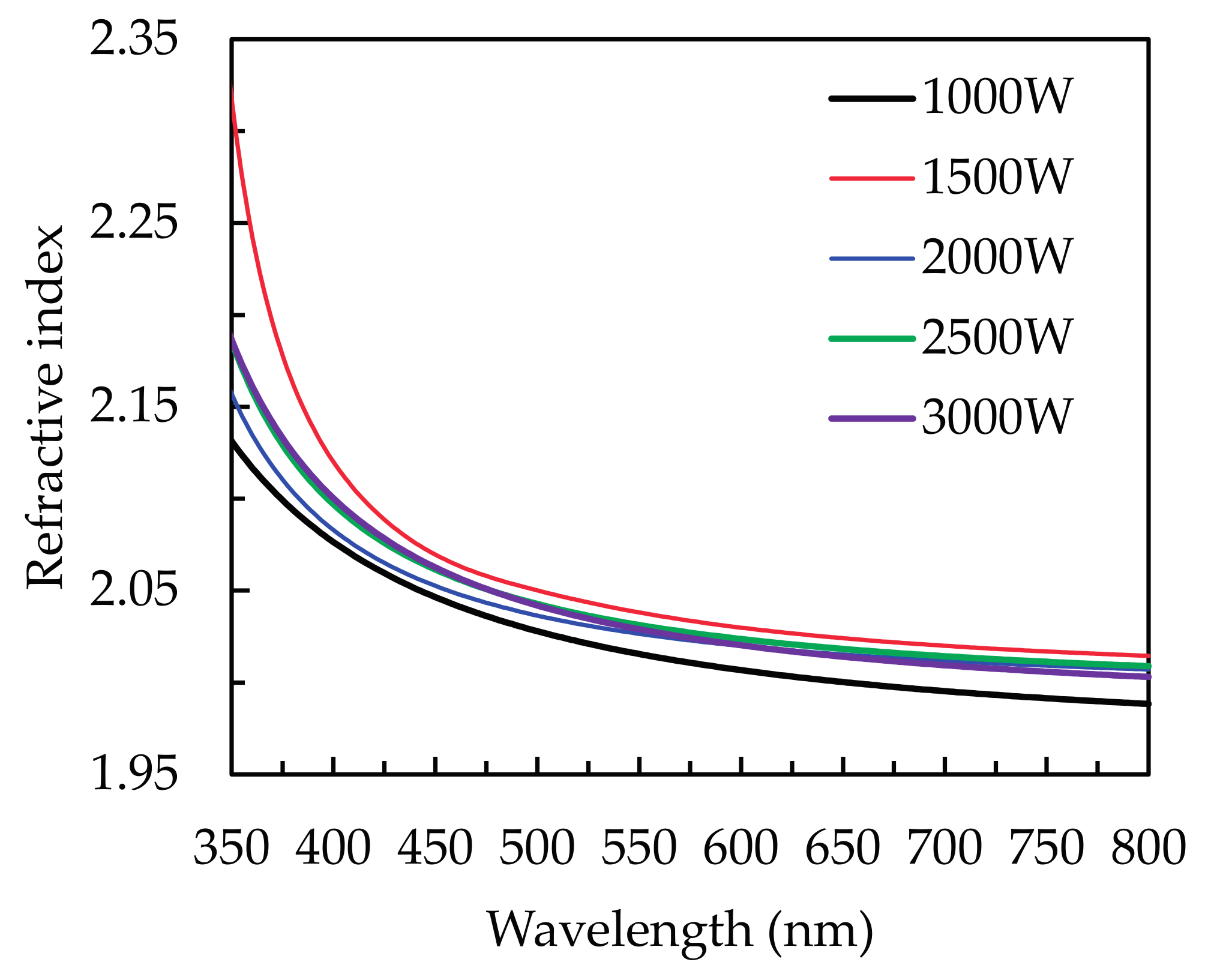
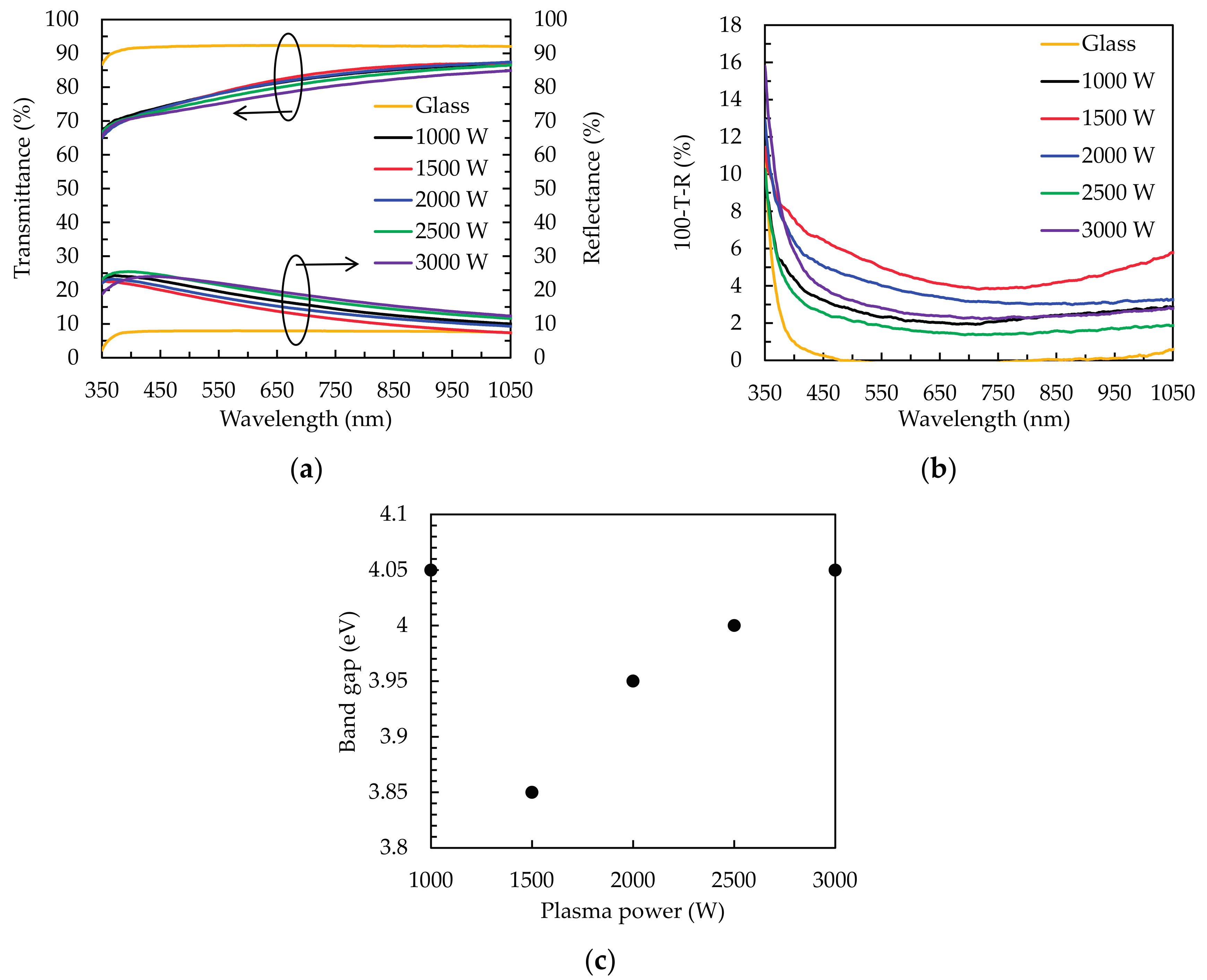
3.2. Optical Properties
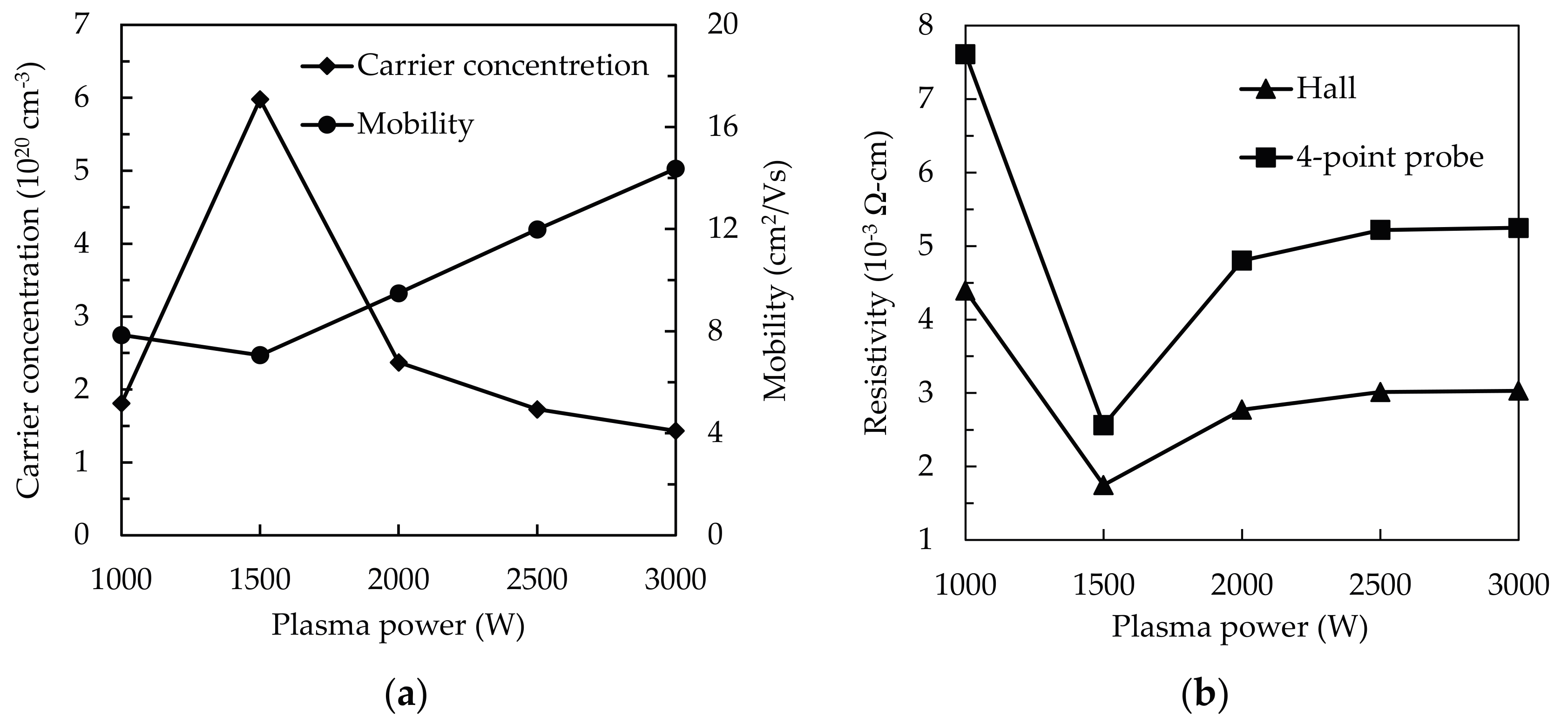
3.3. Electrical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, D.; Jeon, Y.; Kim, M.; Kim, Y.; Kim, S. Electrical Characteristics of SnO2 Thin-Film Transistors Fabricated on Bendable Substrates Using Reactive Magnetron Sputtering. J. Nanosci. Nanotechnol. 2016, 16, 11697–11700. [Google Scholar] [CrossRef]
- Sun, W.; Wang, S.; Li, S.; Miao, X.; Zhu, Y.; Du, C.; Ma, R.; Wang, C. Reactive-Sputtered Prepared Tin Oxide Thin Film as an Electron Transport Layer for Planar Perovskite Solar Cells. Coatings 2019, 9, 320. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, Z.; Ono, L.K.; Jiang, Y.; Son, D.; Hawash, Z.; He, S.; Qi, Y. Scalable Fabrication of Stable High Efficiency Perovskite Solar Cells and Modules Utilizing Room Temperature Sputtered SnO2 Electron Transport Layer. Adv. Funct. Mater. 2019, 29, 1806779. [Google Scholar] [CrossRef]
- Shanker, G.; Prathap, P.; Srivatsa, K.M.K.; Singh, P. Effect of Balanced and Unbalanced Magnetron Sputtering Processes on the Properties of SnO2 Thin Films. Curr. Appl. Phys. 2019, 19, 697–703. [Google Scholar] [CrossRef]
- Howari, H.; Tomsah, I.B.I. Structural, Optical and Ellipsometric Characteristics of PVD Synthesized SnO2 Thin Films on Pt Coated Silicon Wafers. Optik 2017, 144, 467–474. [Google Scholar] [CrossRef]
- Iizuka, K.; Kambara, M.; Yoshida, T. Highly Sensitive SnO2 Porous Film Gas Sensors Fabricated by Plasma Spray Physical Vapor Deposition. Sens. Actuators B Chem. 2012, 173, 455–461. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Spray Pyrolysis Deposition of Undoped SnO2 and In2O3 Films and Their Structural Properties. Prog. Cryst. Growth Charact. Mater. 2017, 63, 1–47. [Google Scholar] [CrossRef]
- Köse, H.; Karaal, Ş.; Aydin, A.O.; Akbulut, H. Structural Properties of Size-Controlled SnO2 Nanopowders Produced by Sol–Gel Method. Mater. Sci. Semicond. Process. 2015, 38, 404–412. [Google Scholar] [CrossRef]
- Kumar, V.; Swart, H.C.; Gohain, M.; Bezuidenhoudt, B.C.B.; van Vuuren, A.J.; Lee, M.; Ntwaeaborwa, O.M. The Role of Neutral and Ionized Oxygen Defects in the Emission of Tin Oxide Nanocrystals for near White Light Application. Nanotechnology 2015, 26, 295703. [Google Scholar] [CrossRef]
- Wang, J.; Ge, J.; Hou, H.; Wang, M.; Liu, G.; Qiao, G.; Wang, Y. Design and Sol–Gel Preparation of SiO2/TiO2 and SiO2/SnO2/SiO2–SnO2 Multilayer Antireflective Coatings. Appl. Surf. Sci. 2017, 422, 970–974. [Google Scholar] [CrossRef]
- Kassem, O.; Saadaoui, M.; Rieu, M.; Viricelle, J.-P. A Novel Approach to a Fully Inkjet Printed SnO 2 -Based Gas Sensor on a Flexible Foil. J. Mater. Chem. C 2019, 7, 12343–12353. [Google Scholar] [CrossRef]
- Kwoka, M.; Ottaviano, L.; Waczyńska, N.; Santucci, S.; Szuber, J. Influence of Si Substrate Preparation on Surface Chemistry and Morphology of L-CVD SnO2 Thin Films Studied by XPS and AFM. Appl. Surf. Sci. 2010, 256, 5771–5775. [Google Scholar] [CrossRef]
- Pan, J.; Ganesan, R.; Shen, H.; Mathur, S. Plasma-Modified SnO2 Nanowires for Enhanced Gas Sensing. J. Phys. Chem. C 2010, 114, 8245–8250. [Google Scholar] [CrossRef]
- Lu, Y.M.; Jiang, J.; Becker, M.; Kramm, B.; Chen, L.; Polity, A.; He, Y.B.; Klar, P.J.; Meyer, B.K. Polycrystalline SnO2 Films Grown by Chemical Vapor Deposition on Quartz Glass. Vacuum 2015, 122, 347–352. [Google Scholar] [CrossRef]
- Verchère, A.; Mishra, S.; Jeanneau, E.; Guillon, H.; Decams, J.-M.; Daniele, S. Heteroleptic Tin(IV) Aminoalkoxides and Aminofluoroalkoxides as MOCVD Precursors for Undoped and F-Doped SnO2 Thin Films. Inorg. Chem. 2020, 59, 7167–7180. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.-Y.; Yuan, K.; Yang, J.-G.; Ma, H.-P.; Wang, T.; Ji, X.-M.; Feng, J.-J.; Devi, A.; Lu, H.-L. Fabrication of Heterostructured P-CuO/n-SnO2 Core-Shell Nanowires for Enhanced Sensitive and Selective Formaldehyde Detection. Sens. Actuators B Chem. 2019, 290, 233–241. [Google Scholar] [CrossRef]
- Ahmed, B.; Anjum, D.H.; Gogotsi, Y.; Alshareef, H.N. Atomic Layer Deposition of SnO 2 on MXene for Li-Ion Battery Anodes. Nano Energy 2017, 34, 249–256. [Google Scholar] [CrossRef]
- Park, H.; Choi, H.; Lee, N.; Jung, C.; Choi, Y.; Song, S.; Choi, Y.; Kim, K.; Kim, J.; Lee, J.; et al. Effect of Single Al2O3 Cycle Insertion with Various Positions in SnO2 Thin Films Using Atomic Layer Deposition. Ceram. Int. 2020, 46, 12782–12787. [Google Scholar] [CrossRef]
- Jeong, S.; Seo, S.; Park, H.; Shin, H. Atomic Layer Deposition of a SnO2 Electron-Transporting Layer for Planar Perovskite Solar Cells with a Power Conversion Efficiency of 18.3%. Chem. Commun. 2019, 55, 2433–2436. [Google Scholar] [CrossRef]
- Su, L.; Zuo, Y.; Xie, J. Scalable Manufacture of Vertical p-GaN/n-SnO2 Heterostructure for Self-powered Ultraviolet Photodetector, Solar Cell and Dual-color Light Emitting Diode. InfoMat 2020, inf2.12127. [Google Scholar] [CrossRef]
- Choi, D.; Maeng, W.J.; Park, J.-S. The Conducting Tin Oxide Thin Films Deposited via Atomic Layer Deposition Using Tetrakis-Dimethylamino Tin and Peroxide for Transparent Flexible Electronics. Appl. Surf. Sci. 2014, 313, 585–590. [Google Scholar] [CrossRef]
- Lee, B.K.; Jung, E.; Kim, S.H.; Moon, D.C.; Lee, S.S.; Park, B.K.; Hwang, J.H.; Chung, T.-M.; Kim, C.G.; An, K.-S. Physical/Chemical Properties of Tin Oxide Thin Film Transistors Prepared Using Plasma-Enhanced Atomic Layer Deposition. Mater. Res. Bull. 2012, 47, 3052–3055. [Google Scholar] [CrossRef]
- Kuang, Y.; Zardetto, V.; van Gils, R.; Karwal, S.; Koushik, D.; Verheijen, M.A.; Black, L.E.; Weijtens, C.; Veenstra, S.; Andriessen, R.; et al. Low-Temperature Plasma-Assisted Atomic-Layer-Deposited SnO2 as an Electron Transport Layer in Planar Perovskite Solar Cells. Acs Appl. Mater. Interfaces 2018, 10, 30367–30378. [Google Scholar] [CrossRef] [PubMed]
- Chistiakova, G.; Mews, M.; Wilks, R.G.; Bär, M.; Korte, L. In-System Photoelectron Spectroscopy Study of Tin Oxide Layers Produced from Tetrakis(Dimethylamino)Tin by Plasma Enhanced Atomic Layer Deposition. J. Vac. Sci. Technol. A Vac. Surf. Film. 2018, 36, 02D401. [Google Scholar] [CrossRef]
- Kim, H.Y.; Nam, J.H.; George, S.M.; Park, J.-S.; Park, B.K.; Kim, G.H.; Jeon, D.J.; Chung, T.-M.; Han, J.H. Phase-Controlled SnO2 and SnO Growth by Atomic Layer Deposition Using Bis(N-Ethoxy-2,2-Dimethyl Propanamido)Tin Precursor. Ceram. Int. 2019, 45, 5124–5132. [Google Scholar] [CrossRef]
- Mai, L.; Zanders, D.; Subaşı, E.; Ciftyurek, E.; Hoppe, C.; Rogalla, D.; Gilbert, W.; de los Arcos, T.; Schierbaum, K.; Grundmeier, G.; et al. Low-Temperature Plasma-Enhanced Atomic Layer Deposition of Tin(IV) Oxide from a Functionalized Alkyl Precursor: Fabrication and Evaluation of SnO2-Based Thin-Film Transistor Devices. ACS Appl. Mater. Interfaces 2019, 11, 3169–3180. [Google Scholar] [CrossRef]
- Profijt, H.B.; Potts, S.E.; van de Sanden, M.C.M.; Kessels, W.M.M. Plasma-Assisted Atomic Layer Deposition: Basics, Opportunities, and Challenges. J. Vac. Sci. Technol. A Vac. Surf. Film. 2011, 29, 050801. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, Y.; Yin, S. Oxygen Vacancies Confined in SnO2 Nanoparticles for Desirable Electronic Structure and Enhanced Visible Light Photocatalytic Activity. Appl. Surf. Sci. 2017, 420, 399–406. [Google Scholar] [CrossRef]
- Ma, D.; Li, Y.; Zhang, P.; Lin, Z. Oxygen Vacancy Engineering in Tin(IV) Oxide Based Anode Materials toward Advanced Sodium-Ion Batteries. ChemSusChem 2018, 11, 3693–3703. [Google Scholar] [CrossRef]
- Ilka, M.; Bera, S.; Kwon, S.-H. Influence of Surface Defects and Size on Photochemical Properties of SnO2 Nanoparticles. Materials 2018, 11, 904. [Google Scholar] [CrossRef]
- Lee, C.; Lieberman, M.A. Global Model of Ar, O2, Cl2, and Ar/O2 High-density Plasma Discharges. J. Vac. Sci. Technol. A Vac. Surf. Film. 1995, 13, 368–380. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Cho, Y.-S.; Liu, T.-X.; Chang, H.-W.; Lien, S.-Y. Optimization of Residual Stress of SiO2/Organic Silicon Stacked Layer Prepared Using Inductively Coupled Plasma Deposition. Surf. Coat. Technol. 2017, 320, 293–297. [Google Scholar] [CrossRef]
- Lien, S.-Y.; Cho, Y.-S.; Hsu, C.-H.; Shen, K.-Y.; Zhang, S.; Wu, W.-Y. Mechanism of Dense Silicon Dioxide Films Deposited under 100 °C via Inductively Coupled Plasma Chemical Vapor Deposition. Surf. Coat. Technol. 2019, 359, 247–251. [Google Scholar] [CrossRef]
- Mackus, A.J.M.; Heil, S.B.S.; Langereis, E.; Knoops, H.C.M.; van de Sanden, M.C.M.; Kessels, W.M.M. Optical Emission Spectroscopy as a Tool for Studying, Optimizing, and Monitoring Plasma-Assisted Atomic Layer Deposition Processes. J. Vac. Sci. Technol. A Vac. Surf. Film. 2010, 28, 77–87. [Google Scholar] [CrossRef]
- Takamatsu, T.; Uehara, K.; Sasaki, Y.; Miyahara, H.; Matsumura, Y.; Iwasawa, A.; Ito, N.; Azuma, T.; Kohno, M.; Okino, A. Investigation of Reactive Species Using Various Gas Plasmas. RSC Adv. 2014, 4, 39901–39905. [Google Scholar] [CrossRef]
- Takechi, K.; Lieberman, M.A. Effect of Ar Addition to an O2 Plasma in an Inductively Coupled, Traveling Wave Driven, Large Area Plasma Source: O2/Ar Mixture Plasma Modeling and Photoresist Etching. J. Appl. Phys. 2001, 90, 3205–3211. [Google Scholar] [CrossRef]
- Berenguer, C.; Katsonis, K. Plasma Reactors and Plasma Thrusters Modeling by Ar Complete Global Models. Int. J. Aerosp. Eng. 2012, 2012, 1–18. [Google Scholar] [CrossRef]
- Marichy, C.; Donato, N.; Willinger, M.-G.; Latino, M.; Karpinsky, D.; Yu, S.-H.; Neri, G.; Pinna, N. Tin Dioxide Sensing Layer Grown on Tubular Nanostructures by a Non-Aqueous Atomic Layer Deposition Process. Adv. Funct. Mater. 2011, 21, 658–666. [Google Scholar] [CrossRef]
- Lim, S.-S. Atomic Layer Deposition of SnO2 Thin Films Using Tetraethyltin and H2O2. Ceram. Int. 2019, 6, 20600–20605. [Google Scholar] [CrossRef]
- Dean, J.A. (Ed.) Lange’s Handbook of Chemistry, 14th ed.; McGraw-Hill: New York, NY, USA, 1992; ISBN 978-0-07-016194-8. [Google Scholar]
- White, T.A.; Moreno, M.S.; Midgley, P.A. Structure Determination of the Intermediate Tin Oxide Sn3O4 by Precession Electron Diffraction. Z. Für Krist. 2010, 225. [Google Scholar] [CrossRef]
- Wu, J.; Xie, Y.; Du, S.; Ren, Z.; Yu, P.; Wang, X.; Wang, G.; Fu, H. Heterophase Engineering of SnO2/Sn3O4 Drives Enhanced Carbon Dioxide Electrocatalytic Reduction to Formic Acid. Sci. China Mater. 2020, 63, 2314–2324. [Google Scholar] [CrossRef]
- Richey, N.E.; de Paula, C.; Bent, S.F. Understanding Chemical and Physical Mechanisms in Atomic Layer Deposition. J. Chem. Phys. 2020, 152, 040902. [Google Scholar] [CrossRef] [PubMed]
- Outemzabet, R.; Doulache, M.; Trari, M. Physical and Photoelectrochemical Properties of Sb-Doped SnO2 Thin Films Deposited by Chemical Vapor Deposition: Application to Chromate Reduction under Solar Light. Appl. Phys. A 2015, 119, 589–596. [Google Scholar] [CrossRef]
- Koo, B.-R.; Bae, J.-W.; Ahn, H.-J. Optoelectronic Multifunctionality of Combustion-Activated Fluorine-Doped Tin Oxide Films with High Optical Transparency. Ceram. Int. 2019, 45, 10260–10268. [Google Scholar] [CrossRef]
- Peelaers, H.; Kioupakis, E.; Van de Walle, C.G. Fundamental Limits on Optical Transparency of Transparent Conducting Oxides: Free-Carrier Absorption in SnO2. Appl. Phys. Lett. 2012, 100, 011914. [Google Scholar] [CrossRef]
- Peelaers, H.; Kioupakis, E.; Van de Walle, C.G. Free-Carrier Absorption in Transparent Conducting Oxides: Phonon and Impurity Scattering in SnO2. Phys. Rev. B 2015, 92, 235201. [Google Scholar] [CrossRef]
- Norek, M.; Putkonen, M.; Zaleszczyk, W.; Budner, B.; Bojar, Z. Morphological, Structural and Optical Characterization of SnO2 Nanotube Arrays Fabricated Using Anodic Alumina (AAO) Template-Assisted Atomic Layer Deposition. Mater. Charact. 2018, 136, 52–59. [Google Scholar] [CrossRef]

| Plasma Power (W) | Composition | Atomic Ratio [O/Sn] | |
|---|---|---|---|
| O (%) | Sn (%) | ||
| 1000 | 64.2 | 35.8 | 1.79 |
| 1500 | 64.2 | 35.8 | 1.79 |
| 2000 | 64.1 | 35.9 | 1.79 |
| 2500 | 64.0 | 36.0 | 1.78 |
| 3000 | 64.2 | 35.8 | 1.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.-H.; Zhang, Z.-X.; Hsu, C.-H.; Wu, W.-Y.; Huang, C.-J.; Lien, S.-Y. Chemical Reaction and Ion Bombardment Effects of Plasma Radicals on Optoelectrical Properties of SnO2 Thin Films via Atomic Layer Deposition. Materials 2021, 14, 690. https://doi.org/10.3390/ma14030690
Huang P-H, Zhang Z-X, Hsu C-H, Wu W-Y, Huang C-J, Lien S-Y. Chemical Reaction and Ion Bombardment Effects of Plasma Radicals on Optoelectrical Properties of SnO2 Thin Films via Atomic Layer Deposition. Materials. 2021; 14(3):690. https://doi.org/10.3390/ma14030690
Chicago/Turabian StyleHuang, Pao-Hsun, Zhi-Xuan Zhang, Chia-Hsun Hsu, Wan-Yu Wu, Chien-Jung Huang, and Shui-Yang Lien. 2021. "Chemical Reaction and Ion Bombardment Effects of Plasma Radicals on Optoelectrical Properties of SnO2 Thin Films via Atomic Layer Deposition" Materials 14, no. 3: 690. https://doi.org/10.3390/ma14030690
APA StyleHuang, P.-H., Zhang, Z.-X., Hsu, C.-H., Wu, W.-Y., Huang, C.-J., & Lien, S.-Y. (2021). Chemical Reaction and Ion Bombardment Effects of Plasma Radicals on Optoelectrical Properties of SnO2 Thin Films via Atomic Layer Deposition. Materials, 14(3), 690. https://doi.org/10.3390/ma14030690








