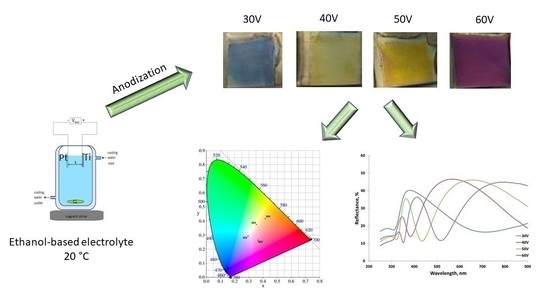
Morphological and Optical Characterization of Colored Nanotubular Anodic Titanium Oxide Made in an Ethanol-Based Electrolyte
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Noh, H.; Liew, S.F.; Saranathan, V.; Mochrie, S.G.J.; Prum, R.O.; Dufresne, E.R.; Cao, H. Structural Color: How Noniridescent Colors Are Generated by Quasi-ordered Structures of Bird Feathers. Adv. Mater. 2010, 22, 2871–2880. [Google Scholar] [CrossRef]
- Rassart, M.; Simonis, P.; Bay, A.; Deparis, O.; Vigneron, J.P. Scale coloration change following water absorption in the beetle Hoplia coerulea (Coleoptera). Phys. Rev. E 2009, 80, 031910. [Google Scholar] [CrossRef] [PubMed]
- Stępniowski, W.J.; Norekm, M.; Michalska-Domańska, M.; Bombalska, A.; Nowak-Stępniowska, A.; Kwaśny, M.; Bojar, Z. Fabrication of anodic aluminum oxide with incorporated chromate ions. Appl. Surf. Sci. 2012, 259, 324–330. [Google Scholar] [CrossRef]
- Macak, J.; Tsuchiya, H.; Schmuki, P. High-Aspect-Ratio TiO2 Nanotubes by Anodization of Titanium. Angew. Chem. Int. Ed. 2005, 44, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Macak, J.M.; Tsuchiya, H.; Taveira, L.; Aldabergerova, S.; Schmuki, P. Smooth anodic TiO2 nanotubes. Angew. Chem. Int. Ed. 2005, 44, 7463–7465. [Google Scholar] [CrossRef]
- Mika, K.; Socha, R.P.; Nyga, P.; Wiercigroch, E.; Malek, K.; Jarosz, M.; Uchacz, T.; Sulka, G.D.; Zaraska, L. Electrochemical synthesis and characterization of dark nanoporous zinc oxide films. Electrochim. Acta 2019, 305, 349–359. [Google Scholar] [CrossRef]
- Syrek, K.; Zaraska, L.; Zych, M.; Sulka, G. The effect of anodization conditions on the morphology of porous tungsten oxide layers formed in aqueous solution. J. Electroanal. Chem. 2018, 829, 106–115. [Google Scholar] [CrossRef]
- Habazaki, H. Growth of Passive Films on Valve Metals and Their Alloys. In Encyclopedia of Interfacial Chemistry Surface Science and Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 250–258. [Google Scholar]
- Chilimoniuk, P.; Michalska-Domańska, M.; Czujko, T. Formation of Nanoporous Mixed Aluminum-Iron Oxides by Self-Organized Anodizing of FeAl3 Intermetallic Alloy. Materials 2019, 12, 2299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Łazińska, M.; Durejko, T.; Michalska-Domańska, M.; Bojar, Z. Characterization of titanium oxide formed on biomedical Ti6Al7Nb alloy fabricated by Laser Engineered Net Shaping (LENS). In Proceedings of the Euro PM2017: International Power Metallurgy Congress and Exhibition, Milan, Italy, 1–4 October 2017. [Google Scholar]
- Stępniowski, W.J.; Choi, J.; Yoo, H.; Oh, K.; Michalska-Domańska, M.; Chilimoniuk, P.; Czujko, T.; Łyszkowski, R.; Jóźwiak, S.; Bojar, Z.; et al. Anodization of FeAl intermetallic alloys for bandgap tunable nanoporous mixed aluminum-iron oxide. J. Electroanal. Chem. 2016, 771, 37–44. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Choi, J.; Yoo, H.; Michalska-Domańska, M.; Chilimoniuk, P.; Czujko, T. Quantitative fast Fourier transform based arrangement analysis of nanoporous anodic oxide formed by self-organized anodization of FeAl intermetallic alloy. Mater. Lett. 2016, 164, 176–179. [Google Scholar] [CrossRef]
- Michalska-Domanska, M.; Stępniowski, W.J.; Jaroszewicz, L.R. Characterization of nanopores arrangement of anodic alumina layers synthesized on low-(AA1050) and high-purity aluminum by two-step anodizing in sulfuric acid with addition of ethylene glycol at low temperature. J. Porous Mater. 2017, 24, 779–786. [Google Scholar] [CrossRef] [Green Version]
- Abrahami, S.; De Kok, J.M.M.; Terryn, H.; Mol, J.M.C. Towards Cr(VI)-free anodization of aluminum alloys for aerospace adhesive bonding applications: A review. Front. Chem. Sci. Eng. 2017, 11, 465–482. [Google Scholar] [CrossRef]
- Abrahami, S.; Hauffman, T.; De Kok, J.M.; Mol, A.; Terryn, H. XPS Analysis of the Surface Chemistry and Interfacial Bonding of Barrier-Type Cr(VI)-Free Anodic Oxides. J. Phys. Chem. C 2015, 119, 19967–19975. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Norek, M.; Stępniowski, W.J.; Budner, B. Fabrication of high quality anodic aluminum oxide (AAO) on low purity aluminum—A comparative study with the AAO produced on high purity aluminum. Electrochim. Acta 2013, 105, 424–432. [Google Scholar] [CrossRef]
- Stępniowski, W.J.; Bojar, Z. Nanoporous anodic aluminum oxide: Fabrication, characterization, and applications. In Handbook of Nanoelectrochemistry: Electrochemical Synthesis Methods, Properties, and Characterization Techniques; Springer: Cham, Switzerland, 2016; pp. 593–646. [Google Scholar]
- Kulkarni, M.; Mazare, A.; Schmuki, P.; Iglic, A. Influence of anodization parameters on morphology of TiO2 nanostructured surfaces. Adv. Mater. Lett. 2016, 7, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Naghizadeh, M.; Ghannadi, S.; Abdizadeh, H.; Golobostanfard, M.R. Effect of Fluoride Concentration and Water Content on Morphology of Titania Nanotubes in Ethylene Glycol Solution. Adv. Mater. Res. 2013, 829, 907–911. [Google Scholar] [CrossRef]
- Michalska-Domańska, M.; Nyga, P.; Czerwiński, M. Ethanol-based electrolyte for nanotubular anodic TiO2 formation. Corros. Sci. 2018, 134, 99–102. [Google Scholar] [CrossRef]
- Valeeva, A.A.; Dorosheva, I.B.; Kozlova, E.A.; Sushnikova, A.A.; Kurenkova, A.Y.; Saraev, A.A.; Schroettner, H.; Rempel, A.A. Solar photocatalysts based on titanium dioxide nanotubes for hydrogen evolution from aqueous solutions of ethanol. Int. J. Hydrogen Energy 2021, 46, 16917–16924. [Google Scholar] [CrossRef]
- Hu, Z.; Cai, J.; Song, G.; Tian, Y.; Zhou, M. Anodic oxidation of organic pollutants: Anode fabrication, process hybrid and environmental applications. Curr. Opin. Electrochem. 2021, 26, 100659. [Google Scholar] [CrossRef]
- Michalska-Domańska, M. An Overview of Anodic Oxides Derived Advanced Nanocomposites Substrate for Surface Enhance Raman Spectroscopy. In Assorted Dimensional Reconfigurable Materials; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/books/assorted-dimensional-reconfigurable-materials/an-overview-of-anodic-oxides-derived-advanced-nanocomposites-substrate-for-surface-enhance-raman-spe (accessed on 20 June 2020). [CrossRef]
- Das, G.; Patra, N.; Gopalakrishnan, A.; Zaccaria, R.P.; Toma, A.; Thorat, S.; Di Fabrizio, E.; Diaspro, A.; Salerno, M. Fabrication of large-area ordered and reproducible nanostructures for SERS biosensor application. Analyst 2012, 137, 1785–1792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gulati, K.; Li, T.; Ivanovski, S. Consume or Conserve: Micro-Roughness of Titanium Implants towards Fabrication of Dual Micro-Nano Topography. ACS Biomater. Sci. Eng. 2018, 4, 3125–3131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, T.; Gulati, K.; Wang, N.; Zhang, Z.; Ivanovski, S. Understanding and augmenting the stability of therapeutic nanotubes on anodized titanium implants. Mater. Sci. Eng. C 2018, 88, 182–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karambakhsh, A.; Afshar, A.; Ghahramani, S.; Malekinejad, P. Pure Commercial Titanium Color Anodizing and Corrosion Resistance. J. Mater. Eng. Perform. 2011, 20, 1690–1696. [Google Scholar] [CrossRef]
- Diamanti, M.; Del Curto, B.; Pedeferri, M. Colored Titanium Oxides: From Jewelry to Biomedical Applications. In Encyclopedia of Interfacial Chemistry; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 99–107. ISBN 9780128098943. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Garbagnoli, P.; Curto, B.; Pedeferri, M. On the Growth of Thin Anodic Oxides Showing Interference Colors on Valve Metals. Curr. Nanosci. 2015, 11, 307–316. [Google Scholar] [CrossRef]
- Van Gils, S.; Mast, P.; Stijns, E.; Terryn, H. Colour properties of barrier anodic oxide films on aluminium and titanium studied with total reflectance and spectroscopic ellipsometry. Surf. Coat. Technol. 2004, 185, 303–310. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Pedeferri, M.P. Effect of anodic oxidation parameters on the titanium oxides formation. Corros. Sci. 2007, 49, 939–948. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Del Curto, B.; Pia Pedeferri, M. Interference Colors of Thin Oxide Layers on Titanium. Color Res. Appl. 2008, 3, 221–228. [Google Scholar] [CrossRef]
- Hassan, F.M.; Nanjo, H.; Venkatachalam, S.; Kanakubo, M.; Ebina, T. Effect of the solvent on growth of titania nanotubes prepared by anodization of Ti in HCl. Electrochim. Acta 2010, 55, 3130–3137. [Google Scholar] [CrossRef]
- Alipal, J.; Lee, T.; Koshy, P.; Abdullah, H.; Idris, M. Evolution of anodised titanium for implant applications. Heliyon 2021, 7, e07408. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Del Curto, B.; Pedeferri, M.P. Anodic oxidation between science and art. Metall. Ital. 2013, 105, 51–55. [Google Scholar]
- Pedeferri, P. Method of Coloring Titanium and Its Alloys Through Anodic Oxidation. European Patent EP 1199385 A2, 24 April 2002. [Google Scholar]
- Xu, Q.; Yang, Y.; Gu, J.; Li, Z.; Sun, H. Influence of Al substrate on the optical properties of porous anodic alumina films. Mater. Lett. 2012, 74, 137–139. [Google Scholar] [CrossRef]
- Xu, Q.; Sun, H.-Y.; Yang, Y.-H.; Liu, L.-H.; Li, Z.-Y. Optical properties and color generation mechanism of porous anodic alumina films. Appl. Surf. Sci. 2011, 258, 1826–1830. [Google Scholar] [CrossRef]
- Yang, S.; Li, H.; Han, W.; Gu, J.; Qi, Y. Study of the fabrication of porous anodic alumina thin films with rainbow rings. Thin Solid Films 2016, 615, 190–194. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Xu, Q.; Wang, Z.-J.; Ren, Y.-X.; Yan, R.-J.; Ma, W.-J.; Zhu, J.-L. The effect of propylene glycol on the optical properties of iridescent porous anodic alumina films. J. Porous Mater. 2017, 25, 1213–1217. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Indacochea, J.; Wang, M. A colorimetric sensor based on anodized aluminum oxide (AAO) substrate for the detection of nitroaromatics. Sens. Actuators B Chem. 2011, 160, 1149–1158. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Xu, Q.; Wang, Z.-J.; Hao, S.-Z.; Sun, C.-X.; Ma, W.-J. Preparation and characterization of anodic alumina films with high-saturation structural colors in a mixed organic electrolyte. Surf. Coat. Technol. 2018, 346, 48–52. [Google Scholar] [CrossRef]
- Kikuchi, T.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Fabrication of Self-Ordered Porous Alumina via Etidronic Acid Anodizing and Structural Color Generation from Submicrometer-Scale Dimple Array. Electrochim. Acta 2015, 156, 235–243. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.-J.; Huang, K.-Y.; Hung, L.-T.; Su, C.-Y. Rapidly fabrication of plasmonic structural color thin films through AAO process in an alkaline solution. Surf. Coat. Technol. 2017, 319, 170–181. [Google Scholar] [CrossRef]
- Yisen, L.; Yi, C.; Zhiyuan, L.; Xing, H.; Yi, L. Structural coloring of aluminum. Electrochem. Commun. 2011, 13, 1336–1339. [Google Scholar]
- Chen, Y.; Santos, A.; Ho, D.; Wang, Y.; Kumeria, T.; Li, J.; Wang, C.; Losic, D. On The Generation of Interferometric Colors in High Purity and Technical Grade Aluminum: An Alternative Green Process for Metal Finishing Industry. Electrochim. Acta 2015, 174, 672–681. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, Q.; Feng, S.; Sun, C.; Peng, Q.; Lan, T. The effect of the voltage waveform on the microstructure and optical properties of porous anodic alumina photonic crystals. Opt. Mater. 2019, 98, 109488. [Google Scholar] [CrossRef]
- Sun, C.; Hao, S.; Wang, Z.; Xu, Q.; Wang, Y.; Peng, Q.; Lan, T. Rapid fabrication of iridescent alumina films supported on an aluminium substrate by high voltage anodization. Opt. Mater. 2020, 104, 109937. [Google Scholar] [CrossRef]
- Roy, P.; Berger, S.; Schmuki, P. TiO2 Nanotubes: Synthesis and Applications. Angew. Chem. Int. Ed. 2011, 50, 2904–2939. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Kunze, J.; Schmuki, P.; Valota, A.T.; LeClere, D.J.; Skeldon, P.; Thompson, G.E. Influence of Water Content on the Growth of Anodic TiO2 Nanotubes in Fluoride-Containing Ethylene Glycol Electrolytes. J. Electrochem. Soc. 2010, 157, C18–C23. [Google Scholar] [CrossRef]
- Lee, K.; Kim, J.; Kim, H.; Lee, Y.; Tak, Y.; Kim, D.; Schmuki, P. Effect of Electrolyte Conductivity on the Formation of a Nanotubular TiO2 Photoanode for a Dye-Sensitized Solar Cell. J. Korean Phys. Soc. 2009, 54, 1027–1031. [Google Scholar] [CrossRef]
- Yoriya, S.; Paulose, M.; Varghese, O.K.; Mor, G.K.; Grimes, C.A. Fabrication of Vertically Oriented TiO2 Nanotube Arrays Using Dimethyl Sulfoxide Electrolytes. J. Phys. Chem. C 2007, 111, 13770–13776. [Google Scholar] [CrossRef]
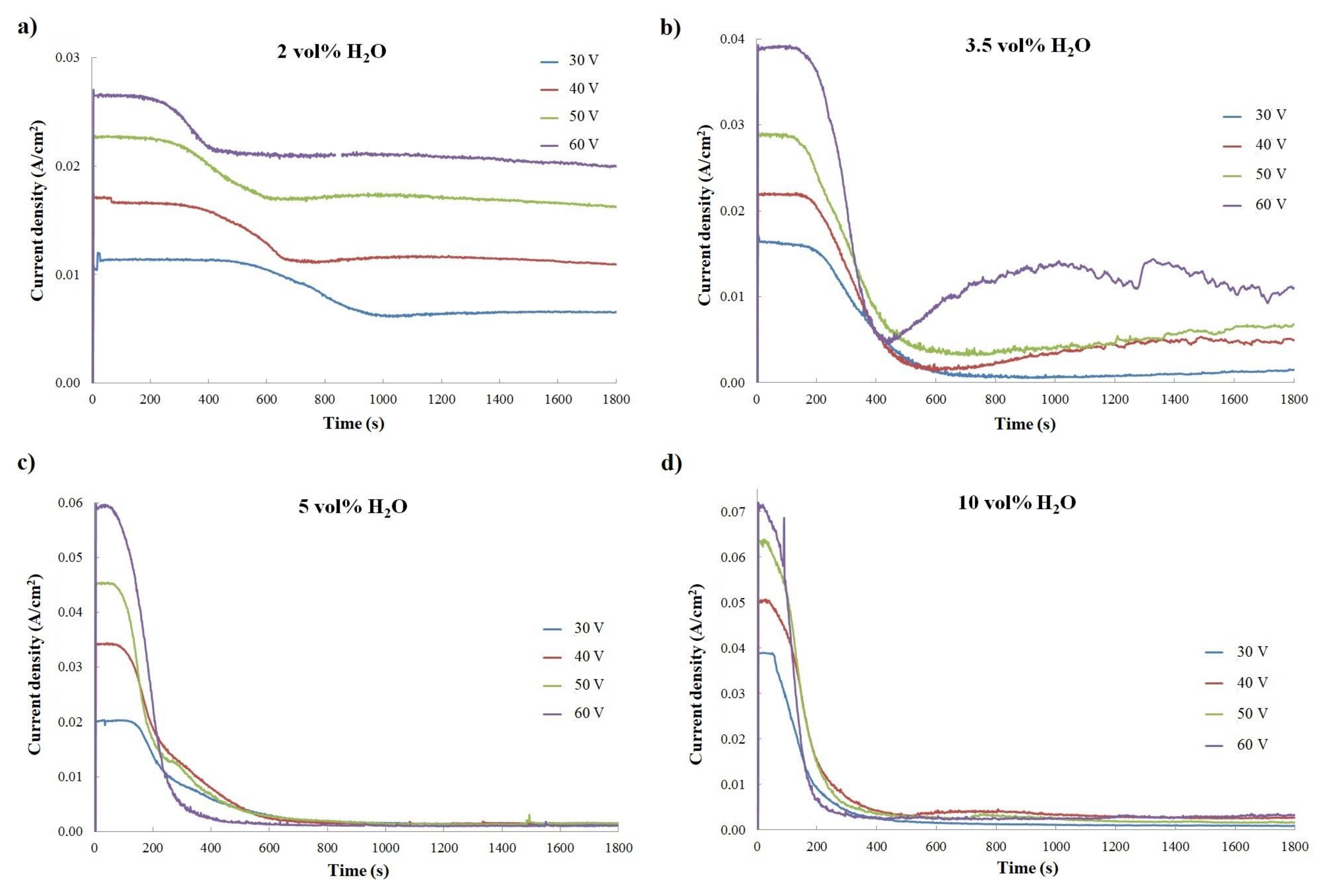
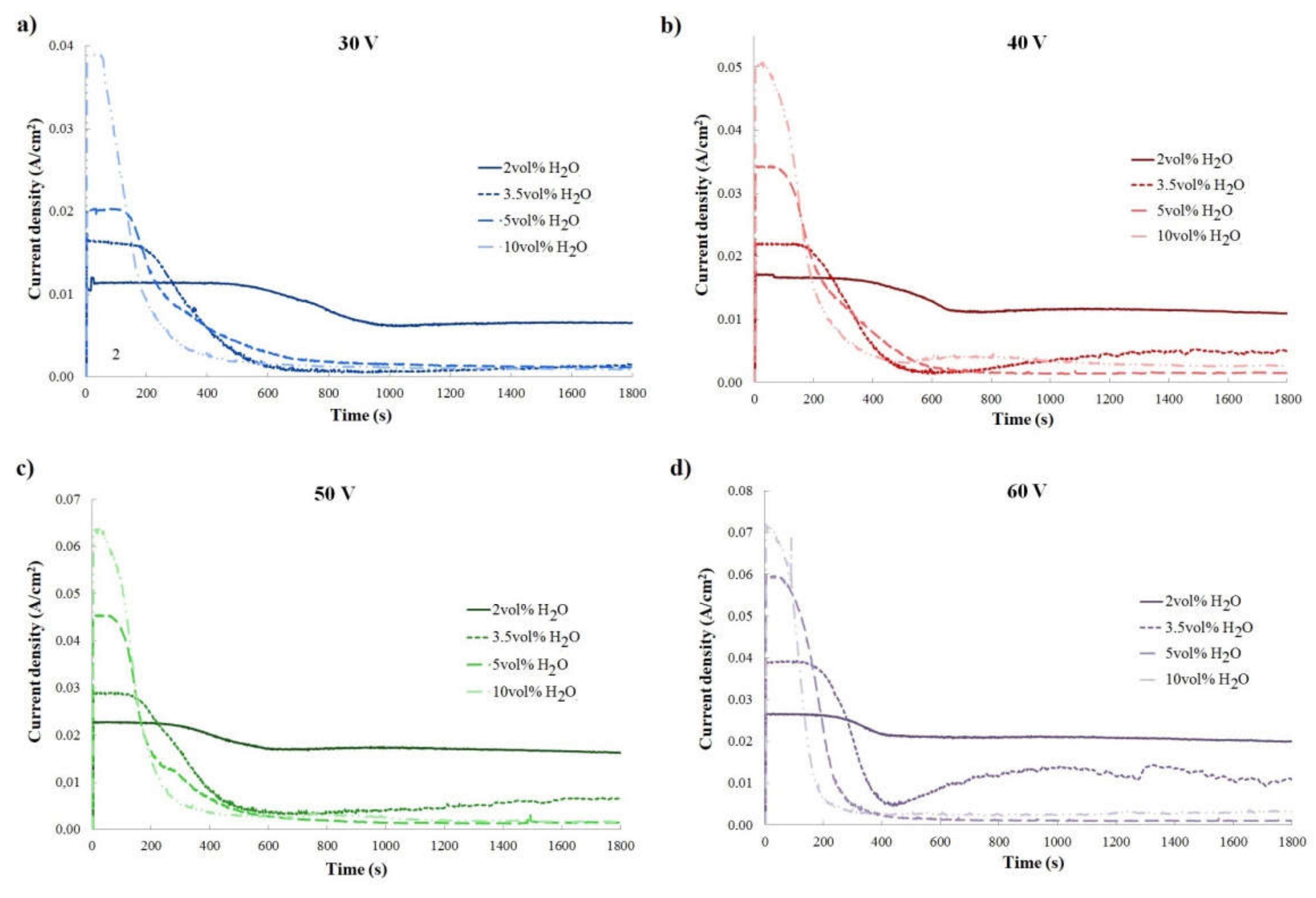

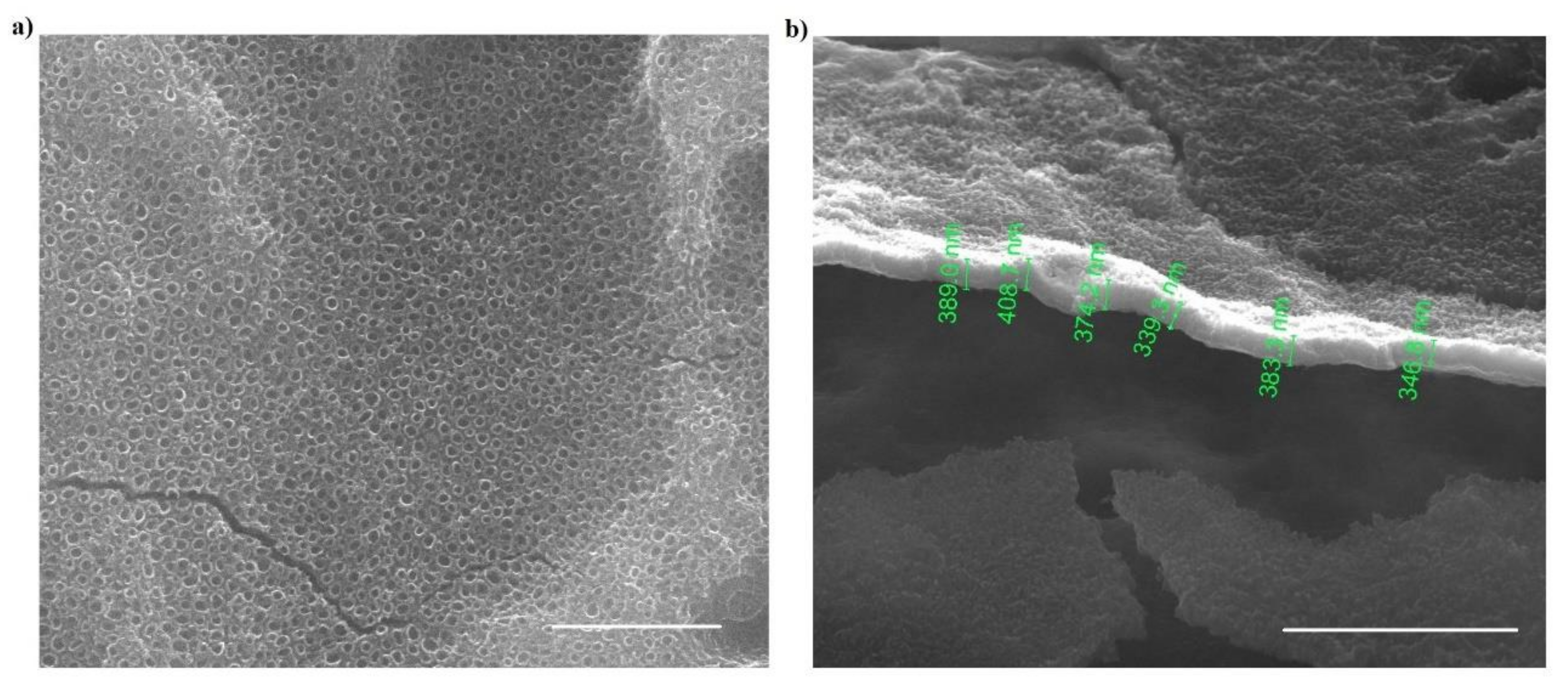
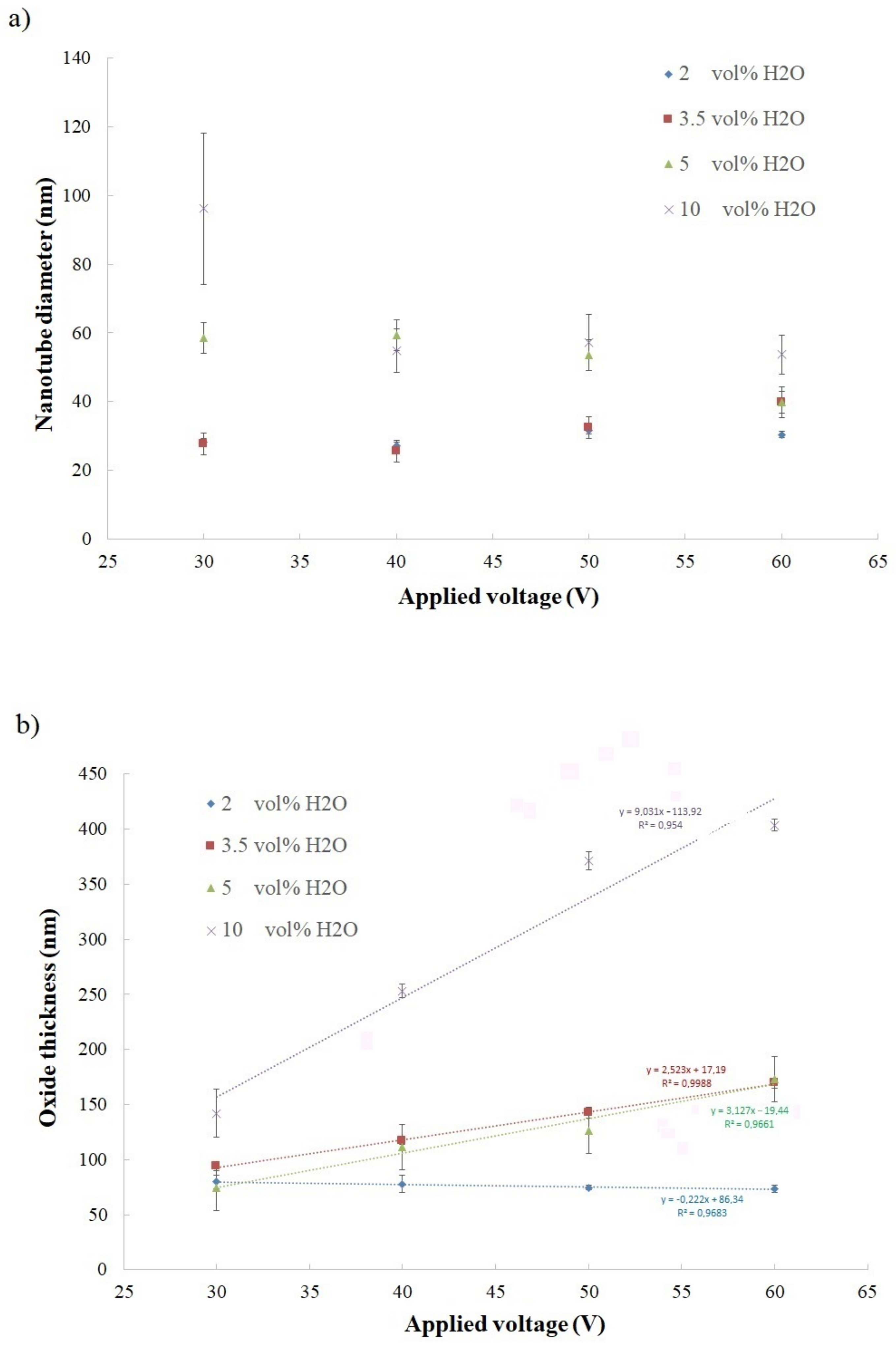
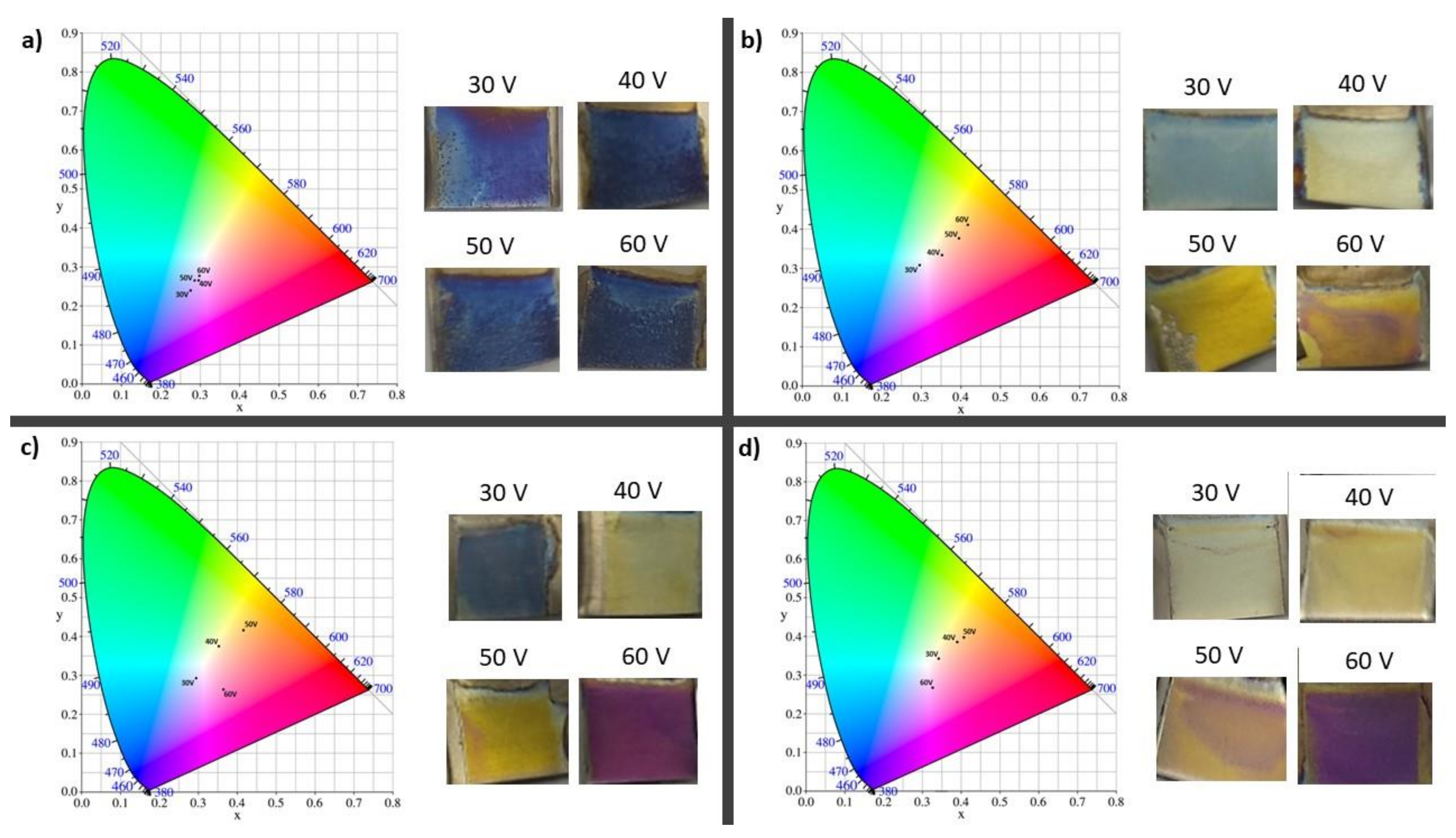
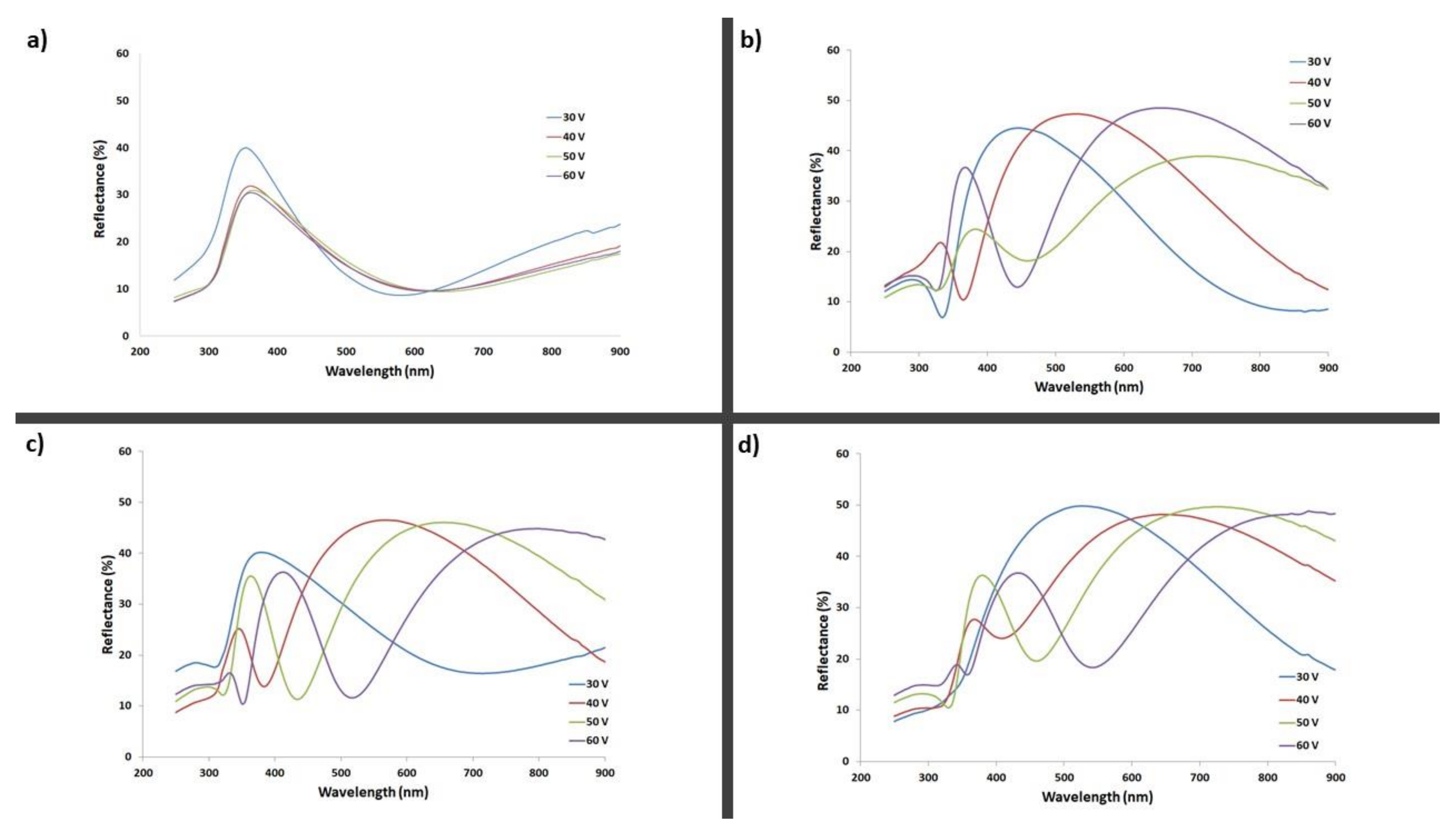
| 2 vol% H2O | 3.5 vol% H2O | 5 vol% H2O | 10 vol% H2O | |
|---|---|---|---|---|
| 30 V | 360 | 450 | 390 | 520 |
| 40 V | 360 | 520 | 550/380 | 620/380 |
| 50 V | 360 | 620/380 | 640/390 | 700/390 |
| 60 V | 360 | 700/390 | 410 | 430 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalska-Domańska, M.; Czerwiński, M.; Łazińska, M.; Dubey, V.; Jakubaszek, M.; Zawadzki, Z.; Kostecki, J. Morphological and Optical Characterization of Colored Nanotubular Anodic Titanium Oxide Made in an Ethanol-Based Electrolyte. Materials 2021, 14, 6992. https://doi.org/10.3390/ma14226992
Michalska-Domańska M, Czerwiński M, Łazińska M, Dubey V, Jakubaszek M, Zawadzki Z, Kostecki J. Morphological and Optical Characterization of Colored Nanotubular Anodic Titanium Oxide Made in an Ethanol-Based Electrolyte. Materials. 2021; 14(22):6992. https://doi.org/10.3390/ma14226992
Chicago/Turabian StyleMichalska-Domańska, Marta, Mateusz Czerwiński, Magdalena Łazińska, Vikas Dubey, Marcin Jakubaszek, Zbigniew Zawadzki, and Jerzy Kostecki. 2021. "Morphological and Optical Characterization of Colored Nanotubular Anodic Titanium Oxide Made in an Ethanol-Based Electrolyte" Materials 14, no. 22: 6992. https://doi.org/10.3390/ma14226992
APA StyleMichalska-Domańska, M., Czerwiński, M., Łazińska, M., Dubey, V., Jakubaszek, M., Zawadzki, Z., & Kostecki, J. (2021). Morphological and Optical Characterization of Colored Nanotubular Anodic Titanium Oxide Made in an Ethanol-Based Electrolyte. Materials, 14(22), 6992. https://doi.org/10.3390/ma14226992