Polymer-Based Nanosystems—A Versatile Delivery Approach
Abstract
1. Introduction
2. Polymers Used as Nanocarriers
2.1. Natural Polymers
2.1.1. Chitosan
2.1.2. Dextran
2.1.3. Alginate
2.1.4. Pullulan
2.1.5. Hyaluronic Acid
2.1.6. Albumin
2.1.7. Poly(γ-Glutamic Acid) (γ-PGA)
2.1.8. Other Natural Polymers
2.2. Synthetic Polymers
2.2.1. Polyethyleneimine (PEI)
2.2.2. Poly (Lactic Acid) (PLA)
2.2.3. Poly (Ethylene Glycol) (PEG)
2.2.4. Poly (Lactic-co-Glycolic Acid) (PLGA)
2.2.5. Poly-ε-Caprolactone (PCL)
2.2.6. Polystyrene (PS)
2.2.7. Dendrimers
2.2.8. Other Synthetic Polymers
3. Polymeric Nanoparticles Synthesis
4. Applications of Polymer-Based Delivery Nanosystems
4.1. Drug Delivery
4.2. Imaging Agent Delivery
4.3. Gene Delivery
4.4. Vaccine Delivery
5. Role of Polymer-Based NPs in Vaccine Development
5.1. Vaccine Adjuvants
5.1.1. Antigen Delivery
5.1.2. Immunomodulation
5.2. COVID-19 Immunization
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Valencia, G.A.; Zare, E.N.; Makvandi, P.; Gutiérrez, T.J. Self-assembled carbohydrate polymers for food applications: A review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2009–2024. [Google Scholar] [CrossRef]
- Aamir, M.; Tolouei-Rad, M.; Giasin, K.; Nosrati, A. Recent advances in drilling of carbon fiber–reinforced polymers for aerospace applications: A review. Int. J. Adv. Manuf. Technol. 2019, 105, 2289–2308. [Google Scholar] [CrossRef]
- Makvandi, P.; Iftekhar, S.; Pizzetti, F.; Zarepour, A.; Zare, E.N.; Ashrafizadeh, M.; Agarwal, T.; Padil, V.V.T.; Mohammadinejad, R.; Sillanpaa, M.; et al. Functionalization of polymers and nanomaterials for water treatment, food packaging, textile and biomedical applications: A review. Environ. Chem. Lett. 2020, 19, 583–611. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M. Protective polymeric films for industrial substrates: A critical review on past and recent applications with conducting polymers and polymer composites/nanocomposites. Prog. Mater. Sci. 2019, 104, 380–450. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, L.; Wang, S. Conjugated Polymer Nanoparticles for Imaging, Cell Activity Regulation, and Therapy. Adv. Funct. Mater. 2018, 29. [Google Scholar] [CrossRef]
- Guo, S.; Fu, D.; Utupova, A.; Sun, D.; Zhou, M.; Jin, Z.; Zhao, K. Applications of polymer-based nanoparticles in vaccine field. Nanotechnol. Rev. 2019, 8, 143–155. [Google Scholar] [CrossRef]
- Bastola, R.; Lee, S. Physicochemical properties of particulate vaccine adjuvants: Their pivotal role in modulating immune responses. J. Pharm. Investig. 2018, 49, 279–285. [Google Scholar] [CrossRef]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, e1800917. [Google Scholar] [CrossRef]
- Kubackova, J.; Zbytovska, J.; Holas, O. Nanomaterials for direct and indirect immunomodulation: A review of applications. Eur. J. Pharm. Sci. 2019, 142, 105139. [Google Scholar] [CrossRef] [PubMed]
- Liang, E.A.J.; Zhao, X. Nanomaterial-based delivery vehicles for therapeutic cancer vaccine development. Cancer Biol. Med. 2021, 18, 352–371. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.; Ng, S.W.; Singh, S.K.; Gulati, M.; Gupta, G.; Chaudhary, S.K.; Hing, G.B.; Collet, T.; MacLoughlin, R.; Löbenberg, R.; et al. Revolutionizing polymer-based nanoparticle-linked vaccines for targeting respiratory viruses: A perspective. Life Sci. 2021, 280, 119744. [Google Scholar] [CrossRef]
- Han, J.; Zhao, D.; Li, D.; Wang, X.; Jin, Z.; Zhao, K. Polymer-Based Nanomaterials and Applications for Vaccines and Drugs. Polymers 2018, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.; Hoemann, C.D. Chitosan immunomodulatory properties: Perspectives on the impact of structural properties and dosage. Futur. Sci. OA 2018, 4, FSO225. [Google Scholar] [CrossRef]
- Liu, Z.; He, J.; Zhu, T.; Hu, C.; Bo, R.; Wusiman, A.; Hu, Y.; Wang, D. Lentinan-Functionalized Graphene Oxide Is an Effective Antigen Delivery System That Modulates Innate Immunity and Improves Adaptive Immunity. ACS Appl. Mater. Interfaces 2020, 12, 39014–39023. [Google Scholar] [CrossRef]
- Milazzo, M.; Gallone, G.; Marcello, E.; Mariniello, M.D.; Bruschini, L.; Roy, I.; Danti, S. Biodegradable Polymeric Micro/Nano-Structures with Intrinsic Antifouling/Antimicrobial Properties: Relevance in Damaged Skin and Other Biomedical Applications. J. Funct. Biomater. 2020, 11, 60. [Google Scholar] [CrossRef] [PubMed]
- Jøraholmen, M.W.; Bhargava, A.; Julin, K.; Johannessen, M.; Škalko-Basnet, N. The Antimicrobial Properties of Chitosan Can Be Tailored by Formulation. Mar. Drugs 2020, 18, 96. [Google Scholar] [CrossRef]
- Shin, M.D.; Shukla, S.; Chung, Y.H.; Beiss, V.; Chan, S.K.; Ortega-Rivera, O.A.; Wirth, D.M.; Chen, A.; Sack, M.; Pokorski, J.K.; et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat. Nanotechnol. 2020, 15, 646–655. [Google Scholar] [CrossRef] [PubMed]
- Pippa, N.; Gazouli, M.; Pispas, S. Recent Advances and Future Perspectives in Polymer-Based Nanovaccines. Vaccines 2021, 9, 558. [Google Scholar] [CrossRef]
- Tzeng, S.Y.; Green, J.J. Polymeric nucleic acid delivery for immunoengineering. Curr. Opin. Biomed. Eng. 2018, 7, 42–50. [Google Scholar] [CrossRef]
- Fadlilah, D.R.; Endarko, E.; Ratnasari, A.; Hozairi, H.; Yusop, Z.; Syafiuddin, A. Enhancement of antibacterial properties of various polymers functionalized with silver nanoparticles. Biointerface Res. Appl. Chem. 2020, 10, 5592–5598. [Google Scholar] [CrossRef]
- Idrees, H.; Zaidi, S.Z.J.; Sabir, A.; Khan, R.U.; Zhang, X.; Hassan, S.-U. A Review of Biodegradable Natural Polymer-Based Nanoparticles for Drug Delivery Applications. Nanomaterials 2020, 10, 1970. [Google Scholar] [CrossRef] [PubMed]
- Avramović, N.; Mandić, B.; Savić-Radojević, A.; Simić, T. Polymeric Nanocarriers of Drug Delivery Systems in Cancer Therapy. Pharmaceutics 2020, 12, 298. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Amanzadi, B.; Mirzaei, E.; Hassanzadeh, G.; Mandaviani, P.; Boroumand, S.; Abdollahi, M.; Abdolghaffari, A.H.; Majidi, R.F. Chitosan-based layered nanofibers loaded with herbal extract as wound-dressing materials on wound model studies. Biointerface Res. Appl. Chem. 2019, 9, 3979–3986. [Google Scholar] [CrossRef]
- Naskar, S.; Sharma, S.; Kuotsu, K. Chitosan-based nanoparticles: An overview of biomedical applications and its preparation. J. Drug Deliv. Sci. Technol. 2018, 49, 66–81. [Google Scholar] [CrossRef]
- Mallick, S.P.; Panda, S.P.; Gayatri, A.; Kunaal, Y.; Naresh, C.; Suman, D.K.; Samineni, J.; Siddiqui, N.; Singh, B.N. Chitosan Oligosaccharide Based Hydrogel: An Insight into the Mechanical, Drug Delivery, and Antimicrobial Studies. Biointerface Res. Appl. Chem. 2020, 11, 10293–10300. [Google Scholar]
- Naskar, S.; Kuotsu, K.; Sharma, S. Chitosan-based nanoparticles as drug delivery systems: A review on two decades of research. J. Drug Target. 2018, 27, 379–393. [Google Scholar] [CrossRef]
- Ullah, F.; Javed, F.; Zakaria, M.R.; Jamila, N.; Khattak, R.; Khan, A.N.; Akil, H.M. Determining the molecular-weight and interfacial properties of chitosan built nanohydrogel for controlled drug delivery applications. Biointerface Res. Appl. Chem. 2019, 9, 4452–4457. [Google Scholar] [CrossRef]
- Azmi, A.A.; Ahyat, N.; Mohamad, F.; Hamzah, S. Synthesis of silver nanoparticles: Double-green approach of using chitosan and microwave technique towards antimicrobial activity against pathogenic bacteria. Biointerface Res. Appl. Chem. 2020, 10, 5918–5922. [Google Scholar] [CrossRef]
- Joshi, B.; Kaur, J.; Khan, E.; Kumar, A.; Joshi, A. Ultrasonic atomizer driven development of doxorubicin-chitosan nanoparticles as anticancer therapeutics: Evaluation of anionic cross-linkers. J. Drug Deliv. Sci. Technol. 2020, 57, 101618. [Google Scholar] [CrossRef]
- Yana, T.; Zhua, S.; Huia, W.; Heb, J.; Liua, Z.; Chengac, J. Chitosan based pH-responsive polymeric prodrug vector for enhanced tumor targeted co-delivery of doxorubicin and siRNA. Carbohydr. Polym. 2020, 250, 116781. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Ahmadi, Z.; Mohamadi, N.; Zarrabi, A.; Abasi, S.; Dehghannoudeh, G.; Tamaddondoust, R.N.; Khanbabaei, H.; Mohammadinejad, R.; Thakur, V.K. Chitosan-based advanced materials for docetaxel and paclitaxel delivery: Recent advances and future directions in cancer theranostics. Int. J. Biol. Macromol. 2020, 145, 282–300. [Google Scholar] [CrossRef]
- Du, X.; Yin, S.; Xu, L.; Ma, J.; Yu, H.; Wang, G.; Li, J. Polylysine and cysteine functionalized chitosan nanoparticle as an efficient platform for oral delivery of paclitaxel. Carbohydr. Polym. 2019, 229, 115484. [Google Scholar] [CrossRef] [PubMed]
- BaŞPinar, Y.; Akbaba, H.; Bayraktar, O. Encapsulation of paclitaxel in electrosprayed chitosan nanoparticles. J. Res. Pharm. 2019, 23, 886–896. [Google Scholar] [CrossRef]
- Jiang, J.; Liu, Y.; Wu, C.; Qiu, Y.; Xu, X.; Lv, H.; Bai, A.; Liu, X. Development of drug-loaded chitosan hollow nanoparticles for delivery of paclitaxel to human lung cancer A549 cells. Drug Dev. Ind. Pharm. 2017, 43, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, M.A.; Madni, A.; Rehman, M.; Rahim, M.A.; Jabar, A. Ionically Cross-Linked Chitosan Nanoparticles for Sustained Delivery of Docetaxel: Fabrication, Post-Formulation and Acute Oral Toxicity Evaluation. Int. J. Nanomed. 2019, 14, 10035–10046. [Google Scholar] [CrossRef]
- Zhang, E.; Xing, R.; Liu, S.; Li, K.; Qin, Y.; Yu, H.; Li, P. Vascular targeted chitosan-derived nanoparticles as docetaxel carriers for gastric cancer therapy. Int. J. Biol. Macromol. 2018, 126, 662–672. [Google Scholar] [CrossRef] [PubMed]
- Vivek, R.; Babu, V.N.; Thangam, R.; Subramanian, K.; Kannan, S. pH-responsive drug delivery of chitosan nanoparticles as Tamoxifen carriers for effective anti-tumor activity in breast cancer cells. Colloids Surfaces B Biointerfaces 2013, 111, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Alhajamee, M.; Marai, K.; Al Abbas, S.M.N.; Tabrizi, M.H. Co-encapsulation of curcumin and tamoxifen in lipid-chitosan hybrid nanoparticles for cancer therapy. Mater. Technol. 2021, 1–12. [Google Scholar] [CrossRef]
- Nair, R.S.; Morris, A.; Billa, N.; Leong, C.-O. An Evaluation of Curcumin-Encapsulated Chitosan Nanoparticles for Transdermal Delivery. AAPS PharmSciTech 2019, 20, 69. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Chitosan-based nanocarriers for encapsulation and delivery of curcumin: A review. Int. J. Biol. Macromol. 2021, 179, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Almutairi, F.M.; El Rabey, H.A.; Tayel, A.A.; Alalawy, A.I.; Al-Duais, M.A.; Sakran, M.I.; Zidan, N.S. Augmented anticancer activity of curcumin loaded fungal chitosan nanoparticles. Int. J. Biol. Macromol. 2019, 155, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Gounden, S.; Danielw, A. Chitosan-Modified Silver Nanoparticles Enhance Cisplatin Activity in Breast Cancer Cells. Biointerface Res. Appl. Chem. 2020, 11, 10572–10584. [Google Scholar] [CrossRef]
- Siavashy, S.; Soltani, M.; Ghorbani-Bidkorbeh, F.; Fallah, N.; Farnam, G.; Mortazavi, S.A.; Shirazi, F.H.; Tehrani, M.H.H.; Hamedi, M.H. Microfluidic platform for synthesis and optimization of chitosan-coated magnetic nanoparticles in cisplatin delivery. Carbohydr. Polym. 2021, 265, 118027. [Google Scholar] [CrossRef] [PubMed]
- Manan, F.A.A.; Yusof, N.A.; Abdullah, J.; Mohammad, F.; Nurdin, A.; Yazan, L.S.; Khiste, S.K.; Al-Lohedan, H.A. Drug Release Profiles of Mitomycin C Encapsulated Quantum Dots–Chitosan Nanocarrier System for the Possible Treatment of Non-Muscle Invasive Bladder Cancer. Pharmaceutics 2021, 13, 1379. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, A.; Amigh, S. In the search of active nanocarriers for delivery of mitomycin C drug. Mater. Adv. 2020, 1, 1909–1919. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; El-Bisi, M.K.; Taha, G.M.; El-Alfy, E.A. Chitosan nanoparticles loaded antibiotics as drug delivery biomaterial. J. Appl. Pharm. Sci. 2015, 5, 85–90. [Google Scholar] [CrossRef]
- Ameeduzzafar; Imam, S.S.; Bukhari, S.N.A.; Ahmad, J.; Ali, A. Formulation and optimization of levofloxacin loaded chitosan nanoparticle for ocular delivery: In-vitro characterization, ocular tolerance and antibacterial activity. Int. J. Biol. Macromol. 2018, 108, 650–659. [Google Scholar] [CrossRef] [PubMed]
- El-Alfy, E.A.; El-Bisi, M.K.; Taha, G.M.; Ibrahim, H.M. Preparation of biocompatible chitosan nanoparticles loaded by tetracycline, gentamycin and ciprofloxacin as novel drug delivery system for improvement the antibacterial properties of cellulose based fabrics. Int. J. Biol. Macromol. 2020, 161, 1247–1260. [Google Scholar] [CrossRef]
- De Andrade, L.F.; Apolinário, A.C.; Rangel-Yagui, C.O.; Stephano, M.A.; Tavares, L.C. Chitosan nanoparticles for the delivery of a new compound active against multidrug-resistant Staphylococcus aureus. J. Drug Deliv. Sci. Technol. 2019, 55, 101363. [Google Scholar] [CrossRef]
- Mohammadi, A.; Hosseini, S.M.; Hashemi, M. Emerging chitosan nanoparticles loading-system boosted the antibacterial activity of Cinnamomum zeylanicum essential oil. Ind. Crop. Prod. 2020, 155, 112824. [Google Scholar] [CrossRef]
- Kahdestani, S.A.; Shahriari, M.H.; Abdouss, M. Synthesis and characterization of chitosan nanoparticles containing teicoplanin using sol–gel. Polym. Bull. 2020, 78, 1133–1148. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-based nanoparticles against bacterial infections. Carbohydr. Polym. 2020, 251, 117108. [Google Scholar] [CrossRef]
- Hassan, Y.A.; Khedr, A.I.; Alkabli, J.; Elshaarawy, R.F.; Nasr, A.M. Co-delivery of imidazolium Zn(II)salen and Origanum Syriacum essential oil by shrimp chitosan nanoparticles for antimicrobial applications. Carbohydr. Polym. 2021, 260, 117834. [Google Scholar] [CrossRef] [PubMed]
- Nemati Shizari, L.; Mohamadpour Dounighi, N.; Bayat, M.; Mosavari, N. A New Amphotericin B-loaded Trimethyl Chitosan Nanoparticles as a Drug Delivery System and Antifungal Activity on Candida albicans Biofilm. Arch. Razi Inst. 2020, 76, 575–590. [Google Scholar]
- Sandhya, M.; Aparna, V.; Raja, B.; Jayakumar, R.; Sathianarayanan, S. Amphotericin B loaded sulfonated chitosan nanoparticles for targeting macrophages to treat intracellular Candida glabrata infections. Int. J. Biol. Macromol. 2018, 110, 133–139. [Google Scholar] [CrossRef] [PubMed]
- El Rabey, H.A.; Almutairi, F.M.; Alalawy, A.I.; Al-Duais, M.A.; Sakran, M.I.; Zidan, N.S.; Tayel, A.A. Augmented control of drug-resistant Candida spp. via fluconazole loading into fungal chitosan nanoparticles. Int. J. Biol. Macromol. 2019, 141, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Loutfy, S.A.; Elberry, M.H.; Farroh, K.Y.; Mohamed, H.T.; Mohamed, A.A.; Mohamed, E.B.; Faraag, A.H.I.; Mousa, S.A. Antiviral Activity of Chitosan Nanoparticles Encapsulating Curcumin Against Hepatitis C Virus Genotype 4a in Human Hepatoma Cell Lines. Int. J. Nanomed. 2020, 15, 2699–2715. [Google Scholar] [CrossRef]
- Donalisio, M.; Leone, F.; Civra, A.; Spagnolo, R.; Ozer, O.; Lembo, D.; Cavalli, R. Acyclovir-Loaded Chitosan Nanospheres from Nano-Emulsion Templating for the Topical Treatment of Herpesviruses Infections. Pharmaceutics 2018, 10, 46. [Google Scholar] [CrossRef] [PubMed]
- Jaber, N.; Al-Remawi, M.; Al-Akayleh, F.; Al-Muhtaseb, N.; Al-Adham, I.S.I.; Collier, P.J. A review of the antiviral activity of Chitosan, including patented applications and its potential use against COVID-19. J. Appl. Microbiol. 2021, n/a. [Google Scholar] [CrossRef] [PubMed]
- Boroumand, H.; Badie, F.; Mazaheri, S.; Seyedi, Z.S.; Nahand, J.S.; Nejati, M.; Baghi, H.B.; Abbasi-Kolli, M.; Badehnoosh, B.; Ghandali, M.; et al. Chitosan-Based Nanoparticles Against Viral Infections. Front. Cell. Infect. Microbiol. 2021, 11, 175. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, J.; Shan, W.; Huang, Y. Developments of mucus penetrating nanoparticles. Asian J. Pharm. Sci. 2015, 10, 275–282. [Google Scholar] [CrossRef]
- Güven, U.M.; Başaran, E. In vitro-in vivo evaluation of olopatadine incorporated chitosan nanoparticles for the treatment of ocular allergy. J. Drug Deliv. Sci. Technol. 2021, 64, 102518. [Google Scholar] [CrossRef]
- Grego, E.A.; Siddoway, A.C.; Uz, M.; Liu, L.; Christiansen, J.C.; Ross, K.A.; Kelly, S.M.; Mallapragada, S.K.; Wannemuehler, M.J.; Narasimhan, B. Polymeric Nanoparticle-Based Vaccine Adjuvants and Delivery Vehicles. In Nanoparticles for Rational Vaccine Design; Gill, H.S., Compans, R.W., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 29–76. [Google Scholar]
- Liu, B.; Wu, Z.; Liu, T.; Qian, R.; Wu, T.; Liu, Q.; Shen, A. Polymeric Nanoparticles Engineered as a Vaccine Adjuvant-Delivery System; IntechOpen: London, UK, 2018. [Google Scholar]
- Mikušová, V.; Mikuš, P. Advances in Chitosan-Based Nanoparticles for Drug Delivery. Int. J. Mol. Sci. 2021, 22, 9652. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Huang, G. Design and application of dextran carrier. J. Drug Deliv. Sci. Technol. 2020, 55, 101392. [Google Scholar] [CrossRef]
- Bachelder, E.M.; Pino, E.N.; Ainslie, K.M. Acetalated Dextran: A Tunable and Acid-Labile Biopolymer with Facile Synthesis and a Range of Applications. Chem. Rev. 2017, 117, 1915–1926. [Google Scholar] [CrossRef]
- Hu, Q.; Lu, Y.; Luo, Y. Recent advances in dextran-based drug delivery systems: From fabrication strategies to applications. Carbohydr. Polym. 2021, 264, 117999. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Johnson, M.M.; Collier, M.A.; Gallovic, M.D.; Bachelder, E.M.; Ainslie, K.M. Tunable degradation of acetalated dextran microparticles enables controlled vaccine adjuvant and antigen delivery to modulate adaptive immune responses. J. Control. Release 2018, 273, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Bakil, S.N.A.; Kamal, H.; Abdullah, H.Z.; Idris, M.I. Sodium Alginate-Zinc Oxide Nanocomposite Film for Antibacterial Wound Healing Applications. Biointerface Res. Appl. Chem. 2020, 10, 6289–6296. [Google Scholar] [CrossRef]
- Jazayeri, S.D.; Lim, H.X.; Shameli, K.; Yeap, S.K.; Poh, C.L. Nano and Microparticles as Potential Oral Vaccine Carriers and Adjuvants Against Infectious Diseases. Front. Pharmacol. 2021, 12, 1399. [Google Scholar] [CrossRef] [PubMed]
- Hasnain, M.S.; Nayak, A.K.; Kurakula, M.; Hoda, M.N. Alginate nanoparticles in drug delivery. In Alginates in Drug Delivery; Nayak, A.K., Hasnain, M.S., Eds.; Academic Press: Cambridge, MA, USA, 2020; Chapter 6; pp. 129–152. [Google Scholar]
- Jin, Z.; Gao, S.; Cui, X.; Sun, D.; Zhao, K. Adjuvants and delivery systems based on polymeric nanoparticles for mucosal vaccines. Int. J. Pharm. 2019, 572, 118731. [Google Scholar] [CrossRef] [PubMed]
- Rajalekshmy, G.P.; Annie Mariya, R.; Rekha, M.R. Pullulan-based nanomaterials in drug delivery applications. In Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications; Bera, H., Hossain, C.M., Saha, S., Eds.; Academic Press: Cambridge, MA, USA, 2021; Chapter 16; pp. 383–404. [Google Scholar]
- Raychaudhuri, R.; Naik, S.; Shreya, A.B.; Kandpal, N.; Pandey, A.; Kalthur, G.; Mutalik, S. Pullulan based stimuli responsive and sub cellular targeted nanoplatforms for biomedical application: Synthesis, nanoformulations and toxicological perspective. Int. J. Biol. Macromol. 2020, 161, 1189–1205. [Google Scholar] [CrossRef] [PubMed]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2019, 151, 1012–1029. [Google Scholar] [CrossRef]
- Arshad, R.; Tabish, T.; Kiani, M.; Ibrahim, I.; Shahnaz, G.; Rahdar, A.; Kang, M.; Pandey, S. A Hyaluronic Acid Functionalized Self-Nano-Emulsifying Drug Delivery System (SNEDDS) for Enhancement in Ciprofloxacin Targeted Delivery against Intracellular Infection. Nanomaterials 2021, 11, 1086. [Google Scholar] [CrossRef]
- Lee, G.Y.; Kim, J.-H.; Choi, K.Y.; Yoon, H.Y.; Kim, K.; Kwon, I.C.; Choi, K.; Lee, B.-H.; Park, J.H.; Kim, I.-S. Hyaluronic acid nanoparticles for active targeting atherosclerosis. Biomaterials 2015, 53, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Paolino, M.; Licciardi, M.; Savoca, C.; Giammona, G.; Modica De Mohac, L.; Reale, A.; Giuliani, G.; Komber, H.; Donati, A.; Leone, G.; et al. Hyaluronan Graft Copolymers Bearing Fatty-Acid Residues as Self-Assembling Nanoparticles for Olanzapine Delivery. Pharmaceutics 2019, 11, 675. [Google Scholar] [CrossRef] [PubMed]
- Behera, S.; Behura, R.; Mohanty, P.; Sahoo, M.; Duggirala, R. Study of Interaction between Bovine Serum Albumin and Dolutegravir Intermediate: Fluorescence and Molecular Docking Analysis. Biointerface Res. Appl. Chem 2021, 11, 13102–13110. [Google Scholar]
- Borah, P.; Mattaparthi, V.S.K. Computational investigation on the role of C-Terminal of human albumin on the dimerization of A beta(1-42) peptide. Biointerface Res. Appl. Chem. 2020, 10, 4944–4955. [Google Scholar] [CrossRef]
- Hirakawa, N.; Ishima, Y.; Kinoshita, R.; Nakano, R.; Chuang, V.T.G.; Ando, H.; Shimizu, T.; Okuhira, K.; Maruyama, T.; Otagiri, M.; et al. Reduction-Responsive and Multidrug Deliverable Albumin Nanoparticles: An Antitumor Drug to Abraxane against Human Pancreatic Tumor-Bearing Mice. ACS Appl. Bio Mater. 2021, 4, 4302–4309. [Google Scholar] [CrossRef]
- Yuan, H.; Guo, H.; Luan, X.; He, M.; Li, F.; Burnett, J.; Truchan, N.; Sun, D. Albumin Nanoparticle of Paclitaxel (Abraxane) Decreases while Taxol Increases Breast Cancer Stem Cells in Treatment of Triple Negative Breast Cancer. Mol. Pharm. 2020, 17, 2275–2286. [Google Scholar] [CrossRef] [PubMed]
- Van De Sande, L.; Graversen, M.; Hubner, M.; Pocard, M.; Reymond, M.; Vaira, M.; Cosyns, S.; Willaert, W.; Ceelen, W. Intraperitoneal aerosolization of albumin-stabilized paclitaxel nanoparticles (Abraxane™) for peritoneal carcinomatosis—A phase I first-in-human study. Pleura Peritoneum 2018, 3, 20180112. [Google Scholar] [CrossRef] [PubMed]
- Youn, Y.S. Albumin-Bound Nanoparticles for Targeted Therapy. In Proceedings of the 6th International Conference on the Development of Biomedical Engineering in Vietnam (BME6), Ho Chi Minh, Vietnam, 27–29 June 2017; Springer: Singapore, 2018; pp. 801–803. [Google Scholar]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Onafuye, H.; Pieper, S.; Mulac, D.; Cinatl, J., Jr.; Wass, M.N.; Langer, K.; Michaelis, M. Doxorubicin-loaded human serum albumin nanoparticles overcome transporter-mediated drug resistance in drug-adapted cancer cells. Beilstein J. Nanotechnol. 2019, 10, 1707–1715. [Google Scholar] [CrossRef]
- Kim, D.; Lee, S.S.; Yoo, W.Y.; Moon, H.; Cho, A.; Park, S.Y.; Kim, Y.-S.; Kim, H.R.; Lee, H.J. Combination Therapy with Doxorubicin-Loaded Reduced Albumin Nanoparticles and Focused Ultrasound in Mouse Breast Cancer Xenografts. Pharmaceuticals 2020, 13, 235. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Yamasaki, K.; Nishi, K.; Taguchi, K.; Otagiri, M. Investigation of anti-tumor effect of doxorubicin-loaded human serum albumin nanoparticles prepared by a desolvation technique. Cancer Chemother. Pharmacol. 2019, 83, 1113–1120. [Google Scholar] [CrossRef]
- Chaiwaree, S.; Prapan, A.; Suwannasom, N.; Laporte, T.; Neumann, T.; Pruß, A.; Georgieva, R.; Bäumler, H. Doxorubicin–Loaded Human Serum Albumin Submicron Particles: Preparation, Characterization and In Vitro Cellular Uptake. Pharmaceutics 2020, 12, 224. [Google Scholar] [CrossRef] [PubMed]
- Qu, N.; Sun, Y.; Li, Y.; Hao, F.; Qiu, P.; Teng, L.; Xie, J.; Gao, Y. Docetaxel-loaded human serum albumin (HSA) nanoparticles: Synthesis, characterization, and evaluation. Biomed. Eng. Online 2019, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Ertugen, E.; Tunçel, A.; Yurt, F. Docetaxel loaded human serum albumin nanoparticles; synthesis, characterization, and potential of nuclear imaging of prostate cancer. J. Drug Deliv. Sci. Technol. 2020, 55, 101410. [Google Scholar] [CrossRef]
- Desale, J.P.; Swami, R.; Kushwah, V.; Katiyar, S.S.; Jain, S. Chemosensitizer and docetaxel-loaded albumin nanoparticle: Overcoming drug resistance and improving therapeutic efficacy. Nanomedicine 2018, 13, 2759–2776. [Google Scholar] [CrossRef]
- Yu, Z.; Li, X.; Duan, J.; Yang, X.-D. Targeted Treatment of Colon Cancer with Aptamer-Guided Albumin Nanoparticles Loaded with Docetaxel. Int. J. Nanomed. 2020, 15, 6737–6748. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pan, J.; Li, H.; Yu, D.; Wu, T.; Wang, L.; Wang, Y.; Zhou, L.; Zheng, S. Albumin based nanomedicine for enhancing tacrolimus safety and lymphatic targeting efficiency. J. Biomed. Nanotechnol. 2019, 15, 1313–1324. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Soudi, T.; Shojaosadati, S.A. Redox responsive curcumin-loaded human serum albumin nanoparticles: Preparation, characterization and in vitro evaluation. Int. J. Biol. Macromol. 2018, 114, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Saleh, T.; Soudi, T.; Shojaosadati, S.A. Aptamer functionalized curcumin-loaded human serum albumin (HSA) nanoparticles for targeted delivery to HER-2 positive breast cancer cells. Int. J. Biol. Macromol. 2019, 130, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Xia, T. Nanomaterial-based vaccine adjuvants. J. Mater. Chem. B 2016, 4, 5496–5509. [Google Scholar] [CrossRef]
- Khalil, I.R.; Burns, A.T.H.; Radecka, I.; Kowalczuk, M.; Khalaf, T.; Adamus, G.; Johnston, B.; Khechara, M.P. Bacterial-Derived Polymer Poly-y-Glutamic Acid (y-PGA)-Based Micro/Nanoparticles as a Delivery System for Antimicrobials and Other Biomedical Applications. Int. J. Mol. Sci. 2017, 18, 313. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Vuksan, V.; Vidgen, E.; Parker, T.; Faulkner, D.; Mehling, C.C.; Garsetti, M.; Testolin, G.; Cunnane, S.C.; et al. Soluble fiber intake at a dose approved by the US Food and Drug Administration for a claim of health benefits: Serum lipid risk factors for cardiovascular disease assessed in a randomized controlled crossover trial. Am. J. Clin. Nutr. 2002, 75, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Xia, N.; Zhao, Q. Carbohydrate-containing nanoparticles as vaccine adjuvants. Expert Rev. Vaccines 2021, 20, 797–810. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Chen, L.; Yang, F.; Cheung, P.C.K. Beta-d-glucan-based drug delivery system and its potential application in targeting tumor associated macrophages. Carbohydr. Polym. 2021, 253, 117258. [Google Scholar] [CrossRef]
- Miyamoto, N.; Mochizuki, S.; Sakurai, K. Designing an immunocyte-targeting delivery system by use of beta-glucan. Vaccine 2018, 36, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Lookian, P.P.; Zhao, D.; Medina, R.; Wang, H.; Zenka, J.; Gilbert, M.R.; Pacak, K.; Zhuang, Z. Mannan-BAM, TLR Ligands, Anti-CD40 Antibody (MBTA) Vaccine Immunotherapy: A Review of Current Evidence and Applications in Glioblastoma. Int. J. Mol. Sci. 2021, 22, 3455. [Google Scholar] [CrossRef] [PubMed]
- El-Emam, S.Z.; Abo El-Ella, D.M.; Fayez, S.M.; Asker, M.; Nazeam, J.A. Novel dandelion mannan-lipid nanoparticle: Exploring the molecular mechanism underlying the potent anticancer effect against non-small lung carcinoma. J. Funct. Foods 2021, 87, 104781. [Google Scholar] [CrossRef]
- Korolenko, T.A.; Bgatova, N.P.; Ovsyukova, M.V.; Shintyapina, A.; Vetvicka, V. Hypolipidemic Effects of β-Glucans, Mannans, and Fucoidans: Mechanism of Action and Their Prospects for Clinical Application. Molecules 2020, 25, 1819. [Google Scholar] [CrossRef] [PubMed]
- Basu, P.; Saha, N.; Saha, T.; Saha, P. Polymeric hydrogel based systems for vaccine delivery: A review. Polymer 2021, 230, 124088. [Google Scholar] [CrossRef]
- Wang, X.; Chang, C.H.; Jiang, J.; Liu, Q.; Liao, Y.; Lu, J.; Li, L.; Liu, X.; Kim, J.; Ahmed, A.; et al. The Crystallinity and Aspect Ratio of Cellulose Nanomaterials Determine Their Pro-Inflammatory and Immune Adjuvant Effects In Vitro and In Vivo. Small 2019, 15, e1901642. [Google Scholar] [CrossRef] [PubMed]
- Čolić, M.; Mihajlović, D.; Mathew, A.; Naseri, N.; Kokol, V. Cytocompatibility and immunomodulatory properties of wood based nanofibrillated cellulose. Cellulose 2015, 22, 763–778. [Google Scholar] [CrossRef]
- Tomić, S.; Kokol, V.; Mihajlović, D.; Mirčić, A.; Čolić, M. Native cellulose nanofibrills induce immune tolerance in vitro by acting on dendritic cells. Sci. Rep. 2016, 6, 31618. [Google Scholar] [CrossRef]
- Afinjuomo, F.; Abdella, S.; Youssef, S.H.; Song, Y.; Garg, S. Inulin and Its Application in Drug Delivery. Pharmaceuticals 2021, 14, 885. [Google Scholar] [CrossRef] [PubMed]
- Gopinath, V.; Saravanan, S.; Al-Maleki, A.R.; Ramesh, M.; Vadivelu, J. A review of natural polysaccharides for drug delivery applications: Special focus on cellulose, starch and glycogen. Biomed. Pharmacother. 2018, 107, 96–108. [Google Scholar] [CrossRef] [PubMed]
- Gálisová, A.; Jirátová, M.; Rabyk, M.; Sticová, E.; Hájek, M.; Hrubý, M.; Jirák, D. Glycogen as an advantageous polymer carrier in cancer theranostics: Straightforward in vivo evidence. Sci. Rep. 2020, 10, 10411. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Han, Y.; Yang, Y.; Zhang, L.; Wang, H.; Shen, Y.; Lai, J.; Chen, J. Phospholipid-Decorated Glycogen Nanoparticles for Stimuli-Responsive Drug Release and Synergetic Chemophotothermal Therapy of Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2020, 12, 23311–23322. [Google Scholar] [CrossRef] [PubMed]
- Nallasamy, P.; Ramalingam, T.; Nooruddin, T.; Shanmuganathan, R.; Arivalagan, P.; Natarajan, S. Polyherbal drug loaded starch nanoparticles as promising drug delivery system: Antimicrobial, antibiofilm and neuroprotective studies. Process Biochem. 2020, 92, 355–364. [Google Scholar] [CrossRef]
- Forouzandehdel, S.; Forouzandehdel, S.; Rezghi Rami, M. Synthesis of a novel magnetic starch-alginic acid-based biomaterial for drug delivery. Carbohydr. Res. 2020, 487, 107889. [Google Scholar] [CrossRef] [PubMed]
- Marto, J.; Ribeiro, H.M.; Almeida, A.J. Starch-based nanocapsules as drug carriers for topical drug delivery. In Smart Nanocontainers; Elsevier: Amsterdam, The Netherlands, 2020; pp. 287–294. [Google Scholar]
- Odeniyi, M.A.; Omoteso, O.A.; Adepoju, A.O.; Jaiyeoba, K.T. Starch nanoparticles in drug delivery: A review. Polim. W Med. 2018, 48, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Kazi, M.; Ahmad, M.Z.; Syed, R.; Alsenaidy, M.A.; Albraiki, S.A. Lignin nanoparticles as a promising vaccine adjuvant and delivery system for ovalbumin. Int. J. Biol. Macromol. 2020, 163, 1314–1322. [Google Scholar] [CrossRef] [PubMed]
- Wijaya, C.J.; Ismadji, S.; Gunawan, S. A Review of Lignocellulosic-Derived Nanoparticles for Drug Delivery Applications: Lignin Nanoparticles, Xylan Nanoparticles, and Cellulose Nanocrystals. Molecules 2021, 26, 676. [Google Scholar] [CrossRef]
- Sun, L.; Xiong, X.; Zou, Q.; Ouyang, P.; Burkhardt, C.; Krastev, R. Design of intelligent chitosan/heparin hollow microcapsules for drug delivery. J. Appl. Polym. Sci. 2017, 134, 44425. [Google Scholar] [CrossRef]
- Wang, X.; Xie, Y.; Jiang, N.; Wang, J.; Liang, H.; Liu, D.; Yang, N.; Sang, X.; Feng, Y.; Chen, R.; et al. Enhanced Antimalarial Efficacy Obtained by Targeted Delivery of Artemisinin in Heparin-Coated Magnetic Hollow Mesoporous Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, J.; Han, Q.; Luo, Q.; Zhang, H.; Wang, Y. Construction of doxorubicin-conjugated lentinan nanoparticles for enhancing the cytotoxocity effects against breast cancer cells. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123657. [Google Scholar] [CrossRef]
- Lin, M.; Dong, L.; Chen, Q.; Xu, H.; Han, X.; Luo, R.; Pu, X.; Qi, S.; Nie, W.; Ma, M.; et al. Lentinan-Based Oral Nanoparticle Loaded Budesonide With Macrophage-Targeting Ability for Treatment of Ulcerative Colitis. Front. Bioeng. Biotechnol. 2021, 9, 678. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Yang, X.; Du, X.; Yang, H.; Liu, Y.; Khan, A.Q.; Zhai, G. Chondroitin sulfate derived theranostic and therapeutic nanocarriers for tumor-targeted drug delivery. Carbohydr. Polym. 2020, 233, 115837. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.R.; Liu, Y.; Yang, H.; Yang, X.; Liu, S.; Ji, J.; Zhai, G. Chondroitin sulfate-based redox-responsive nanoparticles for melanoma-targeted drug delivery. J. Drug Deliv. Sci. Technol. 2020, 60, 102033. [Google Scholar] [CrossRef]
- Shi, X.; Yang, X.; Liu, M.; Wang, R.; Qiu, N.; Liu, Y.; Yang, H.; Ji, J.; Zhai, G. Chondroitin sulfate-based nanoparticles for enhanced chemo-photodynamic therapy overcoming multidrug resistance and lung metastasis of breast cancer. Carbohydr. Polym. 2021, 254, 117459. [Google Scholar] [CrossRef]
- Comberlato, A.; Paloja, K.; Bastings, M.M.C. Nucleic acids presenting polymer nanomaterials as vaccine adjuvants. J. Mater. Chem. B 2019, 7, 6321–6346. [Google Scholar] [CrossRef]
- Gupta, R.; Tandon, A.; Hansen, E.T.; Cebulko, T.C.; Hemmat, Y.J.; Fortune, J.A.; Klibanov, A.M.; Mohan, R.R. Rapid And Substantial Gene Delivery Into Cornea In Vivo And In Vitro With Linearized Polyethyleneimine Nanoparticles. Investig. Ophthalmol. Vis. Sci. 2011, 52, 494. [Google Scholar]
- Mainini, F.; Eccles, M.R. Lipid and Polymer-Based Nanoparticle siRNA Delivery Systems for Cancer Therapy. Molecules 2020, 25, 2692. [Google Scholar] [CrossRef] [PubMed]
- Mendes, L.P.; Sarisozen, C.; Luther, E.; Pan, J.; Torchilin, V.P. Surface-engineered polyethyleneimine-modified liposomes as novel carrier of siRNA and chemotherapeutics for combination treatment of drug-resistant cancers. Drug Deliv. 2019, 26, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Horst, J.D.; De Andrade, P.P.; Duvoisin, C.A.; Vieira, R.D. Fabrication of Conductive Filaments for 3D-printing: Polymer Nanocomposites. Biointerface Res. Appl. Chem. 2020, 10, 6577–6586. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar] [CrossRef] [PubMed]
- Barghi, L.; Farajzadeh, A.; Jahangiri, A. Preparation and evaluation of glibenclamide binary solid dispersions prepared by fusion and solvent-fusion method. Biointerface Res. Appl. Chem. 2019, 9, 4612–4616. [Google Scholar] [CrossRef]
- Lee, H. Molecular Simulations of PEGylated Biomolecules, Liposomes, and Nanoparticles for Drug Delivery Applications. Pharmaceutics 2020, 12, 533. [Google Scholar] [CrossRef] [PubMed]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154, 163–175. [Google Scholar] [CrossRef]
- Bruusgaard-Mouritsen, M.A.; Johansen, J.D.; Garvey, L.H. Clinical manifestations and impact on daily life of allergy to polyethylene glycol (PEG) in ten patients. Clin. Exp. Allergy 2021, 51, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Sellaturay, P.; Nasser, S.; Islam, S.; Gurugama, P.; Ewan, P.W. Polyethylene glycol (PEG) is a cause of anaphylaxis to the Pfizer/BioNTech mRNA COVID-19 vaccine. Clin. Exp. Allergy 2021, 51, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Garvey, L.H.; Nasser, S. Anaphylaxis to the first COVID-19 vaccine: Is polyethylene glycol (PEG) the culprit? Br. J. Anaesth. 2021, 126, e106–e108. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-H.; Stone, C.A.; Jakubovic, B.; Phillips, E.J.; Sussman, G.; Park, J.; Hoang, U.; Kirshner, S.L.; Levin, R.; Kozlowski, S. Anti-PEG IgE in anaphylaxis associated with polyethylene glycol. J. Allergy Clin. Immunol. Pract. 2021, 9, 1731–1733.e3. [Google Scholar] [CrossRef] [PubMed]
- Al-Halifa, S.; Gauthier, L.; Arpin, D.; Bourgault, S.; Archambault, D. Nanoparticle-Based Vaccines Against Respiratory Viruses. Front. Immunol. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Arzani, H.; Adabi, M.; Mosafer, J.; Dorkoosh, F.; Khosravani, M.; Maleki, H.; Nekounam, H.; Kamali, M. Preparation of curcumin-loaded PLGA nanoparticles and investigation of its cytotoxicity effects on human glioblastoma U87MG cells. Biointerface Res. Appl. Chem. 2019, 9, 4225–4231. [Google Scholar] [CrossRef]
- Manoukian, O.S.; Arul, M.R.; Sardashti, N.; Stedman, T.; James, R.; Rudraiah, S.; Kumbar, S.G. Biodegradable Polymeric Injectable Implants for Long-Term Delivery of Contraceptive Drugs. J. Appl. Polym. Sci. 2018, 135, 46068. [Google Scholar] [CrossRef] [PubMed]
- Shakya, A.K.; Nandakumar, K.S. Applications of polymeric adjuvants in studying autoimmune responses and vaccination against infectious diseases. J. R. Soc. Interface 2013, 10, 20120536. [Google Scholar] [CrossRef]
- Tornesello, A.L.; Tagliamonte, M.; Tornesello, M.L.; Buonaguro, F.M.; Buonaguro, L. Nanoparticles to Improve the Efficacy of Peptide-Based Cancer Vaccines. Cancers 2020, 12, 1049. [Google Scholar] [CrossRef]
- Zatorska-Płachta, M.; Łazarski, G.; Maziarz, U.; Foryś, A.; Trzebicka, B.; Wnuk, D.; Chołuj, K.; Karewicz, A.; Michalik, M.; Jamróz, D.; et al. Encapsulation of Curcumin in Polystyrene-Based Nanoparticles—Drug Loading Capacity and Cytotoxicity. ACS Omega 2021, 6, 12168–12178. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, R.; Zaaeri, F.; Rajabi, A.B.; Darbandi-Azar, A.; Faridi-Majidi, R.; Ardestani, M.S. 99mTc-anionic linear globular dendrimer-G2-phenylalanine conjugate: Novel brain tumor SPECT imaging. Biointerface Res. Appl. Chem. 2021, 11, 11244–11255. [Google Scholar]
- Jain, K.; Kesharwani, P.; Gupta, U.; Jain, N.K. Dendrimer toxicity: Let’s meet the challenge. Int. J. Pharm. 2010, 394, 122–142. [Google Scholar] [CrossRef] [PubMed]
- Janaszewska, A.; Lazniewska, J.; Trzepiński, P.; Marcinkowska, M.; Klajnert-Maculewicz, B. Cytotoxicity of Dendrimers. Biomolecules 2019, 9, 330. [Google Scholar] [CrossRef] [PubMed]
- Paolino, M.; Ennen, F.; Lamponi, S.; Cernescu, M.; Voit, B.; Cappelli, A.; Appelhans, D.; Komber, H. Cyclodextrin-Adamantane Host–Guest Interactions on the Surface of Biocompatible Adamantyl-Modified Glycodendrimers. Macromolecules 2013, 46, 3215–3227. [Google Scholar] [CrossRef]
- Tang, T.; Weng, T.; Jia, H.; Luo, S.; Xu, Y.; Li, L.; Zhang, P. Harnessing the layer-by-layer assembly technique to design biomaterials vaccines for immune modulation in translational applications. Biomater. Sci. 2019, 7, 715–732. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Guo, Q.; Chu, T.; Zhang, X.; Wu, Z.; Yu, D. Glucose-sensitive polyelectrolyte nanocapsules based on layer-by-layer technique for protein drug delivery. J. Mater. Sci. Mater. Med. 2014, 25, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Szczepanowicz, K.; Piechota, P.; Węglarz, W.P.; Warszyński, P. Polyelectrolyte nanocapsules containing iron oxide nanoparticles as MRI detectable drug delivery system. Colloids Surf. A Physicochem. Eng. Asp. 2017, 532, 351–356. [Google Scholar] [CrossRef]
- Anandhakumar, S.; Mahalakshmi, V.; Raichur, A.M. Silver nanoparticles modified nanocapsules for ultrasonically activated drug delivery. Mater. Sci. Eng. C 2012, 32, 2349–2355. [Google Scholar] [CrossRef]
- Belbekhouche, S.; Mansour, O.; Carbonnier, B. Promising sub-100 nm tailor made hollow chitosan/poly(acrylic acid) nanocapsules for antibiotic therapy. J. Colloid Interface Sci. 2018, 522, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Mekala, M.; Suganya, K. Characterisation of drug loaded with poly-beta-hydroxyl-butyrate (PHB) nanoparticles onto the cotton gauze for tuberculosis. Afr. J. Pharm. Pharmacol. 2018, 12, 142–150. [Google Scholar] [CrossRef][Green Version]
- Roohi; Zaheer, M.R.; Kuddus, M. PHB (poly-β-hydroxybutyrate) and its enzymatic degradation. Polym. Adv. Technol. 2018, 29, 30–40. [Google Scholar] [CrossRef]
- Rocas, P.; Cusco, C.; Rocas, J.; Albericio, F. On the Importance of Polyurethane and Polyurea Nanosystems for Future Drug Delivery. Curr. Drug Deliv. 2018, 15, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, K.R.; Chaudhari, B.P.; Pokharkar, V.B.; Pawar, A.; Gajbhiye, V. Stimuli-responsive biodegradable polyurethane nano-constructs as a potential triggered drug delivery vehicle for cancer therapy. Int. J. Pharm. 2020, 588, 119781. [Google Scholar] [CrossRef] [PubMed]
- Franco, P.; De Marco, I. The Use of Poly(N-vinyl pyrrolidone) in the Delivery of Drugs: A Review. Polymers 2020, 12, 1114. [Google Scholar] [CrossRef] [PubMed]
- Di Capua, A.; Bejarano, A.; Adami, R.; Reverchon, E. Preparation and characterization of Chilean propolis coprecipitates using Supercritical Assisted Atomization. Chem. Eng. Res. Des. 2018, 136, 776–785. [Google Scholar] [CrossRef]
- Chhouk, K.; Wahyudiono; Kanda, H.; Kawasaki, S.-I.; Goto, M. Micronization of curcumin with biodegradable polymer by supercritical anti-solvent using micro swirl mixer. Front. Chem. Sci. Eng. 2018, 12, 184–193. [Google Scholar] [CrossRef]
- Nair, P.; Navale, G.R.; Dharne, M.S. Poly-gamma-glutamic acid biopolymer: A sleeping giant with diverse applications and unique opportunities for commercialization. Biomass Convers. Biorefinery 2021, 1–19. [Google Scholar] [CrossRef]
- Park, S.-B.; Sung, M.-H.; Uyama, H.; Han, D.K. Poly(glutamic acid): Production, composites, and medical applications of the next-generation biopolymer. Prog. Polym. Sci. 2021, 113, 101341. [Google Scholar] [CrossRef]
- Dai, L.; Si, C.-L. Cellulose-graft-poly(methyl methacrylate) nanoparticles with high biocompatibility for hydrophobic anti-cancer drug delivery. Mater. Lett. 2017, 207, 213–216. [Google Scholar] [CrossRef]
- Prabakaran, S.; Jeyaraj, M.; Nagaraj, A.; Sadasivuni, K.K.; Rajan, M. Polymethyl methacrylate–ovalbumin @ graphene oxide drug carrier system for high anti-proliferative cancer drug delivery. Appl. Nanosci. 2019, 9, 1487–1500. [Google Scholar] [CrossRef]
- Cordeiro, A.P.; Feuser, P.E.; Araújo, P.H.H.; Sayer, C. Encapsulation of Magnetic Nanoparticles and Copaíba Oil in Poly (methyl methacrylate) Nanoparticles via Miniemulsion Polymerization for Biomedical Application. In Macromolecular Symposia; Wiley-VCH: Weinheim, Germany, 2020; p. 2000112. [Google Scholar]
- Vauthier, C.; Bouchemal, K. Methods for the Preparation and Manufacture of Polymeric Nanoparticles. Pharm. Res. 2009, 26, 1025–1058. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Spirescu, V.; Chircov, C.; Grumezescu, A.; Andronescu, E. Polymeric Nanoparticles for Antimicrobial Therapies: An up-to-date Overview. Polymers 2021, 13, 724. [Google Scholar] [CrossRef]
- Saallah, S.; Lenggoro, I.W. Nanoparticles Carrying Biological Molecules: Recent Advances and Applications. KONA Powder Part. J. 2018, 35, 89–111. [Google Scholar] [CrossRef]
- Lee, K.H.; Yang, G.; Wyslouzil, B.E.; Winter, J.O. Electrohydrodynamic Mixing-Mediated Nanoprecipitation for Polymer Nanoparticle Synthesis. ACS Appl. Polym. Mater. 2019, 1, 691–700. [Google Scholar] [CrossRef]
- Tiruwa, R. A review on nanoparticles-preparation and evaluation parameters. Indian J. Pharm. Biol. Res. 2016, 4, 27. [Google Scholar] [CrossRef]
- Jana, U.; Pal, S.; Mohanta, G.P.; Manna, P.K.; Manavalan, R. Nanoparticles: A Potential Approach for Drug Delivery. Res. J. Pharm. Technol. 2011, 4, 1016–1019. [Google Scholar]
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Ying, A.N.J.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and strategies for the synthesis of diverse nanoparticles and their applications: A comprehensive overview. RSC Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Niculescu, A.-G.; Chircov, C.; Bîrcă, A.C.; Grumezescu, A.M. Nanomaterials Synthesis through Microfluidic Methods: An Updated Overview. Nanomaterials 2021, 11, 864. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Rong, F.; Tang, Y.; Li, M.; Feng, T.; Zhou, Q.; Li, P.; Huang, W. Targeted polymer-based antibiotic delivery system: A promising option for treating bacterial infections via macromolecular approaches. Prog. Polym. Sci. 2021, 116, 101389. [Google Scholar] [CrossRef]
- Kumar, R.; Jha, D.; Panda, A.K. Antimicrobial therapeutics delivery systems based on biodegradable polylactide/polylactide-co-glycolide particles. Environ. Chem. Lett. 2019, 17, 1237–1249. [Google Scholar] [CrossRef]
- Gumustas, M.; Sengel-Turk, C.T.; Gumustas, A.; Ozkan, S.A.; Uslu, B. Effect of Polymer-Based Nanoparticles on the Assay of Antimicrobial Drug Delivery Systems. In Multifunctional Systems for Combined Delivery, Biosensing and Diagnostics; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; Chapter 5; pp. 67–108. [Google Scholar]
- Sadrearhami, Z.; Nguyen, T.-K.; Namivandi-Zangeneh, R.; Jung, K.; Wong, E.H.H.; Boyer, C. Recent advances in nitric oxide delivery for antimicrobial applications using polymer-based systems. J. Mater. Chem. B 2018, 6, 2945–2959. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Ariaii, P.; Motamedzadegan, A. Characterization, antioxidant and antibacterial activities of chitosan nanoparticles loaded with nettle essential oil. J. Food Meas. Charact. 2021, 15, 1395–1402. [Google Scholar] [CrossRef]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan nanoparticles loaded with clove essential oil: Characterization, antioxidant and antibacterial activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef] [PubMed]
- Alruwaili, N.K.; Zafar, A.; Imam, S.S.; Alharbi, K.S.; Alotaibi, N.H.; Alshehri, S.; Alhakamy, N.A.; Alzarea, A.I.; Afzal, M.; Elmowafy, M. Stimulus Responsive Ocular Gentamycin-Ferrying Chitosan Nanoparticles Hydrogel: Formulation Optimization, Ocular Safety and Antibacterial Assessment. Int. J. Nanomed. 2020, 15, 4717–4737. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Xu, D.; Sui, G.; Wang, D.; Wu, M.; Han, L.; Mu, H.; Duan, J. Gentamicin decorated phosphatidylcholine-chitosan nanoparticles against biofilms and intracellular bacteria. Int. J. Biol. Macromol. 2020, 156, 640–647. [Google Scholar] [CrossRef]
- George, D.; Maheswari, P.U.; Begum, K.M.M.S. Chitosan-cellulose hydrogel conjugated with L-histidine and zinc oxide nanoparticles for sustained drug delivery: Kinetics and in-vitro biological studies. Carbohydr. Polym. 2020, 236, 116101. [Google Scholar] [CrossRef]
- Ciro, Y.; Rojas, J.; Oñate-Garzon, J.; Salamanca, C.H. Ciro Synthesis, Characterisation and Biological Evaluation of Ampicillin–Chitosan–Polyanion Nanoparticles Produced by Ionic Gelation and Polyelectrolyte Complexation Assisted by High-Intensity Sonication. Polymers 2019, 11, 1758. [Google Scholar] [CrossRef]
- Evangelista, T.F.; Andrade, G.R.; Nascimento, K.N.; dos Santos, S.B.; Santos, M.D.F.C.; D’Oca, C.D.R.M.; Estevam, C.D.S.; Gimenez, I.F.; Almeida, L.E. Supramolecular polyelectrolyte complexes based on cyclodextrin-grafted chitosan and carrageenan for controlled drug release. Carbohydr. Polym. 2020, 245, 116592. [Google Scholar] [CrossRef] [PubMed]
- Scolari, I.R.; Páez, P.L.; Musri, M.M.; Petiti, J.P.; Torres, A.; Granero, G.E. Rifampicin loaded in alginate/chitosan nanoparticles as a promising pulmonary carrier against Staphylococcus aureus. Drug Deliv. Transl. Res. 2020, 10, 1403–1417. [Google Scholar] [CrossRef]
- Kaur, J.; Kour, A.; Panda, J.J.; Harjai, K.; Chhibber, S. Exploring Endolysin-Loaded Alginate-Chitosan Nanoparticles as Future Remedy for Staphylococcal Infections. AAPS PharmSciTech 2020, 21, 233. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, F.; Damoogh, S.; Reis, R.L.; Kundu, S.C.; Mottaghitalab, F.; Farokhi, M. Dual drug delivery system based on pH-sensitive silk fibroin/alginate nanoparticles entrapped in PNIPAM hydrogel for treating severe infected burn wound. Biofabrication 2020, 13, 015005. [Google Scholar] [CrossRef] [PubMed]
- Walvekar, P.; Gannimani, R.; Salih, M.; Makhathini, S.; Mocktar, C.; Govender, T. Self-assembled oleylamine grafted hyaluronic acid polymersomes for delivery of vancomycin against methicillin resistant Staphylococcus aureus (MRSA). Colloids Surf. B Biointerfaces 2019, 182, 110388. [Google Scholar] [CrossRef] [PubMed]
- Falciani, C.; Zevolini, F.; Brunetti, J.; Riolo, G.; Gracia, R.; Marradi, M.; Loinaz, I.; Ziemann, C.; Cossío, U.; Llop, J.; et al. Antimicrobial Peptide-Loaded Nanoparticles as Inhalation Therapy for Pseudomonas aeruginosa Infections. Int. J. Nanomed. 2020, 15, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zeng, J.; Yin, D.; Liao, P.; Ding, S.; Mao, P.; Liu, Y. Synergic fabrication of titanium dioxide incorporation into heparin-polyvinyl alcohol nanocomposite: Enhanced in vitro antibacterial activity and care of in vivo burn injury. Mater. Res. Express 2021, 8, 085012. [Google Scholar] [CrossRef]
- Vrouvaki, I.; Koutra, E.; Kornaros, M.; Avgoustakis, K.; Lamari, F.N.; Hatziantoniou, S. Polymeric Nanoparticles of Pistacia lentiscus var. chia Essential Oil for Cutaneous Applications. Pharmaceutics 2020, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, D.; Exbrayat-Héritier, C.; Rambaud, B.; Megy, S.; Terreux, R.; Verrier, B.; Primard, C. Surface charge modulation of rifampicin-loaded PLA nanoparticles to improve antibiotic delivery in Staphylococcus aureus biofilms. J. Nanobiotechnology 2021, 19, 12. [Google Scholar] [CrossRef] [PubMed]
- Deepika, M.S.; Thangam, R.; Sundarraj, S.; Sheena, T.S.; Sivasubramanian, S.; Kulandaivel, J.; Thirumurugan, R. Co-delivery of Diverse Therapeutic Compounds Using PEG–PLGA Nanoparticle Cargo against Drug-Resistant Bacteria: An Improved Anti-biofilm Strategy. ACS Appl. Bio. Mater. 2020, 3, 385–399. [Google Scholar] [CrossRef]
- Ucak, S.; Sudagidan, M.; Borsa, B.A.; Mansuroglu, B.; Ozalp, V.C. Inhibitory effects of aptamer targeted teicoplanin encapsulated PLGA nanoparticles for Staphylococcus aureus strains. World J. Microbiol. Biotechnol. 2020, 36, 69. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.S.D.M.; Gaspar, L.M.D.A.C.; Rocha, A.M.O.; Da Costa, L.P.; Tada, D.B.; Franceschi, E.; Padilha, F.F. Encapsulation of Red Propolis in Polymer Nanoparticles for the Destruction of Pathogenic Biofilms. AAPS PharmSciTech 2020, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Gürsu, B.Y. Potential antibiofilm activity of farnesol-loaded poly (DL-lactide-co-glycolide)(PLGA) nanoparticles against Candida albicans. J. Anal. Sci. Technol. 2020, 11, 1–10. [Google Scholar]
- Ozkan, G.; Franco, P.; Capanoglu, E.; De Marco, I. PVP/flavonoid coprecipitation by supercritical antisolvent process. Chem. Eng. Process. Process Intensif. 2019, 146, 107689. [Google Scholar] [CrossRef]
- Ma, X.; Lang, J.; Chen, P.; Yang, R. Silver Nanoparticles as an Effective Antimicrobial against Otitis Media Pathogens. AIChE J. 2021. [Google Scholar] [CrossRef]
- Sadrearhami, Z.; Yeow, J.; Nguyen, T.-K.; Ho, K.K.K.; Kumar, N.; Boyer, C. Biofilm dispersal using nitric oxide loaded nanoparticles fabricated by photo-PISA: Influence of morphology. Chem. Commun. 2017, 53, 12894–12897. [Google Scholar] [CrossRef] [PubMed]
- Dickens, E.; Ahmed, S. Principles of cancer treatment by chemotherapy. Surgery 2018, 36, 134–138. [Google Scholar] [CrossRef]
- Bădilă, A.E.; Rădulescu, D.M.; Niculescu, A.-G.; Grumezescu, A.M.; Rădulescu, M.; Rădulescu, A.R. Recent Advances in the Treatment of Bone Metastases and Primary Bone Tumors: An Up-to-Date Review. Cancers 2021, 13, 4229. [Google Scholar] [CrossRef] [PubMed]
- McKenna, M.T.; Weis, J.A.; Brock, A.; Quaranta, V.; Yankeelov, T.E. Precision Medicine with Imprecise Therapy: Computational Modeling for Chemotherapy in Breast Cancer. Transl. Oncol. 2018, 11, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-L.; Chang, M.-C.; Cheng, W.-F. Metronomic chemotherapy and immunotherapy in cancer treatment. Cancer Lett. 2017, 400, 282–292. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, S.; Sharma, D.; Kalia, K.; Tekade, R.K. Tumor microenvironment targeted nanotherapeutics for cancer therapy and diagnosis: A review. Acta Biomater. 2020, 101, 43–68. [Google Scholar] [CrossRef] [PubMed]
- Grewal, I.K.; Singh, S.; Arora, S.; Sharma, N. Polymeric nanoparticles for breast cancer therapy: A comprehensive review. Biointerface Res. Appl. Chem 2021, 11, 11151–11171. [Google Scholar]
- Mariadoss, A.V.A.; Saravanakumar, K.; Sathiyaseelan, A.; Venkatachalam, K.; Wang, M.-H. Folic acid functionalized starch encapsulated green synthesized copper oxide nanoparticles for targeted drug delivery in breast cancer therapy. Int. J. Biol. Macromol. 2020, 164, 2073–2084. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, L.P.; Chandran, T.; Shastri, P.N.; Rizvi, S.A.A.; D’Souza, M.J. Development and evaluation of polycaprolactone based docetaxel nanoparticle formulation for targeted breast cancer therapy. J. Nanoparticle Res. 2020, 22, 372. [Google Scholar] [CrossRef]
- Colpan, R.D.; Erdemir, A. Co-delivery of quercetin and caffeic-acid phenethyl ester by polymeric nanoparticles for improved antitumor efficacy in colon cancer cells. J. Microencapsul. 2021, 38, 381–393. [Google Scholar] [CrossRef]
- Mishra, B.; Chaurasia, S. Design of novel chemotherapeutic delivery systems for colon cancer therapy based on oral polymeric nanoparticles. Ther. Deliv. 2016, 8, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Chen, Y.; He, X.; Yu, Y.; Han, R.; Li, Y.; Yang, C.; Hu, D.; Qian, Z. Polymeric Nanoparticles with ROS-Responsive Prodrug and Platinum Nanozyme for Enhanced Chemophotodynamic Therapy of Colon Cancer. Adv. Sci. 2020, 7, 2001853. [Google Scholar] [CrossRef]
- Sunoqrot, S.; Abujamous, L. pH-sensitive polymeric nanoparticles of quercetin as a potential colon cancer-targeted nanomedicine. J. Drug Deliv. Sci. Technol. 2019, 52, 670–676. [Google Scholar] [CrossRef]
- Shafabakhsh, R.; Yousefi, B.; Asemi, Z.; Nikfar, B.; Mansournia, M.A.; Hallajzadeh, J. Chitosan: A compound for drug delivery system in gastric cancer-a review. Carbohydr. Polym. 2020, 242, 116403. [Google Scholar] [CrossRef] [PubMed]
- Nagaraju, G.P.; Srivani, G.; Dariya, B.; Chalikonda, G.; Farran, B.; Behera, S.K.; Alam, A.; Kamal, M.A. Nanoparticles guided drug delivery and imaging in gastric cancer. Semin. Cancer Biol. 2021, 69, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.; Liang, R.; Ding, D.; Zheng, X.; Zhu, X.; Hu, S.; Wei, H.; Wei, B. A Smart Multifunctional Nanoparticle for Enhanced Near-Infrared Image-Guided Photothermal Therapy Against Gastric Cancer. Int J. Nanomed. 2021, 16, 2897–2915. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, L.; Xie, K.; Wu, J.; Song, P.; Xie, H.; Zhou, L.; Liu, J.; Xu, X.; Shen, Y.; et al. Polylactide-tethered prodrugs in polymeric nanoparticles as reliable nanomedicines for the efficient eradication of patient-derived hepatocellular carcinoma. Theranostics 2018, 8, 3949–3963. [Google Scholar] [CrossRef]
- Gan, H.; Chen, L.; Sui, X.; Wu, B.; Zou, S.; Li, A.; Zhang, Y.; Liu, X.; Wang, D.; Cai, S.; et al. Enhanced delivery of sorafenib with anti-GPC3 antibody-conjugated TPGS-b-PCL/Pluronic P123 polymeric nanoparticles for targeted therapy of hepatocellular carcinoma. Mater. Sci. Eng. C 2018, 91, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, K.; Qin, H.; Zhao, X.; Chen, W.; Xu, L.; Cao, W.; Guo, H. Internal cross-linked polymeric nanoparticles with dual sensitivity for combination therapy of muscle-invasive bladder cancer. J. Nanobiotechnology 2020, 18, 124. [Google Scholar] [CrossRef] [PubMed]
- Sabir, F.; Barani, M.; Rahdar, A.; Bilal, M.; Nadeem, M. How to face skin cancer with nanomaterials: A review. Biointerface Res. Appl. Chem. 2021, 11, 11931–11955. [Google Scholar]
- Ferraz, L.S.; Watashi, C.M.; Colturato-Kido, C.; Pelegrino, M.T.; Paredes-Gamero, E.J.; Weller, R.B.; Seabra, A.B.; Rodrigues, T. Antitumor Potential of S-Nitrosothiol-Containing Polymeric Nanoparticles against Melanoma. Mol. Pharm. 2018, 15, 1160–1168. [Google Scholar] [CrossRef]
- Ramalingam, V.; Varunkumar, K.; Ravikumar, V.; Rajaram, R. Target delivery of doxorubicin tethered with PVP stabilized gold nanoparticles for effective treatment of lung cancer. Sci. Rep. 2018, 8, 3815. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Lin, S.; Yang, F.; Situ, J.; Lin, S.; Luo, Y. Aptamer-Conjugated Multifunctional Polymeric Nanoparticles as Cancer-Targeted, MRI-Ultrasensitive Drug Delivery Systems for Treatment of Castration-Resistant Prostate Cancer. BioMed Res. Int. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Raspantini, G.L.; Luiz, M.T.; Abriata, J.P.; de Oliveira Eloy, J.; Vaidergorn, M.M.; da Silva Emery, F.; Marchetti, J.M. PCL-TPGS polymeric nanoparticles for docetaxel delivery to prostate cancer: Development, physicochemical and biological characterization. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127144. [Google Scholar] [CrossRef]
- Rao, S.V.; Kumar, S.S. Flutamide Loaded Polymeric Nanoparticles for prostate Cancer: A Review. Res. J. Pharm. Technol. 2021, 14, 4501–4503. [Google Scholar] [CrossRef]
- Tiwari, H.; Karki, N.; Pal, M.; Basak, S.; Verma, R.K.; Bal, R.; Kandpal, N.D.; Bisht, G.; Sahoo, N.G. Functionalized graphene oxide as a nanocarrier for dual drug delivery applications: The synergistic effect of quercetin and gefitinib against ovarian cancer cells. Colloids Surf. B Biointerfaces 2019, 178, 452–459. [Google Scholar] [CrossRef]
- Duse, L.; Agel, M.R.; Pinnapireddy, S.R.; Schäfer, J.; Selo, M.A.; Ehrhardt, C.; Bakowsky, U. Photodynamic Therapy of Ovarian Carcinoma Cells with Curcumin-Loaded Biodegradable Polymeric Nanoparticles. Pharmaceutics 2019, 11, 282. [Google Scholar] [CrossRef] [PubMed]
- Jayawardhana, A.M.D.S.; Qiu, Z.; Kempf, S.; Wang, H.; Miterko, M.; Bowers, D.J.; Zheng, Y.-R. Dual-action organoplatinum polymeric nanoparticles overcoming drug resistance in ovarian cancer. Dalton Trans. 2019, 48, 12451–12458. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Amin, S.S.; Hosseini, S.H. Delivery of Hydrophobic Anticancer Drugs by Hydrophobically Modified Alginate Based Magnetic Nanocarrier. Ind. Eng. Chem. Res. 2018, 57, 822–832. [Google Scholar] [CrossRef]
- Ehsanimehr, S.; Najafi Moghadam, P.; Dehaen, W.; Shafiei-Irannejad, V. Synthesis of pH-sensitive nanocarriers based on polyacrylamide grafted nanocrystalline cellulose for targeted drug delivery to folate receptor in breast cancer cells. Eur. Polym. J. 2021, 150, 110398. [Google Scholar] [CrossRef]
- Lu, B.; Lv, X.; Le, Y. Chitosan-Modified PLGA Nanoparticles for Control-Released Drug Delivery. Polymers 2019, 11, 304. [Google Scholar] [CrossRef]
- Prabhuraj, R.S.; Bomb, K.; Srivastava, R.; Bandyopadhyaya, R. Dual drug delivery of curcumin and niclosamide using PLGA nanoparticles for improved therapeutic effect on breast cancer cells. J. Polym. Res. 2020, 27, 133. [Google Scholar] [CrossRef]
- Dacarro, G.; Taglietti, A.; Pallavicini, P. Prussian Blue Nanoparticles as a Versatile Photothermal Tool. Molecules 2018, 23, 1414. [Google Scholar] [CrossRef] [PubMed]
- Thakur, N.S.; Patel, G.; Kushwah, V.; Jain, S.; Banerjee, U.C. Facile development of biodegradable polymer-based nanotheranostics: Hydrophobic photosensitizers delivery, fluorescence imaging and photodynamic therapy. J. Photochem. Photobiol. B Biol. 2019, 193, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Feng, L.; Zhang, G.; Wang, J.; Shen, S.; Li, D.; Yang, X. Semiconducting polymer-based nanoparticles with strong absorbance in NIR-II window for in vivo photothermal therapy and photoacoustic imaging. Biomaterials 2018, 155, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Niculescu, A.-G.; Grumezescu, A.M. Photodynamic Therapy—An Up-to-Date Review. Appl. Sci. 2021, 11, 3626. [Google Scholar] [CrossRef]
- Gheorghe, D.C.; Niculescu, A.-G.; Bîrcă, A.C.; Grumezescu, A.M. Nanoparticles for the Treatment of Inner Ear Infections. Nanomaterials 2021, 11, 1311. [Google Scholar] [CrossRef]
- Szeto, B.; Chiang, H.; Valentini, C.; Yu, M.; Kysar, J.W.; Lalwani, A.K. Inner ear delivery: Challenges and opportunities. Laryngoscope Investig. Otolaryngol. 2020, 5, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Leso, V.; Fontana, L.; Ercolano, M.L.; Romano, R.; Iavicoli, I. Opportunities and challenging issues of nanomaterials in otological fields: An occupational health perspective. Nanomedicine 2019, 14, 2613–2629. [Google Scholar] [CrossRef]
- Jaudoin, C.; Agnely, F.; Nguyen, Y.; Ferrary, E.; Bochot, A. Nanocarriers for drug delivery to the inner ear: Physicochemical key parameters, biodistribution, safety and efficacy. Int. J. Pharm. 2021, 592, 120038. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Lai, J.-Y. Advancing the stimuli response of polymer-based drug delivery systems for ocular disease treatment. Polym. Chem. 2020, 11, 6988–7008. [Google Scholar] [CrossRef]
- Kim, H.M.; Woo, S.J. Ocular Drug Delivery to the Retina: Current Innovations and Future Perspectives. Pharmaceutics 2021, 13, 108. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Yang, L.; Feng, P.; Qiu, H.; Wu, X.; Zhou, M.; Xu, L.; Zhu, Y. Biodegradable Synthetic Polymer-Based Nano-Drug Delivery Systems and Their Applications in Ophthalmic Disease Treatment. Nanosci. Nanotechnol. Lett. 2020, 12, 575–595. [Google Scholar] [CrossRef]
- Zhou, X.; Lv, J.; Li, G.; Qian, T.; Jiang, H.; Xu, J.; Cheng, Y.; Hong, J. Rescue the retina after the ischemic injury by polymer-mediated intracellular superoxide dismutase delivery. Biomaterials 2021, 268, 120600. [Google Scholar] [CrossRef]
- Tiwari, R.; Sethiya, N.K.; Gulbake, A.S.; Mehra, N.K.; Murty, U.S.N.; Gulbake, A. A review on albumin as a biomaterial for ocular drug delivery. Int. J. Biol. Macromol. 2021, 191, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.; Mahmood, S.; Hilles, A.R.; Javed, M.N.; Azmana, M.; Al-Japairai, K.A.S. Polymeric Nanoparticles for Brain Drug Delivery—A Review. Curr. Drug Metab. 2020, 21, 649–660. [Google Scholar] [CrossRef]
- Ansari, R.; Sadati, S.M.; Mozafari, N.; Ashrafi, H.; Azadi, A. Carbohydrate polymer-based nanoparticle application in drug delivery for CNS-related disorders. Eur. Polym. J. 2020, 128, 109607. [Google Scholar] [CrossRef]
- Kempe, K.; Nicolazzo, J.A. Biodegradable Polymeric Nanoparticles for Brain-Targeted Drug Delivery. In Nanomedicines for Brain Drug Delivery; Morales, J.O., Gaillard, P.J., Eds.; Springer: New York, NY, USA, 2021; pp. 1–27. [Google Scholar]
- Ibarra, L.E.; Beaugé, L.; Arias-Ramos, N.; Rivarola, V.A.; Chesta, C.A.; López-Larrubia, P.; Palacios, R.E. Trojan horse monocyte-mediated delivery of conjugated polymer nanoparticles for improved photodynamic therapy of glioblastoma. Nanomedicine 2020, 15, 1687–1707. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.; Duskey, J.T.; Kreuter, J. Nanoparticles as carriers for drug delivery of macromolecules across the blood-brain barrier. Expert Opin. Drug Deliv. 2020, 17, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Duan, Y.; Zhang, Q.; Sun, D.; Fang, R.H.; Liu-Bryan, R.; Gao, W.; Zhang, L. Cartilage-targeting ultrasmall lipid-polymer hybrid nanoparticles for the prevention of cartilage degradation. Bioeng. Transl. Med. 2021, 6, e10187. [Google Scholar] [CrossRef]
- Li, X.; Dai, B.; Guo, J.; Zheng, L.; Guo, Q.; Peng, J.; Xu, J.; Qin, L. Nanoparticle–Cartilage Interaction: Pathology-Based Intra-articular Drug Delivery for Osteoarthritis Therapy. Nano-Micro Lett. 2021, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Colella, F.; Garcia, J.P.; Sorbona, M.; Lolli, A.; Antunes, B.; D’Atri, D.; Barré, F.P.Y.; Oieni, J.; Vainieri, M.L.; Zerrillo, L.; et al. Drug delivery in intervertebral disc degeneration and osteoarthritis: Selecting the optimal platform for the delivery of disease-modifying agents. J. Control. Release 2020, 328, 985–999. [Google Scholar] [CrossRef]
- Zhang, X.; Smith, N.; Webb, A. 1-Medical Imaging. In Biomedical Information Technology; Feng, D.D., Ed.; Academic Press: Burlington, MA, USA, 2008; pp. 3–27. [Google Scholar]
- Huang, W.-Y.; Davis, J.J. Multimodality and nanoparticles in medical imaging. Dalton Trans. 2011, 40, 6087–6103. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.; Ajorlou, E.; Asghari, F.; Pilehvar-Soltanahmadi, Y. An update on nanoparticle-based contrast agents in medical imaging. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1111–1121. [Google Scholar] [CrossRef] [PubMed]
- Azria, D.; Blanquer, S.; Verdier, J.-M.; Belamie, E. Nanoparticles as contrast agents for brain nuclear magnetic resonance imaging in Alzheimer’s disease diagnosis. J. Mater. Chem. B 2017, 5, 7216–7237. [Google Scholar] [CrossRef] [PubMed]
- Vu-Quang, H.; Vinding, M.S.; Nielsen, T.; Ullisch, M.G.; Nielsen, N.C.; Nguyen, D.-T.; Kjems, J. Pluronic F127-Folate Coated Super Paramagenic Iron Oxide Nanoparticles as Contrast Agent for Cancer Diagnosis in Magnetic Resonance Imaging. Polymers 2019, 11, 743. [Google Scholar] [CrossRef] [PubMed]
- Kania, G.; Sternak, M.; Jasztal, A.; Chlopicki, S.; Błażejczyk, A.; Nasulewicz-Goldeman, A.; Wietrzyk, J.; Jasiński, K.; Skórka, T.; Zapotoczny, S.; et al. Uptake and bioreactivity of charged chitosan-coated superparamagnetic nanoparticles as promising contrast agents for magnetic resonance imaging. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Amendola, V.; Guadagnini, A.; Agnoli, S.; Badocco, D.; Pastore, P.; Fracasso, G.; Gerosa, M.; Vurro, F.; Busato, A.; Marzola, P. Polymer-coated silver-iron nanoparticles as efficient and biodegradable MRI contrast agents. J. Colloid Interface Sci. 2021, 596, 332–341. [Google Scholar] [CrossRef]
- Ponsiglione, A.M.; Russo, M.; Torino, E. Glycosaminoglycans and Contrast Agents: The Role of Hyaluronic Acid as MRI Contrast Enhancer. Biomolecules 2020, 10, 1612. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Thorek, D.L.J.; Tsourkas, A. Porous Polymersomes with Encapsulated Gd-Labeled Dendrimers as Highly Efficient MRI Contrast Agents. Adv. Funct. Mater. 2009, 19, 3753–3759. [Google Scholar] [CrossRef] [PubMed]
- Pant, K.; Sedláček, O.; Nadar, R.A.; Hrubý, M.; Stephan, H. Radiolabelled Polymeric Materials for Imaging and Treatment of Cancer: Quo Vadis? Adv. Heal. Mater. 2017, 6, 1601115. [Google Scholar] [CrossRef] [PubMed]
- Gill, M.R.; Menon, J.U.; Jarman, P.J.; Owen, J.; Skaripa-Koukelli, I.; Able, S.; Thomas, J.A.; Carlisle, R.; Vallis, K.A. 111 In-labelled polymeric nanoparticles incorporating a ruthenium-based radiosensitizer for EGFR-targeted combination therapy in oesophageal cancer cells. Nanoscale 2018, 10, 10596–10608. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, N.I.; Miroslavov, A.E.; Alekseev, I.E.; Lumpov, A.A.; Murko, A.Y.; Gavrilova, I.I.; Saprykina, N.N.; Bezrukova, M.A.; Kipper, A.I.; Krasikov, V.D.; et al. Study of N-vinylpyrrolidone-N-vinylformamide copolymers labelled with indium-113m. J. Label. Compd. Radiopharm. 2017, 60, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Zhao, T.; Wang, C.; Nham, K.; Xiong, Y.; Gao, X.; Wang, Y.; Hao, G.; Ge, W.-P.; Sun, X.; et al. PET imaging of occult tumours by temporal integration of tumour-acidosis signals from pH-sensitive 64Cu-labelled polymers. Nat. Biomed. Eng. 2020, 4, 314–324. [Google Scholar] [CrossRef]
- Li, B.; Zhang, X.; Dong, Y. Nanoscale platforms for messenger RNA delivery. WIREs Nanomed. Nanobiotechnology 2019, 11, e1530. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; O’Keeffe-Ahern, J.; Lyu, J.; Pierucci, L.; Zhou, D.; Wang, W. A new developing class of gene delivery: Messenger RNA-based therapeutics. Biomater. Sci. 2017, 5, 2381–2392. [Google Scholar] [CrossRef]
- Yue, Y.; Jin, F.; Deng, R.; Cai, J.; Dai, Z.; Lin, M.C.M.; Kung, H.-F.; Mattebjerg, M.A.; Andresen, T.L.; Wu, C. Revisit complexation between DNA and polyethylenimine—Effect of length of free polycationic chains on gene transfection. J. Control. Release 2011, 152, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shao, B.; He, Z.; Ye, T.; Luo, M.; Sang, Y.; Liang, X.; Wang, W.; Luo, S.; Yang, S. Cationic nanocarriers induce cell necrosis through impairment of Na+/K+-ATPase and cause subsequent inflammatory response. Cell Res. 2015, 25, 237–253. [Google Scholar] [CrossRef] [PubMed]
- Blakney, A.K.; McKay, P.F.; Hu, K.; Samnuan, K.; Jain, N.; Brown, A.; Thomas, A.; Rogers, P.; Polra, K.; Sallah, H.; et al. Polymeric and lipid nanoparticles for delivery of self-amplifying RNA vaccines. J. Control. Release 2021, 338, 201–210. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Bhattacharya, M.; Lee, S.-S. From COVID-19 to Cancer mRNA Vaccines: Moving From Bench to Clinic in the Vaccine Landscape. Front. Immunol. 2021, 12, 2648. [Google Scholar] [CrossRef]
- Rahman, M.; Alharbi, K.S.; Alruwaili, N.K.; Anfinan, N.; Almalki, W.H.; Padhy, I.; Sambamoorthy, U.; Swain, S.; Beg, S. Nucleic acid-loaded lipid-polymer nanohybrids as novel nanotherapeutics in anticancer therapy. Expert Opin. Drug Deliv. 2020, 17, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Bose, R.J.C.; Ravikumar, R.; Karuppagounder, V.; Bennet, D.; Rangasamy, S.; Thandavarayan, R.A. Lipid–polymer hybrid nanoparticle-mediated therapeutics delivery: Advances and challenges. Drug Discov. Today 2017, 22, 1258–1265. [Google Scholar] [CrossRef] [PubMed]
- Vencken, S.; Foged, C.; Ramsey, J.M.; Sweeney, L.; Cryan, S.-A.; MacLoughlin, R.J.; Greene, C.M. Nebulised lipid–polymer hybrid nanoparticles for the delivery of a therapeutic anti-inflammatory microRNA to bronchial epithelial cells. ERJ Open Res. 2019, 5, 00161–02018. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhou, Y.; Chen, J.; Huang, N.; Wang, Z.; Cheng, Y. Gene Therapy for Drug-Resistant Glioblastoma via Lipid-Polymer Hybrid Nanoparticles Combined with Focused Ultrasound. Int J. Nanomed. 2021, 16, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.; Dewangan, H.K. Nanoparticles as adjuvants in vaccine delivery. Crit. Rev. Ther. Drug Carr. Syst. 2020, 37, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Sun, X. Engineering nanoparticulate vaccines for enhancing antigen cross-presentation. Curr. Opin. Biotechnol. 2020, 66, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Simón-Vázquez, R.; Peleteiro, M.; González-Fernández, Á. Polymeric nanostructure vaccines: Applications and challenges. Expert Opin. Drug Deliv. 2020, 17, 1007–1023. [Google Scholar] [CrossRef] [PubMed]
- Rodgers, A.M.; Cordeiro, A.S.; Kissenpfennig, A.; Donnelly, R.F. Microneedle arrays for vaccine delivery: The possibilities, challenges and use of nanoparticles as a combinatorial approach for enhanced vaccine immunogenicity. Expert Opin. Drug Deliv. 2018, 15, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Mehrabi, M.; Montazeri, H.; Mohamadpour Dounighi, N.; Rashti, A.; Vakili-Ghartavol, R. Chitosan-based Nanoparticles in Mucosal Vaccine Delivery. Arch. Razi Inst. 2018, 73, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Sun, Y.; Chen, G.; Rong, G.; Kang, H.; Jin, Z.; Wang, X. Biological evaluation of N-2-hydroxypropyl trimethyl ammonium chloride chitosan as a carrier for the delivery of live Newcastle disease vaccine. Carbohydr. Polym. 2016, 149, 28–39. [Google Scholar] [CrossRef]
- Dhakal, S.; Renu, S.; Ghimire, S.; Shaan Lakshmanappa, Y.; Hogshead, B.T.; Feliciano-Ruiz, N.; Lu, F.; HogenEsch, H.; Krakowka, S.; Lee, C.W.; et al. Mucosal Immunity and Protective Efficacy of Intranasal Inactivated Influenza Vaccine Is Improved by Chitosan Nanoparticle Delivery in Pigs. Front. Immunol. 2018, 9, 934. [Google Scholar] [CrossRef] [PubMed]
- Choi, I.-J.; Kang, A.; Ahn, M.-H.; Jun, H.; Baek, S.-K.; Park, J.-H.; Na, W.; Choi, S.-O. Insertion-responsive microneedles for rapid intradermal delivery of canine influenza vaccine. J. Control. Release 2018, 286, 460–466. [Google Scholar] [CrossRef]
- Tanishita, S.; Ukawa, M.; Tomono, T.; Yoshida, Y.; Tsujioka, T.; Miyata, K.; Tobita, E.; Uto, T.; Baba, M.; Sakuma, S. Cross-Protective Abilities of Hyaluronic Acid Modified with Tetraglycine-l-octaarginine as a Mucosal Adjuvant against Infection with Heterologous Influenza Viruses. Bioconjugate Chem. 2019, 30, 3028–3037. [Google Scholar] [CrossRef]
- Mosafer, J.; Sabbaghi, A.-H.; Badiee, A.; Dehghan, S.; Tafaghodi, M. Preparation, characterization and in vivo evaluation of alginate-coated chitosan and trimethylchitosan nanoparticles loaded with PR8 influenza virus for nasal immunization. Asian J. Pharm. Sci. 2019, 14, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Coucke, D. Development of a Platform for Nasal Delivery of Peptides and Vaccines Using Powder Carriers Based on Starch/Poly (Acrylic) Acid. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2009. [Google Scholar]
- Kilinc, Y.B.; Akdeste, Z.M.; Koc, R.C.; Bagirova, M.; Allahverdiyev, A. Synthesis and characterization of antigenic influenza A M2e protein peptide-poly(acrylic) acid bioconjugate and determination of toxicity in vitro. Bioengineered 2014, 5, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Hamzaoui, A.; Laraba-Djebari, F. Development and evaluation of polymeric nanoparticles as a delivery system for snake envenoming prevention. Biologicals 2021, 70, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, F.; Mohammadpour Dounighi, N.; Avadi, M.R.; Rezayat, M. A New Approach to Antivenom Preparation Using Chitosan Nanoparticles Containing EchisCarinatus Venom as A Novel Antigen Delivery System. Iran. J. Pharm Res. 2017, 16, 858–867. [Google Scholar]
- Lin, L.C.-W.; Chattopadhyay, S.; Lin, J.-C.; Hu, C.-M.J. Advances and Opportunities in Nanoparticle- and Nanomaterial-Based Vaccines against Bacterial Infections. Adv. Healthc. Mater. 2018, 7, 1701395. [Google Scholar] [CrossRef] [PubMed]
- Renu, S.; Han, Y.; Dhakal, S.; Lakshmanappa, Y.S.; Ghimire, S.; Feliciano-Ruiz, N.; Senapati, S.; Narasimhan, B.; Selvaraj, R.; Renukaradhya, G.J. Chitosan-adjuvanted Salmonella subunit nanoparticle vaccine for poultry delivered through drinking water and feed. Carbohydr. Polym. 2020, 243, 116434. [Google Scholar] [CrossRef] [PubMed]
- Renu, S.; Markazi, A.D.; Dhakal, S.; Lakshmanappa, Y.S.; Shanmugasundaram, R.; Selvaraj, R.K.; Renukaradhya, G.J. Oral Deliverable Mucoadhesive Chitosan-Salmonella Subunit Nanovaccine for Layer Chickens. Int. J. Nanomed. 2020, 15, 761–777. [Google Scholar] [CrossRef] [PubMed]
- Meena, J.; Kumar, R.; Singh, M.; Ahmed, A.; Panda, A.K. Modulation of immune response and enhanced clearance of Salmonella typhi by delivery of Vi polysaccharide conjugate using PLA nanoparticles. Eur. J. Pharm. Biopharm. 2020, 152, 270–281. [Google Scholar] [CrossRef]
- Nevagi, R.J.; Khalil, Z.G.; Hussein, W.M.; Powell, J.; Batzloff, M.R.; Capon, R.J.; Good, M.F.; Skwarczynski, M.; Toth, I. Polyglutamic acid-trimethyl chitosan-based intranasal peptide nano-vaccine induces potent immune responses against group A streptococcus. Acta Biomater. 2018, 80, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Skwarczynski, M.; Malcolm, J.M.; Urbani, C.N.; Jia, Z.; Batzloff, M.R.; Good, M.F.; Monteiro, M.J.; Toth, I. Self-adjuvanting polyacrylic nanoparticulate delivery system for group A streptococcus (GAS) vaccine. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 168–173. [Google Scholar] [CrossRef]
- Faruck, M.O.; Zhao, L.; Hussein, W.M.; Khalil, Z.G.; Capon, R.J.; Skwarczynski, M.; Toth, I. Polyacrylate–Peptide Antigen Conjugate as a Single-Dose Oral Vaccine against Group A Streptococcus. Vaccines 2020, 8, 23. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, W.; Cruz, J.G.; Marasini, N.; Khalil, Z.G.; Capon, R.J.; Hussein, W.M.; Skwarczynski, M.; Toth, I. Development of Polyelectrolyte Complexes for the Delivery of Peptide-Based Subunit Vaccines against Group A Streptococcus. Nanomaterials 2020, 10, 823. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.C.; Yang, J.; Hussein, W.M.; Zhao, L.; Wang, X.; Khalil, Z.G.; Capon, R.J.; Toth, I.; Stephenson, R.J. Polyethylenimine: An Intranasal Adjuvant for Liposomal Peptide-Based Subunit Vaccine against Group A Streptococcus. ACS Infect. Dis. 2020, 6, 2502–2512. [Google Scholar] [CrossRef]
- Tada, R.; Suzuki, H.; Ogasawara, M.; Yamanaka, D.; Adachi, Y.; Kunisawa, J.; Negishi, Y. Polymeric Caffeic Acid Acts as a Nasal Vaccine Formulation against Streptococcus pneumoniae Infections in Mice. Pharmaceutics 2021, 13, 585. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Yin, J.; Barkema, H.W.; Chen, L.; Shahid, M.; Szenci, O.; De Buck, J.; Kastelic, J.P.; Han, B. Development of a single-dose recombinant CAMP factor entrapping poly(lactide-co-glycolide) microspheres-based vaccine against Streptococcus agalactiae. Vaccine 2017, 35, 1246–1253. [Google Scholar] [CrossRef]
- Alikhani, Z.; Salouti, M.; Ardestani, M.S. Synthesis and immunological evaluation of a nanovaccine based on PLGA nanoparticles and alginate antigen against infections caused by Pseudomonas aeruginosa. Biomed. Phys. Eng. Express 2018, 4, 045016. [Google Scholar] [CrossRef]
- Azimi, S.; Safari Zanjani, L. Immunization against Pseudomonas aeruginosa using Alg-PLGA nano-vaccine. Iran J. Basic Med. Sci. 2021, 24, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Gonzaga, Z.J.C.; Merakou, C.; DiGiandomenico, A.; Priebe, G.P.; Rehm, B.H.A. A Pseudomonas aeruginosa-Derived Particulate Vaccine Protects against P. aeruginosa Infection. Vaccines 2021, 9, 803. [Google Scholar] [CrossRef]
- Crecente-Campo, J.; Lorenzo-Abalde, S.; Mora, A.; Marzoa, J.; Csaba, N.; Blanco, J.; González-Fernández, Á.; Alonso, M.J. Bilayer polymeric nanocapsules: A formulation approach for a thermostable and adjuvanted E. coli antigen vaccine. J. Control. Release 2018, 286, 20–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Wu, Y.; Kim, Y.B.; Oh, Y.-K. Advances in vaccine delivery systems against viral infectious diseases. Drug Deliv. Transl. Res. 2021, 11, 1401–1419. [Google Scholar] [CrossRef] [PubMed]
- Lindblad, E.B.; Duroux, L. Mineral Adjuvants. Immunopotentiators Mod. Vaccines 2017, 347–375. [Google Scholar] [CrossRef]
- Petrovsky, N.; Aguilar, J.C. Vaccine adjuvants: Current state and future trends. Immunol. Cell Biol. 2004, 82, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Young, A.J. Adjuvants: What a Difference 15 Years Makes! Vet. Clin. Food Anim. Pract. 2019, 35, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Nevagi, R.J.; Skwarczynski, M.; Toth, I. Polymers for subunit vaccine delivery. Eur. Polym. J. 2019, 114, 397–410. [Google Scholar] [CrossRef]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef] [PubMed]
- Wen, R.; Umeano, A.C.; Kou, Y.; Xu, J.; Farooqi, A.A. Nanoparticle systems for cancer vaccine. Nanomedicine 2019, 14, 627–648. [Google Scholar] [CrossRef] [PubMed]
- Wusiman, A.; Gu, P.; Liu, Z.; Xu, S.; Zhang, Y.; Hu, Y.; Liu, J.; Wang, D.; Huang, X. Cationic polymer modified PLGA nanoparticles encapsulating Alhagi honey polysaccharides as a vaccine delivery system for ovalbumin to improve immune responses. Int. J. Nanomed. 2019, 14, 3221–3234. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.J.; Tacken, P.J.; Eich, C.; Rueda, F.; Torensma, R.; Figdor, C.G. Controlled release of antigen and Toll-like receptor ligands from PLGA nanoparticles enhances immunogenicity. Nanomedicine 2017, 12, 491–510. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Duan, J.; Li, X.; Zhu, X.; Chen, Y.; Wang, X.; Sun, H.; Kong, D.; Li, C.; Yang, J. Improved vaccine-induced immune responses via a ROS-triggered nanoparticle-based antigen delivery system. Nanoscale 2018, 10, 9489–9503. [Google Scholar] [CrossRef] [PubMed]
- Saengruengrit, C.; Ritprajak, P.; Wanichwecharungruang, S.; Sharma, A.; Salvan, G.; Zahn, D.R.T.; Insin, N. The combined magnetic field and iron oxide-PLGA composite particles: Effective protein antigen delivery and immune stimulation in dendritic cells. J. Colloid Interface Sci. 2018, 520, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Bussio, J.I.; Molina-Perea, C.; González-Aramundiz, J.V. Lower-Sized Chitosan Nanocapsules for Transcutaneous Antigen Delivery. Nanomaterials 2018, 8, 659. [Google Scholar] [CrossRef]
- Wang, N.; Yang, Y.; Wang, X.; Tian, X.; Qin, W.; Wang, X.; Liang, J.; Zhang, H.; Leng, X. Polydopamine as the Antigen Delivery Nanocarrier for Enhanced Immune Response in Tumor Immunotherapy. ACS Biomater. Sci. Eng. 2019, 5, 2330–2342. [Google Scholar] [CrossRef] [PubMed]
- Miura, R.; Sawada, S.-I.; Mukai, S.-A.; Sasaki, Y.; Akiyoshi, K. Antigen Delivery to Antigen-Presenting Cells for Adaptive Immune Response by Self-Assembled Anionic Polysaccharide Nanogel Vaccines. Biomacromolecules 2019, 21, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.; Bobbala, S.; Karabin, N.; Scott, E. Influences of nanocarrier morphology on therapeutic immunomodulation. Nanomedicine 2018, 13, 1795–1811. [Google Scholar] [CrossRef] [PubMed]
- Dowling, D.J.; Scott, E.A.; Scheid, A.; Bergelson, I.; Joshi, S.; Pietrasanta, C.; Brightman, S.; Sanchez-Schmitz, G.; Van Haren, S.D.; Ninković, J. Toll-like receptor 8 agonist nanoparticles mimic immunomodulating effects of the live BCG vaccine and enhance neonatal innate and adaptive immune responses. J. Allergy Clin. Immunol. 2017, 140, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Rajput, M.K.S.; Kesharwani, S.S.; Kumar, S.; Muley, P.; Narisetty, S.; Tummala, H. Dendritic Cell-Targeted Nanovaccine Delivery System Prepared with an Immune-Active Polymer. ACS Appl. Mater. Interfaces 2018, 10, 27589–27602. [Google Scholar] [CrossRef] [PubMed]
- Widmer, J.; Thauvin, C.; Mottas, I.; Nguyen, V.N.; Delie, F.; Allémann, E.; Bourquin, C. Polymer-based nanoparticles loaded with a TLR7 ligand to target the lymph node for immunostimulation. Int. J. Pharm. 2018, 535, 444–451. [Google Scholar] [CrossRef]
- Chen, X.; Han, W.; Wang, G.; Zhao, X. Application prospect of polysaccharides in the development of anti-novel coronavirus drugs and vaccines. Int. J. Biol. Macromol. 2020, 164, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Slütter, B.; Plapied, L.; Fievez, V.; Alonso Sande, M.; des Rieux, A.; Schneider, Y.-J.; Van Riet, E.; Jiskoot, W.; Préat, V. Mechanistic study of the adjuvant effect of biodegradable nanoparticles in mucosal vaccination. J. Control. Release 2009, 138, 113–121. [Google Scholar] [CrossRef]
- Thomas, C.; Rawat, A.; Hope-Weeks, L.; Ahsan, F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol. Pharm. 2011, 8, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Dewangan, H.K.; Pandey, T.; Maurya, L.; Singh, S. Rational design and evaluation of HBsAg polymeric nanoparticles as antigen delivery carriers. Int. J. Biol. Macromol. 2018, 111, 804–812. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, X.; Jia, J.; Lu, T.; Yang, T.; Wang, L. Potential hepatitis B vaccine formulation prepared by uniform-sized lipid hybrid PLA microparticles with adsorbed hepatitis B surface antigen. Mol. Pharm. 2018, 15, 5227–5235. [Google Scholar] [CrossRef] [PubMed]
- Khademi, F.; Taheri, R.-A.; Yousefi Avarvand, A.; Vaez, H.; Momtazi-Borojeni, A.A.; Soleimanpour, S. Are chitosan natural polymers suitable as adjuvant/delivery system for anti-tuberculosis vaccines? Microb. Pathog. 2018, 121, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Dacoba, T.G.; Omange, R.W.; Li, H.; Crecente-Campo, J.; Luo, M.; Alonso, M.J. Polysaccharide Nanoparticles Can Efficiently Modulate the Immune Response against an HIV Peptide Antigen. ACS Nano 2019, 13, 4947–4959. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Li, Z.; Young, D.J.; Liu, M.; Wu, C.; Wu, Y.L.; Loh, X.J. Toward the prevention of coronavirus infection: What role can polymers play? Mater. Today Adv. 2021, 10, 100140. [Google Scholar] [CrossRef] [PubMed]
- Kisby, T.; Yilmazer, A.; Kostarelos, K. Reasons for success and lessons learnt from nanoscale vaccines against COVID-19. Nat. Nanotechnol. 2021, 16, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Monajjemi, M.; Mollaamin, F.; Shojaei, S. An overview on Coronaviruses family from past to Covid-19: Introduce some inhibitors as antiviruses from Gillan’s plants. Biointerface Res. Appl. Chem. 2020, 10, 5575–5585. [Google Scholar] [CrossRef]
- Chung, J.Y.; Thone, M.N.; Kwon, Y.J. COVID-19 vaccines: The status and perspectives in delivery points of view. Adv. Drug Deliv. Rev. 2021, 170, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Hadi, A.G.; Kadhom, M.; Hairunisa, N.; Yousif, E.; Mohammed, S.A. A Review on COVID-19: Origin, Spread, Symptoms, Treatment, and Prevention. Biointerface Res. Appl. Chem. 2020, 10, 7234–7242. [Google Scholar] [CrossRef]
- Vahedifard, F.; Chakravarthy, K. Nanomedicine for COVID-19: The role of nanotechnology in the treatment and diagnosis of COVID-19. Emergent Mater. 2021, 4, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Hitzky, E.; Darder, M.; Wicklein, B.; Ruiz-Garcia, C.; Martín-Sampedro, R.; del Real, G.; Aranda, P. Nanotechnology Responses to COVID-19. Adv. Heal. Mater. 2020, 9, 2000979. [Google Scholar] [CrossRef] [PubMed]
- Khurana, A.; Allawadhi, P.; Khurana, I.; Allwadhi, S.; Weiskirchen, R.; Banothu, A.K.; Chhabra, D.; Joshi, K.; Bharani, K.K. Role of nanotechnology behind the success of mRNA vaccines for COVID-19. Nano Today 2021, 38, 101142. [Google Scholar] [CrossRef] [PubMed]
- Volpatti, L.R.; Wallace, R.P.; Cao, S.; Raczy, M.M.; Wang, R.; Gray, L.T.; Alpar, A.T.; Briquez, P.S.; Mitrousis, N.; Marchell, T.M.; et al. Polymersomes decorated with SARS-CoV-2 spike protein receptor binding domain elicit robust humoral and cellular immunity. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhang, Q.; Honko, A.; Zhou, J.; Gong, H.; Downs, S.N.; Vasquez, J.H.; Fang, R.H.; Gao, W.; Griffiths, A.; Zhang, L. Cellular Nanosponges Inhibit SARS-CoV-2 Infectivity. Nano Lett. 2020, 20, 5570–5574. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Guo, F.; Zou, Z.; Li, C.; Hong, X.; Zhao, Y.; Wang, C.; Wang, H.; Liu, H.; Yang, P.; et al. Cationic nanoparticles directly bind angiotensin-converting enzyme 2 and induce acute lung injury in mice. Part. Fibre Toxicol. 2015, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh, M.; Sadeghi, S.; Azizi, M.; Rastegari-Pouyani, M.; Pouriran, R.; Haji Molla Hoseini, M. Chitin and chitosan as tools to combat COVID-19: A triple approach. Int. J. Biol. Macromol. 2021, 183, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Stojanowski, J.; Gołębiowski, T. Focus on COVID-19: Antiviral polymers in drugs and vaccines. Polim. W Med. 2020, 50, 75–78. [Google Scholar] [CrossRef] [PubMed]
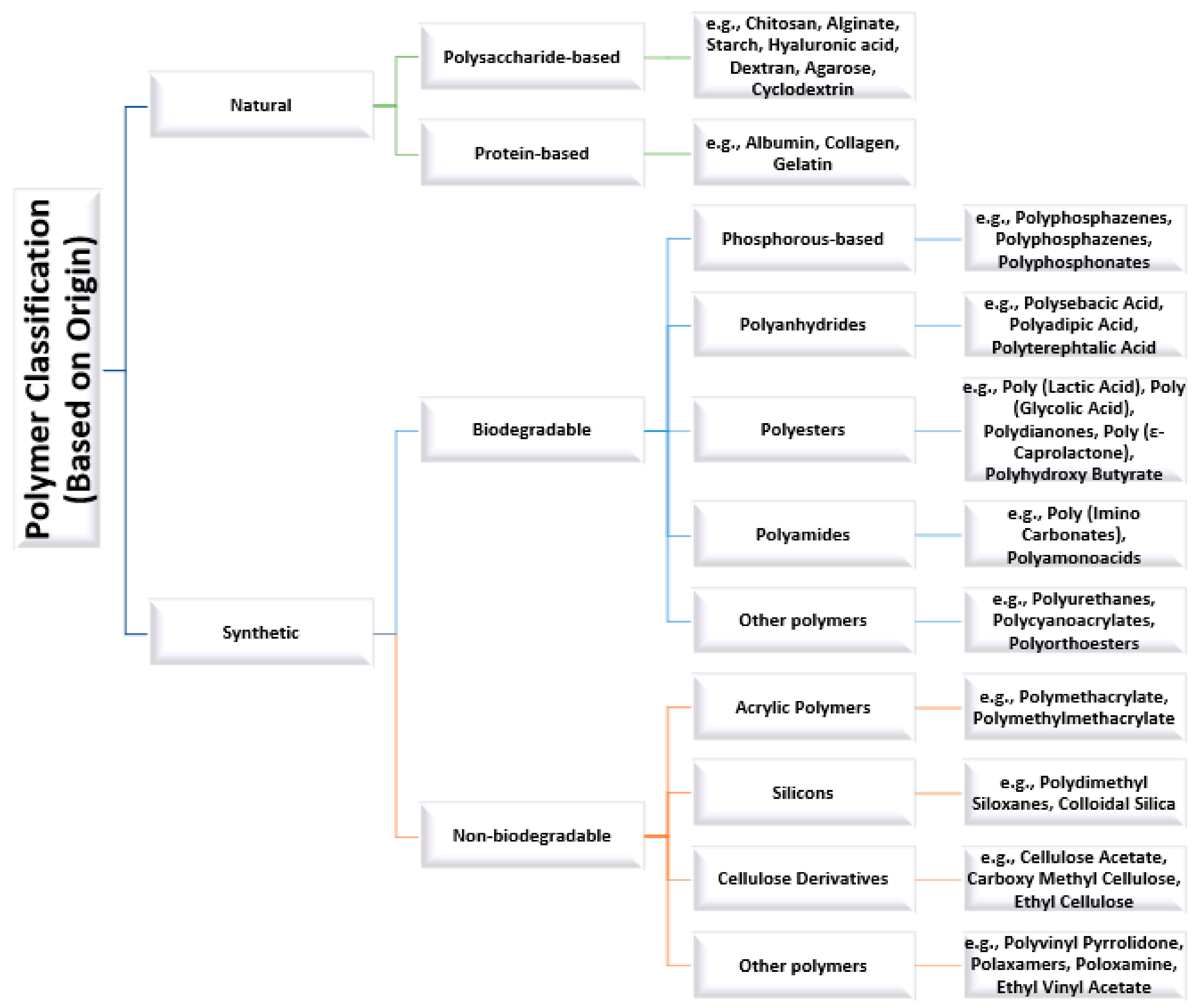

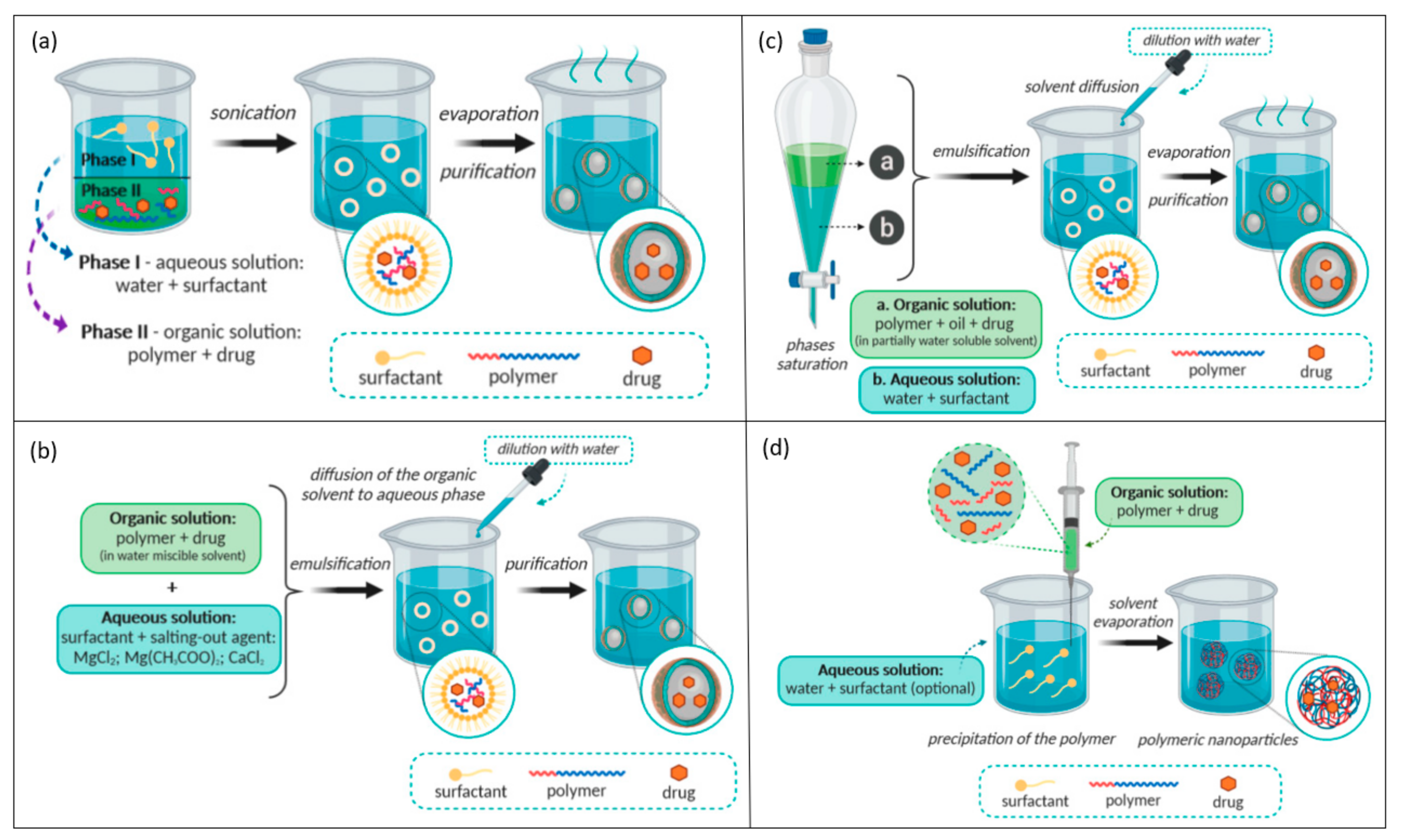
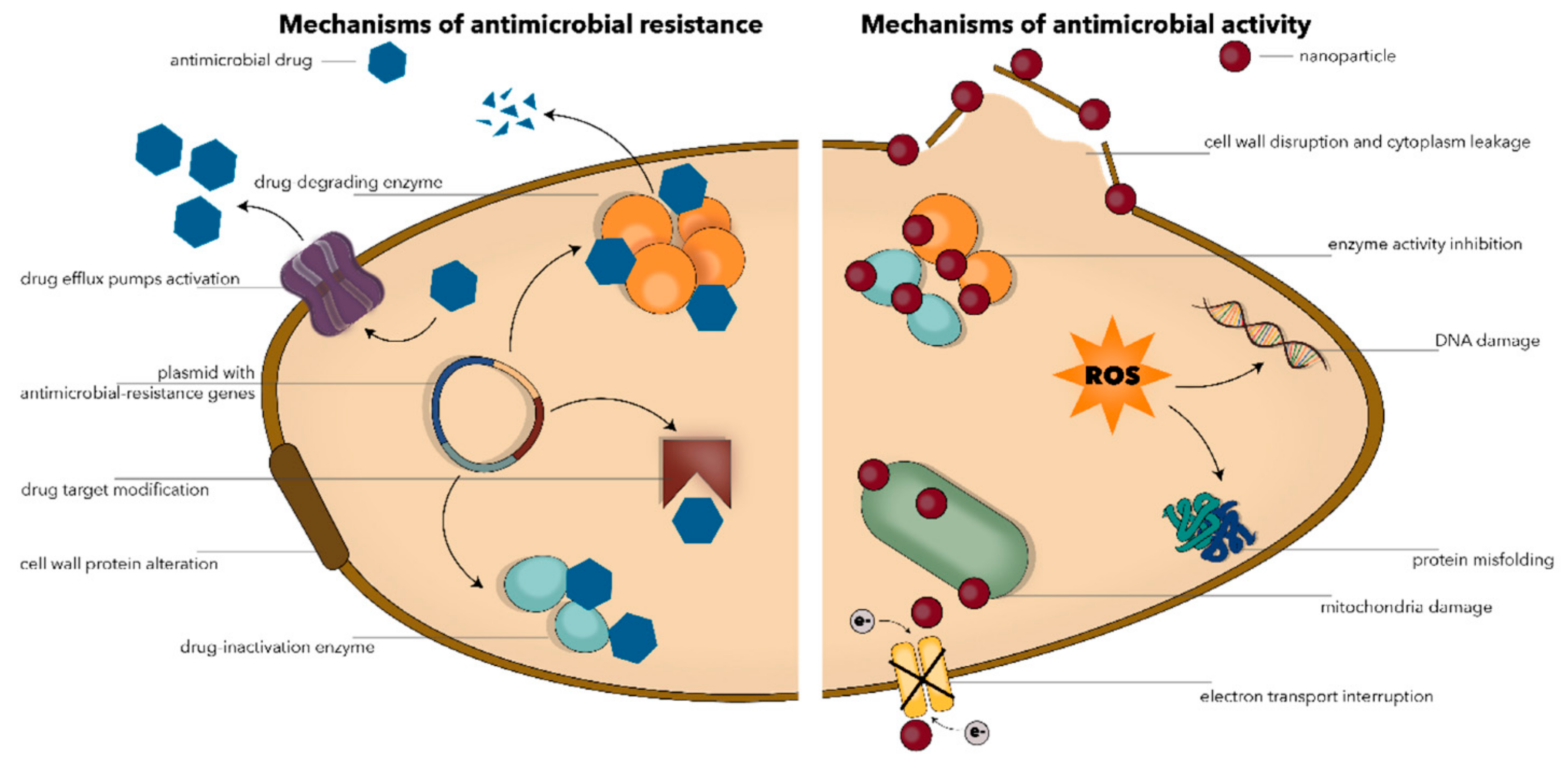
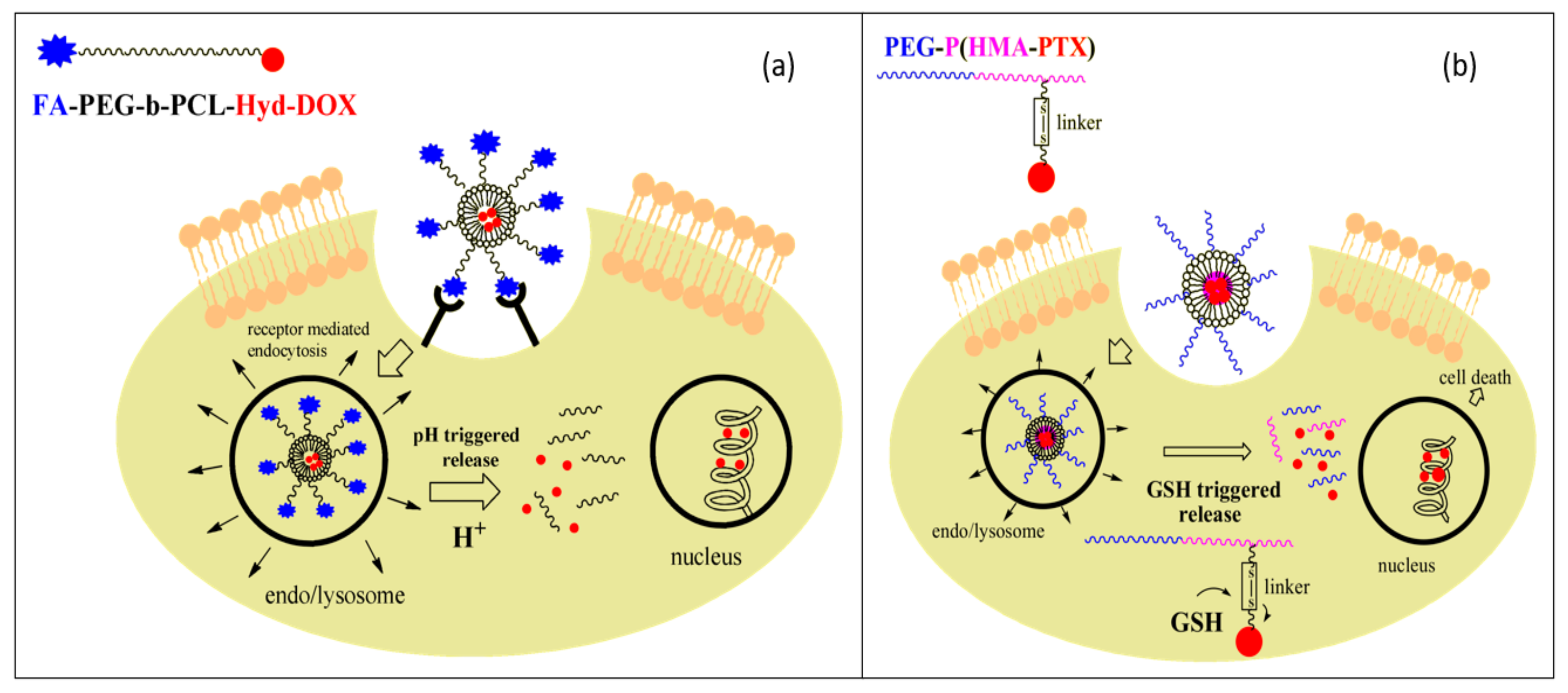

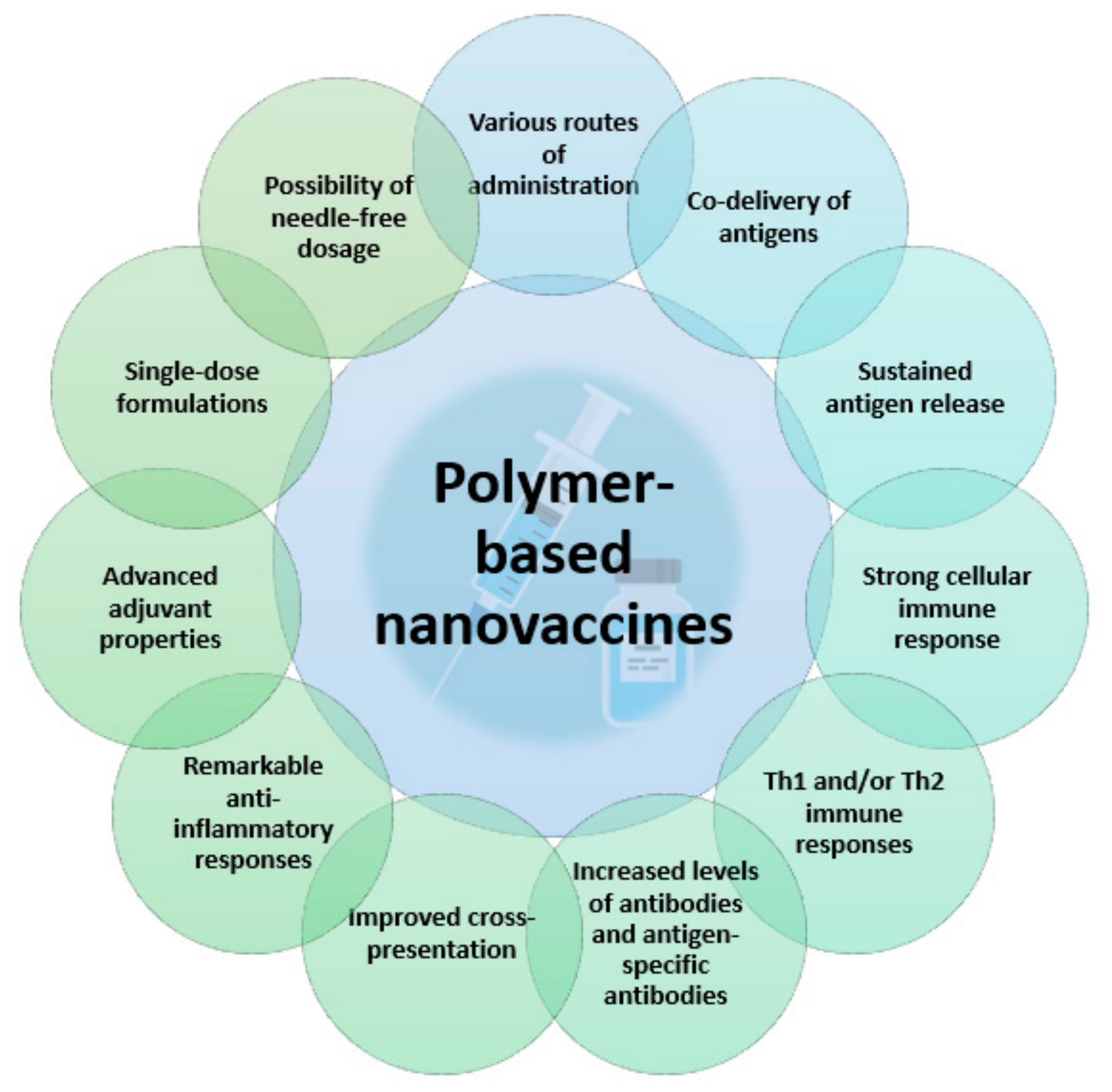
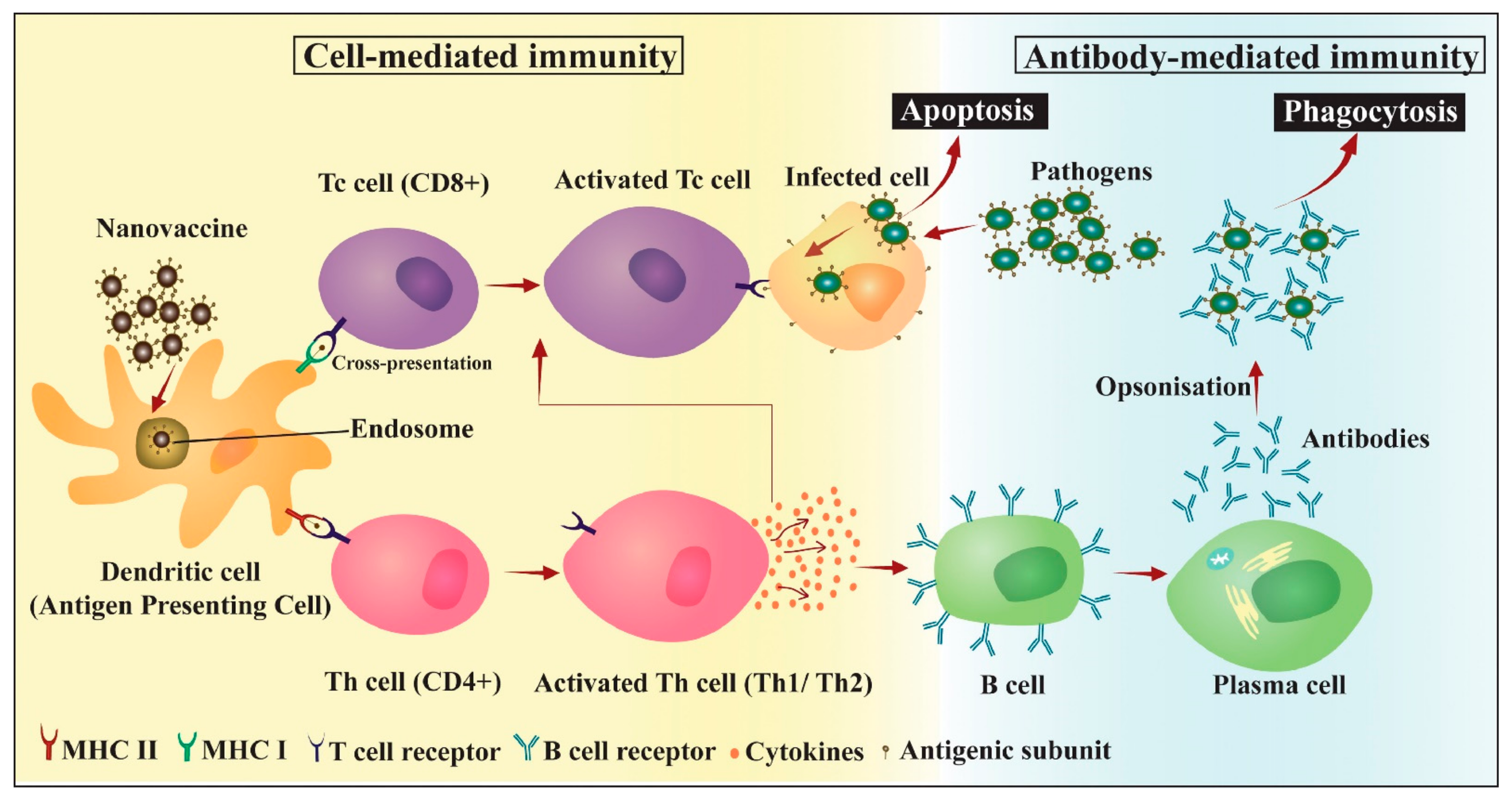
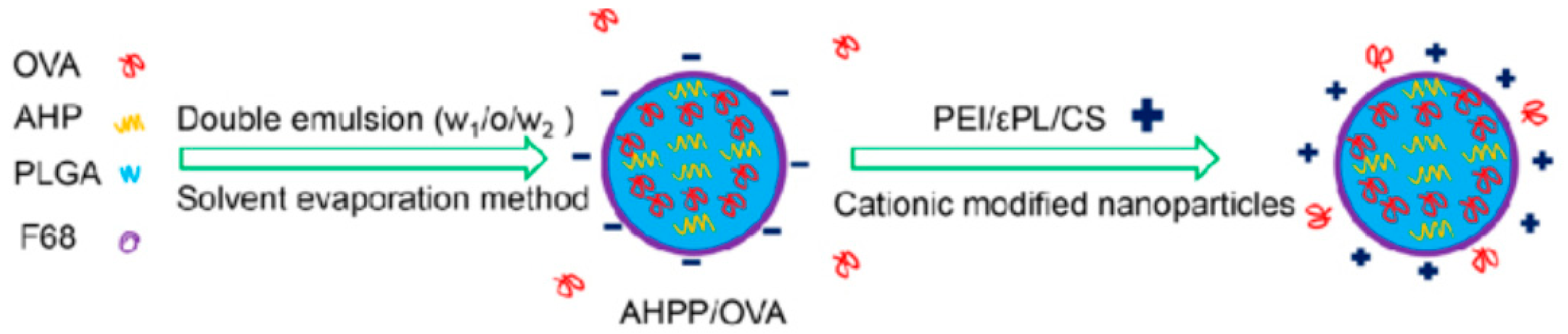

| Delivery System | Results | Refs. |
|---|---|---|
| Antimicrobial agent:Cinnamomum zeylanicum essential oil Polymer: Chitosan Other materials: - | Enhanced antibacterial effect compared to free essential oil for all tested bacteria (Escherichia coli, Erwinia carotovora, and Pseudomonas fluorescens) Highest sensitivity was obtained for P. fluorescens Maximum antibacterial activity was recorded for E. coli | [52] |
| Antimicrobial agent:Origanum syriacum essential oil Polymer: Chitosan Other materials: Zn(II)Salen | Good in vitro release profiles Significant growth suppression of microbial species, in the order Gram-positive bacteria > Gram-negative bacteria > fungi | [55] |
| Antimicrobial agent: Nettle essential oil Polymer: Chitosan Other materials: - | Greater antioxidant activity than free essential oil High antibacterial activity against Staphylococcus aureus and Escherichia coli | [184] |
| Antimicrobial agent: Clove essential oil Polymer: Chitosan Other materials: - | Improved antioxidant and antibacterial activities compared to free essential oil High antibacterial activity against Listeria monocytogenes and Staphylococcus aureus | [185] |
| Antimicrobial agent: N′-((5-nitrofuran-2-yl)methylen)-2-benzhydrazide Polymer: Chitosan Other materials: Polysorbate, Lyoprotectants (lactose, saccharose, glycine) | Potent antibacterial activity against Staphylococcus aureus ATCC 29213, hVISA, and ORSA strains Protective biofilm effect | [51] |
| Antimicrobial agent: Levofloxacin Polymer: Chitosan Other materials: - | High encapsulation and loading Non-irritant and safe formulation for topical ophthalmic use Strong antibacterial activity against Pseudomonas aeruginosa and Staphylococcus aureus | [49] |
| Antimicrobial agent: Gentamycin Polymers: Chitosan, Carbopol 974P Other materials: - | Sustained drug release Safe to the cornea; thus, suitable for ocular delivery Improved patient compliance Antimicrobial susceptibility against Staphylococcus aureus and Escherichia coli | [186] |
| Antimicrobial agent: Gentamycin Polymer: Chitosan Other materials: Phosphatidylcholine | Antibiofilm activity through the damaging and removal of pathogens (Listeria monocytogenes and Pseudomonas aeruginosa) Facilitated antibiotic permeation Neglectable cytotoxicity | [187] |
| Antimicrobial agents: Polyphenol drugs (naringenin, quercetin, curcumin) Polymers: Chitosan, Dialdehyde cellulose Other materials: L-histidine, Zinc oxide NPs | Sustained drug delivery Potent activity antimicrobial activity against Staphylococcus aureus and Trichophyton rubrum | [188] |
| Antimicrobial agent: Ampicillin Polymers: Chitosan, Polyanions Other materials: Phytic acid | High encapsulation efficiency Adequate stability Two-times higher antimicrobial activity than free ampicillin against sensitive and resistant Staphylococcus aureus strains | [189] |
| Antimicrobial agent: Silver sulfadiazine Polymer: Supramolecular polyelectrolyte complexes based on a cyclodextrin-grafted chitosan derivative and carrageenan Other materials: - | Controlled drug delivery (10 times slower drug release than for pure silver sulfadiazine) Strong antibacterial activity against Gram-positive bacteria (Staphylococcus aureus and Enterococcus durans/hirae) and Gram-negative bacteria (Klebsiella pneumoniae and Escherichia coli) | [190] |
| Antimicrobial agents: Rifampicin, Ascorbic acid Polymers: Alginate, Chitosan Other materials: - | Facilitated antibiotic permeation and enhanced cell uptake Significant biocide activity against Staphylococcus aureus strains | [191] |
| Antimicrobial agent: LysMR-5 endolysin Polymers: Alginate, Chitosan Other materials: - | Sustained drug release Biphasic release profile Enhanced bactericidal effect against Staphylococcus aureus | [192] |
| Antimicrobial agent: Vancomycin Polymers: Silk fibroin, Alginate poly(N-isopropylacrylamide) (PNIPAM) Other materials: Growth factor (EGF) | Supported proliferation and growth of fibroblasts Sustained drug release Higher release rate in an alkaline pH compared to neutral pH during 10 days Suitable for severe wound infections | [193] |
| Antimicrobial agent: Vancomycin Polymer: Hyaluronic acid Other materials: Oleylamine | Sustained drug release for 72 h Moderate antibacterial activity against Staphylococcus aureus and methicillin-resistant S. aureus (MRSA) 1.8 times higher MRSA cell death than for free drug administration due to a stronger impact on the bacterial membrane | [194] |
| Antimicrobial agent: Triphala Churna (polyherbal formulation) Polymer: Starch Other materials: - | Excellent antibacterial activity against Salmonella typhi and Shigella dysenteriae Antibiofilm activity against methicillin-resistant Staphylococcus aureus Neuroprotective potential | [117] |
| Antimicrobial agent: SET-M33 peptide Polymer: Dextran Other materials: - | Effective against Pseudomonas aeruginosa Acceptable cytotoxicity Markedly improved lung residence time | [195] |
| Antimicrobial agent: Titanium dioxide Polymers: Heparin, Polyvinyl alcohol Other materials: - | Good antimicrobial activity against Staphylococcus aureus and Escherichia coli Improved wound healing Suitable for burn injuries | [196] |
| Antimicrobial agent:Pistacia lentiscus L. var. chia essential oil Polymer: PLA Other materials: Surfactants (poly(vinyl alcohol—PVA), lecithin—LEC) | Higher encapsulation efficiency was recorded for PLA/PVA NPs than for PLA/LEC NPs A gradual release of the carried agent was noticed for the PLA/PVA NPs, while the PLA/LEC NPs exhibited a more immediate release | [197] |
| Antimicrobial agent: Rifampicin Polymers: PLA, Poly(L-lysine) Other materials: - | High and superficial loading of the antibiotic Effective delivery with a biphasic release profile Slowed particle migration in the Staphylococcus aureus biofilm thickness Improved retention in the biofilm Better antibiotic efficacy than for uncoated particles | [198] |
| Antimicrobial agents: Rutin, Benzamide Polymers: PEG, PLGA Other materials: - | Sustained release of rutin-benzamide for several days Antibacterial activity against Staphylococcus aureus and Pseudomonas aeruginosaAnti-biofilm activity through the disruption of the bacterial membrane and biofilm surface | [199] |
| Antimicrobial agent: Teicoplanin Polymer: PLGA Other materials: Specific aptamers | Targeted drug delivery There were recorded a 32-fold decrease in minimum concentration values for Staphylococcus aureus and a 64-fold decrease for moderately resistant strains, as compared to free teicoplanin | [200] |
| Antimicrobial agent: Red propolis hydroethanolic extract Polymer: PLGA Other materials: - | 96.99% encapsulation efficiency Biofilm inhibitory activity against Staphylococcus aureus and Pseudomonas aeruginosa | [201] |
| Antimicrobial agent: Farnesol Polymer: PLGA Other materials: - | Increased irregular cell morphology, membrane and wall damages, and large vacuoles were noted in Candida albicans cells Inhibited Candida growth and biofilm formation 57% reduced biofilm formation than free farnesol | [202] |
| Antimicrobial agents: Flavonoids (quercetin, rutin) Polymer: PVP Other materials: - | 99.8% entrapment efficiency Higher dissolution rate than unprocessed flavonoids | [203] |
| Antimicrobial agent: Silver nanoparticles Polymer: PVP Other materials: - | Complete eradication of common otitis media pathogens (i.e., Streptococcus pneumoniae and Haemophilus influenzae) No in vitro cytotoxicity | [204] |
| Antimicrobial agent: N-diazeniumdiolates (NONOates) Polymer: Poly(oligo(ethylene glycol)methyl ether methacrylate) (POEGMA) Other materials: Glycidyl methacrylate (GMA) | Pseudomonas aeruginosa biofilm dispersal Worm-like particles are more effective in the long term; spherical NPs are better for faster delivery applications | [205] |
| Delivery System | Results | Refs. |
|---|---|---|
| Chemotherapeutic agent: Mitomycin C Polymer: Chitosan Other materials: Mn:ZnS quantum dots | Diffusion mediated drug release Efficient targeted drug delivery to cancer sites Sustained drug release Effective drug delivery system for non-muscle invasive bladder cancer | [46] |
| Chemotherapeutic agent: Paclitaxel Polymer: Chitosan Other materials: Polystyrene templates | Sustained drug release Good bioavailability Marked inhibition of lung cancer cells proliferation Promoted apoptosis of cancer cells | [36] |
| Chemotherapeutic agent: Cisplatin Polymer: Chitosan Other materials: Silver nanoparticles | High encapsulation efficiency Specificity towards breast cancer cells 80% cancer cell death at less than 10 μg doses Minimal cytotoxicity towards healthy cells | [44] |
| Chemotherapeutic agent: Curcumin Polymer: Chitosan Other materials: - | High encapsulation efficiency The drug was vastly released in the first 5 h, then gradually release up to 90 h Most cancer cells entered apoptosis phase after 72 h of treatment with 150 μM of the drug-carrier system | [43] |
| Chemotherapeutic agents: Tamoxifen, Curcumin Polymer: Chitosan Other materials: Lipid | High encapsulation efficiency High antioxidant effects Inhibitory activity in the proliferation, growth, and migration of cancer cells | [40] |
| Chemotherapeutic agent: Helianthus tuberosus extracts Polymer: Starch Other materials: Copper oxide NPs, Folic acid | High cytotoxicity to human breast cancer cells due to ROS generation, nuclear damage, and reduction in mitochondrial membrane potential Activation of apoptosis-related protein expression Increased penetration in target cells leads to enhanced breast cancer therapy | [212] |
| Chemotherapeutic agent: Betulinic acid Polymers: Cellulose, Polymethyl methacrylate Other materials: - | High drug loading capacity Slow drug release rate Satisfactory antitumor activity both in vitro and in vivo Improved cancer cell cytotoxicity Reduced side-effects risk | [168] |
| Chemotherapeutic agents: Doxorubicin, Paclitaxel Polymer: Alginate Other materials: Oleic acid, Fe3O4 | Increased stability and biocompatibility of the drug-loaded nanocarrier Faster drug release in the acidic medium than in a neutral medium Higher toxicity toward MCF-7 and HeLa cells than free drugs | [233] |
| Chemotherapeutic agent: Doxorubicin Polymers: Cellulose, Polyacrylamide Other materials: Carboxymethyl-β-cyclodextrin, Folic acid | pH-dependent release behavior Targeted drug release High internalization of cellulose-based NPs lead to fast cellular uptake Reduced dose of doxorubicin and subsequently reduced systemic toxicity | [234] |
| Chemotherapeutic agent: Doxorubicin Polymer: Lentinan Other materials: - | pH-responsive drug release Enhanced anticancer effects in breast cancer cells Decreased toxicity against healthy cells | [125] |
| Chemotherapeutic agent: Doxorubicin Polymers: Glycogen, Polypyrrole Other materials: Phospholipids | Efficient specificity and enrichment of hepatocellular carcinoma Controllable drug release to induce cell nucleus damage Synergistic results in combination with photothermal therapy Reduced systemic toxicity Efficient suppression of tumor growth | [116] |
| Chemotherapeutic agent: Doxorubicin Polymer: Albumin Other materials: - | Drug activity was suppressed under physiological pH, but, in the presence of proteolytic enzymes, 40% of the encapsulated doxorubicin was released from the particles Reduced the metabolic activity of lung carcinoma cells after 72 h Up to 98% cell uptake in cancer cell lysosomal compartment | [92] |
| Chemotherapeutic agent: Doxorubicin Polymer: Albumin Other materials: - | Cytotoxicity in colon 26 cancer cultures More pronounced in vivo anti-tumor activity than free drug Suppression of metastasis | [91] |
| Chemotherapeutic agent: Docetaxel Polymer: Albumin Other materials: 131I | 80% of the drug was released at pH 7.4, whereas 93% of docetaxel was released at pH 5.8 Accumulation of drug-carrier system in tumor cells Suitable agent for nuclear imaging and radiotherapy of prostate cancer | [94] |
| Chemotherapeutic agent: Docetaxel Polymer: Albumin Other materials: Nucleolin-targeted aptamers | Sustained drug release Preferential uptake in nucleolin-expressing CT26 colon cancer cells Enhanced antitumor efficacy compared to non-targeted drug delivery Prolonged survival of the CT26-bearing mice | [96] |
| Chemotherapeutic agent: Docetaxel Polymer: Albumin Other materials: - | Higher permeability than free drug Controlled drug release Similar cytotoxicity against A549 cells to free drug Lower systemic toxicity than solvent formulated docetaxel | [93] |
| Chemotherapeutic agent: Curcumin Polymer: Albumin Other materials: | Redox-responsive system and acidic pH-triggered controlled delivery Significantly accelerated drug release in the presence of glutathione Enhanced cellular uptake in MCF-7 cells resulting in higher anticancer efficacy | [98] |
| Chemotherapeutic agent: Paclitaxel Polymer: Chondroitin sulfate Other materials: Quercetin, Chlorin e6 | Redox-responsive system that allows controlled delivery Synergistic results in combination with photodynamic therapy Effective in vivo multidrug resistance inhibition and anti-metastasis efficacy | [129] |
| Chemotherapeutic agent: Docetaxel Polymer: Chondroitin sulfate Other materials: Alpha-tocopherol succinate (TOS), Cystamine | Redox-responsive system that allows controlled delivery Time-dependent qualitative and quantitative uptake by melanoma cells Safe carrier system Enhanced antitumor activity as the drug was delivered accurately, quickly, and thoroughly | [128] |
| Chemotherapeutic agent: Docetaxel Polymers: PCL, Pluronic F108 Other materials: Near infrared dye | Diffusion mediated drug release Increased accumulation of NPs in breast cancer cells Superior targeted drug delivery system | [213] |
| Chemotherapeutic agent: Paclitaxel Polymers: PLGA, Chitosan Other materials: - | Sustained drug release Faster drug release at pH 5.5 than at pH 7.4 Chitosan modification of PLGA NPs leads to increased cellular uptake and cancer cell viability reduction | [235] |
| Chemotherapeutic agents: Curcumin, Niclosamide Polymer: PLGA Other materials: - | Much higher drug release at acidic pH 6.0 than at healthy pH of 7.4 Dual drug-loaded particles exhibited a higher anticancer effect than the bare mixture of drugs without PLGA Effective drug-carrier system for MDA-MB-231 breast cancer cells | [236] |
| Chemotherapeutic agent: Doxorubicin Polymer: PVP Other materials: Gold nanoparticles | Enhanced inhibition of lung cancer cells growth compared to free drug Increased ROS generation Sensitized mitochondrial membrane potential Induced both early and late apoptosis in lung cancer cells Highly upregulated expression of tumor suppressor genes | [226] |
| Chemotherapeutic agents: Quercetin, Gefitinib Polymer: PVP Other materials: Graphene oxide | Acceptable biocompatibility Efficient drug loading Improved drug release Significantly more toxic than individual drug-loaded systems and free drugs toward PA-1 ovarian cancer cells compared to the toxicity toward IOSE-364 cells | [230] |
| Chemotherapeutic agent: Doxorubicin Polymers: PMMA, Ovalbumin (OVA) Other materials: Graphene oxide | Successful loading and controlled drug release Higher swelling ratio of the carrier in acidic medium resulting in increased delivery of the drug at pH 2.8 than at normal pH Anti-cancer effect on gastric cancer cells | [169] |
| Pathogen | Vaccine Formulation | Results | Refs. |
|---|---|---|---|
| Salmonella | Polymer: Chitosan Other materials: Immunogenic outer membrane proteins (OMPs), Flagellin protein | Upregulation of TLRs, and Th1 and Th2 cytokines mRNA expression Enhanced specific systemic IgY and mucosal IgA antibodies responses Reduced Salmonella load in the intestines | [296] |
| Salmonella | Polymer: Chitosan Other materials: OMPs, Flagellin protein | Increased expression of TLR 2, TLR 4, IFN-γ, TGF-β, and Il-4 mRNA expression in chicken cecal tonsils Significantly higher OMPs-specific mucosal IgA production Enhanced lymphocyte proliferation response | [297] |
| Salmonella | Polymer: Poly (lactic acid) Other materials: Vi polysaccharide and r-flagellin of Salmonella typhi | Generated a strong immune response Promoted antibody class switching Produced memory antibody response from single point immunization Enhanced secretion of pro-inflammatory cytokine TNF-α and IL-6, while decreasing IFN-γ production | [298] |
| Streptococcus pyogenes | Polymers: α-Poly-(L-glutamic acid), Trimethyl chitosan (TMC) Other materials: Peptide antigen | Higher systemic and mucosal antibody titers than antigen adjuvanted with standard mucosal adjuvant cholera toxin B subunit or antigen mixed with TMC Reduced bacterial burden in nasal secretions, pharyngeal surface, and nasopharyngeal-associated lymphoid tissue | [299] |
| Streptococcus pyogenes | Polymer: Polyacrylate ester-based dendritic polymer Other materials: J14 peptide | Opsonization of pathogen Self-adjuvanting potential | [300] |
| Streptococcus pyogenes | Polymer: Poly (methyl acrylate) Other materials: B-cell epitope J8, universal T-helper Pan HLA-DR-binding epitope peptide | Strong systemic and mucosal immune responses after a single low-dose immunization Opsonization of pathogen after a second immunization | [301] |
| Streptococcus pyogenes | Polymers: Polyelectrolyte complexes various formulations, including alginate, chondroitin sulfate, dextran, hyaluronic acid or heparin, TMC Other materials: Liposomes | Anionic polymers assisted in eliciting immune responses while also working as complexing agents PEC-heparin system induced higher antigen-specific systemic IgG and mucosal IgA titers than all other tested PECs | [302] |
| Streptococcus pyogenes | Polymer: Polyethyleneimine Other materials: Liposomes Lipidated B-cell epitope, T-helper epitope | Significant mucosal and systemic immunity Production of IgA and IgG antibodies | [303] |
| Streptococcus pneumoniae | Polymer: Polymeric caffeic acid Other materials: Pneumococcal surface protein A (PspA) | Induction of PspA-specific antibody responses in the mucosal and systemic compartments Intranasal vaccination resulted in antigen-dependent protective immunity against a lethal infection of the pathogen | [304] |
| Streptococcus agalactiae | Polymer: Poly(lactic-co-glycolic acid) Other materials: CAMP factor | Induced a sustained increase od antibody titers Mortality and bacteria counts were lower than in the control group No pathological lesions were detected | [305] |
| Pseudomonas aeruginosa | Polymers: Poly(lactic-co-glycolic acid), Alginate Other materials: - | Significant increase in total IgG and IgM antibodies No cytotoxicity in lung, kidney, and liver | [306] |
| Pseudomonas aeruginosa | Polymer: Poly(lactic-co-glycolic acid), Alginate Other materials: - | Significant decrease in the bacterial burden in the spleen Considerably increased opsonic activity | [307] |
| Pseudomonas aeruginosa | Polymer: Polyhydroxyalkanoate Other materials: Selected epitopes | Induced the production of functional antibodies Lead to opsonophagocytic hilling Induced an overall serotype-independent immune response | [308] |
| Escherichia coli | Polymer: Chitosan, Dextran sulfate Other materials: Vitamin E, IutA protein | Improved formulation stability Controlled release of the associated antigen Higher IgG levels than in an alum-adjuvanted vaccine Stable formulation at room temperature for at least 3 months | [309] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculescu, A.-G.; Grumezescu, A.M. Polymer-Based Nanosystems—A Versatile Delivery Approach. Materials 2021, 14, 6812. https://doi.org/10.3390/ma14226812
Niculescu A-G, Grumezescu AM. Polymer-Based Nanosystems—A Versatile Delivery Approach. Materials. 2021; 14(22):6812. https://doi.org/10.3390/ma14226812
Chicago/Turabian StyleNiculescu, Adelina-Gabriela, and Alexandru Mihai Grumezescu. 2021. "Polymer-Based Nanosystems—A Versatile Delivery Approach" Materials 14, no. 22: 6812. https://doi.org/10.3390/ma14226812
APA StyleNiculescu, A.-G., & Grumezescu, A. M. (2021). Polymer-Based Nanosystems—A Versatile Delivery Approach. Materials, 14(22), 6812. https://doi.org/10.3390/ma14226812







