Effectiveness of Direct Pulp Capping Bioactive Materials in Dentin Regeneration: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Study Selection
2.3. Inclusion and Exclusion Criteria
- Clinical controlled trials (CCTs), randomized controlled trials (RCTs), and animal studies;
- Studies on permanent teeth in clinical conditions;
- Direct pulp capping.
- Indirect pulp capping;
- Total pulpotomies;
- Deciduous/primary dentition;
- Studies with insufficient information;
- Non-English publications.
2.4. Data Extraction and Organization
2.5. Quality Assessment
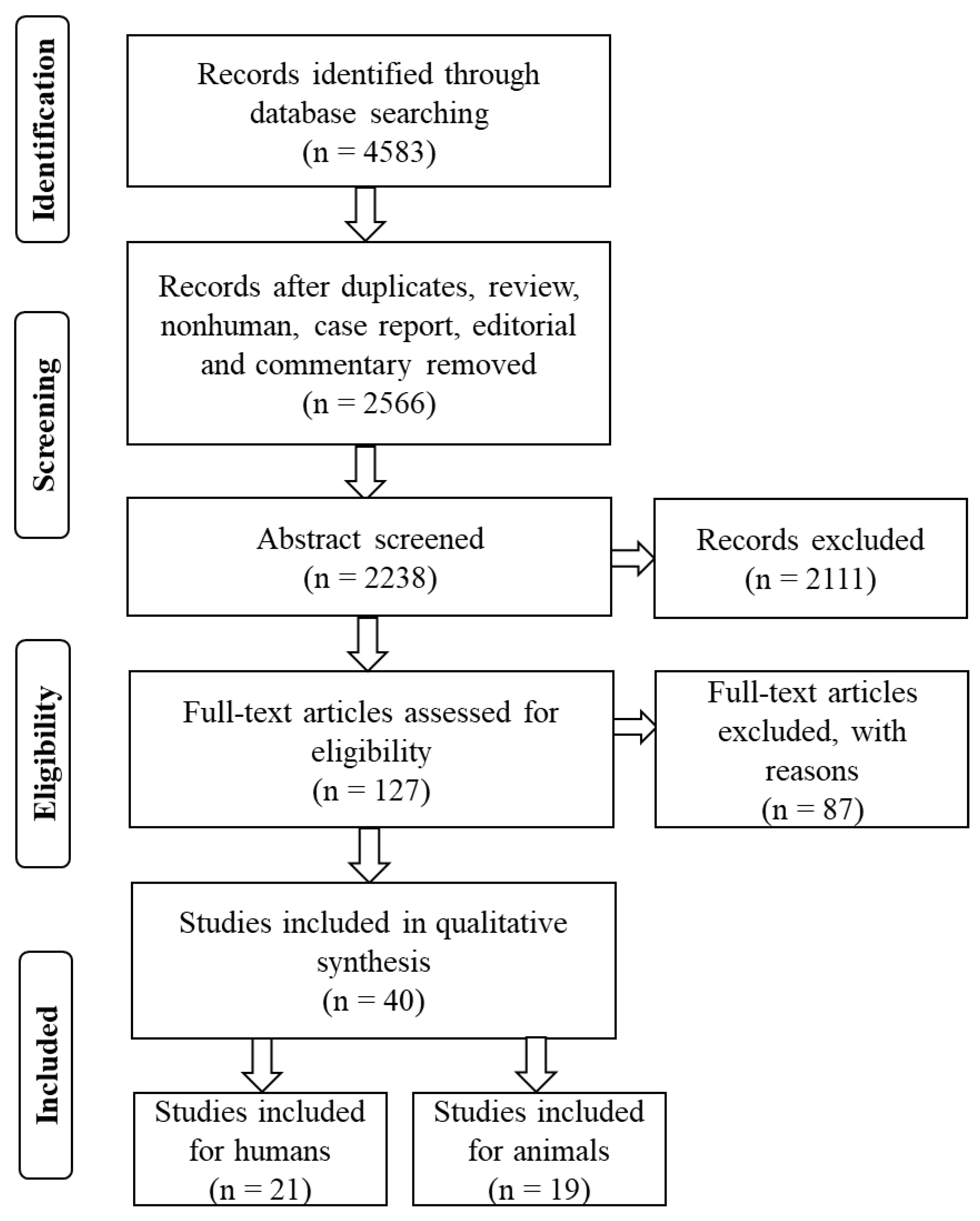
3. Results
3.1. Selection of Studies
3.2. Study Characteristics
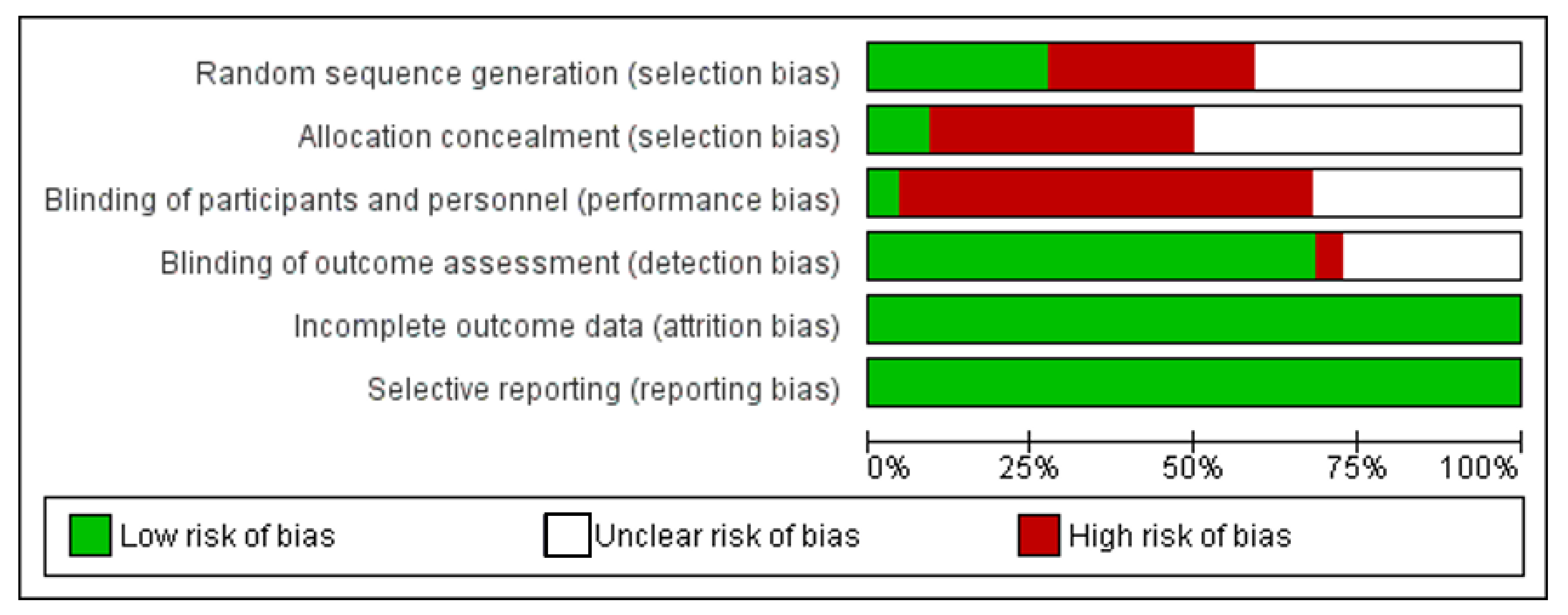
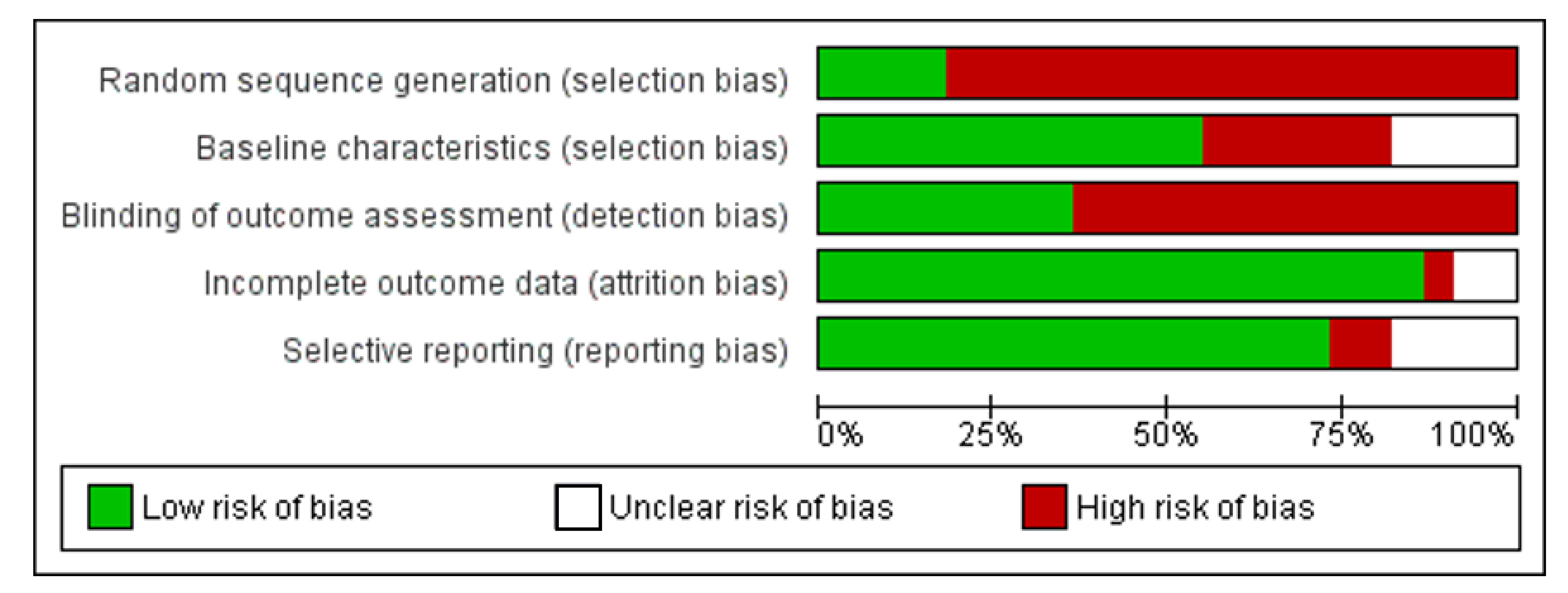
3.3. Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Didilescu, A.C.; Cristache, C.M.; Andrei, M.; Voicu, G.; Perlea, P. The effect of dental pulp-capping materials on hard-tissue barrier formation: A systematic review and meta-analysis. J. Am. Dent. Assoc. 2018, 149, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N.; Okiji, T. Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit Anom. 2016, 56, 144–153. [Google Scholar] [CrossRef]
- Davaie, S.; Hooshmand, T.; Ansarifard, S. Different types of bioceramics as dental pulp capping materials: A systematic review. Ceram. Int. 2021, 47, 20781–20792. [Google Scholar] [CrossRef]
- Islam, R.; Toida, Y.; Chen, F.; Tanaka, T.; Inoue, S.; Kitamura, T.; Yoshida, Y.; Chowdhury, A.F.M.A.; Ahmed, H.M.A.; Sano, H. Histological evaluation of a novel phosphorylated pullulan-based pulp capping material: An in vivo study on rat molars. Int. Endod. J. 2021, 54, 1902–1914. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yang, J.; Zhang, J.; Peng, B. A comparative study of BioAggregate and ProRoot MTA on adhesion, migration, and attachment of human dental pulp cells. J. Endod. 2014, 40, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Toida, Y.; Kawano, S.; Islam, R.; Jiale, F.; Chowdhury, A.A.; Hoshika, S.; Shimada, Y.; Tagami, J.; Yoshiyama, M.; Inoue, S.; et al. Pulpal response to mineral trioxide aggregate containing phosphorylated pullulan-based capping material. Dent. Mater. J. 2021. [CrossRef] [PubMed]
- Çalışkan, M.K.; Güneri, P. Prognostic factors in direct pulp capping with mineral trioxide aggregate or calcium hydroxide: 2-to 6-year follow-up. Clin. Oral Investig. 2017, 21, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Mente, J.; Hufnagel, S.; Leo, M.; Michel, A.; Gehrig, H.; Panagidis, D.; Panagidis, D.; Saure, D.; Pfefferle, T. Treatment outcome of mineral trioxide aggregate or calcium hydroxide direct pulp capping: Long-term results. J. Endod. 2014, 40, 1746–1751. [Google Scholar] [CrossRef] [PubMed]
- Hilton, T.J. Keys to clinical success with pulp capping: A review of the literature. Oper. Dent. 2009, 34, 615–625. [Google Scholar] [CrossRef]
- Utneja, S.; Nawal, R.R.; Talwar, S.; Verma, M. Current perspectives of bio-ceramic technology in endodontics: Calcium enriched mixture cement-review of its composition, properties and applications. Restor. Dent. Endod. 2015, 40, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Łagocka, R.; Lipski, M.; Parafiniuk, M.; Grocholewicz, K.; Sobolewska, E.; Witek, A.; Buczkowska-Radlińska, J. Clinical and histological evaluation of direct pulp capping on human pulp tissue using a dentin adhesive system. BioMed Res. Int. 2016, 2016, 2591273. [Google Scholar] [CrossRef]
- Fernandes, A.M.; Silva, G.A.; Lopes Jr, N.; Napimoga, M.H.; Benatti, B.B.; Alves, J.B. Direct capping of human pulps with a dentin bonding system and calcium hydroxide: An immunohistochemical analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Hörsted-Bindslev, P.; Vilkinis, V.; Sidlauskas, A. Direct capping of human pulps with a dentin bonding system or with calcium hydroxide cement. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2003, 96, 591–600. [Google Scholar] [CrossRef]
- Morotomi, T.; Washio, A.; Kitamura, C. Current and future options for dental pulp therapy. Jpn. Dent. Sci. Rev. 2019, 55, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Brouwer, F.; Schwendicke, A.; Paris, S. Different materials for direct pulp capping: Systematic review and meta-analysis and trial sequential analysis. Clin. Oral Investig. 2016, 20, 1121–1132. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Br. Med. J. 2011, 343, d5928. [Google Scholar] [CrossRef]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Cobanoglu, N.; AlptekIn, T.; Kitagawa, H.; Blatz, M.B.; Imazato, S.; Ozer, F. Evaluation of human pulp tissue response following direct pulp capping with a self-etching adhesive system containing MDPB. Dent. Mat. J. 2021, 40, 689–696. [Google Scholar] [CrossRef]
- Sharma, V.; Nawal, R.R.; Augustine, J.; Urs, A.B.; Talwar, S. Evaluation of Endosequence Root Repair Material and Endocem MTA as direct pulp capping agents: An in vivo study. Aust. Endod. J. 2021. [Google Scholar] [CrossRef]
- Holiel, A.A.; Mahmoud, E.M.; Abdel-Fattah, W.M.; Kawana, K.Y. Histological evaluation of the regenerative potential of a novel treated dentin matrix hydrogel in direct pulp capping. Clin. Oral Investig. 2021, 25, 2101–2112. [Google Scholar] [CrossRef] [PubMed]
- Holiel, A.A.; Mahmoud, E.M.; Abdel-Fattah, W.M. Tomographic evaluation of direct pulp capping using a novel injectable treated dentin matrix hydrogel: A 2-year randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 4621–4634. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, R. Histological Evaluation of Human Pulp Response to Direct Pulp Capping with MTA, CEM Cement, and Biodentine. J. Dent. 2020, 21, 177–183. [Google Scholar]
- Suzuki, M.; Kato, C.; Kawashima, S.; Shinkai, K. Clinical and histological study on direct pulp capping with CO2 laser irradiation in human teeth. Oper Dent. 2019, 44, 336–347. [Google Scholar] [CrossRef]
- Mahendran, K.; Ponnusamy, C.; Maloor, S.A. Histological evaluation of pulpal response to direct pulp capping using statins with α-tricalcium phosphate and mineral trioxide aggregate in human teeth. J. Conserv. Dent. 2019, 22, 441. [Google Scholar] [PubMed]
- Jalan, A.L.; Warhadpande, M.M.; Dakshindas, D.M. A comparison of human dental pulp response to calcium hydroxide and Biodentine as direct pulp-capping agents. J. Conserv. Dent. 2017, 20, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Nowicka, A.; Wilk, G.; Lipski, M.; Kołecki, J.; Buczkowska-Radlińska, J. Tomographic evaluation of reparative dentin formation after direct pulp capping with Ca (OH) 2, MTA, Biodentine, and dentin bonding system in human teeth. J. Endod. 2015, 41, 1234–1240. [Google Scholar] [CrossRef]
- Swarup, S.J.; Rao, A.; Boaz, K.; Srikant, N.; Shenoy, R. Pulpal response to nano hydroxyapatite, mineral trioxide aggregate and calcium hydroxide when used as a direct pulp capping agent: An in vivo study. J. Clin. Pediatr Dent. 2014, 38, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Parolia, A.; Kundabala, M.; Rao, N.N.; Acharya, S.R.; Agrawal, P.; Mohan, M.; Thomas, M. A comparative histological analysis of human pulp following direct pulp capping with Propolis, mineral trioxide aggregate and Dycal. Aust. Dent. J. 2010, 55, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Accorinte, M.L.R.; Loguercio, A.D.; Reis, A.; Costa, C.A.D.S. Response of human pulps capped with different self-etch adhesive systems. Clin. Oral Investig. 2008, 12, 119–127. [Google Scholar] [CrossRef]
- Accorinte, M.L.R.; Loguercio, A.D.; Reis, A.; Carneiro, E.; Grande, R.H.M.; Murata, S.S.; Holland, R. Response of human dental pulp capped with MTA and calcium hydroxide powder. Oper. Dent. 2008, 33, 488–495. [Google Scholar] [CrossRef]
- Accorinte, M.L.R.; Holland, R.; Reis, A.; Bortoluzzi, M.C.; Murata, S.S.; Dezan, E., Jr.; Souza, V.; Alessandro, L.D. Evaluation of mineral trioxide aggregate and calcium hydroxide cement as pulp-capping agents in human teeth. J. Endod. 2008, 34, 1–6. [Google Scholar] [CrossRef]
- Sawicki, L.; Pameijer, C.H.; Emerich, K.; Adamowicz-Klepalska, B. Histological evaluation of mineral trioxide aggregate and calcium hydroxide in direct pulp capping of human immature permanent teeth. Am. J. Dent. 2008, 21, 262–266. [Google Scholar] [PubMed]
- Lu, Y.; Liu, T.; Li, H.; Pi, G. Histological evaluation of direct pulp capping with a self-etching adhesive and calcium hydroxide on human pulp tissue. Int. Endod. J. 2008, 41, 643–650. [Google Scholar] [CrossRef]
- Min, K.S.; Park, H.J.; Lee, S.K.; Park, S.H.; Hong, C.U.; Kim, H.W.; Lee, H.H.; Kim, E.C. Effect of mineral trioxide aggregate on dentin bridge formation and expression of dentin sialoprotein and heme oxygenase-1 in human dental pulp. J. Endod. 2008, 34, 666–670. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N.R.; Duncan, H.F.; Pitt Ford, T.R.; Luder, H.U. Histological, ultrastructural and quantitative investigations on the response of healthy human pulps to experimental capping with mineral trioxide aggregate: A randomized controlled trial. Int. Endod. J. 2008, 41, 128–150. [Google Scholar] [PubMed]
- Silva, G.A.; Lanza, L.D.; Lopes-Júnior, N.; Moreira, A.; Alves, J.B. Direct pulp capping with a dentin bonding system in human teeth: A clinical and histological evaluation. Oper. Dent. 2006, 31, 297–307. [Google Scholar] [CrossRef]
- Iwamoto, C.E.; Adachi, E.; Pameijer, C.H.; Barnes, D.; Romberg, E.E.; Jefferies, S. Clinical and histological evaluation of white ProRoot MTA in direct pulp capping. Am. J. Dent. 2006, 19, 85–90. [Google Scholar] [PubMed]
- Yoon, J.H.; Choi, S.H.; Koh, J.T.; Lee, B.N.; Chang, H.S.; Hwang, I.N.; Oh, W.M.; Hwang, Y.C. Hard tissue formation after direct pulp capping with osteostatin and MTA in vivo. Restor. Dent. Endod. 2021, 46, e17. [Google Scholar] [CrossRef] [PubMed]
- Trongkij, P.; Sutimuntanakul, S.; Lapthanasupkul, P.; Chaimanakarn, C.; Wong, R.H.; Banomyong, D. Pulpal responses after direct pulp capping with two calcium-silicate cements in a rat model. Dent. Mat. J. 2019, 38, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Hanada, K.; Morotomi, T.; Washio, A.; Yada, N.; Matsuo, K.; Teshima, H.; Yokota, K.; Kitamura, C. In vitro and in vivo effects of a novel bioactive glass- based cement used as a direct pulp capping agent. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Okamoto, M.; Komichi, S.; Imazato, S.; Nakatsuka, T.; Sakamoto, S.; Kimoto, K.; Hayashi, M. Application of a direct pulp capping cement containing S- PRG filler. Clin. Oral Investig. 2019, 23, 1723–1731. [Google Scholar] [CrossRef]
- Paula, A.B.; Laranjo, M.; Marto, C.M.; Paulo, S.; Abrantes, A.M.; Fernandes, B.; Casalta-Lopes, J.; Marques-Ferreira, M.; Botelho, M.F.; Carrilho, E. Evaluation of dentinogenesis inducer biomaterials: An in vivo study. J. Appl. Oral Sci. 2019, 28, e20190023. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Pedano, M.S.; Camargo, B.; Hauben, E.; De Vleeschauwer, S.; Chen, Z.; Munck, J.D.; Vandamme, K.; Landuyt, K.V.; Meerbeek, B.V. Experimental tricalcium silicate cement induces reparative dentinogenesis. Dent. Mater. 2018, 34, 1410–1423. [Google Scholar] [CrossRef] [PubMed]
- Trongkij, P.; Sutimuntanakul, S.; Lapthanasupkul, P.; Chaimanakarn, C.; Wong, R.; Banomyong, D. Effects of the exposure site on histological pulpal responses after direct capping with 2 calcium- silicate based cements in a rat model. Restor. Dent. Endod. 2018, 43, e36. [Google Scholar] [CrossRef] [PubMed]
- Shinkai, K.; Taira, Y.; Kawashima, S.; Suzuki, S.; Suzuki, M. Histological evaluation of direct pulp capping with all- in- one adhesives in rat teeth. Dent. Mat. J. 2017, 36, 348–356. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Negm, A.M.; Hassanien, E.E.; Abu-Seida, A.M.; Nagy, M.M. Biological evaluation of a new pulp capping material developed from Portland cement. Exp. Toxicol. Pathol. 2017, 69, 115–122. [Google Scholar] [CrossRef]
- Shi, S.; Bao, Z.F.; Liu, Y.; Zhang, D.D.; Chen, X.; Jiang, L.M.; Zhong, M. Comparison of in vivo dental pulp responses to capping with iRoot BP Plus and mineral trioxide aggregate. Int. Endod. J. 2016, 49, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Taira, Y.; Kato, C.; Shinkai, K.; Katoh, Y. Histological evaluation of direct pulp capping of rat pulp with experimentally developed low-viscosity adhesives containing reparative dentin-promoting agents. J. Dent. 2016, 44, 27–36. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Dong, Y. Evaluation of a bioceramic as a pulp capping agent in vitro and in vivo. J. Endod. 2015, 41, 652–657. [Google Scholar] [CrossRef]
- Tziafa, C.; Koliniotou-Koumpia, E.; Papadimitriou, S.; Tziafas, D. Dentinogenic responses after direct pulp capping of miniature swine teeth with Biodentine. J. Endod. 2014, 40, 1967–1971. [Google Scholar] [CrossRef] [PubMed]
- Danesh, F.; Vahid, A.; Jahanbani, J.; Mashhadiabbas, F.; Arman, E. Effect of white mineral trioxide aggregate compared with biomimetic carbonated apatite on dentine bridge formation and inflammatory response in a dental pulp model. Int. Endod. J. 2012, 45, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Dammaschke, T.; Stratmann, U.; Fischer, R.J.; Sagheri, D.; Schäfer, E. A histologic investigation of direct pulp capping in rodents with dentin adhesives and calcium hydroxide. Quintessence Int. 2010, 41, 62–71. [Google Scholar]
- Cui, C.; Zhou, X.; Chen, X.; Fan, M.; Bian, Z.; Chen, Z. The adverse effect of self-etching adhesive systems on dental pulp after direct pulp capping. Quintessence Int. 2009, 40, 26–34. [Google Scholar]
- Lu, Y.; Liu, T.; Li, X.; Li, H.; Pi, G. Histologic evaluation of direct pulp capping with a self-etching adhesive and calcium hydroxide in beagles. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Koliniotou-Koumpia, E.; Tziafas, D. Pulpal responses following direct pulp capping of healthy dog teeth with dentine adhesive systems. J. Dent. 2005, 33, 639–647. [Google Scholar] [CrossRef]
- Andrei, M.; Vacaru, R.P.; Coricovac, A.; Ilinca, R.; Didilescu, A.C.; Demetrescu, I. The Effect of Calcium-Silicate Cements on Reparative Dentinogenesis Following Direct Pulp Capping on Animal Models. Molecules 2021, 26, 2725. [Google Scholar] [CrossRef]
- Nair, M.; Gurunathan, D. Clinical and radiographic outcomes of calcium hydroxide vs other agents in indirect pulp capping of primary teeth: A systematic review. Int. J. Clin. Pediatr. Dent. 2019, 12, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Petrou, M.A.; Alhamoui, F.A.; Welk, A.; Altarabulsi, M.B.; Alkilzy, M.; Splieth, C.H. A randomized clinical trial on the use of medical Portland cement, MTA and calcium hydroxide in indirect pulp treatment. Clin. Oral Investig. 2014, 18, 1383–1389. [Google Scholar] [CrossRef]
- Li, Z.; Cao, L.; Fan, M.; Xu, Q. Direct Pulp Capping with Calcium Hydroxide or Mineral Trioxide Aggregate: A Meta-analysis. J. Endod. 2015, 41, 1412–1417. [Google Scholar] [CrossRef]
- Zaen El-Din, A.M.; Hamama, H.H.; Abo El-Elaa, M.A.; Grawish, M.E.; Mahmoud, S.H.; Neelakantan, P. The effect of four materials on direct pulp capping: An animal study. Aust. Endod. J. 2020, 46, 249–256. [Google Scholar] [CrossRef]
- Tran, X.V.; Gorin, C.; Willig, C.; Baroukh, B.; Pellat, B.; Decup, F.; Opsahl Vital, S.; Chaussain, C.; Boukpessi, T. Effect of a calcium-silicate-based restorative cement on pulp repair. J. Dent. Res. 2012, 91, 1166–1171. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M. Bone morphogenetic proteins in dentin regeneration for potential use in endodontic therapy. Cytokine Growth Factor Rev. 2005, 16, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Graham, L.; Cooper, P.R.; Cassidy, N.; Nor, J.E.; Sloan, A.J.; Smith, A.J. The effect of calcium hydroxide on solubilisation of bio-active dentine matrix components. Biomaterials 2006, 27, 2865–2873. [Google Scholar] [CrossRef] [PubMed]
- Duque, C.; Hebling, J.; Smith, A.J.; Giro, E.M.; Oliveira, M.F.; de Souza Costa, C.A. Reactionary dentinogenesis after applying restorative materials and bioactive dentin matrix molecules as liners in deep cavities prepared in nonhuman primate teeth. J. Oral Rehabil. 2006, 33, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Walboomers, X.F.; Jansen, J.A. The formation of tertiary dentin after pulp capping with a calcium phosphate cement, loaded with PLGA microparticles containing TGF-beta1. J. Biomed. Mater. Res. A 2008, 85, 439–444. [Google Scholar] [CrossRef]
- Zhu, C.; Ju, B.; Ni, R. Clinical outcome of direct pulp capping with MTA or calcium hydroxide: A systematic review and meta-analysis. Int J. Clin. Exp. Med. 2015, 8, 17055–17060. [Google Scholar] [PubMed]
- Hilton, T.J.; Ferracane, J.L.; Mancl, L. Comparison of CaOH with MTA for Direct Pulp Capping: A PBRN Randomized Clinical Trial. J. Dent. Res. 2013, 92, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Daniele, L. Mineral Trioxide Aggregate (MTA) direct pulp capping: 10 years clinical results. G Ital. Endod. 2017, 31, 48–57. [Google Scholar] [CrossRef]
- Bogen, G.; Kim, J.S.; Bakland, L.K. Direct pulp capping with mineral trioxide aggregate: An observational study. J. Am. Dent. Assoc. 2008, 139, 305–315. [Google Scholar] [CrossRef]
- Laurent, P.; Camps, J.; About, I. BiodentineTM induces TGF-1 release from human pulp cells and early dental pulp mineralization. Int. Endod. J. 2012, 45, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Kunert, M.; Lukomska-Szymanska, M. Bio-inductive materials in direct and indirect pulp capping—A review article. Materials 2020, 13, 1204. [Google Scholar] [CrossRef] [PubMed]
- Dammaschke, T.; Nowicka, A.; Lipski, M.; Ricucci, D. Histological evaluation of hard tissue formation after direct pulp capping with a fast-setting mineral trioxide aggregate (RetroMTA) in humans. Clin. Oral Investig. 2019, 23, 4289–4299. [Google Scholar] [CrossRef]
- Paula, A.; Laranjo, M.; Marto, C.M.; Abrantes, A.M.; Casalta-Lopes, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Ferreira, M.M.; Botelho, M.F.; Carrilho, E. Biodentine™ boosts, WhiteProRoot® MTA increases and Life® suppresses odontoblast activity. Materials 2019, 12, 1184. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Song, Y.S.; Min, K.S.; Kim, S.H.; Koh, J.T.; Lee, B.N.; Chang, H.S.; Hwang, I.N.; Oh, W.M.; Hwang, Y.C. Evaluation of reparative dentin formation of ProRoot MTA, Biodentine and BioAggregate using micro-CT and immunohistochemistry. Restor. Dent. Endod. 2016, 41, 29. [Google Scholar] [CrossRef] [PubMed]
- Giraud, T.; Jeanneau, C.; Rombouts, C.; Bakhtiar, H.; Laurent, P.; About, I. Pulp capping materials modulate the balance between inflammation and regeneration. Dent. Mater. 2019, 35, 24–35. [Google Scholar] [CrossRef]
- Giraud, T.; Jeanneau, C.; Bergmann, M.; Laurent, P.; About, I. Tricalcium silicate capping materials modulate pulp healing and inflammatory activity in vitro. J. Endod. 2018, 44, 1686–1691. [Google Scholar] [CrossRef]
- Long, Y.; Liu, S.; Zhu, L.; Liang, Q.; Chen, X.; Dong, Y. Evaluation of pulp response to novel bioactive glass pulp cap-ping materials. J. Endod. 2017, 43, 1647–1650. [Google Scholar] [CrossRef]
- Simon, S.; Cooper, P.; Smith, A.; Picard, B.; Ifi, C.N.; Berdal, A. Evaluation of a new laboratory model for pulp healing: Preliminary study. Int. Endod. J. 2008, 41, 781–790. [Google Scholar] [CrossRef]
| Author (Year) | Country | Type of Study | Age | Type of Teeth | Experimental Materials | Comparing Materials | Follow-up | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Cobanoglu et al. 2021 [19] | Turkey | Controlled clinical trial | 23–35 | Third molars | Clearfil Protect Bond | Clearfil SE Bond and CH | 90 days | CH group showed better hard-tissue formation than the experimental group. |
| Sharma et al. 2021 [20] | India | Controlled clinical trial | 15–30 | Premolars | Endosequence Root Repair Material and Endocem MTA | ProRoot MTA | 30 days | The mean thickness of dentin-bridge formation in ProRoot MTA was greater than the other two experimental groups. |
| Holiel et al. 2021 [21] | Egypt | Controlled clinical trial | 15–25 | Premolars | Treated dentin matrix hydrogel | Biodentine and MTA | 2 weeks and 2 months | Complete dentin-bridge formation was observed with numerous dentinal tubule lines showing a positive trend to dentin regeneration. |
| Holiel et al. 2021 [22] | Egypt | Randomized clinical trial | 18–40 | Permanent posterior teeth | Treated dentin matrix hydrogel | Biodentine and MTA | 3, 6, 12, 18, and 24 months | Dentin-bridge formation was significantly superior of a higher thickness than Biodentine and MTA. |
| Hoseinifar et al. 2020 [23] | Iran | Randomized clinical trial | 14–25 | Premolars | Calcium-enriched mixture | MTA and Biodentine | 6 weeks | No significant differences were observed between the groups in terms of the dentine bridge formation. |
| Suzuki et al. 2019 [24] | Japan | Controlled clinical trial | 18–33 | Third molars | CO2 laser irradiation | Dycal | 6 and 12 months | Self-etching adhesive system following CO2 laser irradiation without carbonization of the exposed pulp demonstrated dentin-bridge formation that was comparable to Dycal. |
| Mahendran et al. 2019 [25] | India | Controlled clinical trial | 18–24 | Premolars | Simvastatin + α-TCP and atorvastatin + α-TCP | MTA | 7, 30, and 90 days | No significant difference was observed in terms of hard-tissue formation between the groups. |
| Jalan et al. 2017 [26] | India | Randomized clinical trial | 15–25 | Premolars | Biodentine | CH | 45 days | Dentin-bridge formation was significantly thicker and more continuous with Biodentine in comparison to Dycal. |
| Nowicka et al. 2016 [11] | Poland | Controlled clinical trial | 19–30 | Third molars | Single-bond universal | CH | 6 weeks | Single-bond universal showed less dentin-bridge formation than CH. |
| Nowicka et al. 2015 [27] | Poland | Controlled clinical trial | 19–32 | Third molars | MTA, Biodentine, single-bond universal | CH | 6 weeks | MTA and Biodentine groups showed significantly higher dentin-bridge formation than CH and single-bond universal groups. |
| Swarup et al. 2014 [28] | India | Controlled clinical trial | 11–15 | Premolars | Nano hydroxyapatite | MTA, CH | 15 and 30 days | Continuous dentin-bridge formation was observed in the nano hydroxyapatite and MTA groups. Only MTA group showed regular pattern of dentinal tubules. |
| Parolia et al. 2010 [29] | India | Controlled clinical trial | 15–25 | Premolars | Propolis, MTA | Dycal | 15 and 45 days | Propolis and MTA showed more dentin-bridge formation than Dycal group. |
| Accorinte et al. 2008 [30] | Brazil | Controlled clinical trial | 15–30 | Premolars | Clearfil LB 2V and Clearfil SE Bond | CH | 30 and 90 days | Few specimens showed dentin-bridge formation in the experimental group, whereas CH showed dentin-bridge formation almost all the specimens. |
| Accorinte et al. 2008 [31] | Brazil | Controlled clinical trial | 15–30 | Premolars | MTA | CH | 30 and 60 days | CH showed faster hard-tissue formation compared to MTA and a similar response with the hard-tissue bridge in almost all cases was observed. |
| Accorinte et al. 2008 [32] | Brazil | Controlled clinical trial | 15–30 | Premolars | MTA | CH | 30 and 60 days | Dentin-bridge formation was lower in the CH group compared to MTA group. |
| Sawicki et al. 2008 [33] | Poland | Controlled clinical trial | 10–18 | Immature premolars | WMTA | CH | 47–609 days | Complete, thicker, and more solid dentin bridge was observed in the WMTA group when compared with CH. |
| Lu et al. 2008 [34] | China | Controlled clinical trial | 20–25 | Third molars | Clearfil SE Bond | CH | 7, 30, and 90 days | The dentin-bridge formation in the experimental group was significantly lower compared to CH group. |
| Min et al. 2008 [35] | Korea | Controlled clinical trial | 21–50 | Third molars | MTA | CH | 2 months | The thickness of the dentin-bridge formation in the MTA group was statistically greater than CH group. |
| Nair et al. 2006 [36] | UK | Randomized controlled trial | 18–30 | Third molars | MTA | Dycal | 1 week, 1 month, and 3 months | Complete hard-tissue formation was observed in the MTA group, whereas less consistent formation of hard-tissue barrier with numerous tunnel defect was observed in the Dycal group. |
| Silva et al. 2006 [37] | Brazil | Controlled clinical trial | 12–20 | First premolars | Single-bond adhesive system | CH | 30 days | No dentin formation at the exposure area in the single-bong adhesive system group, whereas dentin-bridge formation was observed in the CH group. |
| Iwamoto et al. 2006 [38] | USA | Controlled clinical trial | 18–60 | Third molars | WMTA | CH | 136 ± 24 days | WMTA showed a dentin-bridge formation similar to CH’s. |
| Author (Year) | Country | Animal | Age/ Weight | Type of Teeth | Experimental Materials | Comparing Materials | Follow-up | Outcomes |
|---|---|---|---|---|---|---|---|---|
| Islam et al. 2021 [4] | Japan | Wister rats | 8–9 weeks | Maxillary first molar | Phosphorylated pullulan + MTA | MTA, Super Bond | 1, 3, 7, and 28 days | The experimental group showed more homogenous mineralized tissue formation compared to MTA and Super bond groups. |
| Yoon et al. 2021 [39] | Korea | Sprague-Dawley rats | 6–8 weeks | Maxillary first molar | Osteostatin + ProRoot MTA | ProRoot MTA | 4 weeks | The combined material group showed more mineralized dentin-bridge formation compared to ProRoot MTA group. |
| Trongkij et al. 2019 [40] | Thailand | Wister rats | 8 weeks | Maxillary first molar | Bio-MA | WMTA | 1, 3, and 30 days | Complete dentin-bridge formation was observed in the Bio-MA group which is similar to WMTA. |
| Hanada et al. 2019 [41] | Japan | Wister rats | 9 weeks | Maxillary first molar | Bioactive glass | Dycal and WMTA | 1, 4, and 7 days | Bioactive-glass-based cement induced a significant level of reparative dentin formation, similar to MTA. |
| Takahashi et al. 2019 [42] | Japan | Wister rats | 9 weeks | Maxillary first molars | S-PRG filler | MTA | 1, 2, and 4 weeks | S-PRG filler showed to promote tertiary dentinogenesis, which is similar to MTA. |
| Paula et al. 2019 [43] | Portugal | Wister rats | 12–14 weeks | First mandibular molars | WMTA and Biodentine | Positive control (exposure without treatment) | 3, 7, and 21 days | Mineralized tissue formation was observed in the WMTA and Biodentine group. Biodentine may lead to the formation of pulp calcifications. |
| Li et al. 2018 [44] | Belgium | Minipigs | 33–35 months | Incisors, canines, premolars and molars | Tricalcium silicate cement | ProRoot MTA and TheraCal | 70 days | Complete reparative dentin formation with tubular structures was observed in the tricalcium silicate and ProRoot MTA groups. |
| Trongkij et al. 2018 [45] | Thailand | Wister rats | 8 weeks | Maxillary first molar | Bio-MA | WMTA | 1 and 7 days | Bio-MA can stimulate reparative dentin formation which is similar to WMTA. |
| Shinkai et al. 2017 [46] | Japan | Sprague-Dawley rats | 8–9 weeks | Maxillary first molar | All-in-one adhesives (Clearfil Tri-SBond ND, G Bond Plus, Bond Force, Adper Easy Bond, Xeno V) | MTA | 14 days | MTA group showed complete dentin-bridge formation, whereas all-in-one adhesives group showed incomplete or partial dentin-bridge formation. |
| Negm et al. 2017 [47] | Egypt | Dogs | 4–6 months | Four teeth in three quadrants | Portland cement + 10% calcium hydroxide + 20% bismuth oxide, Portland cement + bismuth oxide | MTA | 3 weeks and 3 months | Addition of calcium hydroxide to Portland cement improves the dentin-bridge formation qualitatively and quantitatively. |
| Shi et al. 2016 [48] | China | Beagle dogs | 8 months | Maxillary and mandibular incisors | iRoot BP Plus | MTA | 3 months | Both experimental groups showed complete calcified bridge formation with no significant difference. |
| Suzuki et al. 2016 [49] | Japan | Sprague-Dawley rats | 6 weeks | Maxillary first molar | Adhesive resin – Primer I, II and III | Dycal | 14, 28, 56, and 112 days | Higher quality of the mineralized tissue formation was observed in the experimental groups. |
| Liu et al. 2015 [50] | China | Wister rats | 180–200 g | Maxillary first molars | iRoot BP Plus | MTA | 1 and 4 weeks | iRoot BP Plus induced the formation of reparative dentin bridge. |
| Tziafa et al. 2014 [51] | Greece | Miniature swine | 18 months | Incisors, canines, premolars and molars | Biodentine | MTA angelus | 3 and 8 weeks | The thickness of hard-tissue bridge formation was significantly higher in the Biodentine group. |
| Danesh et al. 2012 [52] | Tehran | Dogs | 18–24 months | Canine | Biomimetic carbonated apatite | MTA | 7 and 70 days | Biomimetic carbonated apatite did not induce hard-tissue bridge formation. |
| Dammaschke et al. 2010 [53] | Germany | Wister rats | 3 months | Maxillary first molars | Reculcin AquaPrime+ monoBond, ScotchBond 1, Gluma Comfort Bond | CH | 1, 3, 7, and 70 days | CH showed more frequent reparative dentin formation than the experimental groups. |
| Cui et al. 2009 [54] | China | Dog | 1.5 years | Incisor, canine, premolars and first molar | Clearfil SE Bond, Imperva FluoroBond, Prompt L-Pop | Dycal | 7, 14, and 30 days | Hard-tissue formation was observed in the experimental group. |
| Lu et al. 2006 [55] | China | Beagles | 1 year | All teeth | Clearfil SE Bond | CH | 7, 30, and 90 days | Dentin-bridge formation was less in the experimental group than CH. |
| Koliniotou-Koumpia and Tziafas 2005 [56] | Greece | Dog | 2.5–3.5 years | Maxillary and mandibulary molars, premolars, canines, and third incisors | Clearfil SE bond, Prompt L-pop, Etch and prime 3.0, single-bond | Dycal | 7, 21, and 65 days | Continuous hard-tissue bridge formation was totally absence in the experimental groups. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, E.; Yu, J.; Jiang, R.; Liu, X.; Li, X.; Islam, R.; Alam, M.K. Effectiveness of Direct Pulp Capping Bioactive Materials in Dentin Regeneration: A Systematic Review. Materials 2021, 14, 6811. https://doi.org/10.3390/ma14226811
Nie E, Yu J, Jiang R, Liu X, Li X, Islam R, Alam MK. Effectiveness of Direct Pulp Capping Bioactive Materials in Dentin Regeneration: A Systematic Review. Materials. 2021; 14(22):6811. https://doi.org/10.3390/ma14226811
Chicago/Turabian StyleNie, Ermin, Jiali Yu, Rui Jiang, Xiangzhen Liu, Xiang Li, Rafiqul Islam, and Mohammad Khursheed Alam. 2021. "Effectiveness of Direct Pulp Capping Bioactive Materials in Dentin Regeneration: A Systematic Review" Materials 14, no. 22: 6811. https://doi.org/10.3390/ma14226811
APA StyleNie, E., Yu, J., Jiang, R., Liu, X., Li, X., Islam, R., & Alam, M. K. (2021). Effectiveness of Direct Pulp Capping Bioactive Materials in Dentin Regeneration: A Systematic Review. Materials, 14(22), 6811. https://doi.org/10.3390/ma14226811







