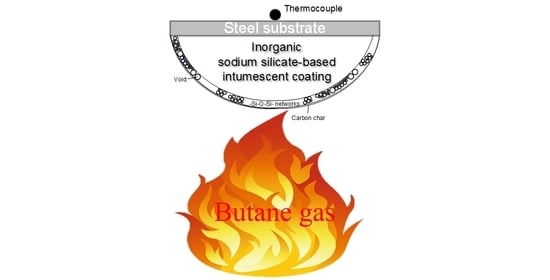
Enhancements on Flame Resistance by Inorganic Silicate-Based Intumescent Coating Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparations of Sodium Silicate Intumescent Flame-Resistance Coating
2.2. Characterization and Measurements
3. Results and Discussion
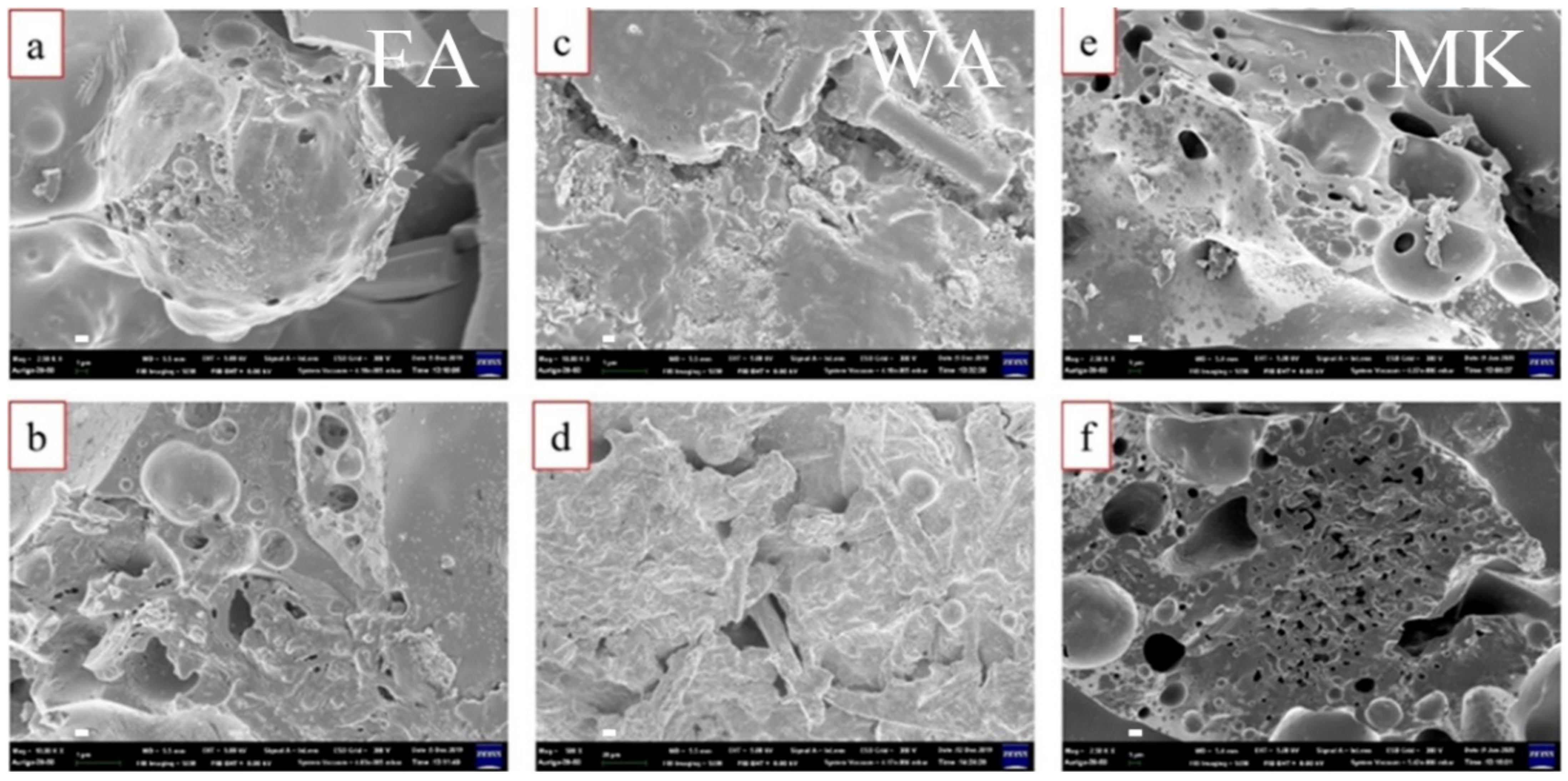
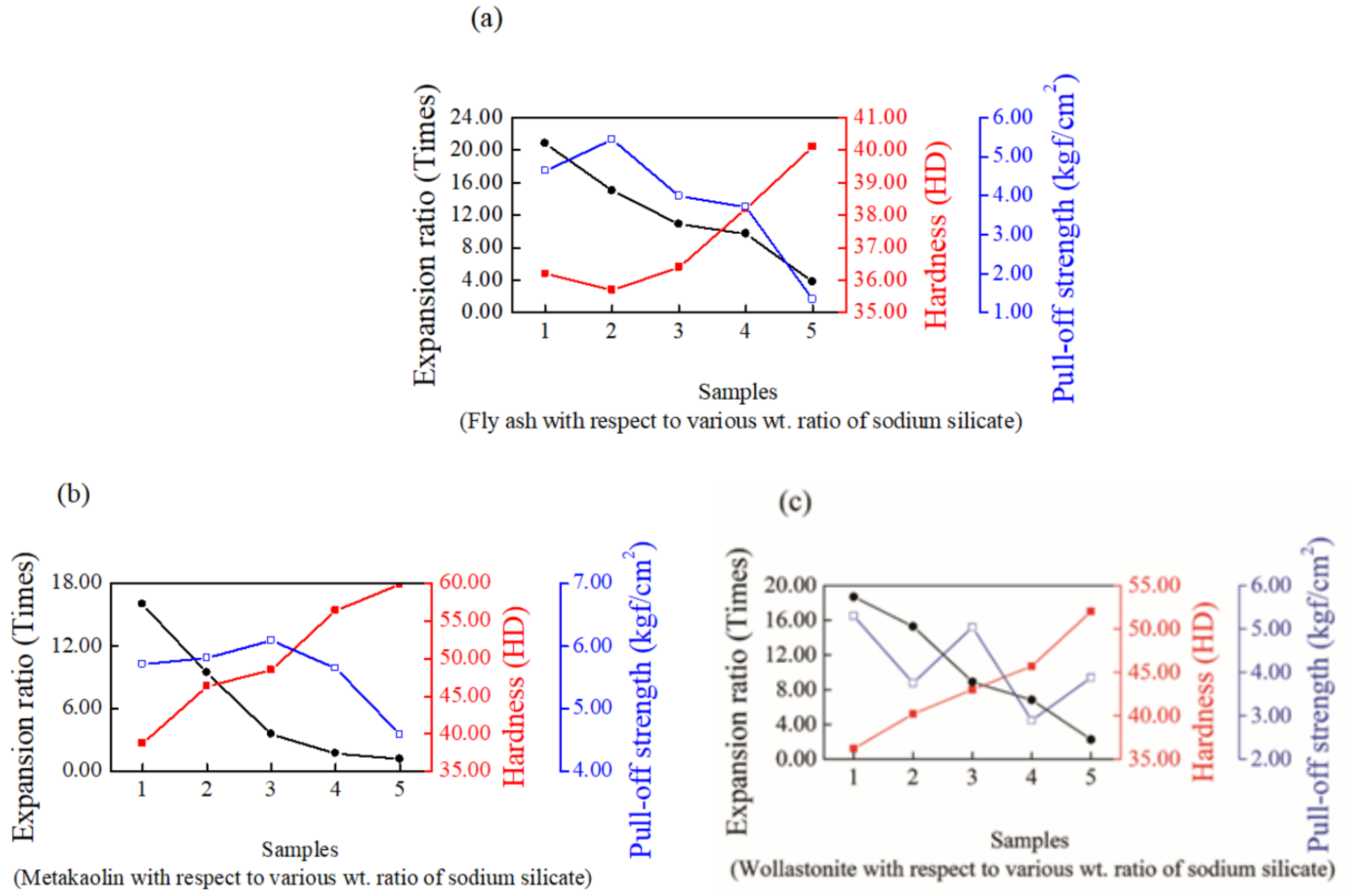
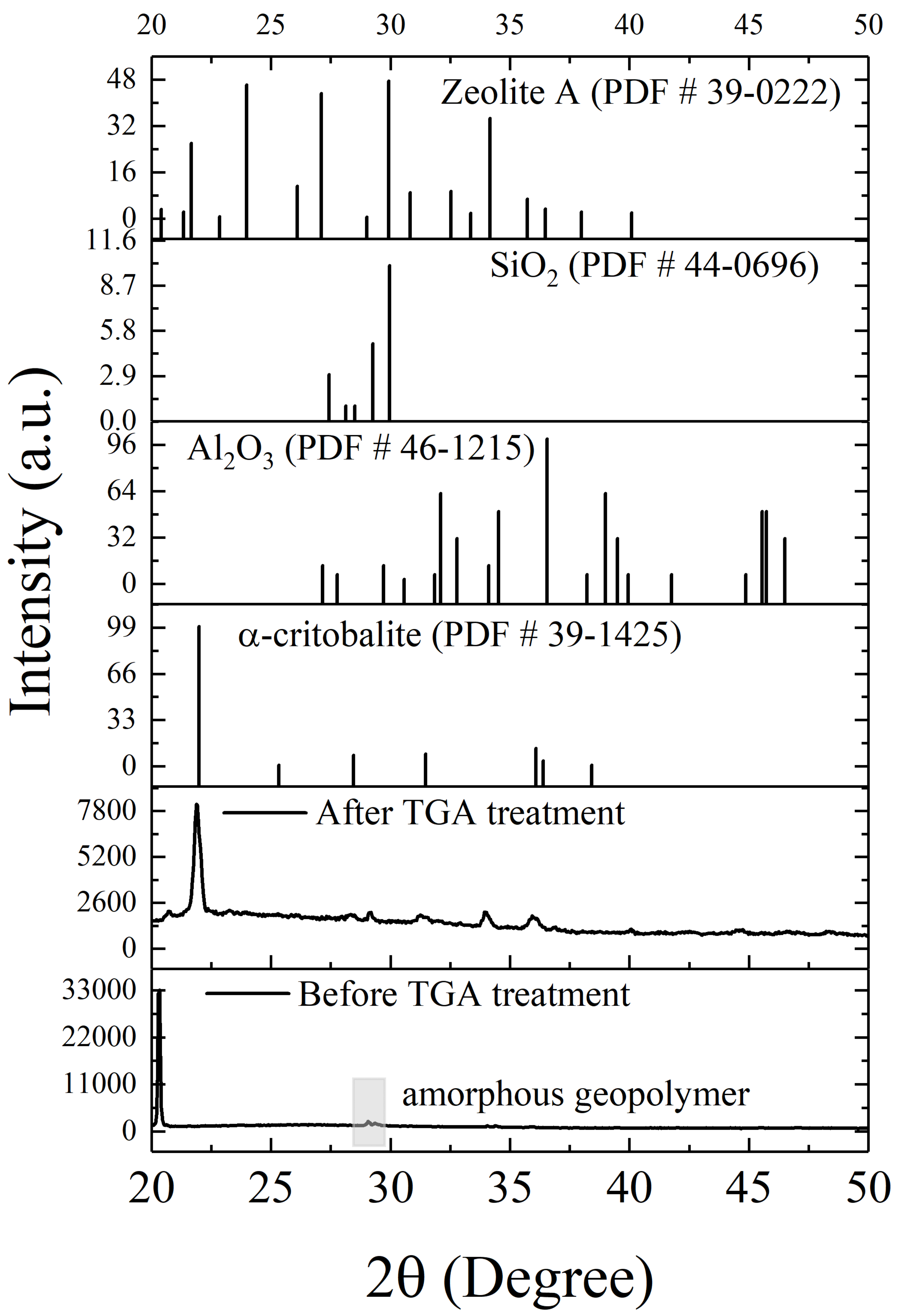
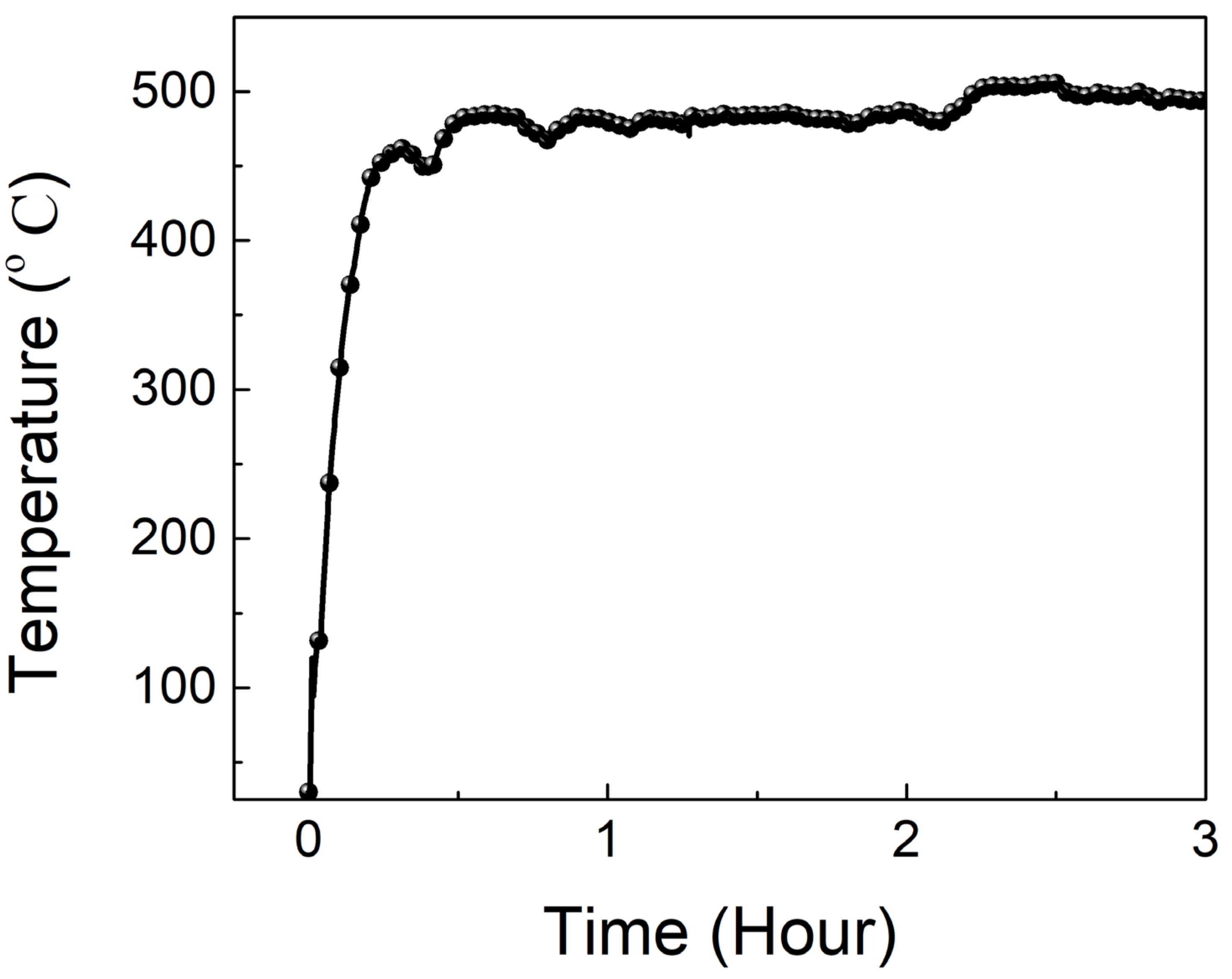
3.1. Properties of Sodium Silicate-Based Intumescent Geopolymer Materials
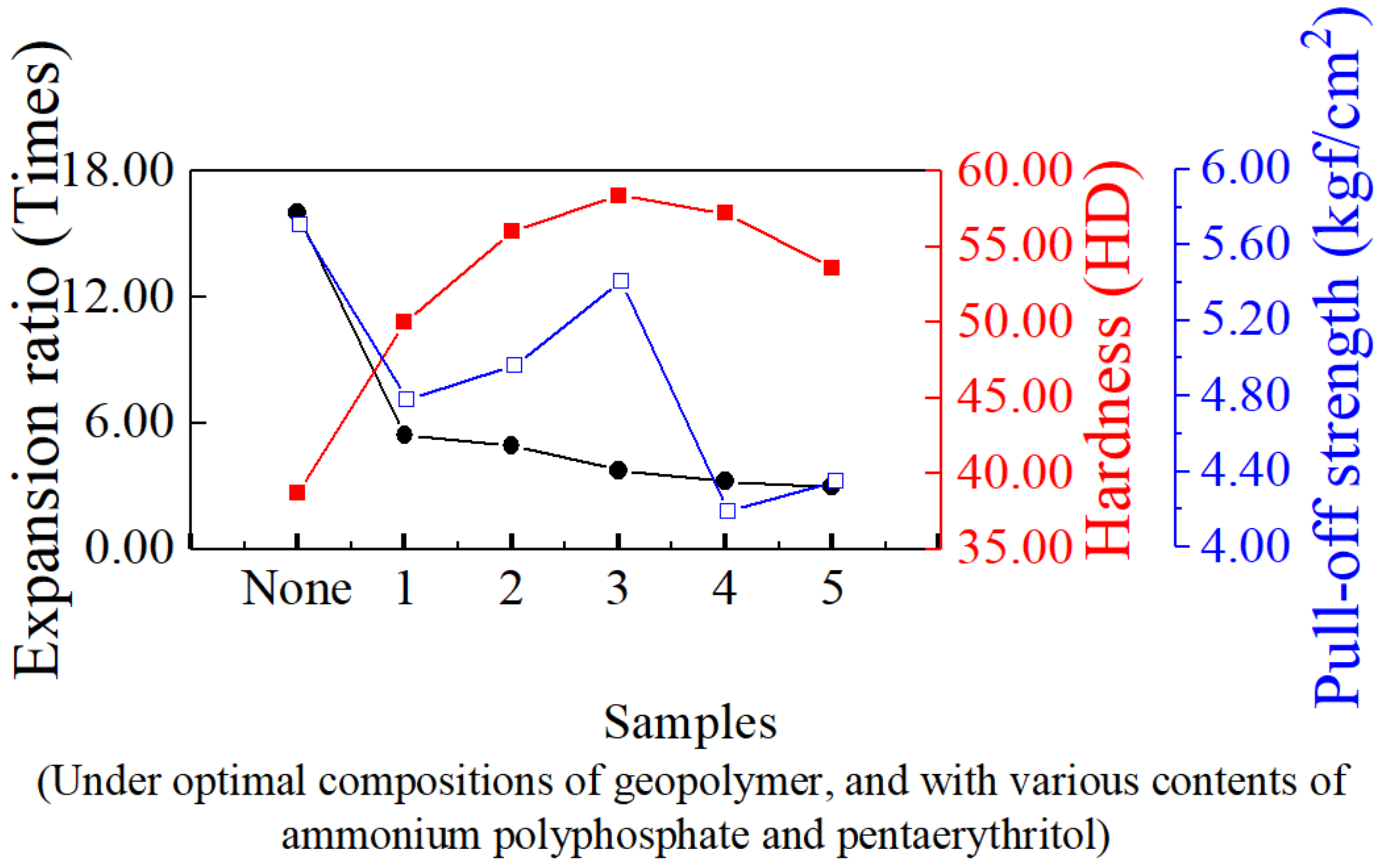
3.2. Effects of Ammonium Polyphosphate and Pentaerythritol on the Physical and Thermal Properties of Intumescent Flame-Resistance Coating Materials
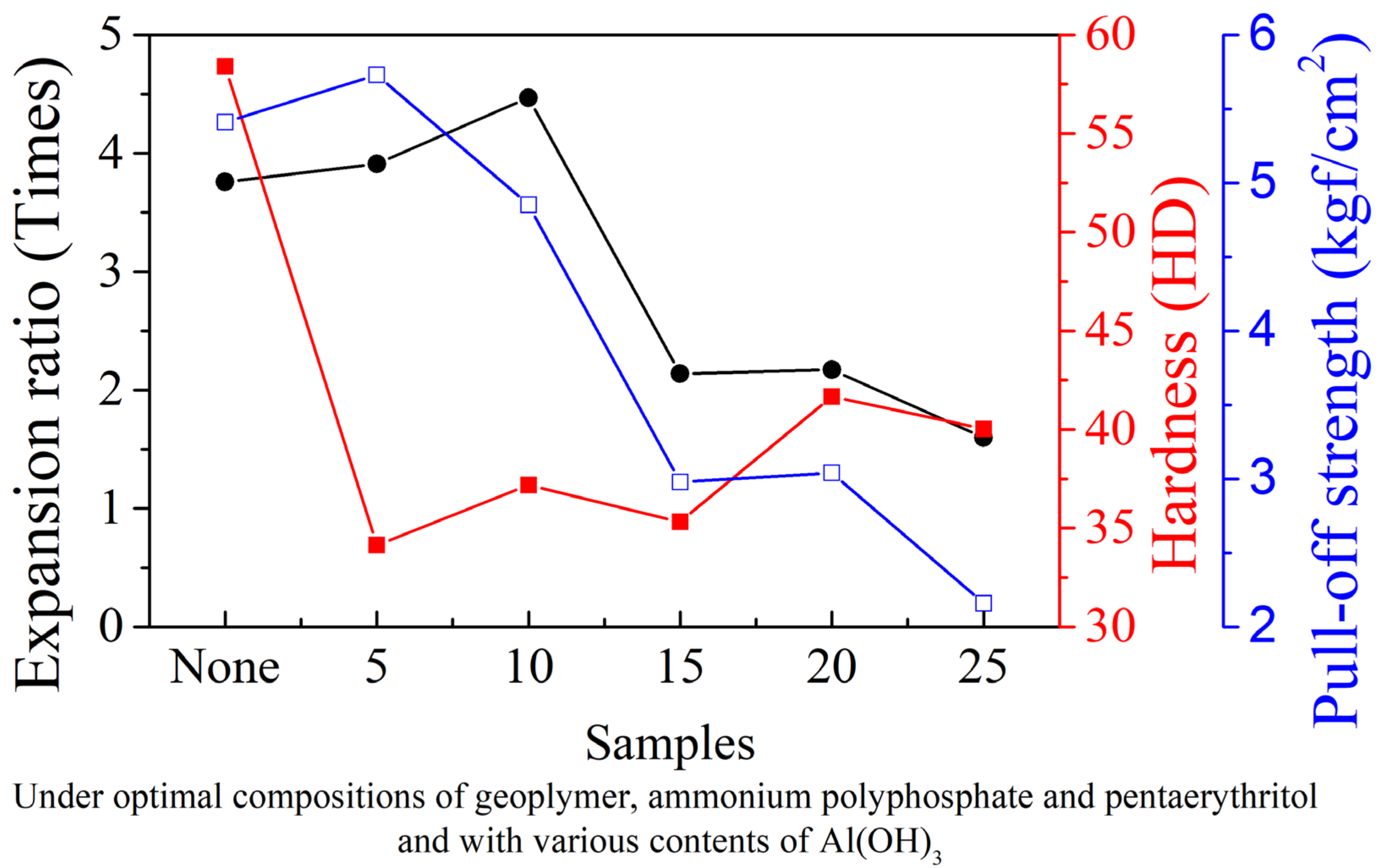
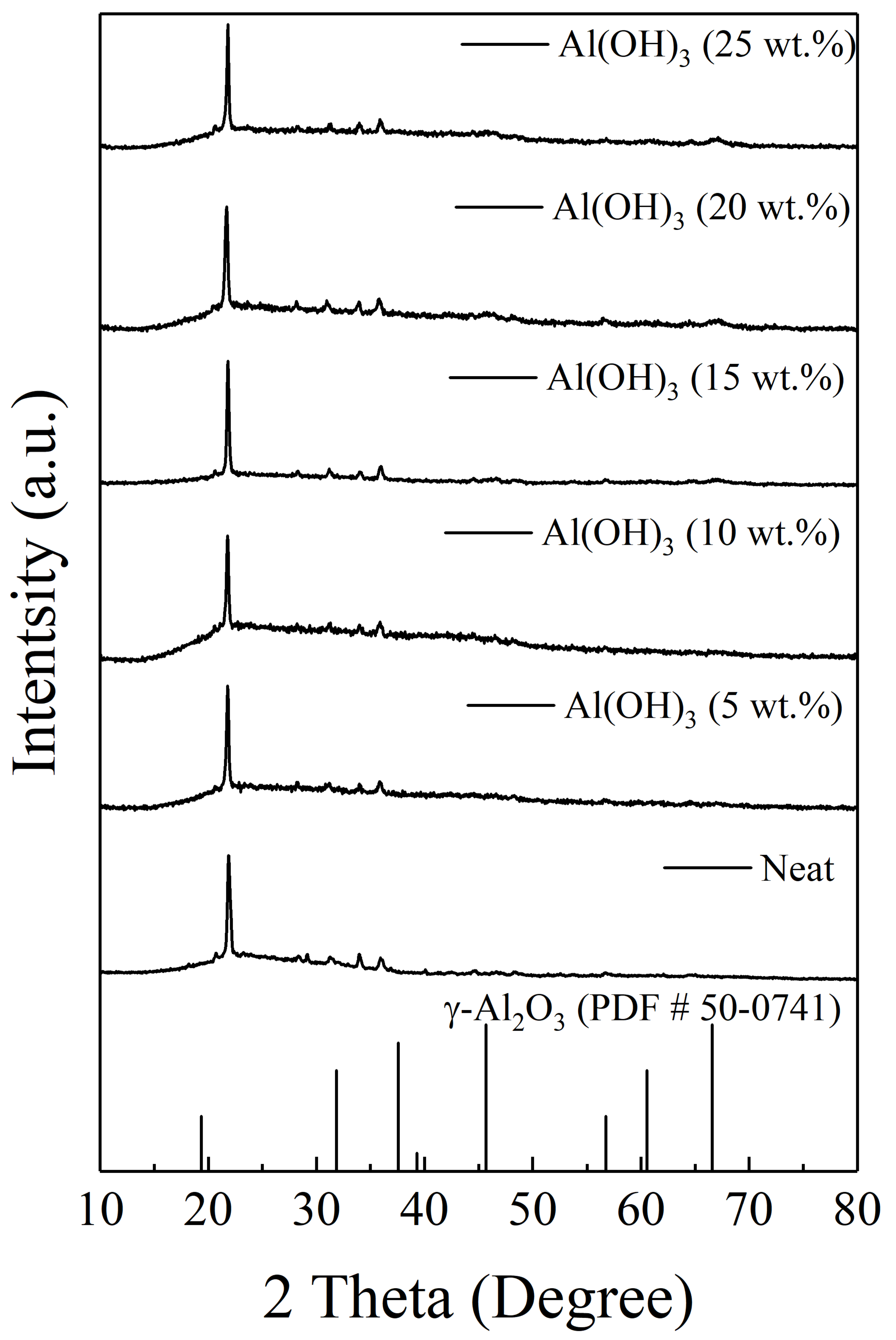
3.3. Effects of Al(OH)3 on the Physical and Thermal Properties of Intumescent Flame-Resistance Coating Materials
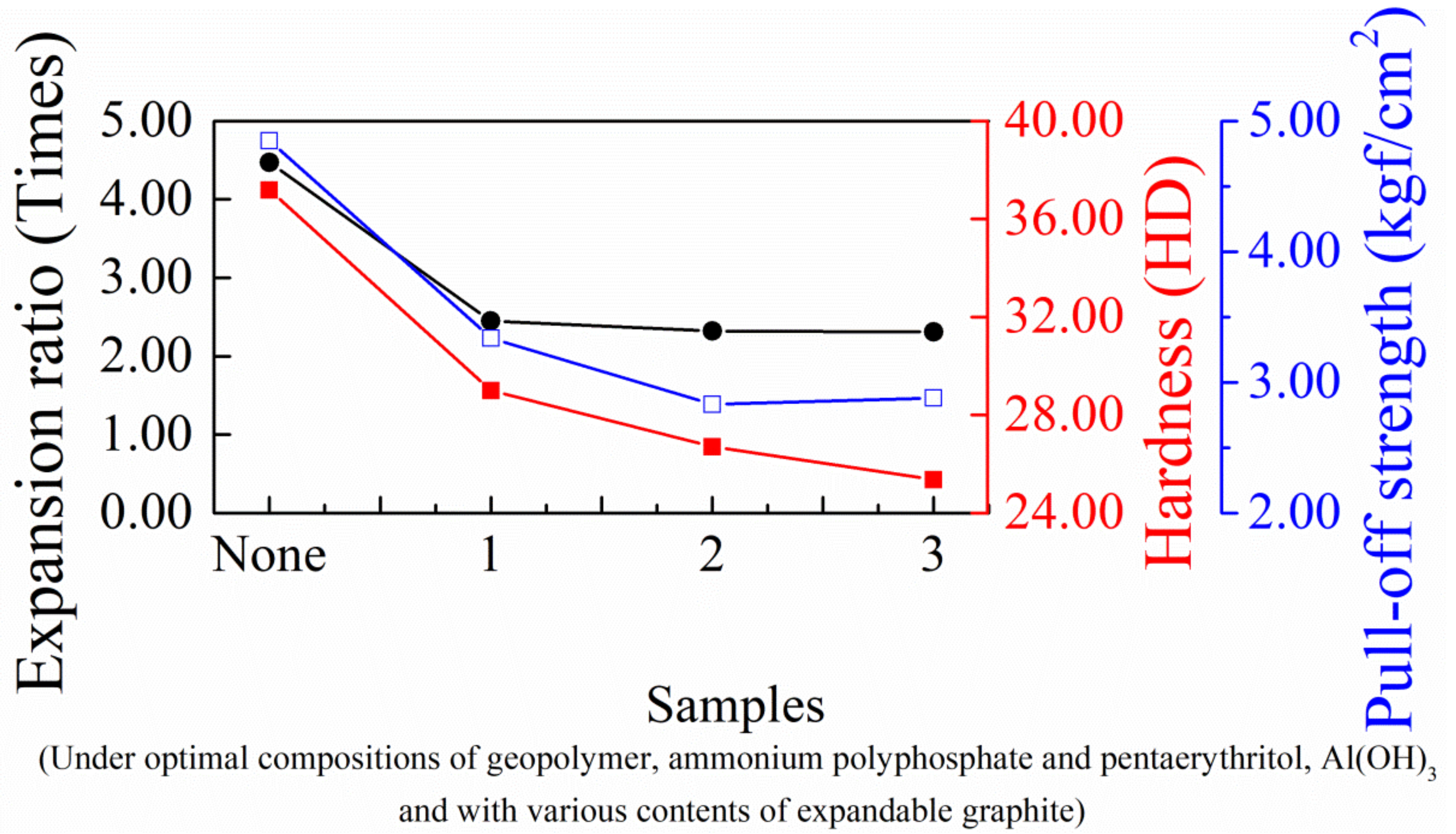
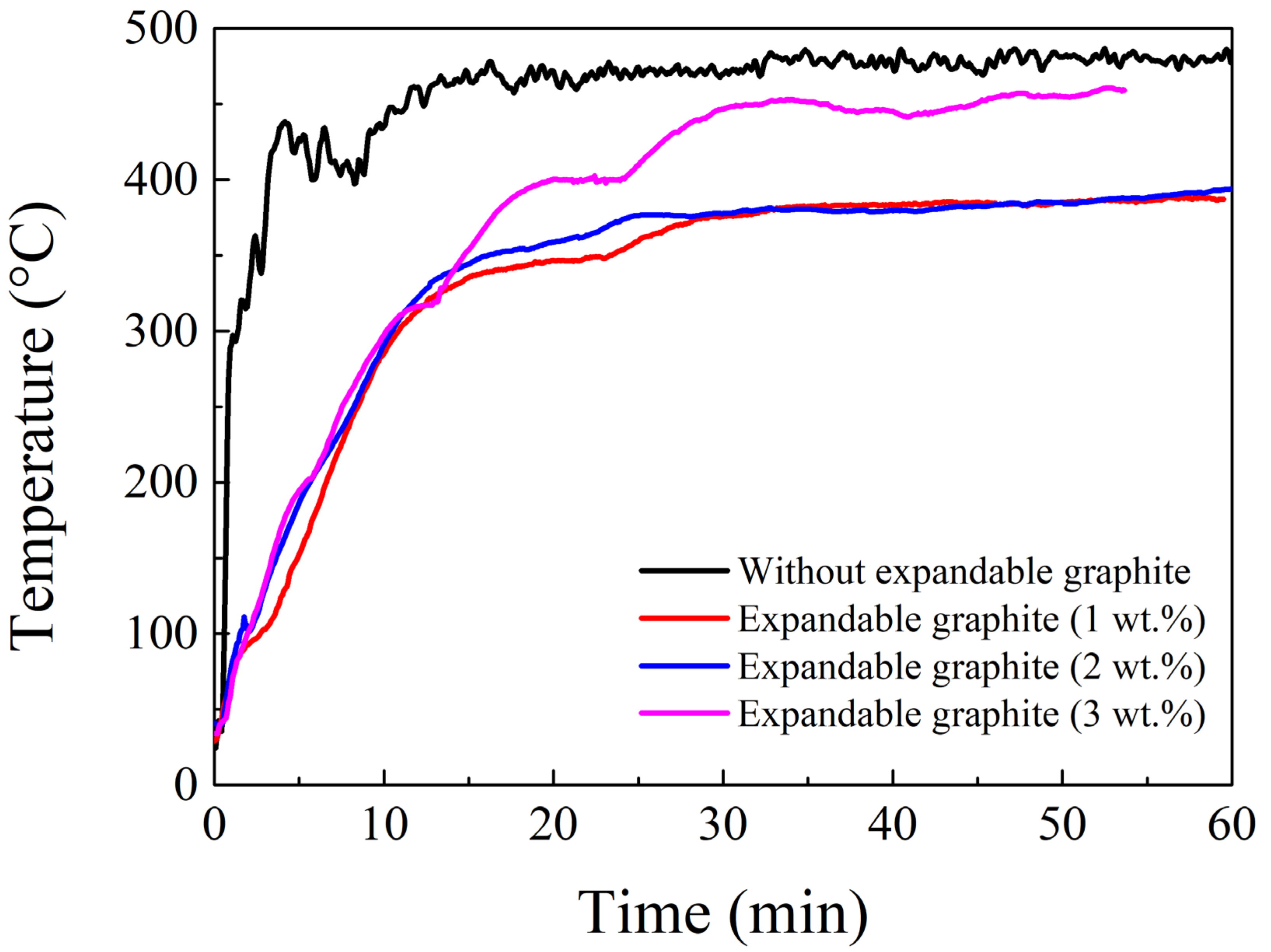
3.4. Effects of Expandable Graphite on the Physical Properties and Flame Testing of Intumescent Flame-Resistant Coating Materials
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Elbasuney, S. Novel multi-component flame retardant system based on nanoscopic aluminium-trihydroxide (ATH). Powder Technol. 2017, 305, 538–545. [Google Scholar] [CrossRef]
- Zhang, P.; Hu, Y.; Song, L.; Ni, J.; Xing, W.; Wang, J. Effect of expanded graphite on properties of high-density polyethylene/paraffin composite with intumescent flame retardant as a shape-stabilized phase change material. Sol. Energy Mater. Sol. Cells 2010, 94, 360–365. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Q.; Shao, D.; Hu, Y.; Song, L.; Gao, W. Magnesium hydroxide and microencapsulated red phosphorus synergistic flame retardant form stable phase change materials based on HDPE/EVA/OMT nanocomposites/paraffin compounds. J. Energy Inst. 2009, 82, 28–36. [Google Scholar] [CrossRef]
- Cai, Y.; Hu, Y.; Song, L.; Lu, H.; Chen, Z.; Fan, W. Preparation and characterizations of HDPE–EVA alloy/OMT nanocomposites/paraffin compounds as a shape stabilized phase change thermal energy storage material. Thermochim. Acta 2006, 451, 44–51. [Google Scholar] [CrossRef]
- Cai, Y.; Wei, Q.; Huang, F.; Lin, S.; Chen, F.; Gao, W. Thermal stability, latent heat and flame retardant properties of the thermal energy storage phase change materials based on paraffin/high density polyethylene composites. Renew. Energy 2009, 34, 2117–2123. [Google Scholar] [CrossRef]
- Cai, Y.B.; Hu, Y.; Song, L.; Kong, Q.H.; Yang, R.; Zhang, Y.P.; Chen, Z.Y.; Fan, W.C. Preparation and flammability of high density polyethylene/paraffin/organophilic montmorillonite hybrids as a form stable phase change material. Energy Convers. Manag. 2007, 48, 462–469. [Google Scholar] [CrossRef]
- Han, J.P.; Liang, G.Z.; Gu, A.J.; Ye, J.H.; Zhang, Z.Y.; Yuan, L. A novel inorganic-organic hybridized intumescent flame retardant and its super flame retarding cyanate ester resins. J. Mater. Chem. A 2013, 1, 2169–2182. [Google Scholar] [CrossRef]
- Puri, R.G.; Khanna, A.S. Intumescent coatings: A review on recent progress. J. Coat. Technol. Res. 2017, 14, 1–20. [Google Scholar] [CrossRef]
- Qian, X.D.; Song, L.; Yuan, B.H.; Yu, B.; Shi, Y.Q.; Hu, Y.; Yuen, R.K. Organic/inorganic flame retardants containing phosphorus, nitrogen and silicon: Preparation and their performance on the flame retardancy of epoxy resins as a novel intumescent flame retardant system. Mater. Chem. Phys. 2014, 143, 1243–1252. [Google Scholar] [CrossRef]
- Weil, E.D. Fire-Protective and Flame-Retardant Coatings—A State-of-the-Art Review. J. Fire Sci. 2011, 29, 259–296. [Google Scholar] [CrossRef]
- Xie, R.C.; Qu, B.J. Expandable graphite systems for halogen-free flame-retarding of polyolefins. I. Flammability characterization and synergistic effect. J. Appl. Polym. Sci. 2001, 80, 1181–1189. [Google Scholar] [CrossRef]
- Li, J.F.; Gao, M.; Zheng, Y.D.; Guan, Y.P.; Yi, D.Q. Effects of Low-Load Boron/Silicon-Based Graphene Oxide on Combustion and Thermal Degradation of Flame-Retardant Unsaturated Polyester Resin. Macromol. Mater. Eng. 2020, 305, 12. [Google Scholar] [CrossRef]
- Moon, H.Y.; Shin, D.G.; Choi, D.S. Evaluation of the durability of mortar and concrete applied with inorganic coating material and surface treatment system. Constr. Build. Mater. 2007, 21, 362–369. [Google Scholar] [CrossRef]
- Iler, R.K. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica; John Wiley & Sons Inc: Hoboken, NJ, USA, 1979; p. 896. [Google Scholar]
- Mulder, C.A. Aerogels, Proceedings of the First International Symposium, Germany, 23–25 September 1985; Fricke, J., Ed.; Springer: Berlin/Heidelberg, Germany, 1986; pp. 68–75. [Google Scholar]
- Uher, C. Thermal Conductivity 25/Thermal Expansion 13; CRC Press: Boca Raton, FL, USA, 2000; p. 391. [Google Scholar]
- Zhang, Z.; Wang, K.; Mo, B.; Li, X.; Cui, X. Preparation and characterization of a reflective and heat insulative coating based on geopolymers. Energy Build. 2015, 87, 220–225. [Google Scholar] [CrossRef]
- Albidah, A.; Abadel, A.; Alrshoudi, F.; Altheeb, A.; Abbas, H.; Al-Salloum, Y. Bond strength between concrete substrate and metakaolin geopolymer repair mortars at ambient and elevated temperatures. J. Mater. Res. Technol. JMRT 2020, 9, 10732–10745. [Google Scholar] [CrossRef]
- Tamburini, S.; Natali, M.; Garbin, E.; Panizza, M.; Favaro, M.; Valluzzi, M.R. Geopolymer matrix for fibre reinforced composites aimed at strengthening masonry structures. Constr. Build. Mater. 2017, 141, 542–552. [Google Scholar] [CrossRef]
- Celik, A.; Yilmaz, K.; Canpolat, O.; Al-Mashhadani, M.M.; Aygormez, Y.; Uysal, M. High-temperature behavior and mechanical characteristics of boron waste additive metakaolin based geopolymer composites reinforced with synthetic fibers. Constr. Build. Mater. 2018, 187, 1190–1203. [Google Scholar] [CrossRef]
- Cheng-Yong, H.; Yun-Ming, L.; Abdullah, M.M.; Hussin, K. Thermal Resistance Variations of Fly Ash Geopolymers: Foaming Responses. Sci. Rep. 2017, 7, 11. [Google Scholar]
- Turker, H.T.; Balcikanli, M.; Durmus, I.H.; Ozbay, E.; Erdemir, M. Microstructural alteration of alkali activated slag mortars depend on exposed high temperature level. Constr. Build. Mater. 2016, 104, 169–180. [Google Scholar] [CrossRef]
- Cheng, T.W.; Chiu, J.P. Fire-resistant geopolymer produced by granulated blast furnace slag. Miner. Eng. 2003, 16, 205–210. [Google Scholar] [CrossRef]
- Watolla, M.B.; Gluth, G.J.; Sturm, P.; Rickard, W.D.; Kruger, S.; Schartel, B. Intumescent Geopolymer-Bound Coatings for Fire Protection of Steel. J. Ceram. Sci. Technol. 2017, 8, 351–364. [Google Scholar]
- Al-Saadi, T.H.; Mahdi, Z.H.; Abdullah, I.T. Foaming Geopolymers Preparation by Alkali Activation of Glass Waste. Rev. Rom. Mat. 2019, 49, 352–360. [Google Scholar]
- Badanoiu, A.I.; Al Saadi, T.H.; Stoleriu, S.; Voicu, G. Preparation and characterization of foamed geopolymers from waste glass and red mud. Constr. Build. Mater. 2015, 84, 284–293. [Google Scholar] [CrossRef]
- Barbosa, V.F.; MacKenzie, K.J. Thermal behaviour of inorganic geopolymers and composites derived from sodium polysialate. Mater. Res. Bull. 2003, 38, 319–331. [Google Scholar] [CrossRef]
- Wu, H.C.; Sun, P. New building materials from fly ash-based lightweight inorganic polymer. Constr. Build. Mater. 2007, 21, 211–217. [Google Scholar] [CrossRef]
- Duan, P.; Yan, C.; Zhou, W.; Luo, W.; Shen, C. An investigation of the microstructure and durability of a fluidized bed fly ash–metakaolin geopolymer after heat and acid exposure. Mater. Des. 2015, 74, 125–137. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, X.; Cao, W.; Gong, L.; Zhang, R.; Zhang, H. Fabrication of adiabatic foam at low temperature with sodium silicate as raw material. Mater. Des. 2015, 88, 1008–1014. [Google Scholar] [CrossRef]
- Glid, M.; Sobrados, I.; Rhaiem, H.B.; Sanz, J.; Amara, A.B. Alkaline activation of metakaolinite-silica mixtures: Role of dissolved silica concentration on the formation of geopolymers. Ceram. Int. 2017, 43, 12641–12650. [Google Scholar] [CrossRef]
- Santos, A.C.; Ortiz-Lozano, J.A.; Villegas, N.; Aguado, A. Experimental study about the effects of granular skeleton distribution on the mechanical properties of self-compacting concrete (SCC). Constr. Build. Mater. 2015, 78, 40–49. [Google Scholar] [CrossRef] [Green Version]
- Kang, F.; Leng, Y.; Zhang, T.Y. Influences of H2O2 on synthesis of H2SO4-GICs. J. Phys. Chem. Solids 1996, 57, 889–892. [Google Scholar] [CrossRef]
- Chen, G.; Weng, W.; Wu, D.; Wu, C.; Lu, J.; Wang, P.; Chen, X. Preparation and characterization of graphite nanosheets from ultrasonic powdering technique. Carbon 2004, 42, 753–759. [Google Scholar] [CrossRef]
- Olson, L.L.; O’melia, C.R. The interactions of Fe(III) with Si(OH)4. J. Inorg. Nucl. Chem. 1973, 35, 1977–1985. [Google Scholar] [CrossRef]
- Fang, K.; Chen, Y.F.; Zhang, S.C.; Sun, H.R.; Sun, X.K.; Yan, D.C.; Tao, L.S. The Study of Heating Mode of an Expansion Insulation Material. Solid State Phenom. 2018, 281, 934–939. [Google Scholar] [CrossRef]
- Gardelle, B.; Duquesne, S.; Vandereecken, P.; Bourbigot, S. Characterization of the carbonization process of expandable graphite/silicone formulations in a simulated fire. Polym. Degrad. Stab. 2013, 98, 1052–1063. [Google Scholar] [CrossRef]
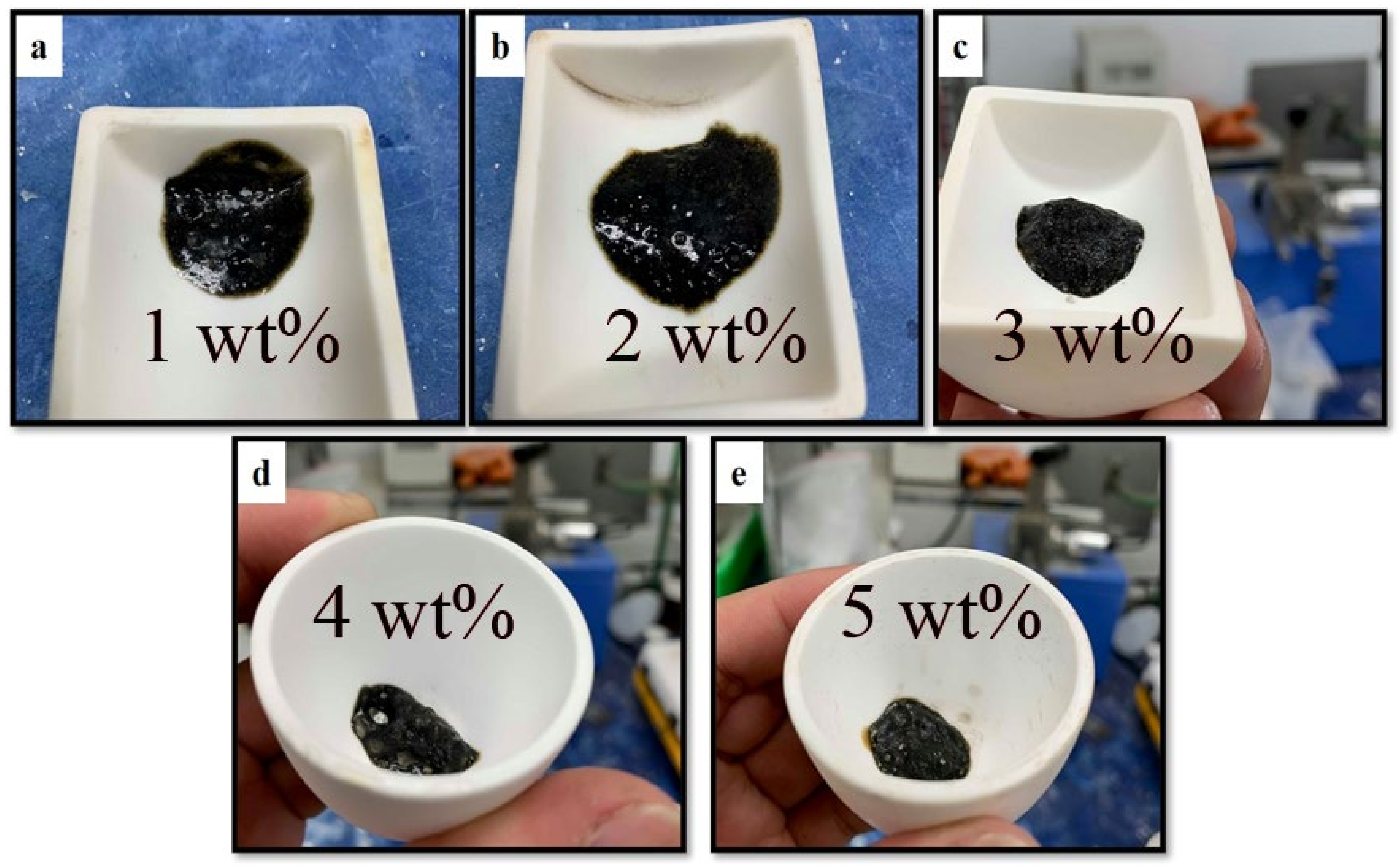
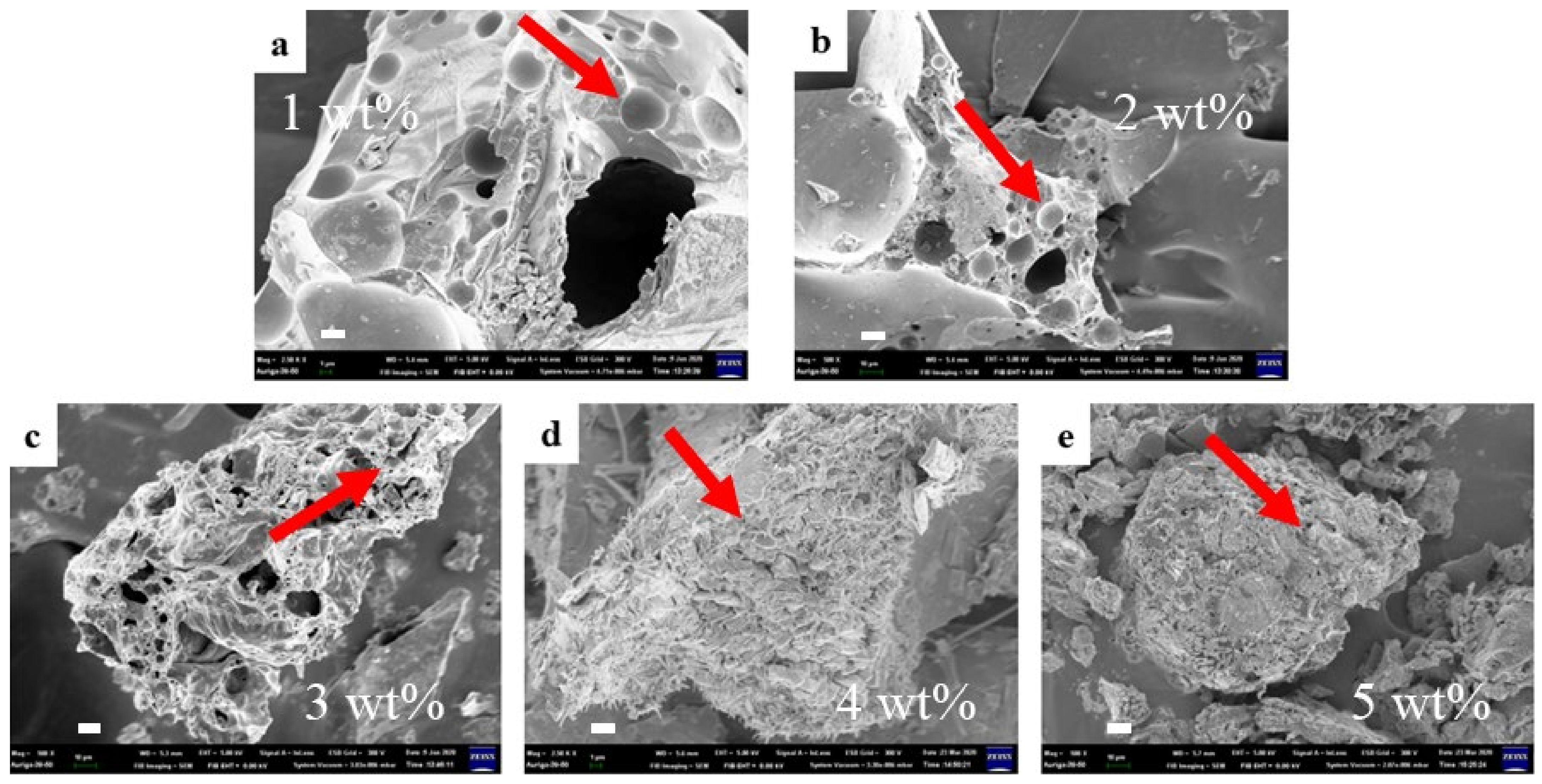


| Sample | L1 (mm) | L2 (mm) | Expansion Rate (Times) |
|---|---|---|---|
| Neat | 5.0 | 18.1 | 3.6 |
| expandable graphite (1 wt.%) | 5.0 | 23.5 | 4.7 |
| expandable graphite (2 wt.%) | 5.0 | 25.2 | 5.1 |
| expandable graphite (3 wt.%) | 5.0 | 21.9 | 4.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, S.-N.; Li, P.-K.; Hsieh, T.-H.; Ho, K.-S.; Hong, Y.-M. Enhancements on Flame Resistance by Inorganic Silicate-Based Intumescent Coating Materials. Materials 2021, 14, 6628. https://doi.org/10.3390/ma14216628
Chen S-N, Li P-K, Hsieh T-H, Ho K-S, Hong Y-M. Enhancements on Flame Resistance by Inorganic Silicate-Based Intumescent Coating Materials. Materials. 2021; 14(21):6628. https://doi.org/10.3390/ma14216628
Chicago/Turabian StyleChen, Sin-Nan, Pei-Kai Li, Tar-Hwa Hsieh, Ko-Shan Ho, and Yu-Meng Hong. 2021. "Enhancements on Flame Resistance by Inorganic Silicate-Based Intumescent Coating Materials" Materials 14, no. 21: 6628. https://doi.org/10.3390/ma14216628
APA StyleChen, S.-N., Li, P.-K., Hsieh, T.-H., Ho, K.-S., & Hong, Y.-M. (2021). Enhancements on Flame Resistance by Inorganic Silicate-Based Intumescent Coating Materials. Materials, 14(21), 6628. https://doi.org/10.3390/ma14216628